Abstract

Mandarins are mostly preferred specie of Citrus genus, and there has been a continuous rise in consumption and global marketing due to having easy-to-peel, attractive flavor, and fresh consumption advantages. However, most of the existing knowledge on quality traits of citrus fruit comes from research conducted on oranges, which are the main products for the citrus juice manufacturing industry. In recent years, mandarin production in Turkey surpassed orange production and took the first place in citrus production. Mandarins are mostly grown in the Mediterranean and Aegean Regions of Turkey. Due to suitable climatic conditions, they are also grown in the microclimatic condition in Rize province located in the Eastern Black Sea region. In this study, we reported the total phenolic content, total antioxidant capacity, and volatiles of 12 Satsuma mandarin genotypes selected from Rize province of Turkey. Considerable differences in the total phenolic content, total antioxidant capacity (2,2-diphenyl-1-picrylhydrazyl assay), and fruit volatile constituent were found among the 12 selected Satsuma mandarin genotypes. The total phenolic content ranged from 3.50 to 22.53 mg of gallic acid equivalent per 100 g of the fruit sample in the selected mandarin genotypes. The total antioxidant capacity was the highest in genotype HA2 as 60.40%, and followed by IB (59.15%) and TEK3 (58.36%), respectively. A total of 30 aroma volatiles were detected from the juice samples of 12 mandarin genotypes by GC/MS, which comprised six alcohols, three aldehydes (including one monoterpene), three esters, one ketone, and one other volatiles. The main volatile compounds were identified in fruits of all Satsuma mandarin genotypes as α-terpineol (0.6–1.88%), linalool (1.1–3.21%), γ-terpinene (4.41–5.5%), β-myrcene (0.9–1.6%), dl-limonene (79.71–85.12%), α-farnesene (1.1–2.44), and d-germacrene (0.66–1.37%). Limonene accounts for most of the aroma compounds (79.71–85.12%) in fruits of all Satsuma genotypes. The genotypes MP and TEK8 had the highest total phenolic content, and HA2, IB, and TEK 3 had the highest antioxidant capacity. The YU2 genotype was found to contain more aroma compounds than the other genotypes. The genotypes selected on the basis of their high bioactive content could be used to develop new Satsuma mandarin cultivars with high human health promoting contents.
Introduction
Citrus is one of the most important and preferred fruit groups grown in the different parts of the world, and the volume of production is constantly increasing. The genus including bright, colorful, fragrant, refreshing, and juicy diverse fruits of orange, pomelo, mandarin, lemon, lime, citron, grapefruit, kumquat, and hybrids are not only delicious for their balanced tart and sweet taste, they are also an essential part of everyday nutrition and have numerous health benefits. Citrus fruits are rich in multiple nutrients such as vitamin C, flavonoids, vitamin A, amino acids, carotenoids, organic acids, fiber, etc., that are needed to form and maintain health.1−3
While world production of citrus in the 1960s was 25,065,867 tons, today it is increased up to 158,488,799 tons. The largest citrus producer in the world is China, followed by Brazil, India, Mexico, and the United States. According to 2021 data, citrus production in Turkey is 5,401,415 tons. With this production value, Turkey ranks second among the Mediterranean countries and seventh in the World.4
In Turkey, citrus can be grown mostly in subtropical regions such as Mediterranean and Aegean region and some suitable ecological conditions such as Black sea regions specifically some microclimatic area (Rize province) that allow economic cultivation of citrus fruits.1
Although scientific research studies on citrus fruit quality traits have focused on mainly oranges due to high economic value, in consumer behavior changes occurred recently and increased demand for mandarins have directly caused further research concentrate on fruit quality traits of mandarins.5−11
Significant developments have been experienced in mandarin production in Turkey in recent years, and it has come to an important position especially in exports. Easy peeling feature, fresh consumption, unique aroma, frost resistance, earliness, seedlessness, etc. characteristics encouraged consumption and production and exceeded the production of oranges. Mandarins shared 36.46% of total citrus production of Turkey and followed by orange (30.67%), lemon (27.33%), and grapefruit (5.47%), respectively. 48.4% of mandarin production is Satsuma (Citrus unshi), 45.28% is other species, 5.87% is Clementine, and 0.45 percent is King.12
The origin and diversity center of Satsuma mandarin (Citrus unshi) is Southeast Asia (China) and introduced to Japan more than 700 years ago, where it is now the major citrus species grown. The specie is introduced to Turkey from Japan in the early 1900s. It entered the Eastern Black Sea Region (Rize province) by way of Batumi and then spread to Mediterranean and Aegean regions of Turkey. During long cultivation period, mutations occurred on Satsuma mandarin, and different types with diverse fruit and tree characteristics are evident.13 Characteristics such as yield, fruit quality, cold resistance, and resistance to several abiotic and biotic factors in citrus have been improved by finding and evaluating random seedlings or mutations.14,15
Mandarins are a good source of nutraceutical compounds and for this reason has a good source of antioxidant capacity. Taste, aroma, color, and texture are the main quality characteristics of mandarin fruits. The aroma constituents of mandarin fruits are a mixture of various chemical classes specifically monoterpenes, sesquiterpenes, alcohols, aldehydes, acids, esters, ketones, and other volatiles.16−24 More recently, increasing global interest in production and consumption of mandarin fruits leads to the intensified need for profiling and determining their bioactive components and aroma profiling.25,26 Mandarins are produced in different continental climates but in general widespread in moderate Mediterranean-like climates, hot and humid tropical regions, and nearly desert-like dry climates. Among mandarins, Satsumas are more resistant to cold and frost and may withstand frost for several weeks.8,26
The main aroma attributes of mandarins are fruity, floral, citrusy, green/grassy, oily, metallic/gummy, herbaceous, and mushroom notes. Similar to oranges, there is not just one particular characteristic compound that imparts the typical mandarin flavor but rather the flavor results from a combination of several different volatiles. Volatile compounds dominate the olfactory perception of mandarin, even demonstrated as key factors that characterize mandarin flavor during sensory evaluation. Thus, extensive research on the aroma compounds of mandarin fruits has been carried out to completely understand aroma profile differences of cultivars.16,18,24,27 Satsuma mandarin has an important place in the markets in Turkey due to its earliness, high flavor, and seedless feature. It is an important specie of Citrus in export. Although Satsuma mandarin is mainly grown in subtropical conditions in the Mediterranean region of Turkey, it is also grown in microclimate areas in the colder Aegean and Eastern Black Sea regions, and it is stated that fruit quality and aroma properties are higher in microclimate areas. Consumers especially prefer Satsuma mandarins grown in these microclimatic areas.
