Abstract
Childhood obesity is one of the biggest public health challenges globally. It is associated with various adverse health consequences throughout life. Prevention and early intervention represent the most reasonable and cost‐effective approaches. Considerable progress has been achieved in the management of obesity in children and adolescents; yet, implementation in the real world remains a challenge. This article aimed to present an overview of the diagnosis and management of obesity in children and adolescents.
Keywords: adolescent, children, diagnosis, management, obesity
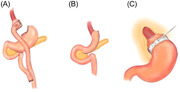
Drawings of three operative procedures in bariatric surgery are presented: (A) Roux‐en‐Y gastric bypass; (B) sleeve gastrectomy; and (C) adjustable gastric band, all of which are normally performed laparoscopically.
Key points
Childhood obesity is one of the biggest public health challenges globally. It is associated with various adverse health consequences throughout life.
The diagnosis of childhood obesity is different from the diagnosis of obesity in adults, and the definition of obesity should be based on age‐ and sex‐specific cutoffs.
Obesity in children is mostly due to prolonged positive energy balance with no underlying pathological cause. However, it is important to identify those with pathological or genetic causes of obesity, as the treatment strategies are different.
While lifestyle and behavior modifications with dietary changes, increased physical activity, and adequate sleep are integral parts of obesity management, the efficacy and sustainability are limited. With emerging evidence on new pharmacological agents, metabolic and bariatric surgery, and fecal microbiota transplant recently, it is hoped that the management of obesity in children and adolescents could be improved in the future.
1. INTRODUCTION
Childhood obesity is one of the biggest public health challenges worldwide. Globally, the age‐standardized prevalence of obesity in children and adolescents aged 5–19 years has increased dramatically in the past four decades: from 4% in 1975 to over 18% in 2016. 1 , 2 With the recent COVID‐19 pandemic and the associated national policies with school closures, lockdowns, and lack of physical activities, the prevalence of childhood obesity has further increased in many parts of the world. 3 , 4 Since obesity is associated with various adverse health consequences throughout life, including type 2 diabetes (T2D), dyslipidemia, and cardiovascular diseases, prevention and early intervention represent the most reasonable and cost‐effective approaches. 5
Childhood obesity has been shown to remain through adulthood with a low rate of spontaneous remission, especially for severely obese children. 6 , 7 , 8 The cumulative effect of increased adiposity spans through the life course, and it has been shown in large birth cohorts that childhood obesity is associated with impaired glucose metabolism and increased carotid intima‐media thickness in adulthood 8 , 9 On the other hand, it has been shown in large‐scale longitudinal studies that adverse effects of childhood obesity on future risk of T2D could be reversed with normalization of body mass index (BMI) before adulthood. 8 , 10 As a result, vigorous management of childhood obesity should be the logical upstream approach to prevent obesity‐related adverse health outcomes in adulthood.
With this background, this article aimed to present an overview of the diagnosis and management of obesity in children and adolescents. In this review, childhood obesity is defined as BMI ≥ 95th percentile for sex and age among children aged less than 19 years.
2. MECHANISMS AND PATHOPHYSIOLOGY OF OBESITY IN CHILDREN AND ADOLESCENTS
The fundamental cause of obesity is a chronically positive energy balance between calories consumed and calories expended, which leads to increased adiposity. Genetics, obesogenic environments, and socioeconomic factors also contribute to the development of obesity. Adipose tissue, as an endocrine organ, is metabolically active and can produce various adipokines and hormones, like, leptin and adiponectin. 11 Adipose tissue is located in multiple sites of our body. Among all the sites, visceral fat is most metabolically active and, if present in excess, could result in increased insulin resistance, T2D, dyslipidemia, and hypertension through the release of free fatty acids and various inflammatory adipokines. 12 , 13 Therefore, the management strategy for childhood obesity should focus on combating the chronically positive energy imbalance and decreasing visceral adiposity.
In addition to a chronically positive energy imbalance, other mechanisms also contribute to childhood obesity. Maternal gestational diabetes has been shown to be associated with childhood obesity in the offspring. 14 This could be partly attributed to shared genetics and shared family lifestyles and environments, but also fetal programming. In utero exposure to maternal hyperglycemia could result in fetal hyperinsulinemia, which, in turn, predisposes children to development of increased adiposity, insulin resistance, and impaired glucose tolerance. 15 Therefore, prevention of gestational diabetes and maternal obesity, preferably starting from preconception, is another important strategy to prevent childhood obesity. 16 At the same time, there is evidence to show that breastfeeding could help in protecting against adverse fat partitioning and higher triglyceride concentrations among children exposed to maternal hyperglycemia in utero. 17 Hence, promotion of breastfeeding for this at‐risk group might modify the risk of development of childhood obesity.
Besides fetal programming during pregnancy, the transgenerational effects of parental nutritional status in early life on the offspring should not be undermined. In a recent longitudinal study, it has been shown that maternal exposure to famine in early life was associated with obesity in their children. This highlights the developmental origins of chronic diseases, in which there is a critical developmental window, when nutritional deficiency could adversely affect the development of diseases in later life and also in their offspring. 18
On the other hand, sleep deprivation has been shown to be associated with obesity in children. 19 , 20 The phenomenon may be related to dysregulated secretion of insulin and various appetite‐related hormones, for example, ghrelin and leptin. 21 In addition, there are also psychological determinants of childhood obesity, and chronic stress and alterations in cortisol secretion might also play a major role in the development and maintenance of excessive body weight. Factors including the parent–child relationship, specific family dynamics, and certain body image distortions have all been described to contribute to the maintenance of obesity. 22 , 23 Understanding the underlying pathophysiology in childhood obesity could provide clues on the most effective strategies to prevent and combat the problem.
3. DIAGNOSIS OF OBESITY IN CHILDREN AND ADOLESCENTS
Since both height and body composition are constantly changing in children and adolescents, the definition of obesity should be based on age‐ and sex‐specific cutoffs. In the past, different definitions based on weight for height and skinfold thickness had been used. In recent years, sex‐ and age‐specific weight for height or BMI percentiles have been widely used to define childhood obesity. However, there is still a lack of a global standard definition of obesity in children. The Centers for Disease Control and Prevention and the Endocrine Society defined obesity as a BMI of ≥95th percentile for age and sex, 24 , 25 while the World Health Organization (WHO) defined obesity using the weight‐for‐height centile—for children under the age of 5 years, obesity is defined as weight for height ≥3 standard deviations above the WHO Child Growth Standards median and for children aged 5–19 years, obesity is defined as ≥2 standard deviations above the WHO Growth Reference median. 26 On the other hand, the International Obesity Task Force developed cutoff values of BMI by age and sex that passed the adult cutoff values of BMI 25.0 (overweight), 30.0 (obesity), and 18.5, 17.0, and 16.0 (thinness grades 1, 2, and 3) at age 18 years. These are based on the BMI data from six countries, namely, the United Kingdom, the United States, the Netherlands, Brazil, Singapore, and Hong Kong of China. The curves were then averaged across countries by age to construct sex‐specific curves for each cutoff. The cutoff for obesity is based on the country‐averaged centile corresponding to the BMI of 30.0 (99.6th centile) at age 18 years. 27
4. CLINICAL ASSESSMENT AND DIFFERENTIAL DIAGNOSIS ON THE UNDERLYING ETIOLOGY OF CHILDHOOD OBESITY
A detailed assessment of history and physical examination are needed to assess the cause of obesity, its related comorbidities, and future risk of complications. A thorough assessment of the history of perinatal course, diet, exercise, screen time, sleeping habits, and psychosocial history should also be performed to facilitate subsequent discussion on lifestyle modifications. These are summarized in Table 1.
