Abstract
Background
Familial hypercholesterolemia (FH) is underrecognized, and its association with coronary artery disease (CAD) remains limited, especially in China. We aimed to investigate the prevalence of FH and its relationship with CAD in a large Chinese cohort.
Methods
FH was defined using the Make Early Diagnosis to Prevent Early Death (MEDPED) criteria. The crude and age‐sex standardized prevalence of FH were calculated based on surveys of the Prediction for Atherosclerotic Cardiovascular Disease Risk in China (China‐PAR) project during 2007−2008. The associations of FH with incident CAD and its major subtypes were estimated with the cohort‐stratified multivariate Cox proportional hazard models based on the data from the baseline to the last follow‐up (2018−2020).
Results
Among 98,885 included participants, 190 participants were defined as FH. Crude and age−sex standardized prevalence and 95% confidence interval (CI) of FH were 0.19% (0.17%–0.22%) and 0.13% (0.10%–0.16%), respectively. The prevalence varied across age groups and peaked in the group of 60–<70 years (0.28%), and the peak prevalence (0.18%) in males was earlier, yet lower than the peak crude prevalence in females (0.41%). During a mean follow‐up of 10.7 years, 2493 cases of incident CAD were identified. After multivariate adjustment, FH patients had a 2.03‐fold greater risk of developing CAD compared to non‐FH participants.
Conclusions
The prevalence of FH was estimated to be 0.19% in the participants, and it was associated with an elevated risk of incident CAD. Our study suggests that early screening of FH has certain public health significance for the prevention of CAD.
Keywords: cohort study, coronary artery disease, familial hypercholesterolemia, prevalence
Key points
This study provided a precise estimate of familial hypercholesterolemia (FH) prevalence and strong evidence of the association between FH and coronary artery disease (CAD), based on a large population.
Our research has important public health implications for the future prevention of FH and CAD.
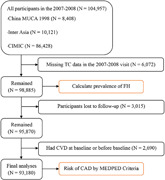
The flow diagram of the study participant selection for the final analysis.
1. INTRODUCTION
Familial hypercholesterolemia (FH) is an autosomal monogenic lipid metabolic disorder, which substantially increases the plasma low‐density lipoprotein cholesterol (LDL‐C) concentrations from birth, leading to premature atherosclerosis. 1 , 2 In the general population, the prevalence of FH is about 0.32% worldwide, 0.30% in Europe, 0.35% in America, 3 and 0.19% in East Asia. 4 In Europe, more than 500,000 children and 200,000 adults were estimated to be affected by FH, yet very few of them receive curative care, 5 leading to an increased burden of cardiovascular disease (CVD) due to accelerated atherosclerosis.
Similarly, the majority of FH patients in China remain unrecognized due to the lack of awareness of FH among physicians and the general public. 6 In addition, insufficient genetic screening and national registration can contribute to the limited data on the prevalence of FH. 7 Currently, two studies have applied modified Dutch Lipid Clinic Network (DLCN) criteria to identify FH patients in Jiangsu Province and rural areas of Henan Province, and the prevalence of FH was estimated to be 0.28% 8 and 0.35%, 9 respectively. A national survey used the Chinese expert consensus on FH diagnosis (CEFH) to identify FH, and the prevalence of FH was estimated to be 0.13%. 10 The results of those studies were inconsistent, and additional studies are needed.
FH and subsequent lifelong LDL‐C exposure aggravate atherosclerosis and increase the risk of early CVD morbidity and mortality. 5 The correlation between FH and coronary artery disease (CAD) varies by geographic location. 11 Patients who had FH have a 3‐ to 13‐fold higher risk of premature CAD in the European population compared with those who had non‐FH. 12 , 13 A cross‐sectional study of Chinese patients undergoing coronary angiography showed that patients with FH have a 1.79‐ to 4.46‐fold higher risk of CAD compared with patients with non‐FH. 14 However, the evidence on the effect of FH on CAD based on the general population is still lacking.
Based on a prospective cohort study conducted in 15 Chinese provinces (China‐PAR project), we estimated the prevalence of FH and analyzed the association between FH and CAD to provide the evidence for guiding the prevention of CAD.
2. METHODS
2.1. Study population
The study design and methods of the China‐PAR project have been reported in detail previously. 15 , 16 The study included three prospective cohorts that were part of the China‐PAR project, comprising the China Multi‐Center Collaborative Study of Cardiovascular Epidemiology (China MUCA‐1998), which employed a cluster random sampling method to select 11 clusters aged 35–59 years in nine provinces, the International Collaborative Study of Cardiovascular Disease in Asia (InterASIA), which used a 4‐stage stratified sampling method with nationally representative samples aged 35–74 years from 10 provinces in China during 2000–2001, and the Community Intervention of Metabolic Syndrome in China and Chinese Family Health Study (CIMIC), which is a large community‐based cohort study, and recruited study participants aged 18 years or more by a 3‐stage cluster random sampling method during 2007–2008. All three cohorts were surveyed from 2007 to 2008 and were followed up in 2012–2015 and 2018–2020 using identical methods of surveys. Participants were included and excluded due to the following reasons: (1) those who participated in the 2007–2008 visit (N = 104,957) were included, (2) participants who were missing data on total cholesterol (TC) (N = 6072) were excluded, (3) individuals who lost to follow‐up (N = 3015) were excluded, and (4) participants having CVD at baseline or before baseline (N = 2690) were excluded, leaving a final sample size of 93,180 participants (Figure 1).
Figure 1.
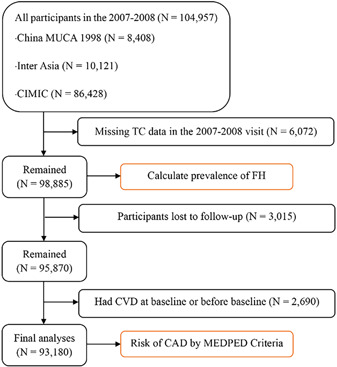
Flow diagram of study participant selection for the final analysis. CAD, coronary artery disease; CVD, cardiovascular diseases; FH, familial hypercholesterolemia; MEDPED, Make Early Diagnosis to Prevent Early Death; TC, total cholesterol.
2.2. Data collection
Trained healthcare staff used a standardized questionnaire under strict quality control to collect demographic characteristics, medical history, and lifestyle risk factors. Smoking was defined as having consumed at least 100 cigarettes throughout a lifetime. We categorized participants into never, former, and current smokers. Current smokers were defined as those who were smoking at the time of the survey, and former smokers were those who ever smoked but had quit at the time of the survey. Alcohol consumption was defined as drinking alcohol at least 12 times during the past year. Education was classified as high school or above or less than high school. Work‐related physical activity was self‐reported by participants. Body weight and height were measured to the nearest 0.5 kg and 0.5 cm, respectively, with lightweight clothing and no shoes. Body mass index (BMI) was calculated as weight (kg) divided by height (m)2. Participants were categorized as normal (BMI < 25 kg/m2) and overweight or obese (BMI ≥ 25 kg/m2).
Participants were asked to avoid smoking, exercising, taking medicine, and drinking caffeine‐containing beverages at least 30 min before BP measurement. Three readings of BP were obtained after participants rested for 5 min. For this analysis, we used the average of the three readings. Hypertension was defined as systolic blood pressure ≥140 mmHg, and/or average diastolic blood pressure ≥90 mmHg, and/or taking antihypertensive medication within the previous 2 weeks. After fasting for at least 10 h, blood samples were drawn from participants to measure glucose and lipid levels, including TC, triglycerides (TG), and high‐density lipoprotein cholesterol (HDL‐C). TC, HDL‐C, and TG levels were analyzed enzymatically in a Hitachi 7060 Clinical Analyzer (Hitachi High‐Technologies Corp.) at the Fuwai Hospital of the Chinese Academy of Medical Sciences. The laboratory participates in the Lipid Standardization Program of the US Centers for Disease Control and Prevention. LDL‐C was calculated using Sampson's equation when triglycerides were <800 mg/dL. 17 , 18 LDL and TC in individuals receiving lipid‐lowering therapy were multiplied by 1.43, corresponding to a 30% average reduction in LDL cholesterol. 19 , 20 Low HDL‐C is defined as HDL‐C less than 40 mg/dL. Diabetes was diagnosed as fasting blood glucose ≥126 mg/dL and/or self‐reported current anti‐diabetes treatment. A family history of CAD was defined as at least a parent or a sibling diagnosed with CAD.
Information on incident CAD during the follow‐up period was collected by interviewing participants or their proxies and further verified by hospital records or death certificates. The central adjudication committee at Fuwai Hospital (Beijing, China) reviewed all medical and death records and determined the final diagnosis. Two adjudication committee members verified events independently, and discrepancies were resolved by discussion involving additional committee members. Causes of death were coded according to ICD‐10 (International Classification of Diseases, 10th revision). CAD included unstable angina, acute myocardial infarction (AMI), and CAD death. Unstable angina was identified as angina pectoris that changes or worsens. AMI was defined as changed biochemical markers of myocardial necrosis accompanied by any one of the following four characteristics: ischemic symptoms, pathological Q waves, ST‐segment elevation or depression, or coronary intervention. CAD death was defined as fatal events secondary to CAD, including fatal MI or other coronary deaths. CAD incidence was defined as the first‐ever CAD event, which was categorized as AMI, and other CAD (unstable angina and other coronary deaths).