There are not enough studies on Satsuma mandarin in Türkiye. In addition, only a small number of genotypes were used in the studies. To the best of our knowledge, the volatile aroma components of Satsuma mandarin juice are poorly studied in Türkiye.28,29 Therefore, the aim of this study is to compare 12 selected Satsuma mandarin genotypes with their bioactive and aroma profile characteristics.
Materials and Methods
Plant Material
In this study, 12 Satsuma mandarin cultivar candidates (genotypes) obtained as a result of clone selection in Rize province in the East Black Sea region among a wide number of Satsuma mandarin genotypes were used as an experimental material. The traits that make it more attractive to consumers for Satsuma mandarins are its higher yield capacity, better sugar/acid ratio, better aroma, higher juice content, lack of seeds, and a larger fruit size. The fruit morphological characteristics of these genotypes were reported before.13 The genotypes found together in a single collection parcel established with 4 × 4 m intervals and grafted on Poncirus trifoliate rootstock. Cultural management procedures were uniformly applied to these trees. Fruit samples were harvested in 2021 from these clones. The fruits were immediately brought to the laboratory under cold chain conditions after harvesting. 30 fruit samples (10 fruits per replicate) were used.
Biochemical Analysis
The 30 fruits for each genotype were randomly divided into three replicates including 10 fruits. Mandarin fruits were manually peeled and squeezed using a simple kitchen press. The obtained fruit juice samples were stored at −20 °C until analysis.
Total Phenolic Content
Total phenols were determined based on the Folin-Ciocalteu reagent procedure of Spanos and Wrolstad30 with slight modifications. Briefly, 50 μL of a juice sample, 100 μL of diluted Folin-Ciocalteu (Sigma), and 1.5 mL of double-distilled water were each added to 2 mL tubes. 50 μL of saturated sodium carbonate (20%) was then added to the mixture and shaken. The samples were kept in a dark room for 2 h. For the control and blank groups, 50 μL of double-distilled water and 50 μL of 80% methanol were used. 250 μL of each sample was analyzed for solution absorbance at 765 nm (Thermo Multi Scan Go, Thermo Scientific, Waltham, MA, USA). Gallic acid was determined using the standard curve prepared at the same time. Quantification was expressed as mg gallic acid equivalents (GAE) per 100 mL of the juice sample.
Total Antioxidant Capacity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging potential of each juice sample was determined according to the method of Cuvelier et al.31 with some modifications. First, 50 μL of the juice sample was added to a 2 mL centrifuge tube (2 mL Eppendorf tube), and then, 1950 μL of 0.06 mM DPPH was added under a dark room condition. The mixture was shaken and left in a dark room at 24 °C for 60 min. 250 μL of the mixture was transferred from the 2 mL centrifuge tube to a vial. 50 μL of double-distilled water and 50 μL (μL) of methanol were used in place of the juice samples for each of the control and blank groups, respectively. The absorbance values of the juice samples were measured at 515 nm in a spectrophotometer (Thermo Multi Scan Go, Thermo Scientific, Waltham, MA, USA), and the results were calculated using the following formula
Analysis of Volatiles
The volatile compounds of the juice samples were analyzed using a HS-SPME/GC/MS techniques. 1 mL of the mandarin juice sample was homogenized and weighed. The samples were placed into the headspace vial, and then, 1 mL of CaCl2 was added to these samples. The mixture was incubated at 40 °C for 30 min. SPME fiber 85 μm CAR/PDMS (carboxene/polydimethylsiloxane; gray) was used for extraction of volatiles. The adsorbed aroma compounds of mandarin juice were analyzed using a Shimadzu GC-2010 Plus gas chromatography-mass spectrometer (GC/MS). An HP Agilent-Innowax column (30 m × 0.25 mm, 0.25 μm film thickness) was used, and Helium was the carrier gas. The injector and detector temperatures were 250 °C. The furnace temperature was held at 60 °C for 10 min and then increased to 280 °C at 4 °C/min and held for 40 min. The flow rate of the carrier gas (helium) was 1 mL/min. The ionization voltage was set at 70 eV and scanned between 35 and 350 amu. Volatile compounds were determined by comparing the mass spectra of standard compounds from the NIST library (US National Institute of Standards and Technology) and the mass spectra of unknown compounds. The retention indices of two columns with different polarities were also compared with the values from the previously published papers. The identified compounds were positively evaluated when the data were confirmed by the mass spectra of the refined standard compounds.
Statistical Analysis
To analyze the significance of genotype, a one-way analysis of variance was performed with SPSS (version 26, USA, 2021), using Duncan’s multiple-range test to compare means at the 95% confidence level. Hierarchical clustering (HCA) and heatmap analysis of 12 mandarin genotypes and aroma compounds were executed with R scripts using the heatmap.2 functions of the ggplots package.32 Principal component analysis (PCA) of 12 mandarin genotypes and aroma compounds was performed with R scripts using the devtools functions of the ggplots package.33
Results and Discussion
Total Phenol Content
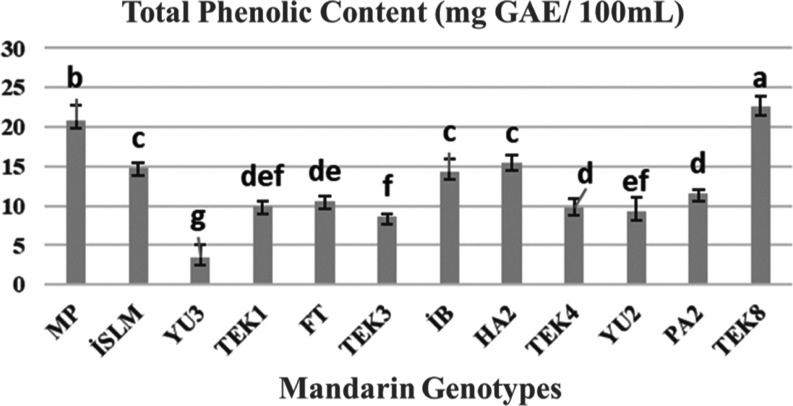
The results of total phenol contents of the 12 selected mandarin juice are presented in Figure 1. Total phenol contents showed significantly different among the juice of selected mandarin genotypes at p 0.05 level. Total phenol content ranged from 3.50 to 22.53 mg GAE/100 mL fresh weight (FW). The juice of YU3 mandarin genotype contained the lowest total phenol (3.50 mg GAE/100 mL FW), and TEK8 had the most (22.53 mg GAE/100 mL FW), followed by MP (21.03 mg GAE/100 mL FW), İSLM (14.93 mg GAE/100 mL FW), İB (14.70 mg GAE/100 mL FW), HA2 (15.80 mg GAE/100 mL FW), PA2 (11.90 mg GAE/100 mL FW), FT (10.80 mg GAE/100 mL FW), TEK1 (10.04 mg GAE/100 mL FW), TEK4 (9.94 mg GAE/100 mL FW), YU2 (9.03 mg GAE/100 mL FW), and TEK3 (8.42 mg GAE/100 mL FW), respectively. Zhang et al.5 reported that the total phenol content of mandarin (Citrus reticulate) juice of 19 samples ranged from 0.228 to 0.328, 0.083 to 0.152, 0.011 to 0.028, and 0.073 to 0.341 mg GAE/100 g dry weight (DW) mL for peels, pulp residues, seeds, and juices, respectively. Sicari et al.34 found that pink grapefruit and yellow grapefruit had the highest total phenolic content (154 and 143 mg/100 mL, respectively), while mandarin had the lowest (96 mg/100 mL). Costanzo et al.9 reported 351 mg GAE/100 g FW total phenol in mandarin (Citrus reticulate Blanco) in Italy. Anticona et al.7 used a number of mandarin hybrid peels for total phenol analysis and reported that the total phenol content varied according to the hybrid mandarin varieties (87–127 mg GAE/100 mL). They found higher total phenol in the “Ortanique” samples compared with the “Clemenvilla” and “Nadorcott” peels. Nipornram et al.35 obtained 14.89 mg GAE/100 mL of total phenol content in the peel of Citrus reticulata Blanco cv. Sainampueng. Singh et al.2 reported that W. Murcott pulp contained higher mean total phenol content than Kinnow pulp (1547 versus. 1279 mg GAE/100 g DW). Maslov Bandic et al.11 used five Satsuma mandarin (Citrus unshiu) grown in Neretva valley and found them ranging from 70.3 mg GAE/100 mL (“Owari”) to 111 mg GAE/mL (“Zorica”). Kelebek and Selli29 found 75 mg GAE/mL total phenolic content in Satsuma mandarin juice. Furthermore, Pyo et al.36 found higher content of Total Phenol Content, 211 mg GAE/mL in Citrus unshiu juice. Chen et al.37 showed significant differences among different mandarin cultivars for the total phenolic content, ranging from 331 to 2346 mg GAE/100 mL DW in peels, from 265 to 1045 mg GAE/100 mL DW in pulps, and from 12 to 137 mg GAE/100 mL FW in juices. They found that Guihuadinanfeng (NF) and Baiju (BJ) had significantly higher total phenolic content than other cultivars tested. This consequence was similar to a previous report by Chen et al. (2010).38 In that study, Manju. Karamandarin and Parson special mandarin were generally the genotypes with higher phenolic contents in fruit tissue. In contrast, the fruit tissue of Shagan had lower phenolic content, with phenolic content in the juice as low as 728 mg GAE/100 g DW. Lee and Kim (2022) found total phenolic content 3120 mg GAE/100 mL dried extract in mandarin juice. Wang et al. (2017) used 35 Citrus reticulate fruit part; the juice sacs had the lowest total phenolic contents (418 mg GAE/100 g DW in CX to 639 mg GAE/100 g DW in Newhall). Our total phenol content results were found to be lower than some studies and similar with some studies. However, difficulties arise in comparisons as previous studies which have been reported mostly on dry sample base. All above results indicate that the total phenolic content is in general dependent on several factors. One of these factors could be the different genetic potential of each variety for the biosynthesis of polyphenols. Maturity, season, fertilizer, geographical origin, storage conditions, soil type, and solar radiation may also be crucial in this regard.5 Phenolic compounds are widely found in horticultural plants and accepted as human health promoting substances.41−44
Figure 1.
Values for the total phenol content (mg GAE/100 mL) in juice of selected mandarin genotypes.Values are given as mean ± standard deviation, n = 3. Results with different letters are significantly different at p < 0.05.
Antioxidant Capacity
Horticultural plants including fruits and vegetables are very diverse groups and exhibit basic components of the human health that strengthen the immune system and had preventive effects on chronic diseases, such as cardiovascular disease, cancer, and diabetes.45 There were different assays that measure antioxidant capacity of horticultural plants. The DPPH assay is commonly used to determine primary antioxidant capacity. DPPH radicals can be reduced by reactions with antioxidant compositions that can donate hydrogen.34
The total antioxidant activity of the juice of selected mandarin genotypes was evaluated by the DPPH method (Figure 2). Genotypes presented statistically significant differences with each other for DPPH values at p = 0.05. The total antioxidant capacity of the mandarin genotypes such as HA2, IB, and TEK3 reached the highest value as 60.40, 59.15, and 58.38%, indicating their importance for promoting human health. Sicari et al. (2016)34 determined the antioxidant activities of citrus species by free-radical scavenging activity (DPPH and ABTS assay methods). In the DPPH and ABTS methods, Nazvel, mandarin, and Castagnaro have the highest value of 66.34, 64.53, and 61.39% DPPH, respectively, while pink grapefruit has the lowest value of 37.32% (DPPH). According to the previously published studies, phenolic compounds control the comprehensive antioxidant capacity of citrus.5,46 In other study, Chen et al.38 reported that Satsuma mandarin had 33.65% DPPH of inhibition. The quality parameters of juices from six citrus species from the north of Iran were studied by Hashempour et al.47 The highest antioxidant activity was observed in “Moro” (blood orange) with 92.16%, and the lowest antioxidant activity content was found in “Marsh” (grapefruit) (39.41%). The authors also found that mandarin had 62% DPHH scavenging activity. Costanzo et al.9 reported lower DPPH activity (21.10%) in mandarin fruits. Singh et al.2 used DPPH assay in two mandarin cultivars and reported DPPH activity from 26.54 to 85.46% during different fruit developmental stages. China Xu et al.48 investigated antioxidant activity of different groups of mandarins (diverse species) and found DPPH value between 23.69 and 61.62%. They also reported DPPH values between 26.31 and 33.65% among C. unshiu genotypes. Chen et al.37 used a large number of mandarins, evaluated by DPPH, ABTS, and FRAP methods to determine the antioxidant capacity. Mandarin genotypes contained high and diverse antioxidant capacity. Wang et al.40 examined and compared four parts, flavedo, albedo, segment membrane, and juice sacs of mandarins in DPPH assay. The juice sacs had the lowest antioxidant capacity, following by the segment membrane. The results are consistent with previous reports,5,11,46 and they indicated that antioxidant capacity of citrus is affected by specie and cultivars. Sampling time, rootstocks, different extraction techniques, geographical regions, harvest, or storage intervals etc. also affect the antioxidant capacity. Our results indicated that local Satsuma mandarin genotypes have excellent antioxidant capacity and should be utilized comprehensively.39
Figure 2.
