Abstract
Background
Emerging data suggest that second-generation influenza vaccines with higher hemagglutinin (HA) antigen content and/or different production methods may induce stronger antibody responses to HA than standard-dose egg-based influenza vaccines in adults. We compared antibody responses to high-dose egg-based inactivated (HD-IIV3), recombinant (RIV4), and cell culture–based (ccIIV4) vs standard-dose egg-based inactivated influenza vaccine (SD-IIV4) among health care personnel (HCP) aged 18–65 years in 2 influenza seasons (2018–2019, 2019–2020).
Methods
In the second trial season, newly and re-enrolled HCPs who received SD-IIV4 in season 1 were randomized to receive RIV4, ccIIV4, or SD-IIV4 or were enrolled in an off-label, nonrandomized arm to receive HD-IIV3. Prevaccination and 1-month-postvaccination sera were tested by hemagglutination inhibition (HI) assay against 4 cell culture propagated vaccine reference viruses. Primary outcomes, adjusted for study site and baseline HI titer, were seroconversion rate (SCR), geometric mean titers (GMTs), mean fold rise (MFR), and GMT ratios that compared vaccine groups to SD-IIV4.
Results
Among 390 HCP in the per-protocol population, 79 received HD-IIV3, 103 RIV4, 106 ccIIV4, and 102 SD-IIV4. HD-IIV3 recipients had similar postvaccination antibody titers compared with SD-IIV4 recipients, whereas RIV4 recipients had significantly higher 1-month-postvaccination antibody titers against vaccine reference viruses for all outcomes.
Conclusions
HD-IIV3 did not induce higher antibody responses than SD-IIV4, but, consistent with previous studies, RIV4 was associated with higher postvaccination antibody titers. These findings suggest that recombinant vaccines rather than vaccines with higher egg-based antigen doses may provide improved antibody responses in highly vaccinated populations.
Keywords: health care personnel, immunogenicity, influenza vaccines
Influenza is associated with 9–41 million illnesses, 140 000–710 000 hospitalizations, and 12 000–52 000 deaths each season in the United States [1]. Health care personnel (HCP) are at increased risk of influenza illness because of occupational exposures and are a priority group recommended for annual influenza vaccination in the United States [2]. Health care employers may also require employees to receive annual influenza vaccination. As a result, HCP in the United States have high vaccination coverage and a history of frequent annual vaccination. For example, an estimated 76% of US HCP were vaccinated against influenza during the 2020–2021 season [3].
Previous studies have found that influenza vaccines may be less effective in populations with a history of frequent influenza vaccination [4] and that antibody responses to influenza vaccines may be blunted with repeated vaccination among HCP [5, 6]. In addition, repeated vaccination with traditional vaccines produced in embryonated eggs may elicit antibodies to egg-adapted epitopes absent in wild-type influenza viruses [7–9].
During the 2018–2019 influenza season, we conducted a randomized, open-label immunogenicity trial among HCP aged 18–64 years comparing receipt of quadrivalent cell culture–based influenza vaccine (ccIIV4) or recombinant influenza vaccine (RIV4) with a conventional egg-based standard-dose quadrivalent inactivated influenza vaccine (SD-IIV4) comparator. Serologic responses to hemagglutinin antigen (HA) were measured against cell-propagated influenza viruses that maintained antigenic similarity with wild-type influenza viruses. RIV4, which contains 45 µg of baculovirus-expressed recombinant HA per strain, elicited higher antibody responses compared with ccIIV4 and SD-IIV4, which each contain 15 µg of HA per strain [10]. To investigate whether higher HA content contributes to improved humoral immune response to RIV4, a nonrandomized, open- and off-label arm was added in the second season to examine the immunogenicity of high-dose egg-based trivalent inactivated influenza vaccine (HD-IIV3), produced in embryonated eggs and containing 60 µg of HA per strain. Responses to HD-IIV3, RIV4, and ccIIV4 were compared with SD-IIV4 to examine whether higher antigen content was associated with higher antibody titers in the second trial season.
METHODS
Trial Design and Participants
The study was conducted among HCP at 2 sites: Baylor Scott & White Health (BSWH) and Kaiser Permanente Northwest (KPNW). HCP participants enrolled in season 1 (2018–2019) of a randomized, open-label influenza vaccine trial [10] were re-consented and enrolled in season 2 (2019–2020). At both study sites in season 2, eligible HCP who were aged 18–64 years at the start of the first season and who had received SD-IIV4 in 2018–2019 were offered enrollment in randomized arms to receive SD-IIV4, RIV4, or ccIIV4. At 1 study site (KPNW), HCP were also recruited and enrolled into an observational study arm to receive open- and off-label HD-IIV3, which is licensed for persons aged ≥65 years in the United States.
To be eligible for enrollment, HCP had to be enrolled in their site's health system/insurance plan for at least 1 month and willing to participate in follow-up for at least 1 month after vaccination. Participants who received or planned to receive any vaccine in the 4 weeks before or after the first study visit, those who reported previous hypersensitivity reactions to influenza vaccinations, and those who were participating in or expected to participate in a study that involved an experimental vaccine, drug, biologic, blood product, or medication were excluded. At KPNW, female HCPs who tested positive by study-administered urine pregnancy test, planned to become pregnant before January 31, 2020, or were nursing were not eligible for participation in the HD-IIV3 arm.
Randomization and Blinding
Study site–specific stratified block randomization was used to ensure even allocations of age groups (18–44 years and 45–65 years) to 3 randomized study arms using REDCap software, as previously described [10]. Participants were randomized 1:1:1 to receive ccIIV4, RIV4, or SD-IIV4. Both participants and study investigators were aware of study arm assignments, but laboratory investigators were blinded until study procedures were completed.
