Abstract
Deficiency of selenium (Se) has been described in a significant number of COVID-19 patients having a higher incidence of mortality, which makes it a pertinent issue to be addressed clinically for effective management of the COVID-19 pandemic. Se nanoparticles (SeNPs) provide a unique option for managing the havoc caused by the COVID-19 pandemic. SeNPs possess promising anti-inflammatory and anti-fibrotic effects by virtue of their nuclear factor kappa-light-chain-stimulator of activated B cells (NFκB), mitogen-activated protein kinase (MAPKs), and transforming growth factor-beta (TGF-β) modulatory activity. In addition, SeNPs possess remarkable immunomodulatory effects, making them a suitable option for supplementation with a much lower risk of toxicity compared to their elemental counterpart. Further, SeNPs have been shown to curtail viral and microbial infections, thus, making it a novel means to halt viral growth. In addition, it can be administered in the form of aerosol spray, direct injection, or infused thin-film transdermal patches to reduce the spread of this highly contagious viral infection. Moreover, a considerable decrease in the expression of selenoprotein along with enhanced expression of IL-6 in COVID-19 suggests a potential association among selenoprotein expression and COVID-19. In this review, we highlight the unique antimicrobial and antiviral properties of SeNPs and the immunomodulatory potential of selenoproteins. We provide the rationale behind their potentially interesting properties and further exploration in the context of microbial and viral infections. Further, the importance of selenoproteins and their role in maintaining a successful immune response along with their association to Se status is summarized.
Keywords: COVID-19, Selenium nanoparticles, Antimicrobial, Antiviral, Anti-inflammatory, Selenoproteins
Graphical abstract
1. Introduction
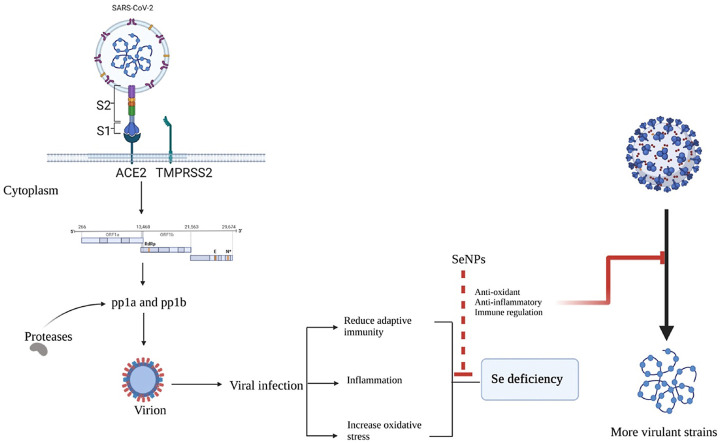
The Coronavirus disease-2019 (COVID-19) originated from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus and emerged as the greatest health emergency of the past century [1]. Currently, multiple emergency use vaccines are available for the prevention and effective management of COVID-19 [2]. Pathologically, the complications of COVID-19 are not restricted to the respiratory system alone. Numerous studies have been reported for its detrimental effects on the nervous, cardiovascular, and gastrointestinal systems [[3], [4], [5], [6]]. The spike proteins of COVID-19 interact with the ACE-2 (angiotensin-converting enzyme-2) receptor of the host which is followed by the release of the viral genome into the host cytoplasm. The single-stranded RNA genome of the virus has open reading frames (ORF) viz; ORF1a and ORF1b genes, which are translated to produce two polyproteins (PPs) namely pp1a and pp1b. These polyproteins are processed or cleaved by proteases resulting in the formation of 16 non-structural proteins, which perform various functions and hijack the host ribosomes for their own protein synthesis. This process is accompanied by assembly and budding into the endoplasmic reticulum and finally, the produced virions are released from the host via exocytosis [[7], [8], [9]]. Patients already affected with diabetes, hypertension, and other metabolic dysfunctions are at an increases risk of morbidity and mortality. Hence, the management of patients with multiple organ dysfunction requires an array of symptomatic medicine to provide relief and reducing the risk of life-threatening complications.
A large number of antiviral, antimicrobials, and anti-parasitic drugs were repurposed for the treatment of COVID-19 with unproven or limited efficacy [10,11]. In fact, drugs like chloroquine and hydroxychloroquine raised serious safety concerns, with most of the trials reporting inadequate therapeutic effects [12,13]. Though multiple vaccines have been authorized for emergency use, the global clinical community is still skeptical about their safety [14,15]. Thus, novel therapeutic options with promising antiviral, antimicrobial, and anti-inflammatory effects are the need of the hour. To this end, nanotechnology offers unique and versatile options to fight against this pandemic [[16], [17], [18]]. The nanotechnology-based tools can be used for effective prevention by using nanomaterial containing disinfectants, for better diagnosis of COVID-19, for superior therapeutic effects of the repurposed drugs and for improving the efficacy of vaccines [[19], [20], [21], [22], [23]].
Selenium (Se) is an essential trace element with diverse physiological effects. Keshan disease is a congestive cardiomyopathy disease that occurs due to Se deficiency in the diet in combination with a mutated strain of Coxsackie virus [24,25]. Moreover, Se deficiency has also been associated with the occurrence of infection or disease progression of other viral infections as well. Beck et al., have described that Se-deficient mice along with the null or low activity of protective glutathione peroxidase 1 (Gpx-1), results in the generation of virulent strains by causing mutations in RNA viruses [26]. This finding supports the explanation for myocarditis-associated mutations in the Coxsackie virus which results in Keshan-disease associated cardiomyopathy [24,25]. It has been shown that a large number of COVID-19 patients suffer from Se deficiency, and high mortality has been shown among such patients [[27], [28], [29], [30]]. Se deficiency is known to support replication, mutation, and emergence of RNA viruses. The risk of lung damage due to oxidative stress is partly counteracted by Se and selenoproteins in the lungs [31,32]. Selenoproteins in the form of selenocysteine maintain multiple physiological functions like redox machinery balance, immune-modulation, and cell signaling. It has been reported that in hospitalized patients of COVID-19 infection, Se deficiency is quite common [27,33]. Khatiwadi et al., revealed that an appropriate dose of Se may serve as supportive therapy in COVID-19 [31], and Zhang et al., showed that Se is linked with the curing rate of COVID-19 [34]. Se nanoparticles (SeNPs) possess potential anti-inflammatory and anti-fibrotic effects, modulate nuclear factor kappa-light-chain-stimulator of activated B cells (NFκB), nuclear factor erythroid 2 (NFE2)-associated factor 2 (Nrf2), redox imbalance, and mitogen-activated protein kinase (MAPKs) [35]. SeNPs have also been shown to possess transforming growth factor-beta (TGF-β) inhibitory activity, a property desirable for halting the progression of organ fibrosis. Further, it elicits remarkable antiviral and antimicrobial effects making them an attractive preclinical candidate for evaluation against COVID-19 [[36], [37], [38], [39], [40]]. These SeNPs can be employed as a holistic approach for the management of COVID-19 and related complications. It can reduce the load of the virus by virtue of its antiviral property, prevent secondary microbial infections by its promising antimicrobial effects, as well as reduce respiratory and other systemic complications by impeding the progression of systemic inflammatory complexities [31,41,42]. Further, SeNPs have been reported to boost the efficacy of vaccines as well. In addition, it has been observed that COVID-19 patients have a deficiency of Se, and supplementation of Se may improve the levels of physiological selenoproteins thus, may aid in recovery from the disease. The current review highlights the role of selenoproteins and SeNPs in the modulation of the immune response against viral and microbial infections. Further, we summarize the unique features and advantages of SeNPs over elemental Se and how SeNPs, may be tested effectively for the management of COVID-19.
2. Physiological role of Se in humans
Se is a vital trace element that is essential for the normal functioning of numerous metabolic pathways in humans and other organisms as well. The quantity of Se present in humans and other organisms is quite variable and typically depends on the geographical location and dietary intake. The recommended minimum dose of Se element is 55 μg/day [43] and is essential to maintain the normal course of physiological and biochemical processes in humans [44,45]. Serum levels of Se among different populations may vary, that depends upon a number of factors like diet, and the amount of dietary Se and also depends on the age of the person [46].
The total amount of Se in a healthy person is ∼3–20 mg. Table 1 enlists the recommended daily limit of Se as per age. The skeletal muscles constitute ∼46.9% of the total amount of Se in humans, while kidneys hold only 4% of total Se [47]. The amount of Se in humans has quite relied on age, the peak Se concentration is attained in adulthood while individuals above the age of 60 years showed a progressive decrease in serum Se levels [48]. Serum level of Se below 85 μg/L is considered to be a deficient state in humans and decreased levels of Se is linked with an increased risk of prostate cancer by 4–5 folds [49,50]. Daily doses in the range of Se with 100–200 μg results in reduced chances of genetic damages [51]. Numerous reports have been published that indicated Se intake may be a crucial factor and can aid in preventing cancer development and treatment as well [52,53]. A randomized double-blinded, placebo-controlled Phase 2 clinical trial was conducted on COVID-19 patients regarding the administration of Se in the form of selenious acid. The results of the clinical trial are not yet released (NCT04869579). The participants who were moderately-ill, severely-ill, or critically ill were given an infusion of selenious acid with a dose of 2000 μg on day 1 as a loading dose, and for the next 2–14 days a maintenance dose was given as infusion comprising of 1000 μg. Many similar clinical trials are being conducted on Se supplementation in COVID-19 patients with doses varying from study to study, in one study Se is given orally at a dose of 15 μg once a day and in another study it is given at a dose of 110 mg as dietary supplement. As there are no concrete evidences available yet, it is hard to conclude which dose, which form of Se and which route of administration is the best for Se supplementation in COVID-19 patients until the results of these trials are made publicly available [54]. Consumption of Se at 200 μg/day in the form of Na2SeO3 results in an increased natural killer (NK) and cytotoxic T cells formation, whereas consuming Se at a dose of 100 μg/day attenuated the symptoms of anxiety and depression [49]. Notably, Se at a dose of 800 μg/day did not reveal any negative result in adults, but a toxic effect of Se has been reported with a dose of 1540–1600 μg Se/day. The severity of symptoms varies from person to person depending upon various factors [55]. Hence, it is difficult to comment if Se species can be of clinical application for COVID-19 and detailed critical investigations are required to arrive at a conclusion.
Table 1.
Daily recommended intake of Se as per age groups.
3. Selenoproteins and their role in redox machinery
The essential role of Se in the biological system is also related to its association with enzymes and proteins. There are several enzymes present in the human body that are Se-dependent where the active central core carries Se in the form of selenocysteine and are called selenoproteins. Selenocysteine is one of the potent antioxidant agents that belongs to the free-radical scavengers class [59]. There are around 25 selenoprotein genes that are known till now, and perform various functions in cells such as in cell signaling, antioxidant, immune modulator, and redox homeostasis. The advances in exploring selenoproteins have contributed to our understanding of the role of Se and its applications related to human health [60]. GPx was the first enzyme that was identified as a selenoprotein which protects the cellular component such as cellular membrane, DNA and also protects the hemoglobin, red blood cells, and fatty acids by eliminating hydrogen peroxide from the body and protects against destructive impacts of oxidation [51,61]. It helps in the prevention of oxidative stress that causes various diseases of development and progression [62]. Downregulation of selenoprotein expression may lead to host susceptibility to oxidative stress (especially endoplasmic reticulum associated stress) and infections as well. The different classes of selenoproteins along with their function in different cells are enlisted in Table 2 .
Table 2.