DPPH scavenging activity (%) in juice of mandarin genotypes. Values are given as mean ± standard deviation, n = 3. Results with different letters are significantly different at p < 0.05.
Volatile Profile
Volatile profiles determined by HS/SPME/GC–MS techniques of 12 Satsuma mandarin genotypes, namely, MP, ISLM, YU3, TEK1, FT, TEK3, IB, HA2, TEK4, YU2, PA2, and TEK8 are given in Table 1. A total of 30 aroma volatiles were detected from the juice samples of 12 mandarin genotypes, which comprised six alcohols, three aldehydes (including one monoterpene), three esters, one ketone, and one other volatiles. In an earlier study, Yu et al.49 detected 28 aroma volatiles in “Ponkan” mandarin. Miyazaki et al.50 evaluated the aroma volatiles of 20 tangerine hybrids and identified over 200 volatiles. Terpene hydrocarbons and oxygenated compounds such as aldehydes, esters, alcohols, and ketones are found the most abundant. As indicated in Table 1, the main volatile compounds of 12 Satsuma mandarin genotypes were α-terpineol, linalool, γ-terpinene, β-myrcene, dl-limonene, α-farnesene, and d-germacrene. The total percentage of the above volatiles ranged from 90.99% (in PA2 genotype) to 96.7% (in HA2 genotype), with limonene and γ-terpinene being the highest in all Satsuma genotypes. When we quantified the volatile compounds according to their chemical classes, we found that the most common group of volatiles in mandarins were monoterpenes, followed by alcohols (mainly terpene alcohols such as linalool, a-terpineol, β-terpineol, and terpinen-4-ol), aldehydes, and esters (Table 1). Hijaz et al.21 found that monoterpenes were the most abundant volatiles and tended to decrease with fruit maturity in four mandarin cultivars, and volatile profiles of the four cultivars were different from each other but similar for both years within a cultivar. Limonene accounts for most of the citrus flavor. Similar to other citrus, limonene was the greatest and key compound in the all mandarins. Limonene was found to be the highest amount in TEK3 while the lowest in the FT genotype (Table 1). Limonene also accounted for 79.71–85.12% of the total volatile compounds in all selected mandarin juices. Barboni et al.51 indicated that limonene, which has a characteristic citrus aroma, is a considerable important aroma compound in mandarin juice. Apart from limonene, γ-terpinene, β-myrcene, β-elemene, and farnesene are the most abundant monoterpenic compounds in Satsuma mandarin fruit. Their odor thresholds were low, and the three compounds have harbeceous, sweety, and piney notes.21 Goldenberg et al.20 quantified the content of 32 volatile compounds in fresh fruit juice of Hernandina mandarins using GC–MS, and they reported that the profile was similar to that of the Dancy and Murcott varieties. Another research group, Perez-Lopez and Carbonell-Barrachina52 studied on two mandarin cultivars in Spain and reported a total 12 volatile compounds. The samples predominantly included d-limonene, myrcene, sabinene, α-pinene, and linalool. For quality criteria, they determined d-limonene, linalool, α-terpineol, and terpinen-4-ol as main parameters. Dharmawan et al.53 determined 41 aroma compounds in the juice of an Asian mandarin which mainly contained terpenes, carbonyls, alcohols, esters, and hydrocarbons, with limonene being the most important compound. They also found that mandarins include mostly α-phellandrene, 1-terpineol, trans-β-terpineol, p-cymen-8-ol, thymol, and traces of β-damascenone, α-farnesene, and α-sinensa. In another study, reported by Yu et al.,54 HS/GC–MS, the volatile aroma compounds in the juices of three different Satsuma mandarin varieties were identified and characterized. Each variety was found to contain between 66 and 73 volatile flavor compounds, and 29 compounds were common to all juices of the three varieties. Yu et al.49 reported that d-limonene was the most abundant volatile compound which accounted for 80.8 and 64.4% of the volatile profile in Murcott and Temple mandarin cultivars, respectively.
Table 1. Volatile Profiles of 12 Various Mandarin Lines Identified by HS/SPME/GC/MS Techniques.
