Abstract
The incorporation of heterocyclic moieties into the standard chemical structure with a biologically active scaffold has become of crucial practice for the construction of pharmacologically potent candidates in the drug arena. Currently, numerous kinds of chalcones and their derivatives have been synthesized using the incorporation of heterocyclic scaffolds, especially chalcones bearing heterocyclic moieties that display improved efficiency and potential for drug production in pharmaceutical sectors. The current Review focuses on recent advances in the synthetic approaches and pharmacological activities such as antibacterial, antifungal, antitubercular, antioxidant, antimalarial, anticancer, anti-inflammatory, antigiardial, and antifilarial activities of chalcone derivatives incorporating N-heterocyclic moieties at either the A-ring or B-ring.
Introduction
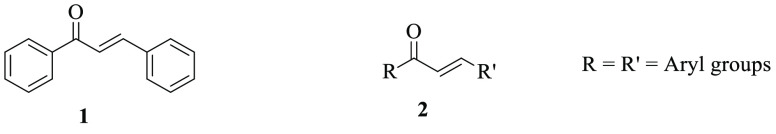
Chalcone scaffolds are privileged chemical structures in the medicinal chemistry sector.1 They are secondary metabolites of plants and found in α,β-unsaturated forms, which have a more thermodynamically stable trans-conformation between two aryl groups.2 Chalcone and its derivatives are the cores of various biologically interesting compounds, and frequently they have been isolated from different medicinal plants such as Dracaena cinnabari, Medicago sativa, and Angelica keiskei (Figure 1).3−6
Figure 1.
General chemical structures of chalcone 1 and its derivative 2.
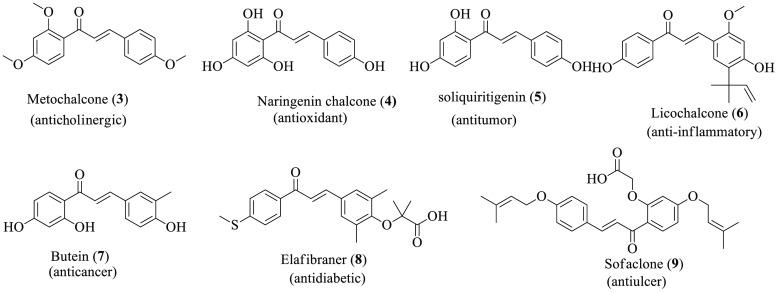
Chemically chalcones are easily prepared using various reaction procedures and strategies. For instance, the named reaction Claisen-Schmidt condensation is one common methodology to prepare the title compound through carbonyl derivative condensation in the presence of base. Additionally, the carbonylative Heck coupling reaction, the Sonogashira isomerization coupling reaction, the continuous flow deuteration reaction, the Suzuki–Miyaura coupling reaction, and solid acid catalyst-mediated reactions are known.7 The precursors of the flavonoids and isoflavones, chalcones serve as promising template scaffolds for synthesizing and developing pharmacologically active compounds in conjugation with other heterocyclic moieties8,9 which have a large role in the sector of medicine to prepare potential drug discovery and improve pharmaceuticals (Figure 2).10
Figure 2.
Chemical structures of chalcone scaffold-based drugs.
Chalcone derivatives incorporating heterocyclic scaffolds are become promising candidates as future drug sources owing to their similar or superior activities compared to those of the standard derivatives.11 To date, the basic chemical structure of chalcone serves as potential source of much research for planning to design and develop various drugs. On top of this, many researchers are exhaustively devoted to synthesizing a chalcone derivative incorporating a heterocyclic scaffold. Chalcones incorporating heterocyclic scaffolds have been reported with various biological and pharmacological activities, such as antioxidant activity,12 antibacterial activity,12 antifungal activity,13 antileishmanial activity,14 anti-inflamatory activity,15 anticancer activity,16 antitubercular activity,17 antiproliferative agents,17 antimalarial activity,18 antiplatelet activity,19 carbonic anhydrase inhibitors,20 an inhibitor of microsomalenzyme glutathione-S-transferases,21 and CYP1 enzyme inhibitors.22
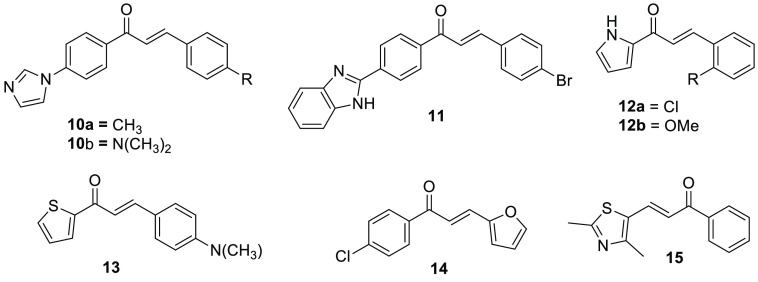
Chalcones with an N-heterocyclic moiety such as pyrrole, imidazole, thiazole, pyrazole, oxazole, isooxazole, pyridine, pyrazoline, indole, benzothiazole, benzimidazole, and quinoline scaffolds play a significant role in the area of medicine.11 Compounds 10, imidazole-based chalcone derivatives, displayed inhibition activity on MAO-A better than the standard drug.23 2-Benzimidazole chalcone (11) with a p-bromo group substituted to the phenyl ring acted as an insect antifeedant agent.24 Williams and co-workers reported a series of pyrrole-based chalcone derivatives and evaluated the biological activity of the CPY1 enzyme inhibition potential. The derivative 12a displayed the most selective inhibition of CYP1B1, and 12b was shown to inhibit both CYP1A1 and CYP1B1 isoforms with minimum IC50 values.25 Among the heterocyclic derivatives, thiophene-based chalcone derivative 13 displayed a calculated inhibitory constant value of approximately 0.64 μM toward the active site of MAO-B.26 Most of the time, furan-containing chalcone derivatives were found to be more active than the others. Derivative 14 displayed high activity against Streptococcus mutans and was the most potent derivative.27 The chalcone derivative 15 containing a difluorophenyl scaffold displayed 2.6× more activity than the standard drug. This suggests that the degree of electronegativity played a key role in modulating the physicochemical properties of the derivative (Figure 3).17
Figure 3.
Chemical structures of heterocycle-based chalcone derivatives.
Thus, the current Review presents the various synthetic protocols used to prepare chalcones incorporated with N-heterocyclic moieties and their wide spectrum of biological activities.
2. Synthesis of Chalcone Bearing an N-Heterocyclic Scaffold
2.1. Chalcone Bearing Pyrrole
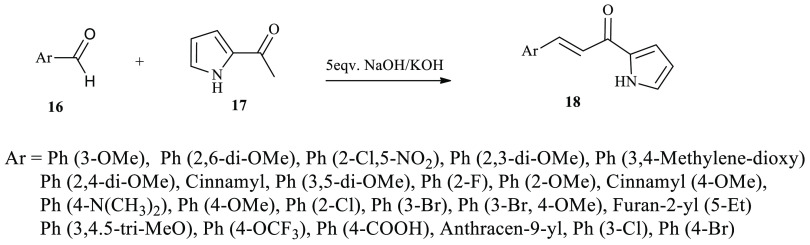
Series of pyrrole-based chalcone derivatives were reported through the classical Claisen–Schimdt condensation reaction protocol.28 The synthesized compounds displayed potential activity on the inhibition of CYP1 isoforms. As depicted in Scheme 1, the reaction afford products of various derivatives 18 starting from aromatic aldehyde derivatives 16 and pyrrole-based acetophenone 17 in basic media. The 2-pyrrole chalcone derivatives 18 were possible to synthesize using either a liquid phase or solid through a grinding method with moderate to good yields.
Scheme 1. General Synthetic Route toward Chalcone Derivatives Incorporating Pyrrole.
Özdemir and co-workers synthesized and reported new potent antimicrobial and anticancer active pyrrole-based chalcone derivatives using the Claisen–Schmidt condensation reaction.28 Using the reaction procedure of the reference, 2-acetyl-1-methylpyrrole 19 and aryl-furfural 20 were subjected to sodium hydroxide in methanol and further stirred at room temperature for about two days. At the completion of the reaction, the final chalcone derivatives 21 were recrystallized from ethano, and the precipitated reaction mixture was filtered, washed, and dried to afford a good yield (Scheme 2).
Scheme 2. General Synthetic Route toward Pyrrole-Based Chalcone Derivatives.
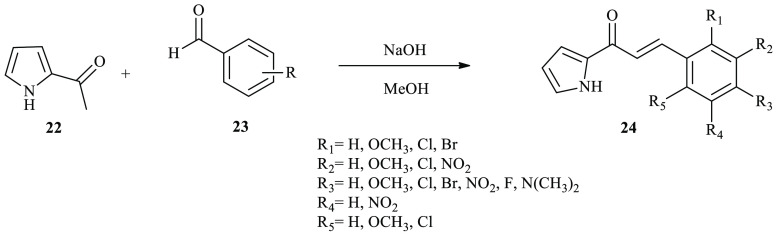
Similarly, Sharma and co-workers’ pyrrole-based chalcone derivatives 24 were synthesized and reported from 2-acetylpyrrole with substituted benzaldehyde derivatives using a condensation reaction under basic conditions.29 During the reaction, substituted benzaldehyde derivative 23 was added to 2-acetylpyrrole 22 that was dissolved in methanol and a 10% aqueous NaOH solution was added, and the reaction mixture was kept in stirred conditions until the completion of the reaction. After the completion of the reaction, the mixture was diluted with distilled water, and precipitated solid was filtered and recrystallized from the EtOH/EtOAc solvent mixture to afford chalcone derivatives 24 (Scheme 3).
Scheme 3. General Synthetic Route toward Chalcone Derivatives Incorporating a Pyrrole Scaffold.
2.2. Chalcones Bearing Imidazole
Sasidharan and co-workers reported a series of 11 imidazole-based chalcone-substituted derivatives using the Claisen–Schmidt condensation reaction between ethanone 25 and various para-substituted aromatic aldehyde derivative 26.30 A mixture of ethanone 25 and para-substituted benzaldehyde 26 in ethanol and aqueous potassium hydroxide was added and stirred at room temperature. The resulting product was kept overnight in a refrigerator, and the solid was filtered off, washed with water, and recrystallized from ethanol and methanol to afford pure imidazole chalcone derivatives 27, as depicted in Scheme 4.
Scheme 4. General Synthetic Route toward Imidazole-Based Chalcone Derivatives.
An antimicrobial active novel series of chalcone-imidazole derivatives 36(a–m) were synthesized and reported through the common Claisen–Schmidt condensation reaction procedure.30 Here, the synthesized derivatives of chalcone further functionalized using the known reaction procedure to afford imidazole-based chalcone derivatives. The synthesized substituted chalcones 28 were mixed with NBS and CCl4 in a round flask in the presence of AIBN. The mixture was refluxed and then filtered and distilled with CCl4 in vacuum to get compounds 29(a–i). Compounds 29 were subsequently further subjected to imidazole to afford compounds 30(j–m) through SN2 nuclephilic substitution reaction. Compounds 32(a–m) were subjected to K2CO3 and KOH with stirring, followed by the addition of imidazole in anhydrous CH3CN at ambient temperature under a nitrogen atmosphere. Finally, the chalcone–imidazole derivatives 33(a–m) were obtained after removal of the solvent following purification using column chromatography (Scheme 5).
Scheme 5. Synthetic Route toward Chalcone Derivatives Incorporating an Imidazole Moiety.
2.3. Chalcones Bearing Thiazole
Kasetti and co-workers reported antioxidant active thiazole-based chalcone derivatives through the common Claisen–Schmidt condensation reaction.31 Here, thiazole-carbaldehyde 34 was dissolved in glacial acetic acid and hydrochloric acid. To this mixture was added aryl or heteroaryl ketone moiety 35, which was previously dissolved in ethanol, and the mixture was refluxed. Once the reaction completed, the precipitate was filtered, washed, and dried, followed by procedural purification by column chromatography to isolate pure thiazole-based chalcone derivatives 36(a–u), as depicted in Scheme 6.
Scheme 6. General Synthesis of Thiazole-Based Chalcone Derivatives.

