Abstract
Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy is a rare autoimmune disorder. Reversible splenial lesion syndrome (RESLES) is a transient clinical-imaging syndrome characterized by specific magnetic resonance imaging (MRI) pattern. A 58-year-old man was admitted with a fever, headache, and confusion for 1 week. Brain MRI showed abnormal leptomeningeal enhancement in the brainstem and high signal intensity on diffusion-weighted MRI of the corpus callosum. Anti-GFAP antibody was positive in the serum and cerebrospinal fluid analysis. This patient significantly improved and had no relapse after glucocorticoid and immune suppressant therapy. A repeated brain MRI revealed the lesion in the corpus callosum and abnormal leptomeningeal enhancement in the brainstem disappeared. Linear perivascular radial enhancement is the characteristic pattern of autoimmune GFAP astrocytopathy which is rarely coexistent with RESLES.
Keywords: autoimmune glial fibrillary acidic protein astrocytopathy, reversible splenial lesion syndrome (RESLES), magnetic resonance imaging, linear perivascular radial enhancement, leptomeningeal enhancement
Introduction
Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy, first described in 2016, is an immune-mediated central nervous system disorder characterized by GFAP antibody (1). This disease mainly involves the meninges, spinal cord, and cerebra, and its predominant clinical syndrome include encephalopathy, myelitis, fever, headache, and epilepsy (2). The characteristic imaging feature of autoimmune GFAP astrocytopathy is linear perivascular radial gadolinium enhancement, but its diagnosis mainly depends on GFAP-IgG in cerebrospinal fluid (2).
Reversible splenial lesion syndrome (RESLES) is a transient clinical-imaging syndrome characterized by the presence of a focal lesion often involving the corpus callosum (3). RESLES was first described to be triggered by antiepileptic drugs, and then it has been reported to be secondary to a variety of factors, including infection, metabolic disorders, and autoimmune diseases (4, 5).
Herein, we report an autoimmune GFAP astrocytopathy concurrent with RESLES in an adult patient. Additionally, we discuss the imaging features of autoimmune GFAP astrocytopathy, with respect to the results of previous studies.
Case presentation
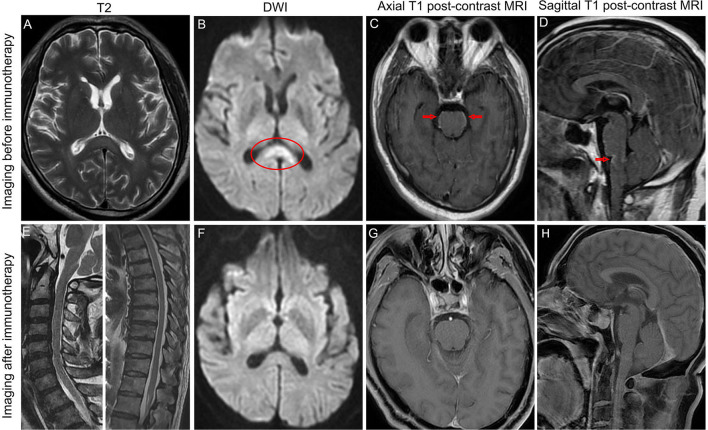
A 58-year-old man presented with a fever (39.8°C), headache, and confusion for 1 week. He has no previous history of other illnesses or medication use. A neurological examination demonstrated neck stiffness, positive Kernig's sign, and motor power grade 4 in both lower extremities. Blood routine examination, C-reactive protein, and procalcitonin were in the normal range. The cerebrospinal fluid (CSF) sample displayed normal open pressure (110 mmH2O), slightly elevated nucleated cells of 80/ul (normal range: 0–10/ul), significantly elevated protein of 158.71 mg/L (normal range: 15–45 mg/dL), and decreased glucose level of 2.15 mmol/L (blood glucose: 7.29 mmol/L). Brain magnetic resonance imaging (MRI) revealed abnormal leptomeningeal enhancement in the brainstem (Figures 1C, D). In addition, brain MRI showed the high signal intensity of the corpus callosum on T2 sequence MRI (Figure 1A) and diffusion-weighted MRI (Figure 1B). Possible tuberculous meningoencephalitis was diagnosed and anti-tuberculosis therapy was started with oral isoniazid, rifampicin, pyrazinamide, and ethambutol.
Figure 1.
Brain MRI revealed hyperintense of the corpus callosum on T2 sequence MRI (A) and diffusion-weighted MRI (B) before immunotherapy. MRI postcontrast axial T1-weighted scans (C) and sagittal T1-weighted scans (D) showed leptomeningeal enhancement of the pons and medulla before immunotherapy. Sagittal T2 sequence MRI showed no hyperintensities throughout the cervical spinal cord and thoracic spinal cord (E). The high signal intensity of the corpus callosum on diffusion-weighted MRI disappeared after immunotherapy (F). MRI postcontrast axial T1-weighted scans and sagittal T1-weighted scans showed that leptomeningeal enhancement of the pons and medulla completely disappeared after immunotherapy (G, H). Arrows indicate leptomeningeal enhancement. Oval shape indicates the lesion in the corpus callosum.
A few days after admission, acute delirium and urinary retention developed. A repeated lumber puncture showed high open pressure (250 mmH2O), normal nucleated cells, significantly elevated protein (119.13 mg/L), and decreased glucose level (2.65 mmol/L).
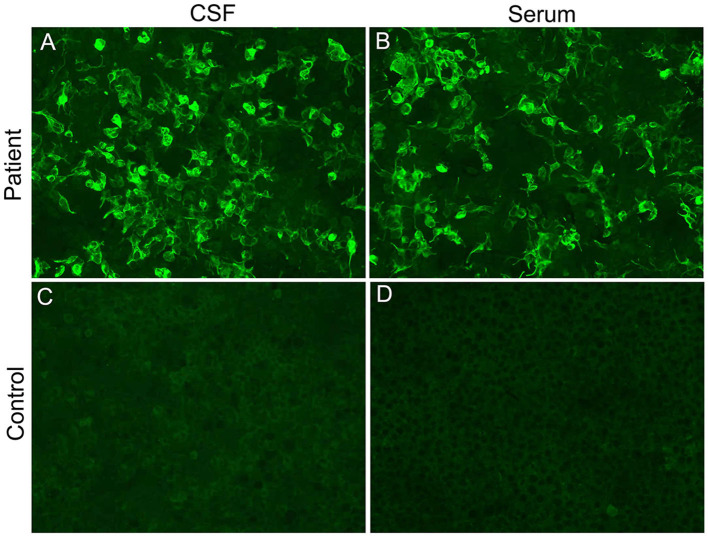
On the 13th day after admission, the patient's condition deteriorated again, with light coma (remaining light reflex and responding to pain) and epilepsy. The neurological examination revealed motor power grade 0 in both lower extremities, tendon areflexia, and limited bilateral abduction. Second-generation sequencing (BGI platform, China) for CSF in this patient revealed no abnormalities. We sent an autoimmune encephalitis panel and demyelinating antibody panel including serum and CSF samples, and the anti-GFAP antibody was positive (1:10) in the serum and CSF analysis (Figure 2). Subsequently, anti-tuberculosis therapy was discontinued and the patient was treated with intravenous 500 mg methylprednisolone per day for 5 days, 240 mg per day for 5 days, 120 mg per day for 5 days, and followed by a slow tapering dose of prednisone over half year. Additionally, the patient received oral immune suppressants (mycophenolate mofetil 500 mg twice daily) for 6 months.
Figure 2.
Cell-based indirect immunofluorescence assays demonstrating positive GFAPα-immunoglobulin G in CSF (A) and serum (B) from our patient. (C, D) represent negative controls.
On the fifth day after intravenous methylprednisolone, the patient's consciousness and motor power improved significantly. After completing glucocorticoid pulse therapy, all symptoms of the patient disappeared except urinary retention, and a repeat brain MRI revealed the lesion in the corpus callosum and abnormal leptomeningeal enhancement in the brainstem disappeared (Figures 1F–H). In addition, the spine MRI showed no T2 hyperintensities throughout the cervical spinal cord and thoracic spinal cord (Figure 1E). For urinary retention, we give oral vitamins B1 and B12, and acupuncture and moxibustion rehabilitation training. At follow-up 1 year after symptom onset, the urinary retention of our patient partly improved, and the patient remained clinically stable and had no relapse.