| compound name | MP (%) | ISLM (%) | YU3 (%) | TEK1 (%) | FT (%) | TEK3 (%) | IB (%) | HA2 (%) | TEK4 (%) | YU2 (%) | PA2 (%) | TEK8 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ketones | |||||||||||||
| 26.558 | neryl acetone | 2 | ND | 0.1 | 0.79 | 0.39 | ND | 0.11 | ND | ND | ND | 0.07 | ND |
| total ketones | 2 | ND | 0.1 | 0.79 | 0.39 | ND | 0.11 | 0.06 | ND | ND | 0.07 | ND | |
| Alcohols | |||||||||||||
| 22.808 | α-terpineol | 1.3 | 1.43 | 0.6 | 1.26 | 1.46 | 1.25 | 1.77 | 0.94 | 2.31 | 1.88 | 0.91 | 1.64 |
| 21.163 | β-terpineol | ND | 0.09 | ND | 0.09 | 0.09 | 0.07 | 0.13 | 0.04 | 0.2 | 0.13 | ND | 0.1 |
| 28.933 | 1-octanol | 0.2 | 0.12 | ND | 0.15 | 0.15 | 0.21 | 0.30 | ND | ND | 0.07 | ND | ND |
| 20.473 | terpinen-4-ol | 0.6 | 0.59 | 0.4 | 0.6 | 0.56 | 0.59 | 0.62 | 0.58 | 0.74 | 0.67 | 0.6 | 0.77 |
| 26.070 | carveol | ND | ND | ND | 0.29 | 0.18 | ND | 0.15 | 0.06 | ND | 0.08 | ND | ND |
| 19.087 | linalool | 1.1 | 2.02 | 2.5 | 3.06 | 1.27 | 1.15 | 1.38 | 2.4 | 3.21 | 2.41 | 1.61 | 2.15 |
| total alcohols | 3.2 | 4.25 | 3.4 | 5.45 | 3.71 | 3.27 | 5.35 | 4.02 | 6.46 | 5.24 | 3.12 | 4.66 | |
| Aldehydes | |||||||||||||
| 25.654 | acetaldehyde | 0.3 | 0.09 | 0.3 | 0.22 | 0.59 | 0.14 | 0.36 | ND | ND | 0.15 | 0.07 | 0.21 |
| 15.006 | nonanal | 0.7 | 0.58 | 0.6 | 1.23 | 1.24 | 0.62 | 0.18 | ND | 0.32 | 0.49 | 0.16 | ND |
| total aldehydes | 1 | 0.67 | 0.9 | 1.45 | 1.83 | 0.76 | 0.54 | ND | 0.32 | 0.64 | 0.23 | 0.21 | |
| Esters | |||||||||||||
| 22.029 | citronellyl acetate | 0.3 | 0.27 | ND | ND | ND | 0.96 | 0.07 | 0.08 | 0 | 0.16 | 0.27 | 0.09 |
| 24.335 | geranyl acetate | 1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.98 |
| 23.604 | neryl acetate | 0.1 | 0.19 | 0.1 | 0.22 | ND | 0.52 | 0.14 | 0.11 | 0.47 | 0.2 | 0.14 | 0.12 |
| total esters | 1.4 | 0.46 | 0.1 | 0.22 | ND | 1.48 | 0.21 | 0.19 | 0.47 | 0.36 | 0.41 | 1.19 | |
| Terpenes | |||||||||||||
| 8.814 | α-terpinene | ND | ND | ND | ND | ND | ND | 0.08 | ND | ND | 0.09 | ND | 0.13 |
| 10.822 | γ-terpinene | 5.4 | 4.27 | 5.3 | 4.73 | 4.72 | 4.86 | 4.59 | 4.54 | 4.41 | 4.36 | ND | 5.15 |
| 17.625 | α-copaene | 0.2 | 0.14 | 0.1 | 0 | 0.11 | 0.12 | 0.18 | 0.14 | 0.15 | 0.16 | ND | 0.1 |
| 22.195 | α-caryophyllene | 0 | 0.36 | ND | ND | ND | ND | 0.3 | 0.27 | 0.3 | 0.36 | 0.34 | 0.25 |
| 4.990 | α-pinene | 0.5 | ND | 0.4 | 0.55 | 0.59 | 0.56 | 0.76 | 0.73 | 0.3 | 0.57 | 0.7 | 0.76 |
| 6.950 | β-pinene | 0.2 | ND | ND | ND | 0.28 | 0.23 | 0.3 | 0.27 | 0.14 | 0.21 | 0.27 | 0.28 |
| 11.873 | α-terpinolene | 0.3 | 0.22 | 0.3 | 0.25 | 0.24 | 0.29 | 0.18 | 0.26 | 0.33 | 0.29 | 0.25 | 0.32 |
| 20.238 | β-elemene | 0.7 | 1.22 | 0.6 | 1.04 | 0.57 | 0.59 | 0.7 | 0.64 | 1.69 | 1.24 | 0.95 | 0.55 |
| 17.070 | δ-elemene | 0.3 | 0.45 | 0.4 | 0.53 | 0.37 | 0.37 | 0.48 | 0.5 | 0.55 | 0.52 | 0.43 | 0.37 |
| 8.607 | β-myrcene | 1.6 | 0.82 | 1.3 | 1.13 | ND | 1.02 | 1.25 | 1.15 | 0.9 | 1.14 | 1.45 | 1.12 |
| 9.713 | a-phellandrene | 0.4 | ND | ND | 0.34 | ND | 0.28 | 0.4 | ND | 0.09 | 0.11 | ND | 0.06 |
| 9.522 | dl-limonene | 81.8 | 84.0 | 85.0 | 79.71 | 85.12 | 83.49 | 81.08 | 83.78 | 80.14 | 80.44 | 84.58 | 82.72 |
| 24.166 | farnesene | 1.3 | ND | 1.1 | 2.08 | 1.07 | 1.19 | 2.09 | 1.58 | 2.27 | 2.44 | 1.55 | 1.46 |
| 26.003 | β-germacrene | 0.2 | 0.17 | 0.1 | ND | ND | ND | 0.2 | 0.17 | ND | 0.19 | 0.11 | 0 |
| 23.171 | d-germacrene | 0.8 | 1.03 | 0.9 | 1.37 | 0.79 | 0.87 | 1.19 | 0.95 | ND | 1.27 | 0.89 | 0.66 |
| 20.379 | caryophyllene | 0 | 0.16 | ND | 0.18 | ND | 0.11 | 0.13 | 0.1 | 0.13 | 0.16 | 0.15 | ND |
| total terpenes | 92 | 94.49 | 95.47 | 91.73 | 93.88 | 93.87 | 93.78 | 95.62 | 92.75 | 93.55 | 96.16 | 93.93 | |
| other compounds | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 36.075 | 2,4-di-tert-butylphenol | ND | 0.12 | ND | 0.33 | 0.16 | 0.25 | ND | 0.1 | ND | 0.19 | ND | ND |
| total other compounds | ND | 0.12 | ND | 0.33 | 0.16 | 0.25 | ND | 0.1 | ND | 0.19 | ND | ND | |
Overall, the compounds detected in the greatest amount in the Satsuma mandarins were identified as limonene, linalool, γ-terpinene, β-myrcene, α-pinene, and octanal. In our study, limonene, γ-terpinene, β-myrcene, β-elemene, and farnesene were considered as characteristic aroma compounds of the all-selected mandarins. Alcohols and aldehydes are the main aroma compounds in citrus fruits. Among the alcohols and aldehydes investigated in this study, linalool and nonanol ranked first in terms of quantity. Nonanol, and specially linalool with fruity aroma, was the most important aroma component in all selected Satsuma mandarins. Both of them could made a big contribution to mandarin aroma. Esters have excessively low thresholds but could not be noticed in this study because of their high volatility. Neryl acetate and Citronellyl acetate were found in most of all Satsuma genotypes, while Geranyl acetate was found only in two mandarin genotypes. These esters with lower amounts would have effects on the flavor of Satsuma mandarin fruit.
