Abstract

Vanadium oxide (V2O5) and carbon spheres (Cs)-doped NiO2 nanostructures (NSs) were prepared using the co-precipitation approach. Several spectroscopic and microscopic techniques, including X-ray diffraction (XRD), UV–vis, FTIR, TEM, and HR-TEM investigations, were used to describe the as-synthesized NSs. The XRD pattern exhibited the hexagonal structure, and the crystallite size of pristine and doped NSs was calculated as 29.3, 32.8, 25.79, and 45.19 nm, respectively. The control sample (NiO2) showed maximum absorption at 330 nm, and upon doping, a redshift was observed, leading to decreased band gap energy from 3.75 to 3.59 eV. TEM of NiO2 shows agglomerated nonuniform nanorods exhibited with various nanoparticles without a specific orientation; a higher agglomeration was observed upon doping. The (4 wt %) V2O5/Cs-doped NiO2 NSs served as superior catalysts with a 94.21% MB reduction in acidic media. The significant antibacterial efficacy was estimated against Escherichia coli by measuring the zone of inhibition (3.75 mm). Besides their bactericidal analysis, V2O5/Cs-doped NiO2 was shown to have a binding score of 6.37 for dihydrofolate reductase and a binding score of 4.31 for dihydropteroate synthase in an in silico docking study of E. coli.
1. Introduction
Rapid growth in population, chemical fertilizers, industrialization, and increased urban wastewater outputs have revealed the most significant environmental issues.1 The primary contaminants in polluted water are waste from several industries, including paper, plastic, leather, food, textile sectors, and organic dyes.2 The most frequently employed cationic dye in industries is methylene blue (MB). The primary adverse effects of this harmful dye in humans include respiratory irritation, teratogenicity, and carcinogenicity.3−5 The dye pollutants are often cleaned using various physical, biological, and chemical techniques such as catalysis, flocculation, absorption, aeration, and photooxidation.6−8 Among them, the dye is broken down by a catalytic process, while nanomaterials are present that are relatively simple, cost-effective, and eco-friendly.6
Metal oxides (MOs) have gained much attention in the catalysis process attributed to their minimal toxicity, high chemical stability, inexpensive, and environmentally favorable qualities.6 MOs have significant adsorption and catalytic properties as well as reactivity and sorption capacity assigned to their large specific surface area and small size. According to the literature, a number of prepared MOs,9 such as La2O3,10 Er2O3,11 Tm2O3,12 and Y2O3,13 are considered chemically stable and toxic and have broad band gaps (∼4.3–7 eV). On the other hand, nickel oxide (NiO2) is a feasible alternative since it is commonly accessible, inexpensive, and electrochemically stable, with a minimal band gap of 3.6–4.0 eV.14 Furthermore, the catalytic abilities and chemical stability of NiO2 can be enhanced by suitable dopants.15
In recent studies, many polymers have been used as dopants for the reduction of MB, such as polyvinylpyrrolidone, polyaniline, polydopamine, poly(N-vinylcaprolactam), and carbon spheres (Cs).16−20 Many researchers used chitosan, cellulose nanocrystal, and poly(acrylic acid) in the MO that degraded MB 88% for 220 min, 70.6% after 30 min, and 65% in 60 min, respectively.21−23 The above-mentioned catalysts/photocatalysts have some drawbacks such as a low photoresponse toward visible light, high cost, and longer reduction time. Among them, Cs is a potential contender in the realm of catalysis due to the availability of various surface functional groups (such as carbonyl, hydroxyl, and carboxylic) with a high specific surface area, superior chemical durability, and high biocompatibility.24,25 Furthermore, V2O5 has excellent redox properties and can be used as a co-catalyst. It has a 3d3-4s2 valence electron layer structure with a remarkable stability, a large surface area, a high energy density, and a high conductivity.26−28
Most of these above-reported catalysts/photocatalysts are related to limitations such as the low photoresponse toward visible light, high cost, and longer degradation time. The proposed material (2 and 4 wt %) V2O5/Cs-doped NiO2 nanostructures (NSs) would be the best candidate for catalytic and antimicrobial activities. The potential of this work is that Cs have a wide surface area and porous structure and promote NiO2 adsorption and reaction, which can improve contact between the NiO2 and the microbes, resulting in a more effective antimicrobial and catalytic activity. Furthermore, adding V2O5 into Cs-doped NiO2 can improve its catalytic and antimicrobial activity by increasing the availability of reactive oxygen species (ROS) and boosting redox reactions. The pathogen Escherichia coli, which broadly spreads through contaminated water and causes diarrhea, kills 1.3 million children globally yearly.29 In this regard, NSs that are tiny, mobile, and conductive prevent bacterial adherence.30,31
The co-precipitation approach has been employed in this study to synthesize V2O5/Cs-doped NiO2 NSs. Characterizations were carried out utilizing techniques such as XRD, FTIR, TEM, HR-TEM, UV–vis, and PL for a detailed study. The prepared ternary system composite can remove organic dyes like MB. Furthermore, their antibacterial experiments against E. coli (Gram-negative) bacteria were carried out through the agar well diffusion method, and the binding propensity of NSs was investigated using molecular docking predictions.
2. Experimental Section
2.1. Materials
Nickel nitrate hexahydrate [Ni(NO3)2·6H2O, 98%] has been purchased from VWR Chemicals, and NaOH glucose and V2O5 were obtained from Sigma-Aldrich (Germany). The entire chemicals were in pure form and used without any further purification.
2.2. Synthesis
2.2.1. Preparation of Cs
The Cs have been synthesized using a hydrothermal carbonization method. Initially, 1 M glucose solution was diluted under steady stirring to achieve a clear and transparent solution. The colloidal solution was placed in a stainless autoclave at 180 °C for 12 h. The obtained precipitates were washed several times using deionized (DI) water and dried overnight at 100 °C (Figure 1a).
Figure 1.

(a, b) Schematic illustration of Cs and V2O5/Cs-doped NiO2 synthesis.
2.2.2. Synthesis of V2O5/Cs-Doped NiO2
NiO2 was prepared through a chemical co-precipitation route using Ni(NO3)2·6H2O as a precursor under continuous stirring at 100 °C. After 30 min, 1 M NaOH was incorporated to maintain pH ≈ 12 and to obtain precipitates. Furthermore, the obtained precipitates have been washed through centrifugation at 7000 rpm for 7 min several times. The washed precipitates were dried at 150 °C for 12 h to sustain the fine powder. The same procedure was carried out to prepare a fixed amount of Cs and different concentrations (2 and 4 wt %) of V2O5-doped NiO2 (Figure 1b).
2.3. Catalytic Activity
The catalytic activity (CA) of NiO2 and (2 and 4 wt %) V2O5/Cs-doped NiO2 NSs for MB reduction was investigated in sodium borohydride (NaBH4). MB and NaBH4 solutions were prepared freshly to perform CA, and 400 μL of NaBH4 was incorporated into the MB solution to check the %age reduction. Afterward, the freshly prepared nanocatalyst solution (400 μL) was introduced into the above solution, and MB color started to change into its leuco form. As a direct result, a UV–vis spectrophotometer was employed to obtain consistent recordings of the absorption variation spectra. A formula was used to calculate the percentage of degradation:
where Co is the initial concentration of MB dye solution and Ct is the specific time concentration after incorporating NSs.
2.4. Isolation and Identification of MDR E. coli
2.4.1. Isolation of E. coli
2.4.1.1. Sample Collection
Raw milk samples have been gathered from the veterinary form sold at different markets, veterinary clinics, and farms in Punjab, Pakistan. The milk was then directly poured into sterile glassware. Raw milk was gathered at 4 °C and transported to the lab immediately. Coliforms discovered in raw milk were enumerated on MacConkey agar. All plates were kept in an incubator for 48 h at 37 °C.
