Abstract
Alveolar epithelial cell (AEC) senescence is a key driver of a variety of chronic lung diseases. It remains a challenge how to alleviate AEC senescence and mitigate disease progression. Our study identified a critical role of epoxyeicosatrienoic acids (EETs), downstream metabolites of arachidonic acid (ARA) by cytochrome p450 (CYP), in alleviating AEC senescence. In vitro, we found that 14,15-EET content was significantly decreased in senescent AECs. Exogenous EETs supplementation, overexpression of CYP2J2, or inhibition of EETs degrading enzyme soluble epoxide hydrolase (sEH) to increase EETs alleviated AECs' senescence. Mechanistically, 14,15-EET promoted the expression of Trim25 to ubiquitinate and degrade Keap1 and promoted Nrf2 to enter the nucleus to exert an anti-oxidant effect, thereby inhibiting endoplasmic reticulum stress (ERS) and alleviating AEC senescence. Furthermore, in D-galactose (D-gal)-induced premature aging mouse model, inhibiting the degradation of EETs by Trifluoromethoxyphenyl propionylpiperidin urea (TPPU, an inhibitor of sEH) significantly inhibited the protein expression of p16, p21, and γH2AX. Meanwhile, TPPU reduced the degree of age-related pulmonary fibrosis in mice. Our study has confirmed that EETs are novel anti-senescence substances for AECs, providing new targets for the treatment of chronic lung diseases.
Keywords: Epoxyeicosatrienoic acids, Alveolar epithelial cells, Senescence, Endoplasmic reticulum stress, Tripartite motif-containing 25
Graphical abstract
Highlights
-
•
EETs alleviate alveolar epithelial cell senescence.
-
•
14,15-EET inhibits endoplasmic reticulum stress through Trim 25/Keap1/Nrf2.
-
•
Inhibiting the degradation of EETs alleviates pulmonary fibrosis in aging mice.
Abbreviations
- ATF6
activating transcription factor 6
- AECs
alveolar epithelial cells
- ARA
arachidonic acid
- CYP
cytochrome P450
- BLM
bleomycin
- D-gal
D-galactose
- EETs
epoxyeicosatrienoic acids
- EPM
elevated plus maze
- ER
endoplasmic reticulum
- ERS
endoplasmic reticulum stress
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- IPF
idiopathic pulmonary fibrosis
- Keap1
kelch-like ECH-associated protein 1
- Nrf2
nuclear factor E2-related factor 2
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- sEH
soluble epoxide hydrolase
- TPPU
trifluoromethoxyphenyl propionylpiperidin urea
- Trim25
Tripartite motif-containing 25
- UPR
unfolded protein response
- β-gal
β-galactosidase
1. Introduction
According to World Health Organization, the number of people over 65 worldwide will exceed 2 billion by 2050 [1]. This demographic shift has led to a sharp rise in diseases such as cancer and lung disease. There is growing evidence that intervention in biological processes that worsen with age can ameliorate or delay many age-related diseases, such as cancer, cardiovascular disease, pulmonary conditions, and dementia [2]. Nowadays, research on the mechanism of tissue and organ aging is a global hotspot. Cell senescence is the core event of tissue and organ aging [3]. Cell senescence is defined as the permanent cycle arrest and dysfunction. Senescent cells can modify the cellular microenvironment through the secretion of a plethora of biologically active products, referred to as the senescence-associated secretory phenotype (SASP). SASP includes pro-inflammatory factors, chemokines, matrix metalloproteinases, and growth factors [4]. Cell senescence not only participates in body aging but also accelerates the process of many diseases, such as atherosclerosis [5], neurodegeneration [6], and chronic lung diseases [7].
Alveolar epithelial cells (AECs) are the prominent cells to maintain the stability of lung function [8] and are also the primary target cells of lung aging. The senescence of AECs has been recognized as a common pathological mechanism in many chronic lung diseases. Single-cell transcriptome analysis has revealed extensive activation of cellular senescence pathways in AECs of idiopathic pulmonary fibrosis (IPF) patients [9]. Eliminating senescent cells inhibits pulmonary fibrosis [10]. Therefore, targeting senescent AECs is essential for the treatment of age-related chronic lung diseases. However, effective strategies to alleviate AEC senescence are still not fully understood.
Epoxyeicosatrienoic acids (EETs), metabolites of arachidonic acid (ARA) mainly through cytochrome P450 2C/2J (CYP2C/2J) metabolic pathway, have many biological activities such as vasodilation, anti-inflammation, and anti-fibrosis [[11], [12], [13]]. While EETs are susceptible to rapid degradation by soluble epoxide hydrolase (sEH) [14]. Research has shown that overexpression of CYP2J2 to increase the production of EETs could reduce the protein expression of p53 and p16 and the number of β-galactosidase (β-gal) stained senescent cells in hydrogen peroxide (H2O2)-treated endothelial cells [15]. In our previous study, we found that inhibition of EETs degradation could attenuate the expression of p53 and p21 in the lungs of bleomycin (BLM)-induced pulmonary fibrosis mice [16]. Therefore, we speculate that EETs may be a critical endogenous anti-cell senescence molecule.
Endoplasmic reticulum stress (ERS)/unfolded protein response (UPR) activation has been implicated in many human diseases, including age-related lung diseases [17]. Impaired ER function leads to the accumulation of misfolded proteins, triggering the activation of the UPR [18]. UPR initially protects cells. However, excessive and/or prolonged ERS results in cell senescence [19]. UPR pathways include the inositol-requiring enzyme 1 pathway, protein kinase-like ER kinase pathway, and activating transcription factor 6 (ATF6) pathway [18]. The study found that ERS is common in the lungs of IPF patients and mainly occurs in AECs [20]. A recent study has shown that ERS is involved in AEC senescence in pulmonary fibrosis mice [21]. In addition, it has been found that EETs could alleviate heart failure in rats by inhibiting ERS [22]. Together, these exciting findings lead us to hypothesize that EETs may have the potential to alleviate cell senescence in AECs by inhibiting ERS.
There is a causal relationship between oxidative stress and ERS [23]. Excessive reactive oxygen species (ROS) interfere with the normal redox balance of the ER, causes ERS, and causes UPR [24]. However, persistent ERS and excess oxidative stress interact and intensify in a positive feedback manner [24]. New evidence suggests that ROS-induced ERS triggers cell senescence [25]. Kelch-like ECH-associated protein 1/Nuclear factor E2-related factor 2 (Keap1/Nrf2) is recognized as the primary signaling pathway of cellular resistance to oxidative stress. Under normal circumstances, Keap1 is bound to Nrf2 in the cytoplasm. Upon oxidative stress, Nrf2 is released from Keap1 and transferred to the nucleus to activate downstream gene expression, including heme oxygenase-1 (HO-1), thus playing an anti-oxidant effect [26]. Studies have shown that increased expression of Nrf2 inhibits ERS [27,28]. Abnormal Nrf2 signaling has been found in a variety of age-related diseases, including pulmonary fibrosis [29].
Tripartite motif-containing (Trim) family members play an essential role in various diseases, including innate immunity, neurodegenerative diseases, and tumorigenesis, mainly by controlling the quality of the proteins [30,31]. Most members of the Trim family have E3 ubiquitin ligase activity. It has been reported that Trim11 effectively eliminates misfolded proteins in the nucleus and cytoplasm and promotes the development of tumors [32]. Trim19 removes misfolded nuclear proteins through sequential SUMOylation and ubiquitination [33]. Trim25 inhibits ERS through Nrf2 and promotes the survival of liver cancer cells [34]. This indicates that the Trims family has the potential to inhibit ERS. While the changes in Trims during the senescence of AECs are unclear, whether ERS is related to Trims needs further exploration.
In this study, we found that EETs content was reduced in senescent AECs while increasing EETs alleviated the senescence of AECs. Mechanically, EETs degraded Keap1 through Trim25 ubiquitination and promoted Nrf2 nuclear translocation to play an anti-oxidant role, thus inhibiting ERS and alleviating AEC senescence. We also found that inhibition of EETs degradation effectively reduced the sensitivity of aged mice to pulmonary fibrosis. Our study suggests that targeting EETs metabolism in AECs is an effective strategy for treating age-related chronic lung disease.
2. Materials and methods
2.1. Animal experiments
The male C57BL/6J mice (8–10 weeks of age, 20–25 g) were provided by Hunan SJA Laboratory Animal Co., Ltd (Hunan, China). And all animal protocols were approved by the Ethics Committee of the Basic Medical School of Central South University (2019–0901).
2.2. Senescent mouse model
C57BL/6 J mice were randomly divided into the Control, D-galactose (D-gal), and D-gal + TPPU groups (TPPU: a specific inhibitor of sEH). Mice in the D-gal and D-gal + TPPU were injected with D-gal (150 mg/kg, D-gal dissolved in saline solution, Sigma‒Aldrich, USA) subcutaneously in the neck and back daily [35,36] and intraperitoneally into TPPU (1 mg/kg, TPPU dissolved in 0.1% ethanol, purity ≥98% HPLC, Sigma-Aldrich) daily. Six weeks later, the mice were anesthetized by intraperitoneal injection of pentobarbital sodium (80 mg/kg) for subsequent experiments.
2.3. Pulmonary fibrosis mouse model
C57BL/6J mice were randomly divided into the BLM, D-gal + BLM, and D-gal + TPPU + BLM groups. Based on the senescent mouse model, mice were intratracheally instilled with BLM (0.5 mg/kg, in 50 μL saline, Nippon Kayaku, Tokyo, Japan). Three weeks later, the mice were anesthetized by intraperitoneal injection of pentobarbital sodium (80 mg/kg) for subsequent experiments.
2.4. Lung histopathology analysis
The right upper lung tissue was used for routine paraffin embedding. Paraffin sections were prepared for HE and Masson staining according to our previous studies [[37], [38], [39]]. The pictures were detected by a microscope (Motic, BA410E, Motic China group CO., LTD. China).
2.5. Elevated plus maze
The elevated plus maze (EPM) consists of two open arms and two closed arms, which cross each other vertically. The arm width is 5 cm, the arm length is 35 cm, the closed arm height is 15 cm, and the height of the maze is about 40 cm above the ground. The experiment was carried out in an environment familiar to the mice. The mice were gently placed in the central area of the instrument, facing the open arm, and the activity of the mice was recorded within 5 min. After testing each mouse, the EPM was washed with a 10% ethanol solution to avoid animal odors or residues interfering with subsequent testing [40].
2.6. Cell culture and treatment
Mouse alveolar epithelial cell lines (MLE12) were purchased from ATCC (USA, CRL-2110). A549 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). MLE12 cells were cultured in DMEM/F12 medium (containing 2% fetal bovine serum, Gibco, USA). A549 cells were cultured in RPMI 1640 medium (containing 10% fetal bovine serum, Gibco). Cells were maintained at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Cells were planted into the 12-well plate (1 × 105 cells/well) and cultured for 24 h until the cell density reached 60%. Then cells were stimulated with BLM (0.01, 0.033, or 0.1 U/mL, Aladdin, Shanghai, China) or H2O2 (0, 100, 250, or 500 μM, Sigma-Aldrich) or Doxorubicin (0, 100, 200, and 400 nM, Aladdin) for 2 h, then washed with PBS twice and further grown with fresh medium for 46 h.
To elevate the EETs content in MLE12 cells, we constructed an adenovirus-CYP2J2 (Lot: VH881244, biosciences, USA). Cells were infected with adenovirus-CYP2J2 (1 × 109 vp/mL) or vector (1 × 109 vp/mL). The cells were washed with fresh complete medium 24 h later and then cultivated for 48 h. Or the sEH inhibitor TPPU (20 μM, SML0750, Sigma-Aldrich) was added 1 h before BLM stimulation. Or exogenous EETs (2.5 μM, Cayman, USA) were added 10 min before BLM stimulation.
To manipulate the Trim25 in MLE12 cells, the lentivirus-mediated sh-Trim25 vector (Lot: 1348BE5) was designed and synthesized by GeneChem (Shanghai, China). MLE12 cells were transfected simultaneously with shTrim25 lentivirus (MOI: 50) and negative control (shCtrl lentivirus, MOI: 50) in the presence of 1 × HitransG P (Lot: REVG003, GeneChem). The cells were washed with fresh complete medium 16 h later and then cultivated for an additional 80 h.
To evaluate the role of ERS in EETs alleviating MLE12 cell senescence, we treated cells with ERS inducer tunicamycin (TM, 0.25 μg/mL, Cat. No.: HY-100523, MedChemExpress, USA) 24 h after the BLM stimulation.
To evaluate the role of Nrf2 in EETs alleviating MLE12 cell senescence, we treated cells with Nrf2 inhibitor ML385 (5 μM, Cat. No.: HY-100523, MedChemExpress, USA) 1 h before the BLM stimulation.