Principal Component Analysis (PCA) of 12 Mandarin Genotypes
In this study, the differences on the bioactive content and aroma profile among the 12 mandarin genotypes were analyzed by PCA (Figure 3). The first (PC1) and second principal components (PC2) represented 29.5 and 25.3% of the total variance, respectively. Terpenes were the most abundant category in each mandarin juice sample, especially dl-Limonene, γ-terpinene, α-pinene, β-pinene, α-terpinolene, β-elemene, δ-elemene, farnesene, and d-germacrene. The concentration of alcohols in mandarin juice was second only to terpenes, with linalool, terpinen-4-ol, α-terpineol, β-terpineol, 1-octanol, and carveol accounting for the all-observed alcohols.
Figure 3.
PCA-Biplot graph for total phenol, total esters, total ketones, total antioxidant, total aldehydes, total other compounds, total alcohols, and total terpenes affected by mandarin genotypes.
In this study, the mandarin aroma compounds were also subjected to HCA and used to explore the heterogeneity between mandarin genotypes (Figure 4). Results showed that the aroma profiles of different 12 mandarins were appropriately divided into two main branches, then were again subdivided into four groups. According to the dendrogram and heatmap obtained, PA2 genotype had the highest terpenes and dl-limonene compound, and it was grouped in one cluster. The aroma component of MP, TEK4, TEK1, IBE, and YU2 was grouped in a second cluster, and TEK8, PA2, TEK3, FT, ISLM, HA2, and YU3 were grouped in the third cluster. The observed clusters can be defined by the similar odor type of the identified odorants. HCA also showed the lowest diversity among the 12 mandarin genotypes due to the significant similarities in the types and amounts of volatiles identified. In addition, the results indicated that the aroma of genotype PA2 was different from that of the other mandarin genotypes. These results were compatible with the PCA analysis, indicating that HCA is an effective tool to validate the rationality of clustering and to reveal the characteristics of each group.55
Figure 4.
Hierarchical clustering (HCA) heat map of aroma components in juices of 12 mandarin genotypes. Each colored cell on the map corresponds to the amount of the aroma component, with samples in the rows and compounds in the columns.
Conclusions
In this study, high yielding and high-quality mandarin genotypes obtained as a result of the first selection study in the Black Sea region were investigated in terms of the total phenolic content, antioxidant capacity, and aroma profile. Significant differences in the total phenolic content, DPPH scavenging activity, and aroma compounds of mandarin genotypes were found. IB, HA2, and TEK3 genotypes showed higher bioactive content. The main volatile compounds of all Satsuma mandarin genotypes were dl-limonene (79.71–85.12%), γ-terpinene (4.41–5.5%), linalool (1.1–3.21%), α-farnesene (1.1–2.44), α-terpineol (0.6–1.88%), β-myrcene (0.9–1.6%), and d-germacrene (0.66–1.37%). The genotypes MP and TEK8 had the highest total phenolic content, and HA2, IB, and TEK 3 had the highest antioxidant capacity. The YU2 genotype was found to contain more aroma compounds than the other genotypes. Results revealed that mandarin genotypes could be differentiated using the metabolite profile.
Author Contributions
Conceptualization, K.Y., B.G., S.E., and O.F.B. Formal analysis, B.G. and O.F.B. Funding acquisition, J.B. Investigation, methodology, and project administration, K.Y. and B.G. Resources, K.Y., B.G., S.E., and O.F.B. Software and supervision, B.G. and J.B. Validation, K.Y. and B.G. Visualization, B.G. and J.B. Writing—original draft, K.Y., B.G., S.E., and O.F.B. Writing—review and editing, K.Y., S.E., and J.B.
The authors declare no competing financial interest.
References
- Canan I.; Gundogdu M.; Seday U.; Oluk C. A.; Karaşahi̇n Z.; Eroglu E. C.; Yazici E.; Unlu M. Determination of antioxidant, total phenolic, total carotenoid, lycopene, ascorbic acid, and sugar contents of citrus species and mandarin hybrids. Turk. J. Agric. For. 2016, 40, 894–899. 10.3906/tar-1606-83. [DOI] [Google Scholar]
- Singh J.; Chahal T. S.; Gill P. S.; Grewal S. K. Changes in phenolics and antioxidant capacities in fruit tissues of mandarin cultivars Kinnow and W. Murcott with relation to fruit development. J. Food Process. Preserv. 2021, 45, e16040 10.1111/jfpp.16040. [DOI] [Google Scholar]
- Saini R. K.; Ranjit A.; Sharma K.; Prasad P.; Shang X.; Gowda K. G. M.; Keum Y.-S. Bioactive Compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. 10.3390/antiox11020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO) . FAOSTAT Database, 2022. Available online:http://www.fao.org/faostat/(accessed on 2 January, 2023).
- Zhang H.; Yang Y. F.; Zhou Z. Q. Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J. Integr. Agric. 2018, 17, 256–263. 10.1016/s2095-3119(17)61664-2. [DOI] [Google Scholar]
- Nawaz R.; Abbasi N. A.; Ahmad Hafiz I.; Khalid A. Impact of climate variables on fruit internal quality of Kinnow mandarin (Citrus nobilis Lour x Citrus deliciosa Tenora) in ripening phase grown under varying environmental conditions. Sci. Hortic. 2020, 265, 109235. 10.1016/j.scienta.2020.109235. [DOI] [Google Scholar]
- Anticona M.; Lopez-Malo D.; Frigola A.; Esteve M. J.; Blesa J. Analysis of polyphenol content and antioxidant capacity of hybrid mandarin peel. Biol. Life Sci. Forum 2021, 6, 25. 10.3390/Foods2021-11100. [DOI] [Google Scholar]
- Bureš M. S.; Maslov Bandic ´ L.; Vlahovicek-Kahlina K. Determination of Bioactive Components in Mandarin Fruits: A Review. Crit. Rev. Anal. Chem. 2022, 1–26. 10.1080/10408347.2022.2035209. [DOI] [PubMed] [Google Scholar]
- Costanzo G.; Vitale E.; Iesce M. R.; Naviglio D.; Amoresano A.; Fontanarosa C.; Spinelli M.; Ciaravolo M.; Arena C. Antioxidant properties of pulp, peel and seeds of phlegrean mandarin (Citrus reticulata Blanco) at different stages of fruit ripening. Antioxidants 2022, 11, 187. 10.3390/antiox11020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Zhang J.-Y.; Shan Y.-X.; Guo C.; He L.; Zhang L.-y.; Ling W.; Liang Y.; Zhong B.-l. Effect of harvest time on the chemical composition and antioxidant capacity of Gannan navel orange Citrus sinensis L. Osbeck ‘Newhall’ juice. J. Integr. Agric. 2022, 21, 261–272. 10.1016/S2095-3119(20)63395-0. [DOI] [Google Scholar]
- Maslov Bandić L.; Vlahovicek-Kahlina K.; Sigurnjak Bureš M.; Sopko Stracenski K.; Jalšenjak N.; Fruk G.; Antolković A. M.; Juric S. Fruit quality of satsuma mandarins from Neretva valley and their flavonoid and carotenoid content. Horticulturae 2023, 9, 383. 10.3390/horticulturae9030383. [DOI] [Google Scholar]
- TUIK , 2022.www.tuik.gov.tr.