2.4.2. Identification and Characterization of Bacterial Isolates
Initial recognition of E. coli was based on the colonial structure by Gram stain and by numerous biochemical investigations with reverence to Bergey’s Manual of Determinative Bacteriology E. coli.32
2.4.2.1. Antibiotic Susceptibility
To determine the antibiotic susceptibility of the sample, the agar well diffusion method was used, which was carried out on Mueller–Hinton agar (MHA).33 The antimicrobial tests were conducted to investigate the E. coli’s resistance to antimicrobial classes such as cephalosporins (ceftriaxone (Cro) 30 μg), aminoglycosides (gentamicin (Gm) 10 μg), quinolones (ciprofloxacin (Cip) 5 μg), carbapenems (imipenem (Imi) 10 μg), penicillins (amoxicillin (A) 30 μg), tetracyclines (tetracycline (Te) 30 μg), and macrolides (azithromycin (Azm) 15 μg).34 Following the McFarland standard, a turbidity level of 0.5 was attained by growing pure E. coli cultures. The bacteria were distributed throughout MHA (Oxoid Limited, Basingstoke, United Kingdom), and antibiotic discs were subsequently positioned at a distance on the surface of the inoculation plate. This was done to prevent interfering with inhibitory zones. The plates were incubated for 48 h at 37 °C, and the data were examined by Diagnostic and Laboratory Standard Institute instructions.35 Bacteria resistant to at least three antibiotics were proclaimed MDR.36
2.4.2.2. Antimicrobial Activity
A good diffusion test evaluated the microbicidal performance of the pure (2 and 4 wt %) V2O5/Cs-doped NiO2 NSs against pathogenic E. coli. MacConkey agar was treated with MDR E. coli at a concentration of 1.5 × 108 CFU/mL (0.5 McFarland standard). To examine the bactericidal capability of pristine and doped NSs, E. coli infection bacteria were successfully isolated from ovine mastitis milk. On MacConkey agar and mannitol salt plates that had been cleaned with 0.5 McFarland of E. coli, piercing wells with an internal diameter of approximately 6 mm were punctured using a sterilized borer. The conventional antibiotic ciprofloxacin (5 mg/50 μL) was employed as a positive control, while 50 μL of DI water functioned as a negative control. After that, the boreholes were filled with pristine and doped NSs at high and low concentrations (1.0 mg/50 μL and 0.5 mg/50 μL), respectively.37 Then, the plates were contaminated aerobically for 12 h at 37 °C, and the inhibited zones around the surroundings of the wellbores were determined utilizing a vernier caliper to determine the antimicrobial properties.
2.4.2.3. Statistical Analysis
Zones of inhibition were used to assess antimicrobial performance and their diameters were subjected to statistical analysis using one-way analysis of variance (ANOVA) in SPSS 20.38
2.5. Molecular Docking Study
2.5.1. Method
The proposed mode of action for V2O5/Cs-doped NiO2 has been predicted using in silico docking, a potential method for determining the distinctive structural characteristic underpinning microbicidal activity. Dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS), two essential enzymes in folate biosynthesis, have been singled out as promising antibiotic targets. The three-dimensional structures of target enzymes were obtained from the Data Bank of Protein and then created using a protein synthesis tool to facilitate the docking of V2O5/Cs-doped NiO2 into the active site. Preferred targets were tagged with the annexation codes 2ANQ (DHFRE.coli)39 and 5U0V (DHPSE.coli).40 The SYBYL-X 2.0 program was used for the docking investigation. As in our previous work,41,42 we used SYBYL-X 2.0 to model compounds in three dimensions and evaluated nanoparticle binding affinities with the residues found in the active sites of certain proteins.
3. Results and Discussion
The phase composition and structural characteristics of prepared NiO2 and (2 and 4 wt %) V2O5/Cs-doped NiO2 NSs were examined using XRD, ranging between 2θ = 20 and 80° (Figure 2a). Diffraction peaks sited at 33.0, 38.5, 52.0, 59.0, 62.6, and 72.8° associated with crystal planes (010), (011), (012), (110), (111), (020), and (112), respectively, well matched with JCPDS card 96-901-1315, having a hexagonal crystal structure and space group p-3m1. The XRD patterns showed no extra peak, confirming the formation of single-phase NiO2. An XRD spectrum of 4 wt % showed an additional diffraction peak of V2O5 at 29.5° (400).43 This result indicates that the doping of V2O5 with low concentrations has a minor effect on the phase structure.44 Consequently, the peaks were shifted toward higher 2θ values, suggesting that the Cs is present in the pristine matrix and filling the void between Ni and O. The XRD peaks shift toward a higher angle corresponding with interstitial site doping.45 In addition, crystalline defects, lattice deformation, domain size dispersion, and crystalline domain size cause peak broadness in the doped samples.46 The peak intensity was diminished upon doping V2O5 compared to the pristine and Cs-doped NiO2. The crystallite size from the most instance peak (010) for pure and doped NiO2 NSs was measured using the Debye–Scherrer formula as 29.3, 32.8, 25.79, and 45.19 nm.
Figure 2.
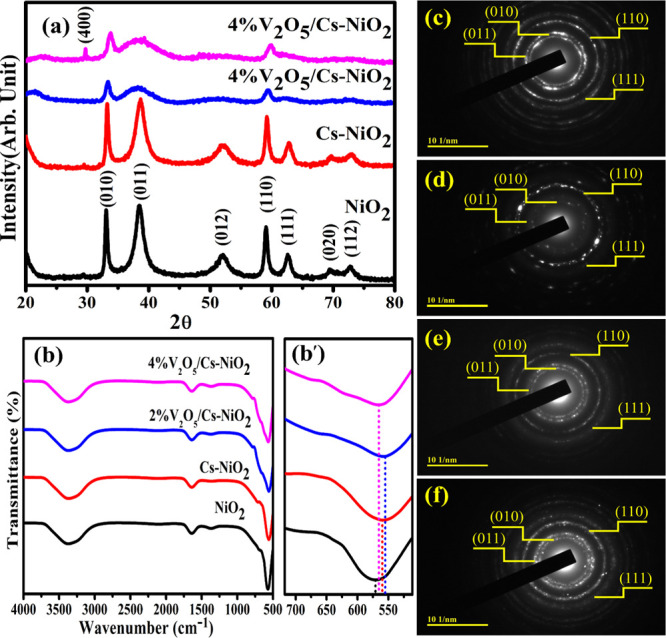
(a) XRD pattern of NSs, (b) FTIR spectra of NSs, (b′) zoom area of the Ni–O band, and SAED pattern of (c) NiO2, (d) Cs-doped NiO2, (e) (2 wt %) V2O5/Cs-doped NiO2, and (f) (4 wt %) V2O5/Cs-doped NiO2.
FTIR spectroscopy used to identify the functional groups (4000 to 1500 cm–1) and the fingerprint regions (1500 to 400 cm–1) on the surfaces of NSs are shown in Figure 2b.47 The O–H stretching vibration causes the broad and intense band at 3373 cm–1. Furthermore, the band observed at 1648 cm–1 were assigned to the bending and stretching vibrations of the −OH group absorbed on the catalyst surface during the FTIR measurement.48 The peak at 1368 cm–1 probably refers to some leftovers of the precursor elements utilized to prepare the NiO2.49 Ni–O bonds are responsible for absorption peaks beneath 1000 cm–1.50 The sharp band at 570 cm–1 was caused by the Ni–O stretching vibrations.51 Upon doping of Cs and V2O5, peaks were shifted toward a lower wavenumber (Figure 2b′). The blue shift of doped and NiO2 NSs was linked to the quantum size effect and the NSs.52 The SAED images used to identify the polycrystalline nature of synthesized pristine and doped NiO2 NSs are shown in Figure 2c–f. Images containing concentric rings that are polycrystalline were categorized as planes (101), (011), (110), and (111), which agree with the XRD results, confirming the crystalline nature of controlled and doped NiO2.
