Abstract
The use of Electronic Nicotine Delivery Systems (ENDS) is increasing in prevalence and popularity. ENDS are a rapidly evolving technology as devices and e-liquid formulations adapt to policy restrictions and market demand To identify the impacts of nicotine formulation and concentration, we exposed female and male C57BL/6J mice to passive electronic vaporization of different nicotine formulations (freebase or salt) and concentrations (1% or 3%) and measured serum nicotine metabolite levels, brain activity by cFos expression, and anxiety-like and motivated behavior using the novelty suppressed feeding test. We found that the 3% freebase nicotine vapor group displayed significantly higher serum nicotine levels than either 1% or 3% nicotine salt formulations, and female mice displayed higher serum nicotine and cotinine levels compared to males. Central amygdala (CeA) activity was significantly elevated in male mice following nicotine vapor exposure, but the increase was not significantly different between nicotine vapor groups. CeA activity in female mice was unaffected. In contrast increased activity in the ventral tegmental area (VTA) was only observed in female mice exposed to 3% nicotine freebase and specifically in the dopaminergic population. Anxiety-like behavior in female mice was relatively unaffected by nicotine vapor exposure, however male mice displayed increased anxiety-like behavior and reduced motivation to feed after vapor exposure, specifically in the 3% freebase group. These results identify important sex differences in the impact of nicotine formulation and concentration on nicotine metabolism, brain region-specific activity and anxiety-like behavior, which may have significant relevance for different consequences of vaping in men and women.
Keywords: Nicotine, Freebase, