Abstract
Across eumetazoans, the ability to perceive and respond to visual stimuli is largely mediated by opsins, a family of proteins belonging to the G protein-coupled receptor (GPCR) superclass. Lineage-specific gains and losses led to a striking diversity in the numbers, types, and spectral sensitivities conferred by visual opsin gene expression. Here, we review the diversity of visual opsins and differences in opsin gene expression from well-studied protostome, invertebrate deuterostome, and cnidarian groups. We discuss the functional significance of opsin expression differences and spectral tuning among lineages. In some cases, opsin evolution has been linked to the detection of relevant visual signals, including sexually selected color traits and host plant features. In other instances, variation in opsins has not been directly linked to functional or ecological differences. Overall, the array of opsin expression patterns and sensitivities across invertebrate lineages highlight the diversity of opsins in the eumetazoan ancestor and the labile nature of opsins over evolutionary time.
Keywords: GPCR, Opsin, Evolution, Invertebrate, C-opsin, R-opsin, Cnidops, Tetraopsin, Xenopsin, Arthropoda, Lophotrochozoa
1. Introduction
Opsins are a family of G protein-coupled receptors (GPCRs) that, among other functions, mediate vision across Eumetazoa. Opsin proteins consist of seven transmembrane helices characterized by a lysine in the seventh helix. This lysine serves as the attachment site for a vitamin A-derived, light-sensitive chromophore that, when covalently bound to an opsin protein, comprise a functional visual pigment. Upon exposure to light, a change in the conformational state of the chromophore, typically 11-cis-retinal to all-trans retinal, drives the activation of the visual pigment (for reviews, see Shichida and Matsuyama, 2009; Terakita, 2005; Tsukamoto and Terakita, 2010).
Visual pigments are sensitive to spectral compositions of light, which are defined by the maximally absorbed wavelength (λmax). While the chromophore alone has a λmax between 377 and 400 nm (Hubbard et al., 1953; Tsukida et al., 1980), the interaction of the chromophore with different opsin proteins leads to a wide range of sensitivities, ranging from 330 nm (ultraviolet light) to 600 nm (red light) (e.g., Bok et al., 2014; Briscoe and Chittka, 2001). Apart from various indirect mechanisms that affect visual sensitivity (e.g., screening pigments, oil droplets etc.), λmax values are largely determined by the distinct interactions between the chromophore and the associated opsin protein.
Phylogenetically, opsins can be divided into several distinct clades (Arendt et al., 2004; Plachetzki et al., 2007; Plachetzki et al., 2010; Porter et al., 2012; Ramirez et al., 2016). Here, we briefly introduce the opsin clades that we discuss throughout this review:
C-opsins: Canonical c-type opsins (hereafter referred to as c-opsins) are characteristically observed in ciliary photoreceptors in the eyes of deuterostomes. Ciliary photoreceptors hyperpolarize in response to light using a Gt (transducin)-mediated signaling cascade and photo-bleach after photoactivation, breaking the association between the opsin and the bound chromophore (see Shichida and Matsuyama, 2009; Terakita, 2005; Tsukamoto and Terakita, 2010).
R-opsins: Canonical r-type opsins (hereafter referred to as r-opsins) are characteristically observed in rhabdomeric photoreceptors in the eyes of protostomes. Rhabdomeric photoreceptors depolarize in response to light using a Gq-type signal transduction pathway and are typically bistable, retaining the chromophore in both the native (dark) and photoactivated (light) states (see Shichida and Matsuyama, 2009; Terakita, 2005; Tsukamoto and Terakita, 2010).
Cnidops: In contrast to r- and c-type opsins, which have broad taxonomic representation in expression patterns, the cnidops clade is composed entirely of cnidarian and ctenophore opsins (Liegertová et al., 2015; Plachetzki et al., 2007; Porter et al., 2012). Cnidops are expressed in retinal ciliary photoreceptors, CNS neurons, and gonad cells (Liegertová et al., 2015; Plachetzki et al., 2007; Suga et al., 2008). Phototransduction, in at least some cnidops, is mediated via a Gs-coupled signaling pathway (Koyanagi et al., 2008b), although cnidops do not universally employ a Gs-signaling cascade (Liegertová et al., 2015).
Group 4/Tetraopsins: The Group 4 opsins or tetraopsins include RGR/retinochromes/peropsins, Go-opsins, and neuropsins (Porter et al., 2012; Ramirez et al., 2016). The opsins within this clade form a monophyletic group with representatives in protostomes and deuterostomes (Ramirez et al., 2016). Among these opsins, we will focus on Go-opsins. Go-opsins function in both ciliary and rhabdomeric photoreceptors (Gühmann et al., 2015; Kojima et al., 1997) and employ a Go-mediated transduction cascade, leading to hyperpolarization in response to light when expressed in ciliary photoreceptors or depolarization when expressed in rhabdomeric photoreceptors (Gühmann et al., 2015; Kojima et al., 1997).
Xenopsins: Xenopsins have been identified in several lophotrochozoan species (Döring et al., 2020; Passamaneck et al., 2011; Ramirez et al., 2016; Rawlinson et al., 2019; Vöcking et al., 2017). Xenopsins are associated with ciliary photoreceptors and employ a similar signaling cascade as c-opsins. However, xenopsins can also be co-expressed with r-opsins in photoreceptor cells that have features of both rhabdomeric and ciliary photoreceptors (Döring et al., 2020; Rawlinson et al., 2019; Vöcking et al., 2017).
Within these major opsin clades, c- and r-opsins form distinct phylogenetic groups that generally correspond to the wavelengths of light that they confer sensitivity to. For r-opsins, these are the long-wavelength (LW), medium-wavelength (MW), and short-wavelength (SW)/ultraviolet (UV) sensitive clades (Porter et al., 2007, 2013). In some lineages (see Sec. 2), duplications of the SW/UV opsin clade form two distinct clades of SW/blue (B) sensitive and ultraviolet (UV) sensitive opsins (Briscoe and Chittka, 2001; Koyanagi et al., 2008a). However, even within opsin clades, a large diversity of visual pigment sensitivity exists. In this review, we discuss the diversity of opsin expression and sensitivities across invertebrate lineages (see Fig. 1 for lineages discussed) and potential ecological and behavioral functions of these changes in vision across species.
Fig. 1.
The invertebrate lineages that are the focus of this review with illustrations of representative species for each group.
2. Visual opsins in arthropods
Arthropoda is divided into three major extant subphyla: Pancrustacea (Insecta + Crustacea), Chelicerata, and Myriapoda. We focus on patterns of opsin expression in Insecta, Crustacea, and Chelicerata (Fig. 1) as studies of myriapod opsins are limited (but see Chipman et al., 2014; Fleming et al., 2018; Kenny et al., 2015).
Arthropods use r-opsins for vision. The arthropod ancestor likely possessed three to five r-opsins, with one UV-sensitive opsin and the remainder sensitive to light in the visible spectrum (Henze and Oakley, 2015; Koyanagi et al., 2008a), representing opsins from the SW, MW, and LW opsin clades (Henze and Oakley, 2015). Opsin duplications and losses diversified patterns of opsin expression within Arthropoda (e.g., Guignard et al., 2022; Henze and Oakley, 2015). For example, while extant insects lack the MW opsin clade that is still present in crustaceans and chelicerates, the insect SW/UV clade has diversified to form distinct SW and UV sensitive clades (Fig. 2) (Henze and Oakley, 2015; Koyanagi et al., 2008a).
Fig. 2.
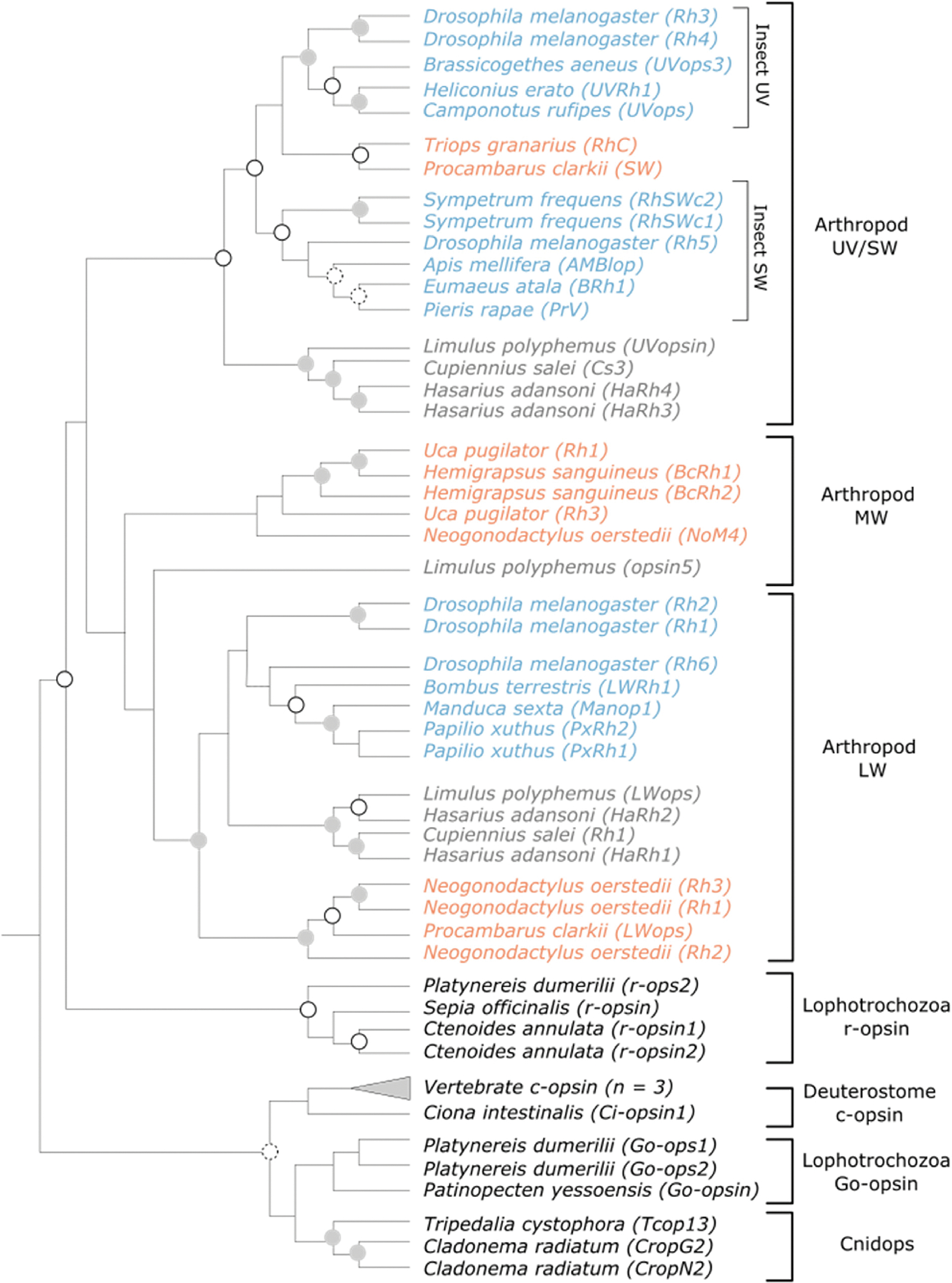
Phylogeny illustrating the relationship between invertebrate opsins genes. Vertebrate c-opsin genes are also included to illustrate their relationship to invertebrate deuterostome opsins (i.e., Ciona intestinalis Ci-opsin1). Color coding within the Arthropod UV/SW, MW, and LW opsin groups insect (blue), crustacean (orange), or chelicerate (grey) lineages. Grey filled circles indicate nodes with >95% bootstrap support, unfilled circles indicate nodes with 90–94% bootstrap support, and circles with dashed outlines indicate nodes with 80–89% bootstrap support. For a full phylogeny including outgroup taxa and bootstrap values, see Supplementary Fig. 1.
In some cases, Arthropod opsin expression, visual sensitivity, and visual behaviors have been particularly well-studied. Below, we discuss opsin expression and potential roles of opsins in the ecology and behavior of these groups.
2.1. Insecta
Insects typically possess at least one copy of the UV, SW, and LW opsin classes (Briscoe and Chittka, 2001; Guignard et al., 2022), conferring sensitivity to UV (~350 nm), blue (~440 nm), and green (~530 nm) wavelengths of light, respectively (Briscoe and Chittka, 2001). Opsin losses and duplications generated diversity within insect opsins, with duplications occurring in all insect opsin classes and losses of the UV, SW, or both in some groups (Guignard et al., 2022).
2.1.1. UV opsins
Most insects possess a single UV opsin copy, with losses only noted in the orders Trichoptera (caddisflies) and Siphonaptera (fleas) (Guignard et al., 2022). UV opsin duplications occurred in some families within Ephemeroptera (mayflies), Hemiptera (true bugs), Raphidioptera (snakeflies), Coleoptera (beetles), Lepidoptera (moths, butterflies), and Diptera (flies) (Almudi et al., 2020; Briscoe et al., 2010; Feuda et al., 2020; Guignard et al., 2022; Lord et al., 2016; Sharkey et al., 2017). UV sensitivity is driven by a single amino acid polymorphism, with a lysine residue in position 90 relative to bovine rhodopsin, while asparagine or glutamate is in this position for blue sensitive opsins (Salcedo et al., 2003).