2.3. Definition of FH
According to the MEDPED criteria, 20 , 21 , 22 FH was defined as LDL‐C ≥ 200 mg/dL or TC ≥ 270 mg/dL for participants under 20 years of age, LDL‐C ≥ 220 mg/dL or TC ≥ 290 mg/dL for those aged 20−29 years, LDL‐C ≥ 240 mg/dL or TC ≥ 340 mg/dL for those aged 30−39 years, and LDL‐C ≥ 260 mg/dL or TC ≥ 360 mg/dL for those over 40 years old.
2.4. Statistical analysis
Characteristics of the study participants were described as mean (standard deviation [SD]) for continuous variables and frequency (%) for categorical variables as appropriate based on the FH or non‐FH. The age‐ and sex‐standardized prevalence of FH was estimated using the direct method based on the Sixth Population Census of China. 23
Person years of follow‐up were calculated from the date of initial examination until the date of CAD occurrence, death, or the date of last follow‐up, whichever occurred first. To address the potential effect variation among subcohorts, multivariate Cox proportional hazard models stratified by cohorts were used to estimate the association of FH with CAD, AMI, and other CAD. Covariates were known risk factors of CAD including age, sex, geographic region (North and South), urbanization (urban and rural), smoking status (never, former, or current smoker), alcohol consumption (yes or no), education level (less than high school or high school or above), work‐related physical activity (vigorous or moderate, light or sedentary, or no job or retirement), BMI, HDL‐C, hypertension (yes or no), family history of CAD (yes or no), and diabetes (yes or no).
Sensitivity analyses were conducted to assess the robustness of our findings. First, we constructed a meta‐analysis for the prevalence of FH stratified by subcohorts using random effect models weighted by sample sizes, considering the heterogeneity between cohorts. Second, we estimated the association between FH and CAD by (1) adding individuals with CVD at baseline and adjusting baseline CVD history in multivariate adjustment; (2) excluding first‐year incident CAD cases after baseline interview; and (3) using Cox competing risk models to consider the impact of non‐CAD deaths.
All analyses were performed using SAS 9.4 (SAS Institute Inc), R software, version 4.1.3 (R Foundation for Statistical Computing). Tests were two‐sided with statistical significance set at p < 0.05.
3. RESULTS
A total of 98,885 participants were included to calculate the prevalence of FH (Figure 1), and 190 participants were defined as FH by MEDPED. At baseline, the mean age of participants was 52.8 ± 12.5 years; 60.2% were female and 50.2% were from northern China. Patients with FH had a higher prevalence of hypertension, diabetes, overweight or obesity, and a family history of CAD (Table 1).
Table 1.
Baseline characteristics of the study participants.
| Variables | Overall | Non‐FH | FH | p‐value |
|---|---|---|---|---|
| No of participants | 98,885 | 98,695 | 190 | |
| Age, mean (SD), year | 52.8 (12.5) | 52.8 (12.5) | 57.8 (9.6) | <0.001 |
| Sex | ||||
| Female | 59,492 (60.2) | 59,354 (60.1) | 138 (72.6) | 0.001 |
| Male | 39,393 (39.8) | 39,341 (39.9) | 52 (27.4) | |
| Urbanization | ||||
| Rural | 91,553 (92.6) | 91,403 (92.6) | 150 (78.9) | <0.001 |
| Urban | 7332 (7.4) | 7292 (7.4) | 40 (21.1) | |
| Geographic region | ||||
| South | 49,243 (49.8) | 49,189 (49.8) | 54 (28.4) | <0.001 |
| North | 49,642 (50.2) | 49,506 (50.2) | 136 (71.6) | |
| Education | ||||
| Less than high school | 85,016 (86.1) | 84,856 (86.1) | 160 (84.2) | 0.522 |
| High school or above | 13,750 (13.9) | 13,720 (13.9) | 30 (15.8) | |
| Smoking status | ||||
| Never or Former | 77,833 (79.0) | 77,673 (79.0) | 160 (84.2) | 0.096 |
| Current | 20,647 (21.0) | 20,617 (21.0) | 30 (15.8) | |