Table 1.
Key elements on assessment of history and physical examination in children and adolescents with obesity.
| History |
| General history |
| ‐Gestation and birth weight |
| ‐History of gestational diabetes |
| ‐Past medical history (history of developmental delay, visual, or hearing impairment might be hints of an underlying genetic obesity syndrome) and history of drug use |
| Growth history |
| ‐Onset of obesity and potential triggering factors identified by parents or child |
| ‐Previous growth parameters, height velocity, and pubertal history |
| Complication screening |
| ‐Specific symptoms |
| Polyuria, polydipsia, and nocturia |
| Hip and knee pain |
| Snoring, daytime lethargy, and early morning headache |
| Headache and visual disturbance (potential pseudotumor cerebri) |
| Acne, hirsutism, and menstrual history (for girls) |
| ‐Psychological effects of obesity |
| ‐Exercise tolerance |
| Family history |
| ‐Family members with the following conditions: |
| Obesity, type 2 diabetes or gestational diabetes, hypertension, fatty liver, dyslipidemia, and other cardiovascular diseases |
| Polycystic ovarian syndrome |
| ‐Home and neighborhood environment |
| ‐Parents' occupation and supervision |
| ‐Household members and relationship |
| ‐Family attitude and commitment toward obesity treatment |
| Lifestyle factors |
| ‐Diet |
| Breakfast consumption |
| Sugary beverage consumption and eating habits, for example, snacking, fast food intake, binge eating |
| 3‐day food diary |
| ‐Physical activity |
| Sports participation |
| 7‐day exercise diary |
| ‐Sedentary behavior |
| Screen time |
| Usual schedule for homework, tutorial classes, and other nonexercise extracurricular activities |
| ‐Sleep routine |
| Physical exam |
| General |
| ‐Anthropometric measurements including waist circumference or waist–hip ratio |
| ‐Blood pressure |
| ‐Pubertal staging |
| ‐Skin |
| Acanthosis nigricans at the neck, axilla, and skin folds |
| Xanthelesmas |
| Acne and hirsutism |
| Striae |
| ‐Short stature, dysmorphism, developmental delay (might be hints of an underlying genetic syndrome) |
| ‐Head and neck |
| Tonsillar size |
| ‐Overall mood and affect |
| Abdomen: Hepatomegaly |
| Endocrine |
| ‐Goiter |
| ‐Signs of Cushing syndrome, including extensive striae, buffalo hump, and plethoric face |
| Neurological: Papilledema and reduced venous pulsations on fundi exam (pseudotumor cerebri) |
| Musculoskeletal |
| ‐Bow legs (Blount disease) |
| ‐Pain in the groin, thigh, or knee area (slipped capital femoral epiphysis) |
Obesity in children is mostly due to prolonged positive energy balance with no underlying pathological cause. However, it is important to identify those with pathological or genetic causes of obesity, as the treatment strategies are different.
One of the key features of endocrine obesity, including hypothyroidism or Cushing syndrome, is stunted height velocity. In other words, a normal or even increased height velocity usually excludes endocrine causes of obesity. 24 One exception is pseudohypoparathyroidism, which could be associated with increased height velocity in early childhood, followed by subsequent stunted height gain and hence short stature at or after the pubertal phase. Therefore, it is generally not recommended to investigate for endocrine cause of obesity unless the child has stunted growth against the background of obesity or ongoing weight gain.
Genetic obesity syndromes are typically associated with extreme early onset of obesity (<5 years), extreme hyperphagia, developmental delay, unique dysmorphism (e.g., characteristic faces in Prader Willi syndrome; syndactyly, brachydactyly, or polydactyly in Bardet–Biedl syndrome [BBS]), or sometimes, visual problems (e.g., retinal dystrophy in BBS, Alstrom syndrome, and TUB deficiency) or hearing impairment (e.g., TUB deficiency). 28 If these clinical features are present, referral to geneticists should be initiated.
Hypothalamic obesity occurs after insult to the medial hypothalamic region, which is critical for satiety regulation and energy balance through neural and humoral connections. It is mostly observed in patients with a history of craniopharyngioma, and sometimes, suprasellar tumors, trauma, or surgical procedures at the hypothalamus. Patients typically have a loss of satiety and a reduced metabolic rate. 29 Therefore, the treatment strategy would be different from that for children with simple obesity.
5. SCREENING OF OBESITY‐ASSOCIATED COMORBIDITIES
Obesity in children and adolescents is associated with various medical and psychological comorbidities, including prediabetes/T2D, prehypertension/hypertension, obstructive sleep apnea, dyslipidemia, fatty liver, polycystic ovarian syndrome, or even early subclinical atherosclerosis. There is also evidence that obesity persists from childhood to adulthood and this persistent excess in adiposity can increase the risk of T2D, hypertension, dyslipidemia, and carotid‐artery atherosclerosis in adulthood. Importantly, the risks of these adverse outcomes among individuals who were obese in childhood and became nonobese by adulthood could be completely reverted to similar outcomes to those who were never obese. 8 , 30 This “reversibility of risk” stresses the importance of early detection and appropriate intervention in the childhood period.
On the other hand, various psychological comorbidities, such as depression, low self‐esteem, and emotional and behavioral disorders, have been reported to be prevalent among obese adolescents. 31 It remains unclear whether these psychological comorbidities are the cause or the consequence of obesity, or they all originated from common susceptible factors that promote both weight gain and psychological disturbances and/or further aggravated because of stigmatization and bullying related to obesity. 31 , 32 , 33 Nonetheless, regular screening with early interventions is essential. The suggested screening tests and assessments are listed in Table 2.
Table 2.
Suggested screening for comorbidities in obese children and adolescents.
| Conditions | Suggested assessment |
|---|---|
| Prediabetes and diabetes mellitus | Urine for glucose |
| HbA1c | |
| Oral glucose tolerance test | |
| Dyslipidemia | Fasting lipid profile |
| Prehypertension and hypertension | Blood pressure standardized according to sex, age, and height percentile |
| Nonalcoholic fatty liver | Liver function test (AST/ALT) |
| Hepatobiliary system ultrasound | |
| Obstructive sleep apnea | Clinical history |
| If there is a positive history, referral to a respirologist should be made for overnight oximetry or polysomnography | |
| Mental health issues | Clinical history, if positive, should lead to referral to a clinical psychologist or psychiatrist |
| Consider use of validated screening tools, for example, PHQ2 for depression in a busy clinic setting | |
| Polycystic ovarian syndrome | Clinical history (oligomenorrhea) |
| Physical exam (hirsutism) | |
| Pelvic ultrasound (look for polycystic ovarian morphology) | |
| Testosterone and luteinizing hormone (LH) |
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; HbA1c, hemoglobin A1c; PHQ2, Patient Health Questionnaire 2.