2.7. Senescence-associated β-galactosidase staining
Senescence-associated β-galactosidase (SA-β-gal) staining kit was from Beyotime Biotechnology (Shanghai, China). Cells were fixed at room temperature for 15 min by adding 0.6 mL of fixation buffer. The staining mixture was added and incubated overnight at 37 °C. The next day, the cells were washed with PBS, and the staining results were observed under a microscope. SA-β-Gal-positive cells (bluish-green color) were counted in three random microscopic fields and expressed as % of total cells.
2.8. Western blot
Lung tissue or cells were added to RIPA lysate (containing protease inhibitors, Solarbio, Beijing, China). The samples were fully lysed at 4 °C for 30 min and centrifuged at 12,000 g for 10 min, and the total protein concentration was determined by the bicinchoninic acid assay (BCA) method. After denaturation at 95 °C for 10 min, 30 μg of protein was added to SDS-PAGE gel for electrophoresis. The protein was transferred to the polyvinylidene fluoride (PVDF) membranes and blocked with 5% skim milk or bull serum albumin (BSA) for 1 h. The primary antibody was incubated overnight at 4 °C. The next day, the membrane was washed with TBST, and the secondary antibody was incubated for 1 h at room temperature. After washing the PVDF membrane with TBST, ECL luminescent solution was added and photographed. Image Lab software was used for statistical analysis. Antibodies used in this study are shown in Table 1.
Table 1.
Antibodies were used in this study.
| Antibodies | Source | Catalog |
|---|---|---|
| Anti-sEH polyclonal antibody | Abcam | ab155280 |
| Anti-CYP2J2 polyclonal antibody | Abcam | Ab151996 |
| Anti- p16 polyclonal antibody | Abcam | ab211542 |
| Anti- p21 polyclonal antibody | Servicebio | GB11153 |
| Anti-p53 polyclonal antibody | Proteintech | 10442-1-AP |
| Anti-γH2AX polyclonal antibody | Boster | BM4841 |
| Anti-ATF6 polyclonal antibody | Proteintech | 24169-1-AP |
| Anti-XBP1s polyclonal antibody | Abcam | Ab220783 |
| Anti-EIF2α polyclonal antibody | Proteintech | 11233-1-AP |
| Anti-p-EIF2α (ser51) polyclonal antibody | CST | 3398 |
| Anti-BAX polyclonal antibody | CST | 2772 |
| Anti-Nrf2 polyclonal antibody | CST | 12,721 |
| Anti–HO–1 polyclonal antibody | Abcam | ab52947 |
| Anti-Keap1 polyclonal antibody | Proteintech | 10503-2-AP |
| Anti-Trim25 polyclonal antibody | Proteintech | 12573-1-AP |
| Anti- Ubiquitin polyclonal antibody | Abclonal | A0162 |
| Anti-Collagen I polyclonal antibody | Abclonal | A21059 |
| Anti-α-SMA polyclonal antibody | CST | #19245 |
| Anti-NLRP3 polyclonal antibody | CST | #15101 |
| Anti-Ki67 polyclonal antibody | Abways | CY5542 |
| Anti-PCNA polyclonal antibody | Proteintech | 10205-2-AP |
| Anti- Histone H3 polyclonal antibody | Servicebio | GB11102 |
| Anti-α-Tubulin polyclonal antibody | Servicebio | GB11200 |
2.9. Real-time PCR
Total RNA was extracted from mouse lung tissues and cells using the RNAiso Plus kit (Takara, Kusatsu, Japan) and reverse transcribed into cDNA. RNA concentration and quality assessed by spectrophotometry (Thermo Fisher Scientific, Waltham, MA, USA). Real-time PCR was performed in a CFX96 Touch™ instrument. Relative expression of genes was calculated by the 2−ΔΔCT method according to our previous study [41]. The profile of Cyps in MLE12 cells was calculated using the 2−ΔCT method. Trims and SASP mRNA were calculated in log2. Primer sequences are shown in Table 2.
Table 2.
Sequences of specific primers were used in this study.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| m-p16 | CTCTGCTCTTGGGATTGGC | GTGCGATATTTGCGTTCCG |
| m-p19 | GAGGCCGGCAAATGATCATAGA | GTGGATACCGGTGGACTGTG |
| m-p21 | GTGAGGAGGAGCATGAATGGA | GAACAGGTCGGACATCACCA |
| m-Cyp2j5 | TGATGGGTTCATCAGCAGGC | CTTGGCTCATCTGGGTTCCAAT |
| m-Cyp2j6 | GGTGCCCTTGTTGTTAGCAC | GGCTAACAAGGAGCCGGTAG |
| m-Cyp2j9 | AGTCAGTCACCGCCTTTGTG | GTCTCATTGCACGCACTCTC |
| m-Cyp2c29 | CCATGGTTGCAGGTAAACCACAT | TCTGTCCCTGCACCAAAGAG |
| m-Cyp2c44 | CAAGGTACCCCGAGTGAAGAA | CACGGCATCTGTATAGGGCA |
| m-Tnf-α | ACCCTCACACTCACAAACCA | ACAAGGTACAACCCATCGGC |
| m-Il-1β | CAGGCAGGCAGTATCACTCA | AGCTCATATGGGTCCGACAG |
| m-Il-8 | ACGTGTTCCAGGACACAACA | CAAACCCTCCCCACCTAACT |
| m-Mcp1 | GTCCCTGTCATGCTTCTGG | GCGTTAACTGCATCTGGCT |
| m-Mmp12 | CTGCTCCCATGAATGACAGTG | AGTTGCTTCTAGCCCAAAGAAC |
| m-Atf6 | GACTCACCCATCCGAGTTGTG | CTCCCAGTCTTCATCTGGTCC |
| m-Keap1 | TCGAAGGCATCCACCCTAAG | CTCGAACCACGCTGTCAATCT |
| m-Nlrp3 | TACGGCCGTCTACGTCTTCT | CGCAGATCACACTCCTCAAA |
| m-Trim1 | AGTTGTTTGAAGACCCCCTTCT | TGTAGGACACTGGAAAGCAGTAA |
| m-Trim2 | TGGACAGTTCAAAAGTCGTTTCG | AATGCTAACCCACTTGTTGTCAT |
| m-Trim3 | GCGTCTCAGCCCTACAAAACA | AAACTCCATTGTCTTGCCTTCA |
| m-Trim5 | AAGAAAGTTCCGAGCCCCTG | GTAGCGTTGAGCCTCTGTGA |
| m-Trim6 | ATGACTTCAACAGTCTTGGTGG | TTCCCAGGCTGATAGGAGGTC |
| m-Trim7 | ACAGAAACAGAATGAGAACCTGG | GCTCAGTGTGCTTTTGAACTCC |
| m-Trim8 | AGGGACACTCGGTGTGTGA | TGTCTGCCGCAAGTCTTCATC |
| m-Trim9 | CTTGGGCAATAACTGAAGGAGG | GCTGGAGTAGAAGTCGGGG |
| m-Trim10 | GGAACACGGGGAGAAAATCTAC | AGACACACGAGACACTTCTGT |
| m-Trim11 | GCCCTCATCTCCGAGCTTG | CGCAGCACTCAATGCAGAG |
| m-Trim13 | TGATGACCCCCGAGTGTTG | TTTCCTTACGGCAGGTAGGAC |
| m-Trim14 | GTGCGTGTGCAGAAGCTAATC | CTGCGTAAACCTTGAGCCTTT |
| m-Trim15 | CCTGAGCGAGACCTACTGTGA | AGAGCTTCTAACCGACTCCTG |
| m-Trim16 | TCTTGGGGCCAGCAGAGTAA | CTCACAGTAGTTCACCATGCAG |
| m-Trim17 | CTTGCCAGACGGTTACAAGAG | CTCAGCCACTTTTGTCAGGAG |
| m-Trim18 | CTGTGACGGCACCTGTCTC | AAACGGCTGACTGTTGGTCTT |
| m-Trim19 | CAGGCCCTAGAGCTGTCTAAG | ATACACTGGTACAGGGTGTGC |
| m-Trim20 | TCATCTGCTAAACACCCTGGA | GGGATCTTAGAGTGGCCCTTC |
| m-Trim21 | GGGAGGAGGTCACCTGTTCTA | CATTACCGTGTTCTTTTGCAGC |
| m-Trim23 | ACCAGAAGCTAATCAGATCCGA | TGGCTCACAGTCAAACTGCTG |
| m-Trim24 | TCAACAGGCCATAAAACAGTGG | GGCACTCGGGACATGAACTG |
| m-Trim25 | GATGAGACGTGGGTCGTCC | TCTGTGTGAGCCATTCCAATTC |
| m-Trim26 | TCGGCCAGTGGATACCTACAT | CTGCACTTGTGATTGTGGGG |
| m-Trim27 | GGAGCAAATCCAGAACCGACT | GCCCCGTTGATGCTGTTATAG |
| m-Trim28 | CGGCGCTATGGTGGATTGT | GGTTAGCATCCTGGGAATCAGAA |
| m-Trim29 | AGAATGGCACTAAAGCAGACAG | AAATAGGCCACTCTTCCCCTC |
| m-Trim30 | CTGTGAGTGCTGATTGTAACCA | ACTCGGCATACAGGGCAGT |
| m-Trim31 | AATACGTTCAGGCCCAATAAGC | GGAGTCACGACACACCACA |
| m-Trim32 | CCTCCGGGAAGTGCTAGAATG | CACTGGCGGCAGATGGTATG |
| m-Trim33 | AGGAAATGTGAACGTCTTCTGC | GGGATTTGGTAATGCTGGGAA |
| m-Trim34 | GTAATAACGGTATCTTGGGCTCC | TGCGTTGTCTAACATCAAACCTT |
| m-Trim35 | TTCCGGGCCAAGTGTAAGAAC | CCAAGTCGTTTGCACCTCA |
| m-Trim36 | GGCTACATTATGGAATTGCTTGC | GGATCAGCGGGTGGGTAAAC |
| m-Trim37 | TCCAAGCTCTGTTGTTTCAGC | TTCCGCCCAACGACAGTTC |
| m-Trim38 | ATGGGCTCAGACTTTAGCACG | CTGTTTTTGGGCTGACATTGC |
| m-Trim39 | AACAGCTAATTGCGGATGTGA | ACAAACTTGACGCTTTTCCGAT |
| m-Trim40 | TCATCTGCTGGTCTTCTCCC | CAGGAGCTCCAAACCCCAAT |
| m-Trim41 | ATGAGCCGCATGTTTTGTCAG | GCCCCTAGTACACAGCAGT |
| m-Trim42 | ATGGAGACGGCTATGTGTGTC | GCACTTACAGTTGGGGTCATT |
| m-Trim43 | TGAAGGACTATAGGCGGTGGA | AGTGTTCACGTCCTATGCGG |
| m-Trim44 | ACGTGTGACGAGTGCGAAC | CCGACCTCGCTCTCTACCTC |
| m-Trim45 | TCAGGCAAGACTCATTGTCCT | ACGGATGTCCACTACTGAGAAT |
| m-Trim46 | GGTGAGGATATGCAGACCTTCA | TTGTGGGTACAAGGCAGCAC |
| m-Trim47 | GGTGAGCCAGATGTTTGCC | TCCCTCTTCGATGAACCCCAT |
| m-Trim50 | CCCATTTGCCTGGAGGTCTTC | CAGGACAGCATAGCTCGGAG |
| m-Trim52 | ATGCAGTCACTTCGGGAAGAA | CTATGGCTATGACCGACCCAC |
| m-Trim54 | GGAGAAGCAGCTCATTTGCC | CCTCCTGAAGACACCGTTGTG |
| m-Trim55 | AAAGCAACTGATCTGTCCCATC | TGTGGGTAAGTACGGGTTAGAG |
| m-Trim56 | CAGCGATTTCCTAGCCTGTAAA | GACCACCGATGTCCAGTTGT |
| m-Trim58 | AGTGGGACTGATGAGTGGGT | AATGAAGCCTCGGGCAGTAG |
| m-Trim59 | ATGCACAATTTTGAGGAGGAGTT | GCAGTTAGGACACTTGAGTGGAA |
| m-Trim60 | GCACAACTTCTGTTTTGCCTG | CAGTCATGTTACGGAACTGGTAG |
| m-Trim61 | CATCTTGCCCCCTGAAAGAAC | GGTCAGCATCAGCGGATCAC |
| m-Trim62 | CTTCGAGGAGTTGCAGAGAGA | GGCGTGAACATAATGCGGTC |
| m-Trim63 | GTGTGAGGTGCCTACTTGCTC | CTGCTCGCAGTAGATGCTCA |
| m-Trim65 | GAGGACGTGGTGACTTGCTC | GCTAGGCATGGGGTTTCGAT |
| m-Trim66 | CTTTGCCTTGTACTGCCCTCT | TTTTCCACGGGCCAAACAAAG |
| m-Trim67 | CCACTCTCTGCGAGCAATG | GCAGGCTCTTGGTAGAGGAC |
| m-Trim68 | TCCCAGAACTTGAGCTACACC | GCTCAGTCTTCTGTCCTTGGA |
| m-Trim69 | AACCACCACCCATTTACCCTC | ACGCCATGAATCCTGGATGC |
| m-Trim71 | CAAGCTGGAGAGCACCATCA | TGGATTTCTTATGTGCCACCTG |
| m-Trim72 | CCGCAGGCTCTAAGCACTAAC | GGTGGCTGAACTAGCCGAT |
| m-Trim75 | TTGGGTACCAACTGTCAGCC | AGACGGACCTTGTCTACAACA |
| m-β-actin | TTCCAGCCTTCCTTCTTG | GGGAGCCAGAGCAGTAATC |
| m-Gapdh | GGGTCCCAGCTTAGGTTCATC | TACGGCCAAATCCGTTCACA |
| h-CYP2J2 | TGCGATGGGCTCTGCTTTAT | GGATCATGGTACCCTTGGGC |
2.10. Immunofluorescent staining
Tissue sections were deparaffinized and endogenous peroxidase was blocked with 3% H2O2. Sections were incubated in 5% albumin bovine V (BSA, Solarbio) for 1 h. The cells were washed three times with PBS and fixed with 4% paraformaldehyde for 15 min. After three washes with PBS, the cells were permeated with 0.1% Triton X-100 for 15 min. After three washes with PBS, the cells were incubated with 5% bovine serum albumin for 30 min at room temperature and then incubated with p21 antibody (1:300, Abcam, Cambridge, MA, USA), p16 antibody (1:200, Abcam), SFTPC antibody (1:200, Abclonal, Wuhan, China), Keap1 antibody (1:300, Proteintech, Wuhan, China), Nrf2 antibody (1:300, Cell Signaling Technology, USA), PCNA antibody (1:200, Proteintech), Ki67 antibody (1:200, Abways, Shanghai, China), or Trim25 antibody (1:200, Proteintech) at 4 °C overnight. The next day, after washing with PBS, fluorescein-labeled secondary antibodies (1:300, Abcam) for immunofluorescence were incubated. Nuclei were counterstained with DAPI (Invitrogen, USA). The samples were washed three times with PBS and photographed with fluorescence microscopy (Nikon, Japan).
2.11. Co-immunoprecipitation (Co-IP) assay
For the detection of the interaction between Keap1 and Trim25 or ubiquitin, cells were washed with PBS three times, collected and lysed with non-Denaturing lysis buffer and protease inhibitor cocktail (MedChemExpress, HY-K0010) on ice for 15 min, then centrifuged at 12,000 rpm for 10 min at 4 °C. At the same time, 40 μL protein A/G Agarose was washed three times with wash buffer, added with anti-Keap1 antibody (1 μg), and incubated for 1 h at room temperature. The protein (1 mg) was transferred to another cold tube and incubated with 40 μL protein A/G Agarose at 4 °C overnight. The next day, the precipitate was collected by centrifugation, washed three times with wash buffer, and added with 50 μL SDS PAGE loading buffer. Protein denaturation was performed at 95 °C for 10 min. The immunoprecipitated proteins were analyzed by Western blotting.
2.12. Targeted metabolomics assay
Untargeted metabolomics assays were performed as previously described [42]. Briefly, untargeted metabolomics assays for glucose were performed by Liquid chromatography-mass spectrometry (Suzhou Panomix Biomedical Technology Co., Ltd.). Metabolomics instrumental analysis was performed on ultra-high-performance liquid chromatography (Vanquish, UPLC, Thermo, USA) and high-resolution mass spectrometry (Q Exactive, Thermo, USA).
2.13. Measurement of ROS
Dilute DCFH-DA (Nanjing Jiancheng Bioengineering Institute, China) with DMEM/F12 at the ratio of 1:1000 to a final concentration of 10 μM. Add 1 mL diluted DCFH-DA solution to each well and incubate for 20–30 min at 37 °C. The MLE12 cells were washed three times with PBS and photographed with fluorescence microscopy (Nikon, Japan).
2.14. Statistical analyses
All data were presented as means ± standard deviation. Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, Inc, San Diego, CA, USA). Multiple group comparisons were made using a one-way analysis of variance. Tukey's test was used as a post hoc test for pairwise comparisons. Comparisons between the two-group were made with an unpaired t-test. Overall survival was compared with the Kaplan-Meier method, and the significance was determined by the log-rank test. Pearson correlations were estimated to examine linear associations between the protein expression. All experiments were independently repeated three times. P < 0.05 was considered statistically significant.
3. Results
3.1. Decreased EETs content is associated with senescence of AECs
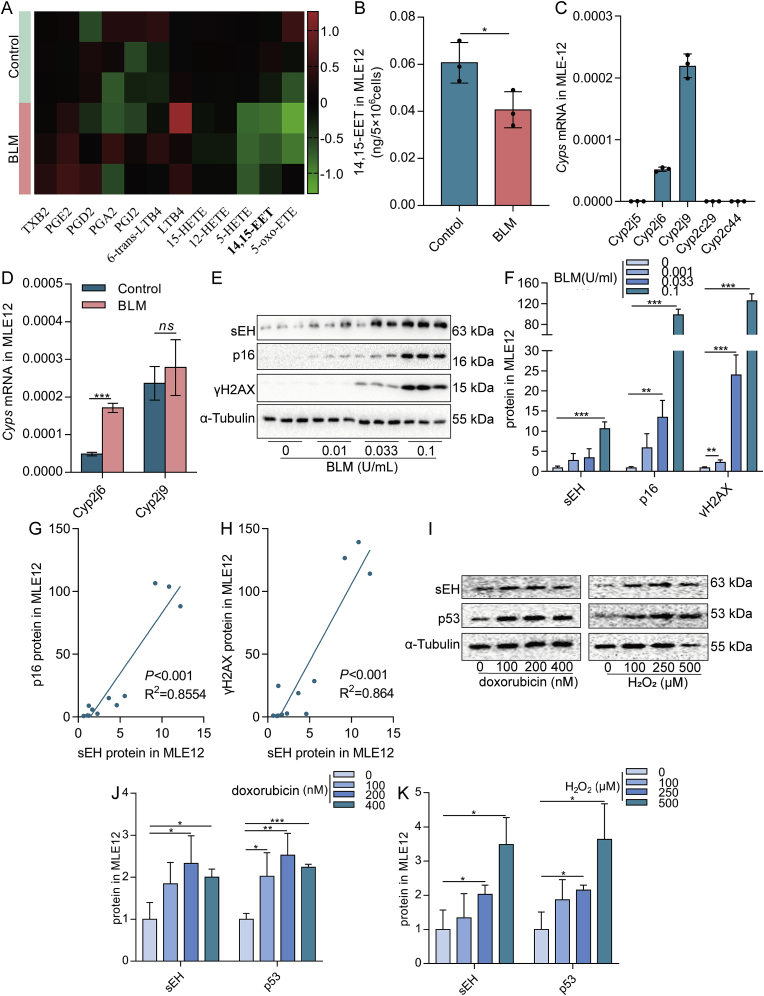
To clarify the changes in EETs in senescent AECs, we first examined changes in EETs content and expression of enzymes related to EETs metabolism. BLM, H2O2, and doxorubicin are commonly used to induce cell senescence [43,44], which were used to establish a model of senescent AECs in vitro in this study. Results showed obvious cell senescence was induced, mainly manifested as increased p16 and p19 mRNA expression, p53 and γH2AX protein expression, and the number of β-gal positive cells (Fig. S1A-G). Then, we evaluated the changes in EETs metabolism. LC-MS/MS revealed a significantly reduced 14,15-EET content in senescent MLE12 cells (Fig. 1A and B). Real-time PCR results showed that the mRNA expression of Cyp2j6 and Cyp2j9 was relatively high in mouse alveolar epithelial cell line MLE12 (Fig. 1C). Therefore, the mRNA expression changes of Cyp2j6 and Cyp2j9 in BLM-induced senescent MLE12 cells were detected. We found that Cyp2j6 mRNA was increased in senescent MLE12 cells, while Cyp2j9 mRNA with the highest expressive abundance did not change (Fig. 1D). However, the EETs degradation enzyme sEH protein expression was significantly increased and positively correlated with the expression of p16 and γH2AX (Fig. 1E–H). The expression of sEH protein in senescent MLE12 induced by H2O2 and doxorubicin was also significantly increased (Fig. 1I–K). We speculated that the increased of Cyp2j6 mRNA presumably compensates due to the faster degradation of EETs. The same results were obtained in A549 cells (Fig. S1H-I). Taken together, these data illustrate that decreased EETs content plays an essential role in the senescent process of AECs.
Fig. 1.
Decreased EETs content is associated with the senescence of AECs. Heatmap showing the levels of arachidonic acid metabolites in MLE12 cells treated with BLM compared to normal saline. The content of metabolites was calculated in log2 (A, n = 3). Absolute quantification of 14,15-EET in MLE12 cells (B, n = 3). Cyps mRNA expression in MLE12 cells was detected by real-time PCR (C, n = 3). MLE12 cells were treated with BLM (0.1 U/mL) for 2 h. Cells were then washed with PBS and subsequently incubated for 22 h. The mRNA expression of Cyp2j6 and Cyp2j9 in MLE12 cells was detected by real-time PCR (D, n = 3). MLE12 cells were treated with different concentrations of BLM (0, 0.01, 0.033, and 0.1 U/mL) for 2 h. Cells were then washed with PBS and subsequently incubated for 46 h. The protein expression of sEH, p16, and γH2AX in MLE12 cells was detected by Western blot (E-F, n = 3). The linear associations between sEH and p16 or γH2AX protein expression were analyzed with Pearson correlations analysis (G–H). MLE12 cells were treated with different concentrations of doxorubicin (0, 100, 200, and 400 nM) or H2O2 (0, 100, 250, and 500 μM) for 2 h. Cells were then washed with PBS and subsequently incubated for 46 h. The protein expression of sEH and p53 in MLE12 cells was detected by Western blot (I–K, n = 3). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. Comparisons between the two-group were made with an unpaired t-test. *P < 0.05, **P < 0.01, and ***P < 0.001.
3.2. Increasing EETs inhibits the senescence of AECs in vitro
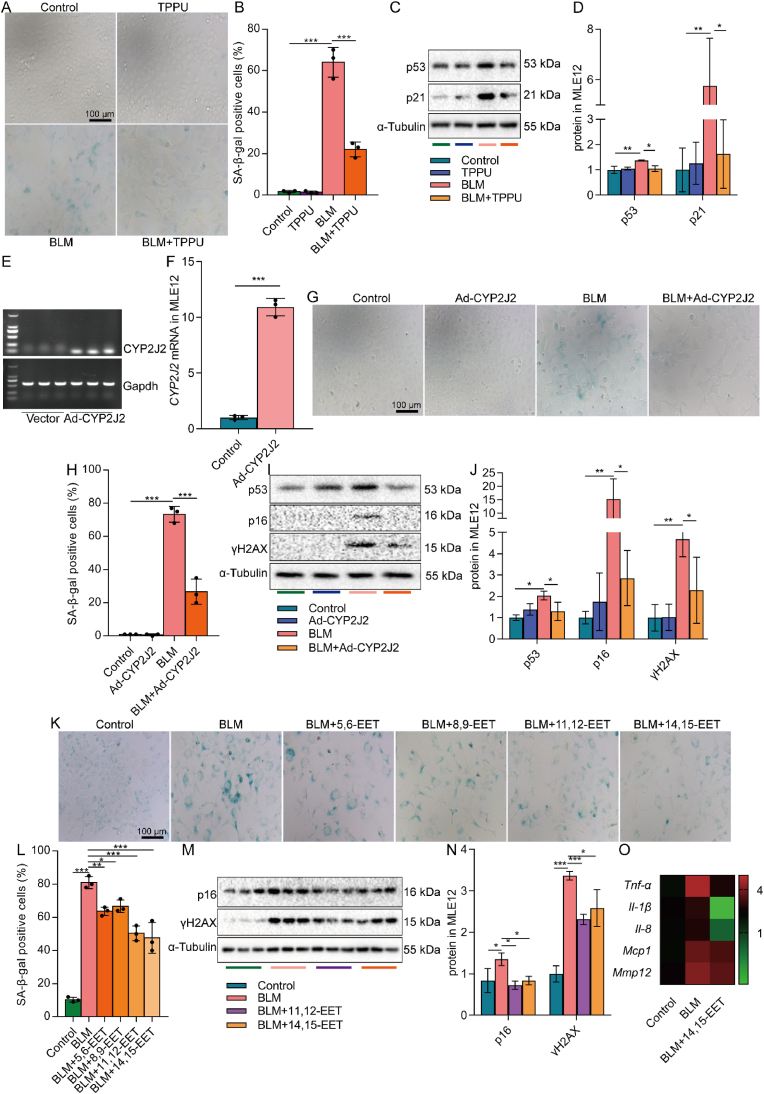
Considering the decreased EETs content during cell senescence in AECs, we carried out experiments to restore the EETs content to investigate the effect of EETs on AEC senescence. MLE12 cells were then respectively treated with sEH inhibitor, overexpression of CYP2J2, or exogenous EETs, which are proven in other studies [45,46]. We first treated MLE12 cells with TPPU, a specific inhibitor of the EETs-degrading enzyme sEH [45]. TPPU significantly reduced the number of BLM-induced β-gal positive cells and reduced the expression of senescence-related p53 and p21 proteins in MLE12 cells (Fig. 2A–D). Secondly, we used adenovirus to overexpress the EETs-synthetase CYP2J2 in MLE12 cells. Ad-CYP2J2 significantly reduced the number of β-galactosidase positive cells and reduced the expression of p53 and p21 protein in MLE12 cells (Fig. 2E–J). EETs have four isomers, namely 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET [47]. Finally, exogenous EETs were administered to MLE12 cells. The results showed that EETs significantly reduced the number of β-gal positive cells in MLE12 cells, among which the effects of 11,12-EET and 14,15-EET were more significant (Fig. 2K-L). Besides, 11,12-EET and 14,15-EET reduced the protein expression of p16 and γH2AX (Fig. 2M−N). What emerges from the results reported here is that EETs have anti-senescence effects on AECs.
