General anesthetics are widely regarded as one of the most important advances in medicine to date. Despite extensive mechanistic studies, the primary targets of general anesthetics remain controversial. Thus, while a large body of evidence supports lipids as being the primary targets, other evidence points toward membrane proteins as the main targets. Here, I wish to highlight a study that we published back in 2011. My main motivation for highlighting that work is twofold. First, it provides deep insight into what I believe is the key step in virtually all lipid-based mechanisms of general anesthetics that have been proposed. Specifically, it shows how general anesthetics can loosen lipid rafts by weakening the affinity between cholesterol and high-melting sphingolipids—the principal components of lipid rafts. Second, I believe that a heightened awareness of such loosening will stimulate new experimental and theoretical studies that may help bring closure to this long-standing lipid/protein debate.
One lipid hypothesis of how general anesthetics operate is that they alter the lateral pressure profile within cell membranes. This alteration is then expected to modify the structure and functioning of membrane proteins.1 A recent experimental study has provided compelling evidence for a much more intricate series of events, in which chloroform and isoflurane activate TWIK-related K+ channels (TREK-1) through disruption of phospholipase D (PLD2) localization to lipid rafts with the subsequent production of signaling lipid phosphatidic acid (PA).2 Although this latter study takes into account a modern view of the lipid organization of cell membranes (where cholesterol-rich lipid domains or “rafts” float in a sea of more fluid lipid regions), exactly how the uptake of these general anesthetics leads to the disruption of lipid rafts and the localization of PLD2 was left unanswered. At a fundamental level, such disruption may be viewed as the main event.
To gain a deep understanding of lipid/anesthetic interactions, we used the nearest-neighbor recognition (NNR) method to quantify the action of chloroform, isoflurane, and halothane on model membranes that mimic the liquid-ordered (Lo) and liquid-disordered (Ld) phases.3−5 At present, the Lo and Ld phases represent the best working models of lipid rafts and the surrounding lipid environment in mammalian cell membranes, respectively.
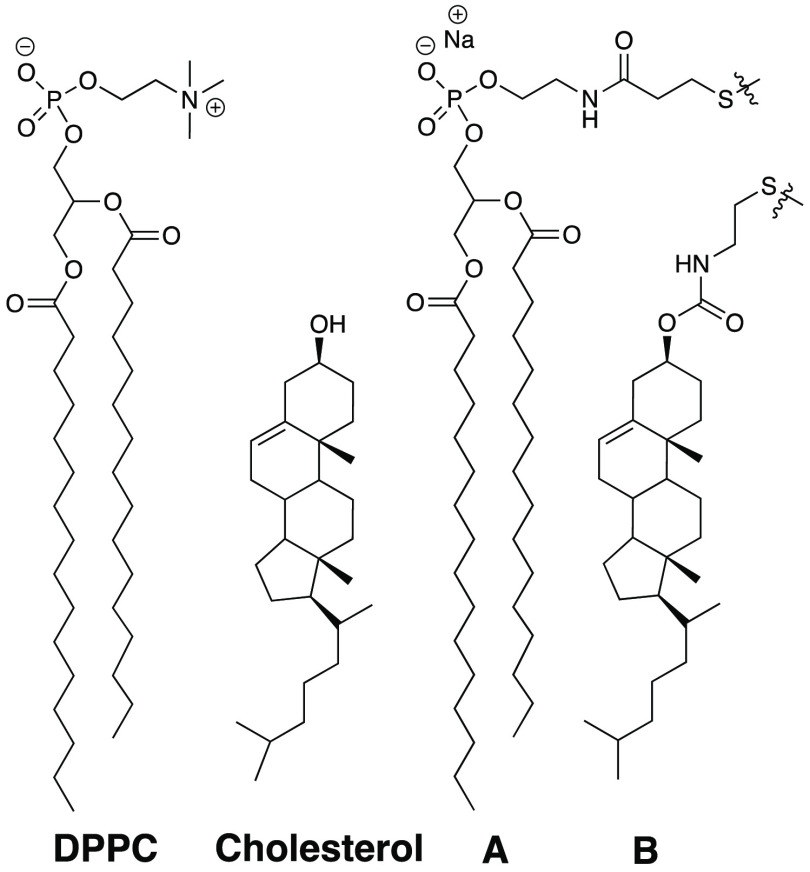
As discussed elsewhere, the NNR method uses exchangeable disulfide-based dimers of mimics of natural phospholipids such as 1,2-dipalmitoyl-sn-glycerol-3-phosphocholine (DPPC) and cholesterol (i.e., A and B, respectively) to quantify the free energy of interaction, ωAB, between them (Chart 1). It is noteworthy that this method is highly sensitive and can detect changes in free energies down to tens of calories per mole. Experimentally, dimer distributions within a given phase are monitored by high-performance liquid chromatography as a function of time until chemical equilibrium has been reached. The equilibrium constant, K, which characterizes the equilibrium between these dimers, AA + BB ⇌ 2AB, then affords a free energy of interaction between A and B via the use of the following equation: ωAB = −1/2RT ln(K/4).3
Chart 1.
