Abstract
Background
Malaria remains a major burden in sub-Saharan Africa (SSA). While an association between poverty and malaria has been demonstrated, a clearer understanding of explicit mechanisms through which socioeconomic position (SEP) influences malaria risk is needed to guide the design of more comprehensive interventions for malaria risk mitigation. This systematic review provides an overview of the current evidence on the mediators of socioeconomic disparities in malaria in SSA.
Methods
We searched PubMed and Web of Science for randomised controlled trials, cohort, case-control and cross-sectional studies published in English between January 1, 2000 to May 31, 2022. Further studies were identified following reviews of reference lists of the studies included. We included studies that either (1) conducted a formal mediation analysis of risk factors on the causal pathway between SEP and malaria infections or (2) adjusted for these potential mediators as confounders on the association between SEP and malaria using standard regression models. At least two independent reviewers appraised the studies, conducted data extraction, and assessed risk of bias. A systematic overview is presented for the included studies.
Results
We identified 41 articles from 20 countries in SSA for inclusion in the final review. Of these, 30 studies used cross-sectional design, and 26 found socioeconomic inequalities in malaria risk. Three formal mediation analyses showed limited evidence of mediation of food security, housing quality, and previous antimalarial use. Housing, education, insecticide-treated nets, and nutrition were highlighted in the remaining studies as being protective against malaria independent of SEP, suggesting potential for mediation. However, methodological limitations included the use of cross-sectional data, insufficient confounder adjustment, heterogeneity in measuring both SEP and malaria, and generally low or moderate-quality studies. No studies considered exposure mediator interactions or considered identifiability assumptions.
Conclusions
Few studies have conducted formal mediation analyses to elucidate pathways between SEP and malaria. Findings indicate that food security and housing could be more feasible (structural) intervention targets. Further research using well-designed longitudinal studies and improved analysis would illuminate the current sparse evidence into the pathways between SEP and malaria and adduce evidence for more potential targets for effective intervention.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40249-023-01110-2.
Keywords: Malaria, Mediation, Socioeconomic disparity, Sub-Saharan Africa
Background
Malaria is considered a disease of poverty [1]. Approximately 90% of all malaria-related morbidity and mortality occur in the world’s poorest regions, such as sub-Saharan Africa (SSA) [2]. Evidence of socioeconomic inequalities in the malaria burden has been consistently documented [1, 3]. Recent systematic reviews show that greater household wealth is associated with reductions in malaria [4–6]. For instance, evidence indicates that the risk of malaria is halved in children from the least poor households compared with those from the poorest households [4, 7]. Previous studies have used different proxies such as education, urbanicity, occupation, housing, and income to define socioeconomic position (SEP) [4, 6]. These proxies are either difficult to measure, have untestable assumptions about the link between indicators and poverty or sometimes comparisons across contexts/settings are not always valid. While household consumption is a better measure than income because it is less affected by inflation, measuring it is time-consuming and subject to bias [8]. A recently validated methodological approach that employs wealth indices derived from household assets, housing and living conditions is rarely used [9].
However, the impact of improved SEP on malaria may be largely indirect [1]. Indeed, studies show that socioeconomic disparities in malaria may be partly explained by factors on the causal pathway, such as improved housing, education, nutrition, food security, and use of insecticide-treated nets (ITN) [1, 10, 11]. If a causal relationship exists between SEP and malaria, then mediating pathways between SEP and malaria may be viable targets for interventions to reduce malaria incidence. Mediation analysis helps to understand whether and to what extent a third (intermediate) variable explains an exposure’s effect by partitioning the total effect of exposure into direct and indirect effects [12, 13]. The mediation analysis is depicted in Fig. 1 where a = coefficient of the path from exposure (E) to mediator (M), b = coefficient of the path from M to outcome (O) and c′ = coefficient of the path from E to O. The path c′ is the direct effect of E on O while the indirect effect of E is through a and b [14].
Fig. 1.
Relationship between exposure E, mediator M and outcome O
The difference method and the product of coefficients method are two conventional approaches biomedical researchers have used to conduct mediation analysis, but they both have drawbacks. The recent developments in the causal inference literature have made it possible to conduct mediation analysis with exposure-mediator interactions, multiple mediators, and counterfactual outcome perspectives [14]. In light of these methodological developments, reviewing the current evidence of the mediators between SEP and malaria provides helpful information to guide future analyses.
Although extant evidence supports the association between SEP and malaria, there is a lack of detailed studies to elucidate the underlying mechanisms behind this association and assess the evidence in light of the recent developments in mediation analysis. To our knowledge, there is no systematic review of studies that apply mediation analyses to investigate the underlying mechanisms between SEP and malaria. There is also a lack of synthesis of studies in which potential mediators have been adjusted for in the analyses. Therefore, this systematic review aims to comprehensively identify, summarize and critically appraise the existing evidence regarding variables that potentially mediate the relationship between SEP and malaria in SSA. Highlights of the review have been provided (Additional file 1: Table S1).
Methods
Search strategy and selection process
This review was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis protocols (PRISMA-P) 2020 statement [15] and registered in PROSPERO in March 2022 (Registration ID: CRD42022312359). We searched PubMed and Web of Science (WoS) for studies published in the English language for the period between January 1, 2000 and June 30, 2020 and updated in May 2022. A search strategy was developed to identify studies reporting on mediators of socioeconomic inequalities in malaria in SSA. A list of search terms and a detailed search strategy are provided (Additional file 1: Table S2).
Inclusion and exclusion criteria
We included all studies that quantitatively assessed mediators of SEP (derived from asset ownership and water and sanitation status) and malaria. We also included studies that reported associations between SEP and laboratory-confirmed malaria and simultaneously included potential mediators as covariates. The study population was not restricted to any age or gender, provided the studies were conducted in SSA [16]. We considered peer-reviewed articles published between January 1, 2000, and May 31, 2022, as eligible for inclusion. Studies that only considered one dimension of SEP, such as income, education, housing, occupation, and those using self-reported malaria or fever as a proxy for malaria, were excluded from this review. We excluded editorials, commentaries, conference abstracts, protocols, case reports and narrative reviews.
Identification of studies and data extraction
The screening was done at different stages. First, the authors (MAF and THH) screened all titles and abstracts of retrieved articles. We evaluated full texts when the abstract was deemed insufficient to draw conclusions. Full texts were then screened by three independent reviewers (STW, MAF, and THH) who extracted all relevant information into a standardized Excel spreadsheet. We also searched existing systematic reviews and reference lists of identified studies in addition to the electronic search. For excluded studies, reasons for exclusion were recorded. In case of discordance, the question of the inclusion of articles was resolved by a discussion with a reviewer panel (EL, JB, DIP). The comprehensive search results were merged, and duplicates were verified and removed.
Two independent reviewers (STW and THH) extracted relevant data from each paper using prepared data extraction forms to summarise evidence after the full text screening. We collected information on the first author's name, country of origin, study designs, study settings, sample size and participant characteristics (age, gender, domain), sampling methods, indicators of malaria and SEP, effect estimates (i.e., odds ratios, risk ratios, highest posterior densities), analysis methods (including mediation), covariates, and limitations. For studies that performed formal mediation analyses, we captured data on the percentage of the total effect that was mediated in each pathway.
Study quality assessment
Two authors (SWT and MAF) undertook quality assessment using an adapted version of the Effective Public Health Practice Project Tool (EPHPP) [17] (Additional file 1: Table S3). We evaluated the quality of individual studies based on participant selection, study design, control of confounding, measurement of outcome, assessment of the exposures, and withdrawals and drop-outs (for longitudinal studies). We rated each item as weak, moderate, or strong according to the quality assessment criteria and determined an overall global rating for the included studies. We categorized studies into strong, moderate, and weak based on the criteria. Studies with no weak component ratings were assigned as “strong”. Those with one weak component ratings were assigned “moderate”, while those two or more weak component ratings received “weak” quality ratings. We resolved any discrepancies through discussion. Details of the ratings are available (Additional file 1: Table S4).
Data synthesis strategy
Due to significant heterogeneity in the studies in terms of study designs, study populations, and settings, a comprehensive narrative synthesis was performed to answer the review’s objective. Study findings have been presented in tabular form, highlighting country, year of study, study population, context, mediators considered, and outcome measurement, among others.