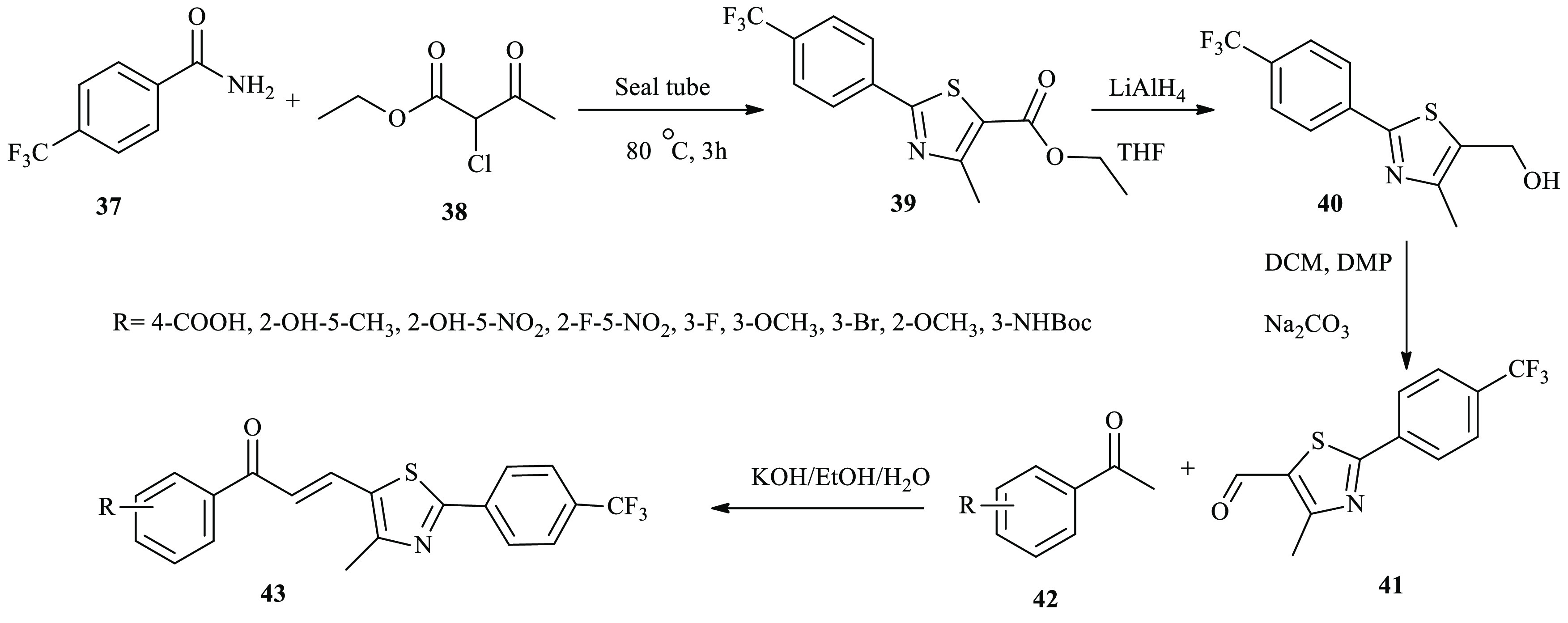
A series of anticancer active thiazole-based chalcone derivatives 43 were reported by Kasetti and co-workers.31 Here, thiazole-carbaldehyde 41 was synthesized in three steps. First, thiazole-carboxylate 39 was formed through subjecting benzamide 37 to 2-chloroacetate 38. The ester moiety of compound 39 was reduced using LiAlH4 to alcohol compound 40, followed by a Dess–Martin oxidation reaction to afford the key target intermediate 41. The synthesized aldehyde was reacted with aryl methyl ketone in ethanol, and KOH was added dropwise to the mixture with continuous stirring. Finally, the mixture was filtered, washed, and dried to get a yellow solid compound, which was further purified using recrystallization with ethanol to get pure yellow solid thiazole-based chalcone derivatives 43 with a good yield, as described in Scheme 7.
Scheme 7. General Synthesis of Thiazole-Based Chalcone Derivatives 43.
Antitumor active thiazole-incorporated chalcone derivatives were reported by Farghaly and co-workers.32 A mixture of 4-acetylthiazole 44 derivative was subjected to the aromatic aldehyde derivatives 45 in anhydrous ethanol. An aqueous solution of NaOH was added dropwise, and the reaction mixture was stirred. The solid formed was collected using filtration and recrystallized from a mixture of ethanol/dioxane solvents to afford the thiazole-based chalcone derivatives 46 (Scheme 8).
Scheme 8. General Synthesis of Thiazole-Based Chalcone Derivatives 46.
2.4. Chalcones Bearing Pyridine
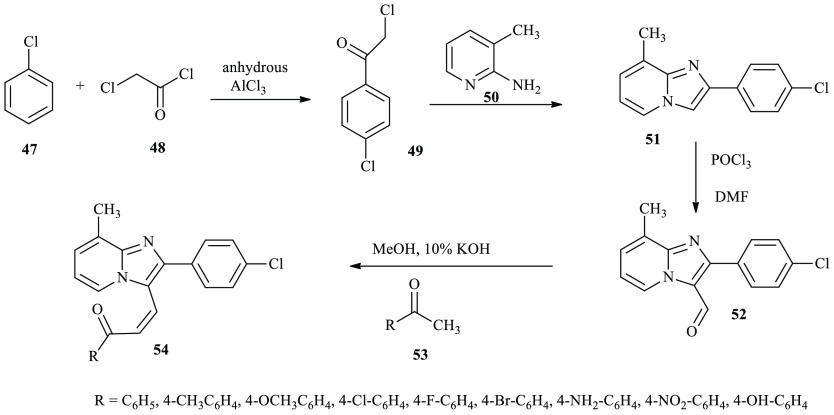
Rupala and co-workers reported a series of imidazo-pyridine derivatives through the condensation of aryl methyl ketone and aryl aldehyde in the presence of alcoholic alkali media.32 The synthesis mixture of pyridine-3-carbaldehyde 52 and ethanone 53 was refluxed in methanol, followed by the addition of NaOH as a catalyst. The crude was poured on to crushed ice, and the resulting product was further recrystallized from dichloromethane to give compound 54, as provide in Scheme 9.
Scheme 9. General Synthesis of Pyridine-Based Chalcone Derivatives.
The synthesis of novel anticancer active chalcone incorporating a pyridine scaffold was reported by Madhavi and co-workers.33,34 The iodopyridine 56 was subjected to a Buchwald coupling reaction with trimethoxyaniline 55 in the presence of dioxane as the solvent and mild base to afford pyridin-3-amine compound 57 in a good yield. The intermediate 57 undergoes Suzuki coupling with a boronic acid 58 using a palladium salt to afford the key skeleton pyridin-3-yl benzaldehyde 59. Finally, the intermediate aldehyde 59 was refluxed with substituted acetophenone derivatives 60(a–j) in ethanol as the solvent and a catalytic amount of piperidine base to afford compounds 61(a–j), as shown in Scheme 10.
Scheme 10. General Synthesis Routes toward Pyridine-Incorporated Chalcone Derivatives 61.

Similarly, the synthesis of a series of anticancer active pyridine-based chalcone derivatives was reported by Durgapal and co-workers from 3-aminomethylpyridine and 4-amino chalcone.35 Procedurally, 4-aminoacetophenone 62 was subjected to aldehyde 63 under basic reaction conditions to afford 4-amino chalcone derivatives 64. Further, 4-amino chalcones 64 react with bromoacetyl bromide in the presence of DCM and TEA with stirring to afford 66. Compounds 66 were further allowed to react with 3-aminomethylpyridine 67 in DCM with TEA base at room temperature to afford various pyridine-based chalcone derivatives 68, as described in Scheme 11.
Scheme 11. General Synthesis Routes toward Chalcone Incorporating a Pyridine Moiety.

2.5. Chalcones Bearing Piperazine
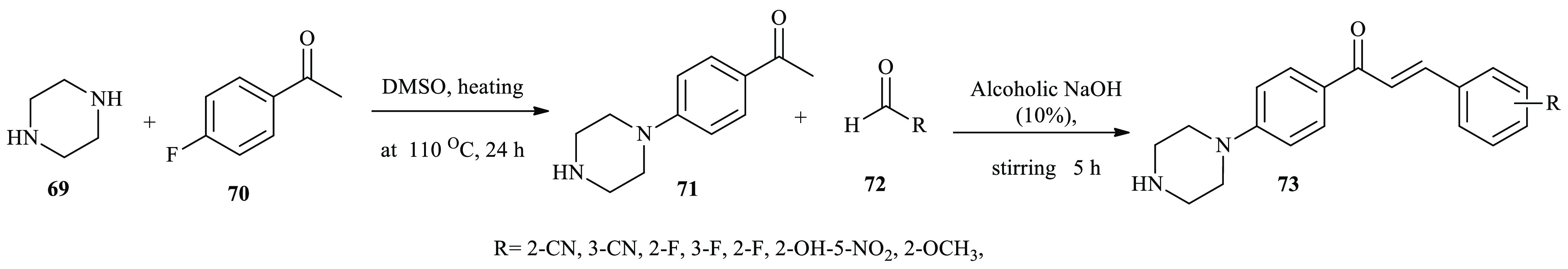
Ahmed and co-workers reported the synthesis of piperazine-chalcone hybrid derivatives as potential vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitors.36 A mixture of a derivative of acetophenone 71 and corresponding aldehyde derivatives 72 was dissolved in 10% alcoholic sodium hydroxide and stirred at room temperature. After the reaction completed, the precipitate was filtered, washed, dried, and recrystallized from ethanol to afford the target compounds 73 (Scheme 12).
Scheme 12. General Synthesis Routes toward Chalcone Derivatives Incorporating Piperazine.
A series of novel chalcone hybrids with piperazine derivatives were synthesized and reported as potent antitumor agents.37 During the preparation of the target compounds, acetophenone 74 reacted with benzaldehyde 75 in the presence of KOH via aldol condensation to give chalcone 76. Subsequently, the key intermediate 78 was prepared through the substitution of the fluorine atom of 76 with piperazine 77. Finally, acylation and sulfonylation of the −NH group with acyl chloride or carboxylic acid and sulfonyl chloride afforded hybrid compounds 84 and 82 in good yields, respectively. Further tertiary amines 83 were synthesized by treatment with 2-bromoacetophenone (Scheme 13).
Scheme 13. General Synthesis Routes Towards a Chalcone–Piperazine Hybrid.

2.6. Chalcones Bearing Indole
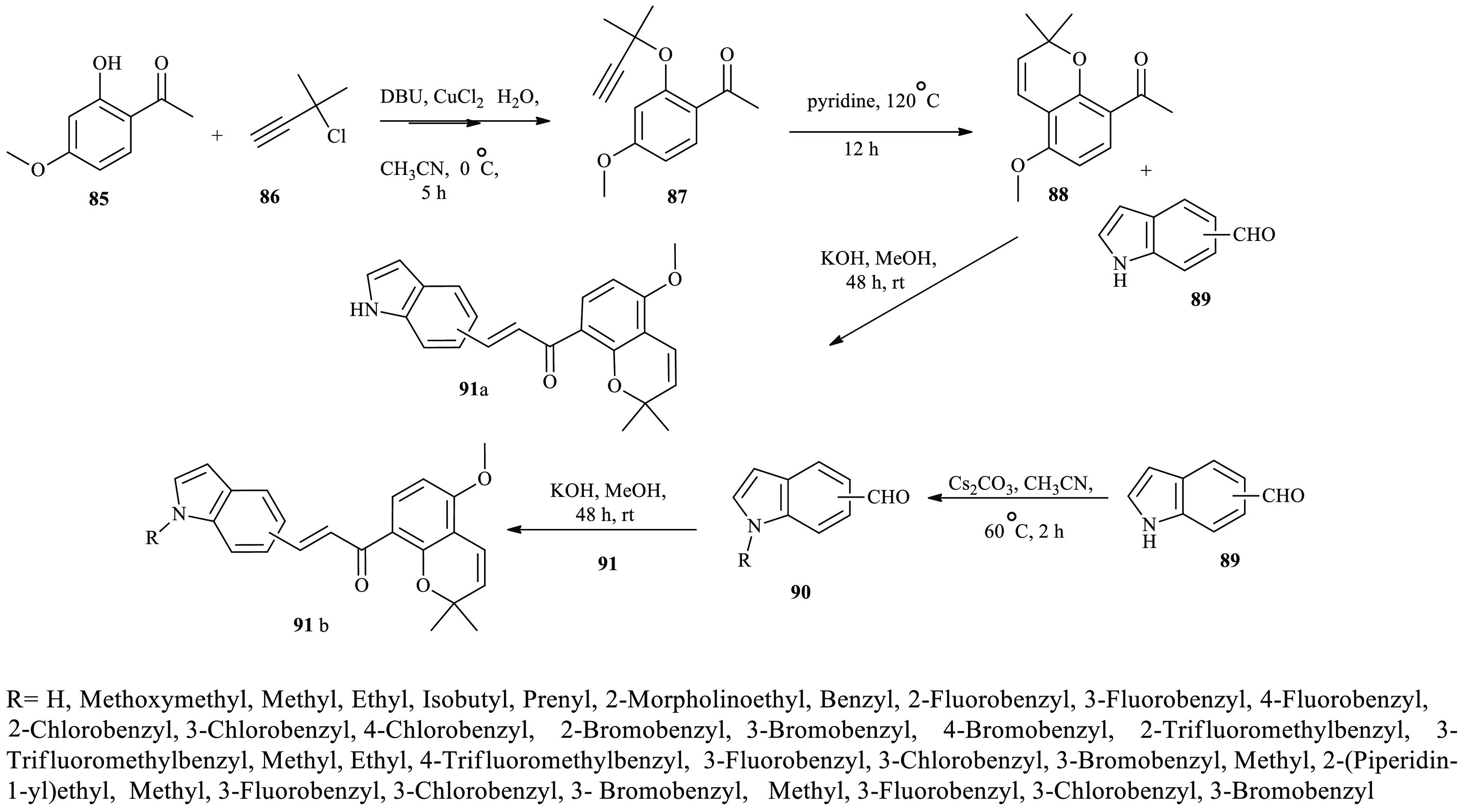
Antiproliferative active compounds based on pyrano-chalcone derivatives containing an indole moiety as a major scaffold were reported by Wang and coworkrs.38 Here, ethanone derivative 85 in acetonitrile was subjected to 3-chloro-3-methyl-1-butyne 86 in the presence of DBU and catalytic copper salt to afford 1,1-dimethylpropargyl ether 87 in a good yield. Further, compound 87 underwent a Claisen rearrangement reaction in the presence of pyridine, leading to key intermediate compound 88. Finally, condensation of compound 88 with N–H indole-aldehyde derivatives 89 or N-alkyl indole aldehyde derivatives 90 under Claisen–Schmidt reaction conditions using the standard reaction procedure provided the desired compounds 91(a and b) (Scheme 14).
