Abstract
The locus coeruleus (LC) is a small noradrenergic brainstem nucleus that plays a central role in regulating arousal, attention, and performance. In the mammalian brain, individual LC neurons make divergent axonal projections to different brain regions, which are distinguished in part by which noradrenaline (NA) receptor subtypes they express. Here, we sought to determine whether similar organizational features characterize LC projections to cortico-basal ganglia (CBG) circuitry in the zebra finch song system, with a focus on the basal ganglia nucleus Area X, the thalamic nucleus DLM, as well as the cortical nuclei HVC, LMAN, and RA. Single and dual retrograde tracer injections reveal that single LC-NA neurons make divergent projections to LMAN and Area X, as well as to the dopaminergic VTA/SNc complex which innervates this CBG circuit. Moreover, in situ hybridization revealed that differential expression of mRNA encoding and adrenoreceptors distinguishes LC-recipient CBG song nuclei. Therefore, LC - NA signaling in the zebra finch CBG circuit employs a similar strategy as in mammals, which could allow a relatively small number of LC neurons to exert widespread yet distinct effects across multiple brain regions.
Keywords: Locus Coeruleus, Songbird, Noradrenaline, Zebra Finch
Introduction
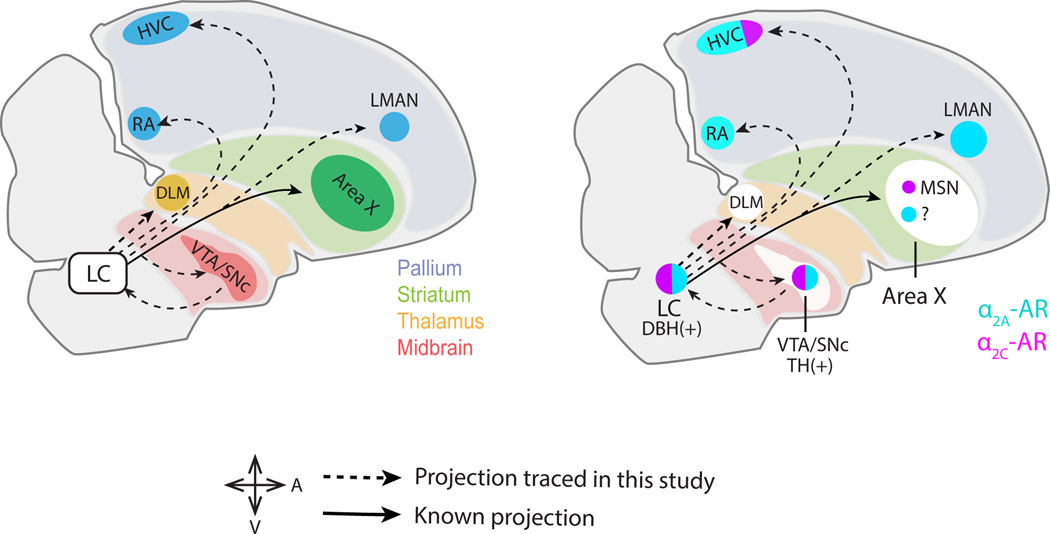
Noradrenergic signaling in the brains of vertebrates is an important regulator of arousal, attention, and performance (Poe et al. 2020; Sara and Bouret 2012). Although noradrenaline (NA) release occurs across the entire brain, the cell bodies of almost all NA-releasing neurons are located in one relatively small pontine nucleus, the locus coeruleus (LC). Thus, one important question is how this small population of NA-releasing neurons exerts a brain-wide influence. Viral-genetic tracing studies in mice revealed that individual LC neurons extend axons that terminate in a large number of different forebrain targets, and this highly divergent architecture is speculated to underlie LC-mediated control of brain states (Schwarz et al. 2015). Another key organizational feature of NA systems in the mouse is the heterogeneous expression patterns of NA receptor subtypes, which allows the LC to exert different effects in different brain regions (Szabadi 2013). Here we sought to explore whether similar organizational features - divergent projections of single LC neurons and heterogeneous NA receptor expression patterns - also characterize how the LC interacts with song-specialized cortico-basal ganglia (CBG) circuitry in the male zebra finch, a small Australian songbird.
Songbirds have proven extremely useful organisms in which to identify neural mechanisms that give rise to the learning and execution of complex vocal behaviors. Male zebra finches learn to sing by copying a tutor song, then use these learned vocalizations as an integral part of a courtship display to attract females. Importantly, adult male zebra finches sing more variable songs when alone but sing more stereotyped songs to females, and this increased stereotypy is more effective as a courtship signal (Woolley and Doupe 2008; Olveczky, Andalman, and Fee 2005). Thus, identifying the neural mechanisms that regulate song variability is important to understanding adult communication and the social context-dependent regulation of song.
Decades of research have delineated a network of brain nuclei that serve a special role in singing and song learning (Nottebohm, Stokes, and Leonard 1976). This song system includes a song motor pathway, which is obligatory for singing, and a song specialized CBG circuit, which comprises the lateral portion of the magnocellular nucleus of the anterior neostriatum (LMAN), the medial portion of the dorsolateral thalamus (DLM), and the basal ganglia region Area X. This CBG circuit is essential to song learning and is a major site where song variability is generated and regulated in adulthood (Olveczky, Andalman, and Fee 2005; Andalman and Fee 2009; Kao, Doupe, and Brainard 2005; Singh Alvarado et al. 2021; Kojima, Kao, and Doupe 2013; Kojima et al. 2018; Goldberg and Fee 2011). Notably, LMAN drives song variability through its connections to the song motor pathway, whereas NA-mediated suppression of Area X activity is a major means by which the adult male zebra finch achieves a highly stereotyped song performance to a nearby female (Singh Alvarado et al. 2021). Here we sought to address whether, in adult male zebra finches, individual LC neurons make divergent projections to LMAN and Area X. We hypothesize that these divergent projections could provide an efficient means for the LC to coordinately control CBG activity and thus regulate song variability.
Activation of the LC can exert differential effects depending on the brain region. In rodents, for example, LC activation leads to improvements in signal-to-noise in cortical regions (Aston-Jones, Rajkowski, and Cohen 2000; Cardin and Schmidt 2004; McBurney-Lin et al. 2019), increased activity correlations in the striatum (Zerbi et al. 2019), and increased excitability in the basolateral amygdala (Giustino et al. 2020). These observations raise the question of how the small numbers of LC neurons with highly divergent axonal projections exert disparate effects in different brain regions. One potential explanation is that different populations of LC-recipient neurons express distinct subtypes of NA receptors. Therefore, beyond establishing whether LC neurons make divergent projections to different CBG nuclei in the adult male zebra finch, we also sought to determine whether LMAN and the Area X express different subtypes of NA receptors. In fact, both and adrenergic receptors (ARs) are densely expressed in several song nuclei of the adult finch, with being more highly expressed in males than females (Riters and Ball 2002). Here, due to their Gi-coupled (inhibitory) signaling and dense expression in CBG regions in mice (Zhang et al. 1999), we focused on how receptor subtypes A and C (- and -AR) were distributed across song-specialized CBG circuitry.
A related issue is whether the LC communicates with neuromodulatory systems that also provide input to CBG circuitry. In fact, dopamine (DA) release from the ventral tegmental area and substantia nigra pars compacta (VTA/SNc) axons into the Area X is necessary for juvenile song copying as well as adult song plasticity (Hisey, Kearney, and Mooney 2018; Xiao et al. 2018), both of which rely on vocal variability to drive adaptive changes in song. Notably, activating NA receptors in the Area X of the adult male suppresses song variability (Singh Alvarado et al. 2021), raising the possibility that these two systems operate in a partially opposing manner. While evidence in rodents indicates that LC activity directly suppresses DA neurons in the VTA (Guiard et al. 2008), whether the VTA/SNc neurons in the finch receive LC input and express NA receptors is unknown. Thus, we also analyzed the anatomical organization of LC axon terminals and NA receptors in the VTA/SNc complex in the adult male zebra finch, while also characterizing the transmitter phenotypes of both LC neurons and VTA/SNc neurons that receive LC input. These studies indicate that individual LC neurons make divergent projections to LMAN and the Area X, that neurons in these two parts of the CBG circuit express different NA receptor subtypes, and that the LC and the VTA/SNc form a recurrent circuit in which LC is equipped to suppress the activity of DA-releasing neurons in the VTA/SNc.
Materials & Methods
Animals
Adult (≥90 days post-hatch) male zebra finches (n = 25) were obtained from the Duke University Medical Center breeding facility. All experimental procedures were in accordance with the NIH guidelines and approved by the Duke University Medical Center Animal Care and Use Committee
Stereotactic Injections
Male zebra finches were food deprived for 30 min and then anesthetized with 2% isoflurane gas before being placed on top of a small heating pad in a custom stereotaxic apparatus. Rate of breathing and stability of the surgical plane were monitored throughout surgery. The feathers over the skull were trimmed and topical anesthetic (0.25% bupivacaine) was applied before an incision was made in the skin from anterior to posterior with a scalpel. Craniotomies were made with a smaller scalpel at a predetermined distance from the bifurcation of the midsagittal sinus. See Table 1 for stereotactic coordinates relative to the midsagittal sinus. The appropriate depth of anatomical targets was confirmed via electrophysiological recording when available (Differential A-C Amplifier 1700, A-M Systems). To inject tracer, a pressure-based system (Drummond Nanoject II) was used. The injections were either a fluorescent dextran amine or a recombinant cholera toxin subunit B tracer: (Dextran-405; CTB-488, CTB-594, CTB-647, Invitrogen #C22841, #C34777, #C34778). We chose our injection volume based on the size, accessibility, and proximity to other structures. Smaller and more ventral targets such as the LC, VTA, LMAN, and DLM received 25 nl of tracer. Larger targets such as Area-X, RA, and HVC received a total injection volume of 45nl in 15nl increments. Injections were spaced at 5-minute intervals to avoid backflow along the pipette. After these injections, the incision site was closed with tissue adhesive, and the bird was allowed to recover from anesthesia under a heat lamp.