Patient and public involvement
No patients were involved in the conceptualisation or conduct of this study due to the nature of the study as a systematic review.
Results
Search results and eligible studies
A total of 4914 articles were obtained after searching literature from the two databases. An extra 10 articles were identified by reviewing references of included studies [18–23] and relevant recent systematic reviews [24–27]. Of the 4924 articles, 537 were found to be duplicates. After screening the titles and abstracts, 217 were retained and determined as eligible for full text review resulting into 176 articles being excluded and leaving 41 articles. A flow chart including details of the article screening process is shown (Fig. 2).
Fig. 2.
PRISMA flow diagram for study screening and selection process. DOI digital object identifier, PMID PubMed identifier, SEP socioeconomic position, WOS web of science
Characteristics of included studies
Forty-one (41) studies were conducted in 20 countries in SSA. The review had eight studies conducted in Tanzania [21, 23, 28–33], and Malawi [23, 34–40] and seven studies from Uganda [1, 22, 23, 25, 41–43], four in Ethiopia [18, 19, 44, 45], three in Kenya [46–48], two in Ghana [27, 49], the Gambia [27, 49], and Burkina Faso [49, 50], Democratic Republic of Congo (DRC) [51, 52], Nigeria [23, 53], Mali [49, 54], Mozambique [55, 56], while Cameroon [57], Equatorial Guinea [24], Angola [23], Liberia [23], Rwanda [23], Senegal [23], Madagascar [23] and South Africa [20] all had one study.
Of the 41 studies, 30 used a cross-sectional design [19, 23–25, 27, 28, 30–40, 42, 45–47, 49–53, 55–58], seven used a cohort design [1, 18, 22, 26, 41, 43, 54], two were case–control studies [20, 48], and two investigations were embedded trials [29, 44]. All studies used objective diagnostic malaria tests, and most studies (26) had children as their population, eight had the general population, six considered both children and their caretakers, and two considered adult women. The characteristics of included studies are included in Table 2.
Table 2.
Studies on socioeconomic position (SEP) and malaria with analysis approaches
| Author year | Analyses performed | Risk estimate (95% CI) and result of mediation (if any) | Adjusted for confounders/mediators | Quality score |
|---|---|---|---|---|
| Siri 2010 | Multivariable logistic regression | OR. wealth percentile [0.8 (0.7–0.9)] | ITN use, mosquito coils, age, location, gender of HH head | Strong |
| Coleman et al. 2010 | Multivariable negative binomial regressions | OR. Reference (the 1st quartile) third [0.24 (0.09–0.65)], and fourth (least poor) [0.27 (0.10–0.79)] | Housing structure, closing windows on retiring | Strong |
| Loha et al. 2012 | Poisson regression | (Beta coefficient = − 0.155, P-value = 0.043) | Age, gender, ITN use, and distance to the breeding place | Strong |
| Clark et al. 2008 | Generalized estimating equations with control for repeated measures | IRR. Reference (SEP quartiles 1 and 2 combined). Third [0.83 (0.62–1.10)], fourth [0.77 (0.56–1.04)] | Age, gender, ITN use, distance to the swamp, household crowding | Strong |
| Snyman et al. 2015 | Negative binomial regression models | IRR. Reference (lowest). Middle [0.91 (0.76–1.1)], highest [0.86 (0.72–1.03)] | Caregiver’s age, education, house construction, location, number of rooms | Moderate |
| Tusting et al. 2016 | Multivariable logistic regression and causal steps approach-simulations and Bootstrapping |
IRR. Reference (lowest), middle [1.12 (0.90–1.40)], highest [1.05 (0.83–1.34)] Housing type explained 24.9% of the SEP effect, and food security explained 18.6% |
Age, gender, level of education, housing typea, food securitya, distance to facility, household size | Strong |
| Wanzirah et al. 2015 | Multivariable logistic regression and negative binomial regression |
OR. Reference (1st tertile) Walukuba: 2nd tertile [0.82 (0.38–1.78)], 3rd tertile [0.83 (0.31–2.18)] Kihihi: 2nd tertile [0.54 (0.28–1.06)], 3rd tertile [0.37 (0.20–0.71)] Nagongera: 2nd tertile [0.72 (0.50–1.04)], 3rd tertile [0.71 (0.47–1.07)] |
Age, gender, house type, floor material, roof material, eaves | Moderate |
| Asante et al. 2013 | Cox proportional hazards regression | HR. Reference (least poor), less poor [1.54 (1.23–1.93)], poor [1.88 (1.50–2.35)], poorer [1.86 (1.50–2.31)], most poor [2.21 (1.77–2.76)] | Housing (thatched roof), location, distance to the health facility, ITN use | Strong |
| Haji et al. 2016 | Multivariable logistic regression | OR. Reference (low), medium [1.51 (0.51–4. 45)], high [0.93 (0.35–2.45)] | Location, ITN use, age of the child, gender, sought advice before, knowledge of malaria | Moderate |
| Kabaghe et al. 2017 | Modified Poisson regression | HPD. SEP [− 0.07 (− 0.11 to − 0.03)] | Age, ITN use, elevation and Normalized Difference Vegetation Index | Weak |
| Mathanga et al. 2015 | Multivariable logistic regression | OR. Reference (poorest), poor [1.08 (0.82–1.42)], medium [1.30, 0.98–1.72)], less poor [1.26 (0.94–1.70)], least poor [0.74 (0.55–0.99)] | Age, gender, ITN use, reported fever, education status, household size, school feeding) | Moderate |
| Sakwe et al. 2019 | Multivariable logistic regression | OR. Development Index [0.76 (0.58–0.99)] | Child age, gender, nutrition status, housing type, HH size, HH head education, and caregiver | Moderate |
| Skarbinski et al. 2011 | Multivariable logistic regression | OR. Reference (least poor), 4th [2.10 (1.45–3.05)], 3rd [2.64 (1.80–3.87)], 2nd [2.84 (2.03–3.97)], 1st [3.46 (2.30–5.21)] | District, ITN use, IRN use | Weak |
| Skarbinski et al. 2012 | Binomial regression modelling | OR. Reference (least poor), 4th [1.19 (0.71–2.00)], 3rd [1.72 (1.09–2.70)], 2nd [1.52 (1.01–2.29)], 1st [1.47 (0.98–2.20)] | IRS use, ITN use, wall material, roof material | Weak |
| Somi et al. 2007 | Probit regression | Coefficients: SEP score based on PCA (numerical) = − 0.04 (P-value = 0.012) | ITN use, age, location, knowledge, eaves | Moderate |
| Somi et al. 2008 | Probit regression | Coefficients: SEP score based on PCA (numerical) = − 0.074 | Age, location, ITN use, HH size, eaves, knowledge | Moderate |
| Ssempiira et al. 2017 | Bayesian geostatistical logistic regression | OR. Reference (poorest), richest [0.19 (0.14, 0.27)], richer [0.52 (0.42, 0.61)], medium [0.77 (0.85, 1.15)], poorer [0.86 (0.72, 1.04)] | Location, ITN use, IRS use, age, mothers’ education, land surface temperature | Moderate |
| Temu et al. 2012 | Multivariable logistic regression | OR. Reference (poorest), 2nd quartile [0.9 (0.7–1.2)], 3rd quartile [0.9 (0.7–1.3)], and 4th quartile [0.5 (0.4–0.7)] | Age, year, ITN use, HH size, house construction, children with current fever | Weak |
| Zoungrana et al. 2014 | Multivariable logistic regression |
OR. Reference (high) Low SEP [4.11 (1.44, 11.75)] |
Age, gender, marital status, education, knowledge, ethnicity, residence, distance, travel time, HH size, decision making | Strong |
| Graves et al. 2009 | Multivariable logistic regression | OR. Asset index [0.79 (0.66—0.94)] | Age, gender, altitude, monthly rain, ITN use, IRS use in the last 12 months | Moderate |
| Mmbando et al. 2011 |
Muitivariate logistic regression Spatial analysis |
OR. Reference (high), medium [1.6(1.3–1.9)], low [1.6 (1.4–1.91)] | Age, ITN use, ITN rate, housing, year, altitude | Moderate |
| Siri 2014 | Multilevel logistic regression | OR. Wealth percentile [0.990 (0.987–0.992)] | Child age, mother’s age, ITN use, country, HH size, location, education, finished windows and ceilings | Moderate |
| Custodio et al. 2009 | Multivariable logistic regression | OR. Reference (low), medium [0.97 (0.29–3.25)], high [0.15 (0.05–0.50)] | ITN use, antimalarials use in pregnancy, age, gender | Moderate |
| de Beaudrap et al. 2011 | Multivariable logistic regression | OR. SEP score [0.75 (0.64–0.89)] | Child age, weight, housing score, ITN use, education and latitude | Moderate |
| Sonko et al. 2014 | Multivariable logistic regression |
For children 6–59 months, OR. Reference (poorest), 2nd [0.40 (0.20–0.70], 3rd [0.5 (0.30–0.90)], 4th [0.30 (0.10–0.60), 5th [0.10 (0.04–0.30)] Children 5–14 years 2nd [0.60 (0.30–1.20]), 3rd [0.70 (0.50–1.10]), 4th [0.20 (0.10–0.50)], 5th [0.30 (0.10–0.60)]. For the general population 2nd [0.80 (0.40–1.30)], 3rd [0.80 (0.50–1.20), 4th (0.40 (0.20–0.80), 5th [0.20 (0.07–0.80)] |
Housing (wall type, roof type, floor type, window type,) age, gender | Moderate |
| Chirombo et al. 2014 | Structured additive logistic regression (Bayesian approach) | OR. Reference (poorest), richest [0.22 (0.14–0.37)], richer (0.42 (0.28–0.64)], medium [0.66 (0.45–0.96)], poorer [1.10 (0.76–1.60)] | ITN use, region, age and location | Weak |
| de Glanville et al. 2019 |
Multivariable logistic regression Mediation analysis using a hierarchical approach |
OR. SEP [0.76 (0.66–0.86)] Minimal mediation by antimalarial use |
Gender, age, access to health care (antimalaria usea) | Moderate |
| Florey et al. 2012 | GEE models with exchangeable correlation matrix and logistic distributions | OR. SEP [0.76 (0.54–1.05)] | Outdoor night activity | Weak |
| Kahabuka et al. 2012 | Multivariable logistic regression | OR. Reference (high), middle [1.00 (0.40–2.80)], and low [1.20 (0.40–3.70)] | Education, parity, hospital travel time, use of near public health facility, source of the first treatment | Weak |
| Ma et al. 2017 | Multivariable logistic regression | OR. SEP [1.20 (0.94–1.50)] | Study site, age, HH size, education, HIV Status, ITN use, phone ownership | Moderate |
| West et al. 2013 | Multivariable logistic regression | OR. Reference (poorest), mild [0.69 (0.34–1.40)], least poor [0.13 (0.05–0.34)] | HH spaying, cluster ITN coverage, age | Moderate |
| Williams et al. 2016 | Multivariable logistic regression | OR. Reference (wealthiest), wealthy [1.82 (0.68–4.83)], medium [0.96 (0.18–5.02)], poor [6.48 (1.68, 25.0), poorest [6.55 (1.27–33.70)] | Education, age, gestation age, gravidity, country | Weak |
| Zgambo et al. 2017 | Multivariable logistic regression |
OR. Reference (richest), 2012 survey: poorest [2.90 (1.60–5.30)], poorer [2.3 (1.10–4.60)], middle [2.50 (1.30–5.00)], richer [1.9 (1.10–3.60)] 2014 survey: poorest [4.7 (1.3–16.2)], poorer [2.9 (0.9–10.0)], middle [2.7 (0.7–10.2)], richer [1.9 (0.4–8.0)] |
ITN use, ITN ownership, IRS, region, location, Gender, child age, altitude, and education of the mother | Moderate |
| Gari T et al. 2016 | A multilevel mixed effects Poisson regression | IRR. Reference (rich). Poor [0.94 (0.35–2.45)], medium [0.70 (0.33–1.50)] | Age, gender, HH head education, ITN use | Strong |
| Liu et al. 2014 | Multivariable negative binomial regressions | IRR. Reference (middle). Poorest [1.316 (0.915–1.891)], poorer [1.292 (0.876–1.905)], richer [1.090 (0.667–1.782)], richest [1.059 (0.533–2.103)] | Age, housing index, regular repellent use, ITN use, location, water source, electricity | Strong |
| Vincenz et al. 2022 | GEE for binary logistic regression | OR. [1.37 (0.99–1.91)] | Maternal age, gravidity, IPTP use, education, season | Weak |
| Mann et al. 2021 | Multivariable logistic regression | OR. Reference (richest), poorest [4.60 (3.05–6.96)], poorer [4.18 (2.81–6.19)], middle [3.27 (2.26–4.71)], richer [2.23 (1.55–3.21)] | Age, gender, residence, education, nutrition (stunting) | Moderate |
| Emina et al. 2021 | Generalized estimating equations with control for repeated measures | OR. Reference (poorest), poorer [1.20 (0.95–1.52)], middle [1.00 (0.77–1.31)], richer [0.69 (0.50–0.96)], richest [0.19 (0.10–0.37)] | Gender, child’s age, mother education, ITN use, sex of HH head, type of residence, province of residence | Moderate |
| Mwaiswelo et al. 2021 | Multivariable logistic regression | OR. Reference (low). Medium [0.54, 0.36–0.83)], upper [0.41(0.25–0.66)] | ITN ownership, HH size, education, district (location) | Weak |
| Mangani et al. 2022 | Multilevel logistic regression | OR. Reference (poorest), poorer [0.80 (0.65–1.00)], middle [0.74 (0.56–0.99)], richer [0.80 (0.62–1.01)], richest [0.64 (0.50–0.81)] | HH wall, roof materials, education, ITN use, child’s age, gender, distance from the irrigation scheme | Moderate |
| Ejigu 2020 | Geostatistical logistic model | OR. Reference (poorest), poorer [0.99 (0.80–1.25)], middle [0.67 (0.53–0.85)], richer [0.52 (0.40–0.69)], richest [0.19 (0.11–0.31)] | Province, mothers’ education, anemia, ITN use, age in months, ITN coverage, malaria incidence | Weak |
CI confidence interval, GEE generalized estimating equations, HH household, HPD highest posterior density, PCA principal component analysis, OR odds ratios, IRR incidence rate ratios, ITN insecticide treated net, IRS indoor residual spraying
aMediators assessed in formal analyses
Measures of SEP
All studies used wealth indices constructed from household assets and other variables to measure SEP per our definition of SEP. Unlike most studies which considered reported SEP in quintiles, quartiles and tertiles for wealth index, some studies also considered SEP as a continuous variable [18, 19, 25, 30, 31, 39, 46, 47, 51, 57], wealth percentiles [23, 48], or as a dichotomous variable [50].
Measures of outcome: malaria
Malaria was assessed as the prevalence or incidence of Plasmodium infection. Twenty-six studies used microscopy to test for malaria, 12 used rapid diagnostic tests (RDTs), 2 used polymerase chain reaction (PCR) and 1 performed histopathology for diagnosis of placental malaria (Table 1).
Table 1.