Fig. 2.
Increasing EETs inhibits the senescence of AECs. MLE12 cells were treated with BLM (0.1 U/mL) for 2 h after TPPU (20 μM) intervention 1 h earlier, and then cultured for 46 h after BLM was removed. SA-β-gal staining was performed 48 h after BLM treatment (A-B, bar = 100 μm). The protein expression of p53 and p21 in MLE12 cells was detected by Western blot (C-D, n = 3). MLE12 cells were treated with BLM (0.1 U/mL) for 2 h after CYP2J2 overexpression, and then cultured for 46 h after BLM was removed. The expression of CYP2J2 mRNA in MLE12 cells 48 h after the adenovirus-CYP2J2 infection was detected by PCR (E-F, n = 3). SA-β-gal staining was performed 48 h after BLM treatment (G-H, bar = 100 μm). The protein expression of p53, p16, and γH2AX in MLE12 cells was detected by Western blot (I-J, n = 3). MLE12 cells were treated with BLM (0.1 U/mL) for 2 h after EETs (2.5 μM) intervention 10 min earlier, and then cultured for 46 h after BLM was removed. SA-β-gal staining was performed 48 h after BLM treatment (K-L, bar = 100 μm). The protein expression of p16 and γH2AX in MLE12 cells was detected by Western blot (M − N, n = 3). The mRNA expression Tnf-α, Il-1β, Il-8, Mcp1, and Mmp12 in MLE12 cells was detected by real-time PCR 24 h after BLM treatment (O, n = 3). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. Comparisons between the two-group were made with an unpaired t-test. *P < 0.05, **P < 0.01, and ***P < 0.001.
Cellular senescence is accompanied by the SASP [48]. Our gene-chip microarray profiling showed that 14,15-EET reduced the mRNA expression of Tnf-α, Il-1β, Il-8, Mcp1, and Mmp12 in SASP components of MLE12 cells treated by BLM (Fig. 2O). Meanwhile, 14,15-EET increased proliferation-related Ki67 expression, a signal of cell arrest, in MLE12 cells (Fig. S2B). These results also suggest that EETs have the effect of alleviating AEC senescence. Interestingly, the anti-senescence effect of EETs was also observed in A549 cells. EETs reduced the number of BLM-induced β-gal positive cells in A549 cells (Fig. S2A). Taken together, these results suggest that EETs alleviate the senescence of AECs, and the effects of 11,12-EET and 14,15-EET are more significant. Therefore, 14,15-EET was adopted for the following study.
3.3. EETs alleviate the senescence of AECs by reducing ERS through Nrf2 anti-oxidant
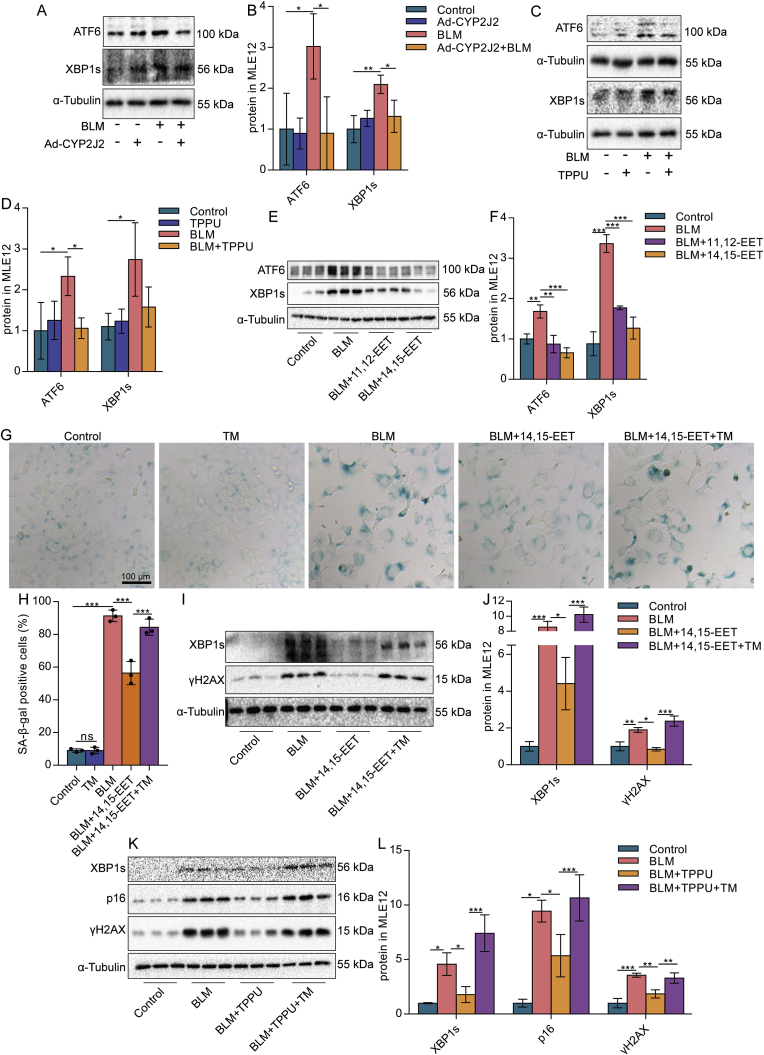
ERS and UPR are indispensable mechanisms of cell senescence [49]. By detecting ERS-UPR-associated ATF6, XBP1s, and p-EIF2α proteins, we found that ERS was enhanced in BLM-treated MLE12 cells (Fig. S3). The expression of XBP1s and ATF6 protein in senescent MLE12 cells could be reduced by Ad-CYP2J2 (Fig. 3A and B), TPPU (Fig. 3C and D), or exogenous EETs (Fig. 3E and F), suggesting that EETs could inhibit ERS. Further, to clarify that EETs alleviate the senescence of AECs by inhibiting ERS, we used an ERS inducer TM. The results showed that low doses of TM (0.25 μg/mL), which activated ERS but was insufficient to induce cellular senescence (Fig. 3G and H), abolished the anti-senescence effects of exogenous EETs and TPPU (Fig. 3G-L). We also found that EETs alleviated a high dose of TM-induced senescence of AECs (Fig. S4). Collectively, these data illustrate that EETs alleviate the senescence of AECs in part by inhibiting ERS.
Fig. 3.
EETs alleviate the senescence of AECs by reducing ERS. MLE12 cells were treated with BLM for 2 h after EETs intervention 10 min earlier, TPPU intervention 1 h earlier, or CYP2J2 overexpression, and then cultured for 46 h after BLM was removed. The protein expression of ATF6 and XBP1s in MLE12 cells was detected by Western blot (A-F, n = 3). MLE12 cells were treated with BLM for 2 h after 10 min of EETs intervention and 1 h after TPPU intervention, respectively. After culturing without BLM for 22 h, the cells were added to TM (0.25 μg/mL) for 24 h. SA-β-gal staining was performed 48 h after BLM treatment (G-H, bar = 100 μm). The protein expression of XBP1s and γH2AX in MLE12 cells was detected by Western blot (I-J, n = 3). The protein expression of XBP1s, p16, and γH2AX in MLE12 cells was detected by Western blot (K-L, n = 3). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. *P < 0.05, **P < 0.01, and ***P < 0.001.
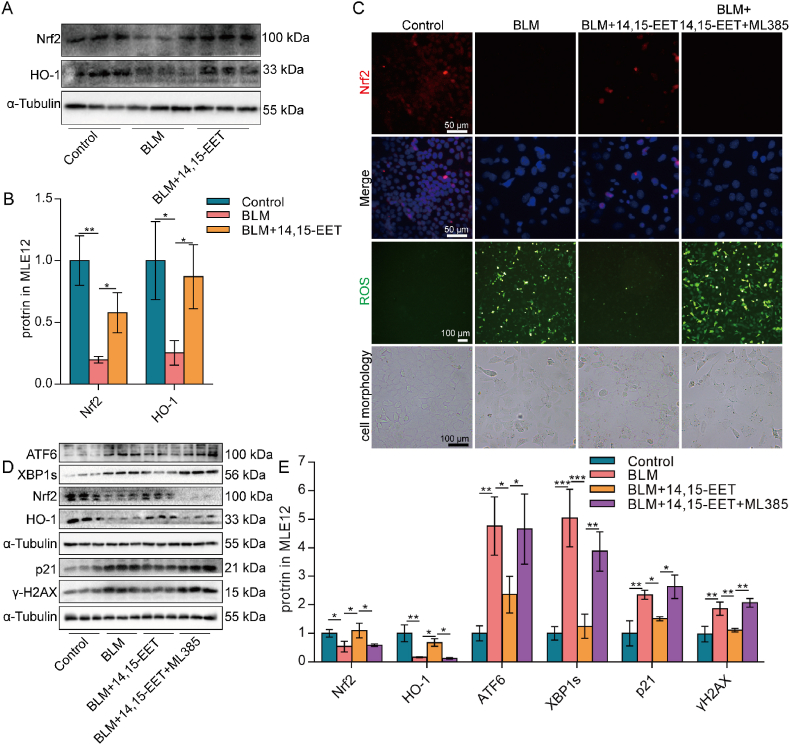
Intracellular oxidation/anti-oxidant imbalance occurs during cell senescence [50]. The multifunctional regulator Nrf2 is considered not only a cytoprotective factor regulating the expression of genes coding for anti-oxidant, anti-inflammatory, and detoxifying proteins but also a potent modulator of species longevity [51]. Our results showed that Nrf2 and its downstream HO-1 protein expression were significantly down-regulated in senescent MLE12 cells (Fig. 4A–C). EETs significantly increased Nrf2 protein expression and nuclear translocation, increased HO-1 protein expression, and decreased ROS content in MLE12 cells (Fig. 4A–C). Inhibition of Nrf2 by ML385 reversed the ability of EETs to reduce ROS in MLE12 cells (Fig. 4C), suggesting that EETs play an anti-oxidant role through Nrf2. Oxidative stress is closely related to ERS, and the production of ROS directly or indirectly affects ER homeostasis and protein folding [52]. It was further observed that after inhibiting Nrf2, the reducing effect of EETs on the protein expression of senescence-related p21, γH2AX, and ERS-UPR-related molecules ATF6 and XBP1s were abrogated in MLE12 (Fig. 4D and E). These data suggest that EETs play an anti-oxidant role through Nrf2, thereby alleviating the ERS and senescence of AECs.
Fig. 4.