The typical λmax of the UV opsin is 330 nm–400 nm (Fig. 3), although the UV opsin can also confer sensitivity to blue light. An example comes from Coleoptera, where some species are sensitive to 420–460 nm light, despite lacking a SW sensitive opsin across the order (Fig. 3C) (Lord et al., 2016; Sharkey et al., 2017). Several amino acid substitutions in the coleopteran UV opsin correspond to known tuning sites in invertebrate and vertebrate species, suggesting conserved mechanisms of opsin tuning across diverse taxa (Lord et al., 2016).
Fig. 3.
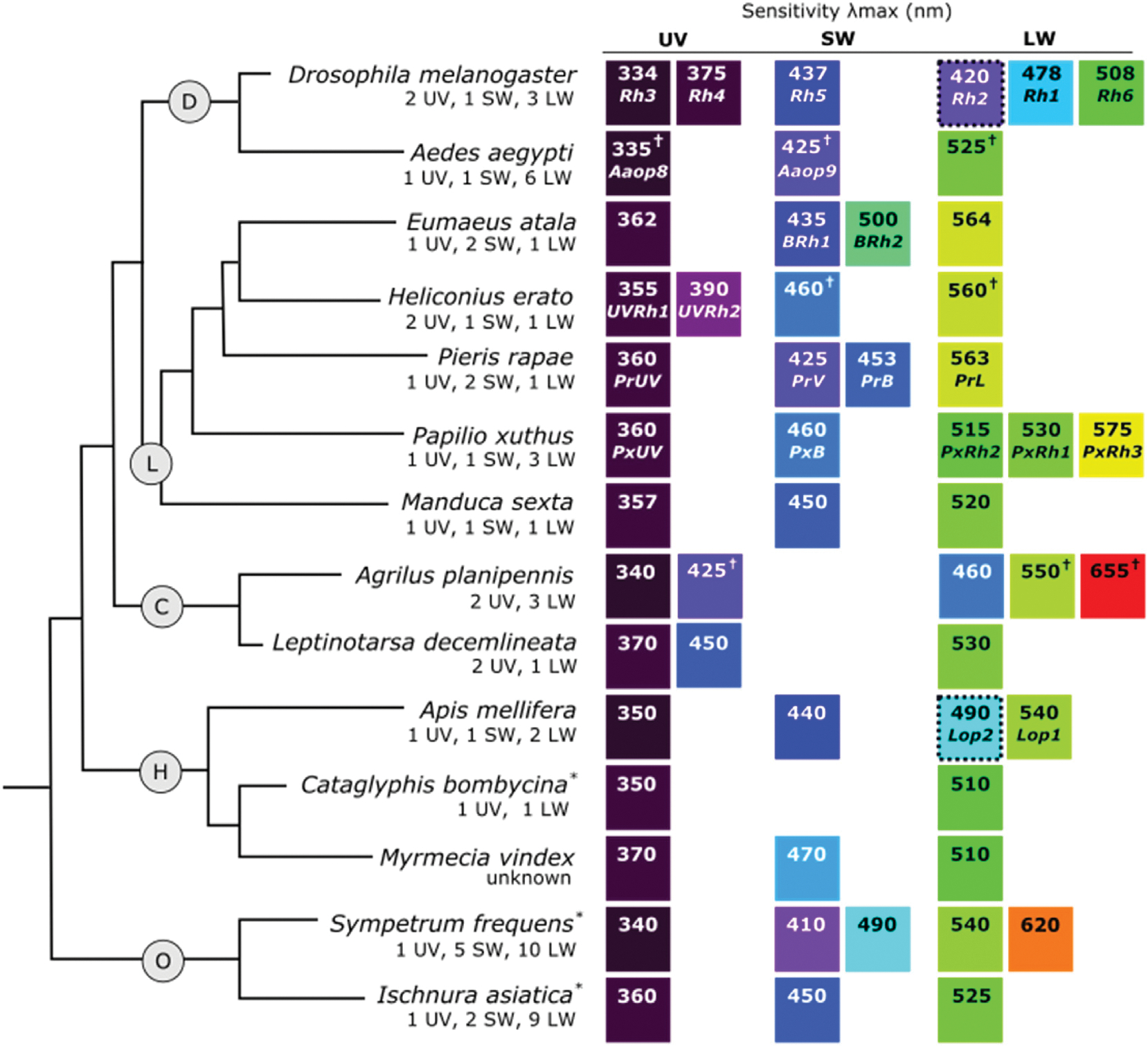
Insect phylogeny with representative species for (D) Diptera, (L) Lepidoptera, (C) Coleoptera, (H) Hymenoptera, and (O) Odonata showing the r-opsin classes and λmax values of recorded visual sensitivities. Number and type of opsins identified from each species is shown below the species name. The color of the squares illustrates an approximation of spectral sensitivity between the UV (dark purple) and visible spectrum, relative to other values. When the opsin associated with a specific sensitivity is known, it is listed below the λmax value. Asterisks (*) indicate λmax values come from a different species of the same genus, a cross (†) symbol indicates that the reported λmax value is the midpoint between a measured range, and a dashed border around a square indicates sensitivity recorded from ocelli. The relationship between insect orders were inferred from Ishiwata et al. (2011) and Misof et al. (2014) and relationships between lepidopteran species were inferred from Timmermans et al. (2014). For λmax references, see Supplemental Table 2.
Specific behaviors involving UV sensitivity include detection and discrimination of flowers (Dyer and Chittka, 2004; Horth et al., 2014), polarized light vision (Brines and Gould, 1979; Schwind, 1991), and orientation (Möller, 2002). UV opsin duplications are often associated with visually guided behavior, including flower detection, hunting, and mate choice (Almudi et al., 2020; McCulloch et al., 2017; McCulloch et al., 2016; Sharkey et al., 2017). For example, the evolution of UV-yellow coloration, based on the pigment 3-hydroxykynurenine (3-OHK), is correlated with the UV duplication event in Heliconius (Fig. 3L, Heliconius erato UVRh1 and UVRh2) (Briscoe et al., 2010). Modeling suggested that the UV duplication functions in conspecific recognition by improving discrimination of 3-OHK coloration from the non-3-OHK based coloration of heterospecific individuals (McCulloch et al., 2017). This corresponds with behavioral observations showing that male H. erato prefer 3-OHK yellow coloration over non-3-OHK yellow coloration in choice assays (Finkbeiner et al., 2017).
2.1.2. SW opsins
An SW opsin is broadly maintained across Insecta, as a recent, widescale study identified SW opsin homologs in over 60% of families (Guignard et al., 2022). Duplications of the SW opsin occurred in several orders, including Lepidoptera and Odonata (dragonflies, damselflies), which will be discussed below. Duplications of the SW opsin are particularly abundant in Paleoptera (Odonata and Ephemeroptera), where most families surveyed have at least 2 SW opsins (Futahashi et al., 2015; Guignard et al., 2022).
The insect SW opsin is typically sensitive to 430–500 nm light (Fig. 3). Amino acid substitutions responsible for sensitivity shifts have been identified from some species of butterflies using site-directed mutagenesis. Spectral tuning in Lycaenid and Pierid butterflies, which have independent duplications of the SW opsin, is influenced by amino acid substitutions at sites 116 and 177 (relative to squid rhodopsin) (Liénard et al., 2021; Wakakuwa et al., 2010). The Pierid Pieris rapae, has a violet-sensitive (PrV: λmax = 425 nm) and blue-sensitive (PrB: λmax = 450 nm) SW opsin with Ala116 + Tyr177 and Ser116 + Phe177, respectively. Double PrB mutants (Ser116Ala + Phe177Tyr) display a short-wavelength shift with approximately the same sensitivity as the PrV opsin (Wakakuwa et al., 2010). In the Lycaenid Eumaeus atala, which has a blue-sensitive SW opsin (BRh1: λmax = 435 nm) with Ala116 and Try177 and blue-green-sensitive SW opsin (BRh2: λmax = 500 nm) with Ser116 and Phe117, the reverse substitutions (Ala116Ser + Tyr177Phe), along with additive effects of additional tuning sites, explains the blue-shift in the sensitivity of BRh2 (Liénard et al., 2021).
Duplications of the SW opsin are hypothesized to function in sexual selection in some lepidopterans. In male Pieris butterflies, filtering of the duplicated SW opsin via pigments within the eye is thought to aid in spectral discrimination among females and males (Fig. 3L) (Arikawa et al., 2005). In Lycaenid butterflies, the presence of two SW opsins has been hypothesized to improve discrimination of the blue wing coloration characteristic of many Lycaenid species (Sison-Mangus et al., 2006). Accordingly, there is high overlap between the λmax of the duplicated SW opsin and reflectance measurements of wing coloration from E. atala butterflies (Liénard et al., 2021), suggesting a role for opsin duplication in the detection of color signals.
Patterns of SW opsin expression changes over developmental stages may also correspond with the saliency of visual signals. For example, the eyes of adult dragonflies express multiple SW opsin copies (three SW opsin genes expressed in Sympetrum frequens and Orthetrum albistylum) while only one SW opsin gene is expressed in nymph eyes (Futahashi et al., 2015). This may correspond to the increased relevance of visual signals for adult dragonflies, which are active flyers and rely on vision for predation and mate detection (Corbert, 1999). Consistent with this, an SW opsin has not been identified in several orders that inhabit low-light environments, and may therefore rely less on vision for their behavior (Guignard et al., 2022; Jadhav and Sharma, 2012; James et al., 2016; Liu, 2021; Vas et al., 1999; Wright et al., 2013).
2.1.3. LW opsins
The insect LW clade can be divided into the LW2a and LW2b opsin groups. LW2a opsins are primarily expressed in the compound eye while LW2b opsins are typically ocelli-specific (Guignard et al., 2022). All insect orders studied have at least one copy of the LW2a opsin, while the LW2b is found in about half of insect orders studied (Guignard et al., 2022). Duplications of LW2b have been identified from some Brachycera (Diptera) and Odonata (Futahashi et al., 2015), although in Drosophila melanogaster, one LW2b copy (Rh1) is expressed in the compound eye. Duplications of the LW2a opsin class are more common, occurring broadly in Odonata and Ephemeroptera, and several families within Hemiptera, Coleoptera, Lepidoptera, Diptera, Strepsiptera (twisted-wing parasites), and Thysanoptera (thrips) (Guignard et al., 2022).
LW sensitivities typically vary from 500 nm to >560 nm (Fig. 3). The sensitivities of LW opsins that are expressed in the ocelli tend to differ from the LW opsins in the compound eye (Aksoy and Camlitepe, 2018; Feiler et al., 1988; Goldsmith and Ruck, 1958; Henze et al., 2012; Menzel and Blakers, 1976; Salcedo et al., 1999). In honeybees (Apis mellifera), LW opsins expressed in the compound eyes have sensitivity with λmax = 540 nm while LW opsins expressed in the ocelli have sensitivity to 490 nm light (Fig. 3H) (Goldsmith and Ruck, 1958; Menzel and Blakers, 1976). In D. melanogaster, Rh2, which is specifically expressed in the ocelli, shares a close phylogenetic relationship with Rh1, which is expressed in the compound eye (Feiler et al., 1988; Mismer et al., 1988; O’Tousa et al., 1985; Pollock and Benzer, 1988). Both Rh1 and Rh2 opsins are phylogenetically grouped with insect LW opsins (Fig. 2) (Guignard et al., 2022), but have short-wavelength shifted sensitivities (Rh1 λmax = 478 nm; Rh2 λmax = 420 nm) relative to the green-sensitive LW opsin (Rh6, λmax = 508 nm) that is expressed in the compound eye (Fig. 3D) (Feiler et al., 1988; Salcedo et al., 1999).
Tuning sites for LW opsins have been identified through experiments in D. melanogaster (Salcedo et al., 2009). Substitutions between Ser and Ala at site 292 (relative to bovine rhodopsin) shifts sensitivity for Rh1 and Rh6. For Rh1, Ala292Ser resulted in a short-wavelength shift in sensitivity, while the reverse substitution in Rh6 shifted sensitivity to longer wavelengths (Salcedo et al., 2009). Similar substitutions between Ala and Ser at this site also affect spectral tuning in many vertebrate species (for review see Hagen, Roberts, & Johnson, this issue). In contrast, sites important for generating differences in vertebrate green- and red-sensitive opsins are invariant in the spectrally diverse LW opsins of the butterfly Papilio glaucus (see Papilio xuthus for sensitivity of a related species, Fig. 3L), suggesting differences in LW tuning mechanisms between insects and vertebrates (Briscoe et al., 2010).
The role of the LW opsin in insect behavior varies across functions performed by the compound eyes and ocelli. For example, the ocelli function in flight regulation, polarized light detection, navigation and orientation, and circadian timing of activity (Guignard et al., 2022; Honkanen et al., 2018; Narendra et al., 2016; Schwarz et al., 2011a,b; Schwarz et al., 2011; Taylor et al., 2016; Wunderer & Jan De Kramer, 1989). In addition to the LW opsin, the ocelli also often express a UV-sensitive opsin (Chappell and Devoe, 1975; Eaton, 1976; Guignard et al., 2021; Henze et al., 2012; Sontag, 1971). Expression of LW and UV opsins in the ocelli may enable discrimination between the predominately green foreground and the sky (Möller, 2002). For LW2b opsins expressed outside of the ocelli, functions include motion vision in D. melanogaster (Heisenberg and Buchner, 1977; Yamaguchi et al., 2008) and light detection in male fig wasps, Ceratosolen solmsi, which lack ocelli (Wang et al., 2013).