6. TREATMENT OF CHILDHOOD OBESITY
The aim of obesity treatment is to ameliorate the chronically positive energy balance. Before a detailed discussion of specific obesity treatment, an agreeable goal should be set with the child/adolescent, parents, and health care worker. In general, a BMI reduction of greater than 0.25 standard‐deviation score (SDS) (which is roughly equivalent to a BMI reduction of 1 kg/m2) is associated with a reduction in cardiovascular risk. 34 As a result, a BMI SDS reduction >0.25 is frequently defined as the criterion of “success” in various interventions for childhood obesity in the literature. However, in the real‐world practice, the exact goal should be individualized and should be specific, realistic, and achievable. It should also be developmentally appropriate and agreed to by the child/adolescent. Sometimes, the goal could be incremental in each clinic visit, with the initial goal not aiming at weight reduction, but merely a change in the weight gain trajectory or lifestyle modifications. Since most of our children are still in the growing phase, a static body weight would indeed translate to improvements in BMI and this might be a more attainable goal to them.
6.1. Lifestyle modifications
With limited licensed antiobesity medications in children and adolescents, obesity treatment in this group is usually achieved through lifestyle modifications. Another unique challenge in managing obesity in adolescents is that, neurocognitively, they are not yet fully mature as to make the best decision. However, they often demand a certain degree of autonomy on their lifestyle choices. For these adolescents to comply with recommendations, the rational of the interventions has to be explained thoroughly and their views and opinions must be respected.
Lifestyle modification is recommended as the first‐line treatment for obesity in children and adolescents. It generally consists of dietary restriction and exercise interventions, sometimes together with the use of behavioral modification techniques. These interventions are usually carried out in outpatient settings, but there is evidence to suggest more efficacious outcomes with in‐patient treatment programs. 35
6.1.1. The role of dietary interventions
Traditionally, caloric restriction is an important element in weight management with both children and adults. Various dietary protocols have been used in children and adolescents with obesity. The most used approach in clinical practice is the “traffic light approach,” in which foods are categorized according to caloric density to guide intake frequency. Foods labeled “green” are of low caloric density and can be consumed freely, high‐calorie foods are labeled “red” and should be eaten rarely, while foods labeled “yellow” are of moderate caloric density and should be eaten judiciously. This approach has been shown to be safe and modestly effective for weight reduction in obese children and adolescents. 36
The other approach is to alter the macronutrient composition. These include a “low‐fat” diet (<35% daily energy from fat), a low‐carbohydrate diet (<50–60 g of carbohydrate per day or <20% daily energy from carbohydrates), and a high‐protein diet (20%–40% daily energy from protein). For example, it has been shown in a recent pilot study that protein‐sparing modified fast, a low‐carbohydrate, high‐protein diet, resulted in clinically significant weight loss (mean weight loss of 3.7 kg, 5.5 kg, and 4.7 kg after 1, 3, and 6 months, respectively) in children and adolescents with severe obesity. 37 A systematic review comparing the effectiveness of these approaches in children and adolescents concluded that improvement in weight status can be achieved with a reduced‐energy diet, irrespective of the macronutrient distribution. 38 Similarly, a randomized clinical trial also compared the effectiveness of carbohydrate‐modified diets with a standard portion‐controlled diet in obese children, and it reported similar efficacy in weight reduction in both groups with caloric restrictions, though adherence to the low‐carbohydrate diet was reported to be an issue. 39 Therefore, the main goal of dietary interventions is to reduce the total energy intake, and the composition should be acceptable to children and adolescents to improve compliance.
Intermittent energy‐restricted diets have gained recent popularity as a weight‐reduction approach in adults and it has been reported in a recent systematic review to be of comparable efficacy to continuous energy restriction for short‐term weight loss in obese adults. 40 However, there is no evidence of its efficacy and safety in obese children and adolescents.
Alternatively, short‐term (4–8 weeks) intensive dietary manipulations with very low‐energy diets (<800 kcal/day) have been demonstrated to be safe and effective in inducing rapid weight loss in obese adolescents. 41 , 42 However, the long‐term outcome is unknown.
Time‐limited eating, due to its simplicity, may be a more practical approach for adolescents with obesity compared with other dietary intervention regimens. This has been shown in a recent small, pilot randomized‐controlled trial to be a feasible approach with a high adherence rate. However, no significant weight loss could be demonstrated. Further studies with larger sample sizes and longer intervention durations would be needed to prove its efficacy. 43
To conclude, none of these approaches has been shown to be superior to the other, and compliance with dietary interventions has been shown to be the most important predictor for weight reductions. 44 It should be noted that little is known about the long‐term safety of these macronutrient‐redistributed diets, intermittent‐fasting protocols, or very low‐energy diets. Since we are dealing with a group of growing individuals, all these special diets should be supervised and monitored intensively by experienced health care professionals. Their micronutrients and overall nutritional intake should be monitored strictly to prevent adverse effects on their growth and pubertal development. Extra attention to their psychological state is also essential to prevent the development of pathological eating habits.
6.1.2. The benefits of physical activity
Physical activity is an integral part of obesity management. In addition to contributing to energy deficit and weight control, it also gives rise to additional health benefits, including improvement in cardiometabolic parameters, improved body composition, physical fitness, and overall psychological well‐being. 45 , 46 A systematic review comparing the effects of a diet‐only intervention with those of a diet plus exercise program or exercise program only among overweight children found that those in the diet plus exercise program groups fared better in terms of greater improvement in fasting insulin and high‐density lipoprotein–cholesterol levels. 46
The general recommendation of “physical activity of at least 60 minutes of daily moderate to vigorous physical activity” for adolescents is usually not practical or achievable in real life. 47 , 48 The determinants of physical activity among youths were shown to be predicted by their attitude toward physical activity, parents' exercise habits, socioeconomic status, staff engagement, enabling school and neighborhood environment, and scheduling physical activity into daily programming. 49 , 50 On the other hand, poor physical fitness, long screen time, and busy schoolwork have been shown to adversely affect the overall activity level. 48 , 49
With all these barriers in mind, instead of merely providing general recommendations on physical activity to our adolescents, special exercise programs, best to be integrated into their busy daily routines, are needed to translate these recommendations into action.
In this digital era, electronic health (eHealth) interventions, for example, wearable activity monitors and smartphone apps, may promote adherence to regular physical activity in children and adolescents. It has been shown to be associated with moderate weight loss in middle/older‐aged adults, though its efficacy seems to be less convincing in children. Further studies are needed to assess this approach to be implemented in routine clinical practice. 51
6.1.3. The importance of sleep
Sleep deprivation results in changes in appetite‐related hormones, for example, orexin, ghrelin, leptin, and insulin secretion. 21 There is ample evidence to support the relationship between sleep deprivation, sleep problems, and childhood obesity, 52 , 53 , 54 and there is also evidence to suggest that it is not just the duration and the quality of sleep that matter; later sleep timings were also associated with obesogenic behaviors in children and would contribute to obesity. 55
Moreover, it has also been shown that sleep deprivation is linked to alterations in energy homeostasis, insulin resistance, and β‐cell function. This is supported by epidemiological cohort studies showing that short sleep duration is a risk factor for developing T2D. 56 Therefore, the importance of adequate sleep duration and healthy sleep patterns should be emphasized as part of lifestyle modifications.