Different classes of selenoproteins along with their function in different cells.
| S. No | Selenoprotein | Function | Tissue localization | Ref. |
|---|---|---|---|---|
| 1. | Glutathione peroxidase-1 (GPx-1) | Antioxidant, inhibits mutations in virus by decreasing the ability of virulence in retrovirus | Present in liver, kidneys, and lungs | [63] |
| 2. | Glutathione peroxidase-2 (GPx-2) | Antioxidant, prevents oxidative stress, maintains integrity of intestinal mucosa, anti-apoptotic activity in colon | Present mainly in liver and gastrointestinal tract | [64] |
| 3. | Glutathione peroxidase-3 (GPx-3) | Decreased lipid hydro peroxides, exhibited antioxidant activity, protects thyroid gland from hydrogen peroxide | Present in extracellular fluid, breast, plasma, liver, placenta heart, gastro-intestinal tract, male reproductive system and kidneys | [65,66] |
| 4. | Glutathione peroxidase-4 (GPx-4) | Antioxidant activity prevents brain from peroxidative damage, converts cholesterol and cholesterol esters into lower toxic analogs, crucial in sperm viability and motility, regulation of follicular helper T cells homeostasis | Present in testes | [[67], [68], [69]] |
| 5. | Glutathione peroxidase-5 (GPx-5) | Unknown | Olfactory lining and embryo | [70] |
| 6. | Glutathione peroxidase-6 (GPx-6) | Unknown | Present in humans only | [71] |
| 7. | Glutathione peroxidase-7 (GPx-7) | Reverses linkage among GPx-7 and cancer cells multiplication | Lumen | [71,72] |
| 8. | Glutathione peroxidase-8 (GPx-8) | Role in protein folding, antioxidant | Liver, kidney | [72] |
| 9. | Iodothyronine deiodinase-1 (DIO1) | Generation of active T3 hormones in thyroid as well as in peripheral tissues. Conversion of inactive thyroxin into active tri-iodothyronine | Mainly in thyroid, kidney, brown fat and liver | [73] |
| 10. | Iodothyronine deiodinase-2 (DIO2) | T3 generation in peripheral tissues, induction of thyroid hormones | Present in central nervous system, heart, brown adipose tissue, pituitary, and skeletal muscle | [74] |
| 11. | Iodothyronine deiodinase-3 (DIO3) | Inhibits presentation of foetus to T3cells, inactivity of thyroid hormones | Found in cerebral cortex, uterus, placenta, skin, fetal, and central nervous system (CNS) | [75] |
| 12. | Thioredoxin reductase 1 (TXNR1) | Antioxidant function, decrease thioredoxin, controls apoptosis, transcription factors, and cell proliferation | Liver, testes | [63] |
| 13. | Thioredoxin reductase 2 (TXNR2) | Cell growth factor in DNA synthesis and inhibition of apoptosis | Liver, kidneys | [63] |
| 14. | Thioredoxin reductase 3 (TXNR3) | Unknown | Present in testes | |
| 15. | Selenoprotein P (SELENOP) | Transport Se, maintain homeostasis, antioxidant activity, possesses ten selenocysteine moiety | Predominantly present in plasma, liver, testes, and brain | [76,77] |
| 16. | Selenoprotein S (SELENOS) | Role in inflammation, clears out mis-folded ER proteins, stimulates apoptosis, ER stress | Liver, kidney | [78] |
| 17. | Selenoprotein N (SELENON) | Redox signaling, calcium homeostasis and muscle development | Liver, kidney | [79] |
| 18. | Selenoprotein W (SELENOW) | Antioxidant activity in lungs, calcium attaching ability, regulates differentiation of osteoclast and inhibition of osteoporosis, regulation of T cells activity | Skeletal muscle, prostate, heart, colon, long bone, brain, liver and brain | [[80], [81], [82]] |
| 19. | Selenoprotein K (SELENOK) | Antioxidant activity, myogenesis, regulation of ER stress | Immune cells and spleen, skeletal muscle | [[83], [84], [85]] |
| 20. | Selenoprotein H (SELENOH) | Gene regulation of glutathione | Brain, and muscle cells | [86] |
| 21. | Selenoprotein R (SELENOR) | Antioxidant, protein repair, methionine metabolism | Kidney and liver cells | [87] |
| 22. | Selenoprotein M (SELENOM) | Antioxidant activity | Neuronal cells | [80] |
| 23. | 15Kd selenoprotein (SEP15) | Role in folding of glycoprotein | Kidney and liver cells | [88] |
| 24. | Selenophosphate synthase 2 (SPS-2) | Selenocysteine biosynthesis from selenophosphates | Kidney, liver | [89] |
3.1. Selenoproteins modulate immunity
Selenoproteins play a crucial role in boosting the immune system as many immune cells express various members of the selenoprotein family [90]. In immune cells, selenoproteins are known to perform the antioxidant function by maintaining redox signaling, oxidative burst, regulating immunity and inflammation, Ca2+ flux, protein folding, and other effector functions of immune cells [91,92]. They can stimulate the regulation of IL-2 receptors that can enhance the response of T and B lymphocytes towards IL-2 thereby modulating the immune cell functioning. It has been reported that selenoproteins play an important role in protecting the cells from oxidative damage and are expressed in a large number in immune cells and tissues [93]. Selenoproteins such as GPx4 exhibit an important role in T cell immunity by inhibiting ferroptotic cell death while GPx3 removes excessive H2O2, hence, maintaining redox homeostasis [92]. Additionally, the neutrophils enhanced the synthesis of the GPx4 in a ROS-dependent manner resulting in cell survival. Similarly, the selenoprotein namely TXNRD1 is one of the highly expressed selenoproteins in macrophages and is crucial in maintaining redox homeostasis in activated macrophages [94]. Two selenoproteins i.e., SELENOK and SELENOS demonstrated a protective role in immunity and inflammation. SELENOS regulates ER stress due to enhanced protein processing along with macrophage activation and also influences circulating inflammatory cytokines (IL-1β, IL-6, TNF-α) in Hashimoto's thyroiditis pathogenesis. Additionally, SELENOK is crucial in ER related protein degradation (ERAD) pathway and maintenance of Ca2+ flux in ER. Numerous immune cell activities are dependent on store operated Ca2+ entry (SOCE) and are affected in SELENOK deficient immune cells; which include migration, proliferation, protection against pathogens and cytokine secretion [90,95]. Moreover, Sep15 mRNA is present in immune cells in abundance and functions by promoting proper protein folding that enhances the efficiency of dietary Se to function properly. The selenoprotein MSRB1 has potential role in macrophage biology that aids in promoting cellular activation by regulating actin polymerization leading into functions like phagocytosis, cytokine secretion and activation of innate immunity [96]. Viral infection results in downregulation of the expression of selenoproteins which in turn leads to the decrease in concentration of Se and thus, making the host vulnerable to infections. Additionally, SARS-CoV-2 infection showed influence on the expression of selenoprotein at mRNA level in Vero cells. SARS-CoV-2 triggers the inflammatory cascade as evident by enhanced IL-6 expression. It reduced the mRNA expression of ferroptosis-associated DNA synthesis-related TXNRD3, GPX4, and endoplasmic reticulum-resident SELENOF, SELENOK, SELENOM and SELENOS. Furthermore, computational modeling predicted an antisense interface between TXNRD3 mRNA and SARS-CoV-2. Findings of this study shows the direct inhibitory property of SARS-CoV-2 replication on the expression of selenoprotein mRNAs, which merits further investigation for the association between dietary Se condition and the impact on SARS-CoV-2 infection [97]. As shown in Table 2, there are several means by which Se, through selenoprotein functions, may counteract COVID-19. Hence, to maintain appropriate Se concentration owing to its beneficial effects, SeNPs might be a useful approach [94].
3.2. Selenoproteins and viral infection
The antioxidant activity of selenoproteins contributes to increasing anti-viral immunity. Selenoproteins such as GPxs, TXNRDs, and ER selenoproteins are known to affect viral pathogenicity by minimizing the oxidative stress caused by the spread of the virus inside the host cell. Selenoproteins are reported to regulate the redox homeostasis in influenza H1N1, coxsackievirus B3, Hantavirus, HIV-1, Polio, influenza A/Bangkok/1/79 (H3N2), hepatitis B & C [98]. In case of infection with lymphocytic choriomeningitis virus (LCMV)-WE strain, the selenoprotein TXNRD1 is crucial for increasing the activated T-cell number in infection [99]. The infection of hepatitis C virus is associated with liver fibrosis and hepatocellular carcinoma (HCC), SELENOM showed upregulation in human HCC cell lines and liver biopsies of patients with HCV-related cirrhosis and are associated with maintaining liver oxidative stress. Selenoproteins can be encoded in the genome of viruses such as in Fowlpox & Molluscum contagiosum, probably preventing them from ROS generated by host phagocytes [99]. Multiple viral families can activate NF-κB by inducing viral proliferation and inhibiting virus-induced apoptosis [99]. As the concentration of 15d-PGJ2 increases, Se/selenoproteins can decline NF-κB activation, thereby minimizing viral replication [100]. Furthermore, in Porcine Circovirus 2 (PCV2), the addition of H2O2 can induce oxidative stress leading to increased viral replication. This effect was prevented by selenoproteins SELENOS and GPx1 [98].
There are reports of a correlation between COVID-19 and selenoproteins. The expression of various selenoproteins namely endoplasmic reticulum selenoproteins, TXNRD3, GPx4, SELENOK, SELENOF, SELENOS, SELENOM remarkably decreased in COVID-19 infected Vero E6 cells while increased expression of IL-6 was noticed [101]. The COVID-19 infection leads to down-regulation of the selenoprotein expression thereby, resulting in increased events of ER-associated stress and mis-folded proteins in ER. Additionally, there is a link between decreased expression of SELENOS and release of inflammatory cytokines [102]. This correlation may be linked to COVID-19 induced significant increase of IL-6 concentrations.
As discussed earlier, Se deficiency results in down-regulation of the selenoprotein expression which in turn leads to weakening of the immune system against infectious diseases. During viral infections, host metabolism can be affected in multiple ways, resulting in dysregulation of redox homeostasis [103]. The viral pathogens give rise to oxidative stress by increasing the ROS production and modification of cellular ROS scavenging systems. As part of the antioxidant defense, selenoproteins, play a crucial role in regulating oxidative stress [96]. However, Se deficiency leads to down-regulation of the expression of selenoproteins resulting in their inability to counteract oxidative stress [94,103,104]. Hence, to overcome the reduced expression of selenoproteins due to deficiency of Se, SeNPs can be an effective means to halt the progression of COVID-19 disease and can be a potent pre-clinical candidate for the management of COVID-19.
4. Selenium based molecules and their antiviral effects
4.1. Ebselen
Ebselen, [2-phenyl-1,2-benzisoselenazol-3(2H)-one] is an organoselenium-based compound that exhibits potential anti-inflammatory, antioxidant, anti-microbial, and cytoprotective properties against mammalian cells [105]. Ebselen showed excellent bactericidal activity against S. aureus (0.125–0.5 μg/mL), E. faecium (0.25–0.5 μg/mL), E. faecalis (0.25–0.5 μg/mL), Streptococcus agalactiae (0.5 μg/mL) and Streptococcus pyogenes (0.5 μg/mL), including multidrug resistance (MDR) clinical isolates of vancomycin and methicillin-resistant strains. On the other hand, ebselen showed modest antimicrobial activity against Gram-negative bacteria like E. coli (32 μg/mL), A. baumannii (16 μg/mL), K. pneumoniae (64 μg/mL), P. aeruginosa (>256 μg/mL) and S. typhimurium (32 μg/mL) [106]. Ebselen and its corresponding analogs were proved to be effectively active against Mtb (10 μg/mL), Bacillus cereus (0.86 μg/mL) and Bacillus subtilis (0.12 μg/mL) [107]. In addition, ebselen has displayed anti-biofilm activity and decreased the already-established staphylococcal biofilm [108].
Ebselen prevents the cellular damage caused by ROS by virtue of its glutathione peroxidase mimetic activity. In yeast, it interferes with ATPase activity and proton-translocation function whereas, in E. coli, it suppresses TRX competitively [105]. Ebselen also possesses potential activity against bacteria where thioredoxin and thioredoxin reductase are crucial for synthesizing DNA and are deficient in glutaredoxin and glutathione reductase [109]. In addition, ebselen demonstrates a remarkable obstacle in developing antibacterial resistance. Altogether, ebselen exhibits a wide range of antibacterial actions against Gram-positive and Gram-negative bacteria either singly or in synergy [108]. All these findings advocate that repurposing ebselen and utilizing it as a potent therapeutic option for the treatment of antibiotic-resistant bacteria.