EETs alleviate ERS and senescence of AECs through Nrf2 anti-oxidant. MLE12 cells were treated with BLM for 2 h after EETs intervention 10 min earlier and cultured for 46 h after BLM was removed. The protein expression of Nrf2 and HO-1 in MLE12 cells was detected by Western blot (A-B, n = 3). The fluorescence intensity of Nrf2 was detected by immunofluorescence (C, bar = 50 μm). The ROS in MLE12 cells were detected by a ROS kit (C, bar = 100 μm). Cell morphology is photographed through a microscope (C, bar = 100 μm). Cells were treated with ML385 (5 μM) for 1 h before the treatment with BLM. ATF6, XBP1s, Nrf2, HO-1, p21, and γH2AX protein expression in MLE12 cells was detected by Western blot (D-E, n = 3). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. *P < 0.05, **P < 0.01, and ***P < 0.001.
3.4. EETs increase the Keap1 ubiquitination level and E3 ubiquitin ligase Trim25 expression
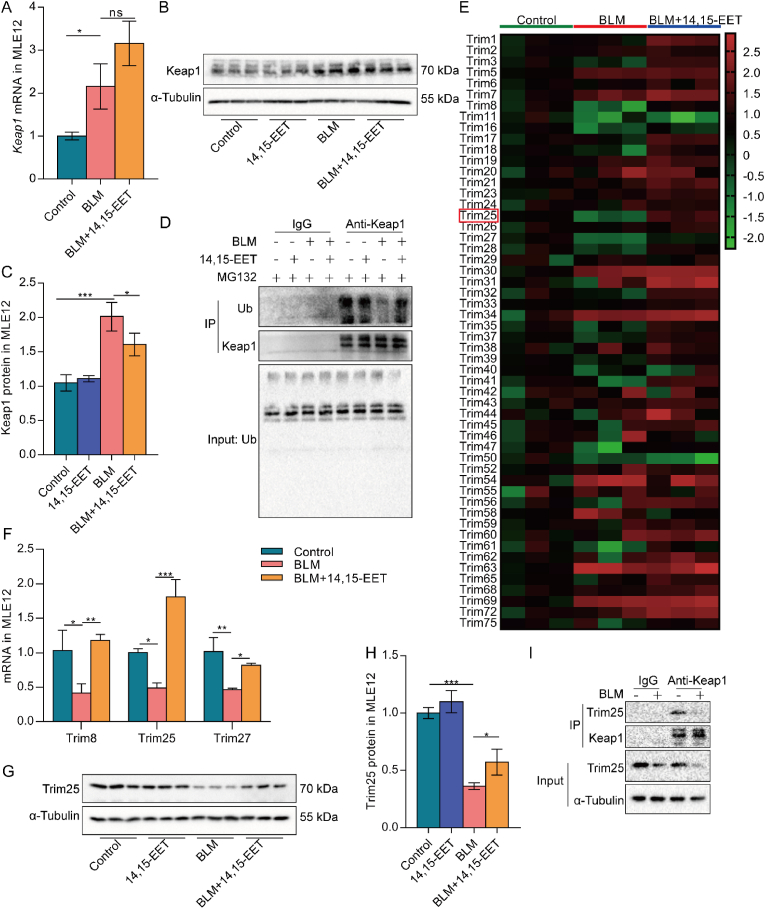
Keap1 is a negative regulator of Nrf2 and can form a polymer with Nrf2 to prevent Nrf2 from entering the nucleus [26]. We detected the Keap1 mRNA and protein expression in BLM-induced senescent MLE12 cells and found that the Keap1 mRNA and protein expression were increased in senescent MLE12 cells. Keap1 mRNA expression was not significantly changed in MLE12 cells in the BLM+14,15-EET group compared with the BLM group, but the protein expression was significantly decreased (Fig. 5A–C). We believe that 14,15-EET regulates Keap1 posttranslationally. A recent study has reported that to maintain homeostasis, hepatoma carcinoma cells degrade Keap1 by ubiquitination to inhibit ERS-related effects [34]. Therefore, we speculated that 14,15-EET decreased Keap1 protein expression might be related to ubiquitination modification. The results showed that the Keap1 ubiquitination level was significantly decreased in senescent MLE12 cells, and 14,15-EET enhanced the Keap1 ubiquitination level (Fig. 5D). These results indicate that 14,15-EET reduces the Keap1 protein level in senescent MLE12 cells by promoting Keap1 ubiquitination.
Fig. 5.
EETs increase Keap1 ubiquitination level and E3 ubiquitin ligase Trim25 expression.Keap1 mRNA expression in MLE12 cells was detected by real-time PCR 24 h after BLM treatment (A, n = 3). Keap1 protein expression was detected by Western blot 48 h after BLM treatment (B–C, n = 3). Keap1 ubiquitination level was detected by Co-IP in MLE12 cells (D). Trims mRNA expression in MLE12 cells was detected by real-time PCR 24 h after BLM treatment (E, n = 3). The mRNA expression of Trim8, Trim25, and Trim27 was detected by real-time PCR (F, n = 3). Trim25 protein expression was detected by Western blot 48 h after BLM treatment (G-H, n = 3). The interaction between Keap1 and Trim25 was detected by Co-IP in MLE12 cells (I). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. *P < 0.05, **P < 0.01, and ***P < 0.001.
Most members of the Trim family possess E3 ubiquitin ligase activity. Accumulating evidence has shown that Trims are involved in regulating protein homeostasis [32,34]. By further detecting the expression of the E3 ubiquitin ligase-valued Trims mRNA in senescent MLE12 cells, we found that 14,15-EET increased the mRNA expression of Trim8, Trim25, and Trim27, among which 14,15-EET increased Trim25 most significantly (Fig. 5E and F). We also detected the downregulation of Trim25 protein expression in senescent MLE12 cells, which was significantly increased by 14,15-EET treatment (Fig. 5G–H, S4A). To determine whether Trim25 protein interacts with Keap1 protein, we carried out Co-IP detection and found that Trim25 protein bound to Keap1 protein in MLE12 cells. However, the binding between Trim25 and Keap1 was decreased in BLM-induced senescent MLE12 cells (Fig. 5I). Overall, these results indicate that EETs increased Keap1 ubiquitination level and E3 ubiquitin ligase Trim25 expression.
3.5. EETs promote Nrf2 into the nucleus by ubiquitination of Keap1 by Trim25
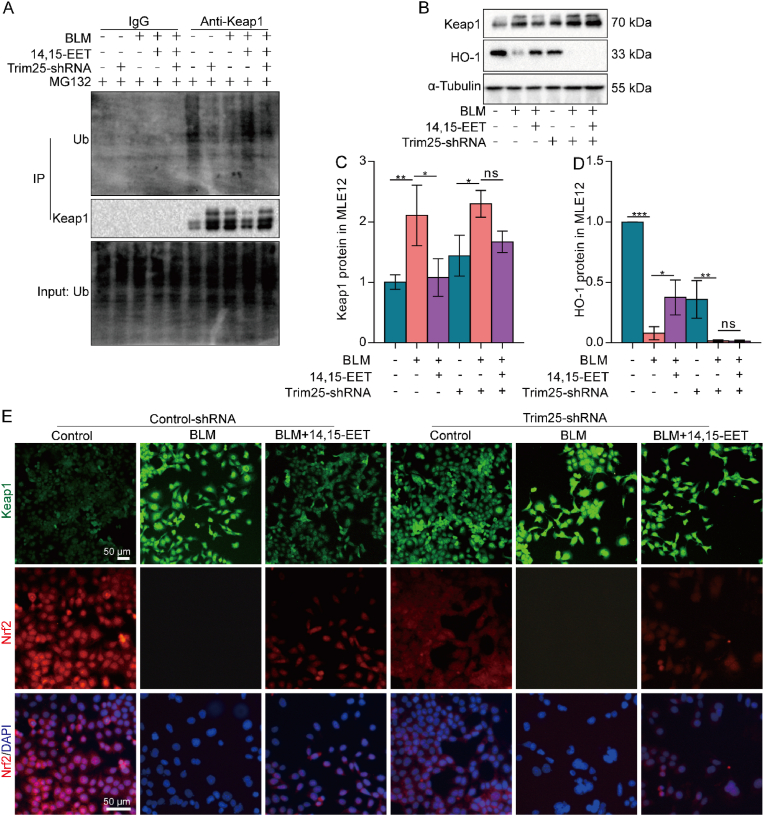
The interaction between Trim25 and Keap1 was reduced, which may be related to the decreased protein expression of Trim25 in senescent MLE12 cells. However, it was not clear whether 14,15-EET promoted Trim25 expression to ubiquitinate Keap1. Therefore, we silenced Trim25 with lentivirus in MLE12 cells for follow-up experiments (Fig. S5). We found that after silencing MLE12 cell Trim25, the ubiquitination degradation of Keap1 promoted by 14,15-EET was canceled (Fig. 6A). The results showed that 14,15-EET promoted Keap1 ubiquitination partly through Trim25. The expression of Keap1 and HO-1 was further detected. The results showed that after Trim25 silencing in MLE12 cells, the effects of 14,15-EET on reducing Keap1 expression and promoting HO-1 expression were offset (Fig. 6B–D). These results indicate that 14,15-EET inhibits Keap1 protein expression and increases HO-1 protein expression through Trim25. We speculated that 14,15-EET degrades Keap1 through Trim25 ubiquitination, which enables Nrf2 to enter the nucleus and promote the expression of HO-1. Therefore, we detected the nuclear translocation of Nrf2 by immunofluorescence. We found that the silenced Trim25, although 14,15-EET could partially increase Nrf2 protein expression, the nuclear translocation of Nrf2 was significantly reduced (Fig. 6E). Altogether, these data suggest that EETs promote Nrf2 entry into the nucleus via ubiquitination of Keap1 in a Trim25-dependent manner.
Fig. 6.
EETs promote Nrf2 into the nucleus by ubiquitination of Keap1 by Trim25. Lentivirus was used to silence Trim25 in MLE12 cells. Keap1 ubiquitination level was detected by Co-IP in MLE12 cells (A). The protein expression of Keap1 and HO-1 in MLE12 cells was detected by Western blot (B-D, n = 3). The fluorescence intensity of Keap1 and Nrf2 was detected by immunofluorescence (E, bar = 50 μm). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. *P < 0.05, **P < 0.01, and ***P < 0.001.
3.6. EETs alleviate AEC senescence by inhibiting ERS through Trim25/Keap1/Nrf2
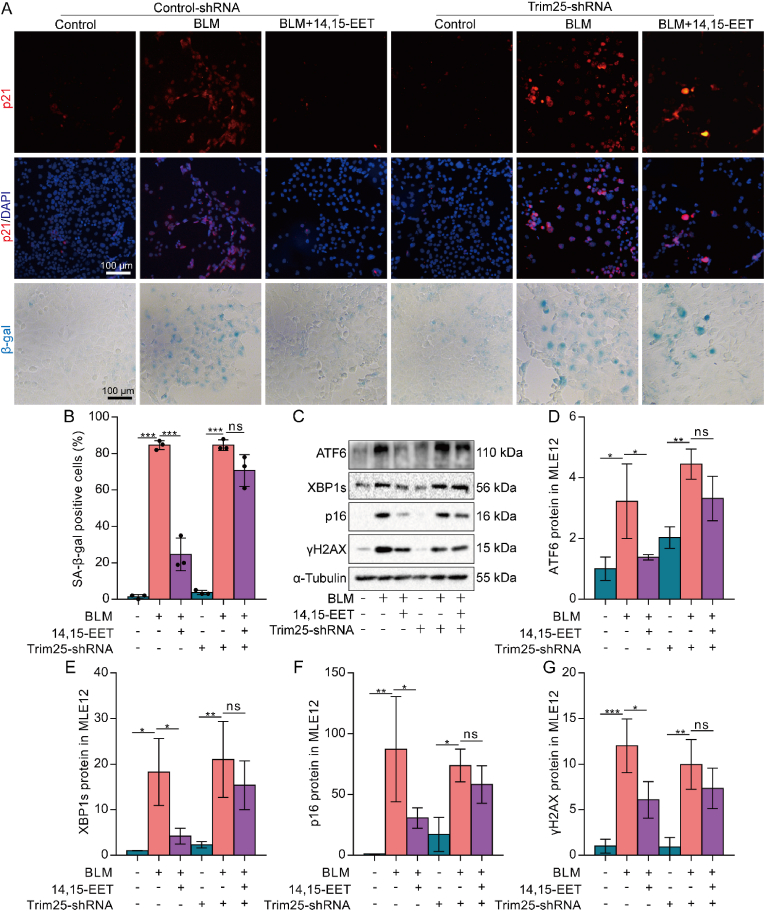
We further observed whether Trim25/Keap1/Nrf2 axis was necessary for EETs to inhibit ERS and alleviate the senescence of AECs. After silencing Trim25 in MLE12 cells, we found that the reducing effect of 14,15-EET on p21 expression was canceled (Fig. 7A). At the same time, the effect of 14,15-EET on the number of β-gal positive cells was also partially offset (Fig. 7A and B). Western blot results showed that after silencing Trim25 in MLE12 cells, the effect of 14,15-EET on reducing the expression of ERS-UPR-related ATF6, XBP1s, and senescent-related p16, γH2AX proteins was eliminated (Fig. 7C–G). Collectively, these results show that EETs could inhibit ERS and alleviate the senescence of AECs through Trim25/Keap1/Nrf2.