The characteristics of included studies in the present review
| Author year; country | Study design | Study population | Sample size, n | Indicator of SEP | Outcome measurement |
|---|---|---|---|---|---|
| Siri 2010; Kenya | Case-control | Cases were children less than 10 years with a hemoglobin level < 8 g/dL and parasite density ≥ 10,000/μl and are normal residents | 906 | Wealth Index (continuous) | Malaria examined by microscopy |
| Coleman et al. 2010; South Africa | Case-control | All Household members (household considered instead of individuals) | 212 | Wealth Index (quartile) | Malaria examined by microscopy |
| Loha et al. 2012; Ethiopia | Cohort | All residents in the Kebele were taken as study subjects. Every household was visited weekly, looking for febrile cases | 8121 | Wealth Index (continuous) | Malaria RDT |
| Clark et al. 2008; Uganda | Cohort | Children aged 1–10 years presenting with fever episode | 601 | Wealth Index (quartile) | Malaria examined by microscopy |
| Snyman et al. 2015; Uganda | Cohort | HIV-exposed and unexposed children of age 4–6 months | 515 | Wealth Index (tertiles) | Malaria examined by microscopy |
| Tusting et al. 2016; Uganda | Cohort | All children aged 6 months to 10 years and their primary caregivers | 333 | Wealth Index (tertile) | Malaria examined by microscopy |
| Wanzirah et al. 2015; Uganda | Cohort | All children aged 6 months to 10 years | 878 | Wealth Index (tertile) | Malaria examined by microscopy |
| Asante et al. 2013; Ghana | Cohort | Infants of mothers enrolled during pregnancy | 1855 | Wealth Index (quintile) | Malaria RDT and Malaria examined by microscopy |
| Vincenz et al. 2022; Mali | Cohort | Mothers who were participants in a cohort study | 249 mothers | Wealth z scores | Placental malaria (histology) |
| Haji et al. 2016; Ethiopia | Cross-sectional | All children under 16 years with symptoms consistent with malaria | 830 | Wealth Index (tertiles) | Malaria examined by microscopy |
| Kabaghe et al. 2017; Malawi | Cross-sectional | Children 6–59 months enrolled | 1016 | Wealth index (continuous) | Malaria RDT |
| Mathanga et al. 2015; Malawi | Cross-sectional | Pupils enrolled in classes 1–8 of participating schools | 2667 | Wealth Index (quintiles) | Malaria examined by microscopy |
| Sakwe et al. 2019; Cameroon | Cross-sectional | Children aged 6 months to 10 years and of both sexes, after full informed consent | 362 | Development Index)—(continuous) | Malaria examined by microscopy |
| Skarbinski et al. 2011; Malawi | Cross-sectional | All household members were asked to participate | 6581 | Wealth Index (quintiles) | Malaria examined by microscopy |
| Skarbinski et al. 2012; Malawi | Cross-sectional | All household members were asked to participate | 884 | Wealth Index (quintiles) | Malaria was examined by microscopy |
| Somi et al. 2007; Tanzania | Cross-sectional | Children and adults | 7657 | Wealth Index (continuous) | Malaria was examined by microscopy |
| Somi 2008; Tanzania | Cross-sectional | General population | 2034 | Wealth index (continuous) | Malaria was examined by microscopy |
| Ssempiira et al. 2017; Uganda | Cross-sectional | Women 15–49 years, Children less than 5 years | 4591 children | Wealth Index (quintiles) | Malaria RDT |
| Temu et al. 2012; Mozambique | Cross-sectional | Children 1–15 years | 8338 children | Wealth Index (quartiles) | Malaria RDT |
| Zoungrana et al. 2014; Burkina Faso | Cross-sectional | All children 0–5 years who had been diagnosed with clinical malaria or produced a paraclinical assessment | 510 | Wealth Index (dichotomous) | Malaria RDT or Malaria examined by microscopy |
| Graves et al. 2009; Ethiopia | Cross-sectional | Children and women | 11,437 | Wealth Index (continuous) | Malaria RDT |
| Mmbando et al. 2011; Tanzania | Cross-sectional | children 0–19 years | 12,298 | Wealth Index (tertile) | Malaria examined by microscopy |
| Siri, 2014; 9 SSA countries | Cross-sectional | Children | 34,139 | Wealth Index (continuous) | Malaria RDT and Malaria examined by microscopy |
| Custodio et al. 2009; Equatorial Guinea | Cross-sectional | Children 0–5 years old | 552 | Wealth Index (tertile) | Malaria examined by microscopy |
| de Beaudrap et al. 2011; Uganda | Cross-sectional | Children 0–5 years old | 2847 | Wealth Index (continuous) | Malaria RDT and Malaria examined by microscopy |
| Sonko et al. 2014; The Gambia | Cross-sectional | Children and the general population | 6–59 months (n = 1248), 5 to 14 years (n = 1987), adults (n = 2306) | Wealth Index (quintile) | Malaria RDT and Malaria examined by microscopy |
| Chirombo et al. 2014; Malawi | Cross-sectional | Children under 5 years | 2093 | Wealth Index (quintiles) | Malaria RDT |
| de Glanville et al. 2019; Kenya | Cross-sectional | Individuals older or equal to 5 years | 2113 | Wealth Index (continuous) | Malaria examined by microscopy |
| Florey et al. 2012; Kenya | Cross-sectional | Children aged 8–17 and adults 18–86 | 223 children and 338 adults | Wealth index (continuous) | Polymerase chain reaction |
| Kahabuka et al. 2012; Tanzania | Cross-sectional | Caretakers who brought their sick children at Korogwe and Muheza district hospitals | 296 | Wealth Index (tertiles) | Malaria RDT |
| Ma et al. 2017; DRC | Cross-sectional | Healthy children aged 2 months to 5 years attending well-child and/or immunization visits | 647 | Wealth index (continuous) | Malaria RDT |
| West et al. 2013; Tanzania | Cross-sectional | Children 6–14 years | 5152 | Wealth index (cluster SEP) (tertiles) | Malaria RDT |
| Williams et al. 2016; Burkina Faso, The Gambia, Ghana and Mali | Cross-sectional | Women enrolled in a trial of intermittent screening and treatment of malaria in pregnancy (ISTP) versus IPTP | 2526 | Wealth Index (quintiles) | Malaria RDT and Polymerase Chain Reaction |
| Zgambo et al. 2017; Malawi | Cross-sectional | Children under the age of five | 2012 Survey (n = 2173), 2014 Survey (n = 2029) | Wealth index (quintiles) | Malaria examined by microscopy |
| Mann et al. 2021; Nigeria | Cross-sectional | Children aged 6–59 months | 12,996 | Wealth index (quintile) | Malaria examined by microscopy |
| Emina et al. 2021; DRC | Cross-sectional | Children aged 6–59 months | 8547 | Wealth index (quintile) | Malaria examined by microscopy |
| Mwaiswelo et al. 2021; Tanzania | Cross-sectional | Children aged 3–59 months | 2340 | Wealth index (tertiles) | Malaria RDT |
| Mangani et al. 2022; Malawi | Cross-sectional | All individuals aged 6 months or older who slept in the house for at least 2 weeks of the previous month | 5829 | Wealth Index (quintiles) | Malaria RDT |
| Ejigu 2020; Mozambique | Cross-sectional | Children of age 6–59 months | 4347 | Wealth Index (quintiles) | Malaria RDT |
| Gari et al. 2016; Ethiopia | Trial | General population | 5309 | Wealth Index (tertiles) | Malaria RDT or Malaria examined by microscopy |
| Liu et al. 2014; Tanzania | Trial | Children (one per household) were followed from 2 to 24 months | 435 | Wealth Index (quintiles) | Malaria examined by microscopy |
DRC Democratic Republic of Congo, IPTP intermittent preventive treatment in pregnancy, RDT rapid diagnostic test, SEP socioeconomic position
Associations between SEP and malaria (regression and mediation analyses)
Most studies employed multivariable logistic regression, followed by Poisson regression. Two studies conducted three mediation analyses. Overall, 39 studies indicated a protective effect of higher SEP on malaria (point effect estimate). Of these, all cross-sectional studies, eight out of nine cohort/case-control studies, and one trial indicated a protective effect. The analyses performed, the effect estimates of SEP on malaria, the confounders adjusted form and quality ratings are shown in Table 2 and Figs. 3 and 4.
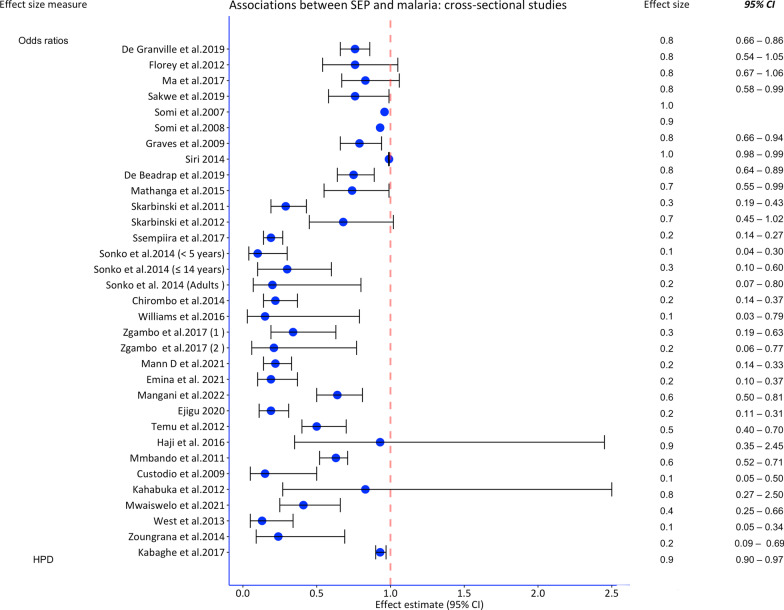
Fig. 3.
