Abstract
Congenital heart disease (CHD) is estimated to affect between 3 to 5% of all newborns. Extra-cardiac malformations are observed in 7 to 50% of patients with CHD. One relatively well-known association that can occur in the context of CHD is VACTERL. Controversy still remains regarding the definition of VATER association and its expansion to VACTERL, the appropriate diagnostic criteria and the overall incidence. We conducted a description of a case series to characterize the cardiac findings present in a cohort of patients meeting the criteria for VACTERL association. 46 of 220 were eligible for inclusion into the study, 67% (31 of 46) had CHD. The most common CHD was VSD, present in 18 of 31 patients (58%). There was no statistically significant association between CHD severity and the presence or absence of other VACTERL CFs, specifically ARM (p=0.18) or as above TEF (p=0.72). CHD presence did not correlate with the presence of TEF or ARM. While this study does not, by design, provide further evidence towards the questions of whether CHD is a defining feature of VACTERL association, the frequency of CHD in our cohort does lend support to it being an important medical consideration in patients with VACTERL association. Based on our experience, we strongly recommend a screening echocardiogram to evaluate for CHD in individuals with a potential diagnosis of VACTERL association.
Keywords: VACTERL, VACTERL Association, VATER, VATER Association, Congenital heart defects
INTRODUCTION
Congenital heart disease (CHD) is estimated to affect 3 to 5% of newborns (Robinson et al, 1993). It comprises about 40% of reported congenital defects and represents one of the most frequent human malformations. Extra-cardiac malformations are observed in 7 to 50% of patients with CHD, the most common being renal anomalies. (Rosa et al, 2012). One association that can occur in the context of CHD is VACTERL, sometimes referred to as VATER association.
In the early 1970s, VATER association was first used a descriptive term referring to the statistically nonrandom co-occurrence of specific congenital anomalies: Vertebral defects, Anal atresia, Tracheo-Esophageal fistula (TEF) with esophageal atresia (EA), Radial and Renal dysplasia (Quan and Smith, 1973). Within the next two years a number of case reports/series suggested that cardiovascular malformations, specifically single umbilical artery (SUA) and cardiac malformations such as ventricular septal defects (VSD), should be included as part of the VATER/VACTERL association. Tetamy et al (1974) described a series of 10 patients with at least three component features (CFs) of VATER association. In their series, cardiovascular malformations were described in 80%, with the most common cardiac defect being a Ventricular Septal Defect (VSD). This group suggested that the V in VATER stands not only for vertebral defects but also vascular anomalies, including VSD and SUA. Further support was provided by data from Nora and Nora (1975) in which 14 of their 15 reported patients had CHD. Shortly after, additional features, such as Cardiac malformations (more generally than just VSD) and Limb abnormalities, were formally included, and the condition was called VACTERL association (Quan and Smith, 1973; Nora and Nora, 1973; Tetamy and Miller, 1974; Nora and Nora, 1975; Khoury et al, 1983; Czeizel and Ludanyi, 1985; Rittler et al, 1996). Overall, a wide variety of CHDs have been described in VACTERL/VATER association, and are estimated to occur in approximately 40–80% of described cohorts, though diagnostic criteria and ascertainment methods admittedly differed greatly between case series (Solomon 2011).
Statistical analysis in the late 1990s and early 2000s of larger cohorts suggested less evidence for the inclusion of cardiovascular and renal anomalies in the association (Kallen et al, 2001; Botto et al, 1997). In a study of 5,260 infants with multiple malformations ascertained through four large malformation monitoring programs, Kallen et al. did not find a higher association of cardiac defects in VATER association compared to infants with multiple malformations. They further suggested an association between EA and heart defects, as well as the combination of upper costovertebral defects, heart malformations, and pre-axial upper limb reduction defects (Kallen et al 2001).