LW opsin duplication is also associated with taxa that have aquatic life stages. LW duplications are common in the orders Odonata (Fig. 3O) and Ephemeroptera and in the suborder Culicomorpha (mosquitoes and lake flies) (Futahashi et al., 2015; Guignard et al., 2022), which have aquatic nymph and terrestrial adult stages. In Culicidae and Odonata, differences in LW opsin expression are observed between nymph and adult stages (Futahashi et al., 2015; Jenkens and Muskavitch, 2015). LW opsin is more highly expressed relative to UV and SW opsin in nymphs, and subsets of LW opsins are differentially expressed between nymphs and adults (Futahashi et al., 2015; Jenkens and Muskavitch, 2015). These differences may be due to a LW-light skew in the aquatic environments inhabited by nymphs (Futahashi et al., 2015; Levine and MacNichol, 1982).
LW opsin duplications also occurred in the family Gerridae (water striders) (Guignard et al., 2022). LW opsins in the water strider Gerris buenoi are predicted to be short wavelength shifted, accounting for the blue-light sensitivity recorded from this species (Armisén et al., 2018) considering that no SW opsins have been identified in this group (Guignard et al., 2022). Like duplications of the UV opsin class following loss of SW opsins in Coleoptera, LW opsin duplications may counteract loss of other opsin classes. In support of this idea, nearly a quarter of duplications within the LW2a/LW2b clades are from families that lack an SW opsin (Guignard et al., 2022).
Extended spectral sensitivities of LW opsins into yellow-red wavelengths function in visual behaviors. For example, Papilio butterflies possess three LW opsin copies, with sensitivity to 515 nm, 530 nm, and 575 nm light (Fig. 3L) (Arikawa et al., 1999; Arikawa et al., 1987; Kitamoto et al., 1998). Visual sensitivity to red wavelengths improves detection of green coloration, improving detection of healthy host plants for egg laying by females (Kelber, 1999). Detection of red light likely benefits the flower pollinating beetle Pygopleurus israelitus, by improving detection of red flowers (Martínez-Harms et al., 2012).
2.2. Crustacea
Crustaceans possess opsins from the SW/UV, MW, and LW opsin clades (Fig. 2). The SW opsins are sensitive to light in the ultraviolet to violet-blue part of the spectrum (λmax = 330 nm–450 nm) (Fig. 4) (Bok et al., 2014; Cronin et al., 1994; Cummins and Goldsmith, 1981; Horch et al., 2002; Porter et al., 2013; Wald, 1968). The MW and LW opsins confer sensitivity to blue (~480 nm) and blue-green (500–530 nm) light, respectively (Fig. 4) (Crandall and Cronin, 1997; Cronin and Marshall, 1989; Porter et al., 2013; Sakamoto et al., 1996). Opsin duplications have been observed in many crustaceans and, in some cases, there have been large opsin gene expansions (e.g., Porter et al., 2020; Zhang et al., 2019).
Fig. 4.
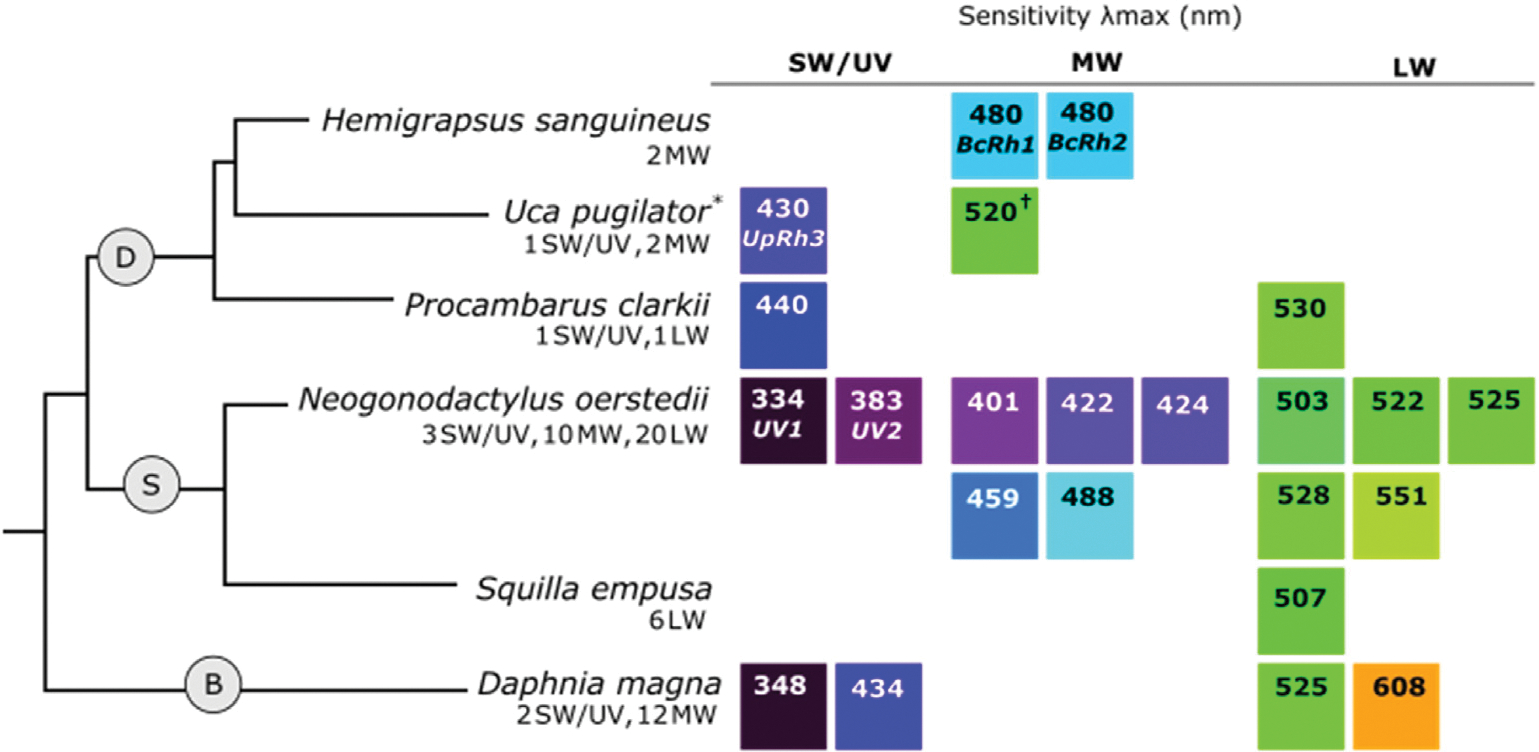
Crustacean phylogeny with representative species for (D) Decapoda, (S) Stomatopoda, and (B) Branchiopoda showing the r-opsin classes and λmax values of recorded visual sensitivities. Number and type of opsins identified from each species is shown below the species name. The color of the squares illustrates an approximation of spectral sensitivity between the UV (dark purple) and visible spectrum, relative to other values. When the opsin associated with a specific sensitivity is known, it is listed below the λmax value. Asterisks (*) indicate λmax values come from a different species of the same genus and a cross (†) symbol indicates that the reported λmax value is the midpoint between a measured range. Relationships between Crustacea were inferred from Chang and Lai (2018) and Wolfe et al. (2019). For λmax references, see Supplemental Table 2.
Compared to insects, less is known about the roles of specific opsins and opsin duplications in behavior in other arthropods. As such, we present the roles of opsin expression in crustaceans in a single section (Sec. 2.2.3) following the description of opsin diversity within the SW, MW, and LW clades.
2.2.1. SW/UV opsins
SW opsins have been identified in various crustaceans, including species from the orders Stomatopoda (mantis shrimp), Decapoda (crabs, lobsters), and Argulidae (fish lice), and from the class Branchiopoda (fairy shrimp) (Brandon et al., 2017; Colbourne et al., 2011; Henze and Oakley, 2015; Kashiyama et al., 2009; Kingston and Cronin, 2015).
The SW/UV opsin clade in Crustacea is sensitive to UV and violet-blue light (Fig. 4). In Daphnia pulex and Daphnia magna, which have duplicated SW/UV opsins with predicted sensitivities to UV (λmax ≈ 350 nm) and blue (λmax ≈ 430 nm) light (Fig. 4B) (Brandon et al., 2017; Smith and Macagno, 1990), amino acid differences were observed in sites associated with spectral tuning in other species (Brandon et al., 2017). Of particular interest is a Ser in the blue sensitive opsin and an Ala in the UV sensitive opsin at position 116, which similarly causes a blue shift in sensitivity in the butterfly P. rapae (Brandon et al., 2017; Wakakuwa et al., 2010).
2.2.2. MW and LW opsins
MW or LW opsins are broadly present in crustaceans (Biscontin et al., 2016; Crandall and Cronin, 1997; Henze and Oakley, 2015; Kingston and Cronin, 2015; Porter et al., 2009; Rajkumar et al., 2010; Sakamoto et al., 1996; Zhang et al., 2019, 2021).
Notable examples of MW and LW duplications in Crustacea have been observed in the Pacific white shrimp Litopenaeus vannamei (Decapoda), stomatopods (e.g., Neogonodactylus oerstedii, Fig. 4S, and species of branchiopods (e.g., Daphnia magna, Fig. 4B). Seven MW and 33 LW opsin genes (in addition to 2 SW) have been identified in L. vannamei, 10 MW and 20 LW opsin genes (in addition to 3 SW) have been identified in N. oerstedii (Porter et al., 2020), and as many as 19 MW opsin genes (sometimes termed long-wave A/B) have been identified in Daphnia species (Brandon et al., 2017; Colbourne et al., 2011; Zhang et al., 2021). In some species with abundant opsin classes, visual sensitivity is likewise diverse, as in N. oerstedii and D. magna (Fig. 4) (Bok et al., 2014; Cronin and Marshall, 1989; Smith and Macagno, 1990). In other cases, opsin duplications do not expand spectral sensitivity. In the brachyuran crab Hemigrapsus sanguineus, two MW opsin classes appear to be sensitive to the same wavelength of light (Fig. 4D) (Sakamoto et al., 1996).
Amino acid changes that shift spectral sensitivity have been identified from several species of crustaceans (Brandon et al., 2017; Crandall and Cronin, 1997; Porter et al., 2020). Many of these substitutions have been implicated in spectral tuning in other species, including vertebrates (Brandon et al., 2017; Porter et al., 2020; Salcedo et al., 2009; Takahashi and Ebrey, 2003; Wakakuwa et al., 2010), suggesting conserved effects of amino acid substitutions on spectral sensitivities across diverse lineages.
2.2.3. Opsins, ecology, and behavior
While many crustaceans are capable of dichromatic vision based on the expression of two different opsins with distinct sensitivities, some species possess numerous spectrally distinct opsins suggesting that these organisms are capable of polychromatic vision (Fig. 4). The role of numerous opsin duplications in color vision is perhaps best studied in stomatopods. Despite possessing 12 distinct color channels, stomatopod vision is generally course, as individuals do not discriminate color differences between 12 and 25 nm (Thoen et al., 2014). These data suggest that stomatopods do not use their abundant opsin classes for color vision using conventional color-opponency mechanisms, but may rather recognize color by incorporating signals from each of their 12 distinct color channels via temporal signals generated by scanning eye movements (Thoen et al., 2014).
In crustaceans, environmental light conditions appear to affect patterns of opsin expression. In L. vannamei, proportionally more LW opsins are expressed in adults (Zhang et al., 2019), which prefer to feed in yellow-red lighting environments (Luchiari et al., 2012) while larvae express higher levels of MW opsin genes, coinciding with the higher availability of green light in planktonic habitats (Zhang et al., 2019). Similarly, stomatopod species inhabiting shallower water, which has greater light availability and spectral richness than deep water, express more opsin classes and have a broader range of spectral sensitivity than species inhabiting deeper waters (Porter et al., 2013, 2020). In contrast, there was no correlation between habitat differences and variation in spectral sensitivity between several species of crayfish (Crandall and Cronin, 1997), suggesting that environmental conditions may not universally influence visual sensitivity.
2.3. Chelicerata
Chelicerates contain opsins from the SW/UV, MW, and LW opsin clades (Fig. 2) (Battelle et al., 2014, 2015, 2016; Morehouse et al., 2017). Corresponding visual sensitivities have been recorded with λmax ≈ 350 nm (Battelle et al., 2014; Nagata et al., 2012; Walla et al., 1996), λmax = 525 nm (Battelle et al., 2014), and λmax = 480–520 nm (Knox et al., 2003; Nagata et al., 2012; Walla et al., 1996) (Fig. 5). Opsin expression has been best studied in Araneae (spiders) and Xiphosura (horseshoe crabs), which will be the primary focus of this section.
Fig. 5.

Chelicerate phylogeny with representative species for (A) Araneae and (X) Xiphosurida showing the r-opsin classes and and λmax values of recorded visual sensitivities. Number and type of opsins identified from each species is shown below the species name. The color of the squares illustrates an approximation of spectral sensitivity between the UV (dark purple) and the visible spectrum, relative to other values. When the opsin associated with a specific sensitivity is known it, is listed below the λmax value. For λmax references, see Supplemental Table 2.
2.3.1. UV opsins
Opsins from the SW/UV opsin class are commonly expressed in Chelicerate species, conferring sensitivity to UV light (Fig. 5). As SW/UV opsins in chelicerates confer sensitivity to UV light (Battelle et al., 2014; Nagata et al., 2012), we will refer to opsins in the SW/UV opsin clade as UV opsins within this section.