Intervention
At enrollment, randomized HCP received 0.5 mL of SD-IIV4 (Fluzone Quadrivalent), ccIIV4 (Flucelvax Quadrivalent), RIV4 (Flublok Quadrivalent), or HD-IIV3 (Fluzone High-Dose); vaccine was given via intramuscular injection into the deltoid muscle of the upper arm. The quadrivalent study vaccines contained antigens representative of the recommended 2019–2020 Northern Hemisphere influenza vaccine strain composition: A/Brisbane/02/2018 (H1N1)pdm09-like virus, A/Kansas/14/2017 (H3N2), B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage), and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage). HD-IIV3 did not contain a B/Yamagata-like virus. Compared with the 2018–2019 influenza vaccine strain composition, 2019–2020 vaccines included updated components for A(H1N1)pdm09 and A(H3N2). During 2019–2020, all 4 components of ccIIV4 and RIV4 were produced from cell-based seed viruses that were not passaged in eggs.
Study Procedures
At enrollment, consented participants completed an online survey, provided 20 mL of venous blood for serologic assays, and received their assigned dose of study vaccine. Participants were scheduled ∼1 month (20–62 days acceptable range) postvaccination for collection of 20 mL of venous blood.
Outcome Measures
The coprimary outcomes were serologic responses to cell-grown vaccine reference viruses by hemagglutination inhibition antibody (HI) assay for influenza A/H3N2, A/H1N1pdm09, influenza B/Yamagata, and influenza B/Victoria vaccine at ∼1 month postvaccination using the following measures: seroconversion rate (SCR), geometric mean titers (GMTs), mean-fold rise (MFR), and GMT ratios comparing postvaccination HI titers by study arm with the SD-IIV4 referent group. SCR was defined as the proportion of participants with either a prevaccination titer of <1:10 and postvaccination titer ≥1:40 or a prevaccination titer ≥1:10 and a ≥4-fold rise between pre- and postvaccination titers. Secondary outcomes were postvaccination HI titers ≥1:40, 1:80, and 1:160 against cell-grown vaccine reference viruses.
Blood Specimen Collection and Testing
Blood was collected in serum separator tubes (Becton Dickinson, catalog #367985) with clot activator gel according to Centers for Disease Control and Prevention guidelines, stored at 4°C for no more than 18 hours, and centrifuged to separate the serum from the clotted blood. Serum was then removed, aliquoted, and stored at −20°C until it was tested.
HI assays were performed using 0.75% guinea pig erythrocytes in the presence of 20 nM of oseltamivir for cell-grown A/Kansas/14/2017 (A/H3N2) virus and 0.5% turkey erythrocytes for the cell-grown A/Idaho/07/2018 (H1N1)pdm09 (A/Brisbane/02/2018-like), B/Colorado/06/2017 (B/Victoria), and B/Phuket/3073/2013 (B/Yamagata) viruses using methods that have been previously described [11]. A/H1N1 and B viruses were propagated in Madin-Darby-Canine-Kidney (MDCK) cells; A/H3N2 viruses were propagated in MDCK-SIAT1 cells. All B antigens were ether treated before HI assays.
Data Analysis
The full analytic population included newly or re-enrolled participants who received an SD-IIV4 product during the 2018–2019 influenza season; analyses of antibody response among re-enrolled participants who received ccIIV4 or RIV4 during season 1 have been reported separately [12]. The per-protocol population comprised eligible HCP who received study vaccine and completed 1-month follow-up with collection of postvaccination sera within the protocol-specified acceptable time interval (20–62 days postvaccination). All analyses presented here are per protocol. GMT ratios were compared between the SD-IIV4 referent group and the HD-IIV3, RIV4, and ccIIV4 arms with analysis of variance (ANOVA). Logistic regression was used to compare rates of seroconversion and proportions of participants with postvaccination HI titers greater than prespecified cutoffs (1:40, 1:80, and 1:160). ANOVA was used to compare 1-month postvaccination HI GMTs and mean-fold GMT rise. All models were adjusted for study site and the log of prevaccination HI titers to the relevant strain; additional covariates, including sex, chronic medical conditions, and immunosuppressive conditions, were considered but were not significant in the outcome models. All statistical tests were 2-tailed. A Bonferroni correction for multiple comparisons was prespecified in the protocol for 7 comparisons of different combinations of influenza vaccines during the 2 trial seasons (3 presented here and 4 in another report [12]); therefore, statistical significance was defined at P = .007, and 99.3% confidence intervals were calculated accordingly. A post hoc sensitivity analysis restricting comparisons to participants from KPNW was performed to examine whether findings differed after eliminating the possible role of unmeasured differences in the 2 site populations. Analyses were conducted with SAS (version 9.3; SAS Institute, Cary, NC, USA).
Patient Consent
The study protocol was reviewed and approved by the institutional review boards (IRBs) of the 2 study sites (BSWH, KPNW) and Abt Associates, which provided site oversight and data management support. The IRB of the Centers for Disease Control and Prevention relied on the IRB review of the BSWH Central Texas IRB. This study is registered in ClinicalTrials.gov, number NCT03722589. Study findings are reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) statement guidelines. An Investigational New Drug (IND) exemption was obtained for the nonrandomized HD-IIV3 arm of this trial from the US Food and Drug Administration Division of Vaccines and Related Products (IND Application #18940). All study participants provided written informed consent.
