Abstract
Introduction:
Pregnant individuals with substance use disorders face complex issues that may serve as barriers to treatment entry and retention. Several professional organizations have established recommendations on comprehensive, collaborative approaches to treatment to meet the needs of this population, but information on real-world application is lacking. Sites participating in the NIDA CTN0080 “Medication treatment for Opioid use disorder in expectant Mothers (MOMs)”—a randomized clinical trial of extended release compared to sublingual buprenorphine among pregnant and postpartum individuals (PPI)—were selected, in part, because they have a collaborative approach to treating PPI with opioid use disorder (OUD). However, organizational differences among sites and how they implement expert recommendations for collaborative care could impact study outcomes.
Methods:
Prior to study launch at each of the 13 MOMs sites, investigators used the Pregnancy and Addiction Services Assessment (PAASA) to collect information about organizational factors. Input from a team of addiction, perinatal, and economic evaluation experts guided the development of the PAASA. Investigators programmed the PAASA into a web-based data system and summarized the resultant site data using descriptive statistics.
Results:
Study sites represented four US census regions. Most sites were specialty obstetrics & gynecology (OB/ GYN) programs providing OUD services (n = 9, 69.2 %), were affiliated with an academic institution (n = 11, 84.6 %), and prescribed buprenorphine in an ambulatory/outpatient setting (n = 11, 84.6 %); all sites offered access to naloxone. Sites reported that their population was primarily White, utilized public insurance, and faced numerous psychosocial barriers to treatment. Although all sites offered many services recommended by expert consensus groups, they varied in how they coordinated these services.
Conclusions:
By providing the organizational characteristics of sites participating in the MOMs study, this report assists in filling the current gap in knowledge regarding similar programs providing services to PPI with OUD. Collaborative care programs such as those participating in MOMs are uniquely positioned to participate in research to determine the most effective models of care and to determine how research can be integrated into those clinical care settings.
Keywords: Opioid use disorder, Organizational factors, Collaborative care, Pregnant, Postpartum
1. Introduction
The ongoing epidemic of opioid use in the United States over the past two decades has impacted many individuals. Pregnant individuals are no exception—the rate of deliveries complicated by opioid use disorder (OUD) has quadrupled with a concomitant rise in neonatal opioid withdrawal (NOWS) (Haight et al., 2018; Hayes & Brown, 2012; Jones et al., 2012; Patrick et al., 2017). Recommended best practice for the treatment of pregnant individuals with OUD utilizes medications for opioid use disorder (MOUD), including methadone and buprenorphine (BUP), combined with counseling and behavior therapies (American College of Obstetricians and Gynecologists, 2017; American Society of Addiction Medicine, 2020; Reddy et al., 2017; Substance Abuse and Mental Health Services Administration, 2018; World Health Organization, 2014b). While effective interventions for OUD exist and can improve outcomes for both the mother and infant, pregnant individuals with substance use disorders (SUDs) face significant challenges that may serve as a barrier to treatment entry and retention (Bedrick et al., 2020; Greenfield et al., 2007; Reising et al., 2019; Substance Abuse and Mental Health Services Administration, 2018; Wilder & Winhusen, 2015). Following delivery, individuals with OUD may face additional challenges that increase their vulnerability for return to non-prescribed use, overdose, and death in the first year postpartum (Schiff et al., 2018; Smid et al., 2020).
In response to the complex issues that pregnant and postpartum individuals (PPI) with OUD experience, the Substance Abuse and Mental Health Services Administration (SAMHSA), American College of Obstetricians and Gynecologists (ACOG), American Society of Addiction Medicine (ASAM), and World Health Organization (WHO) have established recommendations for a comprehensive, collaborative approach to treatment that brings together providers and services from multiple disciplines. Although the intricacies of each set of expert recommendations are beyond the scope of this article, in general the recommendations encompass: 1) Identifying and assessing pregnant individuals with OUD and other SUD by utilizing screening tools, psychosocial assessments, biological toxicology screens; 2) providing MOUD, including naloxone for opioid overdose, rather than opioid withdrawal; 3) providing specialized prenatal care, including physical examination, laboratory testing, imaging, increased pregnancy monitoring; 4) providing a full range of SUD treatment services, including individual and/or group counseling, case management, peer support specialists, mutual support groups, smoking cessation; 5) addressing comorbidities, including testing for infectious disease (HIV, Hepatitis B/C), screening and management of co-occurring medical and behavioral health conditions; 6) providing adequate peripartum pain management; 7) providing ongoing care postpartum, including ongoing treatment for OUD and other SUDs, contraceptive information and access, breast-feeding guidance; 8) providing assessment and care for infants with prenatal exposure to opioids, including management of NOWS, infant developmental assessment, well-baby care; and 9) addressing barriers to receiving services (American College of Obstetricians and Gynecologists, 2017; American Society of Addiction Medicine, 2020; Substance Abuse and Mental Health Services Administration, 2018; World Health Organization, 2014b). Models of care integrating both obstetric and behavioral health treatment increase engagement and improve perinatal outcomes in a cost-effective manner (Crane et al., 2019; Goler et al., 2008; Goodman et al., 2022; Johnson, 2019; Jones et al., 2008; Premkumar et al., 2019). However, we know little about the settings, services delivered, and staff/providers who are implementing this integration of multidisciplinary care for PPI with OUD and to what extent the expert recommendations are being followed. Previous studies suggest that understanding organizational factors such as population characteristics, staffing patterns, size and type of program, funding sources, geographic locations, and adherence to empirically established standards of care may assist in improving treatment delivery for persons with SUD (D’Aunno, 2006; Kimberly & McLellan, 2006; Kramlich et al., 2018; Krans et al., 2019; Meyer & Phillips, 2015; Short et al., 2018). Past research has identified differences in organizational factors as important to the translation of evidence-based treatments into clinical practice (Jacobson et al., 2020; McGovern et al., 2004; McLellan et al., 2003).