Discussion
Reversible splenial lesion syndrome (RESLES) is a rare clinical-imaging syndrome involving the corpus callosum caused by various causes (3). It is characterized by oval, non-enhanced T2 lesions in the splenium of the corpus callosum on MRI, which can completely disappear after a variable period of time (3). This syndrome was related to a variety of factors including antiepileptic drugs, infections, hypoglycemia, hyperglycemia, and electrolyte imbalance (4, 5). Recently, a potential association between autoimmune processes and RESLES has been documented in cases such as N-methyl-D-aspartate receptor encephalitis (6), autoantibodies against voltage-gated potassium channels (7), autoimmune thyroid disease (8), and so on. Herein we report a rare case of autoimmune GFAP astrocytopathy coexistent with RESLES.
Currently, there are no uniform diagnostic criteria or consensus for autoimmune GFAP astrocytopathy. The diagnosis of autoimmune GFAP astrocytopathy may be based on the presence of GFAP antibodies in the CSF and clinical scpectrum (9). The classical phenotype of autoimmune GFAP astrocytopathy is meningoencephalitis or myelomeningoencephalitis but the clinical manifestations are broader, including fever, headache, delirium, seizures, tremor, and so on (2). Our patient presented with fever, headache, confusion, seizures, and myelomeningoencephalitis. Additionally, the initial CSF results suggested tuberculous meningoencephalitis, and then anti-tuberculosis therapy was started. However, an anti-GFAP antibody was detected positive in the serum and CSF analysis in our patient. Furthermore, we excluded autoimmune encephalitis and central nervous system infection through negative autoimmune encephalitis antibodies and negative second-generation sequencing in this patient. Taken together, this patient should be diagnosed with autoimmune GFAP astrocytopathy, and anti-tuberculosis therapy was discontinued.
Our search of the PubMed database focusing on autoimmune GFAP astrocytopathy yielded 10 studies that reported more than 10 cases. The imaging profiles of these reports are summarized in Table 1. The most frequent brain MRI finding of autoimmune GFAP astrocytopathy was hyperintense lesions in the cerebra on T2 sequence such as basal ganglia, thalamus, cerebral white matter, brainstem, and so on. However, it has been reported linear perivascular radial enhancement is a hallmark of autoimmune GFAP astrocytopathy and was observed in 42.3% of patients. Moreover, 29.8% of patients had leptomeningeal enhancement. Longitudinally extensive transverse myelitis (more than three vertebra segments) was the most common finding in autoimmune GFAP astrocytopathy, followed by enhancement of medullary cone surface and intramedullary enhancement. More importantly, autoimmune GFAP astrocytopathy coexistent with RESLES was rarely reported, only one study has summarized this image feature and 4 out of 39 autoimmune GFAP astrocytopathy patients had RESLES (17). Furthermore, four recent cases who had autoimmune GFAP astrocytopathy coincident with RESLES were reported (19–22). However, five sporadic cases including our patient with autoimmune GFAP astrocytopathy and RESLES showed no obvious imaging association between linear perivascular radial enhancement and RESLES. Additionally, RESLES had been reported in cases of anti-Yo encephalitis (23), anti-NMDAR encephalitis (24), and anti-VGKC encephalitis (7), respectively. Taken together with the findings from previous case series, our present findings suggested RESLES may be linked to autoimmune processes and is rarely coexistent with autoimmune GFAP astrocytopathy.
Table 1.
The imaging summarization of autoimmune GFAP astrocytopathy patients.
| Fang et al. (1) | Flanagan et al. (10) | Dubey et al. (11) | Iorio et al. (12) | Long et al. (13) | Yang et al. (14) | Kimura et al. (15) | Fang et al. (16) | Gravier et al. (17) | Liao et al. (18) | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain MRI | |||||||||||
| Hyperintense lesions on T2WI/FLAIR | 9/12 (75%) | 18/32 (56%) | NA | 10/22 (45%) | 8/19 (42.1%) | 9/10 (90%) | 9/14 (64%) | 30/35 (85.7%) | 20/39 (51.3%) | 13/15 (86.6%) | 126/198 (63.6%) |
| Linear perivascular radial enhancement | 6/12 (50%) | 17/32 (53%) | 36/71 (51%) | NA | 8/19 (42.1%) | 4/10 (40%) | 4/9 (44.4%) | 3/35 (8.5%) | 12/38 (31.6%) | 12/15 (80%) | 102/241 (42.3%) |
| Leptomeningeal enhancement | 4/12 (33%) | 7/32 (22%) | NA | NA | NA | NA | 3/9 (33.3%) | 6/35 (17.1%) | 10/38 (26.3%) | 12/15 (80%) | 42/141 (29.8%) |
| RESLES | NA | NA | NA | NA | NA | NA | NA | NA | 4/39 (10.3%) | NA | 4/39 (10.3%) |
| Spinal MRI | |||||||||||
| Longitudinally extensive T2 hyperintensity | 5/7 (71%) | 6/8 (75%) | NA | 3/9 (33%) | 11/16 (68.8%) | 6/9 (66.7%) | NA | 5/35 (14.3%) | 9/11 (81.2%) | 5/15 (33.3%) | 50/110 (45.5%) |
| Intramedullary enhancement | NA | NA | NA | NA | NA | NA | 1/7 (14%) | 3/35 (8.5%) | 4/11 (36.4%) | 7/15 (46.7%) | 15/68 (22.1%) |
| Enhancement of medullary cone surface | NA | NA | NA | NA | NA | NA | 5/7 (71%) | 3/35 (8.5%) | 4/11 (36.4%) | 9/15 (60%) | 21/68 (32.3%) |
GFAP, glial fibrillary acidic protein; MRI, magnetic resonance imaging; NA, not available; FLAIR, fluid-attenuated inversion-recovery sequency; RESLES, reversible splenial lesion syndrome.
Conclusion
In summary, the most frequent brain MRI finding of autoimmune GFAP astrocytopathy was hyperintense lesions in the cerebra on the T2 sequence. However, linear perivascular radial enhancement is a hallmark of autoimmune GFAP astrocytopathy. Autoimmune GFAP astrocytopathy coexistent with RESLES is rare.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL and JH contributed to the conception and design of the study. JL wrote the initial draft of the manuscript. LD and LY contributed to the clinical analysis. JH contributed to the manuscript's revision. All authors read and approved the submitted version.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 82101405).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Fang B, McKeon A, Hinson SR, Kryzer TJ, Pittock SJ, Aksamit AJ, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. (2016) 73:1297–307. 10.1001/jamaneurol.2016.2549 [DOI] [PubMed] [Google Scholar]
- 2.Kunchok A, Zekeridou A, McKeon A. Autoimmune glial fibrillary acidic protein astrocytopathy. Curr Opin Neurol. (2019) 32:452–8. 10.1097/WCO.0000000000000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. (2004) 63:1854–8. 10.1212/01.WNL.0000144274.12174.CB [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Monco JC, Cortina IE, Ferreira E, Martínez A, Ruiz L, Cabrera A, et al. Reversible splenial lesion syndrome (RESLES): what's in a name? J Neuroimaging. (2011) 21:e1–14. 10.1111/j.1552-6569.2008.00279.x [DOI] [PubMed] [Google Scholar]
- 5.Thabet M, Atig A, Fathallah N, Guiga A, Yahia WB, Ouni B, et al. Rituximab-induced mild encephalopathy with a reversible splenial lesion syndrome (MERS): an adverse effect to add to the list. Br J Clin Pharmacol. (2022) 88:2969–72. 10.1111/bcp.15189 [DOI] [PubMed] [Google Scholar]
- 6.Kaminski JA, Pruss H. N-methyl-d-aspartate receptor encephalitis with a reversible splenial lesion. Eur J Neurol. (2019) 26:e68–9. 10.1111/ene.13900 [DOI] [PubMed] [Google Scholar]
- 7.Gilder TR, Hawley JS, Theeler BJ. Association of reversible splenial lesion syndrome (RESLES) with anti-VGKC autoantibody syndrome: a case report. Neurol Sci. (2016) 37:817–9. 10.1007/s10072-015-2464-y [DOI] [PubMed] [Google Scholar]
- 8.De Greef J, Jaumotte C, Quivron B, Derue G. Reversible splenial lesion in auto-immune thyroid disease: a case report. Acta Clin Belg. (2014) 69:208–9. 10.1179/2295333713Y.0000000002 [DOI] [PubMed] [Google Scholar]