The most widely accepted definition requires the presence of at least three component features. Some describe the presence of an anatomically-based upper and lower group of VACTERL/VATER-related anomalies, with cardiac defects in the upper and renal anomalies in the lower group (Kallen et al 2001). Others stress the importance of certain core features: TEF/EA or anorectal malformation (ARM), which must in this view be present to apply the term VACTERL/VATER association (Wijers et al 2010). A consistent requirement is the absence of clinical or laboratory-based evidence of an alternate diagnosis (Solomon 2011). Controversy still remains regarding the definition of VATER association and its expansion to VACTERL, the appropriate diagnostic criteria and the overall incidence (Solomon 2012).
While admitting these controversies, in order to further describe and quantify the type and severity of cardiac anomalies in this group of patients, we aimed to characterize the cardiac findings present in a cohort of patients with VACTERL association.
MATERIALS AND METHODS
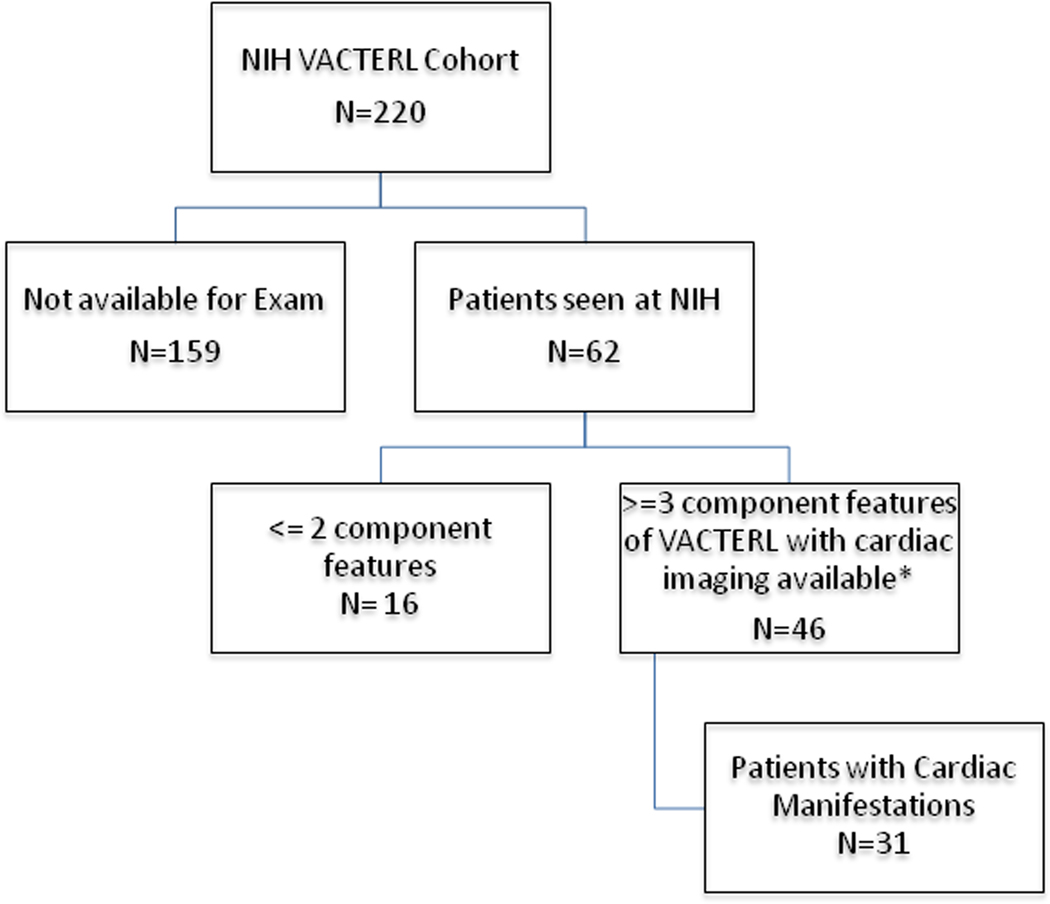
This study was conducted through our National Institutes of Health (NIH)/National Human Genome Research Institute IRB-approved protocol on VACTERL association, with appropriate consent obtained from all participants. For the purpose of this particular inquiry (focusing on cardiac findings), patients were included if they had at least three component features of VACTERL and had available cardiac imaging (echocardiogram) performed either in person at the NIH Clinical Center (along with the rest of their participation in this clinical and genetic study on VACTERL association) or prior imaging documenting a congenital heart defect(s). We defined VACTERL as having at least any 3 of the 6 component features without clinical or genetic evidence for an alternative diagnosis. Individuals were included as part of the cardiac cohort if they had CHD requiring surgical or catheter based intervention during infancy, or if they had CHD by history that closed spontaneously (for example a VSD or ASD however excluding an isolated patent foramen ovale or patent ductus arteriosus – see below). Patients were also excluded if they were unable to come to NIH for clinical exam and imaging, if they had less than 3 component features, if no cardiac imaging was available in the absence of a prior diagnosis of CHD, or they had clinical or laboratory-based evidence of an alternative diagnosis (Figure 1). We excluded minor variants to ensure strict inclusion criteria were met. For example renal anomalies were included if there was evidence of structural malformation; limb anomalies required structural changes such as radial ray malformations, limb length discrepancy, thenar hypoplasia or thumb malformations, and polydactaly. A persistent patent ductus arteriosus (PDA) was documented as present but was not included in as meeting criteria for a CHD if it spontaneously closed or was attributed to prematurity. In