Forest plot of risk estimates from cross-sectional studies assessing the association between socioeconomic position and malaria in Sub-Saharan Africa. CI confidence interval, HPD highest posterior density, SEP socioeconomic position
Fig. 4.
Forest plot of risk estimates from the cohort, case–control studies and trials for the association between socioeconomic position and malaria in Sub-Saharan Africa. CI confidence interval, SEP socioeconomic position
Mediators and mediation methods: association between SEP and malaria
Two studies (a cohort and cross-sectional) investigated putative mediating factors on the path from SEP to malaria. Three pathways were explored: access to healthcare (use of antimalarials) [46], housing quality, and food security [1]. Only one study had a theoretical framework for mediation analysis [1], and no study assessed for interactions or performed sensitivity analyses which are vital to verify findings. Approaches to mediation analysis that overcome the limitations of the difference and the product methods exist and should be used. Details of mediators and methods applied are reported below.
Antimalarial use In a cross-sectional study by de Glanville et al. [46], there was significantly increased use of antimalarials by individuals in wealthier households with relatively lower malaria risk. This study indicated minimal mediation by antimalarial use, although the proportion of the effect mediated was not shown. In the adjusted model, however, both socioeconomic index (OR = 0.76, 95% CI 0.66–0.87) and recent antimalarial use (OR = 0.67, 95% CI 0.46–0.96) were protective against malaria. In this study, the mediation method applied was a regression-based comparison of the models (assessing for attenuation of coefficients) upon inclusion of the mediators.
Housing quality and food security A prospective cohort study by Tusting et al. [1] explored the mediating role of housing and food security on the effect of SEP on malaria incidence in Uganda using the Monte Carlo simulation approach described by Imai [59]. Food security was defined as secure if a household had 3–7 days of meat in the menu compared to only 0–2 times. A theoretical framework guided the mediation analysis in Tusting’s study [1]. In their analysis, housing type explained 24.9% of the effect of SEP, while food security explained 18.6% of the total effect of SEP on malaria risk.
Health expenditure (used as a proxy for treatment-seeking behaviour) was also explored. Due to limited information (data available for only 57% of children), mediation analysis was not robust and reported no mediating role.
Potential mediators in adjusted regression models involving SEP and malaria
Without performing a formal mediation analysis, several studies adjusted for factors that potentially mediate the SEP and malaria relationship. In these studies, SEP was either a covariate or primary independent variable (exposure). These factors satisfy at least two conditions [i.e. (1) have statistically significant relationship between exposure (X) and outcome (Y) in univariable regressions and (2) inclusion of the mediator variable (M) should reduce the direct effect of X on Y] to support mediation, according to Baron and Kenny [60] and warrant testing for the mediating role in future studies.
Use of insecticide-treated mosquito nets
The review identified 26 studies which adjusted for ownership/use of ITNs in the association between SEP and malaria. Of these, 20 studies showed a protective effect on malaria among individuals that used mosquito nets compared to those that did not. Two studies [24, 42] found that ITNs were associated with a higher risk of malaria, while four studies [19, 44, 48, 51] found marginal or no association with malaria risk.
Education attainment
Educational attainment refers to the highest level of education that a person has successfully completed. This includes no formal education, primary/elementary, secondary and tertiary or vocational. Eighteen studies that included educational level [categorized into three levels: none, primary, post primary (11 studies), four levels: none, primary, secondary, post primary (4 studies), two levels: none/primary, post primary (3 studies)], and SEP in models predicting malaria risk were identified. The effect of educational level on malaria risk was mixed with 12 studies [28, 33, 34, 37, 40–42, 51–53, 56, 57] indicating the protective effect of higher educational levels on malaria risk, and six studies [23, 25, 44, 49, 50, 54] showed no significant association with malaria.
Housing quality
Housing was expressed based on quality of specific housing materials (good vs poor); walls (n = 5), roof (n = 5), windows (n = 2), floor (n = 1), or additive housing index (n = 3). Eleven studies included housing quality in the multivariable regression models involving the association between SEP and the prevalence or incidence of malaria. Although eight studies [20, 21, 25–27, 29, 41, 43] indicated a significant protective effect of housing on malaria risk after controlling for SEP, the remaining two studies showed no associations between housing and malaria [23, 57]. There were also mixed associations in the cross-sectional survey by Somi [30], where improved walls were associated with reduced odds of malaria, while improved roofs had no significant effect on the risk of malaria. Rather than looking at the independent effect of roofs, walls, windows, eaves, and ceilings on malaria, most studies assessed the combined effect of these structures. Using these structures, studies grouped houses as poor quality (thatched roofs, dirt floors, completely uncovered windows, no ceilings, rough or mud walls and open eaves) and high quality (iron/tile roofs, concrete/brick walls, closed eaves, screened windows and ceilings).
Indoor residual spraying
The review identified five studies that adjusted for IRS in their multivariable models containing SEP. Of these, four studies showed that IRS was associated with a lower risk of malaria [32, 35, 36, 42]. Graves et al. [19] did not find a relationship between IRS and malaria risk whether assessed at a 6- or 12-month history of IRS.
Health-seeking behaviour
Access to health care was defined in terms of choice of place for healthcare (facility vs home; far vs nearest facility), and duration between symptom onset and seeking of care. Three studies adjusted for self-reported health-seeking behaviours in their associations involving SEP and malaria prevalence [28, 45, 50]. Two studies showed that seeking facility care versus at-home care and receiving treatment for fever symptoms promptly reduced the risk of severe malaria [28, 50]. In contrast, a study in Ethiopia found no significant association between early treatment seeking and malaria risk [45]. It is important to note that these studies used different proxies of health-seeking behaviour, and outcomes were different for example in one study, the outcome was severe malaria and in another, it was uncomplicated malaria.
Antimalarials, nutrition status and outside night activity
The review identified two studies that adjusted for antimalarials [24, 54], four that adjusted for nutritional status [24, 34, 54, 57], and one trial that controlled for repellent use [29]. All these studies highlighted the protective effect of antimalarial use, nutrition, and repellent use, respectively. In addition, one cross-sectional study showed no association between outdoor night activities and malaria [47].
Quality of reviewed studies
Eligible studies were assessed for their methodological quality using the EPHPP tool. Eight (8) studies were rated as having high quality (strong), and 11 studies were rated as having low quality, with the majority of the included studies having a moderate methodological quality (n = 22). Eight of the included studies deemed confounding bias to be a serious threat. These studies only controlled for less than 60% of the significant confounders in the association between SEP and malaria. Six (6) studies were found to have selection bias, and 30 of the 41 studies were cross-sectional, making the claim of mediation only speculative (Additional file 1: Table S4).
Discussion
This systematic review aimed to identify, summarize and critically appraise the existing evidence regarding the variables that potentially mediate the relationship between SEP and malaria in SSA. Our review shows that evidence of mediating pathways between household SES and malaria is sparse and under-researched.
Of the 41 studies, only two assessed mediators of the SEP and malaria path using formal mediation analyses specifically housing quality, food security, antimalarial use. One study showed that a proportion of the total effect of SEP on malaria was mediated through housing and food security, while another showed minimal mediation by antimalarial use. However, each mediator was only investigated in one study, meaning these findings remain inconclusive. Other studies indicated that ITN use, higher education, better nutrition, housing quality, IRS, and repellent use could, to a great extent, protect against malaria. These, however, did not conduct mediation analyses but included potential mediators as covariates. This review provides valuable insights for directed action/interventions to alleviate poverty-related malaria burdens, improve health outcomes of marginalized populations, and contribute to reducing global malaria incidence and mortality rates by at least 90%, as per the global technical strategy for malaria [61] and in line with the Sustainable Development Goals (SDGs), particularly the goal of ending poverty (SDG 1) and achieving universal health coverage (SDG 3). For instance, based on these findings, interventions that target housing improvements and food security could substantially prevent /mitigate malaria risk. It is important to acknowledge that the measurement of SEP varied across different studies and countries, particularly in terms of the included assets, data reduction techniques, and the decision to categorize or not which significantly impacts the comparability of findings, even when there is consistency in the direction of the association between SEP and malaria. We also acknowledge that SEP may have improved or declined in the study areas however all studies (except one) were published within 10 years since recruitment which makes findings relevant.
