Abstract
We describe the successful use of the novel antifungal drug fosmanogepix to treat a chronic case of multidrug-resistant cutaneous Fusarium suttonianum infection in a pediatric patient with STAT3 hyper-IgE syndrome and end-stage kidney disease on peritoneal dialysis.
Treatment of rare mold infections in immunocompromised patients is challenging due to the complexities of host and pathogen. Critical illness, comorbidities, active or previous end-organ damage, and polypharmacy can compound to increase the risks of both infection and treatment. Pathogens are often highly drug-resistant, and new antifungal therapies are needed that are safe, effective, and well tolerated. Fosmanogepix (FMGX) is a novel agent working its way through the antifungal pipeline that is currently in late-stage clinical development for invasive candidiasis, aspergillosis, and other rare mold infections. We describe the compassionate use of FMGX to treat a pediatric patient with signal transducer and activator of transcription 3 (STAT3) autosomal dominant hyper-immunoglobulin E syndrome (STAT3 AD-HIES) and chronic cutaneous fusariosis caused by Fusarium suttonianum. The patient was treated unsuccessfully with multiple antifungal therapies over many years and subsequently presented with end-stage kidney disease secondary to immune complex-mediated glomerulonephritis and amphotericin nephrotoxicity, requiring initiation of peritoneal dialysis. Further treatment of the indolent infection was undertaken after the patient sought clearance from infectious disease before listing for kidney transplant.
CASE REPORT
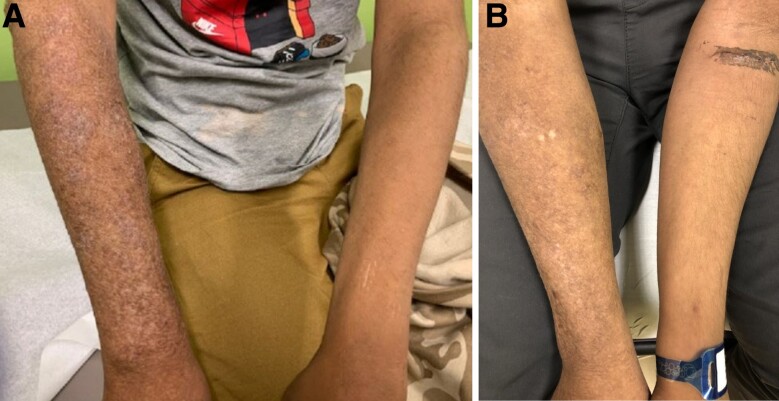
The patient is a 14-year-old Hispanic male diagnosed with signal transducer and activator of transcription 3 (STAT3) autosomal dominant hyper-immunoglobulin E syndrome (STAT3 AD-HIES) primary immunodeficiency in early childhood, whose prior course has been published [1, 2]. At age 3 years of age, he developed a 3 × 3-cm plaque on his right forearm after a dog bite, later diagnosed as Fusarium solani species complex (FSSC) infection. Over the next 8 years, he intermittently received numerous ultimately unsuccessful antifungal therapies, including multiple courses of intravenous (IV) liposomal amphotericin B, and various combination therapies including topical agents and nonpharmacological methods (Table 1). The right forearm lesion waxed and waned in size but ultimately extended from the wrist to the elbow (Figure 1), with persistent growth of FSSC from skin biopsy cultures and development of a small right foot lesion, which was also confirmed by biopsy as FSSC infection. There were no signs or symptoms of systemic infection. The patient experienced acute kidney injury due to amphotericin. At age 12, the patient established care with the Infectious Disease (ID) clinic at our institution, at which time he had been lost to follow-up with ID/Immunology/Dermatology, had been off all antifungal therapy for over 1 year, and was noted to be hypertensive. The FSSC infection appeared as lichenified, erythematous plaques covering the entire dorsal and partial ventral right forearm (Figure 1), and there was a small hyperpigmented patch on the right medial ankle. Antifungal therapy was not immediately reinitiated while awaiting receipt of patient's medical records, given the superficial and chronic nature of the infection and history of treatment failure and intolerance to available antifungals. Less than 2 months later, the patient presented with overt clinical symptoms and laboratory findings of end-stage kidney disease (ESKD). This was confirmed by kidney biopsy showing diffuse tubulointerstitial scarring and inflammation with findings suggestive of immune complex-mediated glomerulonephritis, and the patient was started on peritoneal dialysis (PD). He was referred back to ID clinic 1 year later for pre-kidney transplant clearance.
Table 1.
History of Intermittent Fusarium Treatments Before Fosmanogepix (Age 3–11 Years)
| Therapy | Clinical Response | Isolate MIC |
|---|---|---|
| Itraconazole | None | N/A |
| Itraconazole + terbinafine | None | N/A (itraconazole); >2 (terbinafine) |
| Oral voriconazole × 8 months | Decreased size of RF lesion but discontinued due to hallucinations | 16 |
| Oral posaconazole | None; new lesion developed on RA confirmed as Fusarium | >16 |
| Topical therapies: polyhexamethylene biguanide 0.02%, chlorhexidine, silver sulfadiazine cream, nystatin ointment, terbinafine cream, imiquimod, gentian violet, natamycin 5% ophthalmic solution | None or unsustained short-term improvement | N/A |
| Heat therapy | None | N/A |
| Subcutaneous interferon-gamma in combination with other therapies | None | N/A |
| IV liposomal amphotericin B: intermittent courses on and off through multiple hospitals over the years, including at least 1 course of biweekly dosing × 6 months via port that was discontinued due to nephrotoxicity |
Partially successful, caused AKI | 1 |
| Oral voriconazole + topical amphotericin B | Partially successful; limited course | 16 (voriconazole); 1 (amphotericin) |
| Blue light photodynamic therapy + oral voriconazole + oral terbinafine | Partially successful (not sustained) | 16 (voriconazole); >2 (terbinafine) |
| Oral isavuconazole × 8 months | Progression of RF lesion | >16 |
Abbreviations: AKI, acute kidney injury; IV, intravenous; MIC, minimum inhibitory concentration in micrograms/milliliter; N/A, not applicable; RA, right ankle; RF, right forearm.
Figure 1.
(A) Photo of chronic right forearm skin lesion before fosmanogepix (FMGX) therapy. (B) Photo of right forearm lesion after FMGX therapy. Treatment resulted in resolution of thickened, rough plaques leaving smooth hyperpigmented skin.
Due to the risk of treatment-refractory, multidrug-resistant FSSC infection disseminating or becoming locally invasive with transplant immunosuppression, further treatment of the chronic FSSC infection was pursued. Debridement was not considered due to extent. Cultures and histopathology obtained from skin biopsies at 2 right forearm sites confirmed persistent Fusarium suttonianum (member of FSSC) infection with multidrug resistance, with negative tests from the right ankle (Table 2 and Figure 2). Aspergillus galactomannan and plasma microbial cell-free deoxyribonucleic acid sequencing were negative.
Table 2.
Pathology and Culture Results Including MIC Values for Most Recent Fusarium solani/Fusarium suttonianum Isolates for Different Antifungal Agents With Clinical and CLSI Interpretations
| Fungal Culture Results |
3 Years Before Starting FMGX (RF, Outside Institution): F solani Species Complex |
4 Months Before Starting FMGX, 2 Sites RF: F suttonianum; 1 Site RA: No Fungus Isolated |
2 Months After Starting FMGX, 2 Sites RF: F suttonianum (1 of 2 sites) |
5 Months After Starting FMGX, 2 Sites RF: No Fungus Isolated |
3 Months After Stopping FMGX, 2 Sites RF: No Fungus Isolated |
|||
|---|---|---|---|---|---|---|---|---|
| Drug | MIC | Int. | MIC | Int. | MIC | Int. | … | … |
| Amphotericin B | 1 | NEB | 1 | NEB | 1 | NEB | … | … |
| Caspofungin | >8 | NEB | … | … | … | … | … | … |
| Posaconazole | >16 | NEB | >16 | NEB | … | … | … | … |
| Voriconazole | 16 | NEB | 16 | NEB | … | … | … | … |
| Isavuconazole | >16 | NEB | >16 | NEB | … | … | … | … |
| Terbinafine | >2 | NEB | >2 | NEB | … | … | … | … |
| Olorofim | 1 | NEB | >4 | NEB | … | … | … | … |
| Manogepix | … | … | ≤0.008 | NEB | ≤0.008 | NEB | … | … |
| Time to fungal culture positivity | Unknown | 4 days (2 of 2 sites) | 15 days (1 of 2 sites) | Negative | Negative | |||
| Pathology results | Positive: Granulomatous folliculitis with numerous fungal hyphae in hair follicle (Majocchi's granuloma) | Positive (2 sites RF): Fungal hyphae identified by PAS and GMS stains in a granulomatous dermal inflammatory infiltrate. Negative (1 site RA): Mild perivascular lymphohistiocytic inflammatory infiltrate. No organisms identified by PAS or GMS stains. |
Negative: Superficial and deep perivascular lymphoplasmacytic infiltrate. No organisms identified by PAS or GMS stains. |
Negative: Mild perivascular chronic inflammation. GMS stain negative for fungal elements. |
Negative: Mild superficial dermal perivascular lymphohistiocytic infiltrate. No organisms identified by PAS or GMS stains. |
|||
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; GMS, Grocott methenamine silver stain; FMGX, fosmanogepix; Int., interpretation; MIC, minimum inhibitory concentration in micrograms/milliliter; NEB, no established breakpoints; PAS, periodic acid-Schiff stain; RA, right ankle; RF, right forearm.
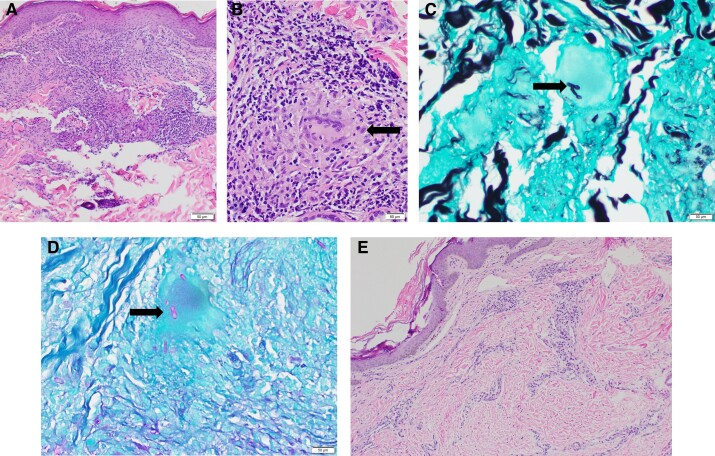
Figure 2.
(A) Pretreatment skin biopsy with granulomatous inflammation. (B) Granuloma (arrow) with giant cell (high power). (C) Grocott methenamine silver stain and (D) Periodic acid-Schiff stain stains of fungal elements in giant cell (arrow, pre-fosmanogepix). (E) Post-fosmanogepix biopsy with minimal chronic inflammation and no granulomas.
After obtaining written informed parental consent and institutional review board approval to acquire fosmanogepix (FMGX) tablets through an expanded access program, the patient started standard adult dosing of 800 mg every morning after completion of nightly PD. The patient was counseled on potential side effects of nausea, vomiting, headache, and dizziness, which were anticipated to be more likely given lower body weight (approximately 50 kg) compared with an adult. Eating breakfast before taking FMGX and premedicating with ondansetron were recommended; however, the patient did not initially follow these recommendations and experienced nausea and vomiting. On day 12 of treatment, FMGX dose was changed to 400 mg twice daily to improve tolerance, and the patient also began premedicating with ondansetron. His symptoms completely resolved but vomiting recurred if he forgot to take ondansetron.