Table 1.
Stereotaxic coordinates and birds used.
| Target | AP (mm) | ML (mm) | DV (mm) | Beak Angle (°) | Successfully targeted injections |
|---|---|---|---|---|---|
| Area X | 5.1, then 1.8–2.0 | 1.2–1.6 | 2.6–2.9 | 43, then 72 | 4 / 6 |
| DLM | 1.75 | 1.2–1.4 | 4.1–4.4 | 37 | 2 / 6 |
| HVC | 0.4 | 2.5 | 0.15–0.3 | 45 | 3 / 7 |
| LC | −0.3 | 0.9–1.0 | 5.6–5.9 | 28 | 2 / 9 |
| LMAN | 4.2 | 2.0 | 1.7–1.75 | 50 | 3 / 9 |
| RA | −1.8 | 2.4 | 1.5 | 90 | 2 / 4 |
| VTA/SNc | 1.65 | 0.5–1.5 | 6.2 | 37 | 2 / 8 |
Antibodies and in situ hybridization probes
Anti Dopamine-beta-hydroxylase (ImmunoStar #22806, RRID: AB_2209751, Rabbit polyclonal) has previously been used in zebra finches to selectively label noradrenergic neurons in LC (Tanaka et al. 2018; Katic, Morohashi, and Yazaki-Sugiyama 2022) and its specificity has been confirmed by western blot (triplet at 72 kD, ImmunoStar), as well as by genetic deletion of DBH. HCR probes were designed based on the following mRNA targets: PPP1R1B (DARPP-32), accession number XM_030256189.2 Alpha-2-c adrenoreceptor (-AR), accession number JQ438775.1. Tyrosine hydroxylase, accession number XM_002198931.3. Alpha-2-a adrenoreceptor (-AR), accession number JQ438773.1. PPP1R1B and -AR have previously been used to label medium spiny neurons and quantify -AR expression (Singh Alvarado et al., 2021). No signal was observed in the absence of HCR probes or fluorescently tagged hairpins alone for any probe type. The TH probe was confirmed to label cell bodies in LC, VTA, and A11, and no cell bodies were labeled in the forebrain. Both -AR and -AR were densely expressed on LC-NA neurons, as has been also reported in rodents (Lee, Rosin, and Van Bockstaele 1998a, [b] 1998).
Immunohistochemistry
One week following these tracer injections, birds were deeply anesthetized with an intraperitoneal injection of pentobarbital solution (Euthasol) and then perfused through the heart with 0.025 M phosphate-buffered saline followed by 4% paraformaldehyde. The brain was then removed from the skull and post-fixed in a cryoprotective formalin sucrose solution (30% sucrose in 4% paraformaldehyde) for 48 hours. The next day consecutive sagittal sections of the cryoprotected brain were cut on a freezing microtome. Slides were washed 3 × 5 minutes in PBS plus 0.025% Triton X-100 with gentle agitation. A subset of sections were treated with a primary antibody overnight at 4 °C in PBS with 1% BSA. Sections were washed again in PBS plus 0.025% Triton X-100 3 × 5 minutes. Sections were then reacted with either anti tyrosine-hydroxylase or anti dopamine-beta-hydroxylase secondary antibody (, 1:1,000, Abcam112; , 1:500, Immunostar #22806) RT (20–25° C) for 1 h at RT in PBS, followed by three 5-minute washes in PBS and mounting onto slides. Sections were imaged with confocal microscope (710 LSM; Zeiss) after coverslipping with Fluoromount-G (SouthernBiotech).
In-situ Hybridization
Brain sections were processed as described above, but PBS was replaced with RNAse-free PBS (i.e, DEPC-PBS), and a 20% RT paraformaldehyde fixation step was performed before mounting. In situ hybridization was performed using hybridization chain reaction (HCR v3.0, Molecular Instruments). Dissected brain samples were post-fixed overnight in 4% PFA at 4 °C, cryoprotected in a 30% sucrose solution in RNAse-free PBS (i.e., DEPC-PBS) at 4 °C for 48 hours, frozen in Tissue-Tek O.C.T. Compound (Sakura), and stored at –80 °C until sectioning. 80-μm thick coronal floating sections were collected into a sterile 24 well plate in DEPC- PBS, fixed again briefly for 5 min. in 4% PFA, then placed in 70% EtOH in DEPC-PBS overnight. Sections were rinsed in DEPC-PBS, incubated for 45 min in 5% SDS in DEPC-PBS, rinsed and incubated in 2x SSCT, pre-incubated in HCR hybridization buffer at 37 °C, and then placed in HCR hybridization buffer containing RNA probes (PPP1R1B / DARPP-32, SLC17A6 / VGLUT2, SLC32A1 / VGAT, TH (custom probes designed by Molecular Technologies)) overnight at 37 °C. The next day, sections were rinsed 4 × 15 minutes at 37 °C in HCR probe wash buffer, twice in 2X SSCT, pre-incubated with HCR amplification buffer, then incubated in HCR amplification buffer containing HCR amplifiers at room temperature for ~48 hours. On the final day, sections were rinsed twice with 2x SSCT, then mounted on slides and coverslipped with Fluoromount-G (Southern Biotech). After drying, slides were imaged on a Zeiss inverted 710 laser scanning confocal microscope (40x magnification).
Image Analysis
Confocal z-stacks were obtained with Leica SP8 710 inverted microscopes. Brightness and contrast were adjusted using ImageJ (Schindelin et al. 2012). ImageJ was used for all processing of z-stacks, including maximum intensity projections, adjustment of brightness and threshold, and changing of look-up tables (e.g., to convert red to magenta). To quantify the number and soma size of neurons, confocal z-stacks were first flattened to a single maximum intensity projection. This maximum intensity projection was then thresholded (otsu method, ImageJ). The image was then binarized, and a mask for the DBH or TH channel was applied.Labeling in the other channels (CTB/Dextran for retrogradely labeled cells) was then manually counted within the masked region. For punctate labeling (such as in the case of RA and DLM injection), we used the Puncta Analyzer plug-in for ImageJ v1.29 after background subtraction with a rolling ball radius of 50 and thresholding (Otsu, ImageJ). Any cell mask with 3 or more of these puncta was considered to be positive for CTB label. For quantifying axon terminal endings that contact soma (referred to as enwrapments in this paper), the DBH channel was maximum projected, and noise was removed with a despeckle filter so as to remove any puncta that could be attributed to background noise. After smoothing with a gaussian filter (3×3 pixels), any area of >8 μm2 were counted as enwrapments, and only 1 enwrapment was counted per neuron soma. We then applied a binary mask to the resulting smoothed enwrapments and counted TH, VGAT, or VGLUT-positive cells that colocalized with enwrapments.
Results
LC projections to the song system
The LC in zebra finches comprises a small number of cells (~1300 total across the two hemispheres, ~600 of which are noradrenergic (Waterman and Harding 2008)) that project extensively throughout the brain. We first investigated the anatomical connectivity of the LC to each nucleus in the CBG circuit (Area X, DLM, LMAN), and to the CBG’s major input and output nuclei in the song motor pathway (HVC (used as a proper name) and the robust nucleus of the arcopallium, RA). We injected small volumes (< 40 nl, see Materials and Methods) of the retrograde tracer cholera toxin subunit B into one of each of these nuclei in different adult (≥90 days post-hatch) male zebra finches (Figure 1), and investigated retrograde labeling within the LC’s boundaries, as defined by expression of the NA-synthetic enzyme dopamine beta hydroxylase (DBH). Injections into any one of these nuclei resulted in retrograde label in DBH-positive LC neurons (Figure 1; Fraction of labeled DBH+ cells: Area X, 73%, (236/324 DBH+ neurons, 6 hemispheres from 3 birds), LMAN, 78%, (215/270 neurons, 5 hemispheres from 3 birds) HVC, 51%, (160/315 neurons, 5 hemispheres from 3 birds) RA, 34%, (82/237 neurons, 3 hemispheres from 2 birds), DLM, 32%, (54/171 neurons, 3 hemispheres from 2 birds)). Even though retrograde labeling from our DLM injections was particularly weak, it was specific to the tracer color channel, suggesting these puncta were due to tracer uptake. Given our small injection volumes, we consider these results to underestimate the amount of retrogradely labeled LC DBH+ neurons, as we only included cases in which the CTB injection was primarily restricted to the target region, even if coverage was partial (see Table 1). These results identify previously undescribed projections from the LC to LMAN and DLM, while extending earlier studies in the canary that showed that the LC projects to HVC and RA (D. Appeltants et al. 2000; Didier Appeltants, Ball, and Balthazart 2002).
