Abstract
Background
Air pollution is a key public health factor with the capacity to induce diseases. The risk of ischemia heart disease (IHD) in those suffering from systemic lupus erythematosus (SLE) from air pollution exposure is ambiguous. This study aimed to: (1) determine the hazard ratio (HR) of IHD after the first-diagnosed SLE and (2) examine the effects of air pollution exposure on IHD in SLE for 12 years.
Methods
This is a retrospective cohort study. Taiwan’s National Health Insurance Research Database and Taiwan Air Quality Monitoring data were used in the study. Cases first diagnosed with SLE in 2006 cases without IHD were recruited as the SLE group. We randomly selected an additional sex-matched non-SLE cohort, four times the size of the SLE cohort, as the control group. Air pollution indices by residence city per period were calculated as the exposure. Life tables and Cox proportional risk models of time-dependent covariance were used in the research.
Results
This study identified patients for the SLE group (n = 4,842) and the control group (n = 19,368) in 2006. By the end of 2018, the risk of IHD was significantly higher in the SLE group than in the control group, and risks peaked between the 6th and 9th year. The HR of incidence IHD in the SLE group was 2.42 times that of the control group. Significant correlations with risk of developing IHD were noted for sex, age, CO, NO2, PM10, and PM2.5, of which PM10 exposure had the highest risk of IHD incidence.
Conclusions
Subjects with SLE were at a higher risk of IHD, especially those in the 6th to 9th year after SLE diagnosis. The advanced cardiac health examinations and health education plan should be recommended for SLE patients before the 6th year after SLE diagnosed.
Keywords: Ischemic heart disease, Systemic lupus erythematosus, Air pollution, Hazard
Introduction
Ischemic heart disease (IHD) is a major cause of mortality worldwide. In total, 20,457 individuals died of IHD in 2020, more than half of which were men (11,809 individuals) and frequently the primary breadwinners for their households [1]. IHD is a serious disease that entails a high probability of sudden death. Hence, families are often caught unprepared, and the effects on the family and society are profound.
Although some IHD-related risk factors cannot be modified, others can be mitigated if a high-risk patient is identified in time to devise a prevention strategy. Studies have indicated that the leading cause of IHD is atherosclerosis of coronary arteries and diminished blood supply, which damages the myocardium [2]. However, recent literature has reported that atherosclerosis itself is a chronic inflammatory condition [3]. The initial pathophysiological mechanism of IHD begins with atherosclerosis, which involves low-grade inflammation of the arterial intima (inner layer). The disease may persist for decades before it progresses to clinical symptoms. This low-grade state of inflammation induces thickening of the coronary artery lining, eventually resulting in varying degrees of arterial lumen stenosis [4]. The chronic inflammation of atherosclerosis promotes the retention of cholesterol-rich low-density lipoprotein (LDL) particles in the arterial wall. Subsequently, oxidative modification of vascular LDL particles promotes an inflammatory response to this endothelial injury. Macrophages ingest the LDL particles and develop into lipid-foam cells.
Further, local inflammation is stimulated. Early atherosclerotic lesions called ‘fatty streaks’ are composed of lipid deposits, cholesterol-laden macrophages, foam cells, and T cells. With disease progression, immune cells interact with resident blood vessel wall cells, eventually forming atherosclerotic plaques [3]. Finally, atherosclerotic plaques are composed of inflammatory cells, cell debris, smooth muscle cells, and cholesterol [4]. This inflammatory-related perspective differs from the previous understanding of the risk factors for IHD. It is therefore desirable to determine whether inflammation-related diseases are associated with IHD.
Inflammation-related diseases include systemic lupus erythematosus (SLE), diabetes, hypertension, and hyperlipidemia. The SLE-mediated relationship between body image and fatigue severity significantly influences a patient’s psychological condition and quality of life (QOL) [5]; as the state of SLE deteriorates, patients’ medical expenses and work disability increase [6, 7]. The diverse symptoms of SLE, such as fatigue severity, impaired body image, depression, and anxiety, impact the individual’s QOL, and not only in the psychological domain [5]. The symptoms associated with SLE can significantly affect patients’ QOL in terms of work and social life [8]. SLE results from a malfunctioning immune system that attacks self-antigens [9]. The actual etiology of SLE remains unknown. Numerous risk factors are associated with immune-related diseases. A complex interaction likely occurs among genetic factors, infectious agents, ultraviolet rays, smoking, and hormonal factors, leading to immune system disturbance and disease manifestation [10]. SLE-related risk factors continue to be investigated.