| Alcohol drinker | ||||
| No | 78,446 (79.4) | 78,288 (79.4) | 158 (83.2) | 0.238 |
| Yes | 20,309 (20.6) | 20,277 (20.6) | 32 (16.8) | |
| Body mass index | ||||
| normal | 63,866 (64.6) | 63,718 (64.7) | 85 (44.7) | <0.001 |
| overweight or obese | 34,932 (35.4) | 34,827 (35.3) | 105 (55.3) | |
| Hypertension | ||||
| No | 61,690 (62.4) | 61,629 (62.5) | 61 (32.1) | <0.001 |
| Yes | 37,108 (37.6) | 36,979 (37.5) | 129 (67.9) | |
| High‐density lipoprotein cholesterol, mean (SD), (mg/dL) | 51.3 (12.8) | 51.3 (12.8) | 53.2 (13.3) | 0.040 |
| Work‐related physical activity | ||||
| Vigorous or moderate level | 53,503 (54.2) | 53,427 (54.2) | 76 (40.0) | <0.001 |
| Light or sedentary level | 22,740 (23.0) | 22,701 (23.0) | 39 (20.5) | |
| No job or retirement | 22,478 (22.8) | 22,403 (22.8) | 75 (39.5) | |
| Diabetes | ||||
| No | 92,104 (93.2) | 91,975 (93.3) | 129 (67.9) | <0.001 |
| Yes | 6706 (6.8) | 6645 (6.7) | 61 (32.1) | |
| Family history of CAD | ||||
| No | 91,333 (92.4) | 91,175 (92.4) | 158 (83.2) | <0.001 |
| Yes | 7552 (7.6) | 7520 (7.6) | 32 (16.8) |
Note: Data are presented as n (%) or mean (SD).
Abbreviations: BMI, body mass index; FH, familial hypercholesterolemia; SBP, systolic blood pressure.
The overall crude and age‐ and sex‐ standardized prevalence of FH was 0.19% (95% confidence interval [CI]: 0.17%–0.22%) and 0.13% (95% CI: 0.10%–0.16%), respectively. There were significant differences in the crude prevalence between age groups (0.27% for age 60 vs. 0.16% for age < 60, p < 0.001) and sex (0.23% for female vs. 0.13% for male, p = 0.001) (Table 2). In the total population, the crude prevalence of FH peaked at the age group of 60–<70 years (0.28%), while in males, the peak crude prevalence (age group of 30–<40 years, 0.18%) was earlier yet lower than the peak crude prevalence in females (age group of 60–<70 years, 0.41%) (Figure 2). There were also significant differences in the prevalence between other subgroups, such as geographic region BMI and diabetes (Table 2).
Table 2.
Prevalence and 95% confidence interval of familial hypercholesterolemia (FH) among participants in subgroups.
| Variable | Participants (n = 98,885) | p‐value |
|---|---|---|
| FH | ||
| Number | 190 | |
| Age | ||
| <60 years | 0.16% (0.13%−0.19%) | <0.001 |
| ≥60 years | 0.27% (0.21%−0.34%) | |
| Sex | ||
| Female | 0.23% (0.19%−0.27%) | 0.001 |
| Male | 0.13% (0.10%−0.17%) | |
| Urbanization | ||
| Rural | 0.16% (0.14%−0.19%) | <0.001 |
| Urban | 0.55% (0.39%−0.74%) | |
| Geographic region | ||
| South | 0.11% (0.08%−0.14%) | <0.001 |
| North | 0.27% (0.23%−0.32%) | |
| Education | ||
| Less than high school | 0.19% (0.16%−0.22%) | 0.522 |
| High school or above | 0.22% (0.15%−0.31%) | |
| Smoking status | ||
| Never or Former smoker | 0.21% (0.17%−0.24%) | 0.096 |
| Current smoker | 0.15% (0.10%−0.21%) | |
| Alcohol drinker | ||
| No | 0.20% (0.17%−0.24%) | 0.238 |
| Yes | 0.16% (0.11%−0.22%) | |
| Body mass index | ||
| Normal | 0.13% (0.11%−0.16%) | <0.001 |
| Overweight or obese | 0.30% (0.25%−0.36%) | |
| Hypertension | ||
| No | 0.10% (0.08%−0.13%) | <0.001 |
| Yes | 0.35% (0.29%−0.41%) | |
| Low high‐density lipoprotein cholesterol | ||
| No | 0.20% (0.17%−0.23%) | 0.455 |
| Yes | 0.17% (0.11%−0.24%) | |
| Work‐related physical activity | ||
| Vigorous or moderate level | 0.14% (0.11%−0.18%) | <0.001 |
| Light or sedentary level | 0.17% (0.12%−0.23%) | |
| No job or retirement | 0.33% (0.26%−0.42%) | |
| Diabetes | ||
| No | 0.14% (0.12%−0.17%) | <0.001 |
| Yes | 0.91% (0.70%−1.17%) | |
| Family history of CAD | ||
| No | 0.17% (0.15%−0.20%) | <0.001 |
| Yes | 0.42% (0.29%−0.60%) |
Note: Data are presented as prevalence and 95% confidence interval.