other words, no patients were diagnosed with CHD based solely on the presence of a self resolving PDA or PFO during the newborn period. Clinical evaluation included genetic/dysmorphologic physical examination and medical/family history (all by BDS), medical photography, echocardiogram, electrocardiogram, chest x-ray, x-rays of the entire spine and limbs, abdominal ultrasound, complete blood count, complete metabolic panel, urinalysis, and other testing (e.g., ophthalmologic examination, other imaging) as clinically indicated.
Figure 1:
*Patients also included based on history of CHD that required surgical or catheter based intervention in infancy, or if they had history of a congenital heart defect that closed spontaneously (excluding PFO, PDA).
All patients, with the exception of infants with surgically corrected congenital heart defects, were evaluated using standard 2D echocardiogram. Two adult patients received further cardiac imaging, one cardiac CT (computed tomography) and another with cardiac MRI (magnetic resonance imaging).
The molecular studies involved to attempt to discern the causes of VACTERL and related disorders currently involve high-density microarrays, candidate gene sequencing, and trio-based exome sequencing. None of the individuals described here have a known molecular etiology for their VACTERL association-type findings.
Statistical analysis was performed using Fisher’s exact test (2-sided) or chi-square analysis as appropriate. P-values < 0.05 were deemed statistically significant.
RESULTS
Forty-six patients (with at least 3 CFs of VACTERL and who had an echocardiogram performed) met criteria for analysis (Table 1), forty-four (96%) of these had either a TEF or ARM. There was no significant difference between males and females, (p=0.24).
Table 1.
GA (Gestational Age), Preterm (<37 weeks gestational age)
| N (number) | P value | ||
|---|---|---|---|
| All VACTERL | 46 | ||
| Males | 27 | 59% | 0.2382 |
| Females | 19 | 41% | |
| GA | |||
| Full term | 44 | 96% | |
| Preterm | 2 | 4% | |
| Pts with 3+ anomalies | 46 | ||
| Pts w/ TEF or ARM | 44 | 96% | |
| Pts w/o TEF or ARM | 2 | ||
| N (number) | P value | ||
| Cardiac Cohort | 31 | 67% | |
| Males | 18 | 58% | 0.3692 |
| Females | 13 | 42% | |
| GA | |||
| Full term | 30 | 97% | |
| Preterm | 1 | 3% | |
| Pts with 3+ anomalies | 31 | ||
| Pts w/ TEF or ARM | 28 | 90% | |
| Pts w/o TEF or ARM | 3 |
Thirty-one (67%) of these 46 patients had a CHD. There was no significant difference regarding gender in the overall cohort nor in the cardiac group (p=0.24, p=0.37, respectively). CHDs ranged from mild and subtle to severe and requiring multiple surgeries and chronic subspecialty care. All of the cohort with CHD had at least 3 CFs, however the majority had 4 or more features (81%), and 90% had either a TEF or ARM. Eighteen (58%) had TEF, 25 (81%) had ARM, and 14 (45%) had both (See Table 2 for details of CHD; see Supplementary table for full clinical details, including related to other organ systems).