- Yazici K.; Goksu B.; Korkmaz O.; Akbulut M.; Bakoglu N.; Gulay T.; Riza O. Breeding of clonal selections of Rize (Satsuma) mandarins. J. Sci. Eng. of Recep Tayyip Erdogan Univ 2020, 1, 1–15. [Google Scholar]
- Cimen B.; Yesiloglu T.; Incesu M.; Yilmaz B. Studies on mutation breeding in citrus: Improving seedless types of ‘Kozan’ common orange by gamma irradiation. Sci. Hortic. 2021, 278, 109857. 10.1016/j.scienta.2020.109857. [DOI] [Google Scholar]
- Cimen B.; Yesiloglu T.; Donmez D.; Aka Kacar Y.; Ercisli S. Recovering triploid citrus hybrids from 2x × 2x sexual crosses with the aid of embryo rescue and flow cytometry in Turkey. Mol. Biol. Rep. 2022, 49, 5625–5634. 10.1007/s11033-022-07555-2. [DOI] [PubMed] [Google Scholar]
- Tietel Z.; Plotto A.; Fallik E.; Lewinsohn E.; Porat R. Taste and aroma of fresh and stored mandarins. J. Sci. Food Agric. 2011, 91, 14–23. 10.1002/jsfa.4146. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Sun Y.; Xi W.; Shen Y.; Qiao L.; Zhong L.; Ye X.; Zhou Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014, 145, 674–680. 10.1016/j.foodchem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Plotto A.; Baldwin E. A.; Bai J.; Huang M.; Yu Y.; Dhaliwal H. S.; Gmitter F. G. Proteomic and metabolomic analyses provide insight into production of volatile and non-volatile flavor components in mandarin hybrid fruit. BMC Plant Biol 2015, 15, 76. 10.1186/s12870-015-0466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg L.; Yaniv Y.; Doron-Faigenboim A.; Carmi N.; Porat R. Diversity among mandarin varieties and natural sub-groups in aroma volatiles compositions. J. Sci. Food Agric. 2016, 96, 57–65. 10.1002/jsfa.7191. [DOI] [PubMed] [Google Scholar]
- Goldenberg L.; Yaniv Y.; Porat R.; Carmi N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2018, 98, 18–26. 10.1002/jsfa.8495. [DOI] [PubMed] [Google Scholar]
- Hijaz F.; Gmitter F. G. Jr.; Bai J.; Baldwin E.; Biotteau A.; Leclair C.; McCollum T. G.; Plotto A. Effect of fruit maturity on volatiles and sensory descriptors of four mandarin hybrids. J. Food Sci. 2020, 85, 1548–1564. 10.1111/1750-3841.15116. [DOI] [PubMed] [Google Scholar]
- Li Z.; Jin R.; Yang Z.; Wang X.; You G.; Guo J.; Zhang Y.; Liu F.; Pan S. Comparative study on physicochemical, nutritional and enzymatic properties of two Satsuma mandarin (Citrus unshiu Marc.) varieties from different regions. J. Food Compos. Anal. 2021, 95, 103614. 10.1016/j.jfca.2020.103614. [DOI] [Google Scholar]
- Sudo M.; Yasuda K.; Yahata M.; Sato M.; Tominaga A.; Mukai H.; Ma G.; Kato M.; Kunitake H. Morphological characteristics, fruit qualities and evaluation of reproductive functions in autotetraploid satsuma mandarin (Citrus unshiu Marcow). Agronomy 2021, 11, 2441. 10.3390/agronomy11122441. [DOI] [Google Scholar]
- Manzoor M.; Hussain S. B.; Anjum M. A.; Naseer M.; Ahmad R.; Ziogas V. Effects of harvest time on the fruit quality of Kinnow and Feutrell’s early mandarins (Citrus reticulata Blanco). Agronomy 2023, 13, 802. 10.3390/agronomy13030802. [DOI] [Google Scholar]
- Feng S.; Niu L. Y.; Suh J. H.; Hung W. L.; Wang Y. Comprehensive metabolomics analysis of mandarins (Citrus reticulata) as a tool for variety, rootstock, and grove discrimination. J. Agric. Food Chem. 2018, 66, 10317–10326. 10.1021/acs.jafc.8b03877. [DOI] [PubMed] [Google Scholar]
- Han S.-H.; Kang S.-B.; Moon Y.-J.; Moon B.-W. Changes in fruit quality, antioxidant activity, and flavonoid content with limited irrigation after full bloom of early-maturing ‘Harye’ Satsuma mandarin in a plastic greenhouse. Hortic. Sci. Technol. 2019, 37, 10–19. 10.12972/kjhst.20190002. [DOI] [Google Scholar]
- Zhang H. P.; Xie Y. X.; Liu C. H.; Chen S. L.; Hu S. S.; Xie Z. Z.; Deng X. X.; Xu J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. 10.1016/j.food-chem.2017.03.040. [DOI] [PubMed] [Google Scholar]
- Elmaci Y.; Altug T. Flavor characterization of three mandarin cultivars (Satsuma, Bodrum, Clemantine) by using GC/MS and flavor profile analysis techniques. J. Food Qual. 2005, 28, 163–170. 10.1111/j.1745-4557.2005.00009.x. [DOI] [Google Scholar]
- Kelebek H.; Selli S. Identification of phenolic compositions and the antioxidant capacity of mandarin juices and wines. J. Food Sci. Technol. 2014, 51, 1094–1101. 10.1007/s13197-011-0606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos G. A.; Wrolstad R. E. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J. Agric. Food Chem. 1990, 38, 1565–1571. 10.1021/jf00097a030. [DOI] [Google Scholar]
- Cuvelier M. E.; Berset C.; Richard H. Antioxidant Constituents in Sage (Salvia officinalis). J. Agric. Food Chem. 1994, 42, 665–669. 10.1021/jf00039a012. [DOI] [Google Scholar]
- Moon K.-W.. In Learn ggplot2 using shiny app; Springer, 2016, pp 255–260.Heatmap [Google Scholar]
- Miyazaki T.; Plotto A.; Baldwin E. A.; Reyes-De-Corcuera J. I.; Gmitter Jr F. G. Aroma characterization of tangerine hybrids by gas-chromatography-olfactometry and sensory evaluation. J. Sci. Food Agric. 2012, 92, 727–735. 10.1002/jsfa.4663. [DOI] [PubMed] [Google Scholar]