Fig 1. Retrograde tracing from various song nuclei.
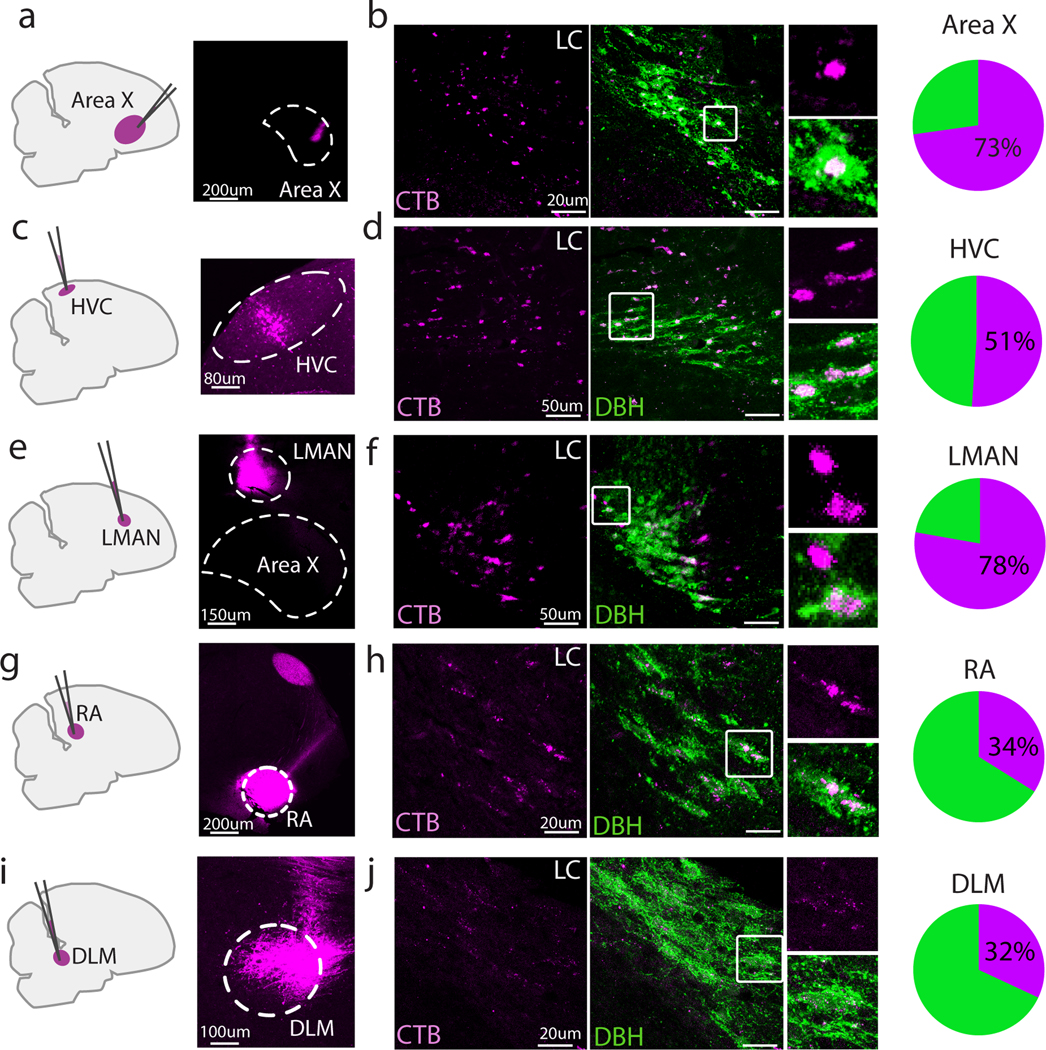
Sagittal section schematic of injection sites (left) and example injection sites (right) into (a) Area X, (c) HVC, (e) LMAN, (g) RA, and (i) DLM. (b), (d), (f), (h), (j): Left, Corresponding retrograde CTB labeling in LC (magenta); Middle, overlay with DBH (green); Right, zoomed in view of the boxed region in the middle panel; Far right, pie charts showing fraction of DBH+ cells labeled with CTB for each injected region.
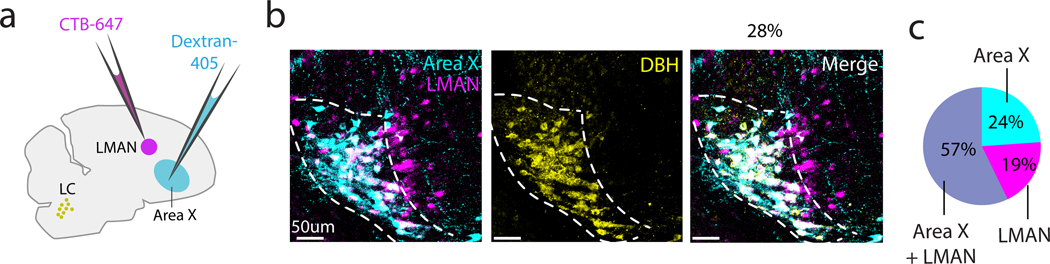
The one-to-many connectivity of LC neurons in mammals led us to ask whether single LC neurons target both Area X and LMAN, which are two major components of the CBG circuit. To answer this question, we injected the fluorescent tracers CTB-647 and dextran-405 into LMAN and Area X respectively and subsequently stained tissue sections containing LC for DBH (Figure 2a). We found that 96% (89/93 neurons, 4 hemispheres from 3 birds, Figure 2b, c) of DBH+ neurons were retrogradely labeled by either one or both tracers. Of these retrogradely labeled neurons, 57% were labeled by both tracers, 24% were labeled from Area X only, and 19% were labeled from LMAN only. Of note, regardless of injection target, we often observed a small number of retrogradely labeled neurons that were negative for DBH and were typically located anterior and dorsal to the DBH+ cells. Thus, a substantial fraction of noradrenergic LC neurons make divergent projections to LMAN and Area X.
Fig 2. Dual retrograde tracing from LMAN and Area X.
(a) Schematic showing CTB-647 (magenta) injection into LMAN, dextran-405 (cyan) injection into Area X, and resulting DBH+ cells in LC (yellow). (b) Left: Resulting retrograde label in LC (magenta, LMAN; cyan, Area X; white, double-labeled cells), Middle: DBH+ cells (yellow) in the same field of view. Right: Merged image, with white denoting triple-labeled cells. (c) Proportion of retrogradely labeled DBH+ cells that were labeled from injections in both LMAN and Area X (dual labeled, grey), only Area X (teal), or only LMAN (magenta).
Contrasting and noradrenergic receptor expression in LMAN and Area X
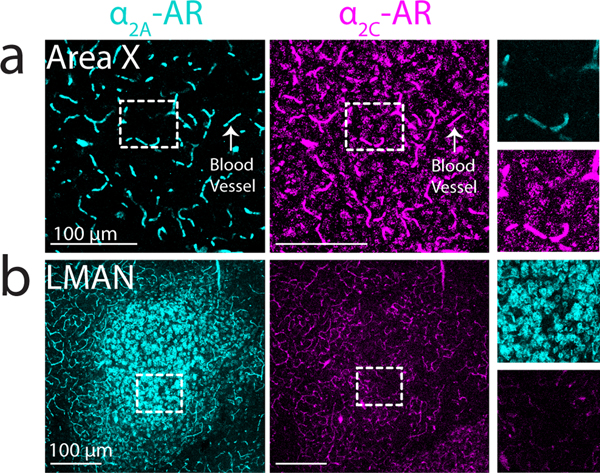
The divergent projections that individual LC neurons make to LMAN and Area X could exert similar or different effects in these two regions depending on the expression patterns of NA receptor subtypes (Szabadi 2013). To test this idea, we used in situ hybridization to examine the expression of mRNA encoding for and adrenergic receptor (-AR and -AR) subtypes in Area X and LMAN. These experiments revealed that -AR mRNA was expressed at high levels in Area X but at relatively low levels in LMAN, whereas -AR mRNA was expressed at high levels in LMAN but at low levels in Area X (Figure 3a, b). Additionally, mRNAs for both receptors were highly expressed on blood vessels across the brain, as previously observed in mammals (Sorriento, Trimarco, and Iaccarino 2011). Higher power images showed that within Area X, -AR but not -AR mRNA is expressed in medium spiny neurons (MSNs, 92.1% positive for -AR, 5.2% positive for -AR), which were identified by their expression of DARPP-32 (Figure 4). In contrast, a sparse population of DARPP-32 negative cells was enriched for -AR (Figure 4b, c). In LMAN, -AR mRNA was highly expressed in neurons with large somas (Figure 3b; median soma diameter of -AR+ cells, 20.6 ± 3.1 um, close to the previously reported size of excitatory LMAN projection neurons (Korsia and Bottjer 1989). Taken together, these results indicate that individual LC neurons send divergent projections to LMAN and Area X, and that neurons in these nuclei differentially express and ARs.
Fig 3. -AR and -AR mRNA expression in Area X and LMAN.
(a) Left: low magnification image showing expression of -AR on Area X cells (both receptors were also observed on blood vessels, arrow). Right: zoom-in of white boxed area. (b) Left: low magnification image showing dense expression of -AR on LMAN cells. Right: zoom-in of white boxed area.