Ebselen prevents LPS induced inflammatory airway and smoking-induced inflammation [110]. BXT-51072 and BXT-51077 are orally active, low molecular weight, organoselenium compounds that have GPx mimetic activity. They augment the rate of peroxide metabolism, restrain inflammation and inhibit oxidative damage by inhibiting the activation of inflammatory mediators [111].
Recently, the protective role of ebselen against SARS-CoV-2 has been described. COVID-19 positive patients were found to possess a reduced level of Se in their body which is correlated to disease associated morbidity and mortality. Notably, ebselen, an organic Se species, has shown to restrain COVID-19 by covalently attaching to COVID-19 virionMpro by binding to cell membranes. Ebselen is most efficient at a dose range of 10 μM in Vero cells infected with COVID-19 infected. Higher Se intake may have potential to increase recovery rate in COVID-19 infections [27,41]. Moreover, Moghaddam et al., reported that Se levels in the serum samples of recovered COVID-19 patients were elevated in comparison with non-survivors ([Se] 53.3 ± 16.2 & 40.8 ± 8.1 μg/L, [Selenoprotein] 3.3 ± 1.3 & 2.1 ± 0.9 mg/L) [112].
In SARS-CoV-2 infected cells in culture, there was reduced expression of several selenoproteins, including those that regulate ER stress, and an increase in the expression of IL-6. This suggests a possible connection between decreased selenoprotein expression and COVID-19-related inflammation. Experimental research has revealed that the selenium compound ebselen is a potent inhibitor of the major SARS-CoV-2 protease required for viral maturation within the host. This study raises the possibility that redox-active selenium species produced at high selenium consumption could block SARS-CoV-2 protease [94]. Presently, there is no FDA approval for the use of ebselen in SARS-CoV2-infection however, there are two phase II clinical trials (NCT04484025 and NCT04483973) with the current status as, “enrolling by invitation for the treatment of moderate and severe SARS-CoV-2 infections”, and no results were posted yet (clinicaltrials.gov).
It has been demonstrated that ebselen co-crystallized with antioxidant enzyme and established a selenylsulphide bond with Cys111 at the dimer-dimer interface, which would further contribute to the reduction of M pro's proteolytic activity [113]. Adjuvant treatment with ebselen improved the therapeutic outcome, it worked on immunity boosting, antioxidant profile in patients suffering from this deadly virus and reduced inflammation of lung in respiratory distress syndrome, and additionally vascular damage and venous thrombosis could be avoided by managing focal ischemic injury [114]. Further, ebselen and its structural analogs inhibited the activity of papain-like protease (PLpro) that plays an inhibitory role in virus replication. The inhibitors of PLpro showed potency in the range of nanomolar. Similarly, ebselen inhibited the virus main protease, Mpro that is involved in replication and gene expression [33,[115], [116], [117], [118]]. However, further detailed studies are warranted before it may be used as a rational approach clinically.
4.2. SeNPs versus elemental form of Se
Se has both inorganic and organic chemical derivatives, including selenite, selenomethyl selenocysteine, and selenomethionine. Numerous studies have been conducted to understand the anti-cancer effects. In general, the inorganic Se compounds exert more genotoxic stress, which may explain why the therapeutic window is smaller, systemic toxicity is higher, and the risk of metastatic burden is high. On the other hand, organic Se compounds have fewer side effects and less systemic effects while having strong anti-tumor activity and an improved capacity to prevent metastasis. Organic Se compounds represent an enormous class of chemically varied nucleophilic molecules. Numerous novel organic Se compounds have been created in an effort to increase efficacy, selectivity, and efficacy while reducing toxicity. Combining all of the aforesaid qualities, organo-selenium compounds are a potential agent for cancer therapies [119]. The Se (IV) species selenite is the most relevant example of an inorganic selenium compound considered a medicinal agent for the treatment of cancer. The study demonstrated considerable cytotoxicity against cancerous cells, including lung, prostate, cervical, ovarian, and colon cancer cells, in human acute myeloid and lymphoblastic leukemia cells and hepatoma and mesothelioma cells. This cytotoxicity was in the low-micromolar range. SeMet, methylselenic acid, the thioselenide selenodiglutathione, the major cellular metabolite of selenite, and selenocyanates are examples of organic compounds. Further, a variety of human cancer cell models found that organic selenium is more effective than inorganic selenium because they are a more potent inhibitor of cell development than selenite [120].
In various clinical trials the dose of selenium treatment for cancer, cardiovascular disorders, and diabetes given were 200 μg/day [[121], [122], [123]]. Further, clinical trials for an HIV virus-infected person given once a day 200 μg selenium tablet [124]. There are various formulations by which selenium is administered to the patient such as in a clinical trial, patients with oral lichen planus (OLP), were instructed to apply the formulation of selenium hydrogel (selenomethionine (SeMet) 14 μg/1g gel) and 200 μg of selenium twice daily, or 400 μg per day, in capsule form [125,126] the concept for topical selenium was inspired by the fact that chronic systemic selenium use has drawbacks, side effects, and a small window between dangerous doses of selenium (˃900 μg/day) and deficient doses (<30 μg/day) [127]. While for use in a variety of nanomedicine disciplines, researchers are developing nanoparticles with both metallic and non-metallic origins. Due to their remarkable biological activity and minimal toxicity, SeNPs have recently attracted a lot of attention as prospective cancer therapeutic payloads. When compared to inorganic and organic Se molecules, SeNPs have greater biocompatibility and bioefficacy. To create new Se-based therapies and theranostics, a plethora of SeNPs have been created in recent years. Non-functionalized SeNPs were produced using several environmental friendly chemical and biotechnological methods, and they demonstrated effectiveness against distinct types of cancer cells in a dose- and time-dependent manner [128,129].
It is well known that nanoparticles offer variable means to decrease toxicity, improve bioactivity, advance targeting of cells, and enhance bioavailability. The profound antioxidant and pro-oxidant activity or bioavailability and toxicity of Se are affected by its chemical forms [130]. As per the antioxidant activities and hematological findings, Bhattacharjee et al., proved that SeNPs have less or no adverse effect in comparison to free forms of organic and inorganic Se. Abnormal renal and hepatic function were highly prominent with selenite treatment than SeNPs as observed in histological findings and confirmed by increased renal and hepatotoxic markers in serum. SeNPs prevented DNA damage and caused lower bone marrow cell death than other forms of Se [131]. In another study, SeNPs showed equal efficacy as compared to Se-methylselenocysteine (SeMSC) which is a native available organic Se product. SeNPs showed similar efficiency in enhancing the enzymatic activities of thioredoxin reductase, glutathione S-transferase, and glutathione peroxidase but had much lower toxicity as observed by survival rate, acute liver injury, median lethal dose, and short-term toxicity [132,133]. Though the studies showing a correlation between the expression of selenoproteins and SeNPs are lacking, it is plausible that the pharmacological effects of SeNPs are mediated by them, with the added advantage of reduced toxicity compared to their elemental counterpart. SeNPs can be used as a promising adjuvant remedy in COVID-19 management with minimal toxicity concerns. SeNPs possess good biocompatibility thereby can be utilized as an antiviral drug. Hence, owing to the beneficial effects of SeNPs, it could be explored as a potential therapeutic agent against COVID-19. Though, there are very few anti-viral experiments using SeNPs against COVID-19. Therefore, there is a need for intense studies in this direction to confirm the role of Se in COVID-19 patients.
4.2.1. Deleterious effects of Se
High selenium exposure reduces the risk of breast cancer, lung cancer, esophageal cancer, gastric cancer, and prostate cancer [134]. However, depending on the element's chemical form, selenium can be toxic to humans [135]. However, on the other hand it has been reported that Se intake can also have the potential to increase the risk of development of cancer. A trial shows that supplementation of selenium at 200 μg/day as a selenized yeast promotes the risk of development of squamous-cell carcinoma and non-melanoma skin cancer. Further, another study also reported that exposure to selenate (7–9 μg/liter) in tap water from 1975 to 1985 increased the incidence of melanoma than in non-selenium-exposed people [[136], [137], [138]].
Moreover, high intake of Se leads to nausea, vomiting, diarrhea, and a garlic-like stench on the breath. In a condition of severe poisoning, cardiac and pulmonary symptoms may appear and may result in death with gun bluing solutions, which frequently contains selenous acid. Ingestion of other forms of Se between 1 and 100 mg Se/kg body weight can also lead to death. Blood levels of 300 μg Se/L and urine levels of 170 μg Se/L exceeding than normal level of 100 μg/L are related to mortality [139].
4.3. Preparation of SeNPs
SeNPs have attracted a lot of attention from researchers due to their unique physical, chemical and biological activity. It can be synthesized through physical methods such as hydrothermal techniques, UV radiation and laser ablation. Further, some chemical and biological methods of SeNPs preparations have also been reported. Chemical synthesis of SeNPs includes various reactions such as catalytic reduction, acid decomposition or precipitation reaction by using sodium dodecyl sulfate, sulfurdioxide, ascorbic acid, glucose etc. However, biosynthesis of SeNPs is a cheap and safe method, and it can be mediated through fungi, bacteria and plants [140]. Moreover, green synthesis of SeNPs has also been reported [141,142].
5. SeNPs as a rational approach against viral and bacterial infections: Implications for the management of COVID-19
5.1. SeNPs may suppress cytokine storm and lung fibrosis in COVID-19
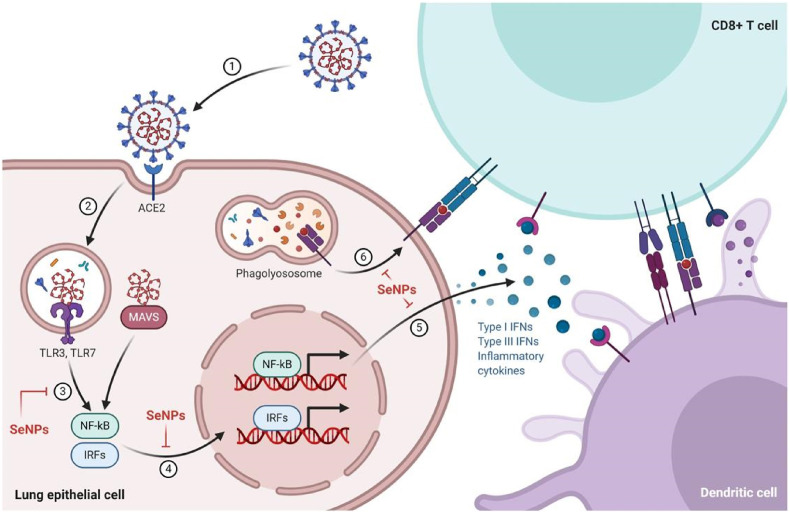
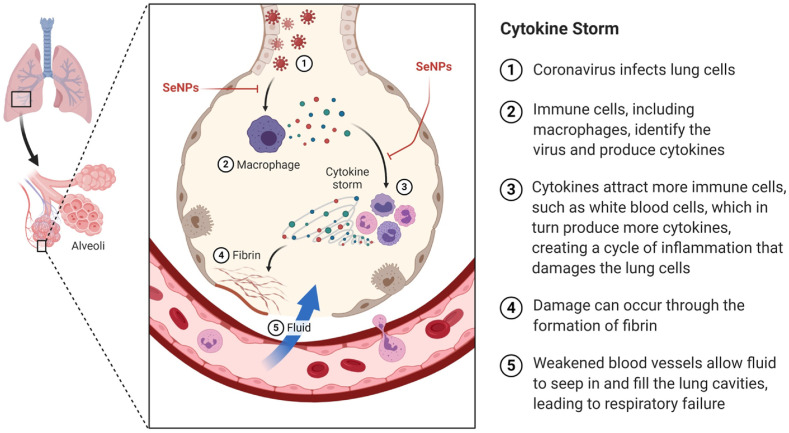
Severe inflammatory response and cytokine storm due to coronavirus infection makes the patient's condition very serious. The SARS-CoV-2 invades the host cells via the ACE2 receptor. The acute immune response against coronaviruses is mediated by the viral RNA-mediated stimulation of the cytoplasmic and endosomal sensors, mitochondrial antiviral-signaling protein (MAVS), and toll-like receptor 3/7 (TLR3/7), respectively. The ACE2 receptors are known to activate the interferon regulatory factors (IRFs) and NFκB which in turn provokes the synthesis and release of the inflammatory cytokines. The dendritic cells (DCs) are known for antigen presentation and then migrate to the lymphoid organs to prime adaptive immunity. After recognizing the antigen on DCs or infected cells surface, CD8+T cells induce apoptosis (Fig. 1 ). The uncontrolled immune response may lead to a massive cytokine storm which can threaten the life of COVID-19 patients (Fig. 2 ). So, therapeutic strategies which can abrogate cytokine storm progression would be of high benefit at this time [143].