Even though healthcare quality has improved for SLE and IHD in the last century, SLE progression to IHD is still unavoidable. The major difference between today and the previous century is air pollution.
This study aimed to: (1) determine the hazard ratio (HR) of IHD after the first-diagnosed SLE and (2) examine the effects of air pollution exposure on ischemic heart disease (IHD) in Systemic lupus erythematosus (SLE) for 12 years. We used data from the National Health Insurance Research Database (NHIRD) and Taiwan Air Quality Index data (AQI) of Taiwan to investigate IHD development in the population newly diagnosed with SLE from 2006 to 2018.
Methods
Study Design and Data source
This study adopted a longitudinal cohort design over 12 years and involved a secondary analysis based on data from the NHIRD of Taiwan. The NHIRD is the largest medical database in Taiwan and contains a wealth of health insurance information. National Health Insurance has covered 99.82% of individuals in Taiwan since 1996. Enrolment is compulsory for most of the population. Therefore, the data can be considered to accurately represent the situation of the population in Taiwan. Medical records, pharmaceutical information, admission and outpatient data, and physical examination records are included in the NHIRD. This study was approved by the Institutional Review Board of China Medical University and Hospital in Taiwan [CMUH111-REC3-040].
Comorbidities and medications
Diseases related to inflammation, including diabetes, hypertension, and hyperlipidemia, were considered potential confounders. Variables (i.e., age and sex) that influenced both SLE and IHD were defined as confounders.
Sampled study cohorts and data Collection
The study population data were extracted from the NHIRD. The inclusion criteria for the study participants were a new diagnosis of SLE in 2006 and an age above 20 years. The exclusion criteria were a diagnosis of SLE or IHD before 2006. We randomly selected an additional sex-matched non-SLE cohort, four times the size of the SLE cohort, as the control group. The SLE disease codes were identified based on the International Classification of Diseases, Ninth Revision (ICD-9) codes 710. IHD includes angina, acute myocardial infarction, and other IHDs. The IHD disease codes were identified based on the ICD-9 codes 410–414.
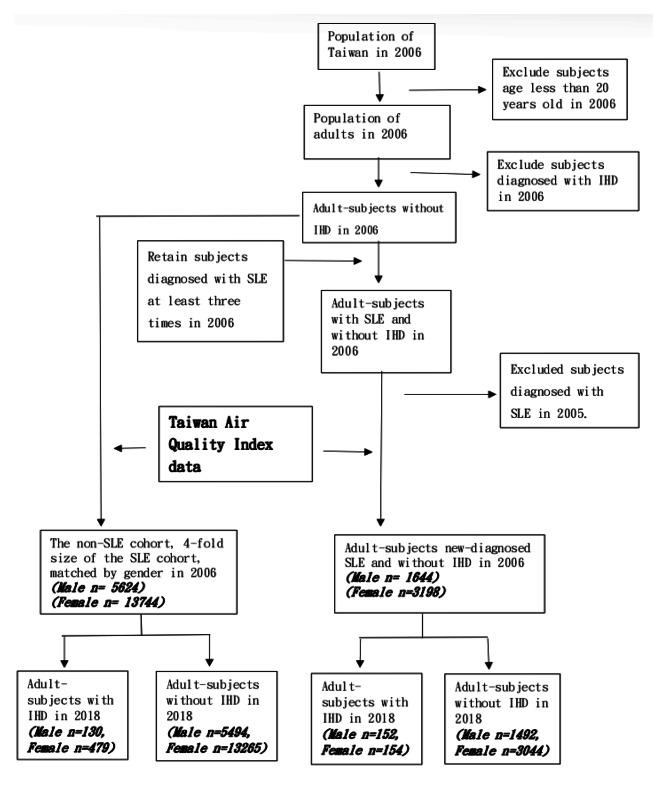
This research focused on adults. SLE and IHD are considered chronic diseases, and subjects with these diseases should visit a doctor at least once a year. Therefore, subjects diagnosed with SLE in 2005 or earlier were excluded. The 4,842 subjects newly diagnosed with SLE and without IHD in 2006 were defined as the “case group” and matched with 19,368 subjects for the control group. We assumed that exposure to air pollution was similar for all subjects in a given area. Patients were linked to the nearest AQI station via their residence, which was used to define their air pollution exposure. We used the residence of patients of both groups to concatenate the data of NHIRD and AQI for subsequent statistical analysis. (Fig. 1).