Due to social factors including residence far from our center and lack of transportation, the patient was admitted monthly for study exams. He had a history of recurrent coagulase-negative Staphylococcus (CONS) PD-associated peritonitis, which persisted despite removal and replacement of his PD catheter. In the course of scheduled study admissions, the patient sometimes presented with fever, abdominal pain, and/or cloudy PD fluid, and peritoneal fluid cultures routinely grew CONS requiring intraperitoneal vancomycin, despite prescription of antistaphylococcal prophylaxis. Monthly laboratory tests included complete blood count with differential and complete metabolic panel. Anemia and electrolyte abnormalities secondary to ESKD and challenges with medication adherence remained grossly unchanged from baseline, and liver function overall remained stable. Monthly EKGs were unremarkable. After 10 weeks of FMGX, repeat skin biopsies from 2 right forearm sites were obtained, one of which grew FSSC although pathology was negative (Table 2). The patient had fluctuating adherence with his chronic kidney disease medications, and after 4 months of FMGX he reported missing multiple doses of FMGX as well. Efforts to aid medication adherence continued. Repeat skin biopsies were obtained after 5 months of FMGX therapy (Table 2). A few weeks later, while awaiting finalization of fungal cultures, the patient experienced severe headache and seizure activity requiring intensive care unit admission. His symptoms were found to be due to acute hypertensive emergency and posterior reversible encephalopathy syndrome related to fluid overload in the setting of peritoneal membrane failure secondary to recurrent peritonitis. He required removal of the PD catheter and transition to intermittent hemodialysis (HD). Fosmanogepix was held until the etiology of his presentation was clearly established, then it was resumed until skin biopsy fungal cultures were negative. The patient ultimately received approximately 6 months total of FMGX, including 1 week on intermittent HD. Three months after stopping FMGX, repeat skin biopsies were obtained and fungal cultures and histopathology remained negative (Table 2 and Figure 2). The patient is currently receiving HD via an arteriovenous fistula created on the left arm. He has been cleared for kidney transplant by ID, pending optimization of medication adherence and finalizing other pretransplant requirements.
Patient Consent Statement
Written informed consent was obtained from the patient's guardian and the patient's verbal assent was obtained. This study was approved by the Institutional Review Board of Children's Healthcare of Atlanta.
DISCUSSION
Rare molds including FSSC often demonstrate high levels of intrinsic resistance to many antifungal agents, contributing to the high rates of morbidity and mortality seen in invasive fungal infections (IFIs) with these pathogens. Globally, antifungal resistance and rare mold infections are on the rise, emerging threats related to increased use of antifungal prophylaxis and treatment in the expanding population of immunosuppressed patients at increased risk of fungal infections [3–5]. Although in vitro antimicrobial susceptibility testing species-specific patterns and patient-specific results are important in guiding therapeutic decisions for patients with rare mold infections, clinical interpretative breakpoints for antifungal minimum inhibitory concentration (MIC) values for Fusarium are not established, and there is often poor correlation between in vitro data and in vivo outcomes [6]. To effectively treat pathogens with intrinsic and induced resistance, new antifungals with extended spectrums of activity and improved toxicity profiles are needed. Fosmanogepix (formerly known as APX001) is a first-in-class antifungal prodrug that is metabolized by systemic phosphatases to the active moiety, manogepix (MGX). It displays highly selective antifungal activity by inhibiting the fungal Gwt1 protein, an enzyme essential for assembly of mannoproteins into cell wall glucan. Disruption of fungal cell wall integrity leads to increased recognition by immune cells and reduces fungal adherence, hyphal formation, biofilm formation, and pathogenicity [7, 8]. Fosmanogepix is active against many major fungal pathogens including Candida species (potent against multidrug-resistant Candida auris but not Candida krusei), Aspergillus (including Aspergillus lentulus and Aspergillus ustus), and rare molds Fusarium and Scedosporium [9]. Despite limited in vitro activity of MGX against most Mucorales, it may have potential for mucormycosis treatment because some efficacy (comparable to isavuconazole) was demonstrated in a murine model [10].
Fosmanogepix is currently in phase II and III clinical trials for treatment of IFIs caused by Candida, Aspergillus, and rare molds. Manogepix is highly bioavailable (>90%) in both oral and IV formulations [8], and it distributes widely into tissues. Nausea and vomiting may be ameliorated by postprandial administration. Manogepix penetrates the central nervous system [11], and headache and dizziness can occur. Manogepix is minimally eliminated by the kidneys and nephrotoxicity has not been observed [12]. Pharmacokinetics/pharmacodynamics (PK/PD) studies in animal models of fungal infection have demonstrated that area under the plasma concentration-time curve over the MIC (AUC/MIC) of FMGX strongly correlates with efficacy [7]. The PK studies in Phase I and II clinical trials showed linear and dose-proportional plasma exposures, with no clinically significant adverse events observed and no dose-limiting toxicities [7]. To date, pediatric PK/PD data have not been published. Although PK blood samples were collected from our patient under the compassionate use agreement, these data are not yet available because samples are being batched for analysis.