6.2. Pharmacological treatment for childhood obesity
While lifestyle modifications with dietary control and physical activity are essential in the management of obesity in children and adolescents, only a modest effect on sustained weight loss is observed, especially in those with severe obesity. This highlights the need for additional evidence‐based treatments, including pharmacotherapy, before consideration of invasive procedures, such as bariatric surgery.
Currently, the only agent that is approved for use in adolescents aged 12–16 years is orlistat. It has been shown to decrease the BMI among adolescents by up to 1.9 kg/m2. 57 , 58 , 59 It works by decreasing gastrointestinal fat absorption by inhibiting of lipase. It must be taken with meals and is associated with major gastrointestinal side effects. Therefore, compliance among adolescents could be a major issue.
Metformin is not an approved drug for obesity treatment, but is approved for the treatment of T2D mellitus for children older than 10 years of age. Its use among obese, nondiabetic children has been shown to result in a modest improvement in BMI of 1.16 kg/m2 over 6–12 months. 60 Despite the very limited weight‐reduction efficacy, it is a relatively safe drug with mainly transient gastrointestinal disturbance. It is also sometimes used in combating the weight gain issue among adolescents taking atypical psychotropic drugs, 61 as well as improving insulin resistance in girls with polycystic ovarian syndrome. 62
In 2020, liraglutide, a glucagon‐like peptide‐1 receptor agonist (GLP‐1RA), was approved by the US Food and Drug Administration (FDA) for the treatment of adolescents with obesity aged 12 years or older. It delays gastric emptying, inhibits postmeal glucagon secretion, and decreases appetite. 63 It also stimulates glucose‐dependent insulin release from β‐cells, and could hence improve glycemic control in those with diabetes. In a randomized, double‐blind trial, the use of liraglutide (3 mg daily) with lifestyle modifications led to a significantly greater reduction in the BMI SDS of −0.22 than placebo. 64 In a recent meta‐analysis, liraglutide was shown to have a higher probability of leading to clinically significant weight loss compared with metformin, orlistat, exenatide, and topiramate. 65 Gastrointestinal adverse events are not uncommon, especially at a higher dose of 3 mg. C‐cell hyperplasia had been reported in animal studies and hence liraglutide is contraindicated in those with a personal or family history of multiple endocrine neoplasia type 2 or familial medullary thyroid carcinoma. 66
While liraglutide has been proven to be useful for obesity treatment in children, compliance with daily injections might an issue. The once‐weekly formulation of the GLP‐1RA, semaglutide, has been approved for obesity treatment in adults, but not yet in children. In a recent double‐blind, randomized, placebo‐controlled trial (Semaglutide Treatment Effect in People with Obesity [STEP] TEENS trial) on adolescents aged 12 to <18 years with obesity, it was shown that, with the use of once‐weekly semaglutide at 2.4 mg for 68 weeks, 73% of the participants in the semaglutide group could achieve a weight loss of more than 5%, as compared to only 18% in the placebo group. The main adverse effects were symptomatic gastrointestinal disturbance. 67 Therefore, it is likely that this drug will be approved for adolescents soon.
In 2022, the US FDA approved Qsymia (phentermine and topiramate extended‐release capsules) for children with obesity aged 12 years and older. 68 It acts as a norepinephrine and gamma‐aminobutyric acid agonist, as well as a glutamate antagonist, leading to suppression of appetite. 69 The approval was based on the positive results from a recent randomized, double‐blind, placebo‐controlled, parallel‐design phase 4 study (ClinicalTrials.gov Identifier: NCT03922945) that evaluated the efficacy and safety of Qsymia in 223 adolescents with obesity. It was shown that individuals taking Qsymia 7.5 mg/46 mg and Qsymia 15 mg/92 mg achieved, on average, 4.8% and 7.1% reduction in their BMI, respectively, while the placebo group gained an average of 3.3% in their BMI. Common adverse reactions included depression, dizziness, arthralgia, pyrexia, influenza, and ligament sprain. 70
On the other hand, there have been further advances in the management of monogenic obesity. Setmelanotide, an MC4R agonist, has been demonstrated to result in weight loss and reduced hunger sensation in individuals with severe obesity due to pro‐opiomelanocortin (POMC) deficiency obesity, leptin receptor (LEPR) deficiency obesity, and BBS. 71 , 72 , 73 Therefore, the US FDA has approved setmelanotide for chronic weight management in children aged 6 years or older with monogenic obesity due to POMC, proprotein convertase subtilisin/kexin type 1, LEPR deficiency, and BBS, which cause severe obesity that begins at an early age. 74
6.3. Metabolic and bariatric surgery (MBS)
With the successful experience in adults, MBS is now also an option for treatment of morbid obesity in adolescents. There is growing evidence on the benefits of MBS in achieving substantial and sustainable weight reduction as well as for remission of comorbidities. 75 , 76
To maximize the benefits and minimize the complications of MBS, optimal patient selection is critical. The selection criteria in adolescents were mainly extrapolated from the experience from adults, with consideration of the ongoing growth and developmental concerns related to age. In the past, pubertal development was included as a selection criterion for MBS. 77 However, the most recent guidelines recommend that neither pubertal development (Tanner stage) nor linear growth should be considered in patients' selection. Eligibility for adolescent MBS includes a BMI of ≥35 kg/m2 (or ≥120% of the 95th percentile) with a clinically significant comorbidity, including obstructive sleep apnea, T2D, idiopathic intracranial hypertension, nonalcoholic steatohepatitis, Blount's disease, slipped upper femoral epiphysis, gastroesophageal reflux disease and hypertension, or a BMI of ≥40 kg/m2 (or 140% of the 95th percentile). 78 Those with medically correctable causes of obesity, ongoing substance abuse, and current/planned pregnancy or those with medical, psychiatric, cognitive, or psychosocial conditions that would prevent adherence to postoperative dietary or medication regimes are contradicted in MBS. 78 Informed consent from the legal guardian as well as consent from the adolescent undergoing MBS is required. Therefore, both the guardian and the adolescent should be informed of the risks and benefits of MBS.
There are various forms of MBS, including laparoscopic adjustable gastric banding (LAGB), Roux‐en‐Y gastric bypass (RYGB), and sleeve gastrectomy (SG) (Figure 1). Each form has its unique characteristics in achieving weight reduction. LAGB is a restrictive procedure that isolates the upper stomach by the placement of an adjustable silicone ring at the entrance to the stomach. In RYGB, a small stomach pouch is created and the rest of the stomach is bypassed. A segment of the jejunum is inserted into the small gastric pouch, which connects to the proximal portion of the jejunum that diverts the bypassed portion of the stomach and the duodenum. SG involves irreversible resection of 85% of the stomach (removal of the fundus and greater curvature), leaving a narrow gastric remnant. 79
Figure 1.
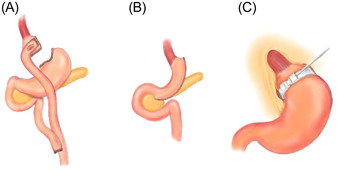
Drawings of three operative procedures in bariatric surgery: (A) Roux‐en‐Y gastric bypass; (B) sleeve gastrectomy; and (C) adjustable gastric band, all of which are normally performed laparoscopically.