To investigate the optical absorption properties of pristine and (2 and 4 wt %) V2O5/Cs-doped NiO2 NSs, a UV–vis spectrophotometer was used (Figure 3a). The significant absorption bands at 330 nm for the pure NiO2 correspond to a band gap energy of 3.75 eV determined from Tauc’s plot approach.14 Optical absorption can be affected by a wide variety of parameters, including the size of the particles and the energy gap. An electronic transition can occur from the valence band to the conduction band in the transition from O (2p) to Ni (3d) in NiO2, resulting in a broad band at 330 nm.53,54 The corresponding peak exhibits the symmetric forbidden transition associated with the electron injection from n to π* transitions. The absorption maxima (λmax) for Cs-NiO2 and (2 and 4 wt %) V2O5/Cs-doped NiO2 have been measured at 335, 340, and 345 nm, respectively. Figure 3a depicts that the absorption wavelength range increased with dopants introducing a redshift. The measured band gap energy values decreased gradually from 3.75 to 3.59 eV attributed to an increase in the crystalline size of NSs after doping (Figure 3b), as described earlier.
Figure 3.
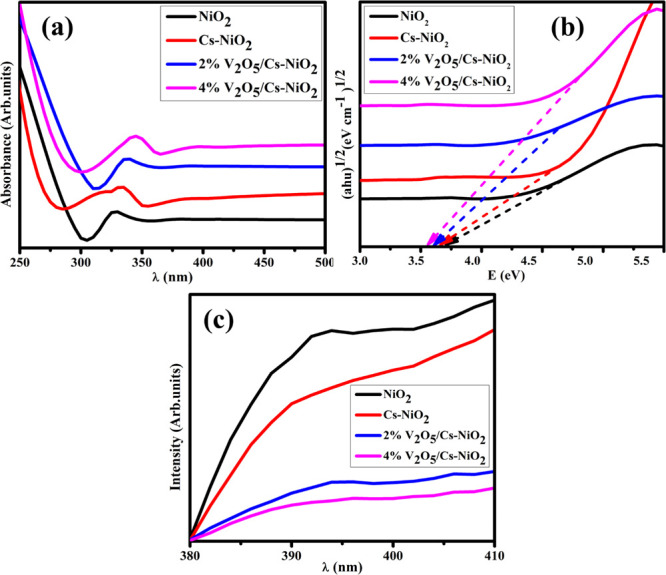
(a) Optical absorption spectra, (b) band gap energy, and (c) PL spectra of pure and doped NSs.
PL spectroscopy has been used to assess the photogenerated electron–hole recombination capability and separation efficiency. The PL emission spectra of undoped and (2 and 4 wt %) V2O5/Cs-doped NiO2 with a range of 380–410 nm are represented in Figure 3c. The PL emission spectra of the synthesized NSs exhibit a significant peak at 393 nm55 with an excitation wavelength of 290 nm at 37 °C. The reduction in peak intensity was found with increased dopants attributed to the difference in the ionic radii.56 As reported, the high-intensity peak of PL confirmed a higher recombination rate of e––h+ and vice versa.57,58 The green shift was observed as the electron–hole recombination decreased, which boosted the CA.59
The elemental composition of NiO2 and (2 and 4 wt %) V2O5/Cs-doped NiO2 NSs was observed by EDS analysis, as presented in Figure 4a–d. Furthermore, EDS assesses the elemental distribution pattern to determine whether there is additional interfacial contact. Strong nickel and oxygen peaks were found, confirming the presence of NiO2 in the produced samples (Figure 4a). A minor peak of sodium was observed caused by NaOH to maintain the pH of the samples. The elemental components of nickel (Ni), vanadium (V), sodium (Na), and oxygen (O) were confirmed in the doped material. On the other hand, the appearance of cesium peaks is linked to the experimental equipment and sample preparation.60
Figure 4.
EDS analysis of (a) NiO2, (b) Cs-NiO2, (c) (2 wt %) V2O5/Cs-doped NiO2, and (d) (4 wt %) V2O5/Cs-doped NiO2 NSs.
The elemental mapping of (4 wt %) V2O5/Cs-doped NiO2 NSs is represented in Figure 5a. The existence of O, Na, V, and Ni for forming pristine and doped NiO2 NSs is confirmed by Figure 5b–e, respectively. The presence of Na was attributed to NaBH4, which was used to maintain the pH, as already mentioned.
Figure 5.
Elemental mapping composition of the synthesized NSs: (a) (4 wt %) V2O5/Cs-doped NiO2, (b) oxygen, (c) sodium, (d) vanadium, and (e) nickel.
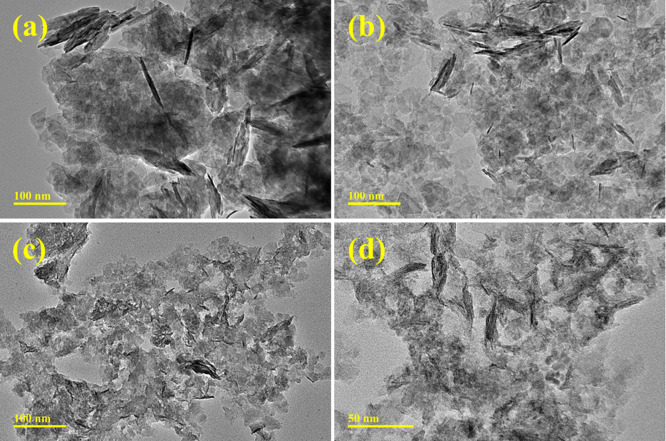
TEM confirmed the morphologies of pristine and (2 and 4 wt %) V2O5/Cs-doped NiO2, as shown in Figure 6a–d. The morphology of NiO2 (Figure 6a) looks like agglomerated nonuniform nanorods with nanoparticles that might partially interact in a multitude of orientations. In contrast, the doping of a fixed amount of Cs into NiO2 caused the production of the sphere, which overlaps the nanorods, indicating a reduction in the size (Figure 6b). The addition of V2O5 (2 and 4 wt %) to the binary system Cs-NiO2 resulted in highly agglomerated and chunk-like morphology (Figure 6c,d). A higher degree of aggregation was observed with increasing amounts of dopants.
Figure 6.
TEM micrographs of (a) NiO2, (b) Cs-doped NiO2, (c) (2 wt %) V2O5/Cs-doped NiO2, and (d) (4 wt %) V2O5/Cs-doped NiO2.
The interlayer d-spacing of the synthesized NiO2 and the V2O5/Cs-doped NSs was measured with HR-TEM, and the results are displayed in Figure 7a–d. The interlayer d-spacing of the NiO2 is about 0.178 nm (Figure 7a). Furthermore, the addition of Cs and (2 and 4 wt %) V2O5 showed considerable interplanar spacings of 0.147, 0.147, and 0.154 nm, respectively, as indicated in Figure 7b–d. The d-spacing that was predicted for pure and doped NiO2 matched the XRD findings as well.
Figure 7.

Interlayer d-spacing of (a) NiO2, (b) Cs-doped NiO2, (c) (2 wt %) V2O5/Cs-doped NiO2, and (d) (4 wt %) V2O5/Cs-doped NiO2.
The CA of pristine and (2 and 4 wt %) V2O5/Cs-doped NiO2 NSs was examined using a UV–vis spectrophotometer in the spectral range of 200–800 nm at various pH levels (neutral, basic, and acidic media). Various pH levels of the dye sludge are regularly released from several industries in fresh water resources. The pH of solution influences the reduction pace and impacts the artificial nanocatalyst. The CA of synthesized samples was affected by the microstructure of the materials, specifically their crystal structures, particle sizes, morphologies, and surface areas. Additionally, the OH– and H+ ions play critical roles in PCA and CA, respectively, and provide more OH– radicals available to decompose the contaminant.
In the catalysis mechanism, the addition of a nanocatalyst and NaBH4 that act as reducing agents to the dye is the most significant factor in the catalytic process (Figure 8). The reducing agent donates an electron to the ongoing reaction, and MB, also called the oxidizing agent, accepts an e– from the reducing agent to carry out its role. During CA, a redox reaction starts in which an electron is transferred from the donor to the acceptor atoms. This leads to the absorption of electrons in MB, which eventually stimulates the synthetic dye to deteriorate. Additionally, MB was tested in the presence of NaBH4, and the oxidation reaction was very slow. Incorporating a nanocatalyst (V2O5/Cs-doped NiO2) into the oxidation–reduction reactions serves as an electron relay and makes it possible for electrons to be transferred from the donor (BH4–) to the acceptor (MB). NSs enhance the adsorption of BH4– ions and the dye molecule by providing more active sites that accelerate their interaction.61,62 The coexistence of a nanocatalyst and a reducing agent enhances the efficiency of degradation.63 As NSs have a high surface-to-volume ratio, facilitating effective dye reduction, their size is another factor influencing the CA.64
Figure 8.