Table 2.
Cardiac patients with VACTERL CF listed and details of cardiac malformation. ToF (Tetralogy of Fallot), VSD (ventricular septal defect), ASD (atrial septal defect), AP (aortopulmonary), s/p (status post), PDA (patent ductus arteriosus), PFO (patent foramen ovale), MPA (main pulmonary artery), AV (atrioventricular), PHTN (pulmonary hyptertension), DORV (double outlet right ventricle), HOL (hours of life), BAV (bicuspid aortic valve), AR (aortic regurgitation), SVC (superior vena cava), PLSVC (persistent superior vena cava), TV (tricuspid valve), TR (tricuspid regurgitation), RCA (right coronary artery), RA (right atrium), RV (right ventricle)
| Gender | Race | Component Features | Findings |
|---|---|---|---|
| F | H:W | VACTR | ToF s/p repair |
| F | N:I | VACTRL | History of VSD/ASD, now with aortic valve sclerosis and aortic regurgitation |
| F | N:W | VCL | Aortopulmonary window s/p repair |
| F | N:W | VACL | ToF s/p repair |
| F | N:W | VACRL | PDA |
| F | N:W | VCR | hypoplastic tranverse aortic arch |
| F | N:W | VAC | ASD, VSD, PFO; Most recently dilated coronary sinus/PLSVC |
| F | N:W | VACTRL | 2VSDs, 1ASD all s/p repair |
| F | N:W | VAC | PDA, ASD, VSD (now resolved) |
| F | N:W | VACTL | ASD/VSD, aortopexy ; now with mildly right dilated MPA, dilated coronary sinus, PLSVC |
| F | N:W | VACR | complete AV canal including VSD/ASD s/p surgery with residual VSD and mitral valve leak |
| F | N:W | VAC | VSD s/p repair, AV block requiring pacemaker |
| F | N:W | VCTR | ASD, VSD, PDA, BAV |
| M | N:M | ACTRL | ASD/VSD closed spontaneously |
| M | N:W | VACTL | VSD/PDA closed spontaneously; s/p aortopexy |
| M | N:W | VACRL | large VSD s/p repair, MR, mild TR; RA/RV dilation, mild PHTN, BAV with mild AR, thickened tricuspid leaflets with moderate to severe TR |
| M | N:W | VACTL | VSD and DORV s/p repair |
| M | N:W | ACTR | DORV and conotruncal septal defect, aortic pulmonary window and aberant left subclavian |
| M | N:W | VCTR | mild restrictive VSD |
| M | N:W | ACTL | VSD s/p spontaneous closure |
| M | N:W | VACRL | Bilateral SVC, Vascular ring s/p surgery @ 5 mos - now with dilated coronary sinus and PLSVC |
| M | N:W | VCTRL | dilated aortic root |
| M | N:W | VACT | 2VSDs surgically closed, ASD/PDA/PFO, mild TR, PLSVC draining into the coronary sinus |
| M | N:W | VACR | mesocardia; Dilated coronary sinus due to persistent left superior vena cava; PFO |
| M | N:W | VACR | BAV, small aortic arch, PLSVC, single anomalous RCA |
| M | N:W | VCR | VSD |
| M | N:W | VACTRL | large VSD, fenestrated ASD and AP window all s/p surgery; BAV, 2 coronary sinuses, mild pulmonary stenosis, history of dysarrhythmias, |
| M | N:W | VACR | VSD, ASD closed spontaneously, mild aortic coarctation - mildly dilated RV, BAV, PFO |
| M | N:W | VACTL | DORV with VSD |
| M | N:W | VACTRL | dilated coronary sinus |
| M | N:W | ACTR | DORV w/ TOF features |
The most common cardiac defect was a VSD, present in 18 of 31 patients (58%). The overwhelming majority had multiple cardiac anomalies including the presence multiple septal defects, patent ductus arteriosus, valvular anomaly or insufficiency. Those with more severe CHD were, as expected, diagnosed in infancy. Severe defects included Tetralogy of Fallot, Double Outlet Right Ventricle, Atrioventricular Canal defect, Aorto-Pulmonary Window, and a Vascular Ring. In total, 14 (45%) patients required surgical or other invasive cardiac interventions. One individual without a known CHD had a new cardiac diagnosis discovered through the NIH echocardiogram, consisting of a Persistent Left Superior Vena Cava (PLSVC) with dilated coronary sinus. This patient underwent cardiac MRI to further characterize these findings. Of the individuals with known cardiac anomalies several had previously undocumented findings or findings unknown to the patients, including bicuspid aortic valve and mesocardia. For a full list of patients and their associated CHD, see Supplemental Table 1a.