Expression of one UV opsin in the eye of the horseshoe crab, L. polyphemus, has been well-characterized (Fig. 5X). Opsin transcripts for three additional opsins belonging to the SW/UV clade have also been identified suggesting additional complexity (Battelle et al., 2016). A UV opsin has also been identified from many species of Araneae spiders, as well as representative species from Scorpiones (scorpions) and Ixodida (ticks) (Morehouse et al., 2017). However, in a survey of 13 species spanning Aranea, about 30% of species were not found to contain opsins from the SW/UV clade (Morehouse et al., 2017). It is still unclear whether these differences represent true losses or simply results of methodological limitations. For example, a SW/UV opsin duplication has been identified from the well-studied jumping spider Hasarius adansoni (Fig. 5A)(Nagata et al., 2012), despite previous studies that identified only one SW/UV opsin copy from this species (Koyanagi et al., 2008a).
2.3.2. MW and LW opsins
Though a MW opsin is expressed in the horseshoe crab L. polyphemus (Fig. 3X)(Battelle et al., 2015, 2016), no MW opsin has been recovered from the majority of other chelicerate species studied to date, with the exception of one species of spider (Morehouse et al., 2017). In contrast, the LW opsin clade is commonly found in chelicerates, with many duplications (Battelle et al., 2015, 2016; Morehouse et al., 2017). LW duplications occurred in Xiphosurida (Battelle et al., 2015, 2016), many Araneae (Koyanagi et al., 2008a; Morehouse et al., 2017), and the scorpion Mesobuthus martensii (Morehouse et al., 2017). In Araneae, one copy of the LW opsin is sensitive to green light (Rh1: λmax = 520 nm) while the second is sensitive to blue light (Rh2: λmax = 480 nm) (Fig. 5A) (Nagata et al., 2012). The Rh1 and Rh2 opsins share about 60% amino acid sequence similarity (Koyanagi et al., 2008a), although specific changes that may underlie sensitivity changes have not been identified to date.
2.3.3. Opsins, ecology, and behavior
The roles of opsin expression are different among chelicerate species. Three spectrally distinct opsins across two classes are found in some Araneae, while three opsin classes confer limited spectral sensitivity to UV and green light in Xiphosurida (Fig. 5). Despite the presence of three spectrally distinct opsins in some spiders, color vision in the acute ‘principal’ eyes of spiders relies only on sensitivity to UV and green light (Blest et al., 1981; DeVoe, 1975; Nagata et al., 2012). Trichromatic vision is nevertheless possible via spectral filtering of green-sensitive opsin, resulting in sensitivity to longer-wavelengths (λmax = 630 nm) (Zurek et al., 2015). UV signals also influence the attractiveness of male jumping spiders Cosmophasis umbratical (Lim et al., 2008), supporting a role of UV sensitivity in sexual behaviors. There is no evidence of color vision in Xiphosurids like L. polyphemus/horseshoe crabs, but dramatic changes in opsin expression in response to light and an internal circadian clock suggest that opsins function in altering photoresponses (Battelle et al., 2015).
Opsin expression appears to correspond to light environment in many chelicerates. In Aranea, UV opsins are absent in most nocturnal species (Morehouse et al., 2017), in line with opsin loss associated with low-light environments in other species (Futahashi et al., 2015; Jacobs, 2013; Sharkey et al., 2017; Sondhi et al., 2021). In contrast, LW opsin expansion is noted in several nocturnal chelicerates. Numerous LW opsin copies are found in species of nocturnal spiders and horseshoe crabs (Battelle et al., 2016; Morehouse et al., 2017). In L. polyphemus, high expression of LW opsins at night (Battelle et al., 2013; Katti et al., 2010) may contribute to increased sensitivity of the lateral eyes to low light (Barlow et al., 1977), aiding in the detection of mates during nocturnal spawning (Barlow et al., 1982).
2.4. Summary of Arthropod opsin expression
Expression of the SW, MW, and LW opsin classes occurs in distinct patterns among arthropod lineages. Despite this, there are several similarities noted between groups as well. UV and MW (or LW) opsins are often present together (Figs. 3–5), meaning that most arthropods have the capacity for dichromatic vision. Many opsin duplications are associated with increased spectral classes, allowing for increased color vision capabilities, and the amino acid substitutions responsible for tuning shifts are sometimes conserved among lineages. Insects represent some of the best studied arthropods in terms of linking opsin expression to behavior and ecology, with some studies in insects finding specific functions attributed to opsin properties. General patterns across arthropods suggest that changes in opsin expression are associated with differences in light environment and that visual sensitivity often appears tuned to environmental lighting conditions.
3. Visual opsins in Lophotrochozoa
Lophotrochozoans are a monophyletic group of protostomes that includes cephalopods, bivalves, and polychaetes (Fig. 1). Rhabdomeric and ciliary photoreceptors have been identified in the visual structures of lophotrochozoans (e.g., McReynolds and Gorman, 1970) expressing many diverse opsin types (Bok et al., 2017a,b; Bok et al., 2017; Kojima et al., 1997). In this section, we discuss r-opsins, c-opsins, Go-opsins, and xenopsins in Mollusca (bivalves, cephalopods) and Polychaeta (annelid worms). Because there are lineage-specific patterns of opsin co-expression between r-, c-, and Go-opsins, we outline expression of these opsin types together. We then discuss emerging data on xenopsins and their roles in behavior.
3.1. R-opsin, C-opsin, and go-opsins
Rhabdomeric photoreceptors, expressing two r-opsins and a Go-opsin, have been found in the larval eyespots and pigment-cup eyes of the polychaete Platynereis dumerilii (Arendt et al., 2002, 2004; Ayers et al., 2018; Gühmann et al., 2015; Randel et al., 2013). The P. dumerilii genome contains a second Go-opsin, however expression of this gene has not been characterized experimentally (Gühmann et al., 2015). R-opsins have also been identified from the rhabdomeric photoreceptors of mollusks (Fig. 6L), with two r-opsins identified in many bivalves (Serb et al., 2013) and one r-opsin identified across several cephalopod species (Chung and Marshall, 2016). A second r-opsin has been identified in the cephalopod Sepia officinalis, although expression of this opsin has not been observed in the eye (Bonadè et al., 2020).
Fig. 6.
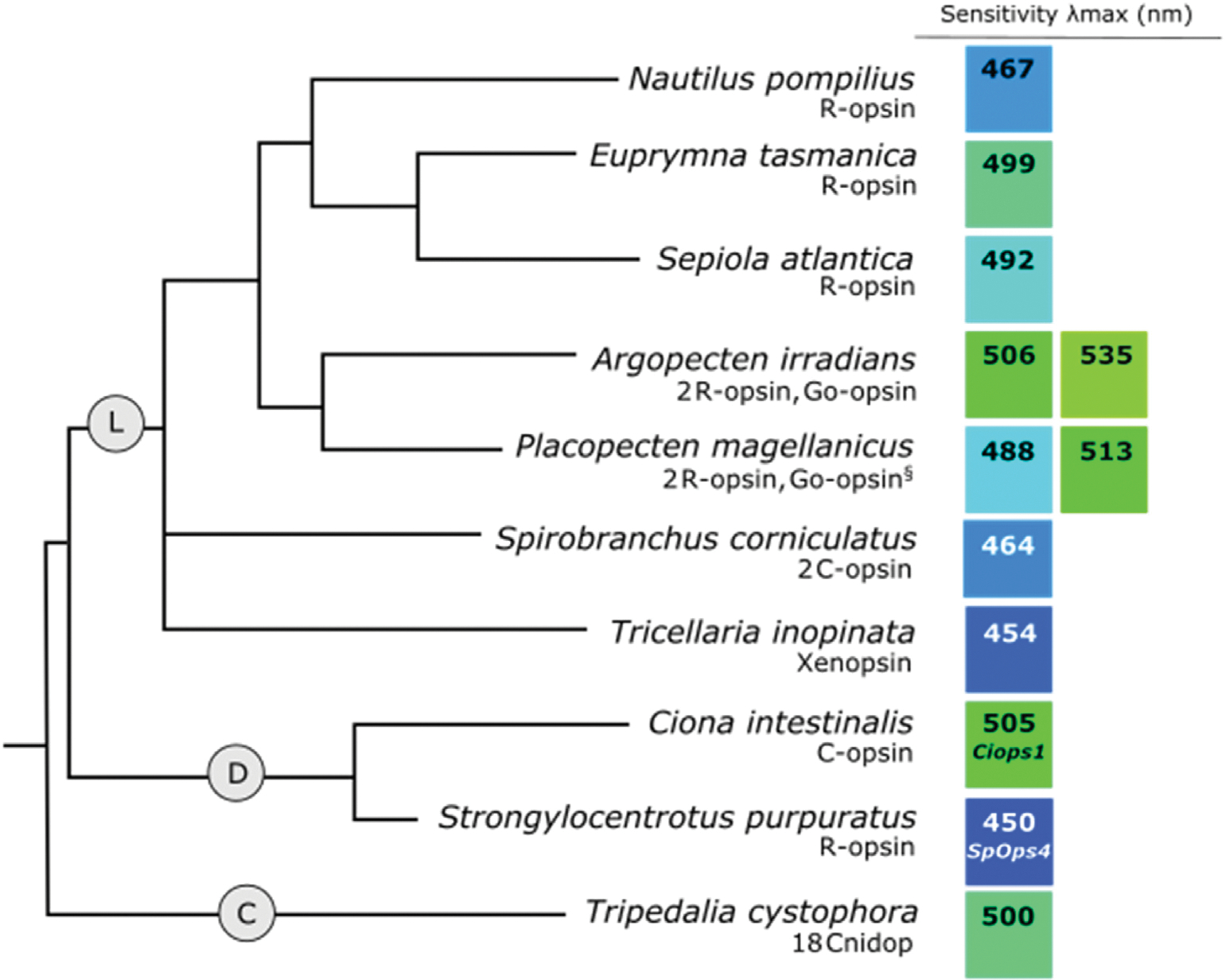
Phylogeny of (L) lophotrochozoan, (D) invertebrate deuterostome, and (C) cnidarian species showing opsin classes with known or putative visual functions and λmax values of recorded visual sensitivities. Number and type of opsins identified from each species is shown below the species name. The color of each square illustrates an approximation of spectral sensitivity relative to other values. When the opsin associated with a specific sensitivity is known, it is listed below the λmax value. The § symbol by the Placopecten magellanicus Go-opsin indicates that this opsin type is assumed to be expressed in the ciliary photoreceptors for this species based on expression of Go-opsin in the ciliary photoreceptors of other closely related species (Kingston et al., 2017; Kojima et al., 1997). Relationships between Lophotrochozoa, Deteruostoma, and Cnidaria were inferred from Valentine and Collins (2000) and relationships within Lophotrochozoa was inferred from (Bleidorn, 2019). For λmax references, see Supplemental Table 2.
Ciliary photoreceptors are also found in polychaetes and bivalves (Barber et al., 1967; Bok et al., 2016; Lawrence and Krasne, 1965; McReynolds and Gorman, 1970). However, the opsins expressed in ciliary photoreceptors differ across groups. While c-type opsins are expressed in ciliary photoreceptors of the radiolar eyes in the polychaetes Spirobranchus corniculatus and Acromegalomma interruptum (Bok et al., 2017a,b; Bok et al., 2017), Go-opsin is expressed in the ciliary photoreceptors of scallops (Kojima et al., 1997).
Opsins appear to play important behavioral and ecological roles in lophotrocozoans. Sessile and eyeless bivalve species generally have fewer r-opsin copies compared to mobile species with eyes (Serb et al., 2013), suggesting functional benefits of multiple opsins in visual tasks and navigation. In P. dumerilii, expression of one of the r-opsins corresponds with the beginning of positive phototaxis behavior in early-stage larvae (Randel et al., 2013). Expression of a second r-opsin occurs at the onset of photoreceptor differentiation in later-stage larvae, suggesting distinct roles of each opsin in development (Randel et al., 2013). In the marine annelid Capitella teleta, r-opsin mediates the strong phototaxis responses of larvae (Neal et al., 2019), which is suggested to aid in dispersal for many marine invertebrates with planktonic, free-swimming larvae (Randel and Jékely, 2016).
Visual sensitivity may also be tuned relative to the light environment. In bivalves, species living in blue-dominated waters are sensitive to shorter wavelengths than species inhabiting green-shifted environments (see A. irradians and P. magellanicus, Fig. 6L) (Speiser et al., 2011). In the polychaeta S. corniculatus, visual sensitivity (λmax = 464 nm) also provides a good match to downwelling environmental light (Fig. 6L) (Bok et al., 2017a,b) and may help with the detection of overhead predators (Nilsson, 1994). In cephalopods, differences in spectral sensitivities match environmental light conditions among decapodiform species (see Nautilus pompilius, Euprymna tasmanica, and Sepiola atlantica Fig. 6L) (Chung and Marshall, 2016).
3.2. Xenopsins
Xenopsins are found in mollusks, brachiopods, rotifers, flatworms, and annelids (Bonadè et al., 2020; Döring et al., 2020; Passamaneck et al., 2011; Ramirez et al., 2016; Rawlinson et al., 2019; Vöcking et al., 2017). In the annelid Malacoceros fuliginosus, xenopsin is co-expressed with r-opsin in the larval eyes (Döring et al., 2020). Identification of a c-opsin sequence from M. fuliginosus makes this the first organisms known to possess r-opsin, c-opsin, and xenopsins together (Döring et al., 2020). Though the roles of xenopsin in vision are not well understood, studies in the bryozoan Tricellaria inopinat, (Fig. 6L), show that xenopsins are involved in phototactic behavior (Döring et al., 2020).
3.3. Summary of Lophotrochozoa opsin expression
Lophotrochozoan vision is characterized by a diversity of opsin types. The use of c-opsin and Go-opsin for vision in Lophotrochozoa contrasts with r-opsin-mediated vision from arthropods and suggests that a diverse array of opsins and photoreceptor types were present in the protostome ancestor. Like in arthropods, visual sensitivity appears to be tuned to environmental lighting conditions and, in some cases, the saliency of visual cues may also explain some opsin duplications.
4. Visual opsins in invertebrate deuterostomes
An analysis of invertebrate deuterostome opsin evolution illustrates the similarities and differences in visual opsin expression between these species, vertebrates, and protostome groups. In this section, we discuss opsin expression and functions in Echinodermata and Tunicata (Chordata). Visual opsin evolution in vertebrates is discussed by Hagen et al. (this issue).
R-, c-, and Go-opsins have been identified in echinoderms and tunicates (e.g., Porter et al., 2012). However, in each case, only a single opsin type has been characterized for a functional role in vision (r-opsin in echinoderms and c-opsin in tunicates), which we discuss below.
4.1. R-opsin
R-opsin (Sp-Opsin4) appears to be the opsin that mediates vision in echinoderms, although c-opsins and Go-opsins have also been identified within this group (Agca et al., 2011; Delroisse et al., 2013; Lesser et al., 2011; Raible et al., 2006; Ullrich-Lüter et al., 2011; Ullrich-Lüter, D’Aniello and Arnone, 2013). Despite the absence of eyes, photoreceptor cells on the tube feet of the purple sea urchin (Strongylocentrotus purpuratus) confer directional vision via the localized expression of Sp-Opsin4, which is likely sensitive to blue light based on tests of phototaxis response (λmax = 450 nm, Fig. 6D) (Ullrich-Lüter et al., 2011). Sp-Opsin4 is also expressed in the optic cushions (optic organs that play a role in phototaxis) of the sea star Asterias rubens, further supporting a role for r-opsin in direction vision in echinoderms (Delroisse et al., 2013; Ullrich-Lüter et al., 2011).
Rhabdomeric photoreceptors have been identified in other deuterostomes (Brandenburger et al., 1973; Gorman et al., 1971; Pantzartzi et al., 2017; Watanabe and Yoshida, 1986). However, the use of r-opsin-expressing rhabdomeric photoreceptors in vision appears to be unique in echinoderms, whereas r-type opsins function in non-visual roles in many deuterostomes (Lacalli, 2004; Panda et al., 2005; Provencio et al., 1998; but see Ecker et al., 2010).
4.2. C-opsin
Though r-opsins have been identified in tunicates (Porter et al., 2012), a single c-opsin identified in ciliary photoreceptors provides putative visual function in larval Ciona intestinalis (Inada et al., 2003; Kusakabe et al., 2001; Kusakabe and Tsuda, 2007). Expression of the c-opsin (λmax = 500 nm) within the ocellus is necessary for the photic behavior of larval Ciona (Inada et al., 2003; Kusakabe and Tsuda, 2007) (Nakagawa et al., 1999) (Fig. 6D). The C. intestinalis c-opsin groups phylogenetically with other deuterostome c-opsins (Fig. 2) and is sister to the vertebrate ancient (VA) opsins identified from teleost fish (Kusakabe et al., 2001; Soni et al., 1998; Soni and Foster, 1997).
4.3. Summary of invertebrate deuterostome opsin expression
Specific opsin types are associated with vision in the two invertebrate deuterostome groups considered here. While echinoderms use a r-opsin to mediate vision, like many protostome species, this contrasts with other deuterostomes that employ c-opsins. Despite differences in opsin type, the spectral sensitivity of the invertebrate deuterostome opsin may function similarly by providing maximal sensitivity to the blue-light environments they inhabit.
5. Cnidops
Cnidops are an opsin class found only in cnidarians and ctenophores (Liegertová et al., 2015; Plachetzki et al., 2007; Porter et al., 2012) that share a close phylogenetic relationship to deuterostome c-opsins and lophotrochozoan opsins (Fig. 2). Nevertheless, the relationships between cnidops and other opsin classes remain unclear (Bielecki et al., 2014; Feuda et al., 2012; Porter et al., 2012; Ramirez et al., 2016).
5.1. Cnidops diversity and functions
Cnidops expression varies greatly among cnidarians (Bielecki et al., 2014; Liegertová et al., 2015; Plachetzki et al., 2007; Suga et al., 2008). cDNAs for two distinct opsins have been identified in the eyeless species Podocoryne carnea, compared to eighteen opsin sequences identified in Cladonema radiatum, seven of which are expressed in the eyes (Suga et al., 2008). In Tripedalia cystophora, eighteen opsin sequences were identified in the rhopalium, the sensory structures containing the eyes (Liegertová et al., 2015). It is unclear if these opsins form visual pigments with distinct spectral sensitivities with physiological evidence suggesting monochromatic vision in the blue-green part of the light spectrum (Fig. 6C) (Coates et al., 2006; Garm et al., 2007).
Opsins often function in extra-ocular photoreception in Cnidaria, with cnidops identified from structures that lack any apparent visual function and from species that lack eyes (Bielecki et al., 2014; Gornik et al., 2021; Suga et al., 2008). For example, a subset of T. cystophora cnidops are expressed in the gonads and tentacles (Liegertová et al., 2015). In the jellyfish Clytia hemisphaerica, a cnidops expressed in the gonads mediates oocyte maturation and spawning behavior (Artigas et al., 2018). Increased expression of Anthozoan specific opsins (which fall outside of the cnidops clade) in gonadal tissue of Aiptasia suggests that opsins may function generally in spawning behavior for cnidarians (Gornik et al., 2021). The large number of opsin genes identified in Cnidaria, even in eyeless and sessile species (e.g. 63 opsin genes identified in Hydra magnipapillata; Suga et al., 2008), indicates an important role of extraocular photoreception in physiological functions, and could contribute to the expansion of opsin genes in these species (for a review see Garm and Ekström, 2010).
6. Conclusion
Invertebrate visual opsins are extremely diverse. While distinct patterns of opsin expression exist across distantly related species, even within closely related lineages, patterns of opsin expression and visual sensitivities vary greatly. The roles of changes to opsin sequence on tuning have been identified in some cases (e.g., Salcedo et al., 2009; Wakakuwa et al., 2010), although there is still much to learn about the roles of sequence changes on spectral tuning. Similarly, the roles of spectral sensitivity variation have been studied within some groups, with findings suggesting general roles of spectral tuning in response to increased saliency of visual cues and environmental light conditions. Exciting advances in genomic techniques have already begun to identify new mechanisms underlying protostome opsin evolution and function (e.g. Liénard et al., 2021; Neal et al., 2019), providing an interesting avenue for future research on invertebrate opsin evolution. Overall, the diversity of opsins spanning protostomes, invertebrate deuterostomes, and cnidaria (this review), and vertebrates (Hagen et al., this issue) highlights the diversity of opsins present in the eumetazoan ancestor and sheds light on the different behavioral and ecological contexts that opsin diversity may influence these groups.
Supplementary Material
Acknowledgements
J.F.D.H. was supported by EMBO ALTF 318-2021. R.J.J. was supported by the National Eye Institute R01EY025598.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ydbio.2022.10.011.
Data availability
No data was used for the research described in the article.
References
- Agca C, Elhajj MC, Klein WH, Venuti JM, 2011. Neurosensory and neuromuscular organization in tube feet of the sea urchin Strongylocentrotus purpuratus. J. Comp. Neurol. 519 (17), 3566–3579. 10.1002/cne.22724. [DOI] [PubMed] [Google Scholar]
- Aksoy V, Camlitepe Y, 2018. Spectral sensitivities of ants-a review. Anim. Biol. Leiden 68 (1), 55–73. 10.1163/15707563-17000119. [DOI] [Google Scholar]
- Almudi I, Vizueta J, Wyatt CDR, de Mendoza A, Marlétaz F, Firbas PN, Feuda R, Masiero G, Medina P, Alcaina-Caro A, Cruz F, Gómez-Garrido J, Gut M, Alioto TS, Vargas-Chavez C, Davie K, Misof B, González J, Aerts S, et al. , 2020. Genomic adaptations to aquatic and aerial life in mayflies and the origin of insect wings. Nat. Commun. 11 (1), 1–11. 10.1038/s41467-020-16284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodf J, 2004. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306 (5697), 869–871. 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- Arendt D, Tessmar K, Medeiros de Campos-Baptista MI, Dorresteijn A, Wittbrodt J, 2002. Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in bilateria. Development 129 (5), 1143–1154. 10.1242/dev.129.5.1143. [DOI] [PubMed] [Google Scholar]
- Arikawa K, Inokuma K, Eguchi E, 1987. Pentachromatic visual system in a butterfly. Naturwissenschaften 74 (6), 297–298. 10.1007/BF00366422. [DOI] [Google Scholar]
- Arikawa K, Scholten DGW, Kinoshita M, Stavenga DG, 1999. Tuning of photoreceptor spectral sensitivities by red and yellow pigments in the butterfly Papilio xuthus. Zool. Sci. 16 (1), 17–24. 10.2108/zsj.16.17. [DOI] [Google Scholar]
- Arikawa K, Wakakuwa M, Qiu X, Kurasawa M, Stavenga DG, 2005. Sexual dimorphism of short-wavelength photoreceptors in the small white butterfly, Pieris rapae crucivora. J. Neurosci. 25 (25), 5935–5942. 10.1523/JNEUROSCI.1364-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armisén D, Rajakumar R, Friedrich M, Benoit JB, Robertson HM, Panfilio KA, Ahn SJ, Poelchau MF, Chao H, Dinh H, Doddapaneni HV, Dugan S, Gibbs RA, Hughes DST, Han Y, Lee SL, Murali SC, Muzny DM, Qu J, et al. , 2018. The genome of the water strider Gerris buenoi reveals expansions of gene repertoires associated with adaptations to life on the water 06 Biological Sciences 0604 Genetics. BMC Genom. 19 (1), 1–16. 10.1186/s12864-018-5163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas GQ, Lapébie P, Leclère L, Takeda N, Deguchi R, Jékely G, Momose T, Houliston E, 2018. A gonad-expressed opsin mediates light-induced spawning in the jellyfish Clytia. Elife 7, e29555. 10.1101/140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers T, Tsukamoto H, Gühmann M, Veedin Rajan VB, Tessmar-Raible K, 2018. A Go-type opsin mediates the shadow reflex in the annelid Platynereis dumerilii. BMC Biol. 16 (1), 1–9. 10.1186/s12915-018-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber VC, Evans EM, Land MF, 1967. The fine structure of the eye of the mollusc Pecten Maximus. Zeitschrift Für Zellforschung 76, 295–312. [PubMed] [Google Scholar]
- Barlow RB, Irl LC, Kass L, 1982. Vision has a role in Limulus mating behaviour. Nature 296 (5852), 65–66. 10.1038/296065a0. [DOI] [PubMed] [Google Scholar]
- Barlow RBJ, Bolanowski SJJ, Brachman ML, 1977. Efferent optic nerve fibers mediate circadian rhythms in the Limulus eye. Science 197 (4298), 86–89. [DOI] [PubMed] [Google Scholar]
- Battelle B-A, Kempler KE, Harrison A, Dugger DR, Payne R, 2014. Opsin expression in Limulus eyes: a UV opsin is expressed in each eye type and co-expressed with a visible light-sensitive opsin in ventral larval eyes. J. Exp. Biol. 217 (17), 3133–3145. 10.1242/jeb.107383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelle B-A, Kempler KE, Parker AK, Gaddie CD, 2013. Opsin1–2, Gqα and arrestin levels at Limulus rhabdoms are controlled by diurnal light and a circadian clock. J. Exp. Biol. 216 (10), 1837–1849. 10.1242/jeb.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelle B-A, Kempler KE, Saraf SR, Marten CE, Dugger DR, Speiser DI, Oakley TH, 2015. Opsins in Limulus eyes: characterization of three visible lightsensitive opsins unique to and co-expressed in median eye photoreceptors and a peropsin/RGR that is expressed in all eyes. J. Exp. Biol. 218 (3), 466–479. 10.1242/jeb.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelle B-A, Ryan JF, Kempler KE, Saraf SR, Marten CE, Warren WC, Minx PJ, Montague MJ, Green PJ, Schmidt SA, Fulton L, Patel NH, Protas ME, Wilson RK, Porter ML, 2016. Opsin repertoire and expression patterns in horseshoe crabs: evidence from the genome of limulus polyphemus (arthropoda: Chelicerata). Genome Biol. Evol. 8 (5), 1571–1589. 10.1093/gbe/evw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki J, Zaharoff AK, Leung NY, Garm A, Oakley TH, 2014. Ocular and extraocular expression of opsins in the rhopalium of Tripedalia cystophora (Cnidaria: cubozoa). PLoS One 9 (6), e98870. 10.1371/journal.pone.0098870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscontin A, Frigato E, Sales G, Mazzotta GM, Teschke M, De Pittà C, Jarman S, Meyer B, Costa R, Bertolucci C, 2016. The opsin repertoire of the Antarctic krill Euphausia superba. Marine Genomics 29, 61–68. 10.1016/j.margen.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Bleidorn C, 2019. Recent progress in reconstructing lophotrochozoan (spiralian) phylogeny. Org. Divers. Evol. 19 (4), 557–566. 10.1007/s13127-019-00412-4. Springer. [DOI] [Google Scholar]