Scheme 14. General Synthesis Routes toward Chalcone Containing an Indole Scaffold.
Previously, the syntheses of novel indole-based chalcone derivatives were reported by Gao and co-workers using two powerful methods, the ultrasound-assisted and solvent-free Claisen Schmidt condensation reactions.39 On one hand, using the ultrasound-assisted method, indole 92 was subjected to aromatic aldehyde 93 in dioxane under basic reaction condition to furnish indole-based chalcone derivatives 94. On the other hand, using the solvent-free grinding method, similarly indole 92 and aromatic aldehydes 93 under basic reaction condition were intimately ground using pestle and mortar at room temperature, and the resulting product was treated with HCl, filtered, and recrystallized from ethanol to provide indole-based derivatives 94. Finally, the ultrasonication procedure proved that efficient promotion and push using the Claisen–Schmidt reaction condensation move the reaction forward in a short reaction time with better yields, as depicted in Scheme 15.
Scheme 15. Ultrasound-Assisted (I) and Solvent-Free (II) Synthesis Protocols for the Preparation of Indole-Based Chalcone Derivatives.
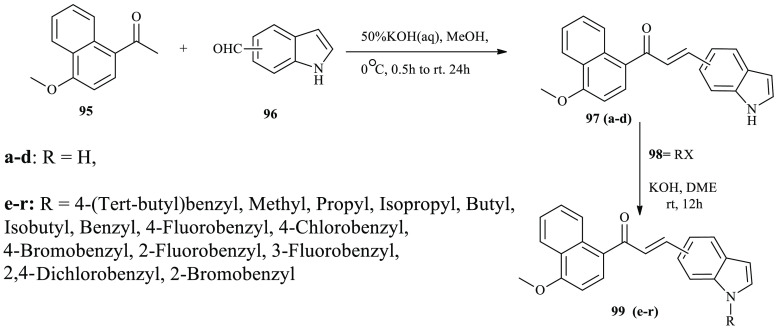
In recent report, various class of chalcone derivatives containing indole and naphthalene moieties were reported as potent anticancer agents.40 Through the Claisen–Schmidt condensation reaction, an aromatic ketone derivative 95 and commercial available indole aldehyde derivatives 96 afford indole-based chalcone derivatives 97(a–d) in moderate to good yields. Indole-based chalcone derivatives 97(a–d) further reacted with alkyl halides 98 under basic conditions to furnish N-alkylated indole-based chalcone derivatives 99(e–r), as depicted in Scheme 16.
Scheme 16. General Synthesis Routes toward Indole-Based Chalcone Derivatives.
2.7. Chalcones Bearing Benzimidazole
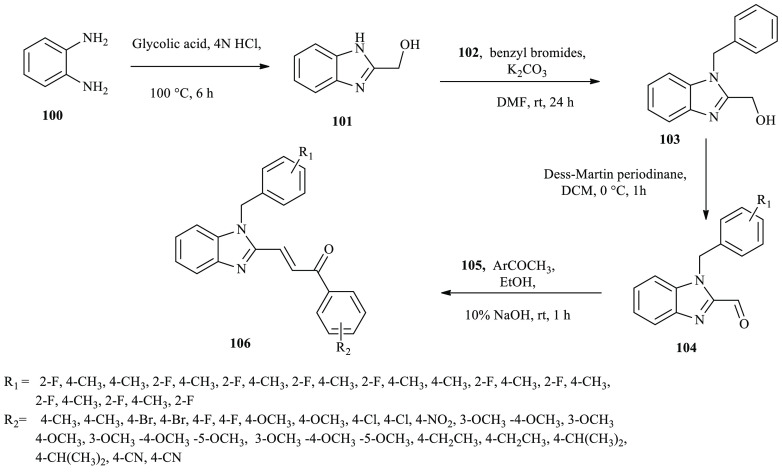
A series of benzimidazole-based chalcone derivatives were synthesized and reported using the Claisen–Schmidt reaction as potential Topo II-targeting anticancer agents.41 Benzimidazole derivative 101 was obtained through refluxing o-phenylenediamine (100) with glycolic acid in the presence of HCl. The substituted benzimidazole-2-methanol (103) was synthesized through the alkylation of compound 101 using a benzyl bromide in the presence of K2CO3. Subsequently, substituted benzimidazole-2-carbaldehyde derivatives 104 were obtained from 103 through oxidation using a Dess–Martin protocol. Compounds 103 were finally prepared from 104 through a Claisen–Schmidt reaction in the presence of appropriate acetophenone derivatives, as depicted in Scheme 17.
Scheme 17. General Synthesis Routes toward Benzimidazole-Based Chalcone Derivatives.
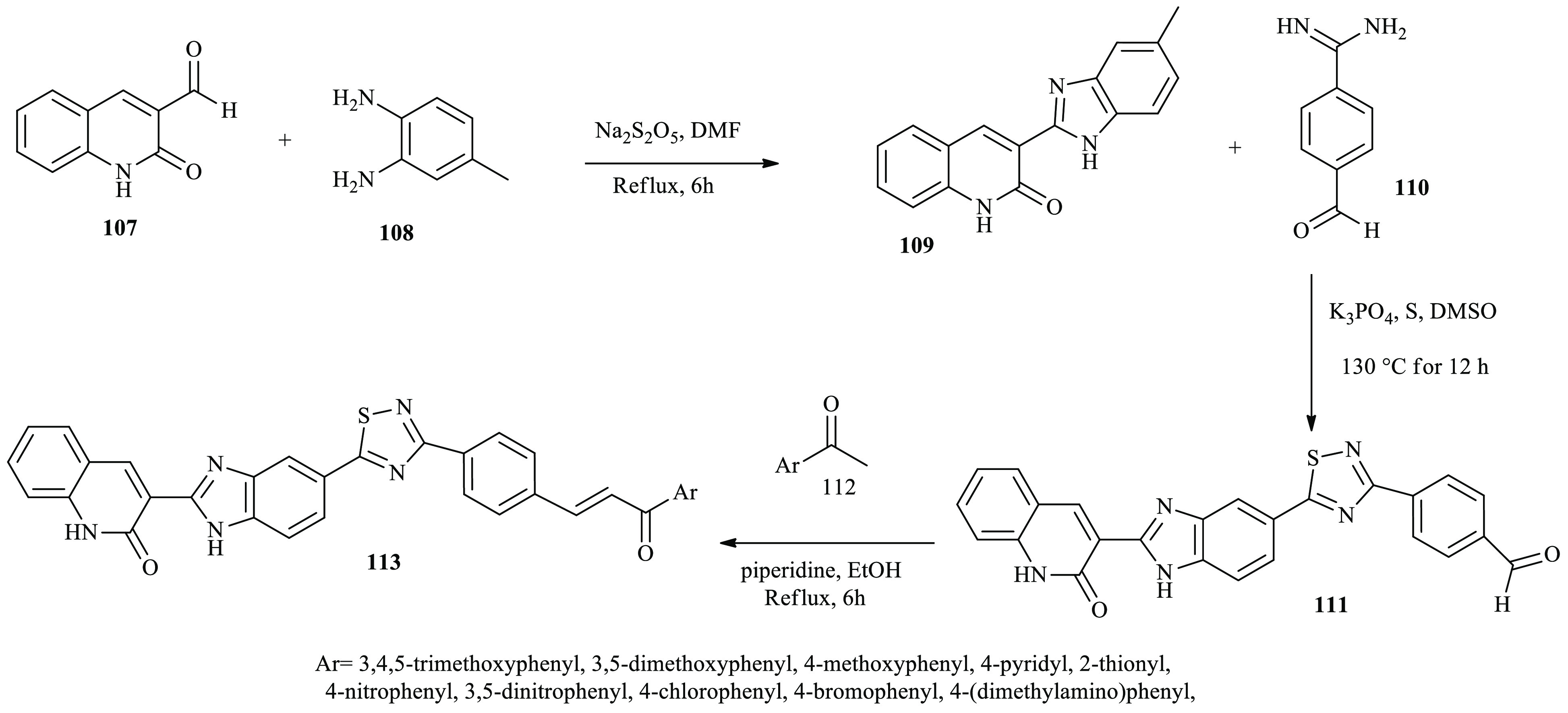
Similarly, Pragathi and co-workers reported a series of anticancer active benzimidazole-based chalcones incorporating quinoline–benzimidazole–thiadiazole heterocyclic scaffolds through the aldol condensation reaction.42 Compound 109 was prepared from 1,2-dihydro-2-oxoquinoline-3-carbaldehyde 107 and benzene-1,2-diamine 108 through a double condensation reaction. Subsequently, the intermediate 109 was reacted with 4-formylbenzamidine hydrochloride 110 to afford derivative 111. Compound 111 further subjected to the aldol condensation reaction with acetophenone derivatives 112(a–j) to furnish benzimidazole-based chalcone derivatives 113(a–j), as depicted in Scheme 18.
Scheme 18. General Synthesis Routes toward Chalcones 113 Incorporating Thiadiazol–Benzimidazol–Quinolinone Heterocyclic Scaffolds.
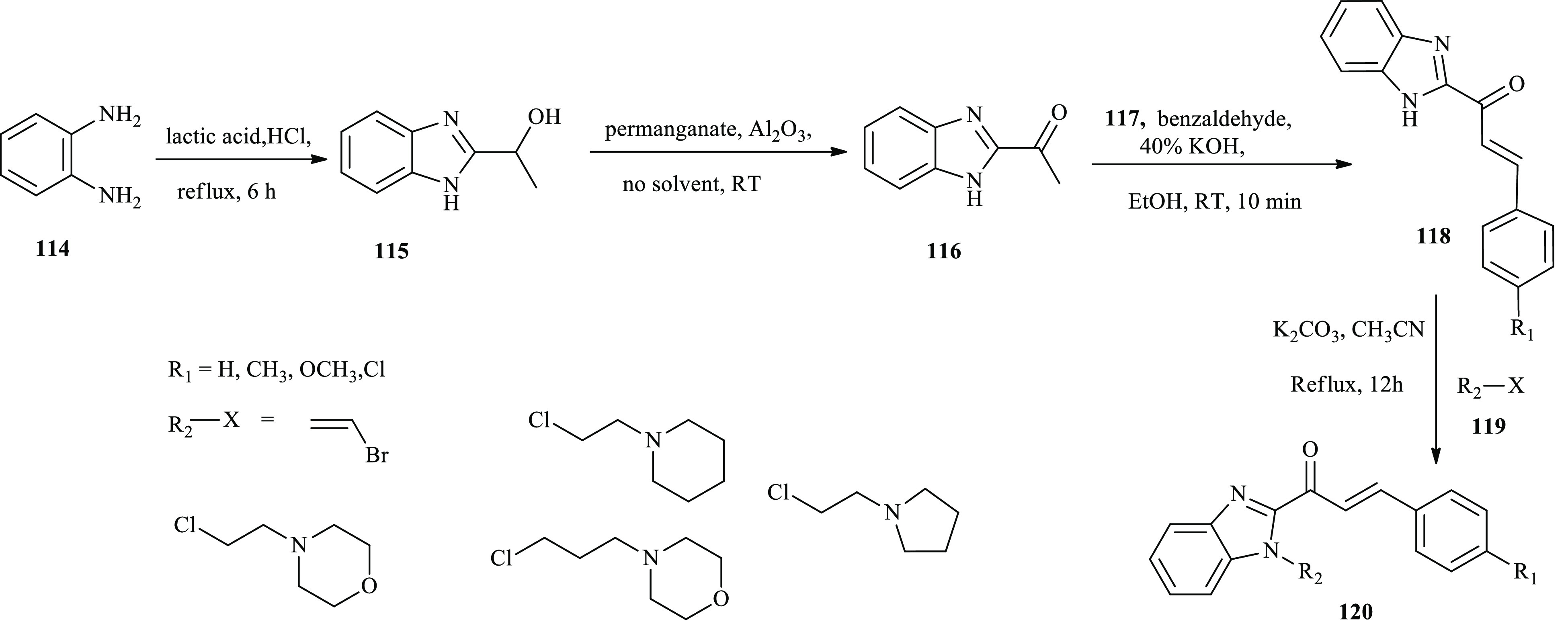
A new series of N-substituted benzimidazole-based chalcone derivatives were reported by Hsieh and co-workers through the conjugation of benzimidazole and aromatic aldehyde derivatives under basic conditions as anticancer agents.43 The reaction of o-phenylenediamine (114) with lactic acid in HCl under reflux reaction conditions prepared benzimidazole derivatives 115, followed by an oxidation reaction in the presence of potassium permanganate as a strong oxidizing agent and solid aluminum oxide to afford compound 116. Benzimidazolyl-aryl chalcone derivatives 118(a–d) were obtained through the aldol condensation reaction of compound 116 with substituted aromatic aldehyde derivatives under basic conditions. Further, benzimidazole–aryl chalcone derivatives 118 subjected to a methylation reaction with the corresponding agent to furnished compound 120, as described in Scheme 19.