Fusarium isolates can have highly variable and species-specific antifungal susceptibilities, and empiric therapy of severe Fusarium infections in immunocompromised hosts often consists of a combination of liposomal amphotericin B and voriconazole for broad coverage until MICs are known [13]. However, FSSC isolates often have elevated amphotericin B and voriconazole MICs [14]. Although MICs are commonly reported, the minimum effective concentration measures inhibition of hyphal extension, versus complete inhibition of growth, and is the standard endpoint used to measure in vitro activity of MGX against filamentous fungi. Manogepix demonstrates good in vitro activity against FSSC isolates [15, 16].
Our patient is unique in that FMGX treatment was sought in the setting of chronic cutaneous Fusarium infection, which was not imminently life-threatening but posed a profound potential risk of dissemination upon proceeding to kidney transplantation. Although fusariosis accounts for less than 1% of IFIs in recipients with solid organ transplant (SOT), and it usually presents as localized infection with favorable outcomes compared with the frequently fatal disseminated infection that occurs in recipients with hematopoietic stem cell transplant (HSCT), overall mortality in SOT remains high ranging from 44% to 67% in various series [17–19]. Our patient's isolate was functionally pan-resistant to currently available antifungals (Table 1 and Table 2). Despite a relatively low MIC [6, 20, 21], amphotericin was not a feasible option due to prior inadequate clinical response and the need to identify an effective, non-nephrotoxic agent that could be used posttransplant if FSSC infection recurred. Our patient previously discontinued voriconazole due to side effects (Table 1). The in vitro activity of another investigational antifungal drug, olorofim, has been evaluated against clinical isolates of FSSC and has shown elevated MIC values [22]. Our patient's olorofim MICs increased from intermediate to resistant over time despite no exposure to this agent (Table 2).
Primary invasive cutaneous Fusarium infections have been described in STAT3 AD-HIES patients [1, 23] who have impaired interleukin (IL)-17 expression and Th17 differentiation leading to defects in the immunological skin barrier. As opposed to the disseminated disease seen in neutropenic patients, the development of chronic, nonsystemic Fusarium skin disease in STAT3 AD-HIES is hypothesized to be due to impaired IL-17-dependent antimicrobial peptide generation at the keratinocyte level, resulting in infection confined to the skin [1]. In some types of primary immunodeficiencies, haploidentical HSCT followed by kidney transplant from the same parent donor has eliminated the need for post-SOT immunosuppression [24]. Although some children with STAT3 AD-HIES have undergone HSCT, this is not established as standard of care. Our patient will remain at higher risk of bacterial and fungal infections post-SOT due to STAT3 AD-HIES. With transplant immune suppression compounding the lack of specific antibodies and poor vaccine antibody response associated with STAT3 AD-HIES, IgG replacement therapy will be indicated posttransplant, and if pneumatoceles are found on pretransplant chest computed tomography then posttransplant mold prophylaxis is recommended.
CONCLUSIONS
To our knowledge, this is the first report of FMGX treatment in a pediatric patient and the first use of FMGX in a patient on peritoneal dialysis. Fosmanogepix appears to be promising for treatment of highly drug-resistant fungal infections.
Acknowledgments
We acknowledge WEP Clinical and Pfizer for providing fosmanogepix through their expanded access program.