RESULTS
Study Enrollment and Participant Baseline Characteristics
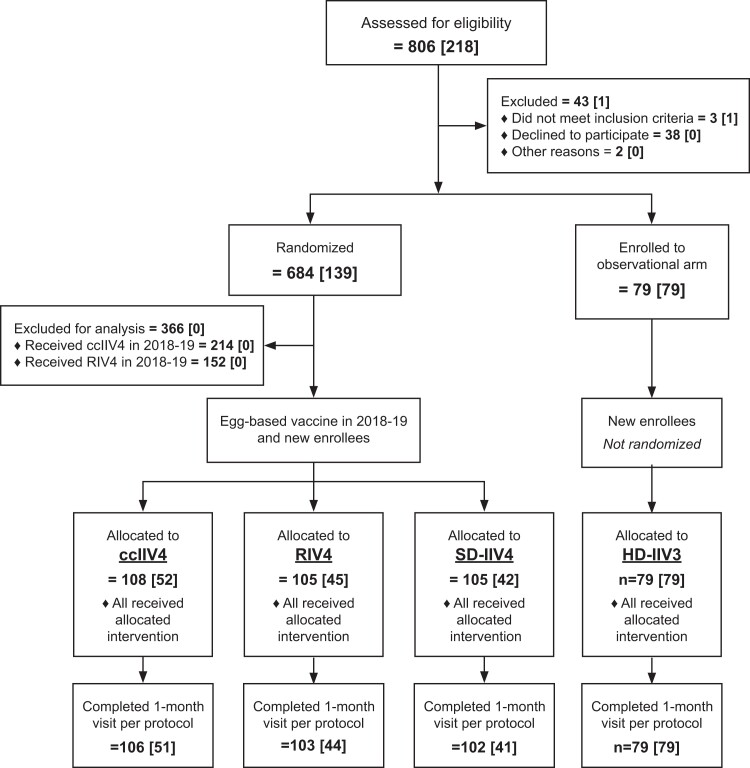
The per-protocol analyses included 390 HCP who received 1 of 4 study vaccines and completed the 1-month postvaccination blood collection (Figure 1); all received an egg-based SD-IIV4 during the previous season. Of the 390 HCP in the per-protocol population, 103 received RIV4, 106 received ccIIV4, 102 received SD-IIV4, and 79 received HD-IIV3 (observational arm at KPNW only). Per-protocol retention rates were >97% for all study arms. The average number of days between enrollment and the 1-month blood collection (SD, range) was 29.2 (6.9, 20–62) days; 110 participants (28.2%) had 1-month draws after 30 days.
Figure 1.
Screening, enrollment, and follow-up at 1 month of health care personnel aged 18–65 years in an open-label immunogenicity trial of cell culture–based, recombinant, and standard-and high-dose conventional egg-based influenza vaccines, 2019–2020 influenza season. “n” indicates total number of participants per level (number of newly enrolled season 2 participants at each level). Abbreviations: ccIIV4, cell culture–based inactivated influenza vaccine represented by Flucelvax Quadrivalent by Seqirus; HD-IIV3, high-dose trivalent inactivated influenza vaccine represented by Fluzone High-Dose by Sanofi Pasteur; RIV4, recombinant inactivated influenza vaccine represented by Flublok Quadrivalent by Sanofi Pasteur; SD-IIV4, standard-dose quadrivalent inactivated influenza vaccine represented by Fluzone Quadrivalent by Sanofi Pasteur.
Across all study arms, participants were similar with respect to age, body mass index, and mean self-rated health status score (Table 1). The proportions of participants who were male, non-Hispanic, White, or had a chronic medical condition were higher in the nonrandomized HD-IIV3 arm compared with the randomized arms. Participants in all study arms reported receiving an average of 5 influenza vaccines during the preceding 6 seasons.
Table 1.
Baseline Characteristics of Trial Participants, Per-Protocol Populationa (n = 390)
| SD-IIV4 | HD-IIV3 | ccIIV4 | RIV4 | |||||
|---|---|---|---|---|---|---|---|---|
| n = 102 | n = 79 | n = 106 | n = 103 | |||||
| n | % | n | % | n | % | n | % | |
| Demographic characteristics | ||||||||
| Age, mean (SD), y | 46 | (11) | 46 | (10) | 45 | (10) | 44 | (11) |
| Age group | ||||||||
| 18–44 y | 47 | (46) | 34 | (43) | 49 | (46) | 48 | (47) |
| 45–65 y | 55 | (54) | 45 | (57) | 57 | (54) | 55 | (53) |
| Female | 88 | (86) | 53 | (67) | 88 | (83) | 89 | (86) |
| White | 81 | (79) | 72 | (91) | 88 | (83) | 89 | (86) |
| Hispanic | 12 | (12) | 4 | (5) | 12 | (11) | 15 | (15) |
| Site | ||||||||
| BSWH | 51 | (50) | 0 | (0) | 53 | (50) | 51 | (50) |
| KPNW | 51 | (50) | 79 | (100) | 53 | (50) | 52 | (50) |
| Baseline health characteristics | ||||||||
| BMI, mean (SD), kg/m2 | 29 | (8) | 29 | (7) | 27 | (6) | 30 | (8) |
| Self-rated health status, mean (SD)b | 2.0 | (0.8) | 2.1 | (0.9) | 1.9 | (0.8) | 2.0 | (0.8) |
| Diagnosed or treated for chronic medical condition during the past 12 mo | 15 | (15) | 20 | (25) | 10 | (9) | 9 | (9) |
| Immunosuppressive condition | 1 | (1) | 5 | (6) | 2 | (2) | 2 | (2) |
| Smokerc | 7 | (7) | 4 | (5) | 3 | (3) | 2 | (2) |
| Prior influenza vaccination receipt | ||||||||
| Total vaccines received during the preceding 6 seasons, mean (SD)d | 5.4 | (1.3) | 5.1 | (1.5) | 5.4 | (1.3) | 5.6 | (1.0) |
Abbreviations: BMI, body mass index; BSWH, Baylor Scott & White Health; ccIIV4, cell culture–based inactivated influenza vaccine represented by Flucelvax Quadrivalent by Seqirus; HCP, health care personnel; HD-IIV3, high-dose trivalent inactivated influenza vaccine represented by Fluzone High-Dose by Sanofi Pasteur; KPNW, Kaiser Permanente Northwest; RIV4, recombinant inactivated influenza vaccine represented by Flublok Quadrivalent by Sanofi Pasteur; SD-IIV4, standard-dose quadrivalent inactivated influenza vaccine represented by Fluzone Quadrivalent by Sanofi Pasteur.