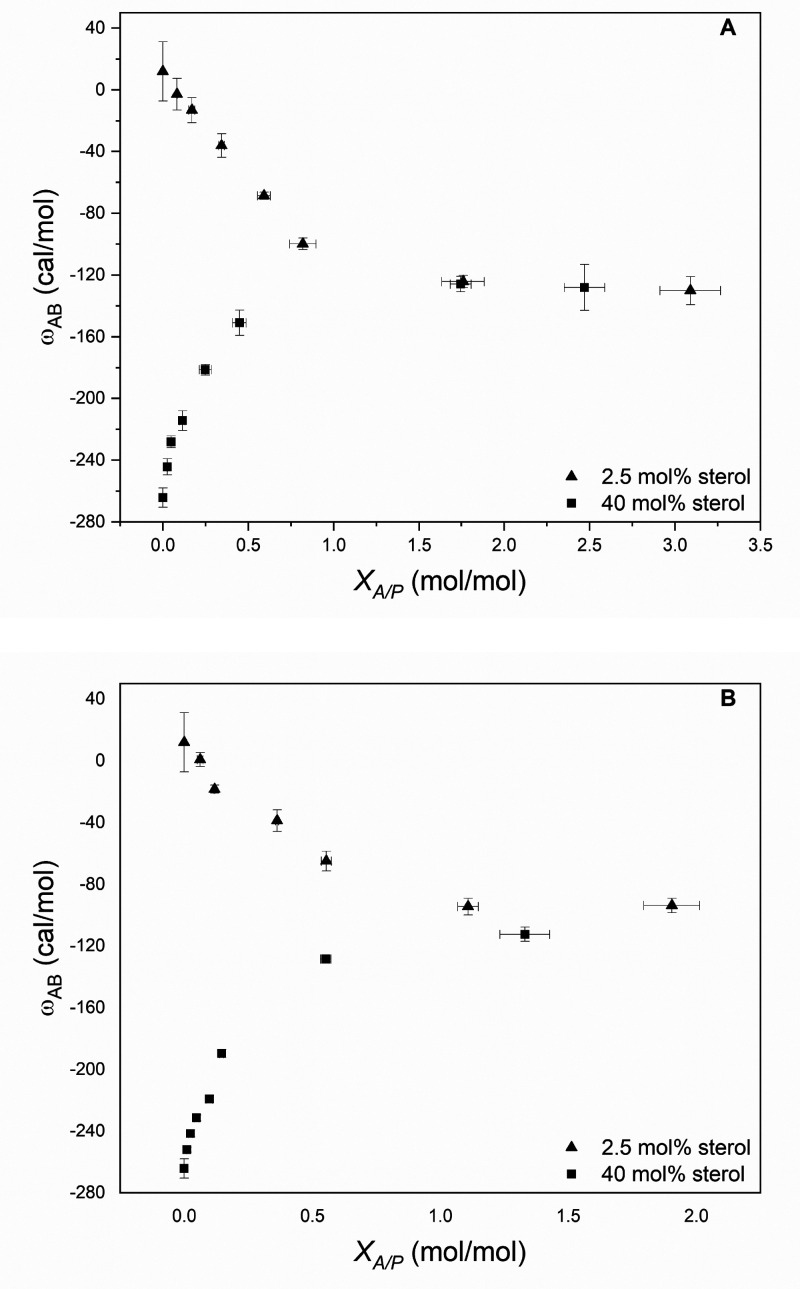
In brief, we found that chloroform, halothane, and isoflurane weaken the affinity betweenAandBin the Lo phase while strengthening their affinity in the Ld phase.5 This behavior is shown for chloroform and halothane in Figure 1 where ωAB is plotted as a function of the molar ratio of membrane-bound anesthetic, A, to phospholipid, P, that is present, i.e., XA/P. The fact that the curves for the Lo and Ld phases converge at a limiting value for XA/P of ca. 1 further implies that 1/1 complexes are being formed between the phospholipid and these anesthetics.
Figure 1.
Plot of free energy of interaction between A and B (i.e., ωAB) in the presence of (A) CHCl3 and (B) CF3CHBrCl (halothane) as a function of XA/P for cholesterol-poor (2.5 mol % sterol) and cholesterol-rich (40 mol % sterol) bilayers made from DPPC and cholesterol. Values of XA/P were determined, experimentally, as a function of the concentration of each anesthetic (not shown). Error bars that are not visible lie within the symbols themselves. Reprinted from ref (5). Copyright 2011 American Chemical Society.
By analogy, the uptake of general anesthetics by lipid rafts is expected to decrease the affinity between cholesterol and high-melting sphingolipids, thereby weakening these rafts and allowing for the disruption of PLD2 localization.
Finally, as noted by others, enantiomer selectivity of certain general anesthetics has been used as evidence to support membrane proteins as being their primary targets.2 However, since sphingolipids and cholesterol are inherently chiral, such selectivity is also fully consistent with lipids as being the primary targets.
The author declares no competing financial interest.
References
- Cantor R. S. The lateral pressure profile in membranes: A physical mechanism of general anesthesia. Biochemistry 1997, 36 (9), 2339–2344. 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- Pavel M. A.; Petersen E. N.; Wang H.; Lerner R. A.; Hansen S. B. Studies on the mechanism of general anesthesia. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (24), 13757–13766. 10.1073/pnas.2004259117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regen S. L. The origin of lipid rafts. Biochemistry 2020, 59 (49), 4617–4621. 10.1021/acs.biochem.0c00851. [DOI] [PubMed] [Google Scholar]
- Regen S. L. Cholesterol’s condensing effect: unpacking a century-old mystery. JACS.Au 2022, 2 (1), 84–91. 10.1021/jacsau.1c00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkyilmaz S.; Almeida P. F.; Regen S. L. Effects of isoflurane, halothane and chloroform on the interactions and lateral organization of lipids in the liquid-ordered phase. Langmuir 2011, 27 (23), 14380–14385. 10.1021/la2035278. [DOI] [PMC free article] [PubMed] [Google Scholar]