Financial support . We received no financial support for the research and authorship of this article. The publication of this article was supported in part by Emory University's Open Access Publishing Fund . This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Contributor Information
Kathryn P Goggin, Division of Infectious Diseases, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Jackson Londeree, Division of Nephrology, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Alexandra F Freeman, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Rouba Garro, Division of Nephrology, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Roshan P George, Division of Nephrology, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
References
- 1. Abbara S, Freeman AF, Cohen JF, et al. Primary invasive cutaneous fusariosis in patients with STAT3 hyper-IgE syndrome. J Clin Immunol 2023; 43:647–52. [DOI] [PubMed] [Google Scholar]
- 2. Belcher IL, Turrentine JE. Multimodal therapy for recalcitrant cutaneous Fusarium solani infection in a patient with hyper-IgE syndrome. Dermatol Arch 2017; 1:35–8. [Google Scholar]
- 3. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 2017; 17:e383–92. [DOI] [PubMed] [Google Scholar]
- 4. Batista BG, Chaves MA, Reginatto P, Saraiva OJ, Fuentefria AM. Human fusariosis: an emerging infection that is difficult to treat. Rev Soc Bras Med Trop 2020; 53:e20200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallabhaneni S, Mody RK, Walker T, Chiller T. The global burden of fungal diseases. Infect Dis Clin North Am 2016; 30:1–11. [DOI] [PubMed] [Google Scholar]
- 6. Al-Hatmi AMS, Curfs-Breuker I, de Hoog GS, Meis JF, Verweij PE. Antifungal susceptibility testing of fusarium: a practical approach. J Fungi (Basel) 2017; 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shaw KJ, Ibrahim AS. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi (Basel) 2020; 6:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rauseo AM, Coler-Reilly A, Larson L, Spec A. Hope on the horizon: novel fungal treatments in development. Open Forum Infect Dis 2020; 7:ofaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfaller MA, Huband MD, Rhomberg PR, Bien PA, Castanheira M. Activities of manogepix and comparators against 1,435 recent fungal isolates collected during an international surveillance program (2020). Antimicrob Agents Chemother 2022; 66:e0102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamoth F, Lewis RE, Kontoyiannis DP. Investigational antifungal agents for invasive mycoses: a clinical perspective. Clin Infect Dis 2022; 75:534–44. [DOI] [PubMed] [Google Scholar]
- 11. Petraitiene R, Petraitis V, Maung BBW, et al. Efficacy and pharmacokinetics of fosmanogepix (APX001) in the treatment of Candida endophthalmitis and hematogenous meningoencephalitis in nonneutropenic rabbits. Antimicrob Agents Chemother 2021; 65:e01795-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfizer, Investigator's Brochure Fosmanogepix, Version 2.0. 2022.
- 13. McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. Mold infections of the central nervous system. N Engl J Med 2014; 371:150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Espinel-Ingroff A, Colombo AL, Cordoba S, et al. International evaluation of MIC distributions and epidemiological cutoff value (ECV) definitions for Fusarium species identified by molecular methods for the CLSI broth microdilution method. Antimicrob Agents Chemother 2016; 60:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Badali H, Patterson HP, Sanders CJ, et al. Manogepix, the active moiety of the investigational agent fosmanogepix, demonstrates in vitro activity against members of the Fusarium oxysporum and Fusarium solani species complexes. Antimicrob Agents Chemother 2021; 65:e02343-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castanheira M, Duncanson FP, Diekema DJ, Guarro J, Jones RN, Pfaller MA. Activities of E1210 and comparator agents tested by CLSI and EUCAST broth microdilution methods against Fusarium and Scedosporium species identified using molecular methods. Antimicrob Agents Chemother 2012; 56:352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lemonovich TL. Mold infections in solid organ transplant recipients. Infect Dis Clin North Am 2018; 32:687–701. [DOI] [PubMed] [Google Scholar]
- 18. Nambiar P, Cober E, Johnson L, Brizendine KD. Fatal Fusarium infection manifesting as osteomyelitis following previous treatment with amphotericin B in a multi-visceral transplant: case report and review of Fusarium infections in solid organ transplantation. Transpl Infect Dis 2018; 20:e12872. [DOI] [PubMed] [Google Scholar]
- 19. Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev 2007; 20:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diekema DJ, Messer SA, Hollis RJ, Jones RN, Pfaller MA. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J Clin Microbiol 2003; 41:3623–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuenca-Estrella M, Gomez-Lopez A, Mellado E, Buitrago MJ, Monzon A, Rodriguez-Tudela JL. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob Agents Chemother 2006; 50:917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Badali H, Cañete-Gibas C, Patterson H, et al. In vitro activity of olorofim against clinical isolates of the Fusarium oxysporum and Fusarium solani species complexes. Mycoses 2021; 64:748–52. [DOI] [PubMed] [Google Scholar]
- 23. Shi M, Lu S, Li J, et al. Cutaneous fusariosis caused by Fusarium lichenicola in a child with hyper-immunoglobulin E syndrome. J Dermatol 2020; 47:181–4. [DOI] [PubMed] [Google Scholar]
- 24. Bertaina A, Grimm PC, Weinberg K, et al. Sequential stem cell-kidney transplantation in Schimke immuno-osseous dysplasia. N Engl J Med 2022; 386:2295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]