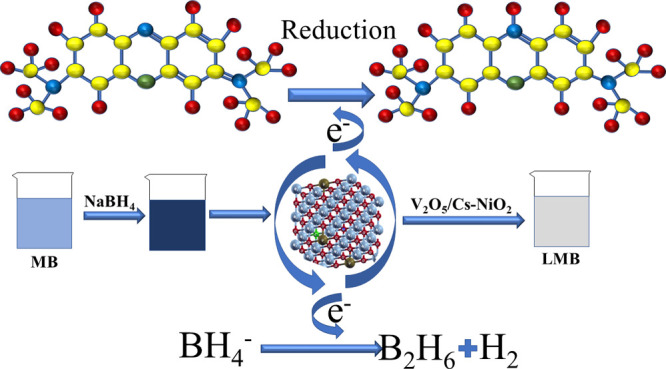
Schematic illustration of the catalytic reduction of dye in the presence of pristine and V2O5/Cs-doped NiO2.
The V2O5/Cs-NiO2 NPs showed outstanding catalytic performance for the reduction of MB dye in the presence of the reducing agent NaBH4, and the findings demonstrate that it exhibited the degradation of MB dye up to 75.13, 81.54, 85.76, and 91.89% in neutral (pH = 7), 59.29, 79.45, 84.22, and 91.01% in basic (pH = 12), and 63.35, 76.1, 87.37, and 94.21% in acidic (pH = 4) media, as seen in Figure 9a–c. The addition of V2O5 improved the CA up to 94.21%, given the ideal level for the reduction of dye exhibited by (4 wt %) V2O5/Cs-NiO2. The literature review and the current experiment study for the reduction of MB can be seen in Table 1. Significant CA against MB dye was observed upon the higher incorporation of catalysts. The comparison study indicates that the recent work has maximum potential against dye reduction. However, their high electrical efficiency was attributed to encouraging the results at acidic pH for (4 wt %) V2O5/Cs-doped NiO2 NSs. The excellent CA in acidic media was ascribed to the enhanced availability of hydrogen (H+) ions for absorption on the NSs horizon.62
Figure 9.

Catalytic potential of the pristine and (2 and 4 wt %) V2O5/Cs-doped NiO2 in (a) neutral, (b) basic, and (c) acidic media.
Table 1. Comparison for the Reduction/Degradation (Red/Deg) of MB from Literature and Current Research.
| nanocatalyst | size (nm) | synthesis method | dye | time (min) | Red/Deg (%) | under light/dark | ref |
|---|---|---|---|---|---|---|---|
| NiO/Ag heterostructure | 25 | hydrothermal | MB | 210 | 70 | light | (65) |
| NiO nanoparticles | 2–5.4 | thermal decomposition | RhB, MB | 300 | RhB: 87MB: 70 | light | (66) |
| NiO-CuO nanoparticles | 14–36 | sol–gel | MB | 26 | 40 | light | (67) |
| NiO/ZnO composite | 28.3 | electrospinning | RhB, MB | 180 | RhB: 59.41MB: 65.43 | light | (68) |
| Cu-doped CNC/NiO composite | 19.1 | co-precipitation | MB | 30 | 70.6 | dark | (21) |
| V2O5/Cs-NiO2 NSs | 45.19 | co-precipitation | MB | 10 | 94.21 | dark | this work |
Since CA directly affects the production of H+ ions in acidic media, the catalyst’s surface becomes more positively charged. Since MB is also a cationic dye, its absorption is inhibited because catalyst surfaces are less receptive to the cationic adsorption of adsorbable families, which reduces the amount of dye that can be absorbed. This occurs due to the increased strength of the electrostatic repulsion force between the positively charged surfaces of the catalyst and the dye.69,70 Generally, the catalyst’s wide surface area or tiny size has provided more active sites to increase CA. Because of the microporosity, the catalyst’s efficiency diminishes when the surface area is big because the reactant cannot diffuse into the binding sites of the catalyst.71
The well diffusion technique was followed to investigate the antibacterial activity observed in vitro, and the synthesized V2O5/Cs-NiO2 NSs samples were tested for E. coli (Gram-negative bacteria) by measuring inhibition zones [49]. Significant inhibitory zones were seen against E. coli for doped and pure NSs, ranging from 1.30 to 3.05 mm in low and 2.25 to 3.75 mm in high concentrations, respectively, as exhibited in Table 2. All the observations were analyzed with ciprofloxacin at a concentration of 5.15 mm for Gram-negative bacteria and DI water at a concentration of 0 mm. At a concentration of 4 wt %, the V2O5/Cs-doped NiO2 demonstrated an effective antibacterial action. As a result of the fact that since the doping concentration is directly related to the size of the inhibition zones, an enhancement in the measurements of the inhibition zones will naturally result in a higher doping concentration. E. coli showed a high antibacterial efficacy when it was in high concentrations. Table 3 shows the literature comparison for the antimicrobial activity of nickel oxide against E. coli.
Table 2. Antimicrobial Potential of NiO2 and (2 and 4 wt %) V2O5/Cs-Doped NiO2 NSs.
|
E. coli inhibition
zone (mm) |
||||
|---|---|---|---|---|
| samples | 0.5 mg/50 μL | 1.0 mg/50 μL | ciprofloxacin | DI water |
| NiO2 | 1.30 | 2.25 | 5.15 | 0 |
| Cs-NiO2 | 1.95 | 2.55 | 5.15 | 0 |
| (2%) V2O5/Cs-NiO2 | 2.15 | 3.05 | 5.15 | 0 |
| (4%) V2O5/Cs-NiO2 | 3.05 | 3.75 | 5.15 | 0 |
Table 3. Literature Comparison of Antimicrobial Activity of Nickel Oxide with the Current Study.
| sample | preparation method | pathogen | % of inhabitation zone | ref |
|---|---|---|---|---|
| graphene oxide-nickel oxide (GO-NiO) nanocomposite | hydrothermal | E. coli | 0 | (72) |
| NiO nanoparticles | green synthesis | E. coli | 27.7 | (73) |
| Cu-doped CNC/NiO nanocrystals | co-precipitation | E. coli | 56.65 | (21) |
| BC-Ag/NiO nanocomposite | hydrothermal | E. coli | 57 | (74) |
| V2O5/Cs-NiO2 NSs | co-precipitation | E. coli | 70 | this work |
The antibacterial activity was linked to a variety of parameters, including the size, shape, and concentration of the particles. Oxidative stress influences the phenotype, size, and mass-to-surface concentration of NSs. This is because nanoparticles quickly release ROS. ROS are responsible for degrading the cytoplasmic contents of the bacterial membrane, which ultimately leads to the death of the bacterium.60 Since NiO2 has a positive surface charge electrostatically attached to the negatively charged cell surface, it restricts cell activity.75 The cell was left lifeless as a result of NiO2 ability to enter the cell and its harmful effects that caused the death of bacteria. The preceding procedure is represented in Figure 10.
Figure 10.

Antibacterial mechanism of produced NiO2 and V2O5/Cs-doped NiO2 schematically.
NSs connect themselves to the cell wall in the form of a helical, disorderly spring and enter the cell membrane, where they build interlinks with the structures of DNA molecules.62 The effectiveness of the antimicrobial agent depends on the crystallite size and the doping concentration, and there is an inverse link between the size of the pure and the size of the V2O5/Cs-doped NiO2 NSs. Because of their diminutive dimensions, the particles actively produce ROS, which, in turn, create a rupture in the bacterial cell membrane and the exposure of the bacterium’s cytoplasmic components, which finally leads to the death of the bacterium. The mechanism for the generation of ROS performed under the presence of visible light can be exhibited as
The mechanism for the response of antimicrobial activity is depicted in Figure 9, where ROS plays an effective part in the death of microbial agents.76 Due to the direct attack of the synthesized materials, the cell wall and cell membrane of the bacteria are damaged, ultimately resulting in the death of the pathogen. NSs that generate intracellular ROS communicate with the nucleic acid of a bacterial cell, which causes single as well as dual-wrecked breakage within the nitrogen base and sugar-phosphate bond of nucleic acid.59
Drug resistance is seen as a serious threat to humanity, and there is a continuing need for effective antibiotics. Metal NSs are commonly believed to have a role in antibacterial activity. Trimethoprim and sulfonamide medicines are known to target the enzymes DHFR and DHPS in the folic acid production pathway.77,78 Here, we tested Cs-doped NiO2 and V2O5/Cs-doped NiO2 for their ability to bind to the DHFR and DHPS enzymes found in E. coli. Potential enzyme inhibitors were identified based on the binding pattern of docked complexes inside the active site of the target enzyme. The best docking zone for DHFRE.coli had H-bonding interactions with Ala-7 and Thr-113 for Cs-doped NiO2 and Ser-49 and Thr-113 for V2O5/Cs-doped NiO2, with binding scores of 3.28 and 6.37, respectively (Figure 11).
Figure 11.

Binding pocket (a) and interaction pattern of Cs-doped NiO2 (b) and V2O5/Cs-doped NiO2 (c) inside active pocket DHFR from E. coli.
In Figure 12, the docking complex of Cs-doped NiO2 for DHPSE.coli exhibited H-bonding with Thr-62 and Asn-115 having a docking score of 2.40. At the same time, V2O5/Cs-doped NiO2 showed H-bonding with Phe-190, Lys-221, and Arg-255 with a binding energy of 4.31. These V2O5/Cs-doped NiO2 NSs were proposed as potential antagonists. Enzyme activity stops when ligand binding prevents the substrate from entering the active site. In silico estimations for selective targets showed their potential binding patterns into the active pocket of the enzyme and proposed those compounds as enzyme inhibitors. V2O5/Cs-doped NiO2 NSs had an enhanced antibacterial action against E. coli.
Figure 12.

Binding pocket (a) and interaction pattern of Cs-doped NiO2 (b) and V2O5/Cs-doped NiO2 (c) inside active pocket DHPS from E. coli.
4. Conclusions
The co-precipitation technique was used to synthesize the (2 and 4 wt %) V2O5/Cs-doped NiO2 NSs. The XRD pattern verified the hexagonal crystal structures and SAED confirmed the polycrystalline nature of the synthesized NSs. The elemental composition and NSs morphology was confirmed by EDS and TEM micrographs. The synthesized NSs were examined as a catalyst for the degradation of MB and its antimicrobial activity against E. coli. The influence of Cs and V2O5 as dopants in NiO2 results in a more efficient and effective catalyst. In principle, Cs provide a wide surface area and porous structure, and V2O5 generates more ROS and boosts the redox reactions. Hence, NiO2 doped with (4 wt %) V2O5/Cs demonstrated a remarkable CA at 91.98, 91.01, and 94.21% in neutral, basic, and acidic media, respectively, against MB reduction. The high bactericidal effectiveness against E. coli with a measured inhibition zone of 3.75 mm at high concentration was observed. In addition, molecular docking analysis suggested that V2O5/Cs-doped NiO2 NSs might block the DHFR and DHPS. This research concludes that (4 wt %) V2O5/Cs-doped NiO2 is an efficient catalytic material for degrading dye and a worthy candidate for antibacterial activities. This study aims to provide new insight into developing a NiO2-based catalyst for bioremediation and CA.
Acknowledgments
W.N. is thankful for the support from Universitat Rovira i Virgili under the Maria Zambrano Programme (reference number: 2021URV-MZ-10), Proyectos de Generación de Conocimiento AEI/MCIN (PID2021-123665OB-I00), and the project reference number of TED2021–129343B-I00. This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R1), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Authors are also thankful for the support from Grant PID2021-123665OB-I00 and TED2021-129343B-I00 funded by MCIN/AEI/ 10.13039/501100011033 and, as appropriate, by “ERDF A way of making Europe”, by the “European Union” or by the “European Union NextGenerationEU/PRTR”.
Data Availability Statement
On-demand accessibility to data is possible.
We would like to express our appreciation to the Higher Education Commission of Pakistan for funding project NRPU-20-17615 (Dr. M.I.).
The authors declare no competing financial interest.
References
- Kookana R. S.; Drechsel P.; Jamwal P.; Vanderzalm J. Urbanisation and emerging economies: Issues and potential solutions for water and food security. Sci. Total Environ. 2020, 732, 139057 10.1016/j.scitotenv.2020.139057. [DOI] [PubMed] [Google Scholar]
- Yang J.; Shojaei S.; Shojaei S. Removal of drug and dye from aqueous solutions by graphene oxide: Adsorption studies and chemometrics methods. npj Clean. Water 2022, 5, 5. 10.1038/s41545-022-00148-3. [DOI] [Google Scholar]
- Islam A.; Teo S. H.; Taufiq-Yap Y. H.; Ng C. H.; Vo D.-V. N.; Ibrahim M. L.; Hasan M. M.; Khan M. A. R.; Nur A. S. M.; Awual M. R. Step towards the sustainable toxic dyes removal and recycling from aqueous solution- A comprehensive review. Resour., Conserv. Recycl. 2021, 175, 105849 10.1016/j.resconrec.2021.105849. [DOI] [Google Scholar]
- Ikram M.; Haider A.; Imran M.; Haider J.; Ul-Hamid A.; Shahzadi A.; Malik R.; Nabgan W.; Nazir G.; Ali S. Graphitic-C3N4/chitosan-doped NiO nanostructure to treat the polluted water and their bactericidal with in silico molecular docking analysis. Int. J. Biol. Macromol. 2022, 277, 962–973. 10.1016/j.ijbiomac.2022.11.273. [DOI] [PubMed] [Google Scholar]