Twenty-eight (90%) of the 34 patients with CHD had either an associated TEF or ARM. Twenty-five (74%) had an ARM (11 with ARM and no associated TEF) , 18 (53%)had a TEF (4 with TEF and no associated ARM), and 14 (41%)) had both an ARM and TEF. We found no statistically significant relationship between the severity of the CHD and the presence a TEF (p=.72), nor did we find that patients with a TEF were more likely to have a cardiac malformation (see below).
There was no statistically significant association between CHD severity and the presence or absence of other VACTERL CFs, specifically ARM (p=0.18) or as above TEF (p~72). TEF or ARMs were not more common in patients with severe (defined as requiring surgical or catheter based intervention) CHD than in the overall cardiac cohort. There was no difference between VACTERL patients with or without CHD when examining the co-existing diagnosis of either ARM (p=.93) or TEF (p=0.76). In other words, CHD presence did not correlate with the presence of TEF or ARM.
DISCUSSION
Cardiac development begins early in embryogenesis with the induction of cardiogenic cells from the embryonic mesoderm, followed by the formation of a linear heart tube, which realigns and undergoes further development into a four-chambered heart. Underlying these changes are several key signaling pathways, forming a complex program of communication between cells and tissues in order to orchestrate their participation in heart development. Among pathways potentially involved in VACTERL pathogenesis and cardiac development are the NOTCH, FGF and Hedgehog signaling networks (Wagner and Siddiqui, 2007; Rochais et al, 2009;Stevenson and Hunter, 2013). Satisfying insight into the developmental biology of cardiac development is far from complete, and remains one of the major research goals in cardiovascular biology because of the potential use of certain therapeutic techniques (e.g., stem cells).
Among congenital anomalies, CHD is the leading cause of infant mortality (Rosa et al, 2012). Non-lethal CHD can result in chronic disability, morbidity, and healthcare costs. A 2005 study including individuals with private insurance estimated medical care costs for an infant with CHD to be nearly $100,000;costs were higher in severe CHD (Boulet et al, 2010). In a population-based study from Atlanta, CHD is estimated to affect 8.1/1000 live births (Reller et al, 2008). In line with our data, the most common CHD found in the Atlanta-based study were VSDs, followed by atrial septal defects (ASDs) (Reller et al 2008). The overall prevalence of CHD is higher in our VACTERL cohort than in the Atlanta CHD cohort. VSDs occurred in 58% of our patients vs. 42% of the Atlanta group. ASDs accounted for 32% of CHD in our VACTERL cohort vs. 13% of the Atlanta CHD cohort. More specific comparisons are limited due to the differences in cohorts and study designs and may be affected by our small sample size. Though theoretically large enough (with p <0.05 and power of 80%) to detect a difference between overall CHD prevalence in the general population vs. our cohort, further specific comparisons might not be adequately powered. For example, our cohort would need to include at least 75 patients for a comparison of VSD prevalence vs. the general population (though only 31 for a comparison of ASD prevalence).