Scheme 19. General Synthesis Routes toward Benzimidazole–Aryl-Based Chalcone Derivatives.
2.8. Chalcones Bearing Benzothiazole
Wang and co-workers reported a series of novel benzothiazole-based chalcone derivatives as potential antibacterial agents.44 Following the standard reaction reported procedure to prepare 4-hydroxy-acetophenone 121, aldosterone condensations were performed under alkali conditions to prepare chalcone derivatives 123 with various aldehyde substitutions.45 Compound 125 was prepared when compound 123 underwent an etherification reaction with 2-chlorobenzothiazole 124 in the presence of acetonitrile as a solvent, potassium carbonate as a catalyst, and reflux, as depicted in Scheme 20.
Scheme 20. General Synthesis Routes toward Benzothiazole-Based Chalcone Derivatives.

Similarly, in recent studies a series of benzothiazole-based chalcones were reported to have potential thymidylate kinase (BmTMK) enzyme inhibition activity.46 Different derivatives of phenol and hexamethylenetetramine were dissolved in TFA at 120 °C with continuous stirring to afford compound 127 in good yields. A dicarbaldehyde compound 127 and different substituted ketone derivatives 128 were dissolved in 10% aq KOH and ethanol, and the resulting solution was refluxed to afford compound 129. A mixture of ortho-substituted chalcone derivatives 129 and 2-hydrazinyl benzothiazole 131 was dissolved in ethanol and stirred at room temperature for 3–4 h to furnish the final benzothiazole-based chalcone derivative 132, as depicted in Scheme 21.
Scheme 21. General Synthesis Routes toward Benzothiazole-Based Chalcone Derivatives.

2.9. Chalcones Bearing Quinoline
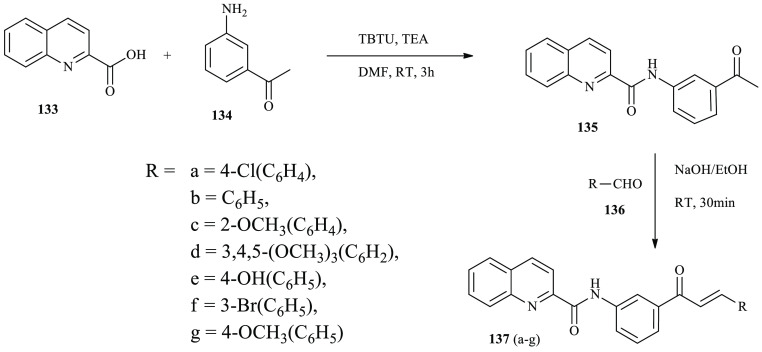
A series of quinoline-based chalcone derivatives were synthesized and reported by Thirumurugan and co-workers through the common aldol condensation reaction.47 Quinoline-based chalcones N-quinoline-2-carboxamides 138 were synthesized using quinoline-2-carboxylic acid 136 subjected to ethanone 137 in TEA using TBTU as the base with reflux for 3 h at room temperature (Scheme 22). Compounds 138 undergo the aldol condensation reaction with benzaldehyde derivatives 139 using NaOH/EtOH at room temperature for 30 min to give quinoline-2-carboxamide derivatives 140(a–g), as shown in Scheme 23.
Scheme 22. General Synthesis Routes toward Chalcones Incorporating a Quinoline Moiety.
Scheme 23. General Synthesis Routes toward Quinoline-Based Chalcone Derivatives.
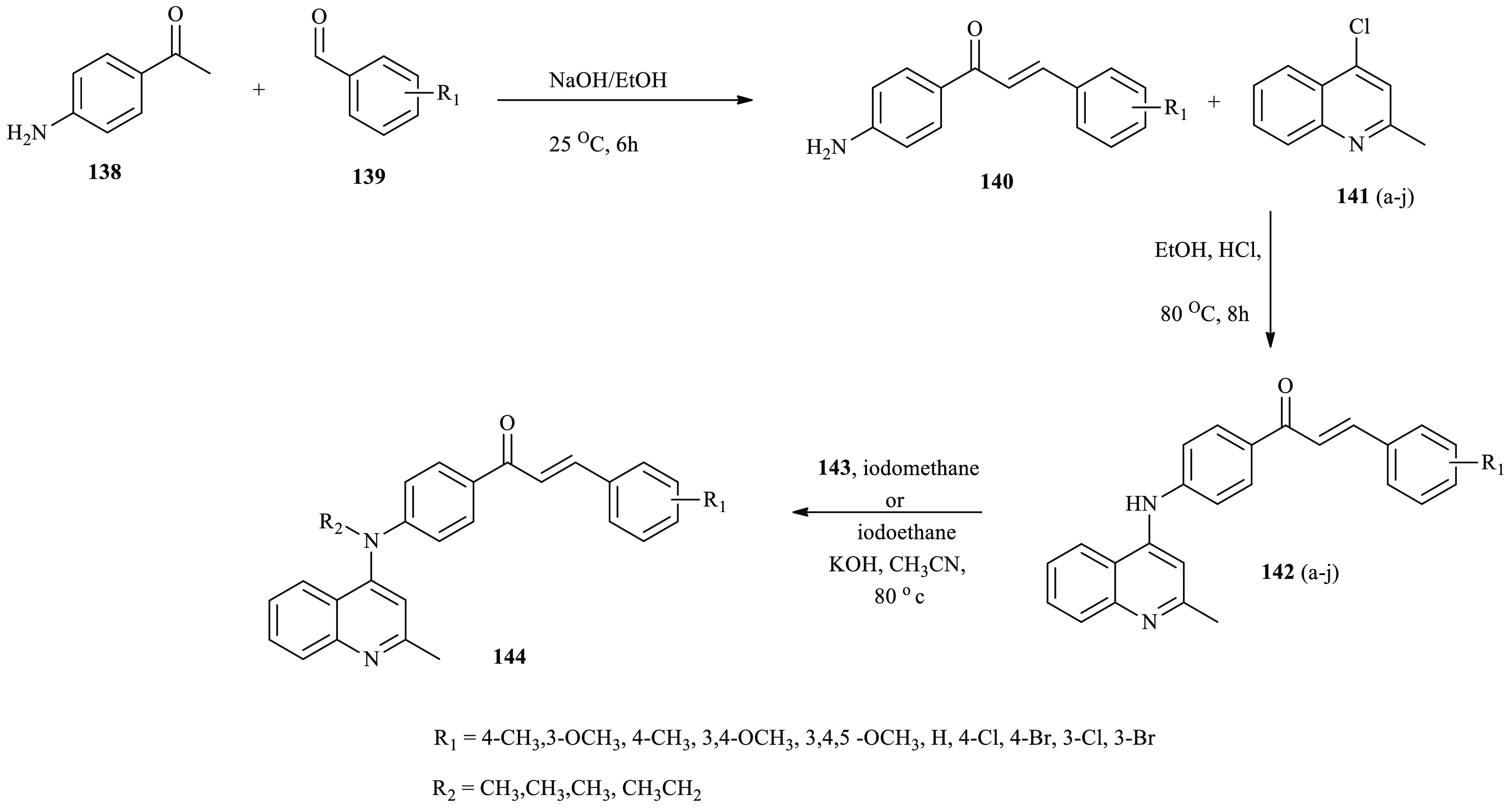
Commonly, quinoline-based chalcone scaffolds are frequently utilized to design novel anticancer agents. A series of quinoline-based chalcone derivatives were synthesized and reported by Guan and co-workers as described.48 Here, 4-aminoacetophenone 138 reacted with aromatic aldehyde derivatives 139(a–j) to afford derivatives 140(a–j). Compounds 140(a–j) were subjected to 4-chloro-2-methylquinoline (141) to afford derivatives 142(a–j). Further, compounds 142 (a, b, e, and f) reacted with iodomethane or iodoethane in the presence of KOH in acetonitrile to afford 144(a, b, e, and f), as depicted in Scheme 23.
3. Biological Activities of Chalcones Bearing an N-Heterocyclic Scaffold
In the synthesis section, chalcone derivatives incorporating heterocyclic scaffolds such as pyrrole, imidazole, thiazole, pyridine, piperazine, indole, benzimidazole, benzothiazole, and quinoline were briefly discussed and their broad spectrum bioactivities were highlighted. In this section, the scope and biological activities of chalcone derivatives incorporating N-heterocyclic scaffolds in the medicinal chemistry arena and therapeutic issues will discussed in detail. The title compound derivatives become impressive, and literature reports stress the significance of the α,β-unsaturated carbonyl moiety to drug target interactions and biological activities.49 Both natural products and synthetic compounds incorporating N-heterocyclic scaffolds displayed a broad band spectrum of pharmaceutical activities such as antibacterial, antifungal, antioxidant, anti-inflammatory, antitubercular, anticancer, antimalarial, and antileishmanial activities.50
3.1. Antibacterial Activity
Chalcone derivatives incorporating heterocyclic scaffolds are basis for the development of antibacterial agents.51 Shaik and co-workers reported the synthesis of novel bioactive isoxazole-based chalcones and their dihydropyrazole derivatives.52 All synthesized derivatives were tested for their antibacterial activities through the serial dilution method against two types of bacterial strain, Staphylococcus aureus and Pseudomonas aeruginosa. All the synthesized compounds exhibited antibacterial activity; in particular, compound 145 containing a 2,4,6-trimethoxyphenyl ring was the most potent agent among the chalcone series, and its antibacterial activity was found to be greater than that of the reference drug ciprofloxacin (Figure 4). Further, the synthesized compounds exhibited antifungal, antioxidant, and anticancer activities.53
Figure 4.
Chemical structures of antibacterial active chalcone derivatives.
Similarly, series of benzimidazole-based chalcone derivatives containing an oxadiazole moiety were reported by Meshram and co-workers through a Claisen–Schimdt condensation reaction.54 The synthesized compounds were evaluated for their efficiency as antibacterial agents against two Gram-positive (Staphylococcus aureus and Streptococcus pyogenes) and two Gram-negative (Escherichia coli and Pseudomonas aeruginosa) strains of bacteria using the broth microdilution method. Although all tested compounds exhibited potent antibacterial activity, compounds 146 and 147 displayed the most potent activities (Figure 4). Chalcone derivatives possessing an oxadiazole ring with a benzimidazole scaffold have displayed enhanced antimicrobial activity due to incorporation of the heterocyclic moieties compared to the parent compounds.
3.2. Antifungal Activity
Osmaniye and co-workers reported the synthesis of imidazole-based chalcones that incorporate pharmacophores through a Claisen–Schmidt condensation reaction from imidazole-acetophenone with the corresponding 4-substituted benzaldehyde derivatives.55 The antifungal activity of the synthesized compounds was evaluated for anticandidal activity against Candida albicans (ATCC 24433), Candida krusei (ATCC 6258), Candida parapsilosis (ATCC 22019), and Candida glabrata (ATCC 90030) in the presence of reference agents ketoconazole and fluconazole. Compound 148 exhibited similar antifungal activity with the reference drug ketoconazole against all Candida species and was evaluated as the most active derivative in the series. In a similar fashion, Sunitha and co-workers reported the synthesis of series of bisisoxazole derivatives blended with chalcone derivatives.56 Antifungal activities of all synthesized derivatives were tested against Microsporum canis, Microsporum gypseum, and Epidermophyton floccosum in 75 and 100 μg/mL concentrations with the reference drug nystatin. Compound 149, 150, and 151 display the highest antifungal activity and also show potent antibacterial activity (Figure 5).