Figure 2.
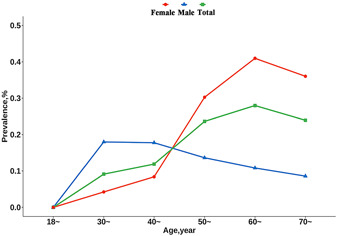
FH prevalence of familial hypercholesterolemia by age and sex. We displayed the age prevalence for total participants, both males and females, among different age groups based on the MEDPED. FH, familial hypercholesterolemia; MEDPED, Make Early Diagnosis to Prevent Early Death.
During the follow‐up of 994,038 person‐years for 93,180 individuals, 2493 CAD were identified. There was a significantly positive association between FH and CAD, with a multivariable‐adjusted hazard ratio (HR) of 2.03 (95% CI: 1.17–3.51) for FH patients compared with individuals without FH. The association of AMI and other CAD with FH was consistent, with the HR of 2.23 (95% CI: 1.11–4.48) and 2.32 (95% CI: 1.15–4.66), respectively (Figure 3).
Figure 3.
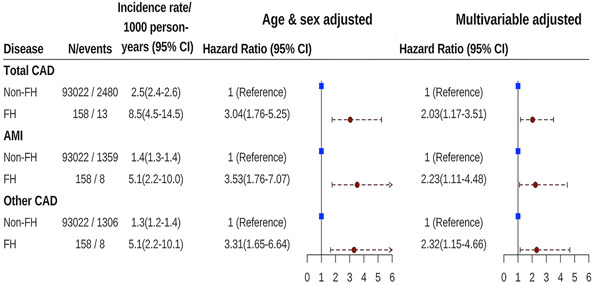
Hazard ratios of CAD associated with FH. The Cox proportional model was adjusted for age, sex, geographic region, urbanization, smoking status, alcohol consumption, education level, work‐related physical activity, body mass index, high‐density lipoprotein cholesterol, hypertension, family history of CAD, and diabetes. AMI, acute myocardial infarction; CAD, coronary artery disease; CI, confidence interval; FH, familial hypercholesterolemia; MEDPED, Make Early Diagnosis to Prevent Early Death.
The sensitivity analyses did not substantially change our results. Despite variations of prevalence across different cohorts, we found generally similar estimates of the combined prevalence (Supporting Information: Table S2 and Figure S1). After adding those with CVD at baseline and adjusting for baseline CVD history, the HR was 2.12 (95% CI: 1.31–3.42) for total CAD. Similar effects were observed in the analyses of excluding incident CAD cases during the first year or using competing risk models to take account of the impact of non‐CAD deaths (Table 3).
Table 3.
Sensitivity analysis for association of CAD incidence with FH.
| Outcome | N/events | Hazard ratio (95% CI) | p‐value |
|---|---|---|---|
| Adding people with CVD at baseline and adjusting baseline CVD history a | |||
| Total CAD | |||
| Non‐FH | 94,386/2666 | 1 (Reference) | |
| FH | 179/17 | 2.12 (1.31−3.42) | 0.002 |
| AMI | |||
| Non‐FH | 94,386/1481 | 1 (Reference) | |
| FH | 179/10 | 2.17 (1.16−4.05) | 0.015 |
| Other CAD | |||
| Non‐FH | 94,386/1383 | 1 (Reference) | |
| FH | 179/10 | 2.37 (1.27−4.44) | 0.007 |
| Excluding incident CAD cases in the first year after baseline b | |||
| Total CAD | |||
| Non‐FH | 92,016/2380 | 1 (Reference) | |
| FH | 157/13 | 2.13 (1.23−3.68) | 0.007 |
| AMI | |||
| Non‐FH | 92,054/1301 | 1 (Reference) | |
| FH | 157/8 | 2.34 (1.16−4.71) | 0.017 |
| Other CAD | |||
| Non‐FH | 92,065/1257 | 1 (Reference) | |
| FH | 157/8 | 2.42 (1.21−4.88) | 0.013 |
| Using Cox competing risk models to consider the impact of non‐CAD deaths | |||
| Total CAD | |||
| Non‐FH | 92,108/2472 | 1 (Reference) | |
| FH | 157/13 | 2.02 (1.15−3.56) | 0.015 |
| AMI | |||
| Non‐FH | 92,108/1355 | 1 (Reference) | |
| FH | 157/8 | 2.23 (1.09−4.57) | 0.028 |
| Other CAD | |||
| Non‐FH | 92,108/1300 | 1 (Reference) | |
| FH | 157/8 | 2.31 (1.14−4.67) | 0.020 |
Abbreviations: AMI, acute myocardial infarction; CAD, coronary artery disease; FH, familial hypercholesterolemia; CVD, cardiovascular diseases.