- Santoso S. P.; Bundjaja V.; Angkawijaya A. E.; Gunarto C.; Go A. W.; Yuliana M.; Tran-Nguyen P. L.; Hsieh C.-W.; Ju Y.-H. One-step synthesis of nitrogen-grafted copper-gallic acid for enhanced methylene blue removal. Sci. Rep. 2021, 11, 12021. 10.1038/s41598-021-91484-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.; Sharif S.; Anjum S.; Imran M.; Ikram M.; Naz M.; Ali S. Preparation of Co and Ni doped ZnO nanoparticles served as encouraging nano-catalytic application. Mater. Res. Express 2019, 6, 1250d5. 10.1088/2053-1591/ab6383. [DOI] [Google Scholar]
- Vázquez-Cuchillo O.; Cruz-López A.; Bautista-Carrillo L. M.; Bautista-Hernández A.; Torres Martínez L. M.; Wohn Lee S. Synthesis of TiO2 using different hydrolysis catalysts and doped with Zn for efficient degradation of aqueous phase pollutants under UV light. Res. Chem. Intermed. 2010, 36, 103–113. 10.1007/s11164-010-0119-4. [DOI] [Google Scholar]
- Thennarasu G.; Sivasamy A. Enhanced visible photocatalytic activity of cotton ball like nano structured Cu doped ZnO for the degradation of organic pollutant. Ecotoxicol. Environ. Saf. 2016, 134, 412–420. 10.1016/j.ecoenv.2015.10.030. [DOI] [PubMed] [Google Scholar]
- Liu F.; Yang J.; Zuo J.; Ma D.; Gan L.; Xie B.; Wang P.; Yang B. Graphene-supported nanoscale zero-valent iron: Removal of phosphorus from aqueous solution and mechanistic study. J. Environ. Sci. 2014, 26, 1751–1762. 10.1016/j.jes.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Balusamy B.; Kandhasamy Y. G.; Senthamizhan A.; Chandrasekaran G.; Subramanian M. S.; Kumaravel T. S. Characterization and bacterial toxicity of lanthanum oxide bulk and nanoparticles. J. Rare Earths 2012, 30, 1298–1302. 10.1016/S1002-0721(12)60224-5. [DOI] [Google Scholar]
- Phung T. H.; Srinivasan D. K.; Steinmann P.; Wise R.; Yu M.-B.; Yeo Y.-C.; Chunxiang Z. High performance metal-insulator-metal capacitors with Er2O3 on ALD SiO2 for RF applications. J. Electrochem. Soc. 2011, 158, H1289. 10.1149/2.085112jes. [DOI] [Google Scholar]
- Wang J.; Ji T.; Zhu Y.; Fang Z.; Ren W. Band gap and structure characterization of Tm2O3 films. J. Rare Earths 2012, 30, 233–235. 10.1016/S1002-0721(12)60029-5. [DOI] [Google Scholar]
- Mudavakkat V. H.; Atuchin V. V.; Kruchinin V. N.; Kayani A.; Ramana C. V. Structure, morphology and optical properties of nanocrystalline yttrium oxide (Y2O3) thin films. Opt. Mater. 2012, 34, 893–900. 10.1016/j.optmat.2011.11.027. [DOI] [Google Scholar]
- Rahdar A.; Aliahmad M.; Azizi Y. NiO nanoparticles: synthesis and characterization. J. Nanostruct. 2015, 5, 145–151. [Google Scholar]
- Hameeda B.; Mushtaq A.; Saeed M.; Munir A.; Jabeen U.; Waseem A. Development of Cu-doped NiO nanoscale material as efficient photocatalyst for visible light dye degradation. Toxin Rev. 2021, 40, 1396–1406. 10.1080/15569543.2020.1725578. [DOI] [Google Scholar]
- Ajitha B.; Ahn C. W.; Yadav P. V. K.; Reddy Y. A. K. Silver nanoparticle embedded polymethacrylic acid/ polyvinylpyrrolidone nanofibers for catalytic application. J. Environ. Chem. Eng. 2021, 9, 106291 10.1016/j.jece.2021.106291. [DOI] [Google Scholar]
- Jamil S.; Ahmad Z.; Ali M.; Rauf Khan S.; Ali S.; Amen Hammami M.; Haroon M.; Saleh T. A.; Janjua R. S. A. M. Synthesis and characterization of polyaniline/nickel oxide composites for fuel additive and dyes reduction. Chem. Phys. Lett. 2021, 776, 138713 10.1016/j.cplett.2021.138713. [DOI] [Google Scholar]
- Lu J.; Fang J.; Li J.; Wang C.; He Z.; Zhu L.; Xu Z.; Zeng H. Polydopamine Nanotubes Decorated with Ag Nanoparticles as Catalyst for the Reduction of Methylene Blue. ACS Appl. Nano Mater. 2020, 3, 156–164. 10.1021/acsanm.9b01861. [DOI] [Google Scholar]
- Ambreen J.; Al-Harbi F. F.; Sakhawat H.; Ajmal M.; Naeem H.; Farooqi Z. H.; Batool N.; Siddiq M. Fabrication of poly (N-vinylcaprolactam-co-acrylic acid)-silver nanoparticles composite microgel with substantial potential of hydrogen peroxide sensing and catalyzing the reduction of water pollutants. J. Mol. Liq. 2022, 355, 118931 10.1016/j.molliq.2022.118931. [DOI] [Google Scholar]
- Hu Z.; Han M.; Chen C.; Zou Z.; Shen Y.; Fu Z.; Zhu X.; Zhang Y.; Zhang H.; Zhao H.; et al. Hollow carbon sphere encapsulated nickel nanoreactor for aqueous-phase hydrogenation-rearrangement tandem reaction with enhanced catalytic performance. Appl. Catal., B 2022, 306, 121140 10.1016/j.apcatb.2022.121140. [DOI] [Google Scholar]
- Ikram M.; Bashir Z.; Haider A.; Naz S.; Ul-Hamid A.; Shahzadi I.; Ashfaq A.; Haider J.; Shahzadi A.; Ali S. Bactericidal action and molecular docking studies of catalytic Cu-doped NiO composited with cellulose nanocrystals. Int. J. Biol. Macromol. 2022, 195, 440–448. 10.1016/j.ijbiomac.2021.12.038. [DOI] [PubMed] [Google Scholar]
- Iqbal S.; Javed M.; Bahadur A.; Qamar M.; Ahmad M.; Shoaib M.; Raheel M.; Ahmed N.; Akbar M. B.; Li H. Controlled synthesis of Ag-doped CuO nanoparticles as a core with poly(acrylic acid) microgel shell for efficient removal of methylene blue under visible light. J. Mater. Sci.: Mater. Electron. 2020, 31, 8423–8435. 10.1007/s10854-020-03377-9. [DOI] [Google Scholar]
- Nandana C. N.; Christeena M.; Bharathi D. Synthesis and characterization of chitosan/silver nanocomposite using rutin for antibacterial, antioxidant and photocatalytic applications. J. Cluster Sci. 2021, 33, 269–279. 10.1007/s10876-020-01947-9. [DOI] [Google Scholar]
- Dou J.; Yin S.; Chong J. Y.; Zhang B.; Han J.; Huang Y.; Xu R. Carbon spheres anchored Co3O4 nanoclusters as an efficient catalyst for dye degradation. Appl. Catal., A 2016, 513, 106–115. 10.1016/j.apcata.2015.12.028. [DOI] [Google Scholar]
- Cheng X.; Fu A.; Li H.; Wang Y.; Guo P.; Liu J.; Zhang J.; Zhao X. S. Sustainable Preparation of Copper Particles Decorated Carbon Microspheres and Studies on Their Bactericidal Activity and Catalytic Properties. ACS Sustainable Chem. Eng. 2015, 3, 2414–2422. 10.1021/acssuschemeng.5b00382. [DOI] [Google Scholar]
- Jin Q.; Shen Y.; Cai Y.; Chu L.; Zeng Y. Resource utilization of waste V2O5-based deNOx catalysts for hydrogen production from formaldehyde and water via steam reforming. J. Hazard. Mater. 2020, 381, 120934 10.1016/j.jhazmat.2019.120934. [DOI] [PubMed] [Google Scholar]
- Levi R.; Bar-Sadan M.; Albu-Yaron A.; Popovitz-Biro R.; Houben L.; Shahar C.; Enyashin A.; Seifert G.; Prior Y.; Tenne R. Hollow V2O5 Nanoparticles (Fullerene-Like Analogues) Prepared by Laser Ablation. J. Am. Chem. Soc. 2010, 132, 11214–11222. 10.1021/ja103719x. [DOI] [PubMed] [Google Scholar]
- Babar B. M.; Pisal K. B.; Sutar S. H.; Mujawar S. H.; Kadam L. D.; Pathan H. M.; Pawar U. T.; Kadam P. M.; Patil P. S. Hydrothermally Prepared Vanadium Oxide Nanostructures for Photocatalytic Application. ES Energy Environ. 2022, 15, 82–91. [Google Scholar]
- Santosham M.; Chandran A.; Fitzwater S.; Fischer-Walker C.; Baqui A. H.; Black R. Progress and barriers for the control of diarrhoeal disease. Lancet 2010, 376, 63–67. 10.1016/S0140-6736(10)60356-X. [DOI] [PubMed] [Google Scholar]