Fig. 7.
EETs alleviate AEC senescence by inhibiting ERS through Trim25/Keap1/Nrf2. Lentivirus was used to silence Trim25 in MLE12 cells. The fluorescence intensity of p21 was detected by immunofluorescence (A, bar = 100 μm). SA-β-gal staining was performed 48 h after BLM treatment (A-B, bar = 100 μm). The protein expression of ATF6, XBP1s, p16, and γH2AX in MLE12 cells was detected by Western blot (C-G, n = 3). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. *P < 0.05, **P < 0.01, and ***P < 0.001.
3.7. Inhibition of EETs degradation alleviates D-gal-induced lung senescence in aging mice
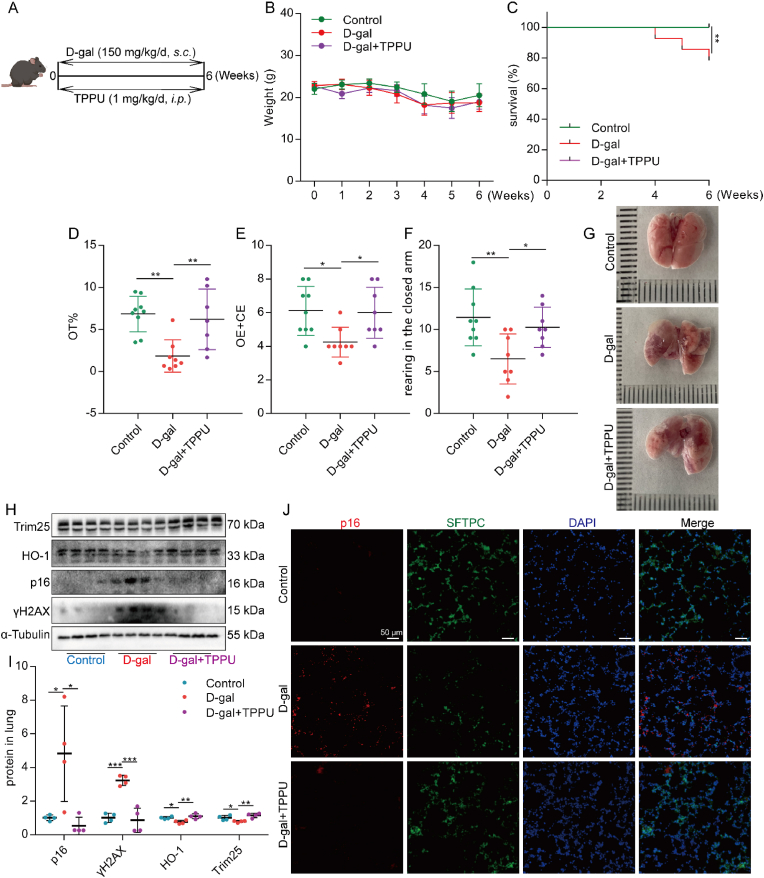
Based on the above studies, we believe that EETs could alleviate the senescence of AECs. Chronic lung disease is often associated with age [53]. Whether EETs can reduce age-related lung senescence is unclear. D-gal is widely used to induce premature aging in mouse models [36,54]. Herein, we used D-gal to induce a mouse model of premature aging and treated it with TPPU, a sEH-specific inhibitor (Fig. 8A). The results showed that TPPU had no significant effect on the body weight of mice (Fig. 8B). While TPPU could significantly improve the survival rate of D-gal premature aging mice (Fig. 8C). Aging mice often show anxiety-like characteristics, and evaluating the degree of anxiety in mice is one of the means to reflect the aging of mice [55]. Through the elevated maze experiment, we found that TPPU significantly increased the time of mice in the open arm, the number of shuttles between the open arm and the closed arm, and the number of mice climbing on the closed arm (Fig. 8D–F), indicating that TPPU could significantly reduce the anxiety degree of D-gal induced aging mice. Further, we observed the effect of TPPU on lung senescence in mice. Lung appearance showed that TPPU alleviated lung structural damage in D-gal-treated mice (Fig. 8G). Western blot analysis showed that the sEH protein was increased in the lungs of mice along with the expression of senescence-related proteins (Fig. S6). TPPU could significantly reduce the expression of senescence-related proteins p16 and γH2AX and increase the expression of Trim25 and HO-1 in the lungs of D-gal-induced premature aging mice (Fig. 8H and I). Immunofluorescence results showed that TPPU reduced the protein expression of lung age-related p16 (Fig. 8J) and p21 (Fig. S6G), including SFTPC-positive AECs. Together, these data illustrate that inhibition of EETs degradation could alleviate D-gal-induced lung senescence in aging mice.
Fig. 8.
Inhibition of EETs degradation alleviates D-gal-induced lung senescence in aging mice. C57BL/6 J mice were given a daily subcutaneous injection of D-gal (150 mg/kg) in the neck and back to establish the aging model and a daily intraperitoneal injection of TPPU (1 mg/kg) (A). Weight change of mice (B, n = 28). The survival rate was expressed as Kaplan Meier survival curves (C, n = 28). Elevated plus-maze test, OT% (Open arm retention time ratio), OE + CE (The total number of times the open and closed arms were entered), and rearing (D–F). The appearance of lung tissue (G). The protein expression of Trim 25, HO-1, p16, and γH2AX in the lung was detected by Western blot (H–I, n = 7). The fluorescence intensity of p16 and SFTPC was detected by immunofluorescence (J, bar = 50 μm). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. Survival data were analyzed using the log-rank test. *P < 0.05, **P < 0.01, and ***P < 0.001.
3.8. Inhibition of EETs degradation alleviates experimental pulmonary fibrosis in aging mice
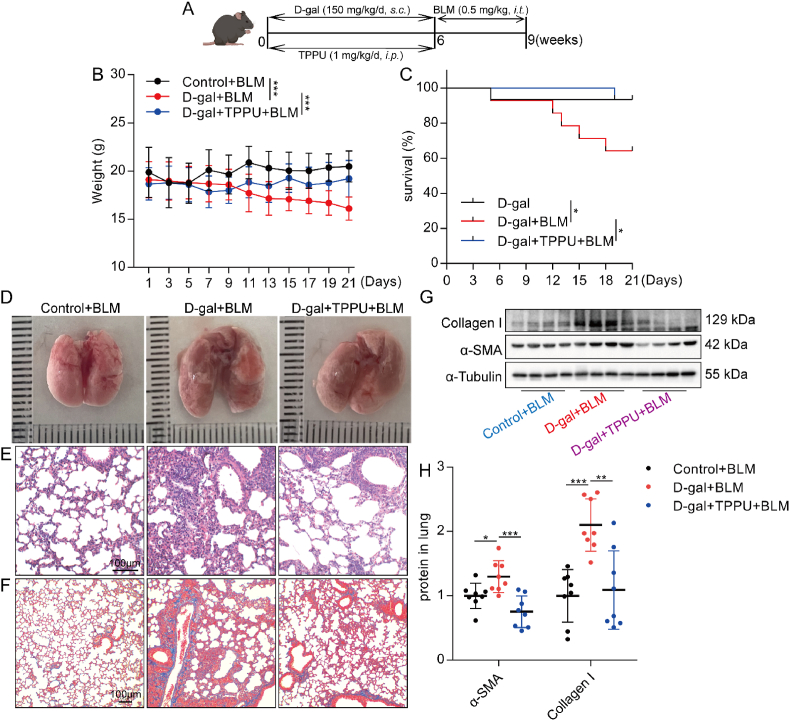
Since inhibition of EETs degradation could alleviate lung senescence in mice, we wondered whether inhibition of EETs degradation reduced age-related chronic lung diseases. BLM at a dose between 1.5 and 5 mg/kg was widely used to induce experimental pulmonary fibrosis in mice [16,56]. To evaluate the effects of EETs on age-relative pulmonary disease, we used low-dose BLM (0.5 mg/kg, this dose of BLM did not induce significant pulmonary fibrosis in young mice) in D-gal-induced aging mice. (Fig. 9A). We found that TPPU-treated aging mice showed lower susceptibility to BLM-induced pulmonary fibrosis. The effect of TPPU treatment was mainly reflected in the increase of body weight (Fig. 9B) and survival rate (Fig. 9C), the reduction of lung tissue structure damage (Fig. 9D and E), the reduction of collagen deposition (Fig. 9F) in the lungs, and the reduction of collagen I and α-SMA protein expression in the lungs (Fig. 9G and H). These results indicate that inhibition of EETs degradation alleviates experimental pulmonary fibrosis in aging mice.
Fig. 9.
Inhibition of EETs degradation alleviates experimental pulmonary fibrosis in aging mice. D-gal-induced aging mice were given a tracheal injection of BLM (0.5 mg/kg) (A). Weight change of mice (B, n = 14–15). The survival rate was expressed as Kaplan Meier survival curves (C, n = 14–15). The appearance of lung tissue (D). HE staining of lung tissue (E, bar = 100 μm). Masson staining of lung tissue (F, bar = 100 μm). The protein expression of Collagen I and α-SMA in the lung was detected by Western blot (I-J, n = 8). Data are expressed as the mean ± SD. Differences among multiple groups were performed using ANOVA. Tukey's test was used as a post hoc test for pairwise comparisons. Survival data were analyzed using the log-rank test. *P < 0.05, **P < 0.01, and ***P < 0.001.
4. Discussion
Cell senescence is known to be involved in the pathogenesis of age-related chronic lung diseases such as COPD and pulmonary fibrosis [7]. Therefore, the selective elimination of senescent cells has been proposed as a therapeutic pathway to restore health in the aging lung. It has been found that anti-aging drugs can selectively remove senescent cells from the tissue microenvironment and inhibit the development of age-related diseases [57]. For example, selectively ablating senescent cells using dasatinib plus quercetin (DQ) alleviates age-related IPF [58]. However, there are few studies on how to delay cellular senescence. In this study, we demonstrated that EETs alleviated the senescence of AECs, and inhibiting the degradation of EETs effectively alleviated age-related pulmonary fibrosis. Mechanically, we found that EETs enhanced Keap1 ubiquitination through Trim25, promoted Nrf2 to enter the nucleus to play an anti-oxidant role, inhibited ERS, and alleviated the senescence of AECs. In conclusion, our study provides new insights into the molecular mechanisms associated with AEC senescence and its role in the pathogenesis of pulmonary fibrosis.
Aging impairs the availability of EETs in peripheral conduit arteries, contributing to the development of endothelial dysfunction [59]. Hak Su Kim et al. found reduced levels of EETs in the lung tissue of IPF patients [60]. This indicates that EETs are negatively associated with age-related diseases. We and others have demonstrated that inhibition of EETs degradation reduces pulmonary fibrosis and COPD in mice [46,61], suggesting the ability of EETs to mitigate chronic age-related lung diseases. More and more studies have confirmed that AEC senescence is the initiating factor of various chronic lung diseases [62,63]. Cell senescence is a complex process that manifests as cell cycle arrest accompanied by changes in SASP gene expression and secretion [64]. Senescent AECs can create an aging microenvironment by secreting SASP, activating macrophages and adjacent cells such as fibroblasts in the lung, and accelerating the process of lung diseases [65]. Using LC-MS/MS, we found that EETs content was significantly reduced in senescent AECs. Increasing EETs slowed down AEC senescence while decreasing SASP expression in senescent AECs. These results collectively reveal that EETs are endogenous anti-aging agents in AECs.
Although the underlying mechanisms of cell senescence remain ambiguous, a recent study indicates that ERS caused by impaired homeostasis is an essential cause of aging and age-related diseases [66]. ERS was found to be one of the causes of epithelial dysfunction in pulmonary fibrosis [21]. Our study confirmed the presence of ERS in senescent AECs. These results suggest that ERS may be a potential mechanism of AEC senescence in IPF. Oxidative stress is one of the major drivers of protein homeostasis imbalance, which can lead to an increase in the number of unfolded proteins in the ER lumen, which in turn induces ERS [67]. Our previous study found that inhibition of EETs degradation has the ability to reduce the level of oxidative stress in the lungs [14]. In this study, we found that EETs reduced intracellular ROS content and ERS levels in senescent AECs. A large number of studies have shown that the key intracellular anti-oxidant factor Nrf2 and its downstream target gene HO-1 have a significant inhibitory effect on ERS [27,28]. In combination with this study, EETs could promote the protein expression of Nrf2 and HO-1 in AECs. Intervention with Nrf2 inhibitor ML385 partially reversed the inhibitory effect of EETs on ERS. These results indicate that EETs can inhibit ERS in aging AECs by exerting an anti-oxidant effect through Nrf2.