The per-protocol population comprised eligible HCP who received study vaccine and had sera drawn and tested at around 1 month postvaccination within the protocol-specified acceptable time interval (20–62 days postvaccination).
Original answer choice converted to numeric scale where 5 = excellent and 1 = poor; 5 missing responses.
Based on questionnaire response, participant currently smokes every day or some days; 5 missing responses.
Based on report of vaccination by participant interview or electronic medical record extraction.
Antibody Responses One Month Postvaccination
Before vaccination, participants in the HD-IIV3 arm had lower baseline HI GMTs against the A(H3N2) vaccine virus, while randomized participants had similar baseline GMTs against all vaccine reference viruses (Table 2). After adjusting for study site and the log of prevaccination HI titers, there were no statistically significant differences in postvaccination SCR, GMT, or MFR against any of the vaccine reference viruses between HD-IIV3 and SD-IIV4 recipients (Table 2; crude outcomes shown in Supplementary Table 1). Adjusted postvaccination SCR, GMT, and MFR were significantly higher among ccIIV4 recipients compared with SD-IIV4 recipients against the A/H1N1pdm09 reference virus only (Table 2). SCRs, GMTs, and MFRs were significantly higher among RIV4 recipients compared with SD-IIV4 recipients against all vaccine reference viruses.
Table 2.
Adjusted Antibody Responses Prevaccination and at One Month Postvaccination by Hemagglutination Inhibition Against Cell-Grown Vaccine Reference Virusesa Among Recipients of High-Dose Egg-Based, Standard-Dose Egg-Based, Cell Culture–Based, and Recombinant Influenza Vaccines, Per-Protocol Populationb (n = 390)
| SD-IIV4 (n = 102) | HD-IIV3 (n = 79) | ccIIV4 (n = 106) | RIV4 (n = 103) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0c | 1 Monthd | P Value* | Day 0c | 1 Monthd | P Value* | Day 0c | 1 Monthd | P Value* | Day 0c | 1 Monthd | P Value* | |
| A/H1N1 | ||||||||||||
| Seroconversion rate | … | 5% (2%–15%) |
Ref | … | 16% (6%–37%) |
.041 | … | 24% (14%–37%) |
<.001 | … | 25% (15%–39%) |
<.001 |
| Geometric mean titer | 29.3 (20.6–41.6) |
39.4 (32.8–47.2) |
Ref | 23.2 (15.5–34.7) | 45.8 (36.4–57.7) |
.164 | 26.2 (18.5–37.0) |
58.3 (48.8–69.7) |
<.001 | 28.6 (20.2–40.6) |
65.7 (54.8–78.7) |
<.001 |
| Mean-fold rise in GMT | … | 1.5 (1.2–1.8) |
Ref | … | 1.7 (1.4–2.1) |
.164 | … | 2.2 (1.8–2.6) |
<.001 | … | 2.4 (2.0–2.9) |
<.001 |
| A/H3N2 | ||||||||||||
| Seroconversion rate | … | 28% (16%–43%) |
Ref | … | 50% (32%–68%) |
.012 | … | 24% (14%–38%) |
.571 | … | 49% (34%–63%) |
.007** |
| Geometric mean titer | 58.9 (43.7–79.5) |
107.4 (84.7–136.1) |
Ref | 26.3 (18.6–37.0) | 115.4 (85.1–156.3) |
.617 | 49.0 (36.5–67.7) |
100.9 (80.0–127.2) |
.610 | 56.5 (41.9–76.1) |
161.3 (127.4–204.1) |
.001 |
| Mean-fold rise in GMT | … | 2.3 (1.8–2.9) |
Ref | … | 2.5 (1.8–3.3) |
.617 | … | 2.1 (1.7–2.7) |
.610 | … | 3.4 (2.7–4.3) |
.001 |
| B/Victoria | ||||||||||||
| Seroconversion rate | … | 4% (4%–13%) |
Ref | … | 11% (3%–31%) |
.126 | … | 8% (3%–18%) |
.284 | … | 21% (12%–35%) |
.001 |
| Geometric mean titer | 32.4 (25.5–41.1) |
48.7 (41.7–57.0) |
Ref | 46.0 (34.8–60.8) | 54.2 (44.3–66.3) |
.263 | 44.4 (35.1–56.1) |
57.6 (49.4–67.1) |
.041 | 40.4 (31.8–41.2) |
78.8 (67.5–92.0) |
<.001 |
| Mean-fold rise in GMT | … | 1.2 (1.0–1.4) |
Ref | … | 1.4 (1.4–1.7) |
.263 | … | 1.4 (1.2–1.7) |
.041 | … | 2.0 (1.7–2.3) |
<.001 |
| B/Yamagata | ||||||||||||
| Seroconversion rate | … | 3% (1%–11%) |
Ref | … | 3% (1%–15%) |
.719 | … | 9% (4%–20%) |
.042 | … | 36% (24%–51%) |
<.001 |
| Geometric mean titer | 40.3 (30.7–52.9) |
54.5 (45.5–65.4) |
Ref | 28.2 (20.2–39.2) | 48.0 (38.1–60.4) |
.237 | 59.0 (49.3–70.5) |
62.0 (50.1–76.7) |
.407 | 48.5 (37.0–63.6) |
100.8 (84.0–120.8) |
<.001 |
| Mean-fold rise in GMT | … | 1.3 (1.1–1.6) |
Ref | … | 1.2 (0.9–1.5) |
.267 | … | 1.4 (1.2–1.7) |
.407 | … | 2.4 (2.0–2.9) |
<.001 |
Abbreviations: ANOVA, analysis of variance; ccIIV4, cell culture–based inactivated influenza vaccine represented by Flucelvax Quadrivalent by Seqirus; GMT, geometric mean titer; HCP, health care personnel; HD-IIV3, high-dose trivalent inactivated influenza vaccine represented by Fluzone High-Dose by Sanofi Pasteur; HI, hemagluttination inhibition; MFR, mean fold rise; RIV4, recombinant inactivated influenza vaccine represented by Flublok Quadrivalent by Sanofi Pasteur; SCR, seroconversion rate; SD-IIV4, standard-dose quadrivalent inactivated influenza vaccine represented by Fluzone Quadrivalent by Sanofi Pasteur.