Fig. 1.
Flow diagram of the sampling process
SLE: Systemic lupus erythematosus IHD: Ischemic heart disease
Statistical analysis
SAS version 9.4 was used to analyze the data. The chi-square test was used to identify differences in patient characteristics by SLE incidence. Life-table method analysis with log-rank tests was performed to examine differences in SLE incidence between male and female patients. Cox proportional hazard regression analyses were performed to analyze the HR of IHD in the SLE and control groups.
Results
Demographical data of subjects in 2006, IHD incidence, and air pollution condition in 2018 are summarized in Table 1. This cohort consisted of 24,210 participants. The study included 4,842 subjects (male n = 1,644, female n = 3,198) with first-diagnosed SLE (SLE group) and 19,368 participants (male n = 5,624, female n = 13,744) without SLE (control group). More than half of the SLE subjects were female. The number of subjects suffering from SLE was higher in the “above 60” age group for both females (24.48%) and men (30.75%). Most of the cohort was without DM, hypertension, or hyperlipidemia. Base on the rule of NHIR in Taiwan, subjects who with the higher socioeconomic status (SS), the higher insurance premium should be pay. The insured amount of NHIR depends on the individual’s SS. Therefore, the SS can be estimated from the insurance amount and a higher-than-average insured amount was defined as high SS in the research.
Table 1.
Distributions of demographic and clinical comorbid status in study cohorts
| SLE | Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||||
| n/mean | (%)/sd | n/mean | (%)/sd | n/mean | (%)/sd | n/mean | (%)/sd | |||||
| In 2006 | ||||||||||||
| Sex | 1301 | 27 | 3541 | 73 | 5196 | 27 | 14,087 | 73 | ||||
| Age | 49.91 | 18.78 | 46.1 | 16.2 | 43.58 | 16.92 | 42.77 | 16.43 | ||||
| 20–29 | 191 | 14.68 | 542 | 15.31 | 1160 | 22.17 | 3260 | 23.09 | ||||
| 30–39 | 222 | 17.06 | 645 | 18.22 | 1048 | 20.02 | 2929 | 20.74 | ||||
| 40–49 | 263 | 20.22 | 733 | 20.7 | 1093 | 20.88 | 2922 | 20.69 | ||||
| 50–59 | 225 | 17.29 | 754 | 21.29 | 861 | 16.38 | 2349 | 16.61 | ||||
| Above 60 | 400 | 30.75 | 867 | 24.48 | 1077 | 20.55 | 2669 | 18.88 | ||||
| DM | no | 1287 | 98.92 | 3520 | 99.41 | 5032 | 96.44 | 13,675 | 96.93 | |||
| yes | 14 | 1.08 | 21 | 0.59 | 207 | 3.56 | 454 | 3.07 | ||||
| HT | no | 1277 | 98.16 | 3502 | 98.9 | 4823 | 92.42 | 13,202 | 93.58 | |||
| yes | 24 | 1.84 | 39 | 1.1 | 416 | 7.58 | 927 | 6.42 | ||||
| HL | no | 100 | 3514 | 99.24 | 5087 | 97.5 | 13,837 | 98.08 | ||||
| yes | 27 | 0.76 | 151 | 2.5 | 292 | 1.92 | ||||||
| SS | 26,850 | 25625.37 | 26205.46 | 23822.19 | ||||||||
| In 2018 | ||||||||||||
| IHD | no | 1492 | 3044 | 5494 | 13,265 | |||||||
| yes | 152 | 154 | 130 | 479 | ||||||||
| Air | CO | 5.16 | 5.7 | 5.32 | 5.64 | |||||||
| pollution | NO2 | 188.87 | 208.5 | 194.04 | 206.06 | |||||||
| O3 | 313.3 | 342.3 | 335.17 | 349.42 | ||||||||
| PM10 | 563.81 | 611.87 | 603.34 | 629.69 | ||||||||
| PM2.5 | 327.88 | 355.53 | 344.2 | 359.81 | ||||||||
| SO2 | 38.9 | 42.06 | 43.11 | 44.97 | ||||||||
SLE: Systemic Lupus Erythematosus IHD: Ischemic heart disease DM: Diabetes Mellitus.