Fig. 1.
Acute Immune Responses to Coronavirus: Coronaviruses are RNA viruses, which get entry into human lung epithelium via angiotensin-converting enzyme-2 (ACE2) receptor and infects them. Viral RNA stimulates the cytoplasmic and endosomal sensors, MAVS and TLR3/7, respectively. These ACE2 receptors activate the Interferon Regulatory Factors (IRFs) and NFκB to provoke inflammatory cytokine synthesis and release, including interferons (IFN). Dendritic cells sample antigens and migrate to lymphoid organs to prime adaptive immunity. After recognizing antigen on DCs or infected cells surface, CD8-T cells induce apoptosis. The figure shows various steps where SeNPs may elicit inhibitory activity. 1. Entry of coronavirus into the lung through ACE2 receptor. 2. Coronavirus starts replication inside the host cell. 3. Stimulation of the cytokine regulatory proteins (NFκB, IRFs). 4. Induced proteins provoke the synthesis of inflammatory cytokines. 5. Release of inflammatory cytokines. 6. Degradation of the receptor. 7. Induction of apoptosis. The Figure was createdwith BioRender.com.
Fig. 2.
Cytokine Storm: Cytokine storm, which is a pathological phenomenon which worsens the COVID-19 patient condition. When cytokines are released without breaks, they may cause damage to the cells which responds to the cytokines and halts the function of multiple organs. The Figure was created with BioRender.com.
SeNPs have been reported to curb the overall inflammatory responses by inhibiting major inflammatory signaling cascades mediated by master players like MAPKs, NFκB and reducing the TNF-α expression [35]. It was shown that Ulva lactuca decorated polysaccharide-based SeNPs formulation suppressed colitis induced by dextran sodium sulfate (DSS) by inhibiting pro-inflammatory cytokines including interleukin (IL)-6, TNF-α through the modulation of NFκB signaling [144]. In an interesting study, photo-dynamically active SeNPs having photosensitive and macrophage-targeting bilayers were shown to effectively tackle macrophage-associated inflammation, an important cell type implicated in acute and chronic inflammation. Further, melatonin-SeNPs conjugation based treatment with different doses improved the function of antioxidant enzymes like superoxide dismutase (SOD), GPX activity, decreased the serum nitrite, splenocyte proliferation, pro-inflammatory cytokines and liver pathological abnormalities [145]. Gangadevi et al., reported that SeNPs block a variety of inflammation and proliferation-related pathways, making them a promising choice for psoriasis treatment. The topical application of SeNPs induces apoptosis by producing reactive oxygen species (ROS) and causing cell cycle arrest by modulating the expressions of MAPKs, STAT3, GSK-3, Akt, PCNA, Ki67, and cyclin-D1 in psoriatic mice [146]. Wallenberg reviewed that Se induces cell death in addition to ROS formation. Se at high concentrations can potentially increase extensive ROS generation and cause oxidative stress [147]. Further, Ren et al., also investigated the anti-inflammatory activity of SeNPs dispersed in phytochemicals over Complete Freund's adjuvant-induced rheumatoid arthritis in rats. SeNPs dramatically reduced the thiobarbituric acid reactive chemicals, COX-2 activity, with the restoration of antioxidant enzyme activities, and the levels of several inflammatory cytokines [148]. Shahabi et al., showed significant beneficial role of SeNPs in bleomycin induced pulmonary fibrosis in a rat model. SeNPs reduced the degree of inflammation, alveolitis and lung architecture as well. SeNPs showed anti-fibrotic effects by down-regulation of TGF-β and TNF-α [149]. Further, Shalby et al., showed the anti-fibrotic property of SeNPs as well as free form of Se by virtue of its membrane stabilizing capacity, free radical scavenging activity, antioxidant potential and anti-inflammatory action [150]. Notably, SeNPs showed superior effects in comparison to elemental Se and the main cause could be attributed to the increased surface area, small particle size, and enhanced bioavailability. Thus, SeNPs could significantly reverse the virus mediated inflammatory cascade as well as potentially inhibit cytokine storm as shown in Fig. 1, Fig. 2.
5.2. Antiviral and antimicrobial effect of SeNPs
Due to their unique antimicrobial and antiviral properties, SeNPs have gained substantial attention in the scientific community. Se is a crucial trace element that is controlled by cellular redox homeostasis and acts as an internal constituent of selenoproteins maintaining fundamental biological activities, such as specific enzyme modulation and ROS elimination. Deficiency of Se in the host body can lead to increased susceptibility to viral infections. COVID-19 is a highly contagious viral disease which is probably more contagious than any other type of flu and has covered the global population rapidly [151]. So, developing a therapeutically viable and effective antiviral treatment is the utmost need of the hour. In this context, SeNPs possess attractive antiviral properties. It has been described that deficiency of Se element leads to increased virus pathogenicity. The deficiency of Se in virus-infected animals displays immune dysfunction, enhanced chemokines including altered cytokine expressions. Se-deficient mice infected with the Coxsackie virus resulted in the development of myocarditis and additional experiments revealed that the alterations in virulence were due to point mutations in the genome of the virus. Hence, in a Se-deficient condition, replication in the host causes a normally benign virus to attain virulence because of mutations in the virus. The scarcity of Se in HIV-infected patients is also linked with disease advancement and with liver cancers induced by the hepatitis C virus. It shows that a suitable amount of Se aids in protecting the host from viral infection [152]. Se deficiency either in serum or plasma ≤85 μg/L, has been related to reduced survival time in HIV-infected patients [153]. Though linkage among low serum or plasma Se exists, lesser CD4+ T cell count and increased viral titer can also be due to the reduced amount of blood Se via acute-phase reaction in individuals with more progressive infection of HIV-1 [154]. The beneficial role of Se supplementation in HIV-infected patients has been shown in two randomized control trials, in which HIV-positive patients supplemented with Se at the dose of 200 μg per day remarkably reduced the frequency of hospital admissions associated with the infection. One more test was conducted where HIV-infected adults having a higher level of Se concentration in serum showed reduction in viral load, HIV disease duration and hepatitis-C virus co-infection [155,156].
Zhong et al., performed an experiment in an anti-EV71 cell model and reported the increased oseltamivir's antiviral activity when it was loaded onto the surfaces of SeNPs to create SeNPs@OT (functionalized antiviral nanoparticles). SeNPs@OT successfully entered human astrocyte U251 cells (host cells) via clathrin-associated endocytosis, suppressing EV71 growth and potentially protecting EV71-infected U251 cells from apoptosis via the mitochondrial pathway. SeNPs@OT also reduced the generation of ROS, which inhibited EV71 activity in EV71-infected U251 cells [157]. Meanwhile, Rojekar et al., developed Etravirine nanostructured lipid carriers and modified them using nano-Se, and the result revealed that the potential for a dual-loaded formulation to target HIV-1 infection synergistically increased intracellular antioxidant balance to improve anti-HIV therapy duration [158]. Arbidol, which is a clinically used anti-viral agent but its use is limited due to drug-resistant viruses. To eliminate this drug resistance issue, Li et al., designed surface altered SeNPs having arbidol (Se@ARB) that exerted a great antiviral effect. SeNPs have an antiviral effect and their decoration with arbidol additively inhibited the H1N1 infection. By down-regulating the activity of hemagglutinin, Se@ARB impeded the association among the H1N1 influenza virus and the host cells. Se@ARB can avert the H1N1 influenza virus from infecting MDCK cells and stop DNA fragmentation and chromatin condensation. Se@ARB treatment resulted in cytoplasmic shrinkage, hampered cell-to-cell communication, and decreased cell numbers. Se@ARB subsequently declined the proliferation of the H1N1 influenza virus and improved the overall cell viability to 85% in comparison to H1N1 influenza virus-infected cells in which cell viability was just 34%. Additionally, Se@ARB repressed the generation of ROS. As evident from the hematoxylin and eosin staining of in vivo experimental probes, Se@ARB prevented the lung injury caused by H1N1 infected mice. TUNEL assay of lung tissue slices showed a high degree of DNA damage but Se@ARB treatment substantially abrogated this effect. Further, histological findings with immunohistochemistry revealed the activation of caspase-3, AKT, and MAPK signaling pathways which were restrained by pharmacological treatment with Se@ARB. Altogether, the findings of this study demonstrated that Se@ARB possesses potential antiviral activity which can be used to tackle the drug resistance observed while clinical therapy of H1N1 influenza virus [37]. Interestingly, SeNPs prevented the infection of Enterovirus A71 (EV-A71) as observed by thiazolyl blue tetrazolium bromide and cytopathic effect in Vero cells. SeNPs impeded the proliferation of Vero cells infected with the EV-A71 virus as evident by the decrease in nucleic acid levels of the virus. Moreover, SeNPs significantly diminished the expression of both caspase-8 and caspase-9 stimulated by the EV-A71 virus. Moreover, SeNPs inhibited the apoptosis of Vero cells triggered by the EV-A71 and reduced the phosphorylation of Jun amino-terminal kinase [159]. SeNPs potentially exhibited antiviral activity against the Dengue virus also. In a recent systematic review a negative correlation of Se deficiency in COVID-19 patients was shown. The systematic review included studies which enrolled COVID-19 patients and took blood samples to assess the Se levels and consistently found Se deficiency in almost all COVID-19 patients indicating that estimating the serum Se is reliable approach for diagnosing Se level in COVID-19 patients. Further, Se levels in the serum of COVID-19 patients were significantly lower than healthy controls that resulted in poor outcome during the treatment. Interestingly, in some patients the Se levels were very high in the urine samples and were associated with severe and fatal cases in contrast to the less severe and already recovered patients [160]. The efficacy of SeNPs is due to the ability to modulate the levels of selenoproteins, the active Se entities of the body. However, it is too early to conclude the clinical application of Se species for COVID-19 management and further detailed investigations are warranted.
Apart from antiviral effects, the antibacterial property of SeNPs have also been described in numerous studies [[161], [162], [163], [164], [165], [166]]. SeNPs were proved to have excellent antimicrobial activity at different doses against different bacterial strains. SeNPs were observed to inhibit replication of Staphylococcus aureus [[167], [168], [169]], Pseudomonas aeruginosa [170,171], and E. coli [172,173], respectively up to 99%. Likewise, SeNPs at a dose of 500 μg/mL were able to hamper the growth of Aspergillus clavatus which is a pathogenic fungus. The antimicrobial potential of SeNPs was found to be equivalent with commercially present Ampicillin antibiotic [174,175]. Moreover, SeNPs were generated by using E. faecalis can be employed as an anti-staphylococcal agent to significantly prevent as well as to treat S. aureus infections [176]. Yang et al., explored the in vitro antimicrobial effect of Qe/CdSe/ZnS (quercetin/cadmium selenide/zinc sulfide) nanoparticles (QCZNPs) against drug-resistant B. subtilis and E. coli. QCZNPs showed distinctly good antimicrobial efficiency than Qe or CdSe NPs [177]. Wang et al., demonstrated the potential of Se coatings on polycarbonate medicinal tools where Se coating remarkably inhibited S. aureus growth to 27% after 72 h in comparison with an uncoated polycarbonate surface [178]. The antimicrobial properties of SeNPs can be effectively harnessed by using disinfectants supplemented with these unique NPs which can be used for effective prevention of this highly contagious viral infection [179,180]. Altogether, these finding demonstrates that SeNPs could be considered as a novel, promising therapeutic strategy against the COVID-19 pandemic and other infections [41,181,182].