Adding baseline CVD history to multivariate adjustment.
Coronary artery disease before baseline interview was excluded.
4. DISCUSSION
In this prospective cohort study in Chinese, the estimated prevalence of FH was 0.19%, which varied within subgroups. FH was an independent risk factor for CAD incidence, with FH patients having a 2.03‐fold higher risk of CAD. These results reflected the burden of FH in Chinese and provided hints for the prevention of CAD.
FH is typically an inherited condition with high plasma TC and LDL‐C caused by mutations in the LDL receptor gene (LDLR), apolipoprotein B‐100 gene (APOB), and proprotein convertase subtilisin/kexin type 9 genes. 11 The lifetime exposure to high LDL‐C further exacerbates atherosclerosis and increases the risk of early CVD morbidity and mortality. 24 Evidence from Canadian Chinese and Chinese FH patients displayed that, even with similar LDLR gene mutations, participants from Canada exhibited higher levels of LDL‐C, as well as a higher prevalence of CAD and tendon xanthoma, 25 demonstrating that the effects of FH‐related gene mutations may vary across a diverse population, dietary patterns, or geographic location. Therefore, the related pathogenic mechanisms and modifiers need to be further explored.
The current diagnostic criteria for FH are not uniform. There are three common clinical scoring systems, including the DLCN criteria, 26 the Simon Broome Register (SBR) criteria, 27 and the MEDPED criteria. Results from the meta‐analyses 3 , 4 showed that compared to the results of the DLCN criteria, the prevalence estimated by the MEDPED criteria is slightly underestimated while overestimated by the SBR criteria. A family history of premature cardiovascular disease and hypercholesterolemia as part of the criteria may be the reason why the screening population based on the DLCN and SBR criteria is more sensitive than the MEDPED criteria. 28 A comparison of different diagnostic criteria for FH 2 is added in Supporting Information: Table S1.
The estimated crude prevalence of FH in our study was slightly lower than that in European and American populations (0.25% [95% CI: 0.14%–0.41%]) 3 and was similar to the results of a meta‐analysis in East Asian (0.19% [95% CI: 0.10%–0.29%]). 4 Although the estimated prevalence of FH in our study was slightly higher than that in a recent nationwide survey (0.13% [95% CI: 0.12%–0.14%]), the age‐sex standardized prevalence was comparable between ours (0.13% [95% CI: 0.10%–0.16%]) and that survey's (0.11% [95% CI: 0.10%–0.12%]). 10 These disparities in crude prevalence of FH could be partly attributed to the differences in geographical regions, survey duration, and demographic characteristics.
As an autosomal dominant disease, the difference in FH prevalence by age might be unexpected, but similar results were reported in other studies. 8 , 9 , 10 We supposed that the disparity of the estimated FH prevalence by age may be due to the impact of age on LDL‐C, which was the result of increased FH gene penetrance with changing environmental factors. 29 In the present study, the sex‐specific FH prevalence showed different trends across age groups, which conforms with a previous study. 9 Differences in the expression of sex hormones were typical in the postmenopausal and late menopausal transition periods, which were associated with lipid and lipoprotein abnormalities, 30 leading to differences in the expression of the FH gene. Additionally, the prevalence of FH also varied across subgroups of the living environment, lifestyle, and urban and rural areas, which was also found in previous studies. 10 , 29 Just as the results from the meta‐analysis of the prevalence, we also found the lowest prevalence in the CIMIC cohort due to the higher proportion of rural and younger individuals.