Fig 4. -AR and -AR mRNA expression within Area X.
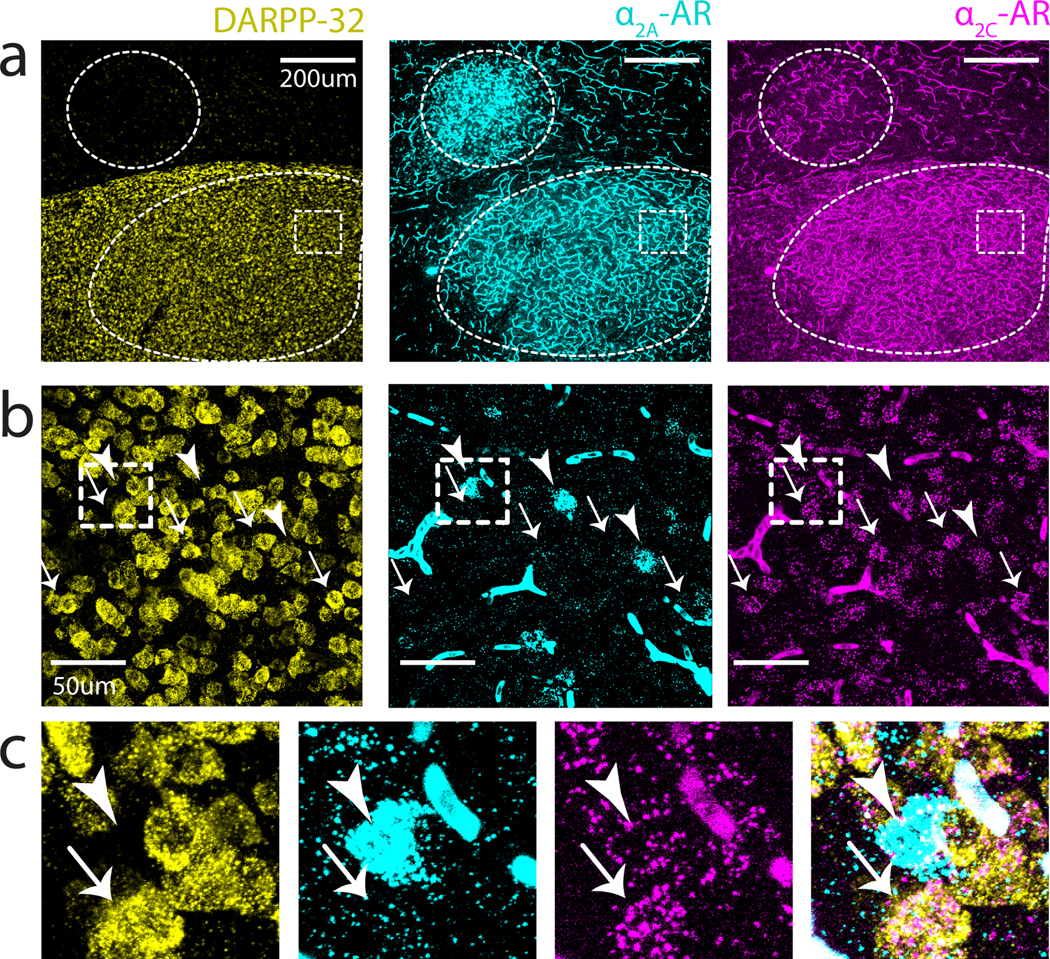
(a) Low power image showing boundaries of LMAN (top) and Area X (bottom) demarcated by dashed outlines. White dashed box depicts area magnified in (b). (b) Higher power image of boxed region in Area X from (a). Sparse clusters of -AR can be observed throughout Area X (arrowheads); cells positive for -AR show high colocalization with DARPP-32 (arrows). (c) Zoomed in view of the boxed region in (b) showing that + cells are largely non-overlapping with DARPP-32(+).
Expression of and adrenergic receptors in VTA/SNc and the LC
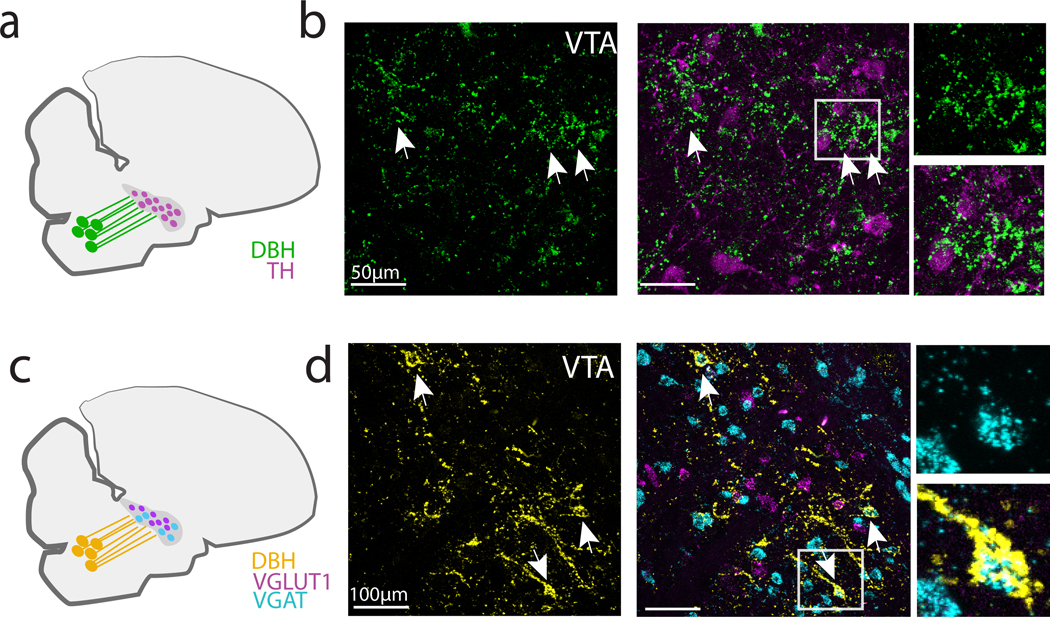
Notably, our in situ hybridization experiments also revealed high expression of -AR and -AR within the VTA/SNc complex, which is a major source of dopamine (DA) input to both Area X and LMAN. To better localize the cell types in the VTA/SNc that express ARs, we added a third in situ probe for the enzyme tyrosine hydroxylase (TH), a marker of catecholaminergic neurons that labels NA-producing neurons in the LC and DA-producing neurons in the VTA. We found that the majority (71%, 160 out of 225, N = 4 hemispheres, 2 birds) of TH+ neurons in the VTA/SNc expressed both and AR mRNA (Figure 5a). Additionally, mRNAs for both and ARs were highly expressed in TH+ LC neurons, indicating that ARs could function as autoreceptors to regulate LC activity (Figure 5b).
Fig 5. -AR and -AR mRNA expression in VTA and LC neurons.
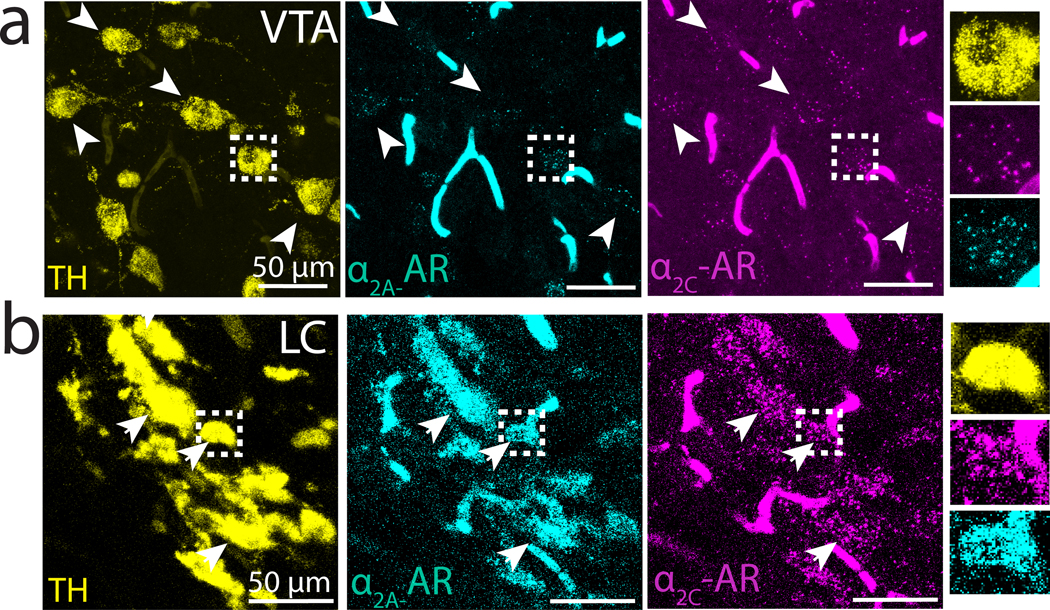
(a) TH+ neurons in VTA (yellow, left) and expression of (cyan, middle) and (magenta, right) mRNA. Arrowheads indicate location of example -AR+ and -AR+ VTA neurons. Rightmost column: magnification of a single cell in boxed region. (b) Similar to (a) but showing -AR and -AR mRNA expression in TH+ neurons in LC.