Hemagglutination inhibition antibody titers were measured against the following cell-grown vaccine reference viruses: A/Kansas/14/2017 (H3N2), A/Idaho/07/2018 (H1N1)pdm09 (A/Brisbane/02/2018-like), B/Colorado/06/2017 (B/Victoria), and B/Phuket/3073/2013 (B/Yamagata) viruses.
The per-protocol population comprised eligible HCP who received study vaccine and had sera drawn and tested at around 1 month postvaccination within the protocol-specified acceptable time interval (20–62 days postvaccination).
Day 0 GMT adjusted for study site with 99.3% CIs.
One-month outcomes adjusted for the log of prevaccination titers and study site with 99.3% CIs.
P values based on logistic regression (SCR) and ANOVA (GMT and MFR) models adjusted for study site and the log of prevaccination titers comparing HD-IIV3, ccIIV4, and RIV4 with SD-IIV4 as the referent group. A Bonferroni correction for multiple comparisons was prespecified in the protocol for 7 comparisons of different combinations of influenza vaccines during the 2 trial seasons (3 presented here and 4 in another report). Therefore, statistical significance was defined at P = .007.
The P value was .0069, which met the threshold for significance before rounding.
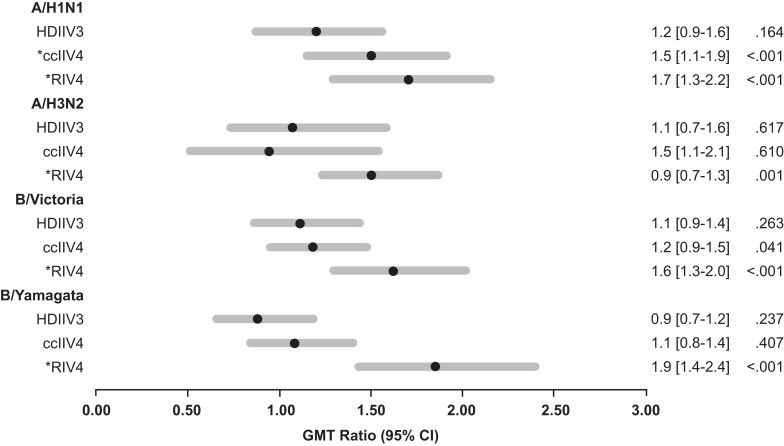
Among HD-IIV3 recipients, adjusted GMT ratios were not significantly greater than 1.0 compared with the SD-IIV4 reference group for any of the vaccine reference viruses (Figure 2). Compared with the SD-IIV4 reference group, the RIV4 group had adjusted GMT ratios against all vaccine reference viruses that were significantly greater than 1.0 and the ccIIV4 group had an adjusted GMT ratio >1.0 against A(H1N1)pdm09 (Figure 2). In sensitivity analyses limited to KPNW participants only, no significant adjusted GMT ratios were observed for the HD-IIV3 or ccIIV4 group compared with the SD-IIV4 reference group; the RIV4 group had adjusted GMT ratios >1.0 against A(H3N2), B/Victoria, and B/Yamagata (Supplementary Table 2).
Figure 2.
Geometric mean titer ratiosa adjusted for the log of prevaccination titers and study site at 1 month postvaccination by hemagglutination inhibitionb by recipients of high-dose egg-based, cell culture–based, and recombinant influenza vaccines compared with recipients of standard-dose egg-based influenza vaccine. aRatio of geometric mean titers at 1 month postvaccination among HD-IIV3 recipients, ccIIV4 recipients, or RIV4 recipients compared with SD-IIV4 recipients. bHemagglutination inhibition antibody titers were measured against the following cell-grown vaccine reference viruses: A/Kansas/14/2017 (H3N2), A/Idaho/07/2018 (H1N1)pdm09 (A/Brisbane/02/2018-like), B/Colorado/06/2017 (B/Victoria), and B/Phuket/3073/2013 (B/Yamagata) viruses. *Indicates significant P value based on ANOVA models adjusted for study site and the log of prevaccination titers comparing HD-IIV3, ccIIV4, and RIV4 with SD-IIV4 as the referent group. A Bonferroni correction for multiple comparisons was prespecified in the protocol for 7 comparisons of different combinations of influenza vaccines during the 2 trial seasons (3 presented here and 4 in another report). Therefore, statistical significance was defined at P = .007. Abbreviations: ANOVA, analysis of variance; ccIIV4, cell culture–based inactivated influenza vaccine represented by Flucelvax Quadrivalent by Seqirus; GMT, geometric mean titer; HD-IIV3, high-dose trivalent inactivated influenza vaccine represented by Fluzone High-Dose by Sanofi Pasteur; RIV4, recombinant inactivated influenza vaccine represented by Flublok Quadrivalent by Sanofi Pasteur; SD-IIV4, standard-dose quadrivalent inactivated influenza vaccine represented by Fluzone Quadrivalent by Sanofi Pasteur.
There were no differences in the proportions of HD-IIV3 and SD-IIV4 recipients with titers greater than 1:40, 1:80, or 1:160 for any vaccine reference virus. However, compared with SD-IIV4 recipients, higher proportions of RIV4 recipients had titers greater than 1:80 and 1:160 against the A(H1N1)pdm09, B/Yamagata, and B/Victoria reference viruses and >1:160 against A(H3N2) (Table 3). There were no differences in the proportions of ccIIV4 compared with SD-IIV4 recipients with titers greater than 1:40, 1:80, or 1:160 for any vaccine reference virus, with the exception of HI titers >1:80 against A/(H1N1)pdm09.
Table 3.
Adjusted Proportion of Participants With Antibody Titers Above Predefined Cutoffs Before Vaccination and at One Month Postvaccination by Hemagglutination Inhibition Against Cell-Grown Vaccine Reference Virusesa Among Recipients of High-Dose Egg-Based, Standard-Dose Egg-Based, Cell Culture–Based, and Recombinant Influenza Vaccines, Per-Protocol Populationb (n = 390)
| SD-IIV4 (n = 102) | HD-IIV3 (n = 79) | ccIIV4 (n = 106) | RIV4 (n = 103) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 Monthc | P Value* | 1 Monthc | P Value* | 1 Monthc | P Value* | 1 Monthc | P Value* | |
| A/H1N1 | ||||||||
| HI titer ≥1:40 | 83% (65%–93%) | Ref | 87% (66%–96%) | .572 | 94% (82%–98%) | .033 | 93% (81%–98%) | .047 |
| HI titer ≥1:80 | 31% (16%–51%) | Ref | 45% (20%–72%) | .253 | 63% (40%–81%) | .005 | 70% (49%–85%) | <.001 |
| HI titer ≥1:160 | 5% (1%–14%) | Ref | 8% (2%–27%) | .252 | 13% (5%–30%) | .018 | 20% (9%–39%) | <.001 |
| A/H3N2 | ||||||||
| HI titer ≥1:40 | 98% (90%–100%) | Ref | 99% (94%–100%) | .363 | 98% (91%–100%) | .938 | 99% (96%–100%) | .061 |
| HI titer ≥1:80 | 82% (67%–91%) | Ref | 80% (63%–91%) | .766 | 76% (61%–86%) | .301 | 94% (84%–98%) | .011 |
| HI titer ≥1:160 | 53% (37%–69%) | Ref | 52% (31%–72%) | .878 | 54% (39%–69%) | .948 | 78% (63%–88%) | .002 |
| B/Victoria | ||||||||
| HI titer ≥1:40 | 90% (79%–96%) | Ref | 86% (68%–95%) | .448 | 92% (80%–97%) | .653 | 96% (89%–99%) | .026 |
| HI titer ≥1:80 | 33% (19%–51%) | Ref | 42% (22%–65%) | .403 | 48% (31%–66%) | .103 | 67% (49%–82%) | <.001 |
| HI titer ≥1:160 | 3% (1%–11%) | Ref | 10% (3%–27%) | .078 | 7% (2%–17%) | .185 | 24% (12%–42%) | <.001 |
| B/Yamagata | ||||||||
| HI titer ≥1:40 | 92% (80%–97%) | Ref | 84% (64%–94%) | .099 | 86% (71%–94%) | .159 | 99% (93%–100%) | .004 |
| HI titer ≥1:80 | 48% (31%–65%) | Ref | 35% (16%–59%) | .238 | 57% (39%–73%) | .337 | 86% (72%–94%) | <.001 |
| HI titer ≥1:160 | 11% (5%–23%) | Ref | 13% (4%–32%) | .764 | 22% (12%–37%) | .039 | 52% (35%–67%) | <.001 |
Abbreviations: ccIIV4, cell culture–based inactivated influenza vaccine represented by Flucelvax Quadrivalent by Seqirus; HCP, health care personnel; HD-IIV3, high-dose trivalent inactivated influenza vaccine represented by Fluzone High-Dose by Sanofi Pasteur; HI, hemagluttination inhibition; RIV4, recombinant inactivated influenza vaccine represented by Flublok Quadrivalent by Sanofi Pasteur; SD-IIV4, standard-dose quadrivalent inactivated influenza vaccine represented by Fluzone Quadrivalent by Sanofi Pasteur.
Hemagglutination inhibition antibody titers were measured against the following cell-grown vaccine reference viruses: A/Kansas/14/2017 (H3N2), A/Idaho/07/2018 (H1N1)pdm09 (A/Brisbane/02/2018-like), B/Colorado/06/2017 (B/Victoria), and B/Phuket/3073/2013 (B/Yamagata) viruses.
The per-protocol population comprised eligible HCP who received study vaccine and had sera drawn and tested at around 1 month postvaccination within the protocol-specified acceptable time interval (20–62 days postvaccination).
One-month proportion adjusted for the log of prevaccination titers and study site with 99.3% CIs.
P values are based on logistic regression models adjusted for study site and the log of prevaccination titers comparing HD-IIV3, ccIIV4, and RIV4 with SD-IIV4 as the referent group. A Bonferroni correction for multiple comparisons was prespecified in the protocol for 7 comparisons of different combinations of influenza vaccines during the 2 trial seasons (3 presented here and 4 in another report). Therefore, statistical significance was defined at P = .007.
DISCUSSION
In this open-label immunogenicity trial in a highly vaccinated population of adults aged 18–65 years in the 2019–2020 influenza season, we found that HD-IIV3 and SD-IIV4 elicited similar antibody responses against HA for all vaccine reference viruses despite the 4-fold higher HA antigen content of HD-IIV3 compared with SD-IIV4. The findings from this trial add to evidence that recombinant HA antigen may elicit higher antibody titers against cell-grown vaccine reference viruses compared with those elicited by egg-based inactivated influenza vaccines. We used cell culture–derived and –propagated antigens in HI assays in this study because these viruses maintain antigenic similarity to wild-type influenza viruses and avoid antigenic changes that can occur during virus adaptation for growth in eggs. Our findings suggest that the improved antibody response to RIV4 may be driven by the recombinant HA antigen rather than higher antigen dose. The findings also suggest that higher egg-based antigen dose alone is not sufficient to increase antibody responses in highly vaccinated adults aged 18–65 years.