HT: Hypertension HL: Hyperlipidemia SS: Socioeconomic status.
CO: carbon monoxide NO2: nitrogen dioxide O3: ozone.
PM10: particulate matter 10 PM2.5: particulate matter 2.5 SO2: sulfur dioxide.
The IHD incidence rate during follow-up for 12 years was 9.2% in men and 4.8% in women in the SLE group, compared to 2.3% and 3.5%, respectively, in the control group. Exposure to air pollution was slightly higher in females in both the SLE and control groups.
Table 2 shows Pearson’s correlation of air pollution indices, CO, NO2, O3, PM10, PM2.5, and SO2. All the air pollution indices were significantly correlated (p < 0.01). Correlations of O3 with CO (r = 0.968, p < 0.01), and PM10 with PM2.5(r = 0.951, p < 0.01) were above 0.9. The lowest correlation was found between PM10 and NO2 (r = 0.408, p < 0.01). The presence of high correlations suggests that collinearity should be considered. We further defined the higher than average of each air pollution index as the “high exposure group” and others as the “low exposure group” for the Cox proportional risk model of time-dependent covariance.
Table 2.
Correlations among air pollution indices
| CO | NO2 | O3 | PM10 | PM2.5 | SO2 | |
|---|---|---|---|---|---|---|
| CO | 1 | 0.968** | 0.544** | 0.465** | 0.449** | 0.652** |
| NO2 | 1 | 0.437** | 0.408** | 0.423** | 0.685** | |
| O3 | 1 | 0.821** | 0.768** | 0.793** | ||
| PM10 | 1 | 0.951** | 0.739** | |||
| PM2.5 | 1 | 0.743** | ||||
| SO2 | 1 |
** p < 0.01
CO: carbon monoxide NO2: nitrogen dioxide O3: ozone
PM10: particulate matter 10 PM2.5: particulate matter 2.5 SO2: sulfur dioxide
The life-table method was used to measure the difference in IHD incidence between the SLE and control groups (Table 3). The estimated hazard curves of those two groups were tested using the log-rank test. Our study showed a statistically significant difference in IHD incidence between the SLE and control groups (log-rank test, p < 0.001). During the follow-up period, the risk of IHD incidence was higher in the SLE group (Fig. 2). The difference in IHD incidence between the SLE and control group peaked between the 6th and 9th years, during which period the hazard ratio of the SLE group was 2.42 times that of the control group (Table 3).
Table 3.
Duration of progression to IHD in the SLE and control group
| Group | Interval (year) |
Log-Rank test | Survival (%) |
Failure (%) |
Survival SE (‰) | Evaluated at the midpoint of the interval | |
|---|---|---|---|---|---|---|---|
| Hazard | Hazard SE | ||||||
| SLE | 490.1264* | ||||||
| 0–3 | 1 | 0 | 0 | 0.001015 | 0.000089 | ||
| 3–6 | 99.7 | 0.30 | 0.266 | 0.002568 | 0.000142 | ||
| 6–9 | 98.9 | 1.07 | 0.499 | 0.005722 | 0.000215 | ||
| 9–12 | 97.3 | 2.75 | 0.796 | 0.002551 | 0.000146 | ||
| 12 | 96.5 | 3.49 | 0.896 | . | . | ||
| Control | |||||||
| 0–3 | 1 | 0 | 0 | 0.000618 | 0.000033 | ||
| 3–6 | 99.8 | 0.19 | 0.099 | 0.001988 | 0.000060 | ||
| 6–9 | 99.2 | 0.78 | 0.203 | 0.002360 | 0.000066 | ||
| 9–12 | 98.5 | 1.48 | 0.280 | 0.001004 | 0.000043 | ||
| 12 | 98.2 | 1.77 | 0.307 | . | . | ||
* p < 0.01
Fig. 2.
The probability of ischemic heart diseases incidence for the SLE and control group
We further evaluated whether comorbidities and air pollution exposure impacted IHD incidence by stratifying the air pollution exposure into two segments (high segment ≥ mean, low segment < mean) in SLE subjects (Table 4) and control subjects (Table 5). The Cox proportional risk model of time-dependent covariance analyses revealed that sex, age, CO, NO2, PM10, and PM2.5 were significantly related to developing IHD in both the SLE and control groups. The highest risk of developing IHD was identified on PM10 exposure in SLE subjects (HR = 66.197, p < 0.01) and in the control group (HR = 108.945, p < 0.01). The risk of developing IHD was significantly higher in the male cohort than the female cohort in both the SLE group (HR = 1.817, p < 0.01) and the control group (HR = 1.609, p < 0.01). In the control group, having diabetes was also associated with a 1.57-fold increase in IHD incidence (p < 0.001). Compared with the SLE group, the prevalence of diabetes was not significantly related to IHD incidence.
Table 4.
The HR of development to IHD in the SLE group under air pollution (N = 4842)
| Parameter Estimate | SE | Chi-Square | HR | 95% HR Confidence | ||
|---|---|---|---|---|---|---|
| Sex | Male | 0.59724 | 0.17771 | 11.2947* | 1.817 | 1.283, 2.574 |
| Female (ref) | 1 | |||||
| Age | 0.10032 | 0.0068 | 217.62* | 1.106 | 1.091, 1.12 | |
| DM | yes | -0.15135 | 1.01579 | 0.0222 | 0.86 | 0.117, 6.294 |
| no(ref) | 1 | |||||
| HT | yes | 0.33328 | 0.46448 | 0.5149 | 1.396 | 0.562, 3.468 |
| no(ref) | 1 | |||||
| HL | yes | 0.19407 | 0.23181 | 0.7009 | 1.214 | 0.771, 1.912 |
| no(ref) | 1 | |||||
| SS | High | 0.56996 | 1.00916 | 0.319 | 1.768 | 0.245, 12.78 |
| Low(ref) | 1 | |||||
| CO | high | 2.56001 | 0.51353 | 24.8512* | 12.936 | 4.728, 35.393 |
| low(ref) | 1 | |||||
| NO2 | high | 3.89938 | 0.43186 | 81.5272* | 49.372 | 21.178, 115.101 |
| low(ref) | 1 | |||||
| O3 | high | -0.0731 | 0.04957 | 2.1761 | 0.929 | 0.843, 1.024 |
| low(ref) | 1 | |||||
| PM10 | high | 4.19263 | 0.51048 | 67.4557* | 66.197 | 24.34, 180.035 |
| low(ref) | 1 | |||||
| PM2.5 | high | 3.54241 | 0.39236 | 81.5125* | 34.55 | 16.013, 74.546 |
| low(ref) | 1 | |||||
| SO2 | high | 0.0304 | 0.19678 | 0.0239 | 1.031 | 0.701, 1.516 |
| low(ref) | 1 |
h: Hazard ratio SE: Standard error *p < 0.01
SLE: Systemic Lupus Erythematosus IHD: Ischemic heart disease DM: Diabetes Mellitus
HT: Hypertension HL: Hyperlipidemia SS: Socioeconomic status
CO: carbon monoxide NO2: nitrogen dioxide O3: ozone
PM10: particulate matter 10 PM2.5: particulate matter 2.5 SO2: sulfur dioxide
Table 5.
The HR of development to IHD in the control group under air pollution (N = 19,368)
| Parameter Estimate | SE | Chi-Square | HR | 95% HR Confidence | ||
|---|---|---|---|---|---|---|
| Sex | Male | 0.4759 | 0.09046 | 27.6763* | 1.609 | 1.348, 1.922 |
| Female (ref) | 1 | |||||
| Age | 0.11337 | 0.00348 | 1058.51* | 1.12 | 1.112, 1.128 | |
| DM | yes | 0.45079 | 0.15837 | 8.102* | 1.57 | 1.151, 2.141 |
| no(ref) | 1 | |||||
| HT | yes | -0.0383 | 0.12335 | 0.0962 | 0.962 | 0.756, 1.226 |
| no(ref) | 1 | |||||
| HL | yes | 0.18626 | 0.23133 | 0.6483 | 1.205 | 0.766, 1.896 |
| no(ref) | 1 | |||||
| SS | High | 0.27921 | 0.45263 | 0.3805 | 1.322 | 0.544, 3.21 |
| Low(ref) | 1 | |||||
| CO | high | 3.0434 | 0.32109 | 89.8381* | 20.976 | 11.179, 39.359 |
| low(ref) | 1 | |||||
| NO2 | high | 4.0207 | 0.26526 | 229.757* | 55.74 | 33.142, 93.747 |
| low(ref) | 1 | |||||
| O3 | high | -0.0428 | 0.02476 | 2.9802 | 0.958 | 0.913, 1.006 |
| low(ref) | 1 | |||||
| PM10 | high | 4.69084 | 0.35732 | 172.345* | 108.945 | 54.083, 219.459 |
| low(ref) | 1 | |||||
| PM2.5 | high | 3.43357 | 0.20312 | 285.757* | 30.987 | 20.811, 46.14 |
| low(ref) | 1 | |||||
| SO2 | high | 4.63071 | 0.38239 | 146.652* | 102.587 | 48.485, 217.061 |
| low(ref) | 1 |
h: Hazard ratio SE: Standard error *p < 0.01
SLE: Systemic Lupus Erythematosus IHD: Ischemic heart disease DM: Diabetes Mellitus
HT: Hypertension HL: Hyperlipidemia SS: Socioeconomic status
CO: carbon monoxide NO2: nitrogen dioxide O3: ozone
PM10: particulate matter 10 PM2.5: particulate matter 2.5 SO2: sulfur dioxide
Discussion
This cohort study analyzed the risk of developing IHD among newly diagnosed SLE subjects from 2006 to 2018. The study results revealed three findings. First, during the follow-up period, the risk of IHD incidence was higher in the SLE group. The difference in IHD incidence between the SLE and control groups peaked between the 6th and 9th year after SLE diagnosis. Second, the peak hazard ratio of IHD in the SLE population occurred during this period. Third, sex, age, CO, NO2, PM10, and PM2.5 significantly contributed to the progression to IHD in SLE patients; PM10 was the highest risk factor for both the SLE and control groups.