The anti-oxidant effect of Nrf2 in the nucleus is limited by the intracellular Keap1 level [68]. Whether EETs promote Nrf2 nuclear translocation is related to Keap1 remains unclear. Interestingly, EETs were found to reduce Keap1 protein levels but not mRNA levels in senescent AECs, suggesting that EETs may regulate post-translational modification of Keap1. It was found that Keap1 could be degraded by ubiquitination, and then Nrf2 could be released [34]. Therefore, we further investigated whether EETs could affect the ubiquitination level of Keap1. The results showed that the Keap1 ubiquitination level was significantly down-regulated in senescent AECs, and 14,15-EET could significantly increase the Keap1 ubiquitination level. These results indicated that EETs could increase the ubiquitination level of Keap1, thereby reducing the protein level of Keap1 and promoting Nrf2 to enter the nucleus to play an anti-oxidant role. How do EETs, as a lipid molecule, promote Keap1 ubiquitination?
Studies have shown that Trim family proteins play an important role in innate immunity, tumorigenesis, and neurodegenerative diseases, mainly by regulating protein quality control [30,31,69]. Trim19 and Trim11 can effectively clear misfolded proteins in the nucleus and cytoplasm and promote tumorigenesis [32,33,70]. In this study, Trims family member Trim25 was found to be significantly down-regulated in senescent AECs. Meanwhile, 14,15-EET could increase the protein expression of Trim25 in senescent AECs. Trim25 has been found to promote colon cancer cell survival by ubiquitylation degradation of Keap1, and the mechanism is related to the inhibition of ERS [34]. By silencing the expression of Trim25, the effect of 14,15-EET on promoting Keap1 ubiquitination and degradation was offset, and the effects of 14,15-EET on promoting Nrf2 nuclear translocation and alleviating ERS and cell senescence were also partially abolished. These results suggest that 14,15-EET alleviates AEC senescence through the Trim25/Keap1/Nrf2 axis. However, how 14,15-EET contributes to increased Trim25 expression remains unclear. It has been reported that EETs exert intracellular biological effects by binding to their intracellular receptors PPARγ, PPARα, or G protein-coupled receptor 40 on the cell membrane, by regulating tyrosine kinase activity or by interacting with inflammatory factors [71]. We have now confirmed that EETs receptor PPARγ primarily expresses in MLE12 cells. However, whether 14,15-EET promotes Trim25 expression by interacting with PPARγ is our next step.
In combination, the data from these in vitro experiments suggest that EETs are a novel anti-aging agent for AECs. Therefore, we further evaluated whether EETs could alleviate age-related lung diseases. We treated D-gal-induced premature aging mice with the sEH inhibitor TPPU. The results suggested that TPPU could alleviate D-gal-induced lung aging, and TPPU intervention could significantly reduce the susceptibility of aged mice to BLM-induced pulmonary fibrosis. Currently, inhibitors of sEH include GSK2256294 A, AUDA, CUDA, TPPU, etc. The study of GSK2256294 A has entered clinical phase II, which is mainly used in treating COPD, diabetes, delayed cerebral hemorrhage, etc. [61,72], indicating that sEH inhibitors have an excellent clinical application prospect. This study will provide a solid experimental basis for the clinical application of sEH inhibitors in the treatment of age-related chronic lung diseases.
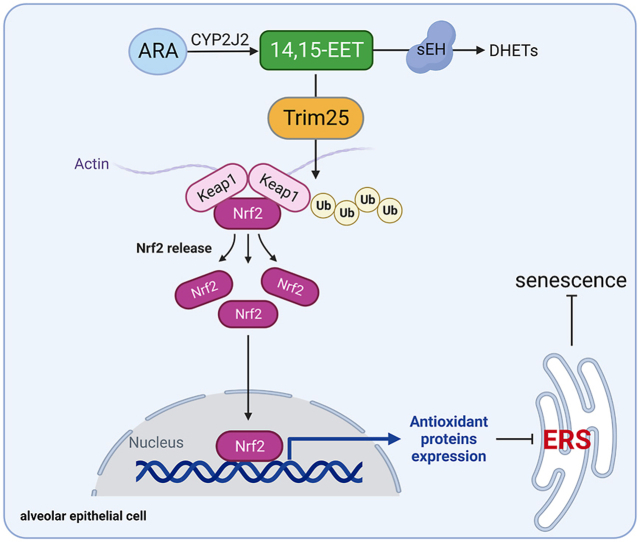
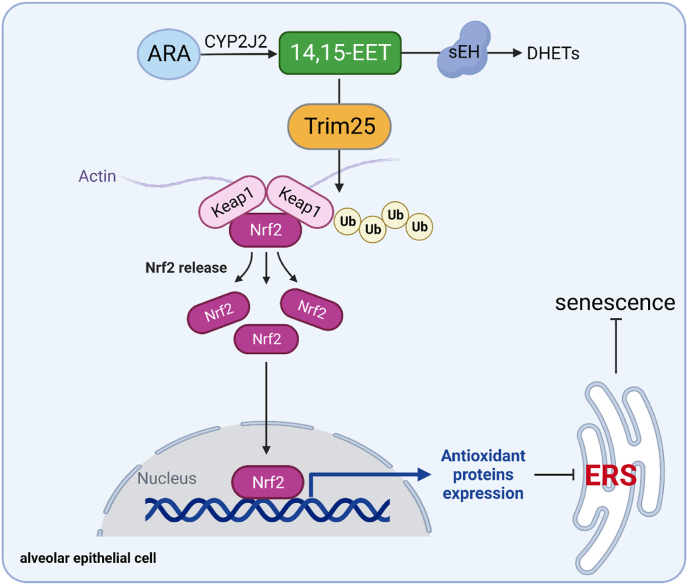
In conclusion, our data demonstrate that EETs degrade Keap1 through Trim25 ubiquitination and promote Nrf2 nuclear translocation to play an anti-oxidant role, inhibit ERS, and attenuate AEC senescence (Fig. 10). We suggest that targeting EETs metabolism in AECs is an effective strategy for treating age-related chronic lung diseases.
Fig. 10.
The schematic diagram for EETs alleviate AEC senescence. 14,15-EET degrades Keap1 through Trim25 ubiquitination and promotes Nrf2 nuclear translocation to play an anti-oxidant role, inhibits ERS, and attenuates AEC senescence.
Author contributions
C.Y.Z., W.J.Z., Y.B.L., J.X.D., N.J., L.J., J.R.H., and H.H.Y. performed the experiments; C.Y.Z., Y.B.L., and Y.Z. analyzed the data; C.X.G., Y.Z., and S.C.M. contributed reagents/materials/analysis tools. C.Y.Z. and Y.Z. wrote the paper; C.X.G., and Y.Z. conceived, designed the experiments, and critically reviewed the manuscript. All authors approved the submitted version.
Ethics statement
All animal protocols were approved by the Ethics Committee of the Basic Medical School of Central South University (2019–0901).
Funding
This study was supported by the National Natural Science Foundation of China (91949110, 82170096, and 82241053).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all the institutions and researchers who contributed to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102765.
Contributor Information
Yong Zhou, Email: zhouyong421@csu.edu.cn.
Cha-Xiang Guan, Email: guanchaxiang@csu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Dzau V.J., Inouye S.K., Rowe J.W., Finkelman E., Yamada T. Enabling healthful aging for all - the national Academy of medicine grand challenge in healthy longevity. N. Engl. J. Med. 2019;381:1699–1701. doi: 10.1056/NEJMp1912298. [DOI] [PubMed] [Google Scholar]
- 2.Schneider J.L., Rowe J.H., Garcia-de-Alba C., Kim C.F., Sharpe A.H., Haigis M.C. The aging lung: physiology, disease, and immunity. Cell. 2021;184:1990–2019. doi: 10.1016/j.cell.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calcinotto A., Kohli J., Zagato E., Pellegrini L., Demaria M., Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol. Rev. 2019;99:1047–1078. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 4.Di Micco R., Krizhanovsky V., Baker D., d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Childs B.G., Baker D.J., Wijshake T., Conover C.A., Campisi J., van Deursen J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendelsohn A.R., Larrick J.W. Cellular senescence as the key intermediate in tau-mediated neurodegeneration. Rejuvenation Res. 2018;21:572–579. doi: 10.1089/rej.2018.2155. [DOI] [PubMed] [Google Scholar]
- 7.Barnes P.J., Baker J., Donnelly L.E. Cellular senescence as a mechanism and target in chronic lung diseases. Am. J. Respir. Crit. Care Med. 2019;200:556–564. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 8.Kamata S., Fujino N., Yamada M., Grime K., Suzuki S., Ota C., Tando Y., Okada Y., Sakurada A., Noda M., et al. Expression of cytochrome P450 mRNAs in Type II alveolar cells from subjects with chronic obstructive pulmonary disease. Pharmacol. Res. Perspect. 2018;6 doi: 10.1002/prp2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyfman P.A., Walter J.M., Joshi N., Anekalla K.R., McQuattie-Pimentel A.C., Chiu S., Fernandez R., Akbarpour M., Chen C.I., Ren Z., et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019;199:1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer M.J., White T.A., Iijima K., Haak A.J., Ligresti G., Atkinson E.J., Oberg A.L., Birch J., Salmonowicz H., Zhu Y., et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017;8 doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan X.X., Rao D.N., Liu Y.Z., Zhou Y., Yang H.H. Epoxyeicosatrienoic acids and fibrosis: recent insights for the novel therapeutic strategies. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X.Q., Duan J.X., Yang H.H., Zhang C.Y., Sun C.C., Guan X.X., Xiong J.B., Zu C., Tao J.H., Zhou Y., et al. Epoxyeicosatrienoic acids inhibit the activation of NLRP3 inflammasome in murine macrophages. J. Cell. Physiol. 2020;235:9910–9921. doi: 10.1002/jcp.29806. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C.Y., Tan X.H., Yang H.H., Jin L., Hong J.R., Zhou Y., Huang X.T. COX-2/sEH dual inhibitor alleviates hepatocyte senescence in NAFLD mice by restoring autophagy through Sirt1/PI3K/AKT/mTOR. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23158267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H.H., Duan J.X., Liu S.K., Xiong J.B., Guan X.X., Zhong W.J., Sun C.C., Zhang C.Y., Luo X.Q., Zhang Y.F., et al. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics. 2020;10:4749–4761. doi: 10.7150/thno.43108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J., Zhao Q., Li M., Duan Q., Zhao Y., Zhang H. The effects of endothelium-specific CYP2J2 overexpression on the attenuation of retinal ganglion cell apoptosis in a glaucoma rat model. Faseb. J. 2019;33:11194–11209. doi: 10.1096/fj.201900756R. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C.Y., Duan J.X., Yang H.H., Sun C.C., Zhong W.J., Tao J.H., Guan X.X., Jiang H.L., Hammock B.D., Hwang S.H., et al. COX-2/sEH dual inhibitor PTUPB alleviates bleomycin-induced pulmonary fibrosis in mice via inhibiting senescence. FEBS J. 2020;287:1666–1680. doi: 10.1111/febs.15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du G., Liu Z., Yu Z., Zhuo Z., Zhu Y., Zhou J., Li Y., Chen H. Taurine represses age-associated gut hyperplasia in Drosophila via counteracting endoplasmic reticulum stress. Aging Cell. 2021;20 doi: 10.1111/acel.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabral-Miranda F., Tamburini G., Martinez G., Ardiles A.O., Medinas D.B., Gerakis Y., Hung M.D., Vidal R., Fuentealba M., Miedema T., et al. Unfolded protein response IRE1/XBP1 signaling is required for healthy mammalian brain aging. EMBO J. 2022;41 doi: 10.15252/embj.2022111952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.H., Lee J. Endoplasmic reticulum (ER) stress and its role in pancreatic beta-cell dysfunction and senescence in type 2 diabetes. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23094843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawson W.E., Crossno P.F., Polosukhin V.V., Roldan J., Cheng D.S., Lane K.B., Blackwell T.R., Xu C., Markin C., Ware L.B., et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;294:L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 21.Borok Z., Horie M., Flodby P., Wang H., Liu Y., Ganesh S., Firth A.L., Minoo P., Li C., Beers M.F., et al. Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2020;201:198–211. doi: 10.1164/rccm.201902-0451OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Ni L., Yang L., Duan Q., Chen C., Edin M.L., Zeldin D.C., Wang D.W. CYP2J2-derived epoxyeicosatrienoic acids suppress endoplasmic reticulum stress in heart failure. Mol. Pharmacol. 2014;85:105–115. doi: 10.1124/mol.113.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin X., Dai Y., Tong X., Xu W., Huang Q., Jin X., Li C., Zhou F., Zhou H., Lin X., et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. 2020;30 doi: 10.1016/j.redox.2020.101431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z., Zhang L., Zhou L., Lei Y., Zhang Y., Huang C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019;25 doi: 10.1016/j.redox.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y.S., Lee D.H., Choudry H.A., Bartlett D.L., Lee Y.J. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol. Cancer Res. 2018;16:1073–1076. doi: 10.1158/1541-7786.MCR-18-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanmugam G., Challa A.K., Litovsky S.H., Devarajan A., Wang D., Jones D.P., Darley-Usmar V.M., Rajasekaran N.S. Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice. Redox Biol. 2019;27 doi: 10.1016/j.redox.2019.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S., Jin S., Chen W., Yu J., Fang P., Zhou G., Li J., Jin L., Chen Y., Chen P., et al. Mangiferin alleviates endoplasmic reticulum stress in acute liver injury by regulating the miR-20a/miR-101a-Nrf2 axis. J. Biochem. 2020;168:365–374. doi: 10.1093/jb/mvaa056. [DOI] [PubMed] [Google Scholar]