HD-IIV3 has been licensed for use in adults aged ≥65 years in the United States since 2009. Data from prelicensure trials and other studies indicate that high-dose egg-based influenza vaccines are more immunogenic [13–17] and may be more efficacious [18, 19] than standard-dose egg-based influenza vaccines in this older adult age group. In contrast, we found no difference in postvaccination antibody titers among HD-IIV3 recipients compared with SD-IIV4 recipients. It is unclear why the 4-fold higher antigen content of HD-IIV3 relative to SD-IIV4 did not elicit an improved antibody response in our study. However, if the relative immunogenicity of HD-IIV3 vs SD-IIV4 differs in older adults compared with adults aged 18–64 years, it may suggest that the immunologic drivers of suboptimal responses to SD-IIV4 vary by age and that different strategies to improve influenza vaccine efficacy may be needed for different age groups.
In this trial, RIV4 produced more robust HA antibody responses to all 4 cell-grown vaccine reference viruses, which is consistent with findings from an increasing number of studies demonstrating that RIV4 is more immunogenic than HD-IIV3 [20–22], ccIIV4 [12, 23], and SD-IIV4 [12, 23]. These findings expand upon the results of the first season of this randomized trial by demonstrating similar findings with updated A(H1N1)pdm09 and A(H3N2) vaccine components. In a separate analysis of data from the second year of this trial, receipt of RIV4 during both trial seasons also elicited more robust immune responses to HA compared with receipt of SD-IIV4 or ccIIV4 during both seasons, except against A(H3N2) [12]. Collectively these findings suggest that RIV4 may result in improved antibody-mediated protection against influenza virus infection among adults compared with other licensed influenza vaccines. Larger efficacy and effectiveness studies could help confirm this hypothesis. Additional studies are needed to examine the breadth of response elicited by recombinant vaccines to antigenically drifted viruses and to assess how the absence of neuraminidase in recombinant vaccines may affect relative efficacy compared with other influenza vaccines.
A limitation of this trial is that participants who received HD-IIV3 were from a single site and were not randomized. HD-IIV3 recipients differed from the rest of the randomized participant population in that they were more frequently male, non-Hispanic, White, had underlying medical conditions, and had lower baseline titers to the cell-grown A/H3N2 vaccine reference virus. To address these differences, we adjusted for study site, which was highly correlated with the other baseline characteristics that differed between vaccine arms, and for baseline prevaccination HI titers; adjusting for other covariates like sex and chronic medical conditions did not affect study findings. We also did a sensitivity analysis restricted to participants from the site with the HD-IIV3 arm. Both approaches resulted in findings consistent with the original unadjusted, full population analysis, though the possibility of residual confounding cannot be completely excluded.
Several additional limitations should be considered when interpreting analysis findings. First, we did not examine durability of response or other measures of immunologic response to the study vaccines, such as antibodies to neuraminidase and markers of cell-mediated immunity. Second, relative difference in HA antibody responses may not translate to relative differences in vaccine efficacy and effectiveness against infection, highlighting the need for larger comparative studies that examine protection against laboratory-confirmed influenza virus infection. Third, we were unable to assess the impact of prior vaccination on serologic response as all participants were vaccinated in the 2018–2019 season as part of the study entry criteria and were highly vaccinated in the preceding 6 seasons. Fourth, findings are limited to a single influenza season and may not be generalizable to other seasons with different vaccine strain compositions. Lastly, study participants in the randomized arms were more likely to be female, non-Hispanic, and White than the underlying patient populations at the 2 study sites, which may further limit generalizability.
In conclusion, high-dose egg-based vaccine with 4-times-higher HA antigen content did not induce more robust antibody responses than standard-dose vaccine, even after accounting for differences in baseline antibody titer. However, participants randomized to receive recombinant influenza vaccine, with 3-times-higher HA antigen content, achieved higher HA antibody titers to all 4 cell-grown vaccine strains than standard-dose egg-based vaccine. These findings suggest that increased egg-based antigen dose alone may be insufficient to improve influenza vaccine efficacy in adults aged 18–65 years. Additional research is needed to elucidate the immune mechanisms behind the increased antibody response associated with recombinant influenza vaccines in highly vaccinated populations.
Supplementary Material
Acknowledgments
From KPNW: Kristi Bays, Kimberley Berame, Cathleen Bourdoin, Kenni Graham, Matt Hornbrook, Willa Jones, Dorothy Kurdyla, Mi Lee, Danielle Millay, Yolanda Prado, Sperry Robinson. From Seqirus, Nedzad Music and Giuseppe Palladino for providing the cell-grown influenza B antigens used for HI testing.
Financial support. This work was supported by the Centers for Disease Control and Prevention (Contract 75D30118F02850).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Contributor Information
Allison L Naleway, Kaiser Permanente Center for Health Research, Portland, Oregon, USA.
Sara S Kim, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Brendan Flannery, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Min Z Levine, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Kempapura Murthy, Baylor Scott & White Health, Temple, Texas, USA.
Suryaprakash Sambhara, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Shivaprakash Gangappa, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Laura J Edwards, Abt Associates, Atlanta, Georgia, USA.
Sarah Ball, Abt Associates, Atlanta, Georgia, USA.
Lauren Grant, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Tnelda Zunie, Baylor Scott & White Health, Temple, Texas, USA.
Weiping Cao, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
F Liaini Gross, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Holly Groom, Kaiser Permanente Center for Health Research, Portland, Oregon, USA.