- Xiang Q.; Yu J.; Jaroniec M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. 10.1039/C1CS15172J. [DOI] [PubMed] [Google Scholar]
- Bhimanapati G. R.; Lin Z.; Meunier V.; Jung Y.; Cha J.; Das S.; Xiao D.; Son Y.; Strano M. S.; Cooper V. R.; et al. Recent Advances in Two-Dimensional Materials beyond Graphene. ACS Nano 2015, 9, 11509–11539. 10.1021/acsnano.5b05556. [DOI] [PubMed] [Google Scholar]
- Bergey D.; JG H.. Bergey’s Manual of Determinative Bacteriology. Baltimore. 9th ed; Williams and Wilkins: USA. 1994. [Google Scholar]
- Bauer A. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. 10.1128/AAC.1.6.451. [DOI] [PubMed] [Google Scholar]
- Adzitey F.; Yussif S.; Ayamga R.; Zuberu S.; Addy F.; Adu-Bonsu G.; Huda N.; Kobun R. Antimicrobial susceptibility and molecular characterization of Escherichia coli recovered from milk and related samples. Microorganisms 2022, 10, 1335. 10.3390/microorganisms10071335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay S.; Sharma M.; Misri J.; Shome B.; Veeraraghavan B.; Ray P.; Ohri V.; Walia K. An integrated surveillance network for antimicrobial resistance, India. Bull. W. H. O. 2021, 99, 562. 10.2471/BLT.20.284406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwalokun B.; Ogunledun A.; Ogbolu D.; Bamiro S.; Jimi-Omojola J. In vitro antimicrobial properties of aqueous garlic extract against multidrug-resistant bacteria and Candida species from Nigeria. J. Med. Food 2004, 7, 327–333. 10.1089/jmf.2004.7.327. [DOI] [PubMed] [Google Scholar]
- Haider A.; Ijaz M.; Imran M.; Naz M.; Majeed H.; Khan J. A.; Ali M. M.; Ikram M. Enhanced bactericidal action and dye degradation of spicy roots’ extract-incorporated fine-tuned metal oxide nanoparticles. Appl. Nanosci. 2020, 10, 1095–1104. 10.1007/s13204-019-01188-x. [DOI] [Google Scholar]
- Haider A.; Ijaz M.; Ali S.; Haider J.; Imran M.; Majeed H.; Shahzadi I.; Ali M. M.; Khan J. A.; Ikram M. Green Synthesized Phytochemically (Zingiber officinale and Allium sativum) Reduced Nickel Oxide Nanoparticles Confirmed Bactericidal and Catalytic Potential. Nanoscale Res. Lett. 2020, 15, 50. 10.1186/s11671-020-3283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield R. L.; Daigle D. M.; Mayer S.; Mallik D.; Hughes D. W.; Jackson S. G.; Sulek M.; Organ M. G.; Brown E. D.; Junop M. S. A 2.13 Å Structure of E. coli Dihydrofolate Reductase Bound to a Novel Competitive Inhibitor Reveals a New Binding Surface Involving the M20 Loop Region. J. Med. Chem. 2006, 49, 6977–6986. 10.1021/jm060570v. [DOI] [PubMed] [Google Scholar]
- Dennis M. L.; Lee M. D.; Harjani J. R.; Ahmed M.; DeBono A. J.; Pitcher N. P.; Wang Z.-C.; Chhabra S.; Barlow N.; Rahmani R.; et al. 8-Mercaptoguanine Derivatives as Inhibitors of Dihydropteroate Synthase. Chem. – Eur. J. 2018, 24, 1922–1930. 10.1002/chem.201704730. [DOI] [PubMed] [Google Scholar]
- Ikram M.; Chaudhary K.; Shahzadi A.; Haider A.; Shahzadi I.; Ul-Hamid A.; Abid N.; Haider J.; Nabgan W.; Butt A. R. Chitosan/starch-doped MnO2 nanocomposite served as dye degradation, bacterial activity, and insilico molecular docking study. Mater. Today Nano 2022, 20, 100271 10.1016/j.mtnano.2022.100271. [DOI] [Google Scholar]
- Shahzadi I.; Islam M.; Saeed H.; Haider A.; Shahzadi A.; Haider J.; Ahmed N.; Ul-Hamid A.; Nabgan W.; Ikram M.; et al. Formation of biocompatible MgO/cellulose grafted hydrogel for efficient bactericidal and controlled release of doxorubicin. Int. J. Biol. Macromol. 2022, 220, 1277–1286. 10.1016/j.ijbiomac.2022.08.142. [DOI] [PubMed] [Google Scholar]
- Abd-Alghafour N.; Ahmed N. M.; Hassan Z.; Almessiere M. A. In Hydrothermal synthesis and structural properties of V2O5 Nanoflowers at low temperatures. J. Phys.: Conf. Ser. 2018, 1083, 012036. [Google Scholar]
- Hu L.; Peng J.; Wang W.; Xia Z.; Yuan J.; Lu J.; Huang X.; Ma W.; Song H.; Chen W.; et al. Sequential Deposition of CH3NH3PbI3 on Planar NiO Film for Efficient Planar Perovskite Solar Cells. ACS Photonics 2014, 1, 547–553. 10.1021/ph5000067. [DOI] [Google Scholar]
- Anand K.; Kaur J.; Singh R. C.; Thangaraj R. Preparation and characterization of Ag-doped In2O3 nanoparticles gas sensor. Chem. Phys. Lett. 2017, 682, 140–146. 10.1016/j.cplett.2017.06.008. [DOI] [Google Scholar]
- Yuan X.; Zhou C.; Jing Q.; Tang Q.; Mu Y.; du A. K. Facile synthesis of g-C3N4 nanosheets/ZnO nanocomposites with enhanced photocatalytic activity in reduction of aqueous chromium (VI) under visible light. Nanomaterials 2016, 6, 173. 10.3390/nano6090173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susha N.; Nandakumar K.; Nair S. S. Enhanced photoconductivity in CdS/betanin composite nanostructures. RSC Adv. 2018, 8, 11330–11337. 10.1039/C7RA13116J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairnar S. D.; Shrivastava V. S. Facile synthesis of nickel oxide nanoparticles for the degradation of Methylene blue and Rhodamine B dye: a comparative study. Journal of Taibah University for Science 2019, 13, 1108–1118. 10.1080/16583655.2019.1686248. [DOI] [Google Scholar]
- López-Ortiz A.; Collins-Martínez V. H.; Hernández-Escobar C. A.; Flores-Gallardo S. G.; Zaragoza-Contreras E. A. Protection of NiO nanoparticles against leaching in acid medium by grafting of polyacrylic acid. Mater. Chem. Phys. 2008, 109, 306–310. 10.1016/j.matchemphys.2007.11.031. [DOI] [Google Scholar]
- Yousaf S.; Zulfiqar S.; Shahi M. N.; Warsi M. F.; Al-Khalli N. F.; Aly Aboud M. F.; Shakir I. Tuning the structural, optical and electrical properties of NiO nanoparticles prepared by wet chemical route. Ceram. Int. 2020, 46, 3750–3758. 10.1016/j.ceramint.2019.10.097. [DOI] [Google Scholar]
- Ngo Y.-L. T.; Hur S. H. Low-temperature NO2 gas sensor fabricated with NiO and reduced graphene oxide hybrid structure. Mater. Res. Bull. 2016, 84, 168–176. 10.1016/j.materresbull.2016.08.004. [DOI] [Google Scholar]
- Wei Z.; Qiao H.; Yang H.; Zhang C.; Yan X. Characterization of NiO nanoparticles by anodic arc plasma method. J. Alloys Compd. 2009, 479, 855–858. 10.1016/j.jallcom.2009.01.064. [DOI] [Google Scholar]
- Barzinjy A. A.; Hamad S. M.; Aydın S.; Ahmed M. H.; Hussain F. H. S. Green and eco-friendly synthesis of Nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J. Mater. Sci.: Mater. Electron. 2020, 31, 11303–11316. 10.1007/s10854-020-03679-y. [DOI] [Google Scholar]
- Barakat A.; Al-Noaimi M.; Suleiman M.; Aldwayyan A. S.; Hammouti B.; Ben Hadda T.; Haddad S. F.; Boshaala A.; Warad I. One step synthesis of NiO nanoparticles via solid-state thermal decomposition at low-temperature of novel aqua (2, 9-dimethyl-1, 10-phenanthroline) NiCl2 complex. Int. J. Mol. Sci. 2013, 14, 23941–23954. 10.3390/ijms141223941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeghi F.; Mozaffari S.; Ghorashi S. M. B. Metal organic framework–derived core-shell CuO@NiO nanosphares as hole transport material in perovskite solar cell. J. Solid State Electrochem. 2020, 24, 1427–1438. 10.1007/s10008-020-04643-w. [DOI] [Google Scholar]