- 28.Naresh Amin K., Rajagru P., Sarkar K., Ganesh M.R., Suzuki T., Ali D., Kunka Mohanram R. Pharmacological activation of Nrf2 by rosolic acid attenuates endoplasmic reticulum stress in endothelial cells. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/2732435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato K., Papageorgiou I., Shin Y.J., Kleinhenz J.M., Palumbo S., Hahn S., Irish J.D., Rounseville S.P., Knox K.S., Hecker L. Lung-targeted delivery of dimethyl fumarate promotes the reversal of age-dependent established lung fibrosis. Antioxidants. 2022;11 doi: 10.3390/antiox11030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatakeyama S. TRIM proteins and cancer. Nat. Rev. Cancer. 2011;11:792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 31.Ozato K., Shin D.M., Chang T.H., Morse H.C., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008;8:849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L., Zhu G., Johns E.M., Yang X. TRIM11 activates the proteasome and promotes overall protein degradation by regulating USP14. Nat. Commun. 2018;9:1223. doi: 10.1038/s41467-018-03499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo L., Giasson B.I., Glavis-Bloom A., Brewer M.D., Shorter J., Gitler A.D., Yang X. A cellular system that degrades misfolded proteins and protects against neurodegeneration. Mol. Cell. 2014;55:15–30. doi: 10.1016/j.molcel.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Tao S., Liao L., Li Y., Li H., Li Z., Lin L., Wan X., Yang X., Chen L. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat. Commun. 2020;11:348. doi: 10.1038/s41467-019-14190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang X., Yan Z., Ma W., Qian Y., Zou X., Cui Y., Liu J., Meng Y. Peroxiredoxin 4 protects against ovarian ageing by ameliorating D-galactose-induced oxidative damage in mice. Cell Death Dis. 2020;11:1053. doi: 10.1038/s41419-020-03253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao J., Liu J., Niu J., Zhang Y., Shen W., Luo C., Liu Y., Li C., Li H., Yang P., et al. Wnt/beta-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell. 2019;18 doi: 10.1111/acel.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H.H., Jiang H.L., Tao J.H., Zhang C.Y., Xiong J.B., Yang J.T., Liu Y.B., Zhong W.J., Guan X.X., Duan J.X., et al. Mitochondrial citrate accumulation drives alveolar epithelial cell necroptosis in lipopolysaccharide-induced acute lung injury. Exp. Mol. Med. 2022;54:2077–2091. doi: 10.1038/s12276-022-00889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong W.J., Liu T., Yang H.H., Duan J.X., Yang J.T., Guan X.X., Xiong J.B., Zhang Y.F., Zhang C.Y., Zhou Y., et al. TREM-1 governs NLRP3 inflammasome activation of macrophages by firing up glycolysis in acute lung injury. Int. J. Biol. Sci. 2023;19:242–257. doi: 10.7150/ijbs.77304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y., Li P., Duan J.X., Liu T., Guan X.X., Mei W.X., Liu Y.P., Sun G.Y., Wan L., Zhong W.J., et al. Aucubin alleviates bleomycin-induced pulmonary fibrosis in a mouse model. Inflammation. 2017;40:2062–2073. doi: 10.1007/s10753-017-0646-x. [DOI] [PubMed] [Google Scholar]
- 40.Liang S., Wang T., Hu X., Luo J., Li W., Wu X., Duan Y., Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 41.Zhong W.J., Yang H.H., Guan X.X., Xiong J.B., Sun C.C., Zhang C.Y., Luo X.Q., Zhang Y.F., Zhang J., Duan J.X., et al. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J. Cell. Physiol. 2019;234:4641–4654. doi: 10.1002/jcp.27261. [DOI] [PubMed] [Google Scholar]
- 42.Glauser G., Grund B., Gassner A.L., Menin L., Henry H., Bromirski M., Schutz F., McMullen J., Rochat B. Validation of the mass-extraction-window for quantitative methods using liquid chromatography high resolution mass spectrometry. Anal. Chem. 2016;88:3264–3271. doi: 10.1021/acs.analchem.5b04689. [DOI] [PubMed] [Google Scholar]
- 43.Huang P., Bai L., Liu L., Fu J., Wu K., Liu H., Liu Y., Qi B., Qi B. Redd1 knockdown prevents doxorubicin-induced cardiac senescence. Aging (Albany NY) 2021;13:13788–13806. doi: 10.18632/aging.202972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Chen R., Li G., Wang Z., Liu J., Liang Y., Liu J.P. FBW7 mediates senescence and pulmonary fibrosis through telomere uncapping. Cell Metabol. 2020;32:860–877 e869. doi: 10.1016/j.cmet.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Ostermann A.I., Herbers J., Willenberg I., Chen R., Hwang S.H., Greite R., Morisseau C., Gueler F., Hammock B.D., Schebb N.H. Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-(1-propanoylpiperidin-4-yl)-3-[4-(trifluoromethoxy)phenyl]urea (TPPU): resulting drug levels and modulation of oxylipin pattern. Prostag. Other Lipid Mediat. 2015;121:131–137. doi: 10.1016/j.prostaglandins.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao J.H., Liu T., Zhang C.Y., Zu C., Yang H.H., Liu Y.B., Yang J.T., Zhou Y., Guan C.X. Epoxyeicosatrienoic acids inhibit the activation of murine fibroblasts by blocking the TGF-beta 1-smad 2/3 signaling in a PPARgamma-dependent manner. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/7265486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell W.B., Imig J.D., Schmitz J.M., Falck J.R. Orally active epoxyeicosatrienoic acid analogs. J. Cardiovasc. Pharmacol. 2017;70:211–224. doi: 10.1097/FJC.0000000000000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuollo L., Antonangeli F., Santoni A., Soriani A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology. 2020;9 doi: 10.3390/biology9120485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baek A.R., Hong J., Song K.S., Jang A.S., Kim D.J., Chin S.S., Park S.W. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp. Mol. Med. 2020;52:2034–2045. doi: 10.1038/s12276-020-00545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faraonio R. Oxidative stress and cell senescence process. Antioxidants. 2022;11 doi: 10.3390/antiox11091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan H., Xu Y., Luo Y., Wang N.X., Xiao J.H. Role of Nrf2 in cell senescence regulation. Mol. Cell. Biochem. 2021;476:247–259. doi: 10.1007/s11010-020-03901-9. [DOI] [PubMed] [Google Scholar]
- 52.Victor P., Sarada D., Ramkumar K.M. Crosstalk between endoplasmic reticulum stress and oxidative stress: focus on protein disulfide isomerase and endoplasmic reticulum oxidase 1. Eur. J. Pharmacol. 2021;892 doi: 10.1016/j.ejphar.2020.173749. [DOI] [PubMed] [Google Scholar]
- 53.GBD Chronic Respiratory Disease Collaborators Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020;8:585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun K., Yang P., Zhao R., Bai Y., Guo Z. Matrine attenuates D-galactose-induced aging-related behavior in mice via inhibition of cellular senescence and oxidative stress. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/7108604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Locker F., Bieler L., Nowack L.M.F., Leitner J., Brunner S.M., Zaunmair P., Kofler B., Couillard-Despres S. Involvement of neuropeptide galanin receptors 2 and 3 in learning, memory and anxiety in aging mice. Molecules. 2021;26 doi: 10.3390/molecules26071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q., Ye W., Liu Y., Niu D., Zhao X., Li G., Qu Y., Zhao Z. S-allylmercapto-N-acetylcysteine ameliorates pulmonary fibrosis in mice via Nrf2 pathway activation and NF-kappaB, TGF-beta 1/Smad 2/3 pathway suppression. Biomed. Pharmacother. 2023;157 doi: 10.1016/j.biopha.2022.114018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewinska A., Adamczyk-Grochala J., Bloniarz D., Olszowka J., Kulpa-Greszta M., Litwinienko G., Tomaszewska A., Wnuk M., Pazik R. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe(3)O(4) nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Justice J.N., Nambiar A.M., Tchkonia T., LeBrasseur N.K., Pascual R., Hashmi S.K., Prata L., Masternak M.M., Kritchevsky S.B., Musi N., et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wils J., Djerada Z., Roca F., Duflot T., Iacob M., Remy-Jouet I., Joannides R., Bellien J. Alteration in the availability of epoxyeicosatrienoic acids contributes with NO to the development of endothelial dysfunction in conduit arteries during aging. Atherosclerosis. 2018;275:239–245. doi: 10.1016/j.atherosclerosis.2018.06.865. [DOI] [PubMed] [Google Scholar]
- 60.Kim H.S., Moon S.J., Lee S.E., Hwang G.W., Yoo H.J., Song J.W. The arachidonic acid metabolite 11,12-epoxyeicosatrienoic acid alleviates pulmonary fibrosis. Exp. Mol. Med. 2021;53:864–874. doi: 10.1038/s12276-021-00618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L., Cheriyan J., Gutterman D.D., Mayer R.J., Ament Z., Griffin J.L., Lazaar A.L., Newby D.E., Tal-Singer R., Wilkinson I.B. Mechanisms of vascular dysfunction in COPD and effects of a novel soluble epoxide hydrolase inhibitor in smokers. Chest. 2017;151:555–563. doi: 10.1016/j.chest.2016.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada Z., Nishio J., Motomura K., Mizutani S., Yamada S., Mikami T., Nanki T. Senescence of alveolar epithelial cells impacts initiation and chronic phases of murine fibrosing interstitial lung disease. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.935114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao C., Guan X., Carraro G., Parimon T., Liu X., Huang G., Mulay A., Soukiasian H.J., David G., Weigt S.S., et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2021;203:707–717. doi: 10.1164/rccm.202004-1274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birch J., Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehmann M., Korfei M., Mutze K., Klee S., Skronska-Wasek W., Alsafadi H.N., Ota C., Costa R., Schiller H.B., Lindner M., et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017;50 doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koloko Ngassie M.L., Brandsma C.A., Gosens R., Prakash Y.S., Burgess J.K. The stress of lung aging: endoplasmic reticulum and senescence tete-a-tete. Physiology. 2021;36:150–159. doi: 10.1152/physiol.00039.2020. [DOI] [PubMed] [Google Scholar]
- 67.Sun N., Yang T., Tang Y., Zhao Y., Wang H., Zhao S., Tan H., Li L., Fan H. Lycopene alleviates chronic stress-induced liver injury by inhibiting oxidative stress-mediated endoplasmic reticulum stress pathway apoptosis in rats. J. Agric. Food Chem. 2022;70:14414–14426. doi: 10.1021/acs.jafc.2c06650. [DOI] [PubMed] [Google Scholar]
- 68.Ulasov A.V., Rosenkranz A.A., Georgiev G.P., Sobolev A.S. Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci. 2022;291 doi: 10.1016/j.lfs.2021.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L., Yang X. TRIM11 cooperates with HSF1 to suppress the anti-tumor effect of proteotoxic stress drugs. Cell Cycle. 2019;18:60–68. doi: 10.1080/15384101.2018.1558870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L., Brewer M.D., Guo L., Wang R., Jiang P., Yang X. Enhanced degradation of misfolded proteins promotes tumorigenesis. Cell Rep. 2017;18:3143–3154. doi: 10.1016/j.celrep.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spector A.A., Fang X., Snyder G.D., Weintraub N.L. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 72.Imig J.D., Hammock B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.