Mediators identified through formal mediation
Our review indicates that housing quality and food security could mediate socioeconomic differences in malaria risk. One cohort study demonstrated that housing quality and food insecurity mediated 24.9% and 18.6% of the effect of SEP on malaria incidence in SSA, respectively [1]. The study also suggested that treatment-seeking behavior could have a mediating role; however, the authors did not perform a robust mediation analysis due to insufficient sample size. The analyses in Tusting’s paper [1] were informed by a conceptual framework that, although commendable, did not operationalize pathways such as access to healthcare and ITNs in their mediation analyses. In another study [46], antimalarial treatment was also found to mediate the association between SEP and malaria. Yet, this study did not provide information on the percentage of total effect mediated and the identifiability assumptions checked, hence requiring a cautious interpretation of the findings [46]. These assumptions are (i) No unmeasured exposure-outcome confounding given covariates, C, (ii) No unmeasured mediator-outcome confounding given C, (iii) No unmeasured exposure-mediator confounding given C, (iv) No effect of exposure that confounds the mediator-outcome relationship [14]. The proportion of the socioeconomic differences in malaria mediated by housing and food security was small (less than 30%), which indicated that other potential mediators could explain part of the effect of SEP. Nevertheless, incremental improvements in housing quality and interventions, such as irrigation and agriculture could promote food security, thereby protecting against malaria.
While previous literature suggests the mediating role of socioeconomic and structural factors in the association between SEP and malaria, research remains limited. Some assumptions for mediation were not always met (including potential reverse causality and identifiability), and many analyses do not consider interactions nor adjust for mediator outcome confounding, which raises concerns about the validity of the mediation effects reported. Future studies need to apply more robust analyses on longitudinal data for which many assumptions may hold.
Potential mediators (with no formal mediation)
Rather than formal mediation analysis, variables were considered potential mediators if their inclusion in the adjusted models resulted into change (reduction) in the SEP coefficient. While it is generally not recommended to control for mediating variables in the causal relationship because conditioning on them introduces bias [62], the attenuation in coefficients of SEP in a multivariable model implies they could be mediators.
Most studies in this review indicated a protective effect on malaria with higher education [28, 34, 37, 41, 42, 51, 57], IRS [32, 35, 36, 42], better housing [20, 21, 25–27, 29, 41, 43], and use of ITNs after adjusting for SEP. Consistent with previous literature, which shows a consistent association between wealth and ITN use and IRS [63–66], a recent systematic review of the effectiveness of ITNs showed a strong protective effect against malaria [67]. In another review, the addition of IRS on averaged reduced malaria parasite prevalence (RR = 0.61, 95% CI 0.42 to 0.88) [68] indicates that interventions targeting IRS and ITNs combined may significantly affect malaria morbidity although this effect may not be observed in all contexts. It is important to note that utilization of IRS and ITNs may be affected by their high costs, low coverage and poor quality of IRS in some settings [69].
Educational attainment is also a well-known predictor of malaria risk [6]. Greater wealth encourages higher educational attainment [70], which may increase individuals’ knowledge of prevention and treatment, decision-making, and access to information [58]. This could encourage the uptake of preventive measures and consequently lower malaria risk. In our review, 12 of 18 studies indeed found an association between higher educational attainment and a lower risk of malaria after adjusting for SEP. However, the evidence regarding the proportion of SEP effect mediated through education is limited.
Further, most studies that adjusted for housing quality found that improved housing was protective against malaria with a reduced coefficient of SEP. Higher SEP makes it easier to acquire better housing (shelters with better roofs, shutters, and eaves), which can then reduce exposure to the biting Anopheles at night by preventing the entry of mosquito vectors. Evidence for the effect of SEP mediated through housing is still forthcoming, with a single study suggesting that improvements in housing could partly explain the protective effect of SEP [1]. In light of this evidence, improving housing or improving accessibility of building materials to households with low SEP could contribute to reduction of malaria burden. However, there is a need to determine potential mediators and their relative contributions to the association between SEP and malaria to inform the design and implementation of targeted socio-structural interventions against malaria.
Strengths, limitations and implications for further research
To the best of our knowledge, this is the first systematic review that has attempted to explore the potential mediators on the path between SEP and malaria in SSA. Unlike recent reviews [4, 6], which included education and housing as proxies for SEP, we defined our exposure (SEP) based on household wealth indices (asset-based indices), which is a more reliable measure of household wealth in low-income countries. However, the review’s findings are not without limitations. First, while the review was comprehensive and involved 41 articles and tens of thousands of participants, we identified only three formal mediation analyses of pathways linking SEP and malaria; hence, uncertainties remain around the relative contribution of several potential mediators. Second, we may have missed other studies because we searched only two databases and also did not search grey literature or studies in languages other than English. Nevertheless, we think this is still a specialized area of academic research, and most of the studies that meet the criteria are most likely to be published in international peer-reviewed journals. Thirdly, most studies had methodological limitations, such as the lack of a conceptual framework, sensitivity analyses, ignorability assumptions, and the use of cross-sectional data, which renders claims of causal mediation speculative because temporarity could not be established. Longitudinal designs are better suited to demonstrate temporality, a key aspect in causal inferences and especially important for studies on SEP and malaria due to the bi-directionality of their relationship [30]. With longitudinal data, more suitable methods, such as VanderWeele’s parametric mediational g-formula, can account for time-varying exposures, mediators and confounding affected by previous exposure could be applied [71].
Conclusions
Our study indicates that a relatively small body of research has tested indirect pathways between SEP and malaria. From reviewed evidence, extant research suggests that housing, food security and recent antimalarial use are likely mediators in the SEP-malaria relationship in SSA. Although other pathways, such as education, IRS and ITN use, nutrition, and health-seeking behaviour, are not fully supported by current evidence, their role cannot be ignored due to their demonstrated protective effect on malaria when modelled with SEP as a covariate. More formal mediation analyses using longitudinal data are needed to overcome methodological limitations, such as cross-sectional data, insufficient confounder adjustment, and limited use of sound conceptual frameworks. This research area holds much potential in informing the design of more effective interventions for malaria control.
Supplementary Information
Additional file 1: Table S1. Highlights of the review. Table S2. Detailed search strategy, conducted on May 31, 2022. Table S3. Quality assessment tool for quantitative studies. Table S4. Assessment of the quality of included studies.
Acknowledgements
We acknowledge the Infectious Epidemiology Department at Bernhard Nocht Institute for Tropical Medicine for their cooperation and support towards completing this article.
Abbreviations
- DRC
Democratic Republic of Congo
- EPHPP
Effective Public Health Practice Project Tool
- GEE
Generalized estimating equations
- HH
Household
- HPD
Highest posterior density
- IPTP
Intermittent preventive treatment in pregnancy
- IRS
Indoor residual spraying
- IRR
Incidence rate ratios
- ITN
Insecticide treated Nets
- OR
Odds ratios
- RDT
Rapid diagnostic test
- PCA
Principal Component Analysis
- SEP
Socioeconomic position
- SSA
Sub-Saharan Africa
Author contributions
JB, DIP, EL and STW conceived the study. THH, MAF and STW spearheaded the review, including conducting database searches, screening review articles, updating the review and critically appraising articles. STW drafted the manuscript, which was critically reviewed by JB, JM, EL and DIP. EL, JB, DIP and JM provided support and mentorship during the development and writing of the review. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
All data relevant to the study are included in this published article and uploaded as Additional file.
Declarations
Ethics approval and consent to participate
This research did not require institutional review approval since all data were publicly available and collected from existing online databases. This research did not involve any human subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tusting LS, Rek J, Arinaitwe E, Staedke SG, Kamya MR, Cano J, et al. Why is malaria associated with poverty? Findings from a cohort study in rural Uganda. Infect Dis Poverty. 2016;5:78. doi: 10.1186/s40249-016-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World malaria report 2020: 20 years of global progress and challenges. WHO. 2020. https://apps.who.int/iris/rest/bitstreams/1321872/retrieve. Accessed 8 Apr 2022.