The NIDA Clinical Trials Network study “Medication treatment for Opioid use disorder in expectant Mothers (MOMs): a pragmatic randomized trial comparing extended-release and daily buprenorphine formulations (CTN0080)” (Winhusen et al., 2020) will evaluate the impact on mother and infant outcomes of treating OUD in PPI with extended-release (XR) BUP, compared to sublingual (SL) BUP. All MOMs sites use a collaborative care model for treating pregnant individuals with OUD, in which prenatal and MOUD services are either: 1) fully integrated into the program or 2) partially provided in-program with remaining services provided through close collaborations with other programs within the organization or provided via linkage/referral to an external provider. Sites participating in the study are diverse in terms of their organizational characteristics and how they implement expert recommendations, which could affect study outcomes. The aim of the current project is to characterize organizational factors and service-delivery methods of sites participating in a large multisite trial of MOUD for PPI.
2. Materials and methods
2.1. Development of the Pregnancy and Addiction Services Assessment (PAASA)
A review of the literature during protocol development failed to identify an instrument for characterizing models of care and other organizational factors for the management of PPI with OUD. Hence, the investigative team developed the Pregnancy and Addiction Services Assessment (PAASA) to characterize the organizational factors associated with the MOMs sites. Investigators reviewed several surveys used to characterize organizational and treatment factors in health care settings associated with SUDs, most notably the Better Outcomes Through Research for Newborns (BORN) survey (Bogen et al., 2017) and the Addiction Treatment Inventory (ATI) (Carise et al., 2000). Members of the study executive team who were experts in research and treatment of PPI with SUD created a comprehensive set of questions specific to the treatment of PPI with OUD, which were piloted with substance use treatment providers, revised, and sent to experts in addictions research and treatment, maternal/fetal medicine, and economic analysis serving on the CTN0080 MOMs protocol development team for review and revision. Developers added specific funding and staffing items to assist with the study’s planned economic analysis. The final version of the survey was programmed into REDCap (Patridge & Bardyn, 2018), hosted by the Center for Clinical and Translational Science and Training at the University of Cincinnati. The survey contained up to 55 questions, depending upon skip patterns, including questions about organizational characteristics, population served, staff characteristics, MOUD type offered, and service delivery. The PAASA addressed most, but not all, expert recommendations for comprehensive care of PPI with OUD. Investigators did not address expert recommendations around peripartum pain management and management of NOWS in the PAASA as these were more relevant to delivery and neonatal care units than to the outpatient programs participating in the study. Most questions were single-answer, although others asked the respondent to check all that applied or to provide total number or percentages. Questions around the provision of MOUD addressed integrated services within the site only. Six questions asked the respondent to identify the OB/GYN, psychosocial addiction treatment, psychiatric/mental health, general medical, and other services provided to patients, and then to indicate which method each used: 1) integrated within the program, 2) collaborative with providers elsewhere within the same organization, or 3) available only through linkage or referral to an outside source. The full survey is available in Appendix 1.
2.2. Data collection
Investigators chose participating sites for the MOMs study based on the extent to which they: 1) provide BUP to pregnant individuals in an office-based setting, 2) offer BUP treatment following delivery for ≥12 months, and 3) have sufficient clinic enrollment to support the target study randomization rate. To be considered a good candidate for the study, a site must have indicated utilizing a model of care in which close collaboration existed between prenatal care and addiction treatment providers and, where possible, treatment was integrated. Twenty-two potential sites submitted Site Selection Surveys, resulting in the selection of 12 sites to participate in the study; the most common reasons for site exclusion were: 1) inadequate enrollment of BUP-SL-maintained pregnant individuals; 2) minimal clinical trials experience; and/or 3) minimal resources to implement the trial (additional information regarding site selection available in Winhusen et al., 2020). The study added a thirteenth site approximately 18 months later. Prior to study initiation, investigators emailed a link to the online survey to representatives from each of the initial 12 sites participating in the CTN0080 MOMs study; the thirteenth site was provided with the link just prior to opening the site for enrollment. Sites will complete the PAASA a second time near the end of participant data collection; the current report describes results from the initial survey only. Respondents reported only on the array of services in their program that are provided to PPI with OUD. Investigators defined “the program” as those services that are included in the organized specialty clinic or billing unit that provides services to PPI with OUD, regardless of whether the physical locations of these services are the same. Upon completion, study staff reviewed surveys for missing data and asked sites to complete missing data points.
2.3. Data analysis
Investigators employed descriptive statistics to summarize PAASA survey responses for all sites, and grouped services according to the expert recommendation with which they were associated. As some sites reported multiple methods for service delivery, investigators summarized responses to those questions based on the most integrative method used by the site (In-Program = Most integrative; Linkage/Referral = Least integrative). The PAASA questions restricted types of MOUD provided to in-program services only.
2.4. Human subjects protection
The main protocol for the CTN0080 MOMs study included administration of the PAASA. The UC College of Medicine Institutional Review Board reviewed and approved this study in accordance with principles outlined in the Declaration of Helsinki.
3. Results
The original 12 sites selected for the MOMS study completed the PAASA in July and August 2019; the study added a thirteenth site, which completed the PAASA in May 2022. All 13 MOMs sites completed the PAASA in its entirety.
3.1. Geographic characteristics
Based on the US Census regional divisions (U.S. Census Bureau, 2022), 5 of the 13 participating sites (38.5 %) were in the West census region (2 Mountain Division, 3 Pacific Division). Four sites (30.8 %) represented the South census region (3 South Atlantic Division, 1 East South-Central Division), and 3 sites (23.1 %) represented the Northeast census region (2 New England Division, 1 Middle Atlantic Division). The remaining site (7.7 %) was in the Midwest census region (East North Central Division). Most programs (n = 8, 61.5 %) were in a major metropolitan area (population 1 million or more), with 3 (23.1 %) located in a large city (population 300,000–999,000) and 2 (15.4 %) were located in a moderately large city (population 100,000–299,999).