It was originally believed that MBS induces weight loss by limiting the amount of food that could be ingested (restriction) and reducing the absorption of ingested calories (malabsorption). Nevertheless, it is now understood that these are not the major mechanisms. In fact, both RYGB and SG lead to an increase in the levels of peptide‐YY and glucagon‐like peptide‐1 (GLP‐1), which are satiety hormones secreted in the L‐cells of the distal small bowel and cause decreased gastric emptying. 80 , 81 SG also results in lower levels of grehlin, a hunger hormone produced in the fundus of the stomach. 82 , 83 , 84 On the other hand, there is also emerging evidence showing that MBS would result in a change in gut microbiome. 85
Therefore, it is not surprising that SG and RYGB are associated with more significant short‐ and long‐term BMI reduction when compared with LAGB in adolescents. When compared with RYGB, SG is less likely to cause malabsorption of micronutrients, nutritional deficiencies, or postoperative bowel obstruction, as it does not involve any reconstruction of the intestinal anatomy. 76 , 84 Therefore, SG is increasingly being performed nowadays, especially in adolescents.
The Teen‐Longitudinal Assessment of Bariatric Surgery consortium is the largest multicenter, prospective observational study involving more than 240 adolescents undergoing LAGB, SG, and RYGB. It reported a mean BMI reduction of 15 kg/m2 at 3 years postoperatively, while remission of comorbidities ranged from 66% for dyslipidemia to 95% for T2D, together with significant improvements in quality‐of‐life measures. 86 Similarly, a recent systematic review and meta‐analysis also reported similar encouraging results in terms of improvements in both BMI and cardiometabolic parameters. 75
With time, more long‐term data has been accumulated regarding the efficacy, safety, and durability of MBS in adolescents. A retrospective study on adolescents who underwent SG in Qatar reported sustainable weight loss effects, with a mean total weight loss of 35.8% and a mean BMI loss of 36% at 5 years of follow‐up. Postoperatively, all patients with prediabetes achieved total remission, and 50% of the patients with T2D were reversed with no relapse after 5 years. 87 A nationwide, prospective, nonrandomized‐controlled study of adolescents with severe obesity undergoing RYGB in Sweden reported substantial weight loss of 36.8 kg in 5 years. 88 Likewise, the US Adolescent Bariatric Surgery study (FABS 5+) also reported significant weight loss after RYGB, with a mean change in BMI of −29.2%, together with significant declines in the prevalence of hypertension, dyslipidemia, and T2D after a mean follow‐up period of 8 years. However, more than 40% of the participants had micronutrient deficiencies, including mild anemia, hyperparathyroidism, or low vitamin B12 levels. 89 This underscored the need for long‐term health maintenance with a regular screening of micronutrient deficiencies.
Notably, when compared with adults undergoing RYGB, despite similar percentages of weight loss 5 years after surgery, adolescents were more likely than adults to achieve remission of T2D and hypertension. 90 Similar findings were obtained on comparing adolescents and adults undergoing SG. 91 This might imply that earlier MBS is associated with better cardiometabolic outcomes.
It should be emphasized that performing MBS does not necessarily guarantee a complete cure of obesity. The patients would still have to make ongoing lifestyle modifications and attend continual follow‐up for monitoring of potential deficiency of trace elements or vitamins due to the procedure. In addition, all female adolescents should be informed of the potential risks associated with pregnancy immediately after the bariatric procedures and they should be counseled on contraception during this period (around 1.5 years following the procedure). 77 These patients should ideally be evaluated and followed up by an experienced multidisciplinary team with a surgeon with expertise in MBS, a pediatrician (usually with a specialty in endocrinology, gastroenterology, or adolescent medicine) to screen for and manage the comorbidities, a registered dietitian to plan for the diet and ensure adequate nutritional intake postoperatively, a physiotherapist to plan for the ongoing personalized exercise program, a clinical psychologist to perform the initial psychological assessment and counsel on the postoperative adjustment, and an overall nurse coordinator.
6.4. Fecal microbiome transfer (FMT)
Emerging evidence is elucidating the relationship between alterations in the gut microbiome in relation to obesity and T2D. Certain specific strains of gut microbiome have been shown to play either a protective or a pathogenic role in the progression of obesity. Therefore, strategies to modify the gut microbiome and reverse metabolic alterations have been developed as a potential therapeutic approach. 92 It works by manipulating the intestinal microbiota by transferring microbiota from a healthy donor into an existing microbial ecosystem. 93 In humans, FMT has been shown in a recent randomized clinical trial on adolescents with obesity to reduce abdominal adiposity and reverse metabolic syndrome, though no effect on weight loss was observed. 94 Further trials are needed to confirm the observations.
7. LONG‐TERM OBESITY MANAGEMENT—A COHESIVE AND STRATEGIC APPROACH
Childhood obesity is a chronic, relapsing condition that requires multidisciplinary and specialist intervention to address its complex pathophysiology. Management includes multimodal lifestyle modifications, regular surveillance of comorbidities, timely initiation of pharmacological agents, and MBS. The care should ideally be delivered by a designated pediatric multidisciplinary team consisting of pediatricians, pediatric surgeons, physiotherapists, dieticians, psychologists, and social workers with close communication with families and schools to develop personalized obesity treatment plans. While this approach has been shown to be effective, 95 given the high prevalence of childhood obesity, its cost‐effectiveness might be doubtful and implementation in the real world could be challenging, especially in resource‐limited countries. Therefore, it is usually reserved for children with morbid obesity.
A comprehensive multisectorial approach, targeting from downstream, midstream to upstream—from hospital‐based programs for children with morbid obesity and comorbidities, to community‐, school‐, or family‐based programs for children with simple obesity, and childhood obesity prevention through public education on healthy food consumption, physical activity, preconception and pregnancy care, and infant diet, and improved urban planning and legislation, might represent a more cohesive strategy in combating this major public health challenge. 95 , 96 , 97 , 98 , 99
8. CONCLUSION
Considerable advances have been made in the management of obesity in children and adolescents. However, implementation in the real world remains a challenge. The broad principle of treatment is to adjust the chronically positive energy balance. While lifestyle and behavior modifications with dietary changes and increased physical activity are the integral part of obesity management, the efficacy and sustainability are limited, especially for those with severe obesity. With emerging evidence on new pharmacological agents, MBS, and recently FMT, it is hoped that obesity management in children and adolescents could be improved in the future. However, it should be highlighted that a cohesive and strategic approach aiming at not just short‐term weight loss but also long‐term behavioral changes and weight maintenance is needed. The approach should be developmentally appropriate for both the physical growth and psychological needs of our children and adolescents. This could best be achieved through orchestrated and multidisciplinary efforts. 100 , 101
AUTHOR CONTRIBUTIONS
Joanna Y. L. Tung reviewed the literature and drafted the manuscript. Kenneth K. Y. Wong prepared the figure on bariatric surgery. Juan Du, Grace W. K. Poon, and Kenneth K. Y. Wong revised the manuscript. All authors approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
None.