Figure 5.
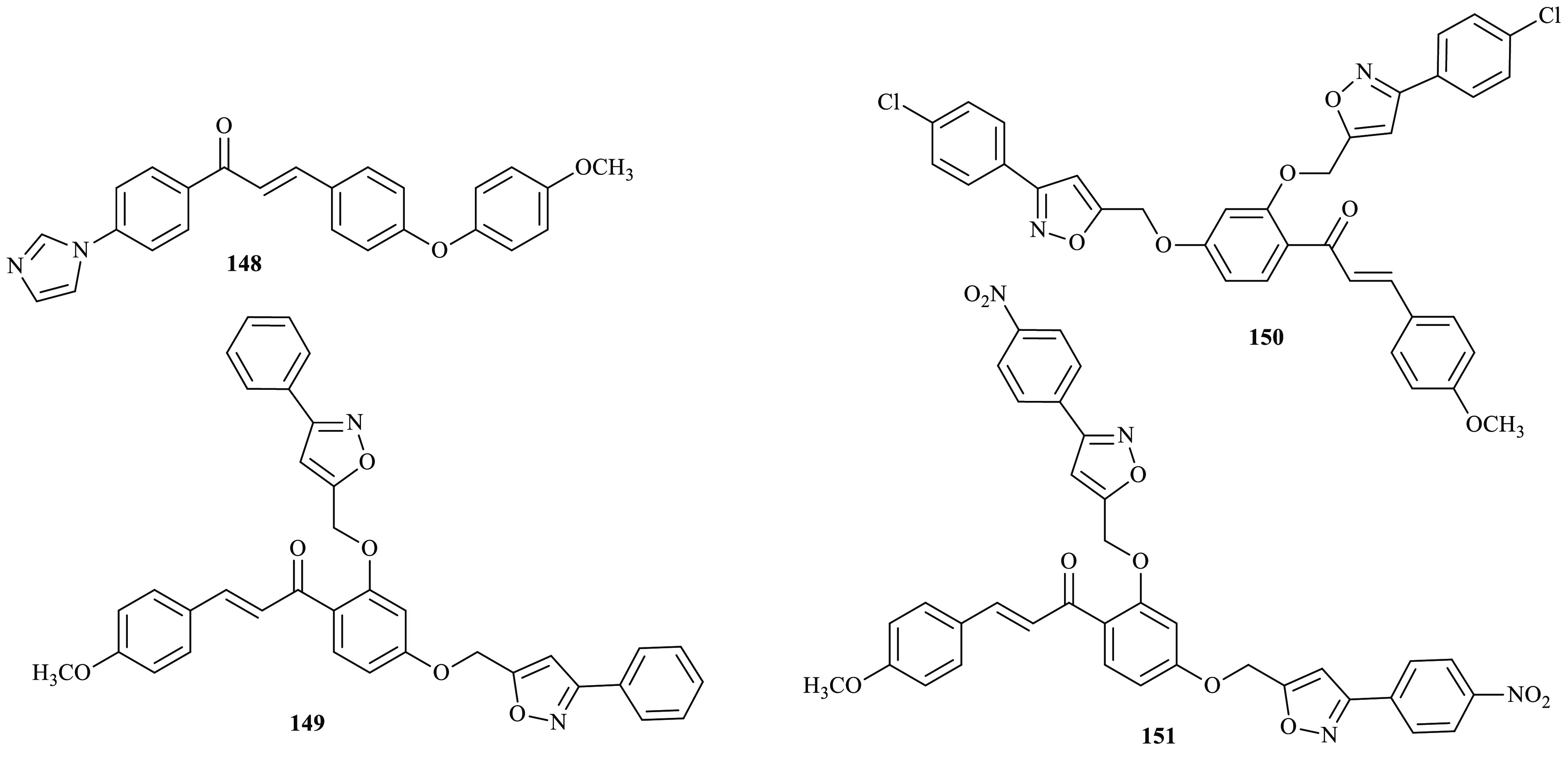
Chemical structures of antifungal active chalcone derivatives.
3.3. Antitubercular Activity
Tuberculosis (TB) is a chronic infectious disease caused predominantly by Mycobacterium tuberculosis. The World Health Organization (WHO) reported that about a third of the world’s population is infected with M. tuberculosis.57 Kasetti and co-workers reported bioactive compounds containing thiazole and chalcone pharmacophores.31 All synthesized compounds were evaluated for their antitubercular activities by MTT assays. Among the tested compounds, the monofluorinated compounds 152 and 153 bearing fluorine atoms at ortho- and para-positions showed activity at MIC 20.68 μM that was 0.81× greater than the pyrazinamide derivative (Figure 6). Solankee and Tailor reported a new series of chalcones bearing a 1,3,5-triazine group that were synthesized by the classical Claisen–Schmidt condensation of a substituted ketone with the corresponding substituted aldehydes.58 The in vitro antitubercular activities of all the newly synthesized compounds were determined using a Lowenstein–Jensen medium against Mycobacterial tuberculosis H37Rv strain relative to the reference drugs isoniazid and rifampicin. Among the compounds, a compound 154 was found to possess the greatest potency against Mycobacterium tuberculosis with 92% inhibition.
Figure 6.

Chemical structures of antitubercular active chalcone derivatives.
3.4. Antioxidant Activity
Recently, Bhat and co-workers reported a novel series of bioactive 1,2,3-triazolyl chalcone derivatives that were synthesized via the Claisen–Schimidt reaction.59 The antioxidant activities of the compounds were further evaluated using the ABTS (2,2′-azino-bis(3-ethyl benzothiazoline-6-sulfonic acid)) antioxidant assay technique. Evaluation of the antioxidant activity revealed that most of the tested compounds exhibited moderate to excellent DPPH and ABTS radical scavenging potential compared to the positive control ascorbic acid. Among the synthesized compounds, 155 and 156 bearing 3,4-dimethyl phenyl and 1,3-(biphenyl)-1H-pyrazole at the third position of chalcone moiety, respectively, were found to be more effective and potent. They further displayed DPPH radical scavenging ability with IC50 values 15.33 and 14.48 μM compared with ascorbic acid with an IC50 value of 12.27 μM at a 31.5 μg/mL concentration by the DPPH radical scavenging activity method. The antioxidant activity of the derivatives 155 and 156 was further illustrated by ABTS assay method with 80.4% and 81.8% inhibition compared to the positive control ascorbic acid with 88.5% inhibition. Similarly, arylic substitutions with pyrazolic chalcones were reported as potent antimicrobial and antioxidant agents by Kumari and co-workers through Claisen–Schmidt condensation.60 The in vitro antioxidant potential of the synthesized compounds was evaluated using the DPPH method, with ascorbic acid as a standard reference. Among the synthesized compounds assayed for antioxidant activity in the DPPH method, compound 157 exhibited good radical scavenging activity an IC50 value of 88.04 μg/mL, and the value of standard drug ascorbic acid was found to be 48 μg/mL (Figure 7).
Figure 7.
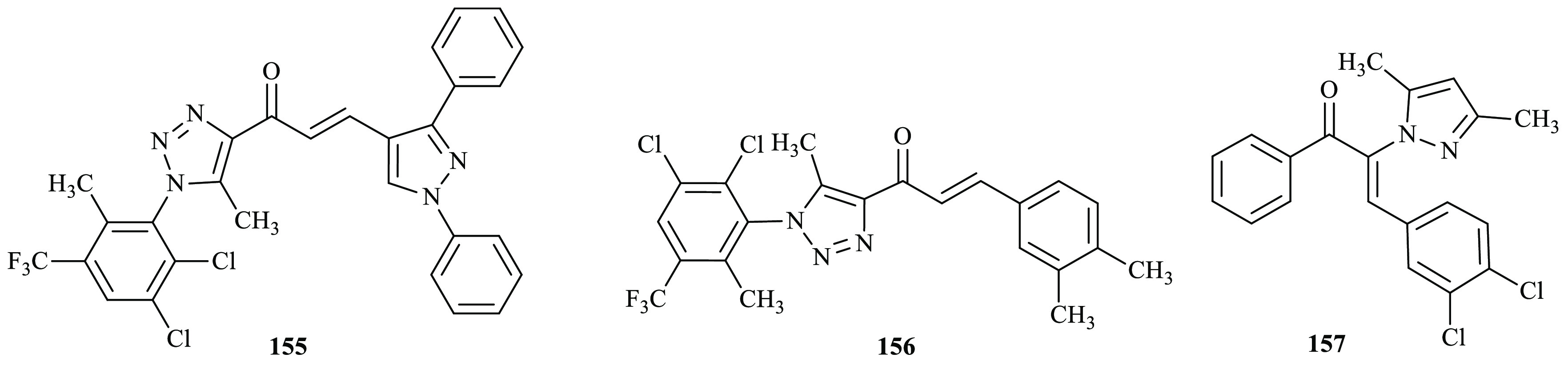
Chemical structures of antioxidant active chalcone derivatives.
3.5. Anticancer Activity
Cancer is one of the most serious of a devastating diseases and a multifactorial disease. In 2020, 19.3 million patients were diagnosed and approximately 10 million deaths were related to cancer.61 These data indicate that the improvement of effective anticancer drugs with minimized side effects is needed. Novel bioactive indole-based chalcone derivatives were reported by Yan and co-workers.62 Antiproliferative activities of all synthesized compounds were evaluated against various human cancer cell lines by MTT assay. Among the tested compounds, compound 158 exhibited the most potent activity, with IC50 values of 3–9 nM against six cancer cells. Similarly, a new series of imidazole-based chalcone derivatives were reported as tubulin inhibitors and anticancer agents.63 The cytotoxic activity of the synthesized compounds was evaluated against four cancer cell lines including MCF7, A549, HepG2, and MCF7/MX by MTT assay. Compounds 159 and 160 exhibited strong cytotoxic activity with IC50 values ranging from 7.05 to 63.43 μM against all four human cancer cells. In previous work, a series of novel chalcones containing quinoline were reported as antiproliferative agents.63 The in vitro antiproliferative efficacy of the prepared compounds was assessed by MTT assays using human chronic myelogenous leukemia cell K562 and compared to that of the reference compound CA-4. Among them, compound 161 exhibited the most potent activity with IC50 values ranging from 0.009 to 0.016 μM in a panel of cancer cell lines. Furthermore, a series of triazole–benzimidazole–chalcone derivatives were reported from the combination of different azide derivatives and substituted benzimidazole terminal alkynes bearing a chalcone moiety.65 The antiproliferative activities of synthesized compounds were examined against two human breast cancer cell lines (T47-D and MDA-MB-231) and one prostate cancer cell line (PC3) using a resazurin-based method. Among the evaluated compounds, 162 exerted highest cytotoxic effects on all the selected PC-3, MDA-MB-231, and T47-D cell lines, with IC50 values of 10.7, 5.89, and 6.23 μM compared to the reference drug doxorubicin (0.13, 1.51, and 0.73 μM), respectively (Figure 8).
Figure 8.