3.2. Organizational characteristics
The sites varied greatly in the number of pregnant individuals with OUD served per year, with a median (IQR) of 50 (35–155). Most sites (n = 9, 69.2 %) were specialty OB/GYN programs for persons with SUDs, with the remainder being specialty addiction programs (n = 3, 23.1 %) or primary care programs for PPI with SUD (n = 1, 7.7 %). Seven of the sites (53.8 %) were hospital ambulatory clinics associated with a multi-site healthcare organization. Of the remaining sites, 3 (23.1 %) were freestanding medical hospitals, 2 (15.4 %) were part of a multi-site SUD treatment organization, and 1 (7.7 %) was a freestanding ambulatory program. Eleven of the 13 sites (84.6 %) were affiliated with an academic health center. On average, sites indicated that 89.3 % (S.D. 11.1) of their PPI patients with OUD were insured by public insurance (Medicare, Medicaid). Sites reported a median (IQR) waiting period of 2 (0–4) days for intake for new pregnant individuals with OUD.
3.3. PPI characteristics
Table 1 provides information on the percentage of racial and ethnic identity reported within the PPI served by the sites. Table 2 provides information on barriers to care found among sites’ PPI.
Table 1.
PPI race/ethnicity characteristics – all sites.
| Number of sites reporting % of population served (n = 13) | 0 % | 1 %−10 % | 11 %−25 % | 26 %−50 % | 50 %−75 % | 76 % or greater |
|---|---|---|---|---|---|---|
| African American/Black | 0 | 9 | 3 | 1 | 0 | 0 |
| American Indian/Alaskan Native | 6 | 7 | 0 | 0 | 0 | 0 |
| Asian | 7 | 6 | 0 | 0 | 0 | 0 |
| Native Hawaiian/other Pacific Islander | 9 | 4 | 0 | 0 | 0 | 0 |
| White/Caucasian | 0 | 0 | 1 | 0 | 4 | 8 |
| More than one race/bi-racial | 0 | 12 | 0 | 1 | 0 | 0 |
| Hispanic | 2 | 10 | 0 | 0 | 0 | 1 |
Table 2.
Barriers to care found among sites’ PPI.
| Barrier type Number and % of sites reporting | All sites (n = 13) |
|
|---|---|---|
| n | % | |
| Homelessness | 12 | 92.3 % |
| Chronic severe mental illness | 13 | 100.0 % |
| History of trauma | 13 | 100.0 % |
| Non-English speaking | 2 | 15.3 % |
| On probation or parole | 13 | 100.0 % |
| Pending legal charges | 13 | 100.0 % |
| Unable to pay for services | 10 | 76.9 % |
| Active domestic violence | 13 | 100.0 % |
| Transportation problems | 13 | 100.0 % |
| Major medical illness | 11 | 84.6 % |
| Lack of childcare | 11 | 84.6 % |
3.4. Provider characteristics
The 13 sites involved in the CTN0080 study employed a wide variety of clinicians and staff, including physicians (OB/GYN, Maternal Fetal Medicine [MFM], psychiatrists, addiction medicine specialists, other), other medical clinicians (nurse practitioners, physician assistants, other nurses), nonmedical clinicians (psychologists, social workers, addictions and mental health counselors), and peer support specialists to provide comprehensive medical, psychosocial, and recovery care services. On average, physicians contributed 1.7 (S.D. 1.2) full-time equivalents (FTEs) to the sites, while other medical clinicians contributed an average of 2.4 (S.D. 1.4) FTEs. Nonmedical clinicians contributed an average of 1.8 (S.D. 1.3) FTEs. On average, sites utilized 5.3 (S.D. 2.9) physicians to prescribe BUP, while 6 sites (46.2 %) also employed nurse practitioners and 2 sites (15.4 %) employed physician assistants to prescribe BUP.
3.5. Implementation of expert recommendations
Study sites integrated many components of the expert recommendations into their programs; as needed, sites coordinated with other clinics within their organization or with external organizations to ensure comprehensive care for their patients. Analysis of sites indicated some degree of variability in how services were integrated into patient care; sites integrated from 10 to 25 of the 29 recommended clinical services assessed by the PAASA into their programs. Table 3 provides detailed information on each site’s method of implementing recommended clinical services; the table includes only services which were specifically mentioned in one or more of the 4 guidance documents. MOUD items reflect integrated MOUD services only. Fig. 1 shows the proportion of recommended non-MOUD clinical services that are fully integrated into care as a function of site. Table 4 provides information about how each site provided additional services to address barriers to treatment.
Table 3.
Site-level adherence to expert recommendations for clinical services.
| Recommendation | Site | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Assess for OUD and Other SUD | |||||||||||||
| Psychosocial assessment | I | I | I | I | I | I | I | I | I | I | I | I | I |
| DUI assessment | R | R | R | N | I | I | N | N | R | R | I | C | R |
| Urine drug screening | I | I | I | I | I | I | I | I | I | I | I | I | I |
| Breathalyzer | I | R | R | N | I | I | N | N | R | C | I | I | R |
| Provide MOUD (in-program only) | |||||||||||||
| Methadone | I | N | N | N | I | I | N | I | N | N | N | N | N |
| Buprenorphine | I | I | I | I | I | I | I | I | I | I | I | I | I |
| Naltrexone with caution | I | N | I | N | N | N | N | I | I | N | N | N | N |
| Naloxone distribution | I | I | I | I | I | I | I | I | I | I | I | I | I |
| Provide specialized prenatal care | |||||||||||||
| Ongoing prenatal care | I | I | I | I | I | I | C | I | I | I | R | I | I |
| Physical exam | I | I | I | I | I | I | I | I | I | I | I | I | I |
| Increased monitoring for high risk | I | I | I | I | I | I | C | I | I | I | R | I | I |
| Lab testing | I | I | I | I | I | I | C | I | I | I | C | I | I |
| Ultrasound | I | C | I | I | R | I | C | C | C | I | R | I | I |
| Provide comprehensive psychosocial substance use disorder treatment | |||||||||||||
| Individual counseling | I | I | I | R | I | I | C | I | C | I | I | I | I |
| Group counseling | I | I | I | R | I | I | C | I | C | C | I | I | I |
| Case management | I | I | I | I | I | I | I | I | I | I | I | I | I |
| Peer support | R | N | I | N | I | I | I | C | I | R | I | I | R |
| Mutual support groups | R | R | C | R | I | I | R | R | R | R | I | R | R |
| Smoking cessation | I | I | I | C | I | I | I | I | I | I | I | I | I |
| Address co-occurring conditions/disorders | |||||||||||||
| Infectious disease testing | I | I | I | I | I | I | I | I | I | I | I | C | I |
| Primary care services | I | I | C | C | R | I | C | C | N | C | R | C | I |
| Mental health assessment | I | I | C | I | I | C | I | I | I | I | I | C | I |
| Individual MH therapy | I | I | C | I | I | I | C | I | C | I | I | C | R |
| Group MH therapy | N | C | I | N | I | C | C | R | C | C | I | C | C |
| MH case management | R | C | I | I | I | I | C | I | I | I | R | C | R |
| Psychiatric crisis services | C | C | C | C | I | C | C | I | I | I | R | C | R |
| Psychiatric medication management | I | I | I | I | N | C | I | I | I | I | I | C | I |
| Integrated SUD-MH | I | C | C | I | I | C | C | I | C | I | I | C | C |
| Provide postpartum care | |||||||||||||
| Postpartum visits | I | I | I | I | I | I | C | I | I | I | R | I | I |
| Breastfeeding guidance | I | I | I | I | I | I | I | I | I | I | R | C | I |
| Family planning/contraception | I | I | I | I | I | I | C | I | I | I | C | I | I |
| Assess and care for opioid-exposed infants | |||||||||||||
| Well-baby care | I | I | C | C | I | I | C | C | I | C | R | C | C |
| Infant developmental assessment | I | I | C | C | R | I | C | C | C | C | R | C | R |
I = integrated into program; C = collaboration with provider elsewhere within organization; R = linkage/referral to external resource; N = not reported.
Fig. 1.
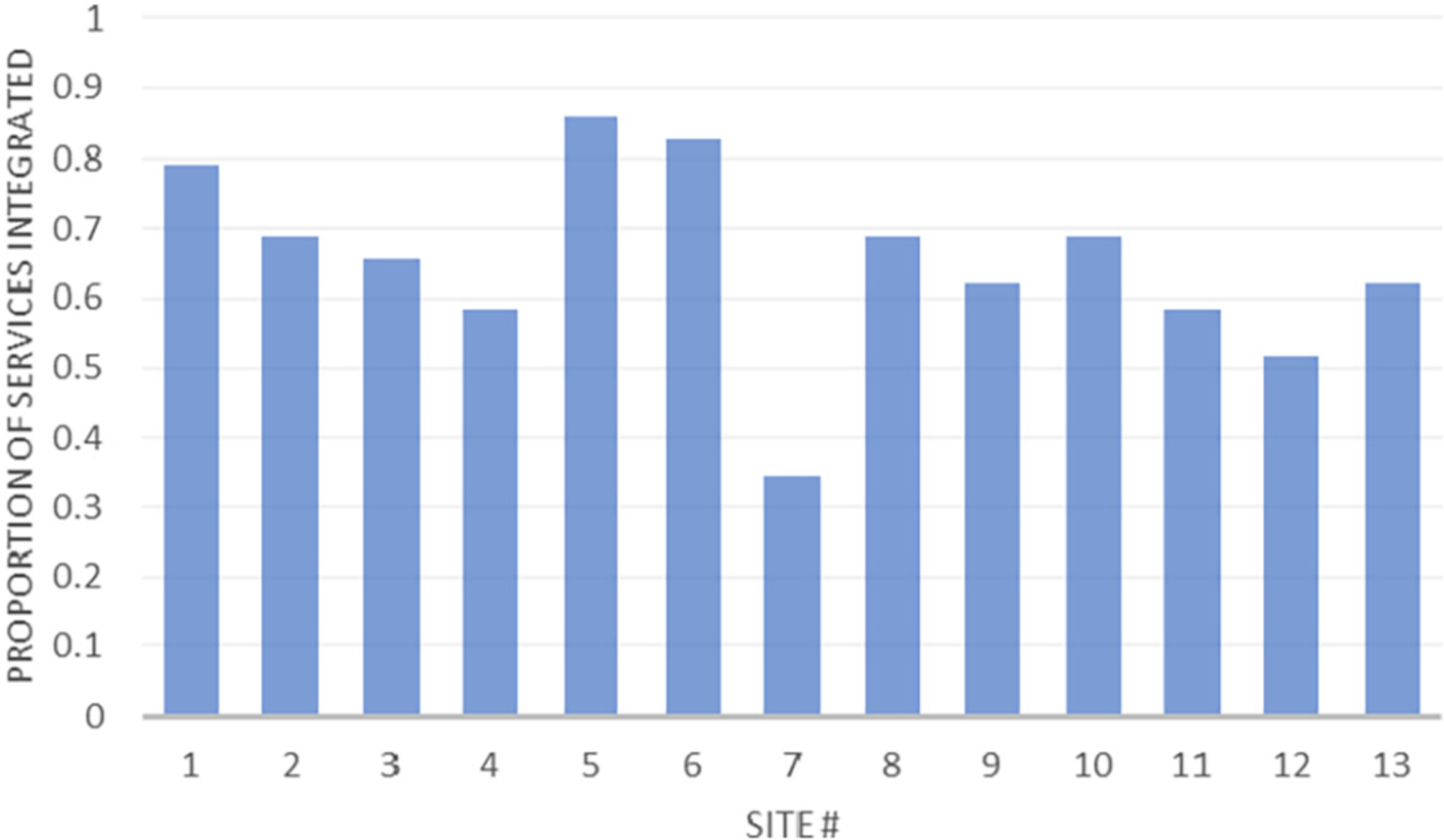
Proportion of recommended services integrated into care as a function of site.
Table 4.
Site-level services to overcome barriers.
| Service | Site | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Transportation assistance | R | C | I | I | I | I | I | C | I | R | C | R | I |
| Financial assistance | C | C | R | R | R | I | I | C | R | C | I | R | I |
| Childcare | R | C | I | N | I | C | I | R | I | R | I | R | R |
| Access to government resources | C | C | I | I | R | I | I | I | C | C | C | I | I |
| Basic needs | R | C | I | R | I | I | I | I | I | C | C | C | I |
| Housing | R | R | I | R | I | I | I | I | C | R | I | C | R |
I = integrated into program; C = collaboration with provider elsewhere within organization; R = linkage/referral to external resource; N = not reported.
3.5.1. Identify PPI with OUD and other SUD
All sites reported screening for illicit substance use utilizing both psychosocial assessments and urine drug screens. Some sites provided screening for driving under the influence (n = 3, 23.1 %) and utilized breathalyzers for alcohol screening (n = 5, 38.5 %) as well.
3.5.2. Provide MOUD
Four (30.8 %) of the 13 sites dispensed methadone through their licensed methadone treatment program (OTP) in addition to providing BUP; 4 (30.8 %) sites also provided naltrexone to patients who were already being treated with naltrexone at the time they became pregnant. Eleven sites (84.6 %) provided both BUP-only (BUP mono) products and products combining BUP and naloxone (BUP/NX), while 1 site (7.7 %) provided only the BUP mono product and 1 site (7.7 %) provided only the BUP/NX product. Three sites (23.1 %) provided injectable BUP and 2 sites (15.4 %) provided implantable BUP. Two sites (15.4 %) dispensed BUP through their OTP while the remaining 11 (84.6 %) sites prescribed BUP in a non-OTP ambulatory/outpatient clinic setting. Most sites performed BUP induction by administering one or more initial doses in person (inpatient or in-clinic), with additional induction doses prescribed/dispensed for home administration. All sites provided patients with naloxone for overdose treatment, with 11 (84.6 %) providing access at the time of program admission; 6 (46.2 %) dispensed naloxone and 7 (53.8 %) provided prescriptions only. Six sites (46.2 %) also dispensed naloxone or provided prescriptions to family members and/or significant others.
3.5.3. Provide specialized prenatal care
At minimum, all sites provided physical examinations to their pregnant individuals; only 2 sites (15.4 %) did not include ongoing prenatal care as part of their program. In addition to the services shown in Table 3, 8 sites (61.5 %) provided in-program birthing classes and 6 sites (46.2 %) reported integrated fetal genetic testing and diagnostic services.
3.5.4. Provide comprehensive substance use disorder treatment
Most sites (n = 12, 92.3 %) offered services in an ambulatory/ outpatient setting. Additionally, 4 (30.8 %) offered intensive outpatient (IOP) or partial hospitalization (PH), and 6 (46.2 %) offered inpatient or residential services. Group services reported by sites included psycho-educational groups, relapse-prevention groups, and addiction-focused group counseling and/or psychotherapy. About half of the sites (n = 7, 53.8 %) reported providing in-program peer support. Three sites (23.1 %) offered family education and/or counseling in addition to services listed in Table 3.
3.5.5. Address comorbid disorders
Four sites (30.8 %) provided primary care services to address co-occurring health concerns, and all but one site (n = 12, 92.3 %) provided testing for infectious diseases commonly associated with substance use such as HIV and hepatitis B/C. Most sites (n = 10, 76.9 %) provided in-program assessment for mental/behavioral health disorders, while the remaining 3 sites coordinated this service elsewhere within their organization. Considerable variation existed in how sites coordinated mental health services for their patients, although most (n = 10, 76.9 %) indicated management of psychiatric medications within their programs.
3.5.6. Provide postpartum care
Most sites (n = 10, 76.9 %) offered all recommended postpartum services as part of their programs. These included postpartum visits, instruction and counseling around breastfeeding, and family planning/ contraceptive services. In addition to services indicated in Table 3, 11 (84.6 %) sites integrated postpartum gynecological care into their programs.
3.5.7. Assess and care for opioid-exposed infants
The PAASA did not include questions specifically aimed at specialized services for opioid-exposed infants or assessment of neonatal opioid withdrawal. However, PAASA questions asked sites to indicate their provision of pediatric services. Sites varied in their approach to services for infants, with 5 sites (38.5 %) providing in-program “well-baby” care and 3 sites (23.1 %) offering developmental assessment services within their program.
3.5.8. Address barriers to services
Sites varied considerably in how they assisted patients with resources to reduce barriers to care. About half of the sites (n = 7, 53.8 %) provided in-program assistance with transportation to clinic appointments; assistance in applying for government resources such as Medicaid, Medicare, or Social Security; and with basic needs such as food, clothing, or infant care supplies. Fewer sites (n = 5, 38.5 %) assisted with providing childcare during clinic visits, while 4 sites assisted patients without insurance in obtaining services through waiving of fees or covering costs through contributions from donors. Six sites (46.2 %) provided assistance with housing needs: 1 site provided emergency shelter only; 2 sites provided long-term recovery/supportive housing only; and 3 sites provided both emergency and long-term housing.
4. Discussion
This description of organizational factors associated with sites participating in the CTN0080 MOMs study assists in filling the current gap in knowledge regarding academic health and community SUD programs providing services to PPI with OUD. It provides a glimpse into differences among the way that these services are coordinated. More precisely, this analysis demonstrates the wealth of information that these comprehensive and experienced programs offer to furthering research on integrative/collaborative care models and methods for implementing expert recommendations for treating this population. For example, a post-hoc analysis is tentatively planned in the MOMs study to explore site characteristics associated with treatment outcome.