Alicia M Fry, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Danielle Hunt, Abt Associates, Atlanta, Georgia, USA.
Zuha Jeddy, Abt Associates, Atlanta, Georgia, USA.
Margarita Mishina, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Meredith G Wesley, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Abt Associates, Atlanta, Georgia, USA.
Sarah Spencer, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Mark G Thompson, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Manjusha Gaglani, Baylor Scott & White Health, Temple, Texas, USA; Texas A&M University, College of Medicine, Temple, Texas, USA.
Fatimah S Dawood, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1.Centers for Disease Control and Prevention. Influenza disease burden . Available at: https://www.cdc.gov/flu/about/burden/index.html. Accessed June 1, 2023.
- 2.Centers for Disease Control and Prevention. Immunization of health care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP) . MMWR Recomm Rep2011; 60 (No. RR-7). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6007a1.htm. Accessed September 9, 2022.
- 3.Centers for Disease Control and Prevention. Influenza vaccination coverage among health care personnel—United States, 2020–21 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/hcp-coverage_1920-21-estimates.htm. Accessed September 9, 2022.
- 4. Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16:1–14. Erratum in: Expert Rev Vaccines 2017; 16:865–6. [DOI] [PubMed] [Google Scholar]
- 5. Gaglani M, Spencer S, Ball S, et al. Antibody response to influenza A(H1N1)pdm09 among healthcare personnel receiving trivalent inactivated vaccine: effect of prior monovalent inactivated vaccine. J Infect Dis 2014; 209:1705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson MG, Naleway A, Fry AM, et al. Effects of repeated annual inactivated influenza vaccination among healthcare personnel on serum hemagglutinin inhibition antibody response to A/Perth/16/2009 (H3N2)-like virus during 2010–11. Vaccine 2016; 34:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu X, Kilbourne ED, Hall HE, Cox NJ. Nonimmunoselected intrastrain genetic variation detected in pairs of high-yielding influenza A (H3N2) vaccine and parental viruses. J Infect Dis 1994; 170:1432–8. [DOI] [PubMed] [Google Scholar]
- 8. Raymond DD, Stewart SM, Lee J, et al. Influenza immunization elicits antibodies specific for an egg-adapted vaccine strain. Nat Med 2016; 22:1465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawood FS, Naleway AL, Flannery B, et al. Comparison of the immunogenicity of cell culture-based and recombinant quadrivalent influenza vaccines to conventional egg-based quadrivalent influenza vaccines among healthcare personnel aged 18–64 years: a randomized open-label trial. Clin Infect Dis 2021; 73:1973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . Manual for the laboratory diagnosis and virological surveillance of influenza. Available at: https://apps.who.int/iris/bitstream/handle/10665/44518/9789241548090_eng.pdf?sequence=1. Accessed September 9, 2002.
- 12. Gaglani M, Kim SS, Naleway AL, et al. Effect of repeat vaccination on immunogenicity of quadrivalent cell-culture and recombinant influenza vaccines among healthcare personnel aged 18–64 years: a randomized, open-label trial. Clin Infect Dis. 2022; 76:e1168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–80. [DOI] [PubMed] [Google Scholar]
- 14. DiazGranados CA, Dunning AJ, Robertson CA, Talbot HK, Landolfi V, Greenberg DP. Efficacy and immunogenicity of high-dose influenza vaccine in older adults by age, comorbidities, and frailty. Vaccine 2015; 33:4565–71. [DOI] [PubMed] [Google Scholar]
- 15. Chang LJ, Meng Y, Janosczyk H, Landolfi V, Talbot HK; QHD00013 Study Group . Safety and immunogenicity of high-dose quadrivalent influenza vaccine in adults ≥65 years of age: a phase 3 randomized clinical trial. Vaccine 2019; 37:5825–34. [DOI] [PubMed] [Google Scholar]
- 16. Samson SI, Leventhal PS, Salamand C, et al. Immunogenicity of high-dose trivalent inactivated influenza vaccine: a systematic review and meta-analysis. Expert Rev Vaccines 2019; 18:295–308. [DOI] [PubMed] [Google Scholar]
- 17. Pepin S, Nicolas JF, Szymanski H, et al. Immunogenicity and safety of a quadrivalent high-dose inactivated influenza vaccine compared with a standard-dose quadrivalent influenza vaccine in healthy people aged 60 years or older: a randomized phase III trial. Hum Vaccin Immunother 2021; 17:5475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 19. Lee JKH, Lam GKL, Shin T, Samson SI, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine 2021; 39(Suppl 1):A24–35. [DOI] [PubMed] [Google Scholar]
- 20. Belongia EA, Levine MZ, Olaiya O, et al. Clinical trial to assess immunogenicity of high-dose, adjuvanted, and recombinant influenza vaccines against cell-grown A(H3N2) viruses in adults 65 to 74 years, 2017–2018. Vaccine 2020; 38:3121–8. [DOI] [PubMed] [Google Scholar]
- 21. Cowling BJ, Perera RAPM, Valkenburg SA, et al. Comparative immunogenicity of several enhanced influenza vaccine options for older adults: a randomized, controlled trial. Clin Infect Dis 2020; 71:1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Alvarado-Facundo E, Vassell R, et al. Comparison of A(H3N2) neutralizing antibody responses elicited by 2018–2019 season quadrivalent influenza vaccines derived from eggs, cells, and recombinant hemagglutinin. Clin Infect Dis 2021; 73:e4312–20. [DOI] [PubMed] [Google Scholar]
- 23. Gouma S, Zost SJ, Parkhouse K, et al. Comparison of human H3N2 antibody responses elicited by egg-based, cell-based, and recombinant protein-based influenza vaccines during the 2017–2018 season. Clin Infect Dis 2020; 71:1447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.