The odds ratio (OR) of FH for myocardial infarction in the Copenhagen general population was 2.7 (95% CI: 1.8–4.2) using the same criteria, 28 and this estimate was comparable with our result (HR: 2.23 [95% CI: 1.11–4.48]). In a cross‐sectional study conducted in Jiangsu Province, China, participants with FH had a 15‐fold higher risk of CVD, compared with those without FH. 8 In Chinese patients undergoing coronary angiography, compared to patients with unlikely FH and without receiving cholesterol‐lowering medication, the risk of CAD was increased by 79% in patients with definite/probable FH and without taking medication and increased by 3.46‐fold in patients with definite/probable FH and taking medication. 14 Those estimates were higher than our result, which may be due to a different study design and population. 20 , 28
Based on a prospective cohort study conducted in 15 Chinese provinces (China‐PAR project), we estimated the FH prevalence and provided strong evidence of the association between FH and CAD. However, our study also had some limitations. First, we lacked information on tendon xanthomas, DNA mutations, and family history, which may have underestimated the prevalence of FH. Second, due to the low FH prevalence, the number of FH participants was limited, which did not support further subgroup analyses of the study. Third, the design of our study is not all based on random sampling, resulting in difficulties in estimating national prevalence. Future studies with larger sample sizes and sufficient information are warranted to obtain precise and nationally representative estimates of FH prevalence.
In conclusion, our study offered estimates of the prevalence of FH in Chinese and provided the evidence of the association between FH and CAD, with significant public health implications for the future prevention of FH and CAD.
AUTHOR CONTRIBUTIONS
Xiapikatijiang Aihaiti completed the project, analyzed the data, interpreted the data, prepared figures and tables, and wrote the manuscript. Xiangfeng Lu conceptualized and designed the study and was the guarantor of this work. All study authors contributed to the interpretation, revision, writing, and finalization of the final submission version of the manuscript.
CONFLICTS OF INTEREST STATEMENT
Professor Xiangfeng Lu and Dongfeng Gu are members of the Chronic Diseases and Translational Medicine editorial board and are not involved in the peer review process of this article. The other authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Institutional Review Board at Fuwai Hospital (Beijing, China) (Approval Number: 2018‐1062). The data collection was carried out after written informed consent was obtained from the participants, including an interviewer‐administered questionnaire, physical examination, and blood sampling.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
For their contributions and participation, the authors would like to thank all the staff and participants of the China‐PAR project.
Aihaiti X, Chen S, Li J, et al. Prevalence of familial hypercholesterolemia and its association with coronary artery disease: a Chinese cohort study. Chronic Dis Transl Med. 2023;9:134‐142. 10.1002/cdt3.69
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request to the corresponding authors.
REFERENCES
- 1. Reiner Ž. Management of patients with familial hypercholesterolaemia. Nat Rev Cardiol. 2015;12(10):565‐575. [DOI] [PubMed] [Google Scholar]
- 2. Berberich AJ, Hegele RA. The complex molecular genetics of familial hypercholesterolaemia. Nat Rev Cardiol. 2019;16(1):9‐20. [DOI] [PubMed] [Google Scholar]
- 3. Hu P, Dharmayat KI, Stevens CAT, et al. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: a systematic review and meta‐analysis. Circulation. 2020;141(22):1742‐1759. [DOI] [PubMed] [Google Scholar]
- 4. Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide prevalence of familial hypercholesterolemia. JACC. 2020;75(20):2553‐2566. [DOI] [PubMed] [Google Scholar]
- 5. Crea F. Screening, diagnosis, and treatment of familial hypercholesterolaemia: a call to action. Eur Heart J. 2022;43(34):3185‐3188. [DOI] [PubMed] [Google Scholar]
- 6. Pang J, Sullivan DR, Harada‐Shiba M, et al. Significant gaps in awareness of familial hypercholesterolemia among physicians in selected Asia‐Pacific countries: a pilot study. J Clin Lipidol. 2015;9(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 7. Chen P, Chen X, Zhang S. Current status of familial hypercholesterolemia in China: a need for patient FH registry systems. Front Physiol. 2019;10:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi Z, Yuan B, Zhao D, Taylor AW, Lin J, Watts GF. Familial hypercholesterolemia in China: prevalence and evidence of underdetection and undertreatment in a community population. Int J Cardiol. 2014;174(3):834‐836. [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Li Y, Liu X, et al. The prevalence and related factors of familial hypercholesterolemia in rural population of China using Chinese modified Dutch Lipid Clinic Network definition. BMC Public Health. 2019;19(1):837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teng H, Gao Y, Wu C, et al. Prevalence and patient characteristics of familial hypercholesterolemia in a Chinese population aged 35‐75 years: results from China PEACE Million Persons Project. Atherosclerosis. 2022;350:58‐64. [DOI] [PubMed] [Google Scholar]