- 3.Teklehaimanot, PaolaMejia A. Malaria and poverty. Ann N Y Acad Sci. 2008;1136:32–37. doi: 10.1196/annals.1425.037. [DOI] [PubMed] [Google Scholar]
- 4.Tusting LS, Willey B, Lucas H, Thompson J, Kafy HT, Smith R, et al. Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet. 2013;382:963–972. doi: 10.1016/S0140-6736(13)60851-X. [DOI] [PubMed] [Google Scholar]
- 5.Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J. 2015;14:209. doi: 10.1186/s12936-015-0724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degarege A, Fennie K, Degarege D, Chennupati S, Madhivanan P. Improving socioeconomic status may reduce the burden of malaria in sub Saharan Africa: a systematic review and meta-analysis. PLoS ONE. 2019;14:e0211205. doi: 10.1371/journal.pone.0211205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krefis AC, Schwarz NG, Nkrumah B, Acquah S, Loag W, Sarpong N, et al. Principal component analysis of socioeconomic factors and their association with malaria in children from the Ashanti Region, Ghana. Malar J. 2010;9:201. doi: 10.1186/1475-2875-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkingham J, Namazie C. Measuring health and poverty: a review of approaches to identifying the poor. DFID. 2002. https://assets.publishing.service.gov.uk/media/57a08d46ed915d622c0018bd/Measuring-health-and-poverty.pdf. Accessed 17 Sept 2021.
- 9.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 10.Fobil JN, Kraemer A, Meyer CG, May J. Neighborhood urban environmental quality conditions are likely to drive malaria and diarrhea mortality in Accra, Ghana. J Environ Public Health. 2011;2011:484010. doi: 10.1155/2011/484010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barat LM, Palmer N, Basu S, Worrall E, Hanson K, Mills A. Do malaria control interventions reach the poor? A view through the equity lens. Am J Trop Med Hyg. 2004;71:174–178. doi: 10.4269/ajtmh.2004.71.174. [DOI] [PubMed] [Google Scholar]
- 12.Liu J. Early health risk factors for violence: conceptualization, review of the evidence, and implications. Aggress Violent Behav. 2011;16:63–73. doi: 10.1016/j.avb.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacKinnon DP. Introduction to statistical mediation analysis. London: Routledge; 2012. [Google Scholar]
- 14.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.World Bank. Sub-Saharan Africa. 2022. https://data.worldbank.org/region/sub-saharan-africa. Accessed 20 June 2020.
- 17.Thomas H, Ciliska D, Micucci S, Wilson‐ABra J, Dobbins M. Effective public health practice project (EPHPP). 1999.
- 18.Loha E, Lindtjørn B. Predictors of Plasmodium falciparum malaria incidence in Chano Mille, South Ethiopia: a longitudinal study. Am J Trop Med Hyg. 2012;87:450–459. doi: 10.4269/ajtmh.2012.12-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graves PM, Richards FO, Ngondi J, Emerson PM, Shargie EB, Endeshaw T, et al. Individual, household and environmental risk factors for malaria infection in Amhara, Oromia and SNNP regions of Ethiopia. Trans R Soc Trop Med Hyg. 2009;103:1211–1220. doi: 10.1016/j.trstmh.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Coleman M, Coleman M, Mabaso ML, Mabuza AM, Kok G, Coetzee M, et al. Household and microeconomic factors associated with malaria in Mpumalanga, South Africa. Trans R Soc Trop Med Hyg. 2010;104:143–147. doi: 10.1016/j.trstmh.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Mmbando BP, Kamugisha ML, Lusingu JP, Francis F, Ishengoma DS, Theander TG, et al. Spatial variation and socio-economic determinants of Plasmodium falciparum infection in northeastern Tanzania. Malar J. 2011;10:145. doi: 10.1186/1475-2875-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark TD, Greenhouse B, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Staedke SG, et al. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J Infect Dis. 2008;198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- 23.Siri JG. Independent associations of maternal education and household wealth with malaria risk in children. Ecol Soc. 2014;19:33. doi: 10.5751/ES-06134-190133. [DOI] [Google Scholar]
- 24.Custodio E, Descalzo MA, Villamor E, Molina L, Sánchez I, Lwanga M, et al. Nutritional and socio-economic factors associated with Plasmodium falciparum infection in children from Equatorial Guinea: results from a nationally representative survey. Malar J. 2009;8:225. doi: 10.1186/1475-2875-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Beaudrap P, Nabasumba C, Grandesso F, Turyakira E, Schramm B, Boum Y, 2nd, et al. Heterogeneous decrease in malaria prevalence in children over a six-year period in south-western Uganda. Malar J. 2011;10:132. doi: 10.1186/1475-2875-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asante KP, Owusu-Agyei S, Cairns M, Dodoo D, Boamah EA, Gyasi R, et al. Placental malaria and the risk of malaria in infants in a high malaria transmission area in Ghana: a prospective cohort study. J Infect Dis. 2013;208:1504–1513. doi: 10.1093/infdis/jit366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonko ST, Jaiteh M, Jafali J, Jarju LBS, D'Alessandro U, Camara A, et al. Does socio-economic status explain the differentials in malaria parasite prevalence? Evidence from The Gambia. Malar J. 2014;13:449. doi: 10.1186/1475-2875-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahabuka C, Kvåle G, Hinderaker SG. Factors associated with severe disease from malaria, pneumonia and diarrhea among children in rural Tanzania—a hospital-based cross-sectional study. BMC Infect Dis. 2012;12:219. doi: 10.1186/1471-2334-12-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JX, Bousema T, Zelman B, Gesase S, Hashim R, Maxwell C, et al. Is housing quality associated with malaria incidence among young children and mosquito vector numbers? Evidence from Korogwe, Tanzania. PLoS ONE. 2014;9:e87358. doi: 10.1371/journal.pone.0087358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somi MF, Butler JR, Vahid F, Njau J, Kachur SP, Abdulla S. Is there evidence for dual causation between malaria and socioeconomic status? Findings from rural Tanzania. Am J Trop Med Hyg. 2007;77:1020–1027. doi: 10.4269/ajtmh.2007.77.1020. [DOI] [PubMed] [Google Scholar]
- 31.Somi MF, Butler JR, Vahid F, Njau JD, Kachur SP, Abdulla S. Use of proxy measures in estimating socioeconomic inequalities in malaria prevalence. Trop Med Int Health. 2008;13:354–364. doi: 10.1111/j.1365-3156.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- 32.West PA, Protopopoff N, Rowland M, Cumming E, Rand A, Drakeley C, et al. Malaria risk factors in North West Tanzania: the effect of spraying, nets and wealth. PLoS ONE. 2013;8:e65787. doi: 10.1371/journal.pone.0065787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mwaiswelo RO, Mmbando BP, Chacky F, Molteni F, Mohamed A, Lazaro S, et al. Malaria infection and anemia status in under-five children from Southern Tanzania where seasonal malaria chemoprevention is being implemented. PLoS ONE. 2021;16:e0260785. doi: 10.1371/journal.pone.0260785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathanga DP, Halliday KE, Jawati M, Verney A, Bauleni A, Sande J, et al. The high burden of malaria in primary school children in Southern Malawi. Am J Trop Med Hyg. 2015;93:779–789. doi: 10.4269/ajtmh.14-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skarbinski J, Mwandama D, Luka M, Jafali J, Wolkon A, Townes D, et al. Impact of health facility-based insecticide treated bednet distribution in Malawi: Progress and challenges towards achieving universal coverage. PLoS ONE. 2011;6:e21995. doi: 10.1371/journal.pone.0021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skarbinski J, Mwandama D, Wolkon A, Luka M, Jafali J, Smith A, et al. Impact of indoor residual spraying with lambda-cyhalothrin on malaria parasitemia and anemia prevalence among children less than five years of age in an area of intense, year-round transmission in Malawi. Am J Trop Med Hyg. 2012;86:997–1004. doi: 10.4269/ajtmh.2012.11-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zgambo M, Mbakaya BC, Kalembo FW. Prevalence and factors associated with malaria parasitaemia in children under the age of five years in Malawi: a comparison study of the 2012 and 2014 Malaria Indicator Surveys (MISs) PLoS ONE. 2017;12:e0175537. doi: 10.1371/journal.pone.0175537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chirombo J, Lowe R, Kazembe L. Using structured additive regression models to estimate risk factors of malaria: analysis of 2010 Malawi malaria indicator survey data. PLoS ONE. 2014;9:e101116. doi: 10.1371/journal.pone.0101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabaghe AN, Chipeta MG, McCann RS, Phiri KS, van Vugt M, Takken W, et