It is well known that subjects with atherosclerosis are susceptible to suffering from IHD, and previous studies have documented that the hazard of atherosclerosis is higher in SLE subjects than in the general population [11]. Some studies have also indicated that IHD incidence increases in subjects with SLE [3, 12, 13]. The disturbance of the immune system and inflammation could promote atherosclerosis and further contribute to IHD [14]. In the present study, the risk of progression to IHD after SLE diagnosis in the SLE group was higher than in the control group, which was consistent with the previous literature.
We found that the highest hazard ratio of IHD occurred in the 6th -9th year after SLE diagnosis and that the hazard ratio in the SLE group was 2.42 times that of the control group. A previous study indicated that subjects with SLE had a higher risk of suffering from cardiovascular diseases compared to the general population, reporting a similar range as in our study (HR 2.67 [95% CI 2.38–2.99]) [15]. In contrast, another study found that the relative risk of myocardial infarction was between five to eight times greater in SLE subjects compared with the general population [11]. However, there was a difference in the study populations, as we recruited not only myocardial infarction patients but also angina patients. The severity and lethality were more serious in myocardial infarctions than in angina, which might contribute to the difference in results.
Our results indicated that the highest HR of IHD occurred in the 6th -9th year after SLE diagnosis and that HR decreased after the 9th year. Previous studies showed that the traditional pathological mechanism could not explain the progression of cardiovascular disease induced by SLE. Vascular events may result from several pathophysiologic mechanisms, including atherosclerosis, primarily thrombotic, and ongoing inflammation [16]. The primary pathogenesis of IHD in the SLE group and the general population is that inflammation due to SLE might strongly contribute to developing IHD. In the absence of immune system problems, traditional risk factors might influence the general population suffering from IHD. Therefore, the IHD incidence differed between SLE subjects and the general population. Inflammation increased with age in the subjects diagnosed with SLE, and theoretically, the risk of progression to IHD was also elevated. Although this study showed that SLE subjects had a higher risk of developing IHD than the control group, the highest HR occurred in the 6th to 9th year after SLE diagnosis. However, the HR gradually declined after the 9th year. A previous study showed a significant difference in the prevalence of cardiovascular disease in subjects of different ethnicities [17], which might contribute to the discrepancy in results between our study and previous research. In addition, since the quality of the medical system in Taiwan is excellent and the case management system for SLE is quite mature, the SLE population may have improved their self-care skills and knowledge in spite of inflammation increasing over the years. This may have prevented an increase in the difference in the HR of IHD between the case and control groups.
Women with diabetes have a greater relative risk of cardiovascular disease compared with their male counterparts [18]. In the present study, diabetes was a significant risk factor in the control subjects who developed IHD, but not a significant risk factor for developing IHD in the SLE group, which is inconsistent with previous findings. Differences in study populations and ethnicity might explain this difference.