- 11. Austin MA. Familial hypercholesterolemia and coronary heart disease: a HuGE association review. Am J Epidemiol. 2004;160(5):421‐429. [DOI] [PubMed] [Google Scholar]
- 12. Benn M, Watts GF, Tybjaerg‐Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the danish general population: prevalence, coronary artery disease, and cholesterol‐lowering medication. J Clin Endocrinol Metab. 2012;97(11):3956‐3964. [DOI] [PubMed] [Google Scholar]
- 13. Khera AV, Won HH, Peloso GM, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. JACC. 2016;67(22):2578‐2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li JJ, Li S, Zhu CG, et al. Familial hypercholesterolemia phenotype in Chinese patients undergoing coronary angiography. Arterioscler Thromb Vasc Biol. 2017;37(3):570‐579. [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Li J, Hu D, et al. Predicting the 10‐year risks of atherosclerotic cardiovascular disease in Chinese population: the China‐PAR project (prediction for ASCVD risk in China). Circulation. 2016;134(19):1430‐1440. [DOI] [PubMed] [Google Scholar]
- 16. Liu F, Li J, Chen J, et al. Predicting lifetime risk for developing atherosclerotic cardiovascular disease in Chinese population: the China‐PAR project. Sci Bull. 2018;63(12):779‐787. [DOI] [PubMed] [Google Scholar]
- 17. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low‐density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vargas‐Vázquez A, Bello‐Chavolla OY, Antonio‐Villa NE, Mehta R, Cruz‐Bautista I, Aguilar‐Salinas CA. Comparative assessment of LDL‐C and VLDL‐C estimation in familial combined hyperlipidemia using Sampson's, Martin's and Friedewald's equations. Lipids Health Dis. 2021;20(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR**STELLAR = Statin Therapies for Elevated Lipid Levels compared Across doses to Rosuvastatin. Trial). Am J Cardiol. 2003;92(2):152‐160. [DOI] [PubMed] [Google Scholar]
- 20. Beheshti S, Madsen CM, Varbo A, Benn M, Nordestgaard BG. Relationship of familial hypercholesterolemia and high low‐density lipoprotein cholesterol to ischemic stroke: Copenhagen General Population Study. Circulation. 2018;138(6):578‐589. [DOI] [PubMed] [Google Scholar]
- 21. Williams RR, Hunt SC, Schumacher MC, et al. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol. 1993;72(2):171‐176. [DOI] [PubMed] [Google Scholar]
- 22. Singh S, Bittner V. Familial hypercholesterolemia—epidemiology, diagnosis, and screening. Curr Atheroscler Rep. 2015;17(2):3. [DOI] [PubMed] [Google Scholar]
- 23. National Bureau of Statistics of China . Population Census of People's Republic of China; 2010. http://www.stats.gov.cn/english/Statisticaldata/CensusData/rkpc2010/indexch.htm
- 24. Chieng D, Pang J, Ellis KL, Hillis GS, Watts GF, Schultz CJ. Elevated lipoprotein(a) and low‐density lipoprotein cholesterol as predictors of the severity and complexity of angiographic lesions in patients with premature coronary artery disease. J Clin Lipidol. 2018;12(4):1019‐1026. [DOI] [PubMed] [Google Scholar]
- 25. Pimstone SN, Sun XM, du Souich C, Frohlich JJ, Hayden MR, Soutar AK. Phenotypic variation in heterozygous familial hypercholesterolemia: a comparison of Chinese patients with the same or similar mutations in the LDL receptor gene in China or Canada. Arterioscler Thromb Vasc Biol. 1998;18(2):309‐315. [DOI] [PubMed] [Google Scholar]
- 26. Umans‐Eckenhausen MA, Defesche JC, Sijbrands EJ, Scheerder RL, Kastelein JJ. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 2001;357(9251):165‐168. [DOI] [PubMed] [Google Scholar]
- 27.Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ. 1991; 303(6807):893896. [DOI] [PMC free article] [PubMed]
- 28. Beheshti S, Madsen CM, Varbo A, Nordestgaard BG. How to identify familial premature myocardial infarction: comparing approaches to identify familial hypercholesterolemia. J Clin Endocrinol Metab. 2019;104(7):2657‐2667. [DOI] [PubMed] [Google Scholar]
- 29. de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016;133(11):1067‐1072. [DOI] [PubMed] [Google Scholar]
- 30. Choi Y, Chang Y, Kim BK, et al. Menopausal stages and serum lipid and lipoprotein abnormalities in middle‐aged women. Maturitas. 2015;80(4):399‐405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data are available upon reasonable request to the corresponding authors.