ACKNOWLEDGMENTS
This study was supported by The Science and Technology Project of Jilin Province (No. 20200201421JC), China.
Tung JYL, Poon GWK, Du J, Wong KKY. Obesity in children and adolescents: overview of the diagnosis and management. Chronic Dis Transl Med. 2023;9:122‐133. 10.1002/cdt3.58
Edited by Yi Cui
DATA AVAILABILITY STATEMENT
None.
REFERENCES
- 1. World Health Organization . Obesity and Overweight: Fact Sheet; 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#cms
- 2. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627‐2642. 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang S, Guo B, Ao L, et al. Obesity and activity patterns before and during COVID‐19 lockdown among youths in China. Clin Obes. 2020;10(6):e12416. 10.1111/cob.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jenssen BP, Kelly MK, Powell M, Bouchelle Z, Mayne SL, Fiks AG. COVID‐19 and changes in child obesity. Pediatrics. 2021;147(5):e2021050123. 10.1542/peds.2021-050123 [DOI] [PubMed] [Google Scholar]
- 5. Darnton‐Hill I, Nishida C, James W. A life course approach to diet, nutrition and the prevention of chronic diseases. Public Health Nutr. 2004;7(1A):101‐121. [DOI] [PubMed] [Google Scholar]
- 6. Patton GC, Coffey C, Carlin JB, et al. Overweight and obesity between adolescence and young adulthood: a 10‐year prospective cohort study. J Adolesc Health. 2011;48(3):275‐280. 10.1016/j.jadohealth.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 7. Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017;377(22):2145‐2153. 10.1056/NEJMoa1703860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Power C, Thomas C. Changes in BMI, duration of overweight and obesity, and glucose metabolism: 45 years of follow‐up of a birth cohort. Diabetes Care. 2011;34(9):1986‐1991. 10.2337/dc10-1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang T, Fan B, Li S, et al. Long‐term adiposity and midlife carotid intima‐media thickness are linked partly through intermediate risk factors. Hypertension. 2023;80(1):160‐168. 10.1161/HYPERTENSIONAHA.122.20217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bjerregaard LG, Jensen BW, Ängquist L, Osler M, Sørensen TIA, Baker JL. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med. 2018;378(14):1302‐1312. 10.1056/NEJMoa1713231 [DOI] [PubMed] [Google Scholar]
- 11. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548‐2556. 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 12. Maffeis C, Morandi A. Body composition and insulin resistance in children. Eur J Clin Nutr. 2018;72(9):1239‐1245. 10.1038/s41430-018-0239-2 [DOI] [PubMed] [Google Scholar]
- 13. Montague CT, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49(6):883‐888. 10.2337/diabetes.49.6.883 [DOI] [PubMed] [Google Scholar]
- 14. Ardıç C, Çolak S, Uzun K, Salı G, Aydemir T, Telatar G. Maternal gestational diabetes and early childhood obesity: a retrospective cohort study. Child Obes. 2020;16(8):579‐585. 10.1089/chi.2020.0183 [DOI] [PubMed] [Google Scholar]
- 15. Fall CHD, Kumaran K. Metabolic programming in early life in humans. Philos Trans R Soc B. 2019;374(1770):20180123. 10.1098/rstb.2018.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saravanan P, Magee LA, Banerjee A, et al. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8(9):793‐800. 10.1016/S2213-8587(20)30161-3 [DOI] [PubMed] [Google Scholar]
- 17. Ong YY, Pang WW, Huang JY, et al. Breastfeeding may benefit cardiometabolic health of children exposed to increased gestational glycemia in utero. Eur J Nutr. 2022;61(5):2383‐2395. 10.1007/s00394-022-02800-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao F, Zhao L, Yang Y, et al. Association between early life famine exposure and metabolic syndrome in adulthood. Nutrients. 2022;14(14):2881. 10.3390/nu14142881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Almulla AA, Zoubeidi T. Association of overweight, obesity and insufficient sleep duration and related lifestyle factors among school children and adolescents. Int J Adolesc Med Health. 2021;34(2):31‐40. 10.1515/ijamh-2021-0041 [DOI] [PubMed] [Google Scholar]
- 20. Guo Y, Miller MA, Cappuccio FP. Short duration of sleep and incidence of overweight or obesity in Chinese children and adolescents: a systematic review and meta‐analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2021;31(2):363‐371. 10.1016/j.numecd.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 21. Liu S, Wang X, Zheng Q, Gao L, Sun Q. Sleep deprivation and central appetite regulation. Nutrients. 2022;14(24):5196. 10.3390/nu14245196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Valk ES, Savas M, van Rossum EFC. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. 2018;7(2):193‐203. 10.1007/s13679-018-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radoszewska J. The psychological determinants of obesity in children and adolescents. Dev Period Med. 2017;21(3):208‐212. 10.34763/devperiodmed.20172103.208212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity‐assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709‐757. 10.1210/jc.2016-2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barlow SE, Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164‐S192. 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 26. de Onis M. Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ. 2007;85(9):660‐667. 10.2471/blt.07.043497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity: extended international BMI cut‐offs. Pediatric Obes. 2012;7(4):284‐294. 10.1111/j.2047-6310.2012.00064.x [DOI] [PubMed] [Google Scholar]
- 28. Farooqi SOR, O'Rahilly S. Genetic obesity syndromes. In: Grant SFA, ed. The Genetics of Obesity. Springer; 2014:23‐32. [Google Scholar]
- 29. Abuzzahab MJ, Roth CL, Shoemaker AH. Hypothalamic obesity: prologue and promise. Horm Res Paediatr. 2019;91(2):128‐136. 10.1159/000496564 [DOI] [PubMed] [Google Scholar]
- 30. Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876‐1885. 10.1056/NEJMoa1010112 [DOI] [PubMed] [Google Scholar]
- 31. Rankin J, Matthews L, Cobley S, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther. 2016;7:125‐146. 10.2147/AHMT.S101631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gibson‐Smith D, Halldorsson TI, Bot M, et al. Childhood overweight and obesity and the risk of depression across the lifespan. BMC Pediatr. 2020;20(1):25. 10.1186/s12887-020-1930-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rankin J, Matthews L, Cobley S, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther. 2018;7:125‐146. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5115694/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reinehr T. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89(5):419‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knechtle B, Karner‐Rezek B, Fenzl M, Schlegel C, Konrad M, Rosemann T. The effects of an 8‐week multicomponent inpatient treatment program on body composition and anaerobic fitness in overweight and obese children and adolescents. Int J Gen Med. 2013;6:159‐166. 10.2147/IJGM.S40187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ho M, Garnett SP, Baur L, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta‐analysis. Pediatrics. 2012;130(6):e1647‐e1671. 10.1542/peds.2012-1176 [DOI] [PubMed] [Google Scholar]