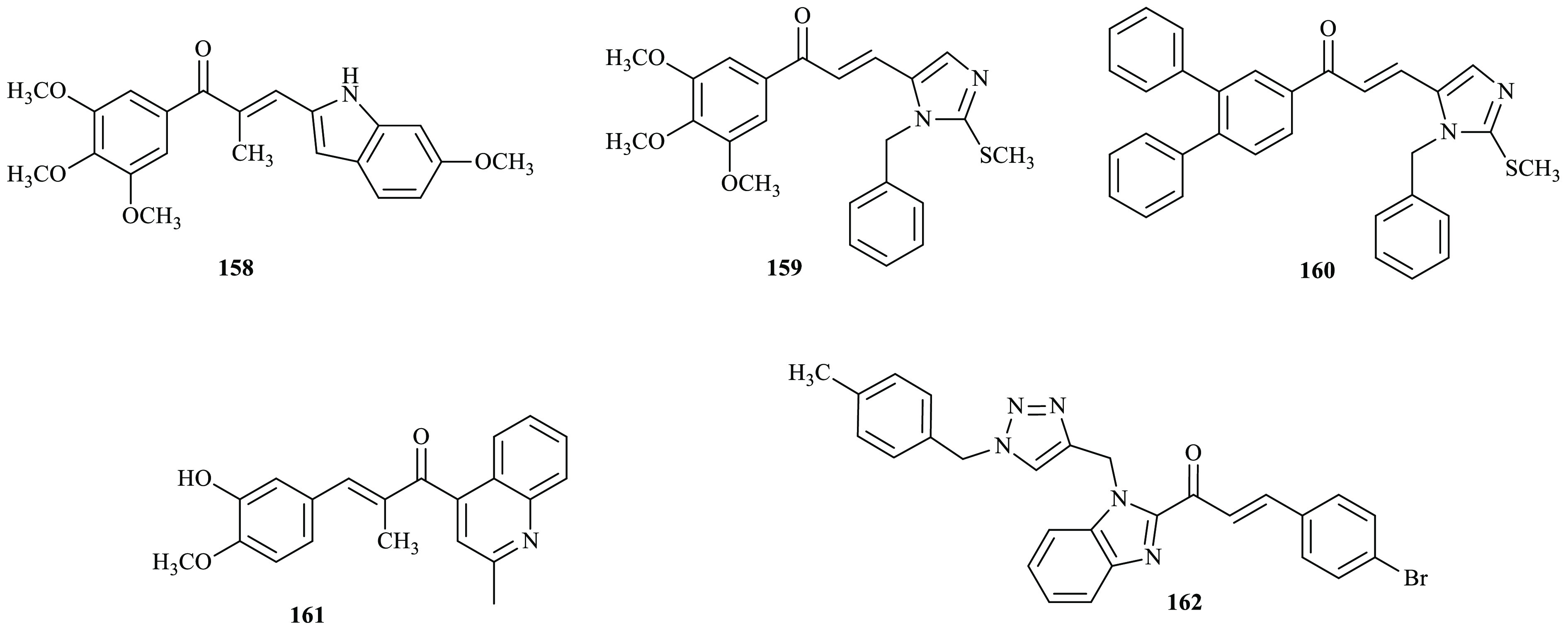
Chemical structures of anticancer active chalcone derivatives.
3.6. Anti-Inflammatory Activity
In previous work, a series of chalcone derivatives bearing a bispiperazine linker were reported by Tang and co-workers via a condensation reaction.66 The synthesized compounds were evaluated for their in vitro anti-inflammatory activity in LPS-induced RAW 264.7, and piperazinochalcone significantly inhibited the production of TNF-α. Most bispiperazine chalcone derivatives exhibited excellent anti-inflammatory activities. Specially, the IC50 values of 163 and 164 were 0.42 and 0.82 μM, respectively, compared to that of the positive control dexamethasone (IC50 < 20 μM). Recently, Özdemir and co-workers were synthesized and reported new indole-based chalcone derivatives through the Claisen–Schmidt condensation reaction.67 Colorimetric COX (ovine) inhibitor screening assay was carried out to evaluate the ability of the compounds to inhibit COX-1 and COX-2 in vitro. Compound 165 (IC50 = 8.6 ± 0.1 μg/mL) and compound 166 (IC50 = 8.1 ± 0.2 μg/mL) were found as the most potent COX-1 inhibitors when compared with indometacin (IC50 = 0.7 ± 0.2 μg/mL). Furthermore, compound 166 exerted a COX-2 inhibitory effect with an IC50 value of 9.5 ± 0.8 μg/mL when compared with indometacin (IC50= 10.0 ± 4.2 μg/mL). Moreover, according to the CCK-8 assay, the cytotoxic doses of compounds 165 (IC50= 32.3 ± 6.7 μg/mL) and 166 (IC50= 51.6 ± 15.3 μg/mL) for NIH/3T3 cells were higher than their effective doses (Figure 9).
Figure 9.
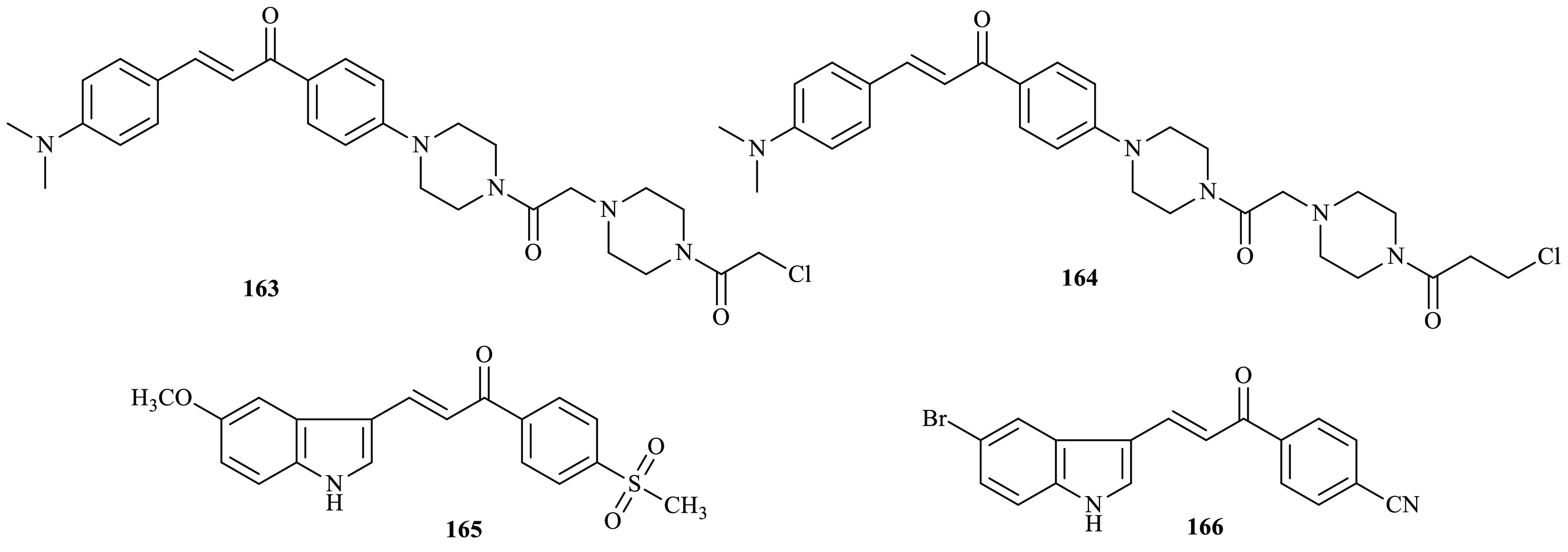
Chemical structures of anti-inflammatory active chalcone derivatives.
3.7. Antimalarial Activity
Earlier malarial reports indicate that it is one of the most prevalent and lethal parasitic diseases in the world, affecting more than 300 million people every year.68 As there is high level of resistance to all the classes of antimalarial compounds, including artemisinin derivatives, it has increased the global malaria burden and is a major threat to malaria control.69 There is an urgent need for the design and development of novel and potent antimalarial drugs, particularly against the Plasmodium falciparum, which causes severe malaria. Accordingly, Jyoti and co-workers reported indolyl–chalcone derivatives.70 All the indolyl–chalcones were evaluated for their in vitro antimalarial activity against P. falciparum, NF54 strain. Antiplasmodial IC50 activity against malaria parasites in vitro provides good screening for identifying the antimalarial potential of the synthesized compounds. Among them, compound 167 was the most active compound against plasmodia with an IC50 value of 2.1 mM/L. In previous work it was reported that novel molecular hybrids were synthesized when the quinoline moiety was coupled with various chalcone derivatives using the appropriate linker.71 The antiplasmodial activities of all synthesized molecular hybrids were evaluated against the drug-sensitive strain (NF54) of P. falciparum using chloroquine as reference drug. Compounds 168, 169, and 170 (Figure 10) with IC50 values of 0.10, 0.10, and 0.11 μM, respectively, were the most active against the plasmodia. These compounds were further tested against the multidrug-resistant K1 strain of P. falciparum. Compound 170 exert about a twofold enhancement in rthe esistivity index (RI = 5.36, PfK1 IC50 = 0.59 μM) relative to Chloroquine, whereas compounds 168 and 169 were less active against the K1 strain with IC50 values of 2.97 and 6 μM, respectively (Figure 10).
Figure 10.

Chemical structures of antimalarial active chalcone derivatives.
3.8. Antifilarial Activity
Lymphatic filariasis is a parasitic infection that causes acute and chronic inflammation.72 It is caused by thread like nematodes Wucheria bancrofti, Brugia malayi, and Brugia timori and spread via mosquitos infected with worm larvae.73 Different research has been carried to get an effective drug for this disease. Sashidhara and co-workers reported a series of chalcone–thiazole derivatives.73 All the synthesized compounds were evaluated for their in vitro activity using motility and MTT 17 reduction assays against microfilaria and female adult worms of B. malayi. Compounds 171 and 172 were found to be effective in 19 killing microfilaria (LC100 = 5 and 10 μM; IC50= 1.8 and 3.5 μM) and adult worms (LC100 = 2.5 and 10 20 μM; IC50 = 0.9 and 3.2 μM); both the compounds also inhibited the MTT reduction potential of 21 adult parasites to 49 and 63%, respectively. The in vivo activity of the tested active compound 172 further exhibits a 100% embryostatic effect (Figure 11).
Figure 11.
Chemical structures of antifilarial active chalcone derivatives.
3.9. Antigiardial Activity
Giardia is a leading cause of infectious gastroenteritis worldwide and is treatable. It is a water-born parasitic disease caused by the Giardia lamblia.74,75 Different researchers were conducting their research to develop a novel drug for overcoming Giardia-born disease. In previous work, novel chalcone derivatives were reported by Bahadur and co-workers that were synthesized via microwave-assisted Claisen–Schmidt condensation.76 The newly synthesized compounds were first tested for their antigiardial activity under anaerobic conditions. Among the tested compounds, only three of them display significant antigiardial activity. These active compounds were tested for their toxicity against Giardia trophozoites under microaerobic conditions and exhibited good activity. Finally, to assess the selectivity of compounds against Giardia, their toxicity toward a mammalian cell line, Caco-2 cells, was tested after 48 h of incubation. According to the IC50 measured on Giardia cells under microaerobic conditions, compounds 173 and 174 show preferential toxicity against parasitic cells (Figure 12).
Figure 12.
Chemical structures of antigiardial active chalcone derivaties.
4. Conclusion
Chalcone derivatives incorporating heterocyclic scaffolds such as pyrrole, imidazole, thiazole, pyridine, piperazine, indole, benzimidazole, benzothiazole, and quinoline are briefly highlighted to give a basic information to the researchers who work with these scaffolds. The above heterocycle-based chalcone derivatives almost all displayed a broad spectrum with a variety of pharmacological activities. Especially, chalcones containing N-heterocyclic scaffolds have become potential candidates for the development of effective medicines and pharmaceutical drugs. Chalcone derivatives incorporating nitrogen heterocyclic biological activities greatly attract the attention of researchers, and currently a lot of papers have been reported to find effective and potent drugs to overcome the influence of different diseases. Conventionally, these chalcones are synthesized through Claisen–Schmidt condensation reactions under basic or acidic media. Currently, findings proved that chalcones containing N-heterocyclic moieties display various pharmacological activities. The most known are antibacterial, antifungal, antitubercular, antioxidant, anti-inflammatory, anticancer, antimarial, antigiardial, and antifilarial activities. This implies that further investigations are needed to design and develop potent, novel, and effective chalcone derivatives with N-heterocyclic scaffold-based drugs.
The authors declare no competing financial interest.
References
- Zhang S.; Li T.; Zhang L.; Wang X.; Dong H.; Li L.; Fu D.; Li Y.; Zi X.; Liu H.-M.; Zhang Y.; et al. A novel chalcone derivative S17 induces apoptosis through ROS dependent DR5 up-regulation in gastric cancer cells. Sci. Rep. 2017, 7 (1), 9873. 10.1038/s41598-017-10400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M. S.; Rahman S. M.; Lee D. U. Synthesis, biological evaluation, quantitative-SAR and docking studies of novel chalcone derivatives as antibacterial and antioxidant agents. Chem. Papers 2015, 69 (8), 1118–1129. 10.1515/chempap-2015-0113. [DOI] [Google Scholar]
- Hailemariam A.; Feyera M.; Deyou T.; Abdissa N. Antimicrobial Chalcones from the Seeds of Persicaria lapathifolia. Biochem. Pharmacol. 2018, 7 (1), 1000237. 10.4172/2167-0501.1000237. [DOI] [Google Scholar]
- Mothana R. A.; Arbab A. H.; ElGamal A. A.; Parvez M. K.; Al-Dosari M. S. Isolation and Characterization of Two Chalcone Derivatives with Anti-Hepatitis B Virus Activity from the Endemic Socotraen Dracaena cinnabari (Dragon’s Blood Tree). Molecules. 2022, 27 (3), 952. 10.3390/molecules27030952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. G.; Li T.; Wei R. R.; Liu W. M.; Sang Z. P.; Song Z. W. Characterization of chalcones from Medicago sativa L. and their hypolipidemic and antiangiogenic activities. Journal of agricultural and food chemistry 2016, 64 (43), 8138–8145. 10.1021/acs.jafc.6b03883. [DOI] [PubMed] [Google Scholar]
- Kil Y. S.; Choi S. K.; Lee Y. S.; Jafari M.; Seo E. K. Chalcones from Angelica keiskei: evaluation of their heat shock protein inducing activities. J. Nat. Prod. 2015, 78 (10), 2481–2487. 10.1021/acs.jnatprod.5b00633. [DOI] [PubMed] [Google Scholar]
- Gomes M. N.; Muratov E. N.; Pereira M.; Peixoto J. C.; Rosseto L. P.; Cravo P. V.; Andrade C. H.; Neves B. J. Chalcone derivatives: promising starting points for drug design. Molecules. 2017, 22 (8), 1210. 10.3390/molecules22081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar Mahapatra D.; Asati V.; Bharti S. K. An updated patent review of therapeutic applications of chalcone derivatives (2014-present). Expert Opinion on Therapeutic Patents 2019, 29 (5), 385–406. 10.1080/13543776.2019.1613374. [DOI] [PubMed] [Google Scholar]
- Sharma P.; Kumar S.; Ali F.; Anthal S.; Gupta V. K.; Khan I. A.; Singh S.; Sangwan P. L.; Suri K. A.; Gupta B. D.; Gupta D. K.; et al. Synthesis and biologic activities of some novel heterocyclic chalcone derivatives. Medicinal Chemistry Research 2013, 22 (8), 3969–3983. 10.1007/s00044-012-0401-7. [DOI] [Google Scholar]
- Raj C. G. D.; Sarojini B. K.; Hegde S.; Sreenivasa S.; Ravikumar Y. S.; Bhanuprakash V.; Revanaiah Y.; Ragavendra R. In vitro biological activities of new heterocyclic chalcone derivatives. Medicinal Chemistry Research 2013, 22 (5), 2079–2087. 10.1007/s00044-012-0193-9. [DOI] [Google Scholar]
- Elkanzi N. A.; Hrichi H.; Alolayan R. A.; Derafa W.; Zahou F. M.; Bakr R. B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega. 2022, 7 (32), 27769–27786. 10.1021/acsomega.2c01779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ünver Y.; Tuluk M.; Kahriman N.; Emirik M.; Bektaş E.; Direkel Ş. New chalcone derivatives with Schiff base-thiophene: synthesis, biological activity, and molecular docking studies. Russian Journal of General Chemistry 2019, 89 (4), 794–799. 10.1134/S107036321904025X. [DOI] [Google Scholar]
- Kiran K.; Sarasija M.; Ananda Rao B.; Namratha V.; Ashok D.; Srinivasa Rao A. Design, Synthesis, and Biological Activity of New Bis-1, 2, 3-triazole Derivatives Bearing Thiophene-Chalcone Moiety. Russian Journal of General Chemistry 2019, 89 (9), 1859–1866. 10.1134/S1070363219090214. [DOI] [Google Scholar]
- Ardiansah B. Chalcones bearing N, O, and S-heterocycles: Recent notes on their biological significances. J. App. Pharm. Sci. 2019, 9 (8), 117–129. 10.7324/JAPS.2019.90816. [DOI] [Google Scholar]
- Mahapatra D. K.; Bharti S. K.; Asati V. Chalcone derivatives: anti-inflammatory potential and molecular targets perspectives. Current topics in medicinal chemistry 2017, 17 (28), 3146–3169. 10.2174/1568026617666170914160446. [DOI] [PubMed] [Google Scholar]
- Jayashree B. S.; Patel H. H.; Mathew N. S.; Nayak Y. Synthesis of newer piperidinyl chalcones and their anticancer activity in human cancer cell lines. Res. Chem. Intermed. 2016, 42 (4), 3673–3688. 10.1007/s11164-015-2238-4. [DOI] [Google Scholar]
- Kasetti A. B.; Singhvi I.; Nagasuri R.; Bhandare R. R.; Shaik A. B. Thiazole–chalcone hybrids as prospective antitubercular and antiproliferative agents: Design, synthesis, biological, molecular docking studies and in silico ADME evaluation. Molecules. 2021, 26 (10), 2847. 10.3390/molecules26102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atukuri D.; S V.; R S.; L V.; R P.; M. M R. Identification of quinoline-chalcones and heterocyclic chalcone-appended quinolines as broad-spectrum pharmacological agents. Bioorganic Chemistry 2020, 105, 104419. 10.1016/j.bioorg.2020.104419. [DOI] [PubMed] [Google Scholar]
- Maatougui A. E.; Yáñez M.; Crespo A.; Fraiz N.; Coelho A.; Ravina E.; Laguna R.; Cano E.; Loza M. I.; Brea J.; Gutiérrez de T. H.; et al. 3-Oxopyridazin-5-yl-Chalcone Hybrids: Potent Antiplatelet Agents That Prevent Glycoprotein IIb/IIIa Activation. ChemistrySelect 2017, 2 (17), 4920–4933. 10.1002/slct.201700243. [DOI] [Google Scholar]
- Tugrak M.; Gul H. I.; Akincioglu H.; Gulcin I. New Chalcone Derivatives with Pyrazole and Sulfonamide Pharmacophores as Carbonic Anhydrase Inhibitors. Letters in Drug Design & Discovery 2021, 18 (2), 191–198. 10.2174/1570180817999201001160414. [DOI] [Google Scholar]
- Joseph M.; Alaxander S. Design, Synthesis and Characterization of Thiophene Substituted Chalcones for Possible Biological. Evaluation.JPRI. 2021, 33 (49B), 55–79. 10.9734/jpri/2021/v33i49B33341. [DOI] [Google Scholar]
- Horley N. J.; Beresford K. J.; Kaduskar S.; Joshi P.; McCann G. J.; Ruparelia K. C.; Williams I. S.; Gatchie L.; Sonawane V. R.; Bharate S. B.; Chaudhuri B. (E)-3-(3, 4, 5-Trimethoxyphenyl)-1-(pyridin-4-yl) prop-2-en-1-one, a heterocyclic chalcone is a potent and selective CYP1A1 inhibitor and cancer chemopreventive agent. Bioorg. Med. Chem. Lett. 2017, 27 (24), 5409–5414. 10.1016/j.bmcl.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Sasidharan R.; Baek S. C.; Leelabaiamma M. S.; Kim H.; Mathew B. Imidazole bearing chalcones as a new class of monoamine oxidase inhibitors. Biomedicine & Pharmacotherapy 2018, 106, 8–13. 10.1016/j.biopha.2018.06.064. [DOI] [PubMed] [Google Scholar]
- Janaki P.; Sekar K. G.; Thirunarayanan G. Synthesis, spectral correlation and insect antifeedant activities of some 2-benzimidazole chalcones. Journal of Saudi Chemical Society 2016, 20 (1), 58–68. 10.1016/j.jscs.2012.11.013. [DOI] [Google Scholar]
- Williams I. S.; Joshi P.; Gatchie L.; Sharma M.; Satti N. K.; Vishwakarma R. A.; Chaudhuri B.; Bharate S. B. Synthesis and biological evaluation of pyrrole-based chalcones as CYP1 enzyme inhibitors, for possible prevention of cancer and overcoming cisplatin resistance. Bioorg. Med. Chem. Lett. 2017, 27 (16), 3683–3687. 10.1016/j.bmcl.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Suresh J.; Mathew G. E.; Haridas A.; Suresh G.; Sabreena P. Synthesis, ADME studies, toxicity estimation, and exploration of molecular recognition of thiophene based chalcones towards monoamine oxidase-A and B. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5 (4), 396–401. 10.1016/j.bjbas.2015.06.003. [DOI] [Google Scholar]
- Zheng C. J.; Jiang S. M.; Chen Z. H.; Ye B. J.; Piao H. R. Synthesis and Anti-Bacterial Activity of Some Heterocyclic Chalcone Derivatives Bearing Thiofuran, Furan, and Quinoline Moieties. Archiv der Pharmazie. 2011, 344 (10), 689–695. 10.1002/ardp.201100005. [DOI] [PubMed] [Google Scholar]
- Özdemir A.; Altıntop M. D.; Sever B.; Gençer H. C.; Kapkaç H. A.; Atlı Ö.; Baysal M. A new series of pyrrole-based chalcones: synthesis and evaluation of antimicrobial activity, cytotoxicity, and genotoxicity. Molecules. 2017, 22 (12), 2112. 10.3390/molecules22122112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P.; Kumar S.; Ali F.; Anthal S.; Gupta V. K.; Khan I. A.; Singh S.; Sangwan P. L.; Suri K. A.; Gupta B. D.; Gupta D. K.; et al. Synthesis and biologic activities of some novel heterocyclic chalcone derivatives. Med. Chem. Res. 2013, 22 (8), 3969–3983. 10.1007/s00044-012-0401-7. [DOI] [Google Scholar]
- Liu Y. T.; Sun X. M.; Yin D. W.; Yuan F. Syntheses and biological activity of chalcones-imidazole derivatives. Res. Chem. Intermed. 2013, 39 (3), 1037–1048. 10.1007/s11164-012-0665-z. [DOI] [Google Scholar]
- Nagasuri R.; Dwivedi J.; Kasetti A. B. Antioxidant Studies of Thiazole Ring Bearing Chalcone Derivatives. Journal of Pharmaceutical Research International. 2021, 33 (59A), 355–359. 10.9734/jpri/2021/v33i59A34280. [DOI] [Google Scholar]
- Farghaly T. A.; Masaret G. S.; Muhammad Z. A.; Harras M. F. Discovery of thiazole-based-chalcones and 4-hetarylthiazoles as potent anticancer agents: Synthesis, docking study and anticancer activity. Bioorganic chemistry 2020, 98, 103761. 10.1016/j.bioorg.2020.103761. [DOI] [PubMed] [Google Scholar]
- Rupala R. G.; Kundariya D. S.; Patel P. K. Synthesis, characterization and biological evaluation of some novel chalcone derivatives containing imidazo [1, 2-a] pyridine moiety. Journal of Chemistry, Environmental Sciences and its Applications 2014, 1 (1), 23–32. 10.15415/jce.2014.11003. [DOI] [Google Scholar]
- Madhavi S.; Sreenivasulu R.; Ansari M.; Ahsan M.; Raju R. Synthesis, biological evaluation and molecular docking studies of pyridine incorporated chalcone derivatives as anticancer agents. Lett. Org. Chem. 2016, 13 (9), 682–692. 10.2174/1570178613666161021105317. [DOI] [Google Scholar]
- Durgapal S. D.; Soni R.; Umar S.; Suresh B.; Soman S. S. 3-Aminomethyl pyridine chalcone derivatives: Design, synthesis, DNA binding and cytotoxic studies. Chemical Biology & Drug Design. 2018, 92 (1), 1279–1287. 10.1111/cbdd.13189. [DOI] [PubMed] [Google Scholar]
- Ahmed M. F.; Santali E. Y.; El-Haggar R. Novel piperazine–chalcone hybrids and related pyrazoline analogues targeting VEGFR-2 kinase; design, synthesis, molecular docking studies, and anticancer evaluation. Journal of enzyme inhibition and medicinal chemistry 2021, 36 (1), 308–319. 10.1080/14756366.2020.1861606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z.; Zheng X.; Qi Y.; Zhang M.; Huang Y.; Wan C.; Rao G. Synthesis and biological evaluation of novel hybrid compounds between chalcone and piperazine as potential antitumor agents. Rsc Advances 2016, 6 (10), 7723–7727. 10.1039/C5RA20197G. [DOI] [Google Scholar]
- Wang G.; Li C.; He L.; Lei K.; Wang F.; Pu Y.; Yang Z.; Cao D.; Ma L.; Chen J.; Sang Y.; et al. Design, synthesis and biological evaluation of a series of pyrano chalcone derivatives containing indole moiety as novel anti-tubulin agents. Bioorg. Med. Chem. 2014, 22 (7), 2060–2079. 10.1016/j.bmc.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Gao W.; Liu R.; Li Y.; Cui P. Two efficient methods for the synthesis of novel indole-based chalcone derivatives. Res. Chem. Intermed. 2014, 40 (8), 3021–3032. 10.1007/s11164-013-1148-6. [DOI] [Google Scholar]