A previous study demonstrated a 50 times greater risk of myocardial infarction in female patients with SLE aged 35–44 years than in women of similar age without SLE in a population-based sample [19]. In our study, female patients in the control group aged 30–59 years were associated with a lower risk of IHD (HR < 1); this age group may include women who have not experienced menopause, and estrogen might thus exert a protective effect on their cardiovascular system. The results of our study are consistent with previous findings that estrogen levels associated with the menstrual cycle may contribute to the delayed onset of cardiovascular diseases in women compared with men [18]. However, patients with SLE from the same age group were at a higher risk of IHD (HR 1.27–2.67), indicating that SLE might increase the susceptibility of this population to IHD.
Conclusions
Subjects with SLE were at a higher risk of IHD, especially those in the 6th to 9th year after SLE diagnosis. Since the highest risk for developing IHD in SLE patients is during this period, it is recommended that advanced cardiac health examinations be included in the health insurance benefits for SLE patients in the 5th to 9th year after SLE diagnosis. At the same time, case managers should make sure that education on diet and exercise is included in the health education plan for first-diagnosed SLE patients. The health education plan could promote a healthier lifestyle for SLE patients to reduce the risk of atherosclerosis or IHD.
This retrospective study used a secondary database search of the NHIR to explore the risk of IHD in first-diagnosed SLE patients. A limitation of this approach was the absence of data on items such as psychosocial issues, clinical measurements, and lifestyle. Future related research on clinical trials should include these data. The results of this study provide a reference for the next phase of clinical trials.
This study has some limitations. First, using the health insurance database, we recruited the subjects only by the ICD-9 code. This database did not include information such as psychosocial information (depression, anxiety, A-type, etc.), health promotion data (smoking and drinking), family history (hereditary disease), and medication adherence. Second, residence status was likely partly inaccurate as subjects might move house. Finally, smoking is one of the risk factors for atherosclerosis. Both direct and second-hand smokers are at risk of nicotine exposure. Unlike the United States and Europe, most areas in Taiwan have a high population density, increasing exposure to a smoking environment even for non-smokers. In the future, relevant clinical studies should identify whether carbon monoxide in exhaled breath can be used in non-smokers to identify smoking status.
Acknowledgements
“Not applicable”.
Abbreviations
- SLE
Systemic Lupus Erythematosus
- IHD
Ischemic heart disease
- DM
Diabetes Mellitus
- HT
Hypertension
- HL
Hyperlipidemia
- CO
carbon monoxide
- NO2
nitrogen dioxide
- O3
ozone
- PM10
particulate matter 10
- PM2.5
particulate matter 2.5
- SO2
sulfur dioxide
Biographies
Pei-Yun Chen
is a RN and assistant professor. The major of Chen is Gerontology and Epidemiology.
Yu-Tse Tsan
is a Clinicians. The major of Tsan is Occupational Medicine.
Chao-Tung Yang
is a Professor. The major of Yang is Computer Science.
Yun-Mei Lee
is a RN and Lecture. The major of Lee is Medicine- Surgery Nursing.
Wen-Chao Ho
is a Professor of Public Health. The major of Ho is public health and Epidemiology.
Shu-Hua Lu
is a RN and Associate Professor. The major of Lu is Medicine- Surgery Nursing.
Authors’ contributions
PYC wrote the main manuscript text, perform the research, and analyzed the data.
YTT, CTY, YM, and WCH interpreted the data.
LLC, WCH, and SHL check the statistical process.
SHL revise the sentence and check the grammar.
All authors read and approved the final manuscript.
Funding
The sources of funding for the research include China Medical University, Taiwan, CMU104-S-52. The role of the funding body in the design of the study and in writing the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article. The data are available from the first author on reasonable request. The first author is Pei-Yun Chen (e-mail: peiyun0203@gmail.com).
Declarations
Ethics approval and consent to participate
The institutional review board (IRB) of China Medical University Hospital in Taiwan approved the study. The committee’s reference number is “CMUH111-REC3-040”. All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from participants.
The licenses from Ministry of Health and Welfare was acquired by our team to access the clinical patient data used in our research. The approval number is H111049.
Consent for publication
“Not applicable”.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wen-Chao Ho, Email: wcho@mail.cmu.edu.tw.
Shu-Hua Lu, Email: shuhua@mail.cmu.edu.tw.
References
- 1.Ministry of Health and Welfare., M. Statistics on the causes of death of Taiwan in 2020. 2021.