- 9.Shan F, Long Y, Qiu W. Autoimmune glial fibrillary acidic protein astrocytopathy: a review of the literature. Front Immunol. (2018) 9:2802. 10.3389/fimmu.2018.02802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flanagan EP, Hinson SR, Lennon VA, Fang B, Aksamit AJ, Morris PP, et al. Glial fibrillary acidic protein immunoglobulin Gas biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann Neurol. (2017) 81:298–309. 10.1002/ana.24881 [DOI] [PubMed] [Google Scholar]
- 11.Dubey D, Hinson SR, Jolliffe EA, Zekeridou A, Flanagan EP, Pittock SJ, et al. Autoimmune GFAP astrocytopathy: Prospective evaluation of 90 patients in 1?year. J Neuroimmunol. (2018) 321:157-63. 10.1016/j.jneuroim.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 12.Iorio R, Damato V, Evoli A, Gessi M, Gaudino S, Di Lazzaro V, et al. Clinical and immunological characteristics of the spectrum of GFAP autoimmunity: a case series of 22 patients. J Neurol Neurosurg Psychiatry. (2018) 89:138–46. 10.1136/jnnp-2017-316583 [DOI] [PubMed] [Google Scholar]
- 13.Long Y, Liang J, Xu H, Huang Q, Yang J, Gao C, et al. Autoimmune glial fibrillary acidic protein astrocytopathy in Chinese patients: a retrospective study. Eur J Neurol. (2018) 25:477–83. 10.1111/ene.13531 [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Xu H, Ding M, Huang Q, Chen B, Yang H, et al. Overlapping autoimmune syndromes in patients with glial fibrillary acidic protein antibodies. Front Neurol. (2018) 9:251. 10.3389/fneur.2018.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura A, Takekoshi A, Yoshikura N, Hayashi Y, Shimohata T. Clinical characteristics of autoimmune GFAP astrocytopathy. J Neuroimmunol. (2019) 332:91–8. 10.1016/j.jneuroim.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Fang H, Hu W, Jiang Z, Yang H, Liao H, Yang L, et al. Autoimmune glial fibrillary acidic protein astrocytopathy in children: A retrospective analysis of 35 cases. Front Immunol. (2021) 12:761354. 10.3389/fimmu.2021.761354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gravier-Dumonceau A, Ameli R, Rogemond V, Ruiz A, Joubert B, Muñiz-Castrillo S, et al. Glial fibrillary acidic protein autoimmunity: a French cohort study. Neurology. (2022) 98:e653–68. 10.1212/WNL.0000000000013087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao H, Chen Q, Zhang M, Chen W. MRI features and evolution of autoimmune glial fibrillary acidic protein astrocytopathy: A retrospective cross-sectional and longitudinal study. Mult Scler Relat Disord. (2022) 58:103512. 10.1016/j.msard.2022.103512 [DOI] [PubMed] [Google Scholar]
- 19.Oger V, Bost C, Salah L, Yazbeck E, Maurey H, Bellesme C, et al. Mild Encephalitis/Encephalopathy with reversible splenial lesion syndrome: an unusual presentation of anti-GFAP astrocytopathy. Eur J Paediatr Neurol. (2020) 26:89–91. 10.1016/j.ejpn.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 20.Nakamura S, Fujioka T, Kawashima S, Kawaguchi T, Mizuno M, Omura M, et al. Self-remitting elevation of adenosine deaminase levels in the cerebrospinal fluid with autoimmune glial fibrillary acidic protein astrocytopathy: a case report and review of the literature. Intern Med. (2021) 60:3031–6. 10.2169/internalmedicine.6457-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issa N, Martin C, Dulau C, Camou F. Severe anti-GFAP meningo-encephalomyelitis following viral infection. Mult Scler Relat Disord. (2020) 45:102448. 10.1016/j.msard.2020.102448 [DOI] [PubMed] [Google Scholar]
- 22.Guo K, Lai X, Liu Y, Zhou D, Hong Z. Anti-glial fibrillary acidic protein antibodies as a cause of reversible splenial lesion syndrome (RESLES): a case report. Neurol Sci. (2021) 42:3903–7. 10.1007/s10072-021-05376-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renard D, Taieb G, Briere C, Bengler C, Castelnovo G. Mild encephalitis/encephalopathy with a reversible splenial, white matter, putaminal, and thalamic lesions following anti-Yo rhombencephalitis. Acta Neurol Belg. (2012) 112:405–7. 10.1007/s13760-012-0080-7 [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Zhang M, Liu D, Wei M, Sheng J, Wang Z, et al. Case report: Autoimmune encephalitis with multiple auto-antibodies with reversible splenial lesion syndrome and bilateral ovarian teratoma. Front Immunol. (2022) 13:1029294. 10.3389/fimmu.2022.1029294 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.