In our study we were able to clinically evaluate and characterize a cohort of VACTERL patients. Our cohort, similar to that of previous reports, CHDs are present in more than 50% of VACTERL patients (though this clearly allows circular logic – see the section on bias below) (Solomon 2011). The severity of CHDs ranged from self-resolving and/or clinically insignificant findings to critical congenital heart disease requiring surgical or catheter based interventions. Unsurprisingly, those with more severe disease were diagnosed in infancy; however a number of patients with known diagnosis of VACTERL association presented to NIH with no prior cardiac evaluation and were found to have abnormal echocardiograms.
Several patients had minor cardiac defects discovered during our study, including a PLSVC and a bicuspid aortic valve. PLSVC is the most common congenital venous anomaly of the thoracic systemic venous return, with an estimated prevalence of approximately 0.3 to 0.5% in the general population and up to 12% of individuals with other forms of CHD, mainly ASD and/or VSD, followed by coarctation of the aorta, transposition of the great vessels, tetralogy of Fallot and anomalous connections of the pulmonary veins. As PLSVC is typically asymptomatic, therefore its true incidence may be underreported and its clinical significance remains poorly described. The most frequently associated extra-cardiac finding with PLSVC is EA. Recent literature details the impact of PLSVC on interventional cardiologists and radiologists, cardiothoracic surgeons, critical care physicians and other physicians who are required to place central venous catheters.. Several case reports have described more critical complications associated with PLSVC such as arrhythmias, cardiogenic shock, thrombosis and cardiac tamponade when pacemaker leads have been inserted via the PLSVC. While these complications are relatively rare, the presence of a PLSVC or dilated coronary sinus should prompt providers to look for other forms of CHD and consider right-sided central venous access (Goyal et al, 2008; Povoski et al, 2011).
Bicuspid aortic valve (BAV) is the most common congenital cardiac abnormality, with an estimated prevalence of 1–2% of the general population. It is commonly associated with aortic arch and root abnormalities, and is believed to have a heritable component, with a 10% chance of a first-degree relative also having a BAV. To date the strongest genetic link involves the NOTCH1 gene and NOTCH signaling pathway. Recent guidance from the American Heart Association and American College of Cardiology recommends that all patients with a first degree relative with BAV be screened for BAV and associated aortopathy. Recent cohort studies (Michelina et al 2008) have highlighted its clinical importance and the high rate of progression to aortic sclerosis, which may eventually require surgical correction (Bonow et al 2006).
Unlike earlier studies, we found no gender difference in our cohort of VACTERL patients with regard to the overall cohort and to the group with CHDs. This lack of a gender difference in our cohort is different from data published by Kallen et al, which suggesteda male predominance in VACTERL patients. Their study also described a primary association between cardiac defects and esophageal atresia, termed “syndrome cardio-oseophagien” (Kallen et al 2001; Worms et al, 1978). We did not find this association, but our inclusion criteria may have resulted in a different population.
The strengths of our study include a cohort of patients who were each examined in person by the same clinical geneticist and through the same study modalities. We were thus perhaps better able to exclude individuals with underlying disease processes that resulted in their phenotypic features similar to VACTERL. We also used stringent inclusion criteria (number of CFs) to allow a slightly more homogeneous population even within an extremely clinically heterogeneous disorder.