- Blest AD, Hardie RC, McIntyre P, Williams DS, 1981. The spectral sensitivities of identified receptors and the function of retinal tiering in the principal eyes of a jumping spider. J. Comp. Physiol. 145 (2), 227–239. 10.1007/BF00605035. [DOI] [Google Scholar]
- Bok MJ, Capa M, Nilsson DE, 2016. Here, there and everywhere: the radiolar eyes of fan worms (Annelida, Sabellidae). Integr. Comp. Biol. 56 (5), 784–795. 10.1093/icb/icw089. [DOI] [PubMed] [Google Scholar]
- Bok MJ, Porter ML, Nilsson DE, 2017a. Phototransduction in fan worm radiolar eyes. Curr. Biol. 27 (14), R698–R699. 10.1016/j.cub.2017.05.093. Cell Press. [DOI] [PubMed] [Google Scholar]
- Bok MJ, Porter ML, Place AR, Cronin TW, 2014. Biological sunscreens tune polychromatic ultraviolet vision in Mantis Shrimp. Curr. Biol. 24 (14), 1636–1642. 10.1016/j.cub.2014.05.071. [DOI] [PubMed] [Google Scholar]
- Bok MJ, Porter ML, Ten Hove HA, Smith R, Nilsson DE, 2017b. Radiolar eyes of serpulid worms (Annelida, serpulidae): structures, function, and phototransduction. Biol. Bull. 233, 39–57. 10.1086/694735. [DOI] [PubMed] [Google Scholar]
- Bonadè M, Ogura A, Corre E, Bassaglia Y, Bonnaud-Ponticelli L, 2020. Diversity of light sensing molecules and their expression during the embryogenesis of the cuttlefish (Sepia officinalis). Front. Physiol. 11. 10.3389/fphys.2020.521989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburger JL, Woolacott RM, Eakin RM, 1973. Fine structure of eyespots in tornarian larvae (Phylum: hemichordata). Z. für Zellforsch. Mikrosk. Anat. 142 (1), 89–102. 10.1007/BF00306706, 1973 142:1. [DOI] [PubMed] [Google Scholar]
- Brandon CS, Greenwold MJ, Dudycha JL, 2017. Ancient and recent duplications support functional diversity of Daphnia opsins. J. Mol. Evol. 84 (1), 12–28. 10.1007/s00239-016-9777-1. [DOI] [PubMed] [Google Scholar]
- Brines ML, Gould JL, 1979. Bees have rules. Science 206 (4418), 571–573. [DOI] [PubMed] [Google Scholar]
- Briscoe AD, Bybee SM, Bernard GD, Yuan F, Sison-Mangus MP, Reed RD, Warren AD, Llorente-Bousquets J, Chiao CC, 2010. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl. Acad. Sci. U.S.A. 107 (8), 3628–3633. 10.1073/pnas.0910085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe AD, Chittka L, 2001. The evolution of color vision in insects. In: Annual Review of Entomology, vol. 46, pp. 471–510. 10.1146/annurev.ento.46.1.471. Annual Reviews 4139 El Camino Way, P.O. Box 10139, Palo Alto, CA 94303–0139, USA. [DOI] [PubMed] [Google Scholar]
- Chang WH, Lai AG, 2018. Comparative genomic analysis of crustacean hyperglycemic hormone (CHH) neuropeptide genes across diverse crustacean species. F1000Research 7, 100. 10.12688/f1000research.13732.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell RL, Devoe RD, 1975. Action spectra and chromatic mechanisms of cells in the median ocelli of dragonflies. J. Gen. Physiol. 65 (4), 399–419. 10.1085/jgp.65.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman AD, Ferrier DEK, Brena C, Qu J, Hughes DST, Schröder R, Torres-Oliva M, Znassi N, Jiang H, Almeida FC, Alonso CR, Apostolou Z, Aqrawi P, Arthur W, Barna JCJ, Blankenburg KP, Brites D, Capella-Gutiérrez S, Coyle M, et al. , 2014. The first myriapod genome sequence reveals conservative arthropod gene content and genome organisation in the centipede Strigamia maritima. PLoS Biol. 12 (11), e1002005. 10.1371/journal.pbio.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Marshall NJ, 2016. Comparative visual ecology of cephalopods from different habitats. Proc. Biol. Sci. 283 (1838), 1–10. 10.1098/rspb.2016.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates MM, Garm A, Theobald JC, Thompson SH, Nilsson DE, 2006. The spectral sensitivity of the lens eyes of a box jellyfish, Tripedalia cystophora (Conant). J. Exp. Biol. 209 (19), 3758–3765. 10.1242/jeb.02431. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Cáceres CE, Carmel L, Casola C, Choi JH, Detter JC, Dong Q, Dusheyko S, Eads BD, et al. , 2011. The ecoresponsive genome of Daphnia pulex. Science 331 (6017), 555–561. 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbert PS, 1999. Dragonflies: Behavior and Ecology of Odonata. Cornell Univ Press, Ithaca, NY. [Google Scholar]
- Crandall KA, Cronin TW, 1997. The molecular evolution of visual pigments of freshwater crayfishes (Decapoda: cambaridae). J. Mol. Evol. 45, 524–534. 10.1007/PL00006257. [DOI] [PubMed] [Google Scholar]
- Cronin TW, Marshall NJ, 1989. Multiple spectral classes of photoreceptors in the retinas of gonodactyloid stomatopod crustaceans. J. Comp. Physiol. 166 (2), 261–275. 10.1007/BF00193471. [DOI] [Google Scholar]
- Cronin TW, Marshall NJ, Quinn CA, King CA, 1994. Ultraviolet photoreception in mantis shrimp. Vis. Res. 34 (11), 1443–1452. 10.1016/0042-6989(94)90145-7. [DOI] [PubMed] [Google Scholar]
- Cummins D, Goldsmith TH, 1981. Cellular identification of the violet receptor in the crayfish eye. J. Comp. Physiol. A 142 (2), 199–202. 10.1007/BF00605738. [DOI] [Google Scholar]
- Delroisse J, Lanterbecq D, Eeckhaut I, Mallefet J, Flammang P, 2013. Opsin detection in the sea urchin Paracentrotus lividus and the sea star Asterias rubens. Cah. Biol. Mar. 54 (4), 721–727. [Google Scholar]
- DeVoe RD, 1975. Ultraviolet and green receptors in principal eyes of jumping spiders. J. Gen. Physiol. 66 (2), 193–207. 10.1085/jgp.66.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring CC, Kumar S, Tumu SC, Kourtesis I, Hausen H, 2020. The visual pigment xenopsin is widespread in protostome eyes and impacts the view on eye evolution. Elife 9, 1–23. 10.7554/ELIFE.55193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AG, Chittka L, 2004. Bumblebee search time without ultraviolet light. J. Exp. Biol. 207 (10), 1683–1688. 10.1242/jeb.00941. [DOI] [PubMed] [Google Scholar]
- Eaton JL, 1976. Spectral sensitivity of the ocelli of the adult cabbage looper moth, Trichoplusia ni. J. Comp. Physiol. 109 (1), 17–24. 10.1007/BF00663432. [DOI] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S, 2010. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67 (1), 49–60. 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiler R, Harris WA, Kirschfeld K, Wehrhahn C, Zuker CS, 1988. Targeted misexpression of a Drosophila opsin gene leads to altered visual function. Nature 333 (6175), 737–741. 10.1038/333737a0. [DOI] [PubMed] [Google Scholar]
- Feuda R, Goulty M, Zadra N, Gasparetti T, Rosato E, Segata N, Rizzoli A, Pisani D, Ometto L, Stabelli OR, 2020. The Diverging Evolutionary History of Opsin Genes in Diptera. BioRxiv. 10.1101/2020.06.29.177931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuda R, Hamilton SC, McInerney JO, Pisani D, 2012. Metazoan opsin evolution reveals a simple route to animal vision. Proc. Natl. Acad. Sci. USA 109 (46), 18868–18872. 10.1073/pnas.1204609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner SD, Fishman DA, Osorio D, Briscoe AD, 2017. Ultraviolet and yellow reflectance but not fluorescence is important for visual discrimination of conspecifics by Heliconius erato. J. Exp. Biol. 220 (7), 1267–1276. 10.1242/JEB.153593. [DOI] [PubMed] [Google Scholar]
- Fleming JF, Kristensen RM, Sørensen MV, Park TYS, Arakawa K, Blaxter M, Rebecchi L, Guidetti R, Williams TA, Roberts NW, Vinther J, Pisani D, 2018. Molecular palaeontology illuminates the evolution of ecdysozoan vision. Proc. Biol. Sci. 285 (1892). 10.1098/rspb.2018.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futahashi R, Kawahara-Miki R, Kinoshita M, Yoshitake K, Yajima S, Arikawa K, Fukatsu T, 2015. Extraordinary diversity of visual opsin genes in dragonflies. Proc. Natl. Acad. Sci. U.S.A. 112 (11), E1247–E1256. 10.1073/pnas.1424670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garm A, Coates MM, Gad R, Seymour J, Nilsson DE, 2007. The lens eyes of the box jellyfish Tripedalia cystophora and Chiropsalmus sp. are slow and color-blind. J. Comp. Physiol.: Neuroethology, Sensory, Neural Behav. Phy. 193 (5), 547–557. 10.1007/s00359-007-0211-4. [DOI] [PubMed] [Google Scholar]
- Garm A, Ekströom P, 2010. Evidence for multiple photosystems in jellyfish. In: International Review of Cell and Molecular Biology, vol. 280. Elsevier Inc, pp. 41–78. 10.1016/S1937-6448(10)80002-4. [DOI] [PubMed] [Google Scholar]
- Goldsmith TH, Ruck PR, 1958. The spectral sensitivities of the dorsal ocelli of cockroaches and honeybees. J. Gen. 41 (6), 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman ALF, McReynolds JS, Barnes SN, 1971. Photoreceptors in primitive chordates: fine structure, hyperpolarizing receptor potentials, and evolution. Science 172 (3987), 1052–1054. 10.1126/science.172.3987.1052. [DOI] [PubMed] [Google Scholar]
- Gornik SG, Bergheim BG, Morel B, Stamatakis A, Foulkes NS, Guse A, 2021. Photoreceptor diversification accompanies the evolution of anthozoa. Mol. Biol. Evol. 38 (5), 1744–1760. 10.1093/molbev/msaa304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gühmann M, Jia H, Randel N, Verasztó C, Bezares-Calderón LA, Michiels NK, Yokoyama S, Jékely G, 2015. Spectral tuning of phototaxis by a go-opsin in the rhabdomeric eyes of platynereis. Curr. Biol. 25, 2265–2271. 10.1016/j.cub.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Guignard Q, Allison JD, Slippers B, 2022. The evolution of insect visual opsin genes with specific consideration of the influence of ocelli and life history traits. BMC Ecol. Evol. 22 (1), 1–9. 10.1186/s12862-022-01960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignard Q, Spaethe J, Slippers B, Strube-Bloss M, Allison JD, 2021. Evidence for UV-green dichromacy in the basal hymenopteran Sirex noctilio (Siricidae). Sci. Rep. 11 (1), 1–10. 10.1038/s41598-021-95107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen JFD, Roberts N, Johnston RJ Jr., n.d. The evolutionary history and spectral tuning of vertebrate visual opsins. Dev. Biol. Submitted to Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M, Buchner E, 1977. The role of retinula cell types in visual behavior of Drosophila melanogaster. J. Comp. Physiol. 117 (2), 127–162. 10.1007/BF00612784. [DOI] [Google Scholar]
- Henze MJ, Dannenhauer K, Kohler M, Labhart T, Gesemann M, 2012. Opsin evolution and expression in Arthropod compound Eyes and Ocelli: insights from the cricket Gryllus bimaculatus. BMC Evol. Biol. 12 (1). 10.1186/1471-2148-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze MJ, Oakley TH, 2015. The dynamic evolutionary history of pancrustacean eyes and opsins. Integr. Comp. Biol. 55 (5), 830–842. 10.1093/icb/icv100. [DOI] [PubMed] [Google Scholar]
- Honkanen A, Saari P, Takalo J, Heimonen K, Weckström M, 2018. The role of ocelli in cockroach optomotor performance. J. Comp. Physiol.: Neuroethology, Sensory, Neural Behav. Phy. 204 (2), 231–243. 10.1007/s00359-017-1235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch K, Salmon M, Forward R, 2002. Evidence for a two pigment visual system in the fiddler crab, Uca thayeri. J. Comp. Physiol.: Neuroethology, Sensory, Neural Behav. Phy. 188 (6), 493–499. 10.1007/s00359-002-0325-7. [DOI] [PubMed] [Google Scholar]