- Reddy B. R.; Harish G.; Reddy C. S.; Reddy P. S. Synthesis and characterization of Cu doped NiO nanoparticles. Int. J. Mod. Eng. Res. 2014, 4, 62–66. [Google Scholar]
- Adeleye A. S.; Conway J. R.; Garner K.; Huang Y.; Su Y.; Keller A. A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. 10.1016/j.cej.2015.10.105. [DOI] [Google Scholar]
- Mafa P. J.; Mamba B. B.; Kuvarega A. T. Construction of hierarchical BiPW12O40/BiOI p–n heterojunction with enhanced visible light activity for degradation of endocrine disrupting Bisphenol A. Sep. Purif. Technol. 2020, 253, 117349 10.1016/j.seppur.2020.117349. [DOI] [Google Scholar]
- Mustajab M.; Ikram M.; Haider A.; Ul-Hamid A.; Nabgan W.; Haider J.; Ghaffar R.; Shahzadi A.; Ghaffar A.; Saeed A. Promising performance of polyvinylpyrrolidone-doped bismuth oxyiodide quantum dots for antibacterial and catalytic applications. Appl. Nanosci. 2022, 12, 2621–2633. 10.1007/s13204-022-02547-x. [DOI] [Google Scholar]
- Khan A. D.; Ikram M.; Haider A.; Ul-Hamid A.; Nabgan W.; Haider J. Polyvinylpyrrolidone and chitosan-doped lanthanum oxide nanostructures used as anti-bacterial agents and nano-catalyst. Appl. Nanosci. 2022, 12, 2227–2239. 10.1007/s13204-022-02471-0. [DOI] [Google Scholar]
- Anjana R.; Geetha N. Degradation Of Methylene Blue using Silver Nanoparticles Synthesized from Cynodon dactylon (L.) Pers. Leaf aqueous extract. Int. J. Sci. Technol. Res. 2019, 8, 225–229. [Google Scholar]
- Ikram M.; Inayat T.; Haider A.; Ul-Hamid A.; Haider J.; Nabgan W.; Saeed A.; Shahbaz A.; Hayat S.; Ul-Ain K.; et al. Graphene Oxide-Doped MgO Nanostructures for Highly Efficient Dye Degradation and Bactericidal Action. Nanoscale Res. Lett. 2021, 16, 56. 10.1186/s11671-021-03516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A.; Ikram M.; Haider A.; Ul-Hamid A.; Haider J.; Shahzadi I.; Nazir G.; Shahzadi A.; Imran M.; Ghaffar A. Evaluation of bactericidal potential and catalytic dye degradation of multiple morphology based chitosan/polyvinylpyrrolidone-doped bismuth oxide nanostructures. Nanoscale Adv. 2022, 4, 2713–2728. 10.1039/D2NA00105E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeen S.; Ikram M.; Haider A.; Haider J.; Ul-Hamid A.; Nabgan W.; Shujah T.; Naz M.; Shahzadi I. Comparative Study of Sonophotocatalytic, Photocatalytic, and Catalytic Activities of Magnesium and Chitosan-Doped Tin Oxide Quantum Dots. ACS Omega 2022, 7, 46428–46439. 10.1021/acsomega.2c05133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S.; Bhattacharjee S.; Ghosh C. K. NiO/Ag heterostructure: enhanced UV emission intensity, exchange interaction and photocatalytic activity. RSC Adv. 2016, 6, 56503–56510. 10.1039/C6RA09432E. [DOI] [Google Scholar]
- Ramesh M.; Rao M. P. C.; Anandan S.; Nagaraja H. Adsorption and photocatalytic properties of NiO nanoparticles synthesized via a thermal decomposition process. J. Mater. Res. 2018, 33, 601–610. 10.1557/jmr.2018.30. [DOI] [Google Scholar]
- Senobari S.; Nezamzadeh-Ejhieh A. A comprehensive study on the enhanced photocatalytic activity of CuO-NiO nanoparticles: designing the experiments. J. Mol. Liq. 2018, 261, 208–217. 10.1016/j.molliq.2018.04.028. [DOI] [Google Scholar]
- Sabzehmeidani M. M.; Karimi H.; Ghaedi M. Electrospinning preparation of NiO/ZnO composite nanofibers for photodegradation of binary mixture of rhodamine B and methylene blue in aqueous solution: central composite optimization. Appl. Organomet. Chem. 2018, 32, e4335 10.1002/aoc.4335. [DOI] [Google Scholar]
- Singh K. P.; Mohan D.; Sinha S.; Tondon G. S.; Gosh D. Color Removal from Wastewater Using Low-Cost Activated Carbon Derived from Agricultural Waste Material. Ind. Eng. Chem. Res. 2003, 42, 1965–1976. 10.1021/ie020800d. [DOI] [Google Scholar]
- Haque E.; Jun J. W.; Jhung S. H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. 10.1016/j.jhazmat.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Ikram M.; Hayat S.; Imran M.; Haider A.; Naz S.; Ul-Hamid A.; Shahzadi I.; Haider J.; Shahzadi A.; Nabgan W.; et al. Novel Ag/cellulose-doped CeO2 quantum dots for efficient dye degradation and bactericidal activity with molecular docking study. Carbohydr. Polym. 2021, 269, 118346 10.1016/j.carbpol.2021.118346. [DOI] [PubMed] [Google Scholar]
- Archana S.; Jayanna B. K.; Ananda A.; Shilpa B. M.; Pandiarajan D.; Muralidhara H. B.; Kumar K. Y. Synthesis of nickel oxide grafted graphene oxide nanocomposites - A systematic research on chemisorption of heavy metal ions and its antibacterial activity. Environ. Nanotechnol., Monit. Manage. 2021, 16, 100486 10.1016/j.enmm.2021.100486. [DOI] [Google Scholar]
- Khodair Z. T.; Ibrahim N. M.; Kadhim T. J.; Mohammad A. M. Synthesis and characterization of nickel oxide (NiO) nanoparticles using an environmentally friendly method, and their biomedical applications. Chem. Phys. Lett. 2022, 797, 139564 10.1016/j.cplett.2022.139564. [DOI] [Google Scholar]
- Kamal T.; Khalil A.; Bakhsh E. M.; Khan S. B.; Chani M. T. S.; Ul-Islam M. Efficient fabrication, antibacterial and catalytic performance of Ag-NiO loaded bacterial cellulose paper. Int. J. Biol. Macromol. 2022, 206, 917–926. 10.1016/j.ijbiomac.2022.03.067. [DOI] [PubMed] [Google Scholar]
- Rakshit S.; Ghosh S.; Chall S.; Mati S. S.; Moulik S. P.; Bhattacharya S. C. Controlled synthesis of spin glass nickel oxide nanoparticles and evaluation of their potential antimicrobial activity: A cost effective and eco friendly approach. RSC Adv. 2013, 3, 19348–19356. 10.1039/c3ra42628a. [DOI] [Google Scholar]
- Ikram M.; Haider A.; Imran M.; Haider J.; Naz S.; Ul-Hamid A.; Nabgan W.; Mustajab M.; Shahzadi A.; Shahzadi I.; et al. Facile synthesis of starch and tellurium doped SrO nanocomposite for catalytic and antibacterial potential: In silico molecular docking studies. Int. J. Biol. Macromol. 2022, 221, 496–507. 10.1016/j.ijbiomac.2022.09.034. [DOI] [PubMed] [Google Scholar]
- Ikram M.; Abbasi S.; Haider A.; Naz S.; Ul-Hamid A.; Imran M.; Haider J.; Ghaffar A. Bimetallic Ag/Cu incorporated into chemically exfoliated MoS2 nanosheets to enhance its antibacterial potential: in silico molecular docking studies. Nanotechnology 2020, 31, 275704 10.1088/1361-6528/ab8087. [DOI] [PubMed] [Google Scholar]
- Hawser S.; Lociuro S.; Islam K. Dihydrofolate reductase inhibitors as antibacterial agents. Biochem. Pharmacol. 2006, 71, 941–948. 10.1016/j.bcp.2005.10.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On-demand accessibility to data is possible.