Anatomical projections between LC and VTA/SNc
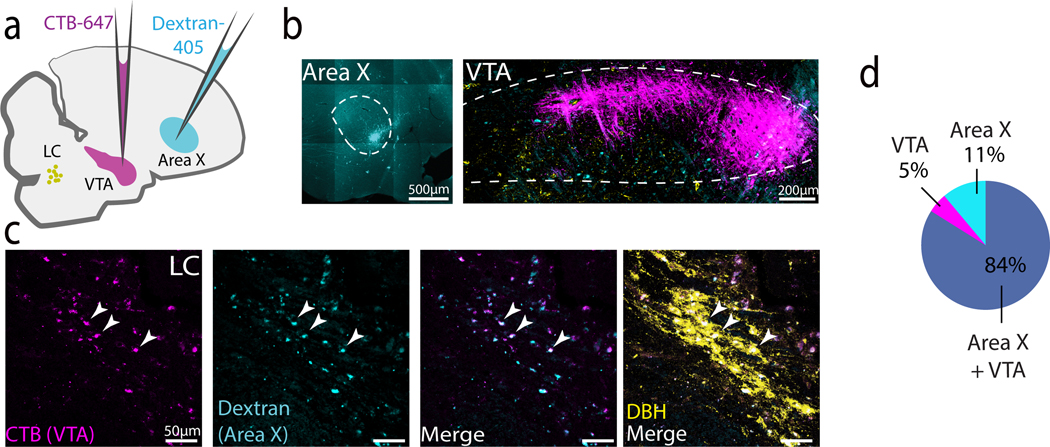
The NA receptor expression patterns that we detected in the VTA/SNc suggest that the LC provides input to these midbrain DA cell groups. Because the VTA/SNc projects to both Area X and LMAN (Bottjer 1993; Person et al. 2008), such an arrangement would enable the LC to exert both direct and indirect effects on the CBG. To test whether the LC is a source of noradrenergic input to the VTA/SNc, we stereotaxically injected the retrograde tracer cholera-toxin B (CTB) into the VTA/SNc complex of adult male zebra finches. Tracer injections that were largely restricted to the VTA/SNc resulted in retrograde labeling in the majority of DBH+ LC cell bodies (80 out of 98 DBH+ neurons, N = 4, hemispheres, 2 birds, Figure 6a-c). Additionally, we consistently observed a strip of retrogradely labeled DBH- neurons dorsal and rostral to the retrogradely-labeled TH+ cells in the LC (Figure 6c, left and middle panels). Thus, the VTA/SNc receives input from DBH+ cells in the LC, as well as from a small population of DBH- cells.
Fig 6. Projections between LC and VTA/SNc.
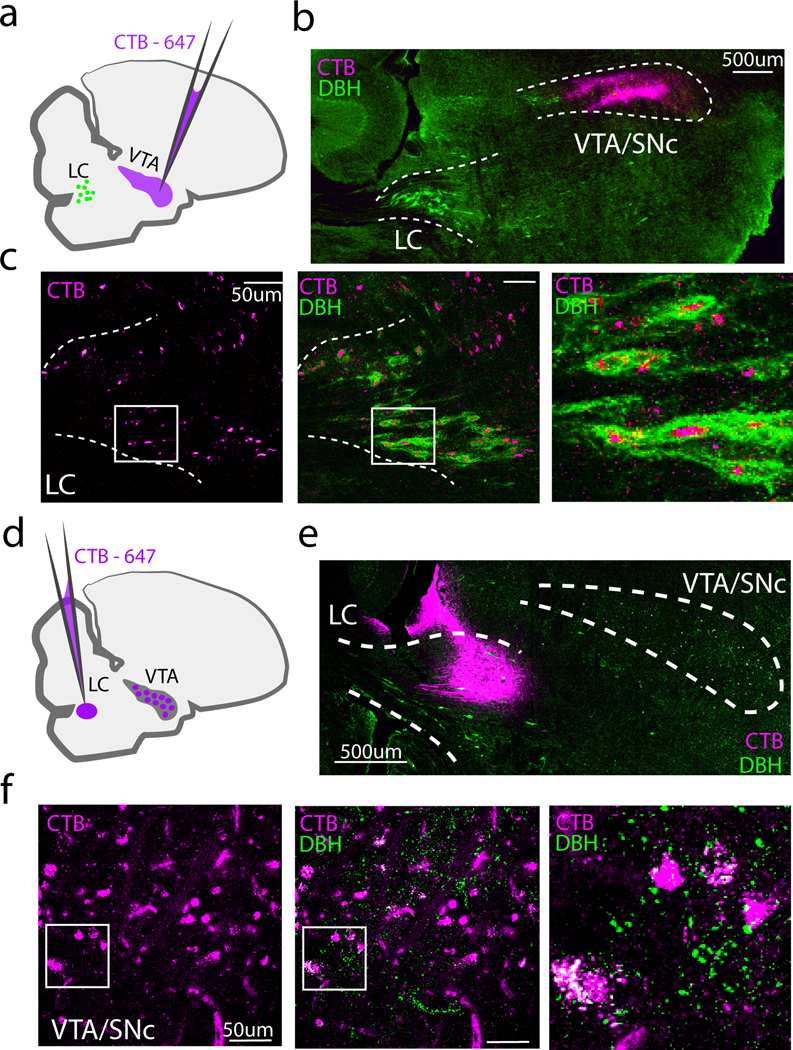
(a) Schematic showing approach for retrograde labeling with CTB-647 (magenta) of DBH+ LC neurons (green) that provide input to the VTA/SNc. A small (10–20nl) injection of CTB-647 was targeted to VTA/SNc. (b) Low power image showing CTB injection in the VTA/SNc. (c) Left: Retrogradely labeled cell bodies (magenta) in LC, middle: merge with DBH immunostaining (green). Right: Zoomed in view of the boxed region in the middle panel. (d) Experimental approach for retrograde labeling of VTA/SNc cells that provide input to the LC. (e) Low power image showing CTB-647 injection in LC and approximate boundaries of the VTA/SNc. (f) Higher power images showing retrogradely labeled cells in VTA/SNc. Left: Retrogradely labeled cell bodies from the CTB-647 injected in LC. Middle: merge with DBH+ fibers in VTA/SNc. Right: Zoomed in view of the boxed region in the middle panel.
We next tested whether the VTA projects to the LC in the adult male zebra finch, as has been shown in mammals (Deutch, Goldstein, and Roth 1986). To avoid lowering our injection pipette through the VTA, we approached the LC through the cerebellum, crossing through the fourth ventricle (See Table 1 and Fig 6d). For birds in which CTB was predominantly limited to the DBH+ boundaries of the LC (N = 2 out of 9), we observed retrogradely labeled cells in the VTA/SNc that overlapped with DBH+ fibers (Figure 6d-f). Taken together, these retrograde tracing experiments indicate that the VTA/SNc and LC are reciprocally connected.
A subset of LC cells that project to the VTA also project to Area X
We next investigated whether individual LC neurons make divergent projections to Area X and the VTA/SNc. Tracer injections of Dextran-405 made in the Area X and CTB in the VTA/SNc resulted in single or double labeling in 82% (96/116) of DBH+ neurons (N = 4 hemispheres, 2 birds, Figure 7a-d). Of these retrogradely labeled neurons, 84% were double labeled, 5% were labeled only from VTA/SNc, and 11% only from Area X. These results indicate that a substantial number of noradrenergic LC neurons project to both the VTA/SNc and Area X.
Fig 7. Individual LC NA neurons project to both VTA/SNc and Area X.
(a) Experimental approach for dual labeling from Area X (Dextran-405, cyan) and VTA/SNc (CTB-647, magenta). LC cell bodies depicted in yellow. (b) Injection sites in Area X and VTA/SNc. (c) High power image of retrograde labeling in LC. From left to right: CTB-647 from VTA/SNc, Dextran-405 from Area X, merge, and overlay with DBH. (d) Proportion of retrogradely labeled DBH+ cells that showed dual labeling (grey), or single label from injections in Area X (teal) or VTA (magenta).
Noradrenergic fibers in VTA/SNc preferentially form appositions with VGAT+ cells.