- Horth L, Campbell L, Bray R, 2014. Wild bees preferentially visit Rudbeckia flower heads with exaggerated ultraviolet absorbing floral guides. Biol. Open 3 (3), 221–230. 10.1242/bio.20146445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R, Gregerman RI, Wald G, 1953. Geometrical isomers of retinene. J. Gen. Physiol. 36 (3), 415–429. 10.1085/jgp.36.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada K, Horie T, Kusakabe T, Tsuda M, 2003. Targeted knockdown of an opsin gene inhibits the swimming behaviour photoresponse of ascidian larvae. Neurosci. Lett. 347 (3), 167–170. 10.1016/S0304-3940(03)00689-X. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Sasaki G, Ogawa J, Miyata T, Su ZH, 2011. Phylogenetic relationships among insect orders based on three nuclear protein-coding gene sequences. Mol. Phylogenet. Evol. 58 (2), 169–180. 10.1016/j.ympev.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, 2013. Losses of functional opsin genes, short-wavelength cone photopigments, and color vision - a significant trend in the evolution of mammalian vision. Vis. Neurosci. 30, 39–53. 10.1017/S0952523812000429. [DOI] [PubMed] [Google Scholar]
- Jadhav SS, Sharma RM, 2012. Insecta : blattodea. Zool. Suev. India Faunda of Maharashtra, State Fauna Series 20 (2), 447–448. [Google Scholar]
- James M, Nandamuri SP, Stahl A, Buschbeck EK, 2016. The unusual eyes of Xenos peckii (Strepsiptera: xenidae) have green- and UV-sensitive photoreceptors. J. Exp. Biol. 219 (24), 3866–3874. 10.1242/jeb.148361. [DOI] [PubMed] [Google Scholar]
- Jenkens AM, Muskavitch MAT, 2015. Crepuscular behavioral variation and profiling of opsin genes in Anopheles gambiae and Anopheles stephensi (Diptera: Culicidae). J. Med. Entomol. 52 (3), 296–307. 10.1093/JME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiyama K, Seki T, Numata H, Goto SG, 2009. Molecular characterization of visual pigments in Branchiopoda and the evolution of opsins in Arthropoda. Mol. Biol. Evol. 26 (2), 299–311. 10.1093/molbev/msn251. [DOI] [PubMed] [Google Scholar]
- Katti C, Kempler K, Porter ML, Legg A, Gonzalez R, Garcia-Rivera E, Dugger D, Battelle B-A, 2010. Opsin co-expression in Limulus photoreceptors: differential regulation by light and a circadian clock. J. Exp. Biol. 213 (15), 2589–2601. 10.1242/jeb.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelber A, 1999. Ovipositing butterflies use a red receptor to see green. J. Exp. Biol. 202 (19), 2619–2630. 10.1242/JEB.202.19.2619. [DOI] [PubMed] [Google Scholar]
- Kenny NJ, Shen X, Chan TTH, Wong NWY, Chan TF, Chu KH, Lam H-M, Hui JHL, 2015. Genome of the rusty millipede, trigoniulus corallinus, illuminates diplopod, myriapod, and arthropod evolution. Genome Biol. Evol. 7 (5), 1280–1295. 10.1093/gbe/evv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston ACN, Chappell DR, Miller HV, Lee SJ, Speiser DI, 2017. Expression of G proteins in the eyes and parietovisceral ganglion of the bay scallop argopecten irradians. Biol. Bull. 233 (1), 83–95. 10.1086/694448. [DOI] [PubMed] [Google Scholar]
- Kingston ACN, Cronin TW, 2015. Short- and long-wavelength-sensitive opsins are involved in photoreception both in the retina and throughout the central nervous system of crayfish. J. Comp. Physiol.: Neuroethology, Sensory, Neural Behav. Phy. 201 (12), 1137–1145. 10.1007/s00359-015-1043-2. [DOI] [PubMed] [Google Scholar]
- Kitamoto J, Sakamoto K, Ozaki K, Mishina Y, Arikawa K, 1998. Two visual pigments in a single photoreceptor cell: identification and histological localization of three mRNAs encoding visual pigment opsins in the retina of the butterfly Papilio xuthus. J. Exp. Biol. 201 (9), 1255–1261. [DOI] [PubMed] [Google Scholar]
- Knox BE, Salcedo E, Mathiesz K, Schaefer J, Chou WH, Chadwell LV, Smith WC, Britt SG, Barlow RB, 2003. Heterologous expression of limulus rhodopsin. J. Biol. Chem. 278 (42), 40493–40502. 10.1074/jbc.M304567200. [DOI] [PubMed] [Google Scholar]
- Kojima D, Terakita A, Ishikawa T, Tsukahara Y, Maeda A, Shichida Y, 1997. A novel G(o)-mediated phototransduction cascade in scallop visual cells. J. Biol. Chem. 272 (37), 22979–22982. 10.1074/jbc.272.37.22979. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Nagata T, Katoh K, Yamashita S, Tokunaga F, 2008a. Molecular evolution of arthropod color vision deduced from multiple opsin genes of jumping spiders. J. Mol. Evol. 66 (2), 130–137. 10.1007/s00239-008-9065-9. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Takano K, Tsukamoto H, Ohtsu K, Tokunaga F, Terakita A, 2008b. Jellyfish vision starts with cAMP signaling mediated by opsin-Gs cascade. Proc. Natl. Acad. Sci. U.S.A. 105 (40), 15576–15580. 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe T, Kusakabe R, Kawakami I, Satou Y, Satoh N, Tsuda M, 2001. Ci-opsin1, a vertebrate-type opsin gene, expressed in the larval ocellus of the ascidian Ciona intestinalis. FEBS (Fed. Eur. Biochem. Soc.) Lett. 506 (1), 69–72. 10.1016/S0014-5793(01)02877-0. [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Tsuda M, 2007. Photoreceptive systems in ascidians. Photochem. Photobiol. 83 (2), 248–252. 10.1562/2006-07-11-ir-965. [DOI] [PubMed] [Google Scholar]
- Lacalli Thurston, C, 2004. Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain Behav Evol. 64 (3), 148–162. 10.1159/000079744. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Krasne FB, 1965. Annelid ciliary photoreceptors. Science 148 (3672), 965–966. 10.1126/science.148.3672.965. [DOI] [PubMed] [Google Scholar]
- Lesser MP, Carleton KL, Böttger SA, Barry TM, Walker CW, 2011. Sea urchin tube feet are photosensory organs that express a Rhabdomeric-like Opsin and PAX6. Proc. Biol. Sci. 278 (1723), 3371–3379. 10.1098/rspb.2011.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JS, MacNichol EF, 1982. Color vision in fishes. Sci. Am. 246 (2), 140–149. 10.1038/scientificamerican0282-140. [DOI] [Google Scholar]
- Liegertová M, Pergner J, Kozmiková I, Fabian P, Pombinho AR, Strnad H, Pačes J, Vlček Č, Bartůněk P, Kozmik Z, 2015. Cubozoan genome illuminates functional diversification of opsins and photoreceptor evolution. Sci. Rep. 5 (1), 1–19. 10.1038/srep11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liénard MA, Bernard GD, Allen A, Lassance JM, Song S, Childers RR, Yu N, Ye D, Stephenson A, Valencia-Montoya WA, Salzman S, Whitaker MRL, Calonje M, Zhang F, Pierce NE, 2021. The evolution of red color vision is linked to coordinated rhodopsin tuning in lycaenid butterflies. Proc. Natl. Acad. Sci. U.S.A. 118 (6), e2008986118. 10.1073/pnas.2008986118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MLM, Li J, Li D, 2008. Effect of UV-reflecting markings on female mate-choice decisions in Cosmophasis umbratica, a jumping spider from Singapore. Behav. Ecol. 19 (1), 61–66. 10.1093/beheco/arm100. [DOI] [Google Scholar]
- Liu H, 2021. Biology and ecology of the northern walkingstick, Diapheromera femorata (say) (phasmatodea: diapheromerinae): a review. J. Appl. Entomol. 145 (7), 635–647. 10.1111/jen.12902. [DOI] [Google Scholar]
- Lord NP, Plimpton RL, Sharkey CR, Suvorov A, Lelito JP, Willardson BM, Bybee SM, 2016. A cure for the blues: opsin duplication and subfunctionalization for short-wavelength sensitivity in jewel beetles (Coleoptera: buprestidae). BMC Evol. Biol. 16 (1), 1–17. 10.1186/s12862-016-0674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchiari AC, Marques AO, Freire FAM, 2012. Effects of substrate colour preference on growth of the shrimp litopenaeus vannamei (BOONE, 1931) (Decapoda, Penaeoidea). Crustaceana 85 (7), 789–800. 10.1163/156854012X650232. [DOI] [Google Scholar]
- Martínez-Harms J, Vorobyev M, Schorn J, Shmida A, Keasar T, Homberg U, Schmeling F, Menzel R, 2012. Evidence of red sensitive photoreceptors in Pygopleurus israelitus (Glaphyridae: Coleoptera) and its implications for beetle pollination in the southeast Mediterranean. J. Comp. Physiol. 198 (6), 451–463. 10.1007/s00359-012-0722-5. [DOI] [PubMed] [Google Scholar]
- McCulloch KJ, Osorio D, Briscoe AD, 2016. Sexual dimorphism in the compound eye of Heliconius erato: a nymphalid butterfly with at least five spectral classes of photoreceptor. J. Exp. Biol. 219 (15), 2377–2387. 10.1242/jeb.136523. [DOI] [PubMed] [Google Scholar]
- McCulloch KJ, Yuan F, Zhen Y, Aardema ML, Smith G, Llorente-Bousquets J, Andolfatto P, Briscoe AD, 2017. Sexual dimorphism and retinal mosaic diversification following the evolution of a violet receptor in butterflies. Mol. Biol. Evol. 34 (9), 2271–2284. 10.1093/molbev/msx163. [DOI] [PubMed] [Google Scholar]
- McReynolds JS, Gorman ALF, 1970. Photoreceptor potentials of opposite polarity in the eye of the scallop, Pecten irradians. J. Gen. Physiol. 56 (3), 376–391. 10.1085/jgp.56.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R, Blakers M, 1976. Colour receptors in the bee eye - morphology and spectral sensitivity. J. Comp. Physiol. 108 (1), 11–13. 10.1007/BF00625437. [DOI] [Google Scholar]
- Mismer D, Michael WM, Laverty TR, Rubin GM, 1988. Analysis of the promoter of the Rh2 opsin gene in Drosophila melanogaster. Genetics 120, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C, Frandsen PB, Ware J, Flouri T, Beutel RG, Niehuis O, Petersen M, Izquierdo-Carrasco F, Wappler T, Rust J, Aberer AJ, Aspöck U, Aspöck H, Bartel D, et al. , 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346 (6210), 763–767. 10.1126/science.1257570. [DOI] [PubMed] [Google Scholar]
- Möller R, 2002. Insects could exploit UV-green contrast for landmark navigation. J. Theor. Biol. 214 (4), 619–631. 10.1006/jtbi.2001.2484. [DOI] [PubMed] [Google Scholar]
- Morehouse NI, Buschbeck EK, Zurek DB, Steck M, Porter ML, 2017. Molecular evolution of spider vision: new opportunities, familiar players. Biol. Bull. 233, 21–38. 10.1086/693977. [DOI] [PubMed] [Google Scholar]
- Nagata T, Koyanagi M, Tsukamoto H, Saeki S, Isono K, Shichida Y, Tokunaga F, Kinoshita M, Arikawa K, Terakita A, 2012. Depth perception from image defocus in a jumping spider. Science 335, 469–471. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Miyamoto T, Ohkuma M, Tsuda M, 1999. Action spectrum for the photophobic response of Ciona intestinalis (Ascidieacea, Urochordata) larvae implicates retinal protein. Photochem. Photobiol. 70 (3), 359–362. 10.1111/j.1751-1097.1999.tb08149.x. [DOI] [PubMed] [Google Scholar]
- Narendra A, Ramirez-Esquivel F, Ribi WA, 2016. Compound eye and ocellar structure for walking and flying modes of locomotion in the Australian ant, Camponotus consobrinus. Sci. Rep. 6, 1–10. 10.1038/srep22331. November 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal S, De Jong DM, Seaver EC, 2019. CRISPR/CAS9 mutagenesis of a single r-opsin gene blocks phototaxis in a marine larva. Proc. Biol. Sci. (1904), 286. 10.1098/rspb.2018.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson DE, 1994. Eyes as optical alarm systems in fan worms and ark clams. Phil. Trans. Biol. Sci. 346 (1316), 195–212. 10.1098/rstb.1994.0141. [DOI] [Google Scholar]
- O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML, 1985. The Drosophila ninaE gene encodes an opsin. Cell 40 (4), 839–850. 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T, 2005. Illumination of the melanopsin signaling pathway. Science 307 (5709), 600–604. 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- Pantzartzi CN, Pergner J, Kozmikova I, Kozmik Z, 2017. The opsin repertoire of the European lancelet: a window into light detection in a basal chordate. Int. J. Dev. Biol. 61 (10–12), 763–772. 10.1387/ijdb.170139zk. [DOI] [PubMed] [Google Scholar]
- Passamaneck YJ, Furchheim N, Hejnol A, Martindale MQ, Lüter C, 2011. Ciliary photoreceptors in the cerebral eyes of a protostome larva. EvoDevo 2 (1), 1–18. 10.1186/2041-9139-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachetzki DC, Degnan BM, Oakley TH, 2007. The origins of novel protein interactions during animal opsin evolution. PLoS One 2 (10), 1–9. 10.1371/journal.pone.0001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachetzki DC, Fong CR, Oakley TH, 2010. The evolution of phototransduction from an ancestral cyclic nucleotide gated pathway. Proc. Biol. Sci. 277 (1690), 1963–1969. 10.1098/rspb.2009.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JA, Benzer S, 1988. Transcript localization of four opsin genes in the three visual organs of Drosophila; RH2 is ocellus specific. Nature 333 (6175), 779–782. 10.1038/333779a0. [DOI] [PubMed] [Google Scholar]