One important weakness of this study is several types of selection bias. First, since we accepted the standard definition of VACTERL association as requiring 3 CFs (of which one could be CHD), estimating the prevalence of CHD in VACTERL association or attempting to analyze whether CHD should be included as part of VACTERL is clearly specious. Thus, our goal was simply to describe the range of CHD in VACTERL after accepting the premise that CHD are part of VACTERL association. Second, our patients primarily represent a highly-motivated group of individuals who seek studies on rare disorders and who have the ability to take time for study participation. Given their interest in furthering research and knowledge regarding VACTERL, one might hypothesize that they are more severely affected, thereby over-representing the prevalence of the associated CFs. Conversely, this study by nature only includes participants who survive the neonatal period and who are stable enough for travel and study participation, which may lead to a more mildly-affected cohort. Third, we excluded patients who did not have a full cardiac evaluation to include an echocardiogram. This may have increased our selection bias, falsely elevating the incidence of CHD in the entire cohort. However, echocardiogram is now considered a standard part of the initial evaluation of patients suspected of having VACTERL association or other midline defects. We attempted to limit this bias by inviting all interested VACTERL patients to come to NIH for evaluation (which included a standard 2D echocardiogram) and including them in the overall cohort if the had any 3 of the component features. Last, for some individuals, we relied on reported history with regard to presence of specific congenital heart defects due to either inability to obtain imaging on day of visit, surgical correction, or spontaneous closure. This potential bias may have falsely increased the overall prevalence of cardiac defects in our cohort.
While this study does not, by design, provide further evidence towards the questions of whether CHD is a defining feature of VACTERL association, the frequency of CHD in our cohort does lend support to it being an important medical consideration in patients with VACTERL association. Further, we found no statistically significant association between the different malformations making it difficult to predict which patients may have cardiac disease. Based on our experience, we strongly recommend a screening echocardiogram to evaluate for CHD in individuals with a potential diagnosis of VACTERL association.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported (in part) by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. Pertaining to Dr. Cunningham, the views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, nor the US Government. Dr. Solomon would like to thank Dr. Max Muenke for his support and mentorship. All of the authors would like to express their deepest gratitude to the patients and families who participate in their research on VACTERL association and related disorders.
REFERENCES
- Amorim LF, Pires CA, Campos AS, Aguiar RA, Tiburcio JD, Siqueira AL, Mota CC, Aguiar MJ. 2008. Presentation of Congenital Heart Disease diagnosed at birth: analysis of 29,770 infants. J Pediatr (Rio J) 84:83–90 [DOI] [PubMed] [Google Scholar]
- Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Page RL, Riegel B. 2006. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 114:e84–e231. [DOI] [PubMed] [Google Scholar]
- Botto LD, Khoury MJ, Mastroiacovo P, Castilla EE, Moore CA, Skjaerven R, Mutchinick EM, Borman B, Cocchi G, Czeizel AE, Goujard J, Irgens LM, Lancaster PA, Martinez-Frias ML, Merlob P, Ruusinen A, Stoll C, Sumiyoshi Y. 1997. The spectrum of congenital anomalies of the VATER association: An international study. Am J Med Genet 71:8–15 [DOI] [PubMed] [Google Scholar]
- Boulet SL, Gross SD, Riehle-Colarusso T, Correa-Villasenor A. 20102. Health care costs of congenital heart disease. In: Wyszynski DF, Correa-Villasenor A, Graham TP, editors. Congenital Heart Defects: From Origin to Treatment. New York: Oxford University Press, p 493–501 [Google Scholar]
- Czeizel A, Ludanyi I. 1985. An aetiological study of the VACTERL-association. Eur J Pediatr 144(4):331–337 [DOI] [PubMed] [Google Scholar]
- Goyal SK, Punnam SR, Verma G, Ruberg FL. 2008. Persistent left superior vena cava: a case report and review of literature. Cardiovasc Ultrasound 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen K, Mastroiacovo P, Castilla EE, Robert E, Kallen B. 2001. VATER Non-random association of congenital malformations: Study based on data from four malformation registers. Am J Med Genet 101:26–32 [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Cordero JF, Greenberg F, James LM, Erickson JD. 1983. A population study of the VACTERL association: evidence for its etiologic heterogeneity. Pediatrics 71(5):815–820 [PubMed] [Google Scholar]
- Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, Pellikka PA, Tajik AJ, Enriquez-Sarano M. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 117(21):2776–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora AH, Nora JJ. 1975. A syndrome of multiple congenital anomalies associated with teratogenic exposure. Arch Environ Health 30:17–21 [DOI] [PubMed] [Google Scholar]
- Nora JJ, Norah AH. 1973. Birth defects and oral contraceptives. Lancet 1:941–942 [DOI] [PubMed] [Google Scholar]
- Povoski SP, Khabiri H. 2011. Persistent left superior vena cava: Review of the literature, clinical implications, and relevance of alterations in thoracic central venous anatomy as pertaining to the general principles of central venous access device placement and venography in cancer patients. World J Surg Oncol 9:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan L, Smith DW. 1973. The VATER association. Vetebral defects, Anal atresia, T-E fistula with esophageal atresia, Radial and Renal dysplasia: A spectrum of associated defects. J Pediatr 82(1):104–107 [DOI] [PubMed] [Google Scholar]
- Reller MD, Strickland MJ, Riegle-Colarusso T, Mahle WT, Correa A. 2008. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr 153(6):807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittler M, Paz JE, Castilla EE. 1996. VACTERL association, epidemiologic definition and delineation. Am J Med Genetic 63(4):529–536 [DOI] [PubMed] [Google Scholar]
- Rochais F, Mesbach K, Kelly RG. 2009. Signaling pathways controlling second heart field development. Circ Res 104:933–942 [DOI] [PubMed] [Google Scholar]
- Robinson A, Linden MG. Clinical genetics handbook. Boston, USA: Blackwell Science; 1993. [Google Scholar]
- Rosa RC, Rosa RF, Zen PR, Paskulin GA. 2012. Congenital heart defects and extracardiac malformations. Rev Paul Pediatr 31(2):243–251 [DOI] [PubMed] [Google Scholar]
- Solomon BD. 2011. VACTERL/VATER association. Orphanet J Rare Dis 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Bear KA, Kimonis V, de Klein A, Scott DA, Shaw-Smith C, Tibboel D, Reutter H, Giampietro PF. 2012. Clinical Geneticists’ views of VACTERL/VATER association. Am J Med Genet Part A 158A:3087–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RE, Hunter AG. 2013. Considering the embryopathogenesis of VACTERL association. 4(1–2):7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetamy SA, Miller JD. 1974. Extending the scope of the VATER association: Definition of the VATER syndrome. J Pediatr 85(3): 345–349 [DOI] [PubMed] [Google Scholar]
- Wagner M, Siddiqui MA. 2007. Signal transduction in early heart development (I): Cardiogenic Induction and Heart Tube Formation. Exp Biol Med 232(7):852–865. Review. [PubMed] [Google Scholar]
- Wijers CH, de Blaauw I, Marcelis CL, Wihnen RM, Brunner H, Midrio P, Gamba P, Clementi M, Jenetzky E, Zwink M, Reutter H, Bartels E, Grasshoff-Derr S, Holland-Cunz S, Hosie S, Marzheuser S, Schmiedeke E, Cretolle C, Sarnacki S, Levitt MA, Knoers NV, Roeleveld N, van Rooij IA. Research perspectives in the etiology of congenital anorectal malformations using data of the International Consortium on Anorectal Malformations: evidence for risk factors across different populations. Pediatr Surg Int 2010. 26:1093–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worms A-M, Beley G, Couronne A-M, Prevot J, Pernot C. 1978. Syndromes “cardio-oesophagiens” confenitaux. Arch F Pediatr 35:863–869 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.