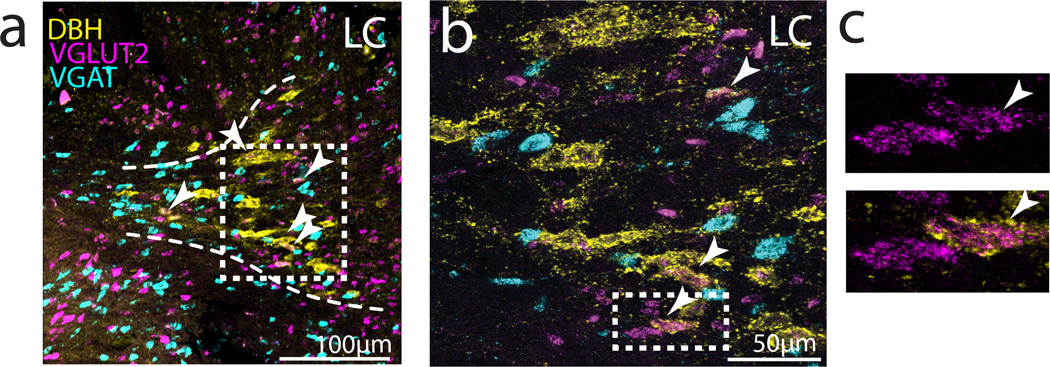
Upon closer investigation, we also noticed that DBH axons formed dense appositions, or enwrapments, around cell bodies within the VTA/SNc complex, especially around TH-negative cell bodies (Figure 8a, b). To better characterize which cells in the VTA/SNc complex were targeted by these DBH+ enwrapments, we performed in situ hybridization for vesicular glutamate and GABA transporters (VGLUT2 and VGAT) and TH, to identify glutamatergic, GABAergic and dopaminergic neurons, respectively, followed by immunohistochemistry to label DBH+ axons in the VTA/SNc region. We found that only 14% of these DBH+ enwrapments occurred near TH+ neurons (3/21 DBH+ enwrapments, n = 4 hemispheres, 2 birds, Figure 8a, b). In contrast, the majority (78%) of DBH+ enwrapments were juxtaposed to VGAT+ cell bodies, with only a small minority (~10%) occurring near VGLUT2+ neurons (32/42 enwrapments on VGAT+ cells, 4/42 onto VGLUT+ cells, n = 4 hemispheres, 2 birds, Figure 8c, d). These results suggest that LC axons preferentially synapse on GABAergic cells of the VTA/SNc complex.
Fig 8. DBH+ terminals form perisomatic appositions onto VGAT+ neurons in VTA/SNc.
(a) DBH+ terminals in VTA/SNc were visualized in combination with TH (b) Left: High power images of VTA/SNc showing DBH terminals (green) and enwrapments (arrowheads). Middle: DBH+ terminals and in situ labeling for TH mRNA. Right: Magnification of boxed region showing enwrapments near TH mRNA-expressing neurons. (c) DBH+ terminals in VTA/SNc were visualized in combination with VGAT+ and VGLUT2+ mRNA in VTA/SNc neurons. (d) Left: High power images of VTA/SNc showing DBH+ terminals (yellow) and enwrapments (arrowheads). Middle: DBH+ terminals and VGAT+ cell bodies in VTA/SNc. Right: Magnification of boxed region showing TH- enwrapment onto a single VGAT+ neuron.
A subset of LC neurons co-express TH and VGLUT2 mRNA
Here we have focused on the expression of NA receptors in the CBG and VTA/SNc complex, but an emerging body of evidence indicates that catecholaminergic neurons can co-release other neurotransmitters such as glutamate and GABA (Tritsch, Granger, and Sabatini 2016; Stuber et al. 2010). To investigate whether LC neurons in the finch could also release these neurotransmitters, we performed in situ hybridization for the vesicular glutamate and GABA transporters VGLUT2 and VGAT in tissue sections containing LC NA neurons, which we identified using a third in situ probe for TH. We observed that approximately 30% (28/96 neurons, N = 4 hemispheres, 2 birds) of TH+ LC neurons expressed VGLUT2, but none (0/96 neurons) expressed VGAT (Figure 9). Therefore, LC neurons in the finch have the potential to release glutamate as well as NA on their postsynaptic targets.
Fig 9. Expression of VGLUT2 and VGAT mRNA in DBH+ LC neurons.
(a) Left: Low power image of immunohistochemical labeling for DBH (yellow), and in situ hybridization labeling of VGLUT2 (magenta) and VGAT (cyan) mRNA expression in LC. Arrowheads point to cells that are positive for DBH and VGLUT2. (b) Higher power image of boxed region in (a). (c) Zoom in on example LC-NA cells that co-express VGLUT2 and DBH.
Discussion
LC projections to the zebra finch song system
Despite its small size, the LC is known to influence fundamental aspects of behavior, such as wakefulness and attention. One way the LC achieves such influence is through highly divergent axonal projections, an anatomical feature that enables individual LC neurons to communicate with a wide array of brain regions. In support of this idea, recent studies have used input- and output-specific viral tracing to demonstrate that LC neurons in the mouse receive inputs from many brain regions and send axonal projections to many destinations (Schwarz et al. 2015). Here we show a similar anatomical organization in the songbird for LC’s outputs (summarized in Figure 10a) to different components of song specialized CBG circuitry, whereby individual LC neurons make divergent projections to Area X , LMAN and the VTA/SNc complex. Therefore, similar to the mouse, individual LC neurons in the finch broadcast their signals to midbrain, basal ganglia, and cortical regions.
Fig 10. Summary of findings for this study.
(a) Parasagittal diagram of the male zebra finch brain highlighting LC connectivity with forebrain song nuclei and the VTA/SNc observed in this study. (b) Summary of and receptor expression in the forebrain song nuclei, VTA/SNc and the LC.
In addition, we also found that retrograde tracer injections made into three other forebrain song nuclei (RA, HVC, DLM) resulted in labeling that was largely restricted to LC DBH+ cells. These results stand in contrast to previous tracing studies in canaries, which found very sparse LC projections to HVC and RA (D. Appeltants et al. 2000; Didier Appeltants, Ball, and Balthazart 2002), and other tracing work identifying a projection from the LC to Area X in zebra finches (Castelino, Diekamp, and Ball 2007). More broadly, our results show that LC’s divergent connectivity extends beyond the CBG, to encompass midbrain, thalamic, and pallial regions.
Differential and AR expression in the CBG circuit
Our in situ hybridization experiments further demonstrated dense expression of the Gi-coupled adrenergic receptors and in LMAN and Area X respectively, suggesting that NA release onto these areas could act synergistically to inhibit CBG output. Within Area X, NA-mediated activation of -ARs directly suppresses SN activity, presumably leading to reduced pause variability in downstream pallidal neurons (Hessler and Doupe 1999; Woolley et al. 2014). In addition, NA application suppresses excitatory transmission from LMAN to the motor output nucleus RA (Sizemore and Perkel 2008), presumably via activation of -ARs on LMAN terminals. Thus, NA signaling across LMAN and Area X could work synergistically to reduce vocal variability during directed song.
Connectivity between LC and VTA/SNc
Anatomical tracing revealed reciprocal connectivity between LC and VTA/SNc, and dual tracing experiments further showed that individual VTA/SNc-projecting LC neurons also project to Area X. We also identified two potential ways in which LC activity could modulate VTA/SNc activity. First, noradrenergic terminals within VTA/SNc preferentially formed enwrapments onto VGAT+ neurons, presumably allowing the LC to modulate activity of VTA/SNc interneurons, potentially through the co-release of glutamate. Second, TH+ VTA/SNc neurons co-express the two Gi-coupled adrenergic receptors and (Figure 10b). Therefore, the LC could suppress the activity of VTA/SNc DA neurons both indirectly through local GABA-releasing interneurons and directly through inhibitory AR receptors on DA neurons.
Functional and behavioral implications of LC connectivity
The widespread and divergent connectivity pattern of LC neurons we observed here, which had not been previously described in songbirds, is well suited to “switch” a large part of the song system into different states depending on behavioral context, motivational factors and endocrine cues. For example, zebra finches change their song properties or body movements depending on whether they are practicing, performing (Kao, Doupe, and Brainard 2005; Sossinka and Böhner 1980), tutoring (Chen, Matheson, and Sakata 2016), or being tutored (Bart B. Houx, Cate, and Enja Feuth 2000), and many of these behavioral changes are associated with changes in neural activity and immediate early gene (IEG) expression in song CBG nuclei (Jarvis et al. 1998; Kao, Wright, and Doupe 2008; Woolley et al. 2014), and in the LC and VTA/SNc complex (Lynch, Diekamp, and Ball 2008; Chen, Matheson, and Sakata 2016; Riters et al. 2004). In support of a role for LC in facilitating brain-wide changes that control context-dependent changes to singing and other related behaviors, interfering with noradrenergic signaling through lesions (Castelino and Ball 2005; Barclay, Harding, and Waterman 1996) or treatment with -AR agonists (Tobari et al. 2014, 2021) affects courtship behaviors and their corresponding neural signatures (Castelino and Ball 2005; Barclay, Harding, and Waterman 1996), and LC stimulation affects social context-sensitive song features (Sheldon et al. 2020).
Relevance to courtship singing and song variability
The songs of male zebra finches are faster, less variable and more vigorous when they are directed to a female as compared to when they are produced in social isolation (undirected) (Sossinka and Böhner 1980; Kao, Doupe, and Brainard 2005). These acoustic changes are readily detected by female receivers, who strongly prefer directed songs over undirected songs (Woolley and Doupe 2008). Moreover, changes in song production during directed singing are thought to be caused by changes in neural activity, particularly within the CBG. Previous evidence suggests that D1 receptors play a role in this context-dependent switch (Leblois and Perkel 2012; Leblois, Wendel, and Perkel 2010), and DA levels are slightly higher during directed song than during solo song (Sasaki et al. 2006), though recent work challenges this notion (Gadagkar, Puzerey, and Goldberg 2019). Despite the initial establishment of the DA system as a primary region of interest, the LC-NA system has also been implicated in the context-dependent control of song features. Systemic lesions of the noradrenergic system largely abolish context-dependent differences in immediate early gene (IEG) expression in Area X (Castelino and Ball 2005)). Furthermore, pharmacologic activation of noradrenergic receptors can also selectively suppress glutamate release from LMAN axon terminals onto song premotor neurons in RA, allowing for further context-dependent regulation of the CBG pathway’s influence on song variability (Sheldon et al. 2020; Sizemore and Perkel 2008). More recently, we showed that pharmacologic blockade of alpha-adrenergic receptors in the CBG increases pitch variability of directed songs to the degree of undirected singing, while agonism of the same receptors results in reduced pitch variability during undirected singing. In contrast to an earlier study (Leblois, Wendel, and Perkel 2010), our more recent study found that selective pharmacologic blockade of D1 receptors did not alter song variability in either context, potentially due to the different time course of drug application used in these studies (days vs hours). While additional experiments will be needed to better understand how DA and NA act in the CBG to influence song variability, catecholaminergic signaling in this pathway is important to how song is employed to facilitate courtship.