Given that previous research has identified significant challenges facing PPI with OUD in accessing care, we find it encouraging that results from the PAASA indicate that study sites, which were selected in part because of the comprehensive nature of their services, offer many elements described in the coordinated collaborative care models proposed by expert consensus groups (American College of Obstetricians and Gynecologists, 2017; American Society of Addiction Medicine, 2020; Substance Abuse and Mental Health Services Administration, 2018; World Health Organization, 2014b). Even these exemplary sites, however, were unable to integrate the full range of recommended services within their programs. Further, the number of integrated services did not appear to be associated with the type of program. The site integrating the largest number of clinical services (Site #5) was one of the nonacademic specialty SUD treatment programs. The site integrating the largest number of services to address barriers (Site #7) was a specialty OB/GYN program. Given that these exemplary sites do not fully integrate all recommended services, a collaborative care model may be as effective as an integrative care model. If true, the use of effective collaborations could assist programs in maximizing resources to ensure that the PPIs they serve effectively meet all expert recommendations regardless of whether they are able to integrate them into their in-program service delivery. A 2017 executive summary of a workshop by the National Institute of Child Health and Human Development identified numerous research gaps and opportunities for screening and management of PPI with OUD (Reddy et al., 2017). Research instruments collecting information about specific services and how they are coordinated, such as the PAASA, can assist in exploring which array of services are most critical for PPI with OUD, and how best to implement those models. Programs implementing collaborative care models, such as the ones in this study, are uniquely positioned to provide recruitment sites for further research on these questions.
The finding that most sites had very low rates of racial and ethnic minority patient populations is consistent with recent findings of disparity in receipt of MOUD, particularly buprenorphine, during pregnancy for persons of color compared with White, non-Hispanic individuals (Hansen et al., 2013; Krans et al., 2019; Lagisetty et al., 2019; Schiff et al., 2020). Such disparities limit the generalizability of results to the full population of PPI with OUD and indicate barriers that must be addressed for sites participating in randomized clinical trials. In the CTN0080 MOMs study, sites are specifically focusing on recruitment and retention efforts that assist in breaking down barriers for persons of color.
Opioid overdose is a leading cause of death in PPI (Bagley et al., 2018; Mehta et al., 2016; Schiff et al., 2018; Smid et al., 2019; Smid et al., 2020); and overdose education with naloxone distribution is recommended for the prevention of opioid overdose (American Society of Addiction Medicine, 2016; US Department of Health and Human Services, 2018; World Health Organization, 2014a). Although all sites provide access to naloxone for all PPI with OUD in their program, some do not offer it as part of the standard intake process, and many of those that do offer access at intake do so using prescriptions that must be filled elsewhere. To reduce the risk of opioid overdose in PPIs with OUD, further research should determine effective methods for increasing direct access to naloxone as they present for care.
The findings presented here have limitations. Most notably, the 13 sites participating in CTN0080 MOMs were chosen in large part due to their strong collaborative care model, significant treatment resources, and experience with clinical trials; we do not know to what extent they represent other programs in the United States that are treating the population of PPI with OUD. Further, the sample size is small (n = 13) and 11 of the 13 sites were specialized practices affiliated with academic health centers serving a large number of PPIs. Consequently, the majority of sites in this study represent a narrowly focused practice type rather than representing the broad range of program structures serving this population. Because of these limitations, readers should take caution in interpreting the findings. In addition, the information collected provides relatively sparse details on the organizations involved. Prior research in examining organizational factors associated with substance use treatment has typically utilized a mixed-methods approach, combining extensive surveys with interviews and other qualitative methods. By relying solely on this brief survey, the current data provide only a high-level view of the participating sites.
5. Conclusion
The current article provides some preliminary information about types of providers and staff along with their respective effort/time allocations, services offered, and population served within academic health centers and large community-based SUD treatment organizations that are providing treatment for PPI with OUD using a collaborative care model, which is lacking in the existing literature. Sites selected to participate in the CTN0080 MOMs study are providing a comprehensive array of medical, counseling, harm reduction (e.g., naloxone), case management, and psychosocial recovery support services in accordance with recommendations for multidisciplinary comprehensive care and are taking steps to reduce barriers to care for this population. Further research should more fully explore the organizational factors utilized in care for PPI with OUD to determine the most effective/cost-effective models of care and how best to implement and expand access to those models. Collaborative care programs, such as those participating in the CTN0080 MOMs study, are uniquely positioned to serve as recruiting sites for such research.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the contributions of the CTN0080 Protocol Development Team for their critical review of the PAASA, Lindsay Bybee for her programming of the PAASA, and Daniel Lewis for his assistance in extracting the PAASA data from the database.
Role of the funding source
This work was supported by the National Institutes of Health through the NIH HEAL Initiative under award numbers UG1DA013732 to the University of Cincinnati (T. John Winhusen), UG1DA049444 (Adam Gordon and Gerald Cochran), UG1DA015831 (Roger Weiss and Gail D’Onofrio). The Publications Committee of the National Drug Abuse Treatment Clinical Trials Network reviewed and gave approval for submission of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Footnotes
CRediT authorship contribution statement
Frankie B. Kropp: Conceptualization, Methodology, Formal analysis, Writing – original draft, Project administration. Marcela C. Smid: Investigation, Writing – review & editing. Michelle R. Lofwall: Conceptualization, Writing – review & editing. Elisha M. Wachman: Investigation, Writing – review & editing. Peter R. Martin: Investigation, Writing – review & editing. Sean M. Murphy: Conceptualization, Methodology, Writing – review & editing. Christine M. Wilder: Conceptualization, Methodology, Investigation, Writing – review & editing. T. John Winhusen: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
Dr. Smid serves as a medical consultant for Gilead Science Inc. for hepatitis C treatment in pregnancy and Organon on postpartum hemorrhage management in a low resource setting. Her institution received funding from Alydia Health/Organon for research activities. She receives research support from the CDC and NIDA. Dr. Lofwall reports having consulted for Titan Pharmaceuticals Inc. and Camurus, and Dr. Murphy reports having consulted for Sandoz Inc., Nature Sacred Inc., and West Virginia Perinatal Partnership Inc. outside of the submitted work. All other authors have no potential conflicts of interest to report.
Trial registration
Clinical Trials.gov http://www.clinicaltrials.gov; Identifiers NCT03918850, NCT03911739, NCT03911466.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.josat.2023.209030.