5.3. The efficacy of SeNPs to scavenge detrimental reactive oxygen species
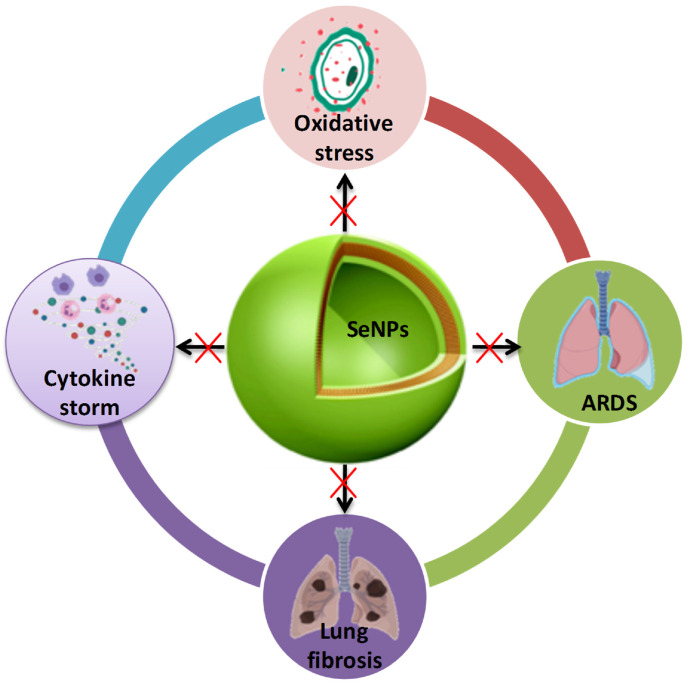
Oxidative stress has a profound negative impact on the pathogenesis of COVID-19 that adds up to the disease-related co-morbidities. Due to disturbed redox status, normal homeostasis between antioxidants and pro-oxidants is imbalanced which triggers cellular damage and has been related to augmenting COVID-19 related systemic complications. Macromolecular damage and restrained thiol redox trail cause imbalanced redox activity resulting in aberrant cellular signaling which makes the disease more aggressive [183]. Because of its potent antioxidant capability, SeNPs restore the imbalanced oxidative stress and, thus, can potentially reverse or halt the oxidative stress-mediated changes in different diseases [179,180]. SeNPs have been reported to restore lipid peroxidation, improve physiological glutathione levels which makes it a strong candidate to be tested for efficacy against COVID-19 management [35,41,181,182]. In a novel study, glutathione (GSH)-anchored SeNPs (G@SeNPs) with different enantiomers were used to explore its antioxidant property in palmitic acid-induced oxidative stress in insulinoma cells (INS-1E). Cytotoxicity assay revealed a remarkable safety profile of the SeNPs even at high concentrations. After treatments with G@SeNPs, ROS levels were significantly reduced in palmitic acid-induced stress indicating a potent antioxidant profile of SeNPs [184].
Further, the hepatoprotective activity of SeNPs against acetaminophen (APAP)-induced hepatic injury has been reported. Oral administration of APAP overdose impelled the remarkable increase in liver function biomarkers and increased hepatic lipid peroxidation, with reduced hepatic SOD, catalase, glutathione (GSH) content, and glutathione reductase (GR) activity and significantly stimulated the DNA impairment in hepatocytes, in comparison to control rats. SeNPs intervention upgrades the hepatic antioxidant defense process and reduced the cellular sensitivity towards DNA impairment, improved the liver function and oxidative stress by modulating the activity of SOD, catalase, and GSH, and declines in hepatic DNA fragmentation indicating the potent anti-oxidant profile of SeNPs [185]. Fig. 3 shows various proposed pharmacological effects of SeNPs of potential interest for the management of COVID-19. No doubt there remains a risk of adverse effects upon modulation of any biological process in the body. In COVID-19 patients, excess generation of ROS/RNS have been reported widely and reducing their levels proved as a beneficial outcome in infected patients. Generation of ROS leads to cellular damage and death which has close association with inflammation, neurological disorders, cancer, and other maladies. Small-molecule antioxidants containing sulfur in their chemical skeletal and selenium can reduce oxidative damage. The antioxidant properties of sulfur compounds are commonly evaluated with regards to Se antioxidant properties; nevertheless, sulfur and Se antioxidant actions can be relatively distinct, with each utilizing diverse antioxidant mechanism pathways to prevent oxidative cellular damage. Sulfur and Se based compounds, adapt ROS scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms to balance redox homeostasis processes. But reducing the ROS/RNS below the normal levels causes imbalance between these processes leading to altered cellular functions [186]. Hence, further detailed investigations on Se species are warranted for any future clinical application.
Fig. 3.
Potential (probable) benefits of SeNPs against COVID-19.
5.4. SeNPs as an immunomodulator
Selenoproteins play a crucial role and are required for activated T-cell functioning. T cells are highly susceptible to oxidative stress, and deficiency of selenoproteins in T cells leads to attenuated proliferation response to T-cell-receptor stimulation. Patients having heterozygous defects in the selenocysteine (Sec) insertion sequence binding protein 2 (SBP2) have been found to have a reduced ability to synthesize most of the selenoproteins. These individuals show reduced lymphocyte count and attenuated T cells proliferation after polyclonal stimulation, emphasizing the significance of selenoproteins in the orchestration of an efficacious immune response [187].
Interactions among IL-2 and its receptor regulate T-cell proliferation [188]. Clinical studies reported the association among lymphocyte proliferation and Se supplementation, led by improved expression of the high-affinity IL-2 receptor [189]. Mice fed with high Se supplementation demonstrated enlarged expression of both IL-2 and the high-affinity IL-2 receptor chain along with increased T-cell signaling and CD4+ T-cell functions. It was observed that high Se supplementation changed the Th1–Th2 balance towards Th1, resulting in enhanced expression of CD40 ligand and interferon-γ. Such a transition effect would be highly beneficial against antiviral immune or anti-tumor responses which rely on powerful Th1 immunity [115,188,190,191].
Patients having immuno-compromised status are more prone to COVID-19-related complications. Patients suffering from other diseases are more vulnerable to this infection, especially the older people which have compromised immunity [192]. Thus, boosting the immune system by a pharmacotherapeutic might be of immense value. Earlier, SeNPs were proved to elicit a strong immune response in breast cancer. After 20 days of tumor induction, serum samples were collected to estimate the cytokine levels. During the complete study weight of mice and tumor growth along with delayed-type hyper (DTH) sensitivity response was observed. Treatment with SeNPs significantly increased the levels of serum IFN-γ, IL-2, IL-12 and reduced the TGF-β and tumor volume. Further, additional intense DTH responses and longer survival rate was observed in comparison to control and tumor lysate vaccine [193]. In a similar study, a 200 μg/day dose of SeNPs shrunk the tumor and extended the survival time [194]. Interestingly, the fact that deficiency of Se is one of the features of a large group of COVID-19 patients and increases the risk of fatality. Hence, the use of SeNPs for supplementation may be an effective option for boosting immunity and reducing the risk of mortality [41,[179], [180], [181], [182]]. Further, SeNPs can be appraised as an adjuvant in vaccines to effectively boost the immune response. Se has been reported to hamper cancer cell growth by counteracting the harmful effect of aflatoxins, thus, reducing the teratogenic potential of this toxin [195,196]. Potentially, the anti-carcinogenic potential of Se leads to the initiation of various changes such as metabolizing carcinogens, altering the interaction among carcinogens and DNA, improvement of glutathione concentration and the process of detoxification, reducing the metabolic reactions in tumor cells, alterations in cell membrane permeability, and boosting the immune system [197,198].
Despite concrete results from in vitro and in vivo studies that Se plays an imperative role in immunity, however, its indication in human beings is limited [188,199]. Se supplementation has prominent immunostimulant effects in Se-enriched individuals as it provides an improved proliferation rate of activated T cells, enhanced natural killer cell activity, and cytotoxic lymphocyte-mediated tumor cytotoxicity activity. The immune response is generally weak in old age people and during cancer therapy [115,153,200]. Se supplementation in old age volunteers with 400 μg/day remarkably improved the total T-cell count by 27% in comparison to the placebo, chiefly due to boosting subsets of CD4+ T cells and enhanced NK cells cytotoxicity. Se supplementation of 100 μg for 6 months significantly augmented the proliferative response to antigen challenge [200]. Similarly, Se supplementation (200 μg) in patients having squamous-cell carcinoma of the neck and head during radiation or surgery resulted in a significant enhancement in cell-mediated immune sensitivity in both cases during and after the treatment period. However, a decreased immune response was observed in the patients who were given a placebo [191]. A study in which subjects having fairly low Se status were supplemented with 2 different doses of Se (50 μg and 100 μg per day) and were challenged with an oral, live, attenuated poliovirus and were found to clear the virus more swiftly than placebo control subjects [115].
Luo et al., reported that the Se-HEP loaded PLGA NPs and Se modified HEP-PLGA NPs dramatically increased macrophage phagocytic activity, as well as CD40 and CD86 expression. Furthermore, peritoneal macrophages were stimulated with Se-HEP-PLGA and HEP-PLGA-Se NPs, which enhanced NO, TNF-α, IL-1, and IL-6 levels. The effects of Se-HEP-PLGA on the expression of co-stimulatory molecules, NO secretions, and cytokines were the best of all. These findings suggested that Se-HEP-PLGA could boost macrophage activation and could be used as a HEP delivery strategy to produce powerful immune responses [201]. Additionally, Se synergies with vitamin E exhibits a strong anti-oxidant effect. In metabolic pathways, sulfur-based amino acids such as methionine and cysteine are also linked. Se, combined with vitamin E, which is also a potent anti-oxidant, guards the organs against the devastating effects of ROS. At the cellular level, they protect from the mitochondrial dysfunction, maintain mitochondrial membrane potential, and prevent the microsomal membranes from fatty acids oxidation. Combined management of Se with vitamin E results in an effective immunostimulatory effect [202]. However, there is no conclusive evidence that Se species may be of potential clinical relevance, and detailed investigations are warranted.
6. Possibility of SeNPs for the preparation of novel drug delivery systems for COVID-19 therapy
The path of drug delivery and dose formulation of a drug is the main deciding factor for disease treatment. Hence, it is essential to formulate an appropriate dose formulation to attain improved therapeutic results, and in this regard nanotechnology offers a plethora of platforms for exploitation [130,180,203]. Primarily, COVID-19 attacks the lungs, making them inefficient in functioning; thus, locally targeted drug delivery to the lungs may be an effective therapeutic approach [204]. SeNPs can be formulated as a theranostic agent with simultaneous tracking using fluorescent dyes and therapeutic benefits at the same time. In addition, SeNPs provide a versatile platform for devising effective diagnostic tools for the screening kits as the polymerase chain reaction (PCR) based diagnosis is extremely costly and time taking [181,182]. To achieve this objective, aerosol-based mouth spray formulation is a potential strategy that is easily manageable and imparts rapid relief for a particular period of time. Aerosols can directly target the lungs and decrease the extremity of infections caused by COVID-19, resulting in improved patient health outcomes. It is advised to repeatedly consume the dose by inhalational spray; it may not be compliant in all sets of patients. In such situation, sustained release SeNPs formulation can be injected directly i.v. that will have 100% bioavailability and increased clinical response. Furthermore, SeNPs can be delivered into a thin film and can be formulated as transdermal patches that may deliver the SeNPs for a long duration. Further, adding other anti-inflammatory drugs along with SeNPs would act as dual synergistic approach for the management of COVID-19 therapy [41,179,205,206]. Fig. 4 shows the schematic representation of the proposed routes of administration.