- Wang G.; Peng Z.; Li Y. Synthesis, anticancer activity and molecular modeling studies of novel chalcone derivatives containing indole and naphthalene moieties as tubulin polymerization inhibitors. Chem. Pharm. Bull. 2019, 67 (7), 725–728. 10.1248/cpb.c19-00217. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Zhang W.; Peng Y.; Jiang Z. H.; Zhang L.; Du Z. Design, synthesis and anti-tumor activity of novel benzimidazole-chalcone hybrids as non-intercalative topoisomerase II catalytic inhibitors. Molecules. 2020, 25 (14), 3180. 10.3390/molecules25143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragathi Y. J.; Veronica D.; Anitha K.; Rao M. V. B.; Raju R. R. Synthesis and biological evaluation of chalcone derivatives of 1,2,4-thiadiazol-benzo[d]imidazol-2-yl)quinolin-2 (1H)-one as anticancer agents. Chemical Data Collections 2020, 30, 100556. 10.1016/j.cdc.2020.100556. [DOI] [Google Scholar]
- Hsieh C.-Y.; Ko P.-W.; Chang Y.-J.; Kapoor M.; Liang Y.-C.; Chu H.-L.; Lin H.-H.; Horng J.-C.; Hsu M.-H. Design and synthesis of benzimidazole-chalcone derivatives as potential anticancer agents. Molecules. 2019, 24 (18), 3259. 10.3390/molecules24183259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Li P.; Jiang S.; Chen Y.; Su S.; He J.; Chen M.; Zhang J.; Xu W.; He M.; Xue W. Synthesis and antibacterial evaluation of novel chalcone derivatives containing a benzothiazole scaffold. Monatshefte für Chemie-Chemical Monthly 2019, 150 (6), 1147–1154. 10.1007/s00706-019-02399-2. [DOI] [Google Scholar]
- Karki R.; Song C.; Kadayat T. M.; Magar T. B. T.; Bist G.; Shrestha A.; Na Y.; Kwon Y.; Lee E. S. Topoisomerase I and II inhibitory activity, cytotoxicity, and structure–activity relationship study of dihydroxylated 2, 6-diphenyl-4-aryl pyridines. Bioorganic & medicinal chemistry 2015, 23 (13), 3638–3654. 10.1016/j.bmc.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Sashidhara K. V.; Avula S. R.; Doharey P. K.; Singh L. R.; Balaramnavar V. M.; Gupta J.; Misra-Bhattacharya S.; Rathaur S.; Saxena A. K.; Saxena J. K. Designing, synthesis of selective and high-affinity chalcone-benzothiazole hybrids as Brugia malayi thymidylate kinase inhibitors: in vitro validation and docking studies. Eur. J. Med. Chem. 2015, 103, 418–428. 10.1016/j.ejmech.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Thirumurugan C.; Vadivel P.; Lalitha A.; Lakshmanan S. Synthesis, characterization of novel quinoline-2-carboxamide based chalcone derivatives and their molecular docking, photochemical studies. Synth. Commun. 2020, 50 (6), 831–839. 10.1080/00397911.2020.1720737. [DOI] [Google Scholar]
- a Guan Y. F.; Liu X. J.; Yuan X. Y.; Liu W. B.; Li Y. R.; Yu G. X.; Tian X. Y.; Zhang Y. B.; Song J.; Li W.; Zhang S. Y. Design, Synthesis, and Anticancer Activity Studies of Novel Quinoline-Chalcone Derivatives. Molecules. 2021, 26 (16), 4899. 10.3390/molecules26164899. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhao P. L.; Liu C. L.; Huang W.; Wang Y. Z.; Yang G. F. Synthesis and fungicidal evaluation of novel chalcone-based strobilurin analogues. Journal of agricultural and food chemistry 2007, 55 (14), 5697–5700. 10.1021/jf071064x. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Dhawan S.; Girase P. S.; Awolade P.; Shinde S. R.; Karpoormath R.; Singh P. Recent Advances in Chalcone-Based Anticancer Heterocycles: A Structural and Molecular Target Perspective. Curr. Med. Chem. 2021, 28 (33), 6805–6845. 10.2174/0929867328666210322102836. [DOI] [PubMed] [Google Scholar]
- Venkataramireddy V.; Shankaraiah V.; Rao A. T.; Kalyani C.; Narasu M. L.; Varala R.; Jayashree A. Synthesis and anti-cancer activity of novel 3-aryl thiophene-2-carbaldehydes and their aryl/heteroaryl chalcone derivatives. Rasayan J. Chem. 2016, 9 (1), 31–39. [Google Scholar]
- Reddy P. V.; Hridhay M.; Nikhil K.; Khan S.; Jha P. N.; Shah K.; Kumar D. Synthesis and investigations into the anticancer and antibacterial activity studies of β-carboline chalcones and their bromide salts. Bioorg. Med. Chem. Lett. 2018, 28 (8), 1278–1282. 10.1016/j.bmcl.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A.; Asiri A. M.; Al-Ghamdi N. S. M.; Asad M.; Zayed M. E.; Elroby S. A.; Aqlan F. M.; Wani M. Y.; Sharma K. Microwave assisted synthesis of chalcone and its polycyclic heterocyclic analogues as promising antibacterial agents: In vitro, in silico and DFT studies. J. Mol. Struct. 2019, 1190, 77–85. 10.1016/j.molstruc.2019.04.046. [DOI] [Google Scholar]
- Shaik A.; Bhandare R. R.; Palleapati K.; Nissankararao S.; Kancharlapalli V.; Shaik S. Antimicrobial, antioxidant, and anticancer activities of some novel isoxazole ring containing chalcone and dihydropyrazole derivatives. Molecules. 2020, 25 (5), 1047. 10.3390/molecules25051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshram G. A.; Vala V. A. Synthesis, characterization, and antimicrobial activity of benzimidazole-derived chalcones containing 1, 3, 4-oxadiazole moiety. Chem. Heterocycl. Compd. 2015, 51 (1), 44–50. 10.1007/s10593-015-1653-1. [DOI] [Google Scholar]
- Osmaniye D.; Kaya Cavusoglu B.; Saglik B. N.; Levent S.; Acar Cevik U.; Atli O.; Ozkay Y.; Kaplancikli Z. A. Synthesis and anticandidal activity of new imidazole-chalcones. Molecules. 2018, 23 (4), 831. 10.3390/molecules23040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunitha V.; Kumar A. K.; Mahesh M.; Shankaraiah P.; Jalapathi P.; Lincoln C. Synthesis and antimicrobial evaluation of Bis-3, 5-disubstituted isoxazoles based chalcones. Russian Journal of General Chemistry 2018, 88 (9), 1904–1911. 10.1134/S1070363218090232. [DOI] [Google Scholar]
- Gomes M. N.; Braga R. C.; Grzelak E. M.; Neves B. J.; Muratov E.; Ma R.; Klein L. L.; Cho S.; Oliveira G. R.; Franzblau S. G.; Andrade C. H. QSAR-driven design, synthesis and discovery of potent chalcone derivatives with antitubercular activity. European journal of medicinal chemistry 2017, 137, 126–138. 10.1016/j.ejmech.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solankee A.; Tailor R. An efficient synthesis of some new chalcone, acetyl pyrazoline and amino pyrimidine bearing 1,3,5-triazine nucleus as potential antimicrobial and antitubercular agent. Chem. Int. 2016, 2 (4), 189–200. [Google Scholar]
- Bhat M.; G. K N.; P D.; N H.; Pai K S. R.; Biswas S.; S. K P. Design, synthesis, characterization of some new 1, 2, 3-triazolyl chalcone derivatives as potential anti-microbial, anti-oxidant and anti-cancer agents via a Claisen–Schmidt reaction approach. RSC Adv. 2016, 6 (102), 99794–99808. 10.1039/C6RA22705H. [DOI] [Google Scholar]
- Kumari S.; Paliwal S. K.; Chauhan R. An improved protocol for the synthesis of chalcones containing pyrazole with potential antimicrobial and antioxidant activity. Current Bioactive Compounds 2018, 14 (1), 39–47. 10.2174/1573407212666161101152735. [DOI] [Google Scholar]
- Suma V. R.; Sreenivasulu R.; Rao M. V. B.; Subramanyam M.; Ahsan M. J.; Alluri R.; Rao K. R. M. Design, synthesis, and biological evaluation of chalcone-linked thiazole-imidazopyridine derivatives as anticancer agents. Medicinal Chemistry Research 2020, 29 (9), 1643–1654. 10.1007/s00044-020-02590-9. [DOI] [Google Scholar]
- Yan J.; Chen J.; Zhang S.; Hu J.; Huang L.; Li X. Synthesis, evaluation, and mechanism study of novel indole-chalcone derivatives exerting effective antitumor activity through microtubule destabilization in vitro and in vivo. Journal of medicinal chemistry 2016, 59 (11), 5264–5283. 10.1021/acs.jmedchem.6b00021. [DOI] [PubMed] [Google Scholar]
- Oskuei S. R.; Mirzaei S.; Jafari-Nik M. R.; Hadizadeh F.; Eisvand F.; Mosaffa F.; Ghodsi R. Design, synthesis and biological evaluation of novel imidazole-chalcone derivatives as potential anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 2021, 112, 104904. 10.1016/j.bioorg.2021.104904. [DOI] [PubMed] [Google Scholar]
- Djemoui A.; Naouri A.; Ouahrani M. R.; Djemoui D.; Lahcene S.; Lahrech M. B.; Boukenna L.; Albuquerque H. M.; Saher L.; Rocha D. H.; Monteiro F. L.; et al. A step-by-step synthesis of triazole-benzimidazole-chalcone hybrids: Anticancer activity in human cells+. J. Mol. Struct. 2020, 1204, 127487. 10.1016/j.molstruc.2019.127487. [DOI] [Google Scholar]
- Tang Y. L.; Zheng X.; Qi Y.; Pu X. J.; Liu B.; Zhang X.; Li X. S.; Xiao W. L.; Wan C. P.; Mao Z. W. Synthesis and anti-inflammatory evaluation of new chalcone derivatives bearing bispiperazine linker as IL-1β inhibitors. Bioorganic chemistry 2020, 98, 103748. 10.1016/j.bioorg.2020.103748. [DOI] [PubMed] [Google Scholar]
- Özdemir A.; Altıntop M. D.; Turan-Zitouni G.; Çiftçi G. A.; Ertorun İ.; Alataş Ö.; Kaplancıklı Z. A. Synthesis and evaluation of new indole-based chalcones as potential antiinflammatory agents. European journal of medicinal chemistry 2015, 89, 304–309. 10.1016/j.ejmech.2014.10.056. [DOI] [PubMed] [Google Scholar]
- Global Tuberculosis Report 2013; World Health Organization: Geneva, Switzerland, 2013; pp 222–225. [Google Scholar]
- Thillainayagam M.; Pandian L.; Murugan K. K.; Vijayaparthasarathi V.; Sundaramoorthy S.; Anbarasu A.; Ramaiah S. In silico analysis reveals the anti-malarial potential of quinolinyl chalcone derivatives. J. Biomol. Struct. Dyn. 2015, 33 (5), 961–977. 10.1080/07391102.2014.920277. [DOI] [PubMed] [Google Scholar]
- Jyoti; Gaur R.; Kumar Y.; Cheema H. S.; Kapkoti D. S.; Darokar M. P.; Khan F.; Bhakuni R. S. Synthesis, molecular modelling studies of indolyl chalcone derivatives and their antimalarial activity evaluation. Nat. Prod. Res. 2021, 35 (19), 3261–3268. 10.1080/14786419.2019.1696788. [DOI] [PubMed] [Google Scholar]
- Vinindwa B.; Dziwornu G. A.; Masamba W. Synthesis and evaluation of Chalcone-Quinoline based molecular hybrids as potential anti-malarial agents. Molecules. 2021, 26 (13), 4093. 10.3390/molecules26134093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourens G. B.; Ferrell D. K. Lymphatic filariasis. Nursing Clinics 2019, 54 (2), 181–192. 10.1016/j.cnur.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Sashidhara K. V.; Rao K. B.; Kushwaha V.; Modukuri R. K.; Verma R.; Murthy P. K. Synthesis and antifilarial activity of chalcone–thiazole derivatives against a human lymphatic filarial parasite, Brugia malayi. European journal of medicinal chemistry 2014, 81, 473–480. 10.1016/j.ejmech.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Waldram A.; Vivancos R.; Hartley C.; Lamden K. Prevalence of Giardia infection in households of Giardia cases and risk factors for household transmission. BMC Infect. Dis. 2017, 17, 486. 10.1186/s12879-017-2586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo A. A.; Hanevik K.; Almirall P.; Cimerman S.; Alfonso M. Management of chronic Giardia infection. Expert review of anti-infective therapy 2014, 12 (9), 1143–1157. 10.1586/14787210.2014.942283. [DOI] [PubMed] [Google Scholar]
- Bahadur V.; Mastronicola D.; Tiwari H. K.; Kumar Y.; Falabella M.; Pucillo L. P.; Sarti P.; Giuffrè A.; Singh B. K. O2-Dependent Efficacy of Novel Piperidine-and Piperazine-Based Chalcones against the Human Parasite Giardia intestinalis. Antimicrob. Agents Chemother. 2014, 58 (1), 543–549. 10.1128/AAC.00990-13. [DOI] [PMC free article] [PubMed] [Google Scholar]