- 37. Eneli I, Xu J, Tindall A, et al. Using a revised protein‐sparing modified fast (rPSMF) for children and adolescents with severe obesity: a pilot study. Int J Environ Res Public Health. 2019;16(17):3061. 10.3390/ijerph16173061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gow ML, Ho M, Burrows TL, et al. Impact of dietary macronutrient distribution on BMI and cardiometabolic outcomes in overweight and obese children and adolescents: a systematic review. Nutr Res. 2014;72(7):453‐470. 10.1111/nure.12111 [DOI] [PubMed] [Google Scholar]
- 39. Kirk S, Brehm B, Saelens BE, et al. Role of carbohydrate modification in weight management among obese children: a randomized clinical trial. J Pediatr. 2012;161(2):320‐327. 10.1016/j.jpeds.2012.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris L, Hamilton S, Azevedo LB, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta‐analysis. JBI Database Syst Rev Implementation Rep. 2018;16(2):507‐547. 10.11124/JBISRIR-2016-003248 [DOI] [PubMed] [Google Scholar]
- 41. Gow ML, Baur LA, Johnson NA, Cowell CT, Garnett SP. Reversal of type 2 diabetes in youth who adhere to a very‐low‐energy diet: a pilot study. Diabetologia. 2017;60(3):406‐415. 10.1007/s00125-016-4163-5 [DOI] [PubMed] [Google Scholar]
- 42. Lowe MR, Butryn ML, Thomas JG, Coletta M. Meal replacements, reduced energy density eating, and weight loss maintenance in primary care patients: a randomized controlled trial. Obesity. 2014;22(1):94‐100. 10.1002/oby.20582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vidmar AP, Naguib M, Raymond JK, et al. Time‐limited eating and continuous glucose monitoring in adolescents with obesity: a pilot study. Nutrients. 2021;13(11):3697. 10.3390/nu13113697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gow M, Garnett S, Baur L, Lister N. The effectiveness of different diet strategies to reduce type 2 diabetes risk in youth. Nutrients. 2016;8(8):486. 10.3390/nu8080486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poitras VJ, Gray CE, Borghese MM, et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school‐aged children and youth. Appl Physiol Nutr Metab. 2016;41(6 Suppl 3):S197‐S239. 10.1139/apnm-2015-0663 [DOI] [PubMed] [Google Scholar]
- 46. Ho M, Garnett SP, Baur LA, et al. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta‐analysis of randomized trials. JAMA Pediatr. 2013;167(8):759‐768. 10.1001/jamapediatrics.2013.1453 [DOI] [PubMed] [Google Scholar]
- 47. Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247‐257. 10.1016/S0140-6736(12)60646-1 [DOI] [PubMed] [Google Scholar]
- 48. World Health Organization . Global Recommendations on Physical Activity for Health. Geneva WHO; 2012. [PubMed] [Google Scholar]
- 49. Yeung DCS, Yuan X, Hui SSC, Feresu SA. Determinants of moderate to vigorous physical activity and obesity in children: a structural equation modeling analysis. World J Pediatr. 2016;12(2):170‐176. 10.1007/s12519-015-0057-8 [DOI] [PubMed] [Google Scholar]
- 50. Woods AJ, Probst YC, Norman J, et al. Correlates of physical activity and sedentary behaviour in children attending before and after school care: a systematic review. BMC Public Health. 2022;22(1):2364. 10.1186/s12889-022-14675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dobbie LJ, Tahrani A, Alam U, James J, Wilding J, Cuthbertson DJ. Exercise in obesity—the role of technology in health services: can this approach work? Curr Obes Rep. 2022;11(3):93‐106. 10.1007/s13679-021-00461-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kanellopoulou A, Notara V, Magriplis E, et al. Sleeping patterns and childhood obesity: an epidemiological study in 1,728 children in Greece. J Clin Sleep Med. 2021;17(5):1093‐1101. 10.5664/jcsm.9160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chaput J‐P, McHill AW, Cox RC. The role of insufficient sleep and circadian misalignment in obesity. Nat Rev Endocrinol. 2022;19:82‐97. https://pubmed-ncbi-nlm-nih-gov.eproxy.lib.hku.hk/36280789/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fu J, Wang Y, Li G, et al. Childhood sleep duration modifies the polygenic risk for obesity in youth through leptin pathway: the Beijing child and adolescent metabolic syndrome cohort study. Int J Obes. 2019;43(8):1556‐1567. 10.1038/s41366-019-0405-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Skjåkødegård HF, Danielsen YS, Frisk B, et al. Beyond sleep duration: sleep timing as a risk factor for childhood obesity. Pediatr Obes. 2021;16(1):e12698. 10.1111/ijpo.12698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2022;252(2):125‐141. 10.1530/JOE-21-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maahs D, de Serna DG, Kolotkin RL, et al. Randomized, double‐blind, placebo‐controlled trial of orlistat for weight loss in adolescents. Endoc Pract. 2006;12(1):18‐28. 10.4158/EP.12.1.18 [DOI] [PubMed] [Google Scholar]
- 58. McDuffie JR, Calis KA, Uwaifo GI, et al. Three‐month tolerability of orlistat in adolescents with obesity‐related comorbid conditions. Obes Res. 2002;10(7):642‐650. 10.1038/oby.2002.87 [DOI] [PubMed] [Google Scholar]
- 59. Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293(23):2873‐2883. 10.1001/jama.293.23.2873 [DOI] [PubMed] [Google Scholar]
- 60. McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178‐184. 10.1001/jamapediatrics.2013.4200 [DOI] [PubMed] [Google Scholar]
- 61. Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double‐blind, placebo‐controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072‐2079. 10.1176/ajp.2006.163.12.2072 [DOI] [PubMed] [Google Scholar]
- 62. Allen HF, Mazzoni C, Heptulla RA, et al. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2005;18(8):761‐768. [DOI] [PubMed] [Google Scholar]
- 63. Lee YS, Jun HS. Anti‐diabetic actions of glucagon‐like peptide‐1 on pancreatic beta‐cells. Metabolism. 2014;63(1):9‐19. 10.1016/j.metabol.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 64. Kelly AS, Auerbach P, Barrientos‐Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117‐2128. 10.1056/NEJMoa1916038 [DOI] [PubMed] [Google Scholar]
- 65. Zhao G, Zhang Q, Wu F, Yin S, Xie Y, Liu H. Comparison of weight loss and adverse events of obesity drugs in children and adolescents: a systematic review and meta‐analysis. Expert Rev Clin Pharmacol. 2022;15(9):1119‐1125. 10.1080/17512433.2022.2117152 [DOI] [PubMed] [Google Scholar]
- 66. Ryan PM, Seltzer S, Hayward NE, Rodriguez DA, Sless RT, Hawkes CP. Safety and efficacy of glucagon‐like peptide‐1 receptor agonists in children and adolescents with obesity: a meta‐analysis. J Pediatr. 2021;236:137‐147.e13. 10.1016/j.jpeds.2021.05.009 [DOI] [PubMed] [Google Scholar]
- 67. Weghuber D, Barrett T, Barrientos‐Pérez M, et al. Once‐weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387(24):2245‐2257. 10.1056/NEJMoa2208601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. The U.S. Food and Drug Administration . FDA Approves Treatment for Chronic Weight Management in Pediatric Patients Aged 12 Years and Older; 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-chronic-weight-management-pediatric-patients-aged-12-years-and-older