Fig. 4.
Diagramatic representation of route of administration of SeNPs to combat COVID-19.
7. Conclusions
Owing to their ability to curb inflammation by modulating NFκB, Nrf2, TGF-β, and MAPKs, SeNPs can halt the progression of lung injury and fibrosis. In addition, it may suppress systemic complications as well. Further, the immunomodulatory activity may aid in improving viral defense by supplementation. As it possesses attractive antiviral and antimicrobial effects, SeNPs can inhibit the progression of diseases as well as reduce the threat of secondary infections. We have highlighted the importance of SeNPs and selenoproteins and their potential for further exploration in the context of emerging microbial and viral infections, including COVID-19.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the team of Department of Veterinary Pharmacology and Toxicology, College of Veterinary Science, Hyderabad.
Data availability
No data was used for the research described in the article.
References
- 1.Sanders J.M., et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 2.Salvi R., Patankar P. Biomedicine & Pharmacotherapy; 2020. Emerging Pharmacotherapies for COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long B., et al. The American journal of emergency medicine; 2020. Cardiovascular Complications in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger J.R. COVID-19 and the nervous system. J. Neurovirol. 2020:1. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koralnik I.J., Tyler K.L. COVID‐19: a global threat to the nervous system. Ann. Neurol.88. 2020;1:1–11. doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaafarani H.M., et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann. Surg. 2020;272(2):e61–e62. doi: 10.1097/SLA.0000000000004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allawadhi P., et al. Decorin as a possible strategy for the amelioration of COVID-19. Med. Hypotheses. 2021;152 doi: 10.1016/j.mehy.2021.110612. 110612-110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh V., et al. Critical neurological features of COVID-19: role of imaging methods and biosensors for effective diagnosis. Sensors International. 2021;2 doi: 10.1016/j.sintl.2021.100098. 100098-100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prajapat M., et al. Drug targets for corona virus: a systematic review. Indian J. Pharmacol. 2020;52(1):56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clancy C.J., Nguyen M.H. Clinical Infectious Diseases; 2020. COVID-19, Superinfections and Antimicrobial Development: what Can We Expect? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White N.J., et al. COVID-19 prevention and treatment: a critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Broek M., et al. Netherlands Heart Journal; 2020. Chloroquine-induced QTc Prolongation in COVID-19 Patients; p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11(7):1–6. doi: 10.1038/s41419-020-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burki T.K. The Lancet Respiratory Medicine; 2020. The Russian Vaccine for COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorp H.H. American Association for the Advancement of Science; 2020. A Dangerous Rush for Vaccines. [DOI] [PubMed] [Google Scholar]
- 16.Ikram M., et al. Photocatalytic and bactericidal properties and molecular docking analysis of TiO2 nanoparticles conjugated with Zr for environmental remediation. RSC Adv. 2020;10(50):30007–30024. doi: 10.1039/d0ra05862a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikram M., et al. Promising performance of chemically exfoliated Zr-doped MoS2 nanosheets for catalytic and antibacterial applications. RSC Adv. 2020;10(35):20559–20571. doi: 10.1039/d0ra02458a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allawadhi P., et al. Silver nanoparticle based multifunctional approach for combating COVID-19. Sensors International. 2021;2 doi: 10.1016/j.sintl.2021.100101. 100101-100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khurana A., et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38 doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khurana I., et al. Can bilirubin nanomedicine become a hope for the management of COVID-19? Med. Hypotheses. 2021;149 doi: 10.1016/j.mehy.2021.110534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalkal A., et al. Sensing and 3D printing technologies in personalized healthcare for the management of health crises including the COVID-19 outbreak. Sensors International. 2022;3 doi: 10.1016/j.sintl.2022.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalkal A., et al. Allium sativum derived carbon dots as a potential theranostic agent to combat the COVID-19 crisis. Sensors International. 2021;2 doi: 10.1016/j.sintl.2021.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allawadhi P., et al. Biomedical applications of polysaccharide nanoparticles for chronic inflammatory disorders: focus on rheumatoid arthritis, diabetes and organ fibrosis. Carbohydrate Polym. 2022;281 doi: 10.1016/j.carbpol.2021.118923. [DOI] [PubMed] [Google Scholar]
- 24.Beck M.A., Levander O.A., Handy J. Selenium deficiency and viral infection. J. Nutr. 2003;133(5 Suppl 1) doi: 10.1093/jn/133.5.1463S. 1463s-7s. [DOI] [PubMed] [Google Scholar]
- 25.Lei C., et al. Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin. Chim. Acta. 2009;399(1–2):102–108. doi: 10.1016/j.cca.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Beck M.A., Handy J., Levander O.A. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12(9):417–423. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghaddam A., et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander J., et al. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bermano G., et al. Selenium and viral infection: are there lessons for COVID-19? Br. J. Nutr. 2020:1–37. doi: 10.1017/S0007114520003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatiwada S., Subedi A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19) Current Nutrition Reports. 2021;10(2):125–136. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harthill M. Review: micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011;143(3):1325–1336. doi: 10.1007/s12011-011-8977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Z., et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., et al. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020;111(6):1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khurana A., et al. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 36.Li Y., et al. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 2017;12:5733. doi: 10.2147/IJN.S140939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., et al. Inhibition of H1N1 influenza virus-induced apoptosis by selenium nanoparticles functionalized with arbidol through ROS-mediated signaling pathways. J. Mater. Chem. B. 2019;7(27):4252–4262. [Google Scholar]
- 38.Lin Z., et al. Inhibition of H1N1 influenza virus by selenium nanoparticles loaded with zanamivir through p38 and JNK signaling pathways. RSC Adv. 2017;7(56):35290–35296. [Google Scholar]
- 39.Huang X., et al. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016;30:397–407. doi: 10.1016/j.actbio.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 40.Huang T., et al. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale. 2019;11(31):14937–14951. doi: 10.1039/c9nr04424h. [DOI] [PubMed] [Google Scholar]
- 41.He L., et al. Using nano-selenium to combat coronavirus disease 2019 (COVID-19)? Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medhi R., et al. Nanoparticle-based strategies to combat COVID-19. ACS Appl. Nano Mater. 2020;3(9):8557–8580. doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- 43.Galan-Chilet I., et al. Plasma selenium levels and oxidative stress biomarkers: a gene-environment interaction population-based study. Free Radic. Biol. Med. 2014;74:229–236. doi: 10.1016/j.freeradbiomed.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Krohn R.M., et al. High-selenium lentil diet protects against arsenic-induced atherosclerosis in a mouse model. J. Nutr. Biochem. 2016;27:9–15. doi: 10.1016/j.jnutbio.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Pophaly S.D., et al. Selenium enrichment of lactic acid bacteria and bifidobacteria: a functional food perspective. Trends Food Sci. Technol. 2014;39(2):135–145. [Google Scholar]
- 46.Post M., et al. Serum selenium levels are associated with age-related cataract. Ann. Agric. Environ. Med. 2018;25(3):443–448. doi: 10.26444/aaem/90886. [DOI] [PubMed] [Google Scholar]
- 47.Lyons M.P., Papazyan T.T., Surai P.F. Selenium in food chain and animal nutrition: lessons from nature -review. Asian-Australas. J. Anim. Sci. 2007;20(7):1135–1155. [Google Scholar]
- 48.Tamari Y., Kim E.S. Longitudinal study of the dietary selenium intake of exclusively breast-fed infants during early lactation in Korea and Japan. J. Trace Elem. Med. Biol. 1999;13(3):129–133. doi: 10.1016/S0946-672X(99)80002-9. [DOI] [PubMed] [Google Scholar]
- 49.Zwolak I., Zaporowska H. Selenium interactions and toxicity: a review. Selenium interactions and toxicity. Cell Biol. Toxicol. 2012;28(1):31–46. doi: 10.1007/s10565-011-9203-9. [DOI] [PubMed] [Google Scholar]
- 50.Hsueh Y.M., et al. Levels of plasma selenium and urinary total arsenic interact to affect the risk for prostate cancer. Food Chem. Toxicol. 2017;107(Pt A):167–175. doi: 10.1016/j.fct.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 51.Kieliszek M., Błażejak S. Selenium: significance, and outlook for supplementation. Nutrition. 2013;29(5):713–718. doi: 10.1016/j.nut.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Murdolo G., et al. Selenium and cancer stem cells. Adv. Cancer Res. 2017;136:235–257. doi: 10.1016/bs.acr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Kieliszek M., Błażejak S., Kurek E. Binding and conversion of selenium in Candida utilis ATCC 9950 yeasts in bioreactor culture. Molecules. 2017;22(3) doi: 10.3390/molecules22030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balboni E., et al. Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies. J. Trace Elem. Med. Biol. 2022;71 doi: 10.1016/j.jtemb.2022.126956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoffaneller R., Morse N.L. A review of dietary selenium intake and selenium status in europe and the Middle East. Nutrients. 2015;7(3):1494–1537. doi: 10.3390/nu7031494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kipp A.P., et al. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015;32:195–199. doi: 10.1016/j.jtemb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Monsen E.R. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet Assoc. 2000;100(6):637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 58.Kieliszek M. Selenium⁻Fascinating microelement, properties and sources in food. Molecules. 2019;24(7) doi: 10.3390/molecules24071298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kieliszek M., et al. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019;46(2):1797–1808. doi: 10.1007/s11033-019-04630-z. [DOI] [PubMed] [Google Scholar]
- 60.Hariharan S., Dharmaraj S. Selenium and selenoproteins: it's role in regulation of inflammation. Inflammopharmacology. 2020;28(3):667–695. doi: 10.1007/s10787-020-00690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kieliszek M., et al. Equilibrium modeling of selenium binding from aqueous solutions by Candida utilis ATCC 9950 yeasts. 3 Biotech. 2018;8(9):388. doi: 10.1007/s13205-018-1415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Additives E.Panel o., et al. Assessment of the application for renewal of authorisation of selenomethionine produced by Saccharomyces cerevisiae CNCM I-3060 (selenised yeast inactivated) for all animal species. EFSA J. 2018;16(7) doi: 10.2903/j.efsa.2018.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reeves M., Hoffmann P. The human selenoproteome: recent insights into functions and regulation. Cell. Mol. Life Sci. 2009;66(15):2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Florian S., et al. Loss of GPx2 increases apoptosis, mitosis, and GPx1 expression in the intestine of mice. Free Radic. Biol. Med. 2010;49(11):1694–1702. doi: 10.1016/j.freeradbiomed.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yant L.J., et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 2003;34(4):496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 66.Schmutzler C.M., Schomburg B., Hoang-Vu L., Herzog C., V. Hohrle J. Selenoproteins of the thyroid gland: expression, localization and possible function of glutathione peroxidase 3. Biol. Chem. 2007;388(10):1053–1059. doi: 10.1515/BC.2007.122. [DOI] [PubMed] [Google Scholar]
- 67.Maiorino M., et al. Distinct promoters determine alternative transcription of gpx-4 into phospholipid-hydroperoxide glutathione peroxidase variants. J. Biol. Chem. 2003;278(36):34286–34290. doi: 10.1074/jbc.M305327200. [DOI] [PubMed] [Google Scholar]
- 68.Papp L.V., Holmgren A., Khanna K.K. Selenium and selenoproteins in health and disease. Antioxidants Redox Signal. 2010;12(7):793–795. doi: 10.1089/ars.2009.2973. [DOI] [PubMed] [Google Scholar]