al. Adaptive geostatistical sampling enables efficient identification of malaria hotspots in repeated cross-sectional surveys in rural Malawi. PLoS ONE. 2017;12:e0172266. doi: 10.1371/journal.pone.0172266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangani C, Frake AN, Chipula G, Mkwaila W, Kakota T, Mambo I, et al. Proximity of residence to irrigation determines malaria risk and Anopheles abundance at an irrigated agroecosystem in Malawi. Am J Trop Med Hyg. 2021;106:283–292. doi: 10.4269/ajtmh.21-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyman K, Mwangwa F, Bigira V, Kapisi J, Clark TD, Osterbauer B, et al. Poor housing construction associated with increased malaria incidence in a cohort of young Ugandan children. Am J Trop Med Hyg. 2015;92:1207–1213. doi: 10.4269/ajtmh.14-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ssempiira J, Nambuusi B, Kissa J, Agaba B, Makumbi F, Kasasa S, et al. Geostatistical modelling of malaria indicator survey data to assess the effects of interventions on the geographical distribution of malaria prevalence in children less than 5 years in Uganda. PLoS ONE. 2017;12:e0174948. doi: 10.1371/journal.pone.0174948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanzirah H, Tusting LS, Arinaitwe E, Katureebe A, Maxwell K, Rek J, et al. Mind the gap: house structure and the risk of malaria in Uganda. PLoS ONE. 2015;10:e0117396. doi: 10.1371/journal.pone.0117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gari T, Kenea O, Loha E, Deressa W, Hailu A, Balkew M, et al. Malaria incidence and entomological findings in an area targeted for a cluster-randomized controlled trial to prevent malaria in Ethiopia: results from a pilot study. Malar J. 2016;15:145. doi: 10.1186/s12936-016-1199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haji Y, Fogarty AW, Deressa W. Prevalence and associated factors of malaria among febrile children in Ethiopia: a cross-sectional health facility-based study. Acta Trop. 2016;155:63–70. doi: 10.1016/j.actatropica.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 46.de Glanville WA, Thomas LF, Cook EAJ, Bronsvoort BMDC, Wamae NC, Kariuki S, et al. Household socio-economic position and individual infectious disease risk in rural Kenya. Sci Rep. 2019;9:2972. doi: 10.1038/s41598-019-39375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Florey LS, King CH, Van Dyke MK, Muchiri EM, Mungai PL, Zimmerman PA, et al. Partnering parasites: evidence of synergism between heavy Schistosoma haematobium and Plasmodium species infections in Kenyan children. PLoS Negl Trop Dis. 2012;6:e1723. doi: 10.1371/journal.pntd.0001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siri JG, Wilson ML, Murray S, Rosen DH, Vulule JM, Slutsker L, et al. Significance of travel to rural areas as a risk factor for malarial anemia in an urban setting. Am J Trop Med Hyg. 2010;82:391–397. doi: 10.4269/ajtmh.2010.09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams J, Njie F, Cairns M, Bojang K, Coulibaly SO, Kayentao K, et al. Non-falciparum malaria infections in pregnant women in West Africa. Malar J. 2016;15:53. doi: 10.1186/s12936-016-1092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoungrana A, Chou YJ, Pu C. Socioeconomic and environment determinants as predictors of severe malaria in children under 5 years of age admitted in two hospitals in Koudougou district, Burkina Faso: a cross sectional study. Acta Trop. 2014;139:109–114. doi: 10.1016/j.actatropica.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Ma C, Claude KM, Kibendelwa ZT, Brooks H, Zheng X, Hawkes M. Is maternal education a social vaccine for childhood malaria infection? A cross-sectional study from war-torn Democratic Republic of Congo. Pathog Glob Health. 2017;111:98–106. doi: 10.1080/20477724.2017.1288971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emina JBO, Doctor HV, Yé Y. Profiling malaria infection among under-five children in the Democratic Republic of Congo. PLoS ONE. 2021;16:e0250550. doi: 10.1371/journal.pone.0250550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mann DM, Swahn MH, McCool S. Undernutrition and malaria among under-five children: findings from the 2018 Nigeria demographic and health survey. Pathog Glob Health. 2021;115:423–433. doi: 10.1080/20477724.2021.1916729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincenz C, Dolo Z, Saye S, Lovett JL, Strassmann BI. Risk factors for placental malaria, sulfadoxine-pyrimethamine doses, and birth outcomes in a rural to urban prospective cohort study on the Bandiagara Escarpment and Bamako, Mali. Malar J. 2022;21:110. doi: 10.1186/s12936-022-04125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Temu EA, Coleman M, Abilio AP, Kleinschmidt I. High prevalence of malaria in Zambezia, Mozambique: the protective effect of IRS versus increased risks due to pig-keeping and house construction. PLoS ONE. 2012;7:e31409. doi: 10.1371/journal.pone.0031409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ejigu BA. Geostatistical analysis and mapping of malaria risk in children of Mozambique. PLoS ONE. 2020;15:e0241680. doi: 10.1371/journal.pone.0241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakwe N, Bigoga J, Ngondi J, Njeambosay B, Esemu L, Kouambeng C, et al. Relationship between malaria, anaemia, nutritional and socio-economic status amongst under-ten children, in the North Region of Cameroon: a cross-sectional assessment. PLoS ONE. 2019;14:e0218442. doi: 10.1371/journal.pone.0218442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mmbando BP, Segeja MD, Msangeni HA, Sembuche SH, Ishengoma DS, Seth MD, et al. Epidemiology of malaria in an area prepared for clinical trials in Korogwe, north-eastern Tanzania. Malar J. 2009;8:165. doi: 10.1186/1475-2875-8-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15:309. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 60.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 61.WHO . Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015. [Google Scholar]
- 62.VanderWeele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology. 2012;23:1–9. doi: 10.1097/EDE.0b013e31823aca5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macintyre K, Keating J, Sosler S, Kibe L, Mbogo CM, Githeko AK, et al. Examining the determinants of mosquito-avoidance practices in two Kenyan cities. Malar J. 2002;1:14. doi: 10.1186/1475-2875-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Njama D, Dorsey G, Guwatudde D, Kigonya K, Greenhouse B, Musisi S, et al. Urban malaria: primary caregivers’ knowledge, attitudes, practices and predictors of malaria incidence in a cohort of Ugandan children. Trop Med Int Health. 2003;8:685–692. doi: 10.1046/j.1365-3156.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 65.Wafula ST, Mendoza H, Nalugya A, Musoke D, Waiswa P. Determinants of uptake of malaria preventive interventions among pregnant women in eastern Uganda. Malar J. 2021;20:5. doi: 10.1186/s12936-020-03558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Onwujekwe O, el Malik F, Mustafa SH, Mnzavaa A. Do malaria preventive interventions reach the poor? Socioeconomic inequities in expenditure on and use of mosquito control tools in Sudan. Health Policy Plan. 2006;21:10–16. doi: 10.1093/heapol/czj004. [DOI] [PubMed] [Google Scholar]
- 67.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 68.Pryce J, Medley N, Choi L. Indoor residual spraying for preventing malaria in communities using insecticide-treated nets. Cochrane Database Syst Rev. 2022;1:CD012688. doi: 10.1002/14651858.CD012688.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mumbengegwi DR, Sturrock H, Hsiang M, Roberts K, Kleinschmidt I, Nghipumbwa M, et al. Is there a correlation between malaria incidence and IRS coverage in western Zambezi region, Namibia? Public Health Action. 2018;8:S44–S49. doi: 10.5588/pha.17.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goesch JN, Schwarz NG, Decker ML, Oyakhirome S, Borchert LB, Kombila UD, et al. Socio-economic status is inversely related to bed net use in Gabon. Malar J. 2008;7:60. doi: 10.1186/1475-2875-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.VanderWeele TJ, TchetgenTchetgen EJ. Mediation analysis with time varying exposures and mediators. J R Stat Soc Ser B Stat Methodol. 2017;79:917–938. doi: 10.1111/rssb.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Highlights of the review. Table S2. Detailed search strategy, conducted on May 31, 2022. Table S3. Quality assessment tool for quantitative studies. Table S4. Assessment of the quality of included studies.
Data Availability Statement
All data relevant to the study are included in this published article and uploaded as Additional file.