- 2.Shao C, et al. Coronary artery disease: from mechanism to clinical practice. Adv Exp Med Biol. 2020;1177:1–36. doi: 10.1007/978-981-15-2517-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Wirtz PH, von Känel R. Psychological stress, inflammation, and Coronary Heart Disease. Curr Cardiol Rep. 2017;19(11):111. doi: 10.1007/s11886-017-0919-x. [DOI] [PubMed] [Google Scholar]
- 4.Ambrose JA, Singh M. Pathophysiology of coronary artery disease leading to acute coronary syndromes F1000Prime Rep, 2015. 7: p. 08. [DOI] [PMC free article] [PubMed]
- 5.Pereira MG, et al. Quality of life in patients with systemic lupus erythematosus: the mediator role of psychological morbidity and disease activity. Psychol Health Med. 2020;25(10):1247–57. doi: 10.1080/13548506.2020.1728350. [DOI] [PubMed] [Google Scholar]
- 6.Houri Levi E, et al. Coexistence of ischemic heart disease and rheumatoid arthritis patients-A case control study. Autoimmun Rev. 2016;15(4):393–6. doi: 10.1016/j.autrev.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto ST, Valim V, Fisher BA. Health-related quality of life and costs in Sjögren’s syndrome. Rheumatology (Oxford); 2019. [DOI] [PubMed]
- 8.Di Cara M, et al. Quality of life in patients with multiple sclerosis and caregivers. Predictive factors: an observational study. J Clin Neurosci. 2020;78:242–5. doi: 10.1016/j.jocn.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan R, Raina V, Nandi SP. Prevalence of Autoimmune Diseases and its Challenges in diagnosis. Crit Rev Immunol. 2019;39(3):189–201. doi: 10.1615/CritRevImmunol.2019031798. [DOI] [PubMed] [Google Scholar]
- 10.Ortona E, et al. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita. 2016;52(2):205–12. doi: 10.4415/ANN_16_02_12. [DOI] [PubMed] [Google Scholar]
- 11.Tazi Mezalek Z, et al. [Atherosclerosis in systemic lupus erythematosus] Presse Med. 2014;43(10 Pt 1):1034–47. doi: 10.1016/j.lpm.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Kostopoulou M, et al. Cardiovascular Disease in systemic Lupus Erythematosus: recent data on epidemiology, risk factors and Prevention. Curr Vasc Pharmacol. 2020;18(6):549–65. doi: 10.2174/1570161118666191227101636. [DOI] [PubMed] [Google Scholar]
- 13.Luni FK, et al. Risk of ischemic heart disease in patients with Sjögren’s Syndrome. Am J Med Sci. 2017;354(4):395–8. doi: 10.1016/j.amjms.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira CB, Kaplan MJ. Cardiovascular disease risk and pathogenesis in systemic lupus erythematosus. Semin Immunopathol. 2022;44(3):309–24. doi: 10.1007/s00281-022-00922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbhaiya M, et al. Comparative risks of Cardiovascular Disease in patients with systemic Lupus Erythematosus, Diabetes Mellitus, and in General Medicaid recipients. Volume 72. Arthritis Care & Research; 2020. pp. 1431–9. 10. [DOI] [PMC free article] [PubMed]
- 16.Gustafsson JT, Svenungsson E. Definitions of and contributions to cardiovascular disease in systemic lupus erythematosus. Autoimmunity. 2014;47(2):67–76. doi: 10.3109/08916934.2013.856005. [DOI] [PubMed] [Google Scholar]
- 17.Khoddam H, Alemi Z, Modanloo M. Comparison of prevalence and risk factors of Acute Coronary Syndrome in patients with different ethnicity: a cross-sectional study. Ethiop J Health Sci. 2021;31(5):1011–8. doi: 10.4314/ejhs.v31i5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young L, Cho L. Unique cardiovascular risk factors in women. Heart. 2019;105(21):1656–60. doi: 10.1136/heartjnl-2018-314268. [DOI] [PubMed] [Google Scholar]
- 19.Durante A, Bronzato S. The increased cardiovascular risk in patients affected by autoimmune diseases: review of the various manifestations. J Clin Med Res. 2015;7(6):379–84. doi: 10.14740/jocmr2122w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The data are available from the first author on reasonable request. The first author is Pei-Yun Chen (e-mail: peiyun0203@gmail.com).