- 69.Yao Y., et al. Selenium–GPX4 axis protects follicular helper T cells from ferroptosis. Nat. Immunol. 2021;22(9):1127–1139. doi: 10.1038/s41590-021-00996-0. [DOI] [PubMed] [Google Scholar]
- 70.Ashton K., et al. Methods of assessment of selenium status in humans: a systematic review. Am. J. Clin. Nutr. 2009;89(6):2025S–2039S. doi: 10.3945/ajcn.2009.27230F. [DOI] [PubMed] [Google Scholar]
- 71.Brigelius-Flohé R., et al. The yin and yang of nrf2-regulated selenoproteins in carcinogenesis. International journal of cell biology. 2012;2012 doi: 10.1155/2012/486147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Espada S., et al. The purinergic P2Y13 receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic. Biol. Med. 2010;49(3):416–426. doi: 10.1016/j.freeradbiomed.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 73.Tinggi U. Selenium: its role as antioxidant in human health. Environ. Health Prev. Med. 2008;13(2):102–108. doi: 10.1007/s12199-007-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu J., Holmgren A. Selenoproteins. Journal of Biological Chemistry. 2009;284(2):723–727. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- 75.Rasmussen L.B., et al. Selenium status, thyroid volume, and multiple nodule formation in an area with mild iodine deficiency. Eur. J. Endocrinol. 2011;164(4):585–590. doi: 10.1530/EJE-10-1026. [DOI] [PubMed] [Google Scholar]
- 76.Mostert V., et al. Modulation of selenoprotein P expression by TGF‐β1 is mediated by Smad proteins. Biofactors. 2001;14(1‐4):135–142. doi: 10.1002/biof.5520140118. [DOI] [PubMed] [Google Scholar]
- 77.Burk R.F., Hill K.E. Selenoprotein P—expression, functions, and roles in mammals. Biochim. Biophys. Acta Gen. Subj. 2009;1790(11):1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer H.A., et al. Selenoprotein P status correlates to cancer-specific mortality in renal cancer patients. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arbogast S., Ferreiro A. Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxidants Redox Signal. 2010;12(7):893–904. doi: 10.1089/ars.2009.2890. [DOI] [PubMed] [Google Scholar]
- 80.Yao H.-D., et al. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim. Biophys. Acta Gen. Subj. 2013;1830(4):3112–3120. doi: 10.1016/j.bbagen.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Kim H., et al. Selenoprotein W ensures physiological bone remodeling by preventing hyperactivity of osteoclasts. Nat. Commun. 2021;12(1):2258. doi: 10.1038/s41467-021-22565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang L.J., et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn's disease. Immunity. 2021;54(8):1728–1744.e7. doi: 10.1016/j.immuni.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y., et al. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J. Nutr. 2012;142(8):1410–1416. doi: 10.3945/jn.112.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang S., et al. Vol. 50. 2022. (Selenoprotein K Protects Skeletal Muscle from Damage and Is Required for Satellite Cells-Mediated Myogenic Differentiation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang S., et al. Selenoprotein K protects skeletal muscle from damage and is required for satellite cells-mediated myogenic differentiation. Redox Biol. 2022;50 doi: 10.1016/j.redox.2022.102255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehta S.L., et al. Overexpression of human selenoprotein H in neuronal cells enhances mitochondrial biogenesis and function through activation of protein kinase A, protein kinase B, and cyclic adenosine monophosphate response element-binding protein pathway. Int. J. Biochem. Cell Biol. 2013;45(3):604–611. doi: 10.1016/j.biocel.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu Y.J., Diamond A.M. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63(12):3347–3351. [PubMed] [Google Scholar]
- 88.Karthik L., et al. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hart D.J., et al. Selenium concentration and speciation in biofortified flour and bread: retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem. 2011;126(4):1771–1778. doi: 10.1016/j.foodchem.2010.12.079. [DOI] [PubMed] [Google Scholar]
- 90.Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9):1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 2014;94(3):739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang Z., Rose A.H., Hoffmann P.R. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal. 2012;16(7):705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiremidjian-Schumacher L., Roy M. Selenium and immune function. Zeitschrift fur Ernahrungswissenschaft. 1998;37:50–56. [PubMed] [Google Scholar]
- 94.Zhang J., et al. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moghadaszadeh B., Beggs A.H. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology. 2006;21:307–315. doi: 10.1152/physiol.00021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9) doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y., et al. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food Chem. Toxicol. 2021;153 doi: 10.1016/j.fct.2021.112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guillin O.M., Vindry C., Ohlmann T. Selenium, Selenoproteins and Viral Infection. 2019;11(9) doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muri J., et al. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. 2018;9(1):1851. doi: 10.1038/s41467-018-04274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qian F., Misra S., Prabhu K.S. Selenium and selenoproteins in prostanoid metabolism and immunity. Crit. Rev. Biochem. Mol. Biol. 2019;54(6):484–516. doi: 10.1080/10409238.2020.1717430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y., et al. bioRxiv; 2020. SARS-CoV-2 Suppresses mRNA Expression of Selenoproteins Associated with Ferroptosis, Endoplasmic Reticulum Stress and DNA Synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Curran J.E., et al. Genetic variation in selenoprotein S influences inflammatory response. Nat. Genet. 2005;37(11):1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- 103.Guillin O.M., et al. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rayman M.P. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 105.Chan G., et al. Evaluation of the antimicrobial activity of ebselen: role of the yeast plasma membrane H+-ATPase. J. Biochem. Mol. Toxicol. 2007;21(5):252–264. doi: 10.1002/jbt.20189. [DOI] [PubMed] [Google Scholar]
- 106.Thangamani S., Younis W., Seleem M.N. Repurposing clinical molecule ebselen to combat drug resistant pathogens. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gustafsson T.N., et al. Ebselen and analogs as inhibitors of Bacillus anthracis thioredoxin reductase and bactericidal antibacterials targeting Bacillus species, Staphylococcus aureus and Mycobacterium tuberculosis. Biochimica et Biophysica Acta (BBA) - General Subjects. 2016;1860(6):1265–1271. doi: 10.1016/j.bbagen.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 108.Thangamani S., Younis W., Seleem M.N. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci. Rep. 2015;5(1) doi: 10.1038/srep11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu J., et al. Inhibition of bacterial thioredoxin reductase: an antibiotic mechanism targeting bacteria lacking glutathione. Faseb. J. 2013;27(4):1394–1403. doi: 10.1096/fj.12-223305. [DOI] [PubMed] [Google Scholar]
- 110.Duong C., et al. Glutathione peroxidase-1 protects against cigarette smoke-induced lung inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299(3):L425–L433. doi: 10.1152/ajplung.00038.2010. [DOI] [PubMed] [Google Scholar]
- 111.Moutet M., et al. Glutathione peroxidase mimics prevent TNFα- and neutrophil-induced endothelial alterations. Free Radical Biol. Med. 1998;25:270–281. doi: 10.1016/s0891-5849(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 112.Moghaddam A., et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7) doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amporndanai K., et al. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat. Commun. 2021;12(1):3061. doi: 10.1038/s41467-021-23313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haritha C.V., Sharun K., Jose B., Ebselen A new candidate therapeutic against SARS-CoV-2. Int. J. Surg. 2020;84:53–56. doi: 10.1016/j.ijsu.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Broome C.S., et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004;80(1):154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- 116.Menéndez C.A., et al. Molecular characterization of ebselen binding activity to SARS-CoV-2 main protease. Sci. Adv. 2020;6(37):eabd0345. doi: 10.1126/sciadv.abd0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sies H., Parnham M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020;156:107–112. doi: 10.1016/j.freeradbiomed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weglarz-Tomczak E., et al. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci. Rep. 2021;11(1):3640. doi: 10.1038/s41598-021-83229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gandin V., et al. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018;127:80–97. doi: 10.1016/j.freeradbiomed.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 120.Fernandes A.P., Gandin V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta Gen. Subj. 2015;1850(8):1642–1660. doi: 10.1016/j.bbagen.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 121.Kohler L.N., et al. Selenium and type 2 diabetes: systematic review. Nutrients. 2018;10(12):1924. doi: 10.3390/nu10121924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alehagen U., Alexander J., Aaseth J. Supplementation with selenium and coenzyme Q10 reduces cardiovascular mortality in elderly with low selenium status. A secondary analysis of a randomised clinical trial. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jacobs E.T., et al. Selenium supplementation and insulin resistance in a randomized, clinical trial. BMJ Open Diabetes Research & Care. 2019;7(1) doi: 10.1136/bmjdrc-2018-000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kamwesiga J., et al. Effect of selenium supplementation on CD4+ T-cell recovery, viral suppression and morbidity of HIV-infected patients in Rwanda: a randomized controlled trial. AIDS. 2015;29(9):1045–1052. doi: 10.1097/QAD.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qataya P.O., et al. Selenium: a sole treatment for erosive oral lichen planus (Randomized controlled clinical trial) Oral Dis. 2020;26(4):789–804. doi: 10.1111/odi.13285. [DOI] [PubMed] [Google Scholar]
- 126.agha-hosseini F., et al. Efficacy of IMOD in the treatment of oral lichen planus—efficacy of IMOD in oral lichen planus. Open J. Stomatol. 2011;1 [Google Scholar]
- 127.Ashton K., et al. Methods of assessment of selenium status in humans: a systematic review. Am. J. Clin. Nutr. 2009;89(6):2025s–2039s. doi: 10.3945/ajcn.2009.27230F. [DOI] [PubMed] [Google Scholar]
- 128.Wang D., et al. Encapsulated nanoepigallocatechin-3-gallate and elemental selenium nanoparticles as paradigms for nanochemoprevention. Int. J. Nanomed. 2012;7:1711–1721. doi: 10.2147/IJN.S29341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luo H., et al. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf. B Biointerfaces. 2012;94:304–308. doi: 10.1016/j.colsurfb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 130.Patra J.K., et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bhattacharjee A., Basu A., Bhattacharya S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus. 2019;62(3):259–268. [Google Scholar]
- 132.Zhang J., Wang X., Xu T. Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2007;101(1):22–31. doi: 10.1093/toxsci/kfm221. [DOI] [PubMed] [Google Scholar]
- 133.Wang H., Zhang J., Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007;42(10):1524–1533. doi: 10.1016/j.freeradbiomed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 134.Cai X., et al. Selenium exposure and cancer risk: an updated meta-analysis and meta-regression. Sci. Rep. 2016;6 doi: 10.1038/srep19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nuttall K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006;36(4):409–420. [PubMed] [Google Scholar]
- 136.Rataan A.O., et al. Potential role of selenium in the treatment of cancer and viral infections. Int. J. Mol. Sci. 2022;23(4) doi: 10.3390/ijms23042215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Duffield-Lillico A.J., et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J. Natl. Cancer Inst. 2003;95(19):1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 138.Vinceti M., et al. Excess melanoma incidence in a cohort exposed to high levels of environmental selenium. Cancer Epidemiol. Biomarkers Prev. 1998;7(10):853–856. [PubMed] [Google Scholar]
- 139.Hadrup N., Ravn-Haren G. Acute human toxicity and mortality after selenium ingestion: a review. J. Trace Elem. Med. Biol. 2020;58 doi: 10.1016/j.jtemb.2019.126435. [DOI] [PubMed] [Google Scholar]
- 140.Wadhwani S.A., et al. Biogenic selenium nanoparticles: current status and future prospects. Appl. Microbiol. Biotechnol. 2016;100(6):2555–2566. doi: 10.1007/s00253-016-7300-7. [DOI] [PubMed] [Google Scholar]