References
- American College of Obstetricians and Gynecologists. (2017). Committee opinion no. 711. Opioid use and opioid use disorder in pregnancy. Obstetrics & Gynecology, 130, e81–e94. 10.1097/AOG.0000000000002235 [DOI] [PubMed] [Google Scholar]
- American Society of Addiction Medicine. (2016). Public policy statement on the use of naloxone for the prevention of opioid overdose deaths. Retrieved 6/6/2022 from https://www.asam.org/docs/default-source/public-policy-statements/use-of-naloxonefor-the-prevention-of-opioid-overdose-deaths-final.pdf.
- American Society of Addiction Medicine. (2020). The ASAM National Practice Guideline for the treatment of opioid use disorder: 2020 focused update. Journal of Addiction Medicine, 14(2S Supplement 1), 1–91. 10.1097/ADM.0000000000000633 [DOI] [PubMed] [Google Scholar]
- Bagley SM, Cabral H, Saia K, Brown A, Lloyd-Travaglini C, Walley AY, & Rose- Jacobs R (2018). Frequency and associated risk factors of non-fatal overdose reported by pregnant women with opioid use disorder. Addiction Science & Clinical Practice, 13(1), 26. 10.1186/s13722-018-0126-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrick BS, O’Donnell C, Marx CM, Friedman H, Carter EB, Stout MJ, & Kelly JC (2020). Barriers to accessing opioid agonist therapy in pregnancy. American Journal of Obstetrics & Gynecology MFM, 2(4), Article 100225. 10.1016/j.ajogmf.2020.100225 [DOI] [PubMed] [Google Scholar]
- Bogen DL, Whalen BL, Kair LR, Vining M, & King BA (2017). Wide variation found in care of opioid-exposed newborns. Academic Pediatrics, 17(4), 374–380. 10.1016/j.acap.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carise D, McLellan AT, & Gifford LS (2000). Development of a “treatment program” descriptor: The Addiction Treatment Inventory. Substance Use & Misuse, 35 (12–14), 1797–1818. 10.3109/10826080009148241 [DOI] [PubMed] [Google Scholar]
- Crane D, Marcotte M, Applegate M, Massatti R, Hurst M, Menegay M, Mauk R, & Williams S (2019). A statewide quality improvement (QI) initiative for better health outcomes and family stability among pregnant women with opioid use disorder (OUD) and their infants. Journal of Substance Abuse Treatment, 102, 53–59. 10.1016/j.jsat.2019.04.010 [DOI] [PubMed] [Google Scholar]
- D’Aunno T (2006). The role of organization and management in substance abuse treatment: Review and roadmap. Journal of Substance Abuse Treatment, 31(3), 221–233. 10.1016/j.jsat.2006.06.016 [DOI] [PubMed] [Google Scholar]
- Goler NC, Armstrong MA, Taillac CJ, & Osejo VM (2008). Substance abuse treatment linked with prenatal visits improves perinatal outcomes: A new standard. Journal of Perinatology, 28(9), 597–603. 10.1038/jp.2008.70 [DOI] [PubMed] [Google Scholar]
- Goodman DJ, Saunders EC, Frew JR, Arsan C, Xie H, Bonasia KL, Flanagan VA, Lord SE, & Brunette MF (2022). Integrated vs nonintegrated treatment for perinatal opioid use disorder: Retrospective cohort study. American Journal of Obstetrics & Gynecology MFM, 4(1), Article 100489. 10.1016/j.ajogmf.2021.100489 [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, & Miele GM (2007). Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug and Alcohol Dependence, 86(1), 1–21. 10.1016/j.drugalcdep.2006.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight SC, Ko JY, Tong VT, Bohm MK, & Callaghan WM (2018). Opioid use disorder documented at delivery hospitalization - United States, 1999–2014. MMWR Morbidity and Mortality Weekly Report, 67(31), 845–849. 10.15585/mmwr.mm6731a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HB, Siegel CE, Case BG, Bertollo DN, DiRocco D, & Galanter M (2013). Variation in use of buprenorphine and methadone treatment by racial, ethnic, and income characteristics of residential social areas in New York City. The Journal of Behavioral Health Services & Research, 40(3), 367–377. 10.1007/s11414-013-9341-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M, & Brown M (2012). Epidemic of prescription opiate abuse and neonatal abstinence. JAMA: Journal of the American Medical Association, 307(May 9, 2012), 1974–1975. 10.1001/jama.2012.4526 [DOI] [PubMed] [Google Scholar]
- Jacobson N, Horst J, Wilcox-Warren L, Toy A, Knudsen HK, Brown R, Haram E, Madden L, & Molfenter T (2020). Organizational facilitators and barriers to medication for opioid use disorder capacity expansion and use. The Journal of Behavioral Health Services & Research, 47(4), 439–448. 10.1007/s11414-020-09706-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E (2019). Models of care for opioid dependent pregnant women. Seminars in Perinatology, 43(3), 132–140. 10.1053/j.semperi.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Jones HE, O’Grady KE, Malfi D, & Tuten M (2008). Methadone maintenance vs. methadone taper during pregnancy: Maternal and neonatal outcomes. The American Journal on Addictions, 17(5), 372–386. 10.1080/10550490802266276 [DOI] [PubMed] [Google Scholar]
- Jones HE, Heil SH, Baewert A, Arria AM, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, & Fischer G (2012). Buprenorphine treatment of opioid-dependent pregnant women: A comprehensive review. Addiction, 107 (Supplement 1), 5–27. 10.1111/j.1360-0443.2012.04035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly JR, & McLellan AT (2006). The business of addiction treatment: A research agenda. Journal of Substance Abuse Treatment, 31(3), 213–219. 10.1016/j.jsat.2006.06.018 [DOI] [PubMed] [Google Scholar]
- Kramlich D, Kronk R, Marcellus L, Colbert A, & Jakub K (2018). Rural postpartum women with substance use disorders. Qualitative Health Research, 28(9), 1449–1461. 10.1177/1049732318765720 [DOI] [PubMed] [Google Scholar]