- Porter ML, Awata H, Bok MJ, Cronin TW, 2020. Exceptional diversity of opsin expression patterns in Neogonodactylus oerstedii (Stomatopoda) retinas. Proc. Natl. Acad. Sci. U.S.A. 117 (16), 8948–8957. 10.1073/pnas.1917303117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW, Robinson PR, 2012. Shedding new light on opsin evolution. Proc. Biol. Sci. 279 (1726), 3–14. 10.1098/rspb.2011.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ML, Bok MJ, Robinson PR, Cronin TW, 2009. Molecular diversity of visual pigments in Stomatopoda (Crustacea). Vis. Neurosci. 26 (3), 255–265. 10.1017/S0952523809090129. [DOI] [PubMed] [Google Scholar]
- Porter ML, Cronin TW, McClellan DA, Crandall KA, 2007. Molecular characterization of crustacean visual pigments and the evolution of pancrustacean opsins. Mol. Biol. Evol. 24 (1), 253–268. 10.1093/molbev/msl152. [DOI] [PubMed] [Google Scholar]
- Porter ML, Speiser DI, Zaharoff AK, Caldwell RL, Cronin TW, Oakley TH, 2013. The evolution of complexity in the visual systems of stomatopods: insights from transcriptomics. Integr. Comp. Biol. 53 (1), 39–49. 10.1093/icb/ict060. [DOI] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Pär Hayes W, Rollag MD, 1998. Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. U.S.A. 95 (1), 340–345. 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible F, Tessmar-Raible K, Arboleda E, Kaller T, Bork P, Arendt D, Arnone MI, 2006. Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 300 (1), 461–475. 10.1016/j.ydbio.2006.08.070. [DOI] [PubMed] [Google Scholar]
- Rajkumar P, Rollmann SM, Cook TA, Layne JE, 2010. Molecular evidence for color discrimination in the Atlantic sand fiddler crab, Uca pugilator. J. Exp. Biol. 213 (24), 4240–4248. 10.1242/jeb.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MD, Pairett AN, Pankey MS, Serb JM, Speiser DI, Swafford AJ, Oakley TH, 2016. The last common ancestor of most bilaterian animals possessed at least nine opsins. Genome Biol. Evol. 8 (12), 3640–3652. 10.1093/gbe/evw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randel N, Bezares-Calderón LA, Gühmann M, Shahidi R, Jékely G, 2013. Expression dynamics and protein localization of rhabdomeric opsins in platynereis larvae. Integr. Comp. Biol. 53 (1), 7–16. 10.1093/icb/ict046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randel N, Jékely G, 2016. Phototaxis and the origin of visual eyes. Phil. Trans. Biol. Sci. 371 (1685). 10.1098/rstb.2015.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson KA, Lapraz F, Ballister ER, Terasaki M, Rodgers J, McDowell RJ, Girstmair J, Criswell KE, Boldogkoi M, Simpson F, Goulding D, Cormie C, Hall B, Lucas RJ, Telford MJ, 2019. Extraocular, rod-like photoreceptors in a flatworm express xenopsin photopigment. Elife 8. 10.7554/eLife.45465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Hisatomi O, Tokunaga F, Eguchi E, 1996. Two opsins from the compound eye of the crab Hemigrapsus sanguineus. J. Exp. Biol. 199 (2), 441–450. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Farrell DM, Zheng L, Phistry M, Bagg EE, Britt SG, 2009. The green-absorbing Drosophila Rh6 visual pigment contains a blue-shifting amino acid substitution that is conserved in vertebrates. J. Biol. Chem. 284 (9), 5717–5722. 10.1074/jbc.M807368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo E, Huber A, Henrich S, Chadwell LV, Chou WH, Paulsen R, Britt SG, 1999. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell- specific Rh5 and Rh6 rhodopsins. J. Neurosci. 19 (24), 10716–10726. 10.1523/jneurosci.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo E, Zheng L, Phistry M, Bagg EE, Britt SG, 2003. Molecular basis for ultraviolet vision in invertebrates. J. Neurosci. 23 (34), 10873–10878. 10.1523/jneurosci.23-34-10873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Albert L, Wystrach A, Cheng K, 2011a. Ocelli contribute to the encoding of celestial compass information in the Australian desert ant melophorus bagoti. J. Exp. Biol. 214 (6), 901–906. 10.1242/jeb.049262. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Wystrach A, Cheng K, 2011b. A new navigational mechanism mediated by ant ocelli. Biol. Lett. 7 (6), 856–858. 10.1098/rsbl.2011.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwind R, 1991. Polarization vision in water insects and insects living on a moist substrate. J. Comp. Physiol. Sensory Neural Behav. Physiol. 169 (5), 531–540. 10.1007/BF00193544. [DOI] [Google Scholar]
- Serb JM, Porath-Krause AJ, Pairett AN, 2013. Uncovering a gene duplication of the photoreceptive protein, opsin, in scallops (bivalvia: pectinidae). Integr. Comp. Biol. 53 (1), 68–77. 10.1093/icb/ict063. [DOI] [PubMed] [Google Scholar]
- Sharkey CR, Fujimoto MS, Lord NP, Shin S, McKenna DD, Suvorov A, Martin GJ, Bybee SM, 2017. Overcoming the loss of blue sensitivity through opsin duplication in the largest animal group, beetles. Sci. Rep. 7 (1), 1–10. 10.1038/s41598-017-00061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichida Y, Matsuyama T, 2009. Evolution of opsins and phototransduction. Phil. Trans. Biol. Sci. 364 (1531), 2881–2895. 10.1098/rstb.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sison-Mangus MP, Bernard GD, Lampel J, Briscoe AD, 2006. Beauty in the eye of the beholder: the two blue opsins of lycaenid butterflies and the opsin gene-driven evolution of sexually dimorphic eyes. J. Exp. Biol. 209 (16), 3079–3090. 10.1242/jeb.02360. [DOI] [PubMed] [Google Scholar]
- Smith KC, Macagno ER, 1990. UV photoreceptors in the compound eye of Daphnia magna (Crustacea, Branchiopoda). A fourth spectral class in single ommatidia. J. Comp. Physiol. 166 (5), 597–606. 10.1007/BF00240009. [DOI] [PubMed] [Google Scholar]
- Sondhi Y, Ellis EA, Bybee SM, Theobald JC, Kawahara AY, 2021. Light environment drives evolution of color vision genes in butterflies and moths. Commun. Biol. 4 (1), 1–11. 10.1038/s42003-021-01688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni BG, Foster RG, 1997. A novel and ancient vertebrate opsin. FEBS (Fed. Eur. Biochem. Soc.) Lett. 406 (3), 279–283. 10.1016/S0014-5793(97)00287-1. [DOI] [PubMed] [Google Scholar]
- Soni BG, Philp AR, Foster RG, Knox BE, 1998. Novel retinal photoreceptors [3]. Nature 394 (6688), 27–28. 10.1038/27794. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Sontag C, 1971. Spectral sensitivity studies on the visual system of the praying mantis, Tenodera sinensis. J. Gen. Physiol. 57 (1), 93–112. 10.1085/jgp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser DI, Loew ER, Johnsen S, 2011. Spectral sensitivity of the concave mirror eyes of scallops: potential influences of habitat, self-screening and longitudinal chromatic aberration. J. Exp. Biol. 214 (3), 422–431. 10.1242/jeb.048108. [DOI] [PubMed] [Google Scholar]
- Suga H, Schmid V, Gehring WJ, 2008. Evolution and functional diversity of jellyfish opsins. Curr. Biol. 18 (1), 51–55. 10.1016/j.cub.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Ebrey TG, 2003. Molecular basis of spectral tuning in the newt short wavelength sensitive visual pigment. Biochemistry 42 (20), 6025–6034. 10.1021/bi020629+. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Ribi W, Bech M, Bodey AJ, Rau C, Steuwer A, Warrant EJ, Baird E, 2016. The dual function of orchid bee ocelli as revealed by X-ray microtomography. Curr. Biol. 26 (10), 1319–1324. 10.1016/j.cub.2016.03.038. [DOI] [PubMed] [Google Scholar]
- Terakita A, 2005. The opsins. Genome Biol. 6 (3), 1–9. 10.1186/gb-2005-6-3-213. BioMed Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen HH, How MJ, Chiou TH, Marshall J, 2014. A different form of color vision in mantis shrimp. Science 343 (6169), 411–413. 10.1126/science.1245824. [DOI] [PubMed] [Google Scholar]
- Timmermans MJTN, Lees DC, Simonsen TJ, 2014. Towards a mitogenomic phylogeny of Lepidoptera. Mol. Phylogenet. Evol. 79 (1), 169–178. 10.1016/j.ympev.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Terakita A, 2010. Diversity and functional properties of bistable pigments. Photochem. Photobiol. Sci. 9 (11), 1435–1443. 10.1039/c0pp00168f. [DOI] [PubMed] [Google Scholar]
- Tsukida K, Masahara R, Ito M, 1980. High-performance liquid chromatographic analysis of cis-trans stereoisomeric 3-dehydroretinals in the presence of retinal isomers. J. Chromatogr. A 192 (2), 395–401. 10.1016/S0021-9673(80)80014-8. [DOI] [Google Scholar]
- Ullrich-Lüter EM, D’Aniello S, Arnone MI, 2013. C-opsin expressing photoreceptors in echinoderms. Integr. Comp. Biol. 53 (1), 27–38. 10.1093/icb/ict050. [DOI] [PubMed] [Google Scholar]
- Ullrich-Lüter EM, Dupont S, Arboleda E, Hausen H, Arnone MI, 2011. Unique system of photoreceptors in sea urchin tube feet. Proc. Natl. Acad. Sci. U.S.A. 108 (20), 8367–8372. 10.1073/pnas.1018495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JW, Collins AG, 2000. The significance of moulting in Ecdysozoan evolution. Evol. Dev. 2 (3), 152–156. 10.1046/j.1525-142X.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- Vas J, Ábrahám L, Markó V, 1999. Study of nocturnal and diurnal activities of lacewings (Neuropteroidea: Raphidioptera, Neuroptera) by suction trap. Acta Phytopathol. Entomol. Hung. 34 (1–2), 149–152. [Google Scholar]
- Vöcking O, Kourtesis I, Tumu SC, Hausen H, 2017. Co-expression of xenopsin and rhabdomeric opsin in photoreceptors bearing microvilli and cilia. Elife 6, 1–26. 10.7554/eLife.23435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakakuwa M, Terakita A, Koyanagi M, Stavenga DG, Shichida Y, Arikawa K, 2010. Evolution and mechanism of spectral tuning of Blue-absorbing visual pigments in butterflies. PLoS One 5 (11), e15015. 10.1371/journal.pone.0015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G, 1968. Single and multiple visual systems in arthropods. J. Gen. Physiol. 51 (2), 125–156. 10.1085/jgp.51.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walla P, Barth FG, Eguchi E, 1996. Spectral sensitivity of single photoreceptor cells in the eyes of the ctenid spider cupiennius salei keys. Zool. Sci. 13 (1), 199–202. 10.2108/zsj.13.199. [DOI] [Google Scholar]
- Wang B, Xiao J-H, Bian S-N, Niu L-M, Murphy RW, Huang D-W, 2013. Evolution and expression plasticity of opsin genes in a fig pollinator, ceratosolen solmsi. PLoS One 8 (1), e53907. 10.1371/journal.pone.0053907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Yoshida M, 1986. Morphological and histochemical studies on Joseph cells of amphioxus, Branchiostoma belcheri Gray. Exp. Biol. 46 (2), 67–73. https://europepmc.org/article/med/3817116. [PubMed] [Google Scholar]
- Wolfe JM, Breinholt JW, Crandall KA, Lemmon AR, Lemmon EM, Timm LE, Siddall ME, Bracken-Grissom HD, 2019. A phylogenomic framework, evolutionary timeline and genomic resources for comparative studies of decapod crustaceans. Proc. Biol. Sci. 286 (1901). 10.1098/rspb.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DR, Pytel AJ, Houghton DC, 2013. Nocturnal flight periodicity of the caddisflies (Insecta: Trichoptera) in a large Michigan river. J. Freshw. Ecol. 28 (4), 463–476. 10.1080/02705060.2013.780187. [DOI] [Google Scholar]
- Wunderer H, Jan De Kramer J, 1989. Dorsal ocelli and light-induced diurnal activity patterns in the arctiid moth Creatonotos transiens. J. Insect Physiol. 35 (2), 87–95. 10.1016/0022-1910(89)90041-3. [DOI] [Google Scholar]
- Yamaguchi S, Wolf R, Desplan C, Heisenberg M, 2008. Motion vision is independent of color in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 105 (12), 4910–4915. 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Blair D, Wolinska J, Ma X, Yang W, Hu W, Yin M, 2021. Genomic regions associated with adaptation to predation in Daphnia often include members of expanded gene families. Proc. Biol. Sci. 288 (1955). 10.1098/rspb.2021.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yuan J, Sun Y, Li S, Gao Y, Yu Y, Liu C, Wang Q, Lv X, Zhang X, Ma KY, Wang X, Lin W, Wang L, Zhu X, Zhang C, Zhang J, Jin S, Yu K, et al. , 2019. Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat. Commun. 10 (1), 1–14. 10.1038/s41467-018-08197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek DB, Cronin TW, Taylor LA, Byrne K, Sullivan MLG, Morehouse NI, 2015. Spectral filtering enables trichromatic vision in colorful jumping spiders. Curr. Biol. 25 (10), R403–R404. 10.1016/j.cub.2015.03.033. Cell Press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.