- 141.He H., et al. Green synthesis of ultrasmall selenium nanoparticles (SeNPs) using Hericium erinaceus polysaccharide (HEP) as nanozymes for efficient intracellular antioxidation. Mater. Lett. 2022;317 [Google Scholar]
- 142.Ren L., et al. Preparation and growth-promoting effect of selenium nanoparticles capped by polysaccharide-protein complexes on tilapia. J. Sci. Food Agric. 2021;101(2):476–485. doi: 10.1002/jsfa.10656. [DOI] [PubMed] [Google Scholar]
- 143.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu C., et al. One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: crossflow filtration operation and membrane fouling analysis. Chemical Engineering and Processing - Process Intensification. 2017;120:20–26. [Google Scholar]
- 145.Wang H., et al. Melatonin-selenium nanoparticles protects liver against immunological injury induced by bacillus Calmette-Guérin and lipopolysaccharide. Acta Pharmacol. Sin. 2005;26(6):745–752. doi: 10.1111/j.1745-7254.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 146.Gangadevi V., et al. Selenium nanoparticles produce a beneficial effect in psoriasis by reducing epidermal hyperproliferation and inflammation. J. Nanobiotechnol. 2021;19(1):101. doi: 10.1186/s12951-021-00842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wallenberg M., et al. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell Mol. Med. 2014;18(4):671–684. doi: 10.1111/jcmm.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ren S.X., et al. Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019;25:991–1000. doi: 10.12659/MSM.912545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shahabi R., et al. Protective and anti-inflammatory effect of selenium nano-particles against bleomycin-induced pulmonary injury in male rats. Drug Chem. Toxicol. 2019:1–9. doi: 10.1080/01480545.2018.1560466. [DOI] [PubMed] [Google Scholar]
- 150.Shalby A.B., et al. Antifibrotic candidates of Selenium nanoparticles and selenium in the experimental model. J. Appl. Pharmaceut. Sci. 2017;7(9):191–198. [Google Scholar]
- 151.Allawadhi P., et al. Potential of electric stimulation for the management of COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110259. 110259-110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beck M.A. In: Selenium: its Molecular Biology and Role in Human Health. Hatfield D.L., editor. Springer US; Boston, MA: 2001. Selenium as an antiviral agent; pp. 235–245. [Google Scholar]
- 153.Rayman M.P. The importance of selenium to human health. Lancet. 2000;356(9225):233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 154.Drain P.K., et al. Low serum albumin and the acute phase response predict low serum selenium in HIV-1 infected women. BMC Infect. Dis. 2006;6:85. doi: 10.1186/1471-2334-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Burbano X., et al. Impact of a selenium chemoprevention clinical trial on hospital admissions of HIV-infected participants. HIV Clin. Trials. 2002;3(6):483–491. doi: 10.1310/A7LC-7C9V-EWKF-2Y0H. [DOI] [PubMed] [Google Scholar]
- 156.Hurwitz B.E., et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch. Intern. Med. 2007;167(2):148–154. doi: 10.1001/archinte.167.2.148. [DOI] [PubMed] [Google Scholar]
- 157.Zhong J., et al. Functionalized selenium nanoparticles enhance the anti-EV71 activity of oseltamivir in human astrocytoma cell model. Artif. Cells, Nanomed. Biotechnol. 2019;47(1):3485–3491. doi: 10.1080/21691401.2019.1640716. [DOI] [PubMed] [Google Scholar]
- 158.Rojekar S., et al. Dual loaded nanostructured lipid carrier of nano-selenium and Etravirine as a potential anti-HIV therapy. Int. J. Pharm. 2021;607 doi: 10.1016/j.ijpharm.2021.120986. [DOI] [PubMed] [Google Scholar]
- 159.Li Y., et al. Inhibition of Enterovirus A71 by selenium nanoparticles interferes with JNK signaling pathways. ACS Omega. 2019;4(4):6720–6725. [Google Scholar]
- 160.Fakhrolmobasheri M., et al. COVID-19 and selenium deficiency: a systematic review. Biol. Trace Elem. Res. 2022;200(9):3945–3956. doi: 10.1007/s12011-021-02997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lin W., et al. The advancing of selenium nanoparticles against infectious diseases. Front. Pharmacol. 2021;12(1971) doi: 10.3389/fphar.2021.682284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hariharan H., et al. Microbial synthesis of selenium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett. 2012;9(12):509–515. [Google Scholar]
- 163.Wang Q., Larese-Casanova P., Webster T.J. Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int. J. Nanomed. 2015;10:2885. doi: 10.2147/IJN.S78466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu W., et al. Selenium nanoparticles incorporated into titania nanotubes inhibit bacterial growth and macrophage proliferation. Nanoscale. 2016;8(34):15783–15794. doi: 10.1039/c6nr04461a. [DOI] [PubMed] [Google Scholar]
- 165.Medina Cruz D., Mi G., Webster T.J. Synthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus aureus, methicillin‐resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa. J. Biomed. Mater. Res. 2018;106(5):1400–1412. doi: 10.1002/jbm.a.36347. [DOI] [PubMed] [Google Scholar]
- 166.Yazhiniprabha M., Vaseeharan B. In vitro and in vivo toxicity assessment of selenium nanoparticles with significant larvicidal and bacteriostatic properties. Mater. Sci. Eng. C. 2019;103 doi: 10.1016/j.msec.2019.109763. [DOI] [PubMed] [Google Scholar]
- 167.Tran P.A., Webster T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011;6:1553. doi: 10.2147/IJN.S21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cihalova K., et al. Staphylococcus aureus and MRSA growth and biofilm formation after treatment with antibiotics and SeNPs. Int. J. Mol. Sci. 2015;16(10):24656–24672. doi: 10.3390/ijms161024656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sonkusre P., Cameotra S.S. Biogenic selenium nanoparticles inhibit Staphylococcus aureus adherence on different surfaces. Colloids Surf. B Biointerfaces. 2015;136:1051–1057. doi: 10.1016/j.colsurfb.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 170.Liu J., et al. Synthesis and investigations of ciprofloxacin loaded engineered selenium lipid nanocarriers for effective drug delivery system for preventing lung infections of interstitial lung disease. J. Photochem. Photobiol. B Biol. 2019;197 doi: 10.1016/j.jphotobiol.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 171.Srivastava N., Mukhopadhyay M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioproc. Biosyst. Eng. 2015;38(9):1723–1730. doi: 10.1007/s00449-015-1413-8. [DOI] [PubMed] [Google Scholar]
- 172.Guisbiers G., et al. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016;11:3731. doi: 10.2147/IJN.S106289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tran P.A., et al. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology. 2015;27(4) doi: 10.1088/0957-4484/27/4/045101. [DOI] [PubMed] [Google Scholar]
- 174.Sowndarya P., Ramkumar G., Shivakumar M.S. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cell Nanomed. Biotechnol. 2017;45(8):1490–1495. doi: 10.1080/21691401.2016.1252383. [DOI] [PubMed] [Google Scholar]
- 175.Srivastava N., Mukhopadhyay M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioproc. Biosyst. Eng. 2015;38(9):1723–1730. doi: 10.1007/s00449-015-1413-8. [DOI] [PubMed] [Google Scholar]
- 176.Shoeibi S., Mashreghi M. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J. Trace Elem. Med. Biol. 2017;39:135–139. doi: 10.1016/j.jtemb.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 177.Yang X., et al. Quercetin loading CdSe/ZnS nanoparticles as efficient antibacterial and anticancer materials. J. Inorg. Biochem. 2017;167:36–48. doi: 10.1016/j.jinorgbio.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 178.Wang Q., Webster T.J. Nanostructured selenium for preventing biofilm formation on polycarbonate medical devices. J. Biomed. Mater. Res. 2012;100(12):3205–3210. doi: 10.1002/jbm.a.34262. [DOI] [PubMed] [Google Scholar]
- 179.Khatiwada S., Subedi A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19) Current Nutrition Reports. 2021:1–12. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tyagi P.K., et al. Contribution of nanotechnology in the fight against COVID-19. Biointerface Research in Applied Chemistry. 2020;11(1):8233–8241. [Google Scholar]
- 181.Zhang J., et al. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020 doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu Q., et al. Selenium (Se) plays a key role in the biological effects of some viruses: implications for COVID-19. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Laforge M., et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang Y., et al. Chirality-driven transportation and oxidation prevention by chiral selenium nanoparticles. Angew. Chem. Int. Ed. 2020;59(11):4406–4414. doi: 10.1002/anie.201910615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Amin K., et al. Antioxidant and hepatoprotective efficiency of selenium nanoparticles against acetaminophen-induced hepatic damage. Biol. Trace Elem. Res. 2017:175. doi: 10.1007/s12011-016-0748-6. [DOI] [PubMed] [Google Scholar]
- 186.Battin E.E., Brumaghim J.L. Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009;55(1):1–23. doi: 10.1007/s12013-009-9054-7. [DOI] [PubMed] [Google Scholar]
- 187.Schoenmakers E., et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J. Clin. Invest. 2010;120(12):4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hoffmann F.W., et al. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J. Nutr. 2010;140(6):1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Roy M., et al. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol. Trace Elem. Res. 1994;41(1–2):103–114. doi: 10.1007/BF02917221. [DOI] [PubMed] [Google Scholar]
- 190.Hoffmann P.R. Mechanisms by which selenium influences immune responses. Arch. Immunol. Ther. Exp. 2007;55(5):289–297. doi: 10.1007/s00005-007-0036-4. [DOI] [PubMed] [Google Scholar]
- 191.Kiremidjian-Schumacher L., et al. Selenium and immunocompetence in patients with head and neck cancer. Biol. Trace Elem. Res. 2000;73(2):97–111. doi: 10.1385/BTER:73:2:97. [DOI] [PubMed] [Google Scholar]
- 192.Jayawardena R., et al. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metabol. Syndr.: Clin. Res. Rev. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yazdi M.H., et al. Adjuvant effect of biogenic selenium nanoparticles improves the immune responses and survival of mice receiving 4T1 cell antigens as vaccine in breast cancer murine model. J. Nanosci. Nanotechnol. 2015;15(12):10165–10172. doi: 10.1166/jnn.2015.11692. [DOI] [PubMed] [Google Scholar]
- 194.Sakr T.M., Korany M., Katti K.V. Selenium nanomaterials in biomedicine—an overview of new opportunities in nanomedicine of selenium. J. Drug Deliv. Sci. Technol. 2018;46:223–233. [Google Scholar]
- 195.Cai Z., Zhang J., Li H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019;31(8):1035–1047. doi: 10.1007/s40520-018-1086-7. [DOI] [PubMed] [Google Scholar]
- 196.Kieliszek M., Lipinski B. Pathophysiological significance of protein hydrophobic interactions: an emerging hypothesis. Med. Hypotheses. 2018;110:15–22. doi: 10.1016/j.mehy.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 197.Hatfield D.L., et al. In: Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Collins J.F., editor. Academic Press; Boston: 2017. Chapter 38 - selenium and cancer; pp. 463–473. [Google Scholar]
- 198.Woo J., Lim W. Anticancer effect of selenium. emj. 2017;40(1):17–21. [Google Scholar]
- 199.Carlson B.A., et al. Role of selenium-containing proteins in T-cell and macrophage function. Proc. Nutr. Soc. 2010;69(3):300–310. doi: 10.1017/S002966511000176X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008;52(11):1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Luo Y., et al. Designing selenium polysaccharides-based nanoparticles to improve immune activity of Hericium erinaceus. Int. J. Biol. Macromol. 2020;143:393–400. doi: 10.1016/j.ijbiomac.2019.12.061. [DOI] [PubMed] [Google Scholar]
- 202.Sodhi S., Sharma A., Brar R.S. A protective effect of vitamin E and selenium in ameliorating the immunotoxicity of malathion in chicks. Vet. Res. Commun. 2006;30(8):935–942. doi: 10.1007/s11259-006-2503-5. [DOI] [PubMed] [Google Scholar]
- 203.Benet L.Z. Effect of route of administration and distribution on drug action. J. Pharmacokinet. Biopharm. 1978;6(6):559–585. doi: 10.1007/BF01062110. [DOI] [PubMed] [Google Scholar]
- 204.De Virgiliis F., Di Giovanni S. Lung innervation in the eye of a cytokine storm: neuroimmune interactions and COVID-19. Nat. Rev. Neurol. 16. 2020;11:645–652. doi: 10.1038/s41582-020-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Allawadhi P., et al. Silver nanoparticle based multifunctional approach for combating COVID-19. Sensors International. 2021;2 doi: 10.1016/j.sintl.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Allawadhi P., et al. Nanoceria as a possible agent for the management of COVID-19. Nano Today. 2020;35 doi: 10.1016/j.nantod.2020.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.