- Sicari V.; Pellicanò T. M.; Giuffrè A. M.; Zappia C.; Capocasale M. Bioactive compounds and antioxidant activity of citrus juices produced from varieties cultivated in Calabria. J. Food Meas. Charact. 2016, 10, 773–780. 10.1007/s11694-016-9362-8. [DOI] [Google Scholar]
- Nipornram S.; Tochampa W.; Rattanatraiwong P.; Singanusong R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. 10.1016/j.foodchem.2017.08.114. [DOI] [PubMed] [Google Scholar]
- Pyo Y. H.; Jin Y. J.; Hwang J. Y. Comparison of the Effects of Blending and Juicing on the Phytochemicals Contents and Antioxidant Capacity of Typical Korean Kernel Fruit Juices. Prev. Nutr. Food Sci. 2014, 19, 108–114. 10.3746/pnf.2014.19.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Wang D.; Tan C.; Hu Y.; Sundararajan B.; Zhou Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local citrus cultivars. Plants 2020, 9, 196. 10.3390/plants9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Yuan K.; Liu H. Phenolic contents and antioxidant activities in ethanol extracts of Citrus reticulata blanco cv. Ougan fruit. Int. J. Food, Agric. Environ. 2010, 8, 150–155. [Google Scholar]
- Lee S.; Kim H. J. Antioxidant activities of premature and mature mandarin (Citrus unshiu) peel and juice extracts. Food Sci. Biotechnol. 2022, 31, 627–633. 10.1007/s10068-022-01064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Qian J.; Cao J.; Wang D.; Liu C.; Yang R.; Li X.; Sun C. Antioxidant capacity, anticancer ability and flavonoids composition of 35 citrus (Citrus reticulata Blanco) varieties. Molecules 2017, 22, 1114. 10.3390/molecules22071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawadi P.; Shrestha R.; Mishra S.; Bista S.; Raut J. K.; Joshi T. P.; Bhatt L. R. Nutritional value and antioxidant properties of Viburnum mullaha Buch.-Ham. Ex D. Don fruit from central Nepal. Turk. J. Agric. For. 2022, 46, 781–789. 10.55730/1300-011x.3041. [DOI] [Google Scholar]
- Tahmaz H.; Soylemezoglu G. Selected phenolics and antioxidant capacities: From Bogazkere (Vitis vinifera L.) grape to pomace and wine. Turk. J. Agric. For. 2022, 46, 623–631. 10.55730/1300-011X.3031. [DOI] [Google Scholar]
- Sarker U.; Oba S.; Ercisli S.; Assouguem A.; Alotaibi A.; Ullah R. Bioactive phytochemicals and quenching activity of radicals in selected drought-resistant amaranthus tricolor vegetable amaranth. Antioxidants 2022, 11, 578. 10.3390/antiox11030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker U.; Hossain M. N.; Oba S.; Ercisli S.; Marc R. A.; Golokhvast K. S. Salinity stress ameliorates pigments, minerals, polyphenolic profiles, and antiradical capacity in lalshak. Antioxidants 2023, 12, 173. 10.3390/antiox12010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mateos A.; Vauzour D.; Krueger C. G.; Shanmuganayagam D.; Reed J.; Calani L.; Mena P.; Del Rio D.; Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. 10.1007/s00204-014-1330-7. [DOI] [PubMed] [Google Scholar]
- Ghasemi K.; Ghasemi Y.; Ebrahimzadeh M. A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [PubMed] [Google Scholar]
- Hashempour A.; Sharifzadeh K.; Bakhshi D.; Ghazvini R. F.; Ghasemnezhad M.; Mighani H. Variation in total phenolic, ascorbic acid and antioxidant activity of citrus fruit of six species cultivated in north of Iran. Int. J. Agric:Res. Rev. 2013, 3, 1–5. [Google Scholar]
- Xu G.; Liu D.; Chen J.; Ye X.; Ma Y.; Shi J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. 10.1016/j.foodchem.2007.06.046. [DOI] [Google Scholar]
- Yu Y.; Bai J.; Chen C.; Plotto A.; Baldwin E. A.; Gmitter F. G. Comparative analysis of juice volatiles in selected mandarins, mandarin relatives and other citrus genotypes. J. Sci. Food Agric. 2018, 98, 1124–1131. 10.1002/jsfa.8563. [DOI] [PubMed] [Google Scholar]
- Miyazaki T.; Plotto A.; Goodner K.; Gmitter F. G. Jr. Distribution of aroma volatile compounds in tangerine hybrids and proposed inheritance. J. Sci. Food Agric. 2011, 91, 449–460. 10.1002/jsfa.4205. [DOI] [PubMed] [Google Scholar]
- Barboni T.; Muselli A.; Luro F.; Desjobert J.-M.; Costa J. Influence of processing steps and fruit maturity on volatile concentrations in juices from clementine, mandarin, and their hybrids. Eur. Food Res. Technol. 2010, 231, 379–386. 10.1007/s00217-010-1283-x. [DOI] [Google Scholar]
- Pérez-López A. J.; Carbonell-Barrachina Á.A. Volatile odour components and sensory quality of fresh and processed mandarin juices. J. Sci. Food Agric. 2006, 86, 2404–2411. 10.1002/jsfa.2631. [DOI] [Google Scholar]
- Dharmawan J.; Kasapis S.; Curran P.; Johnson J. R. Characterization of volatile compounds in selected citrus fruits from asia. Part I: Freshly-Squeezed Juice. Flavour Fragr J 2007, 22, 228–232. 10.1002/ffj.1790. [DOI] [Google Scholar]
- Qiao Y.; Xie B. j.; Zhang Y.; Zhou H. y.; Pan S. y. Study on aroma components in fruit from three different satsuma mandarin varieties. Agric. Sci. China 2007, 6, 1487–1493. 10.1016/S1671-2927(08)60012-7. [DOI] [Google Scholar]
- Perez-Cacho P. R.; Rouseff R. L. Fresh squeezed orange juice odor: A review. Crit. Rev. Food Sci. 2008, 48, 681–695. 10.1080/10408390701638902. [DOI] [PubMed] [Google Scholar]