- 69. Jordan J, Astrup A, Engeli S, Narkiewicz K, Day WW, Finer N. Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity. J Hypertens. 2014;32(6):1178‐1188. 10.1097/HJH.0000000000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vivus Inc . Qsymia. Package Insert; 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022580s021lbl.pdf
- 71. Haws R, Brady S, Davis E, et al. Effect of setmelanotide, a melanocortin‐4 receptor agonist, on obesity in Bardet–Biedl syndrome. Diabetes Obes Metab. 2020;22(11):2133‐2140. 10.1111/dom.14133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Clément K, van den Akker E, Argente J, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single‐arm, open‐label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8(12):960‐970. 10.1016/S2213-8587(20)30364-8 [DOI] [PubMed] [Google Scholar]
- 73. Haws RM, Gordon G, Han JC, Yanovski JA, Yuan G, Stewart MW. The efficacy and safety of setmelanotide in individuals with Bardet–Biedl syndrome or Alström syndrome: phase 3 trial design. Contemp Clin Trials Commun. 2021;22:100780. 10.1016/j.conctc.2021.100780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. The U.S. Food and Drug Administration . FDA Approves Treatment for Weight Management in Patients with Bardet–Biedl Syndrome Aged 6 or Older; 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-weight-management-patients-bardet-biedl-syndrome-aged-6-or-older
- 75. Pedroso FE, Angriman F, Endo A, et al. Weight loss after bariatric surgery in obese adolescents: a systematic review and meta‐analysis. Surg Obes Relat Dis. 2018;14:413‐422. 10.1016/j.soard.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 76. Paulus GF, de Vaan LEG, Verdam FJ, Bouvy ND, Ambergen TAW, van Heurn LWE. Bariatric surgery in morbidly obese adolescents: a systematic review and meta‐analysis. Obes Surg. 2015;25(5):860‐878. 10.1007/s11695-015-1581-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Michalsky M, Reichard K, Inge T, Pratt J, Lenders C. ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis. 2012;8(1):1‐7. 10.1016/j.soard.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 78. Pratt JSA, Browne A, Browne NT, et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis. 2018;14(7):882‐901. 10.1016/j.soard.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weiss AL, Mooney A, Gonzalvo JP. Bariatric surgery. Adv Pediatr. 2017;64(1):269‐283. 10.1016/j.yapd.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 80. Dirksen C, Jørgensen NB, Bojsen‐Møller KN, et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux‐en‐Y gastric bypass. Int J Obes. 2013;37(11):1452‐1459. 10.1038/ijo.2013.15 [DOI] [PubMed] [Google Scholar]
- 81. Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10(10):575‐584. 10.1038/nrgastro.2013.119 [DOI] [PubMed] [Google Scholar]
- 82. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide‐YY levels after Roux‐en‐Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401‐407. 10.1097/SLA.0b013e318156f012 [DOI] [PubMed] [Google Scholar]
- 83. Hutch CR, Sandoval D. The role of GLP‐1 in the metabolic success of bariatric surgery. Endocrinology. 2017;158(12):4139‐4151. 10.1210/en.2017-00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wasserman H, Inge TH. Bariatric surgery in obese adolescents: opportunities and challenges. Pediatr Ann. 2014;43(9):e230‐e236. 10.3928/00904481-20140825-10 [DOI] [PubMed] [Google Scholar]
- 85. Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight‐loss intervention. Nature Med. 2017;23(7):859‐868. 10.1038/nm.4358 [DOI] [PubMed] [Google Scholar]
- 86. NCBI . Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents; 2018. https://www.ncbi.nlm.nih.gov/pubmed/26544725 [DOI] [PMC free article] [PubMed]
- 87. El‐Matbouly MA, Khidir N, Touny HA, El Ansari W, Al‐Kuwari M, Bashah M. A 5‐year follow‐up study of laparoscopic sleeve gastrectomy among morbidly obese adolescents: does it improve body image and prevent and treat diabetes. Obes Surg. 2018;28(2):513‐519. 10.1007/s11695-017-2884-2 [DOI] [PubMed] [Google Scholar]
- 88. Olbers T, Beamish AJ, Gronowitz E, et al. Laparoscopic Roux‐en‐Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5‐year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017;5(3):174‐183. 10.1016/S2213-8587(16)30424-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Inge TH, Jenkins TM, Xanthakos SA, et al. Long‐term outcomes of bariatric surgery in adolescents with severe obesity (FABS‐5+): a prospective follow‐up analysis. Lancet Diabetes Endocrinol. 2017;5(3):165‐173. 10.1016/S2213-8587(16)30315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Inge TH, Courcoulas AP, Jenkins TM, et al. Five‐year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med. 2019;380(22):2136‐2145. 10.1056/NEJMoa1813909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Khidir N, El‐Matbouly MA, Sargsyan D, Al‐Kuwari M, Bashah M, Gagner M. Five‐year outcomes of laparoscopic sleeve gastrectomy: a comparison between adults and adolescents. Obes Surg. 2018;28(7):2040‐2045. 10.1007/s11695-018-3139-6 [DOI] [PubMed] [Google Scholar]
- 92. Aron‐Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health‐pathophysiology and therapeutic strategies. Gastroenterology. 2021;160(2):573‐599. 10.1053/j.gastro.2020.10.057 [DOI] [PubMed] [Google Scholar]
- 93. Kang Y, Cai Y. Gut microbiota and obesity: implications for fecal microbiota transplantation therapy. Hormones. 2017;16(3):223‐234. 10.14310/horm.2002.1742 [DOI] [PubMed] [Google Scholar]
- 94. Leong KSW, Jayasinghe TN, Wilson BC, et al. Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA Network Open. 2020;3(12):e2030415. 10.1001/jamanetworkopen.2020.30415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Marten KA, Allen DB, Rehm J, Vanderwall C, Peterson AL, Carrel AL. A multidisciplinary approach to pediatric obesity shows improvement postintervention. Pediatr . 2022;S1876‐2859(22):00556‐3. 10.1016/j.acap.2022.10.019 [DOI] [PubMed]
- 96. Persaud A, Castro I, Simione M, et al. Multi‐sector stakeholder's perceptions of determinants of successful implementation of a pediatric weight management intervention. Front Public Health. 2022;10:954063. 10.3389/fpubh.2022.954063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fisher L, Dahal M, Hawkes S, Puri M, Buse K. Barriers and opportunities to restricting marketing of unhealthy foods and beverages to children in Nepal: a policy analysis. BMC Public Health. 2021;21(1):1351. 10.1186/s12889-021-11257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wu T, Yang S, Liu M, et al. Urban sprawl and childhood obesity. Obesity Reviews. 2021;22(Suppl 1):e13091. 10.1111/obr.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wickramasinghe K, Chatterjee S, Williams J, et al. Childhood overweight and obesity abatement policies in Europe. Obes Rev. 2021;22(Suppl 6):e13300. 10.1111/obr.13300 [DOI] [PubMed] [Google Scholar]
- 100. Ip P, Ho FKW, Louie LHT, et al. Childhood obesity and physical activity‐friendly school environments. J Pediatr. 2017;191:110‐116. 10.1016/j.jpeds.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 101. Wong SH, Huang WY, Cerin E, Gao Y, Lai PC, Burnett A. Home and neighbourhood environment: association with children's physical activity and obesity‐related dietary behaviour. Hong Kong Med J = Xianggang Yi Xue Za Zhi. 2016;22(Suppl 6 (6)):43‐47. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