Hypothesis for opposing roles of NA and DA in context-dependent singing
Given the interactions between the dopaminergic and noradrenergic systems described here, a parsimonious explanation is that NA and DA act in opposing manners to drive context-dependent differences in neural activity and behavior. For example, in the presence of a female, increased LC activity could result in release of NA in VTA/SNc, which could suppress DA neuron activity directly via adrenergic receptors, or indirectly through glutamate release onto GABA-releasing interneurons. This would be in concordance with previous observations showing that in the VTA/SNc, GABAergic interneurons but not dopaminergic neurons, display increased IEG expression after directed singing (Hara et al. 2007). Such a reduction in VTA/SNc activity could then lead to decreased excitability in downstream areas such as Area X or LMAN. This suppression would be augmented by the direct effect of NA release onto the CBG, as indicated by the inhibitory effect of NA on Area X MSNs (Singh Alvarado et al. 2021). Indeed, opposite effects of NA and DA have been reported before in mouse MSNs (Zhang et al. 1999). Thus, based on our results we propose the hypothesis that such oppositional neuromodulatory systems may act on CBG circuitry to drive context-dependent changes to song. Testing these ideas will ideally require improved viral genetic control over the NA and DA systems in finches and will yield important advances to our understanding of how neuromodulatory systems interact to enable flexible production of complex behaviors such as learned birdsongs.
Key Points:
The locus coeruleus projects to most forebrain song nuclei (HVC, RA, LMAN, Area X, DLM).
Individual LC neurons make divergent projections to different parts of cortico-BG circuitry, and to the VTA/SNc complex.
Different subtypes of noradrenergic receptors are expressed in different parts of the cortico-BG circuitry.
Noradrenergic LC neurons form enwrapments on VGAT+ cells in the VTA/SNc
These features likely enable the LC to exert direct and indirect effects on cortico-BG circuitry that are relevant to the control of vocal variability and learning.
Funding:
National Institutes of Health, Grant/Award Numbers: R01NS099288, R01NS118424. George Barth Geller Fund (R.M.), and a Broad Predoctoral Fellowship (J.S.A.).
Footnotes
Conflicts of interest: Authors report no conflicts of interest.
Data availability:
All data is available upon reasonable request to the authors.
References
- Andalman Aaron S., and Fee Michale S. 2009. “A Basal Ganglia-Forebrain Circuit in the Songbird Biases Motor Output to Avoid Vocal Errors.” Proceedings of the National Academy of Sciences of the United States of America 106 (30): 12518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, and Ball GF 2000. “Identification of the Origin of Catecholaminergic Inputs to HVc in Canaries by Retrograde Tract Tracing Combined with Tyrosine Hydroxylase Immunocytochemistry.” Journal of Chemical Neuroanatomy 18 (3): 117–33. [DOI] [PubMed] [Google Scholar]
- Appeltants Didier, Ball Gregory F., and Balthazart Jacques. 2002. “The Origin of Catecholaminergic Inputs to the Song Control Nucleus RA in Canaries.” Neuroreport 13 (5): 649–53. [DOI] [PubMed] [Google Scholar]
- Aston-Jones Gary, Rajkowski Janusz, and Cohen Jonathan. 2000. “Locus Coeruleus and Regulation of Behavioral Flexibility and Attention.” In Progress in Brain Research, 126:165–82. Elsevier. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF, and Waterman SA 1996. “Central DSP-4 Treatment Decreases Norepinephrine Levels and Courtship Behavior in Male Zebra Finches.” Pharmacology, Biochemistry, and Behavior 53 (1): 213–20. [DOI] [PubMed] [Google Scholar]
- Houx Bart B., Ten Cate Carel, and Feuth Enja. 2000. “Variations in Zebra Finch Song Copying: An Examination of the Relationship with Tutor Song Quality and Pupil Behaviour.” Behaviour 137 (10): 1377–89. [Google Scholar]
- Bottjer SW 1993. “The Distribution of Tyrosine Hydroxylase Immunoreactivity in the Brains of Male and Female Zebra Finches.” Journal of Neurobiology 24 (1): 51–69. [DOI] [PubMed] [Google Scholar]
- Cardin Jessica A., and Schmidt Marc F. 2004. “Noradrenergic Inputs Mediate State Dependence of Auditory Responses in the Avian Song System.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 24 (35): 7745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino Christina B., and Ball Gregory F. 2005. “A Role for Norepinephrine in the Regulation of Context-Dependent ZENK Expression in Male Zebra Finches (Taeniopygia Guttata).” The European Journal of Neuroscience 21 (7): 1962–72. [DOI] [PubMed] [Google Scholar]
- Castelino Christina B., Diekamp Bettina, and Ball Gregory F. 2007. “Noradrenergic Projections to the Song Control Nucleus Area X of the Medial Striatum in Male Zebra Finches (Taeniopygia Guttata).” The Journal of Comparative Neurology 502 (4): 544–62. [DOI] [PubMed] [Google Scholar]
- Chen Yining, Matheson Laura E., and Sakata Jon T. 2016. “Mechanisms Underlying the Social Enhancement of Vocal Learning in Songbirds.” Proceedings of the National Academy of Sciences of the United States of America 113 (24): 6641–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Goldstein M, and Roth RH 1986. “Activation of the Locus Coeruleus Induced by Selective Stimulation of the Ventral Tegmental Area.” Brain Research 363 (2): 307–14. [DOI] [PubMed] [Google Scholar]
- Gadagkar Vikram, Puzerey Pavel A., and Goldberg Jesse H. 2019. “Dopamine Neurons Change Their Tuning according to Courtship Context in Singing Birds.” Cold Spring Harbor Laboratory. 10.1101/822817. [DOI]
- Giustino Thomas F., Ramanathan Karthik R., Totty Michael S., Miles Olivia W., and Maren Stephen. 2020. “Locus Coeruleus Norepinephrine Drives Stress-Induced Increases in Basolateral Amygdala Firing and Impairs Extinction Learning.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 40 (4): 907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Jesse H., and Fee Michale S. 2011. “Vocal Babbling in Songbirds Requires the Basal Ganglia-Recipient Motor Thalamus but Not the Basal Ganglia.” Journal of Neurophysiology 105 (6): 2729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard Bruno P., Mostafa El Mansari Zul Merali, and Blier Pierre. 2008. “Functional Interactions between Dopamine, Serotonin and Norepinephrine Neurons: An in-Vivo Electrophysiological Study in Rats with Monoaminergic Lesions.” The International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum 11 (5): 625–39. [DOI] [PubMed] [Google Scholar]
- Hara Erina, Kubikova Lubica, Hessler Neal A., and Jarvis Erich D. 2007. “Role of the Midbrain Dopaminergic System in Modulation of Vocal Brain Activation by Social Context.” The European Journal of Neuroscience 25 (11): 3406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, and Doupe AJ 1999. “Singing-Related Neural Activity in a Dorsal Forebrain-Basal Ganglia Circuit of Adult Zebra Finches.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 19 (23): 10461–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisey Erin, Kearney Matthew Gene, and Mooney Richard. 2018. “A Common Neural Circuit Mechanism for Internally Guided and Externally Reinforced Forms of Motor Learning.” Nature Neuroscience 21 (4): 589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, and Nottebohm F. 1998. “For Whom the Bird Sings: Context-Dependent Gene Expression.” Neuron 21 (4): 775–88. [DOI] [PubMed] [Google Scholar]
- Kao Mimi H., Doupe Allison J., and Brainard Michael S. 2005. “Contributions of an Avian Basal Ganglia–forebrain Circuit to Real-Time Modulation of Song.” Nature. 10.1038/nature03127. [DOI] [PubMed]
- Kao Mimi H., Wright Brian D., and Doupe Allison J. 2008. “Neurons in a Forebrain Nucleus Required for Vocal Plasticity Rapidly Switch between Precise Firing and Variable Bursting Depending on Social Context.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 28 (49): 13232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic Jelena, Morohashi Yuichi, and Yazaki-Sugiyama Yoko. 2022. “Neural Circuit for Social Authentication in Song Learning.” Nature Communications 13 (1): 4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Satoshi, Kao Mimi H., and Doupe Allison J. 2013. “Task-Related ‘Cortical’ Bursting Depends Critically on Basal Ganglia Input and Is Linked to Vocal Plasticity.” Proceedings of the National Academy of Sciences of the United States of America 110 (12): 4756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Satoshi, Kao Mimi H., Doupe Allison J., and Brainard Michael S. 2018. “The Avian Basal Ganglia Are a Source of Rapid Behavioral Variation That Enables Vocal Motor Exploration.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 38 (45): 9635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsia S, and Bottjer SW 1989. “Developmental Changes in the Cellular Composition of a Brain Nucleus Involved with Song Learning in Zebra Finches.” Neuron 3 (4): 451–60. [DOI] [PubMed] [Google Scholar]