- Krans EE, Kim JY, James AE 3rd, Kelley D, & Jarlenski MP (2019). Medication-assisted treatment use among pregnant women with opioid use disorder. Obstetrics & Gynecology, 133(5), 943–951. 10.1097/AOG.0000000000003231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetty PA, Ross R, Bohnert A, Clay M, & Maust DT (2019). Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry, 76(9), 979–981. 10.1001/jamapsychiatry.2019.0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern MP, Fox TS, Xie H, & Drake RE (2004). A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. Journal of Substance Abuse Treatment, 26(4), 305–312. 10.1016/j.jsat.2004.03.003 [DOI] [PubMed] [Google Scholar]
- McLellan AT, Carise D, & Kleber HD (2003). Can the national addiction treatment infrastructure support the public’s demand for quality care? Journal of Substance Abuse Treatment, 25(2), 117–121. 10.1016/S0740-5472(03)00156-9 [DOI] [PubMed] [Google Scholar]
- Mehta PK, Bachhuber MA, Hoffman R, & Srinivas SK (2016). Deaths from unintentional injury, homicide, and suicide during or within 1 year of pregnancy in Philadelphia. American Journal of Public Health, 106(12), 2208–2210. 10.2105/AJPH.2016.303473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, & Phillips J (2015). Caring for pregnant opioid abusers in Vermont: A potential model for non-urban areas. Preventive Medicine, 80, 18–22. 10.1016/j.ypmed.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Schiff DM, & Committee On Substance use Prevention. (2017). A public health response to opioid use in pregnancy. Pediatrics, 139(Mar 2017), Article e20164070. 10.1542/peds.2016-4070 [DOI] [PubMed] [Google Scholar]
- Patridge EF, & Bardyn TP (2018). Research electronic data capture (REDCap). Journal of the Medical Library Association, 106(1), 142–144. 10.5195/jmla.2018.319 [DOI] [Google Scholar]
- Premkumar A, Grobman WA, Terplan M, & Miller ES (2019). Methadone, buprenorphine, or detoxification for management of perinatal opioid use disorder: A cost-effectiveness analysis. Obstetrics & Gynecology, 134(5), 921–931. 10.1097/AOG.0000000000003503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy UM, Davis JM, Ren Z, & Greene MF (2017). Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: Executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstetrics & Gynecology, 130(1), 10–28. 10.1097/aog.0000000000002054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reising VA, Bergren MD, & Bennett A (2019). Care and treatment recommendations for pregnant women with opioid use disorder. MCN: The American journal of Maternal/ChildNursing, 44(4), 212–218. 10.1097/NMC.0000000000000538 [DOI] [PubMed] [Google Scholar]
- Schiff DM, Nielsen T, Terplan M, Hood M, Bernson D, Diop H, Bharel M, Wilens TE, LaRochelle M, Walley AY, & Land T (2018). Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstetrics and Gynecology, 132(2), 466–474. 10.1097/AOG.0000000000002734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff DM, Nielsen T, Hoeppner BB, Terplan M, Hansen H, Bernson D, Diop H, Bharel M, Krans EE, Selk S, Kelly JF, Wilens TE, & Taveras EM (2020). Assessment of racial and ethnic disparities in the use of medication to treat opioid use disorder among pregnant women in Massachusetts. JAMA Network Open, 3(5), Article e205734. 10.1001/jamanetworkopen.2020.5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short VL, Hand DJ, MacAfee L, Abatemarco DJ, & Terplan M (2018). Trends and disparities in receipt of pharmacotherapy among pregnant women in publically funded treatment programs for opioid use disorder in the United States. Journal of Substance Abuse Treatment, 89, 67–74. 10.1016/j.jsat.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Smid MC, Stone NM, Baksh L, Debbink MP, Einerson BD, Varner MW, Gordon AJ, & Clark EAS (2019). Pregnancy-associated death in Utah: Contribution of drug-induced deaths. Obstetrics & Gynecology, 133(6), 1131–1140. 10.1097/AOG.0000000000003279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid MC, Maeda J, Stone NM, Sylvester H, Baksh L, Debbink MP, Varner MW, & Metz TD (2020). Standardized criteria for review of perinatal suicides and accidental drug-related deaths. Obstetrics & Gynecology, 136(4), 645–653. 10.1097/AOG.0000000000003988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2018). Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18–5054. Retrieved 11/19/2019 from https://store.samhsa.gov/sites/default/files/d7/priv/sma18-5054.pdf. [Google Scholar]
- U.S. Census Bureau. (2022). Economic census geographies. Retrieved 5/27/2022 from https://www.census.gov/programs-surveys/economic-census/geographies.html.
- US Department of Health and Human Services. (2018). Naloxone: The opioid reversal drug that saves lives. Retrieved 6/6/2022 from https://www.hhs.gov/opioids/sites/default/files/2018-12/naloxone-coprescribing-guidance.pdf.
- Wilder C, & Winhusen T (2015). Pharmacological management of opioid use disorder in pregnant women. CNS Drugs, 29, 625–636. 10.1007/s40263-015-0273-8 [DOI] [PubMed] [Google Scholar]
- Winhusen T, Lofwall M, Jones HE, Wilder C, Lindblad R, Schiff DM, Wexelblatt S, Merhar S, Murphy SM, Greenfield SF, Terplan M, Wachman EM, Kropp F, Theobald J, Lewis M, Matthews AG, Guille C, Silverstein M, & Rosa C (2020). Medication treatment for opioid use disorder in expectant mothers (MOMs): Design considerations for a pragmatic randomized trial comparing extended-release and daily buprenorphine formulations. Contemporary Clinical Trials, 93, Article 106014. 10.1016/j.cct.2020.106014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2014a). Community management of opioid overdose. Retrieved 6/6/2022 from https://www.who.int/publications/i/item/9789241548816. [PubMed]
- World Health Organization. (2014b). Guidelines for the identification and management of substance use and substance use disorders in pregnancy. Retrieved 6/17/2022 from https://www.ncbi.nlm.nih.gov/books/NBK200701/. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