- Leblois Arthur, and Perkel David J. 2012. “Striatal Dopamine Modulates Song Spectral but Not Temporal Features through D1 Receptors.” The European Journal of Neuroscience 35 (11): 1771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois Arthur, Wendel Benjamin J., and Perkel David J. 2010. “Striatal Dopamine Modulates Basal Ganglia Output and Regulates Social Context-Dependent Behavioral Variability through D1 Receptors.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 30 (16): 5730–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Rosin DL, and Van Bockstaele EJ 1998a. “Ultrastructural Evidence for Prominent Postsynaptic Localization of alpha2C-Adrenergic Receptors in Catecholaminergic Dendrites in the Rat Nucleus Locus Coeruleus.” The Journal of Comparative Neurology 394 (2): 218–29. [PubMed] [Google Scholar]
- Lee A, Rosin DL, and Van Bockstaele EJ 1998b. “alpha2A-Adrenergic Receptors in the Rat Nucleus Locus Coeruleus: Subcellular Localization in Catecholaminergic Dendrites, Astrocytes, and Presynaptic Axon Terminals.” Brain Research 795 (1–2): 157–69. [DOI] [PubMed] [Google Scholar]
- Lynch Kathleen S., Diekamp Bettina, and Ball Gregory F. 2008. “Catecholaminergic Cell Groups and Vocal Communication in Male Songbirds.” Physiology & Behavior 93 (4–5): 870–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney-Lin Jim, Lu Ju, Zuo Yi, and Yang Hongdian. 2019. “Locus Coeruleus-Norepinephrine Modulation of Sensory Processing and Perception: A Focused Review.” Neuroscience and Biobehavioral Reviews 105 (October): 190–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, and Leonard CM 1976. “Central Control of Song in the Canary, Serinus Canarius.” The Journal of Comparative Neurology 165 (4): 457–86. [DOI] [PubMed] [Google Scholar]
- Olveczky Bence P., Andalman Aaron S., and Fee Michale S. 2005. “Vocal Experimentation in the Juvenile Songbird Requires a Basal Ganglia Circuit.” PLoS Biology 3 (5): e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person Abigail L., Gale Samuel D., Farries Michael A., and Perkel David J. 2008. “Organization of the Songbird Basal Ganglia, Including Area X.” The Journal of Comparative Neurology 508 (5): 840–66. [DOI] [PubMed] [Google Scholar]
- Poe Gina R., Foote Stephen, Eschenko Oxana, Johansen Joshua P., Bouret Sebastien, Gary Aston-Jones Carolyn W. Harley, et al. 2020. “Locus Coeruleus: A New Look at the Blue Spot.” Nature Reviews. Neuroscience 21 (11): 644–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters Lauren V., and Ball Gregory F. 2002. “Sex Differences in the Densities of Alpha 2-Adrenergic Receptors in the Song Control System, but Not the Medial Preoptic Nucleus in Zebra Finches.” Journal of Chemical Neuroanatomy 23 (4): 269–77. [DOI] [PubMed] [Google Scholar]
- Riters Lauren V., Teague Donald P., Schroeder Molly B., and Cummings Sydney E. 2004. “Vocal Production in Different Social Contexts Relates to Variation in Immediate Early Gene Immunoreactivity within and outside of the Song Control System.” Behavioural Brain Research 155 (2): 307–18. [DOI] [PubMed] [Google Scholar]
- Sara Susan J., and Bouret Sebastien. 2012. “Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal.” Neuron 76 (1): 130–41. [DOI] [PubMed] [Google Scholar]
- Sasaki Aya, Sotnikova Tatyana D., Gainetdinov Raul R., and Jarvis Erich D. 2006. “Social Context-Dependent Singing-Regulated Dopamine.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 26 (35): 9010–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin Johannes, Ignacio Arganda-Carreras Erwin Frise, Kaynig Verena, Longair Mark, Pietzsch Tobias, Preibisch Stephan, et al. 2012. “Fiji: An Open-Source Platform for Biological-Image Analysis.” Nature Methods 9 (7): 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Lindsay A., Miyamichi Kazunari, Gao Xiaojing J., Beier Kevin T., Weissbourd Brandon, DeLoach Katherine E., Ren Jing, et al. 2015. “Viral-Genetic Tracing of the Input-Output Organization of a Central Noradrenaline Circuit.” Nature 524 (7563): 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon Zachary P., Castelino Christina B., Glaze Christopher M., Bibu Steve P., Yau Elvina, and Schmidt Marc F. 2020. “Regulation of Vocal Precision by Noradrenergic Modulation of a Motor Nucleus.” Journal of Neurophysiology 124 (2): 458–70. [DOI] [PubMed] [Google Scholar]
- Alvarado Singh, Jonnathan Jack Goffinet, Michael Valerie, Liberti William 3rd, Hatfield Jordan, Gardner Timothy, Pearson John, and Mooney Richard. 2021. “Neural Dynamics Underlying Birdsong Practice and Performance.” Nature, October. 10.1038/s41586-021-04004-1. [DOI] [PMC free article] [PubMed]
- Sizemore Max, and Perkel David J. 2008. “Noradrenergic and GABA B Receptor Activation Differentially Modulate Inputs to the Premotor Nucleus RA in Zebra Finches.” Journal of Neurophysiology 100 (1): 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorriento Daniela, Trimarco Bruno, and Iaccarino Guido. 2011. “Adrenergic Mechanism in the Control of Endothelial Function.” Translational Medicine @ UniSa 1 (September): 213–28. [PMC free article] [PubMed] [Google Scholar]
- Sossinka Roland, and Jörg Böhner. 1980. “Song Types in the Zebra Finch Poephila Guttata Castanotis 1.” Zeitschrift Für Tierpsychologie 53 (2): 123–32. [Google Scholar]
- Stuber Garret D., Hnasko Thomas S., Britt Jonathan P., Edwards Robert H., and Bonci Antonello. 2010. “Dopaminergic Terminals in the Nucleus Accumbens but Not the Dorsal Striatum Corelease Glutamate.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 30 (24): 8229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadi Elemer. 2013. “Functional Neuroanatomy of the Central Noradrenergic System.” Journal of Psychopharmacology 27 (8): 659–93. [DOI] [PubMed] [Google Scholar]
- Tanaka Masashi, Sun Fangmiao, Li Yulong, and Mooney Richard. 2018. “A Mesocortical Dopamine Circuit Enables the Cultural Transmission of Vocal Behaviour.” Nature 563 (7729): 117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobari Yasuko, Masuzawa Ami, Harada Norika, Suzuki Kenta, and Meddle Simone L. 2021. “Noradrenergic Alpha-2A Receptor Activation Suppresses Courtship Vocalization in Male Japanese Quail.” Behavioural Brain Research 414 (September): 113513. [DOI] [PubMed] [Google Scholar]
- Tobari Yasuko, You Lee Son Takayoshi Ubuka, Hasegawa Yoshihisa, and Tsutsui Kazuyoshi. 2014. “A New Pathway Mediating Social Effects on the Endocrine System: Female Presence Acting via Norepinephrine Release Stimulates Gonadotropin-Inhibitory Hormone in the Paraventricular Nucleus and Suppresses Luteinizing Hormone in Quail.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 34 (29): 9803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch Nicolas X., Granger Adam J., and Sabatini Bernardo L. 2016. “Mechanisms and Functions of GABA Co-Release.” Nature Reviews. Neuroscience 17 (3): 139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman Susanna A., and Harding Cheryl F. 2008. “Neurotoxic Effects of DSP-4 on the Central Noradrenergic System in Male Zebra Finches.” Behavioural Brain Research 188 (2): 271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley Sarah C., and Doupe Allison J. 2008. “Social Context-Induced Song Variation Affects Female Behavior and Gene Expression.” PLoS Biology 6 (3): e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley Sarah C., Rajan Raghav, Joshua Mati, and Doupe Allison J. 2014. “Emergence of Context-Dependent Variability across a Basal Ganglia Network.” Neuron 82 (1): 208–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Lei, Chattree Gaurav, Francisco Garcia Oscos Mou Cao, Wanat Matthew J., and Roberts Todd F. 2018. “A Basal Ganglia Circuit Sufficient to Guide Birdsong Learning.” Neuron 98 (1): 208–21.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbi Valerio, Amalia Floriou-Servou Marija Markicevic, Vermeiren Yannick, Sturman Oliver, Privitera Mattia, von Ziegler Lukas, et al. 2019. “Rapid Reconfiguration of the Functional Connectome after Chemogenetic Locus Coeruleus Activation.” Neuron 103 (4): 702–18.e5. [DOI] [PubMed] [Google Scholar]
- Zhang W, Klimek V, Farley JT, Zhu MY, and Ordway GA 1999. “alpha2C Adrenoceptors Inhibit Adenylyl Cyclase in Mouse Striatum: Potential Activation by Dopamine.” The Journal of Pharmacology and Experimental Therapeutics 289 (3): 1286–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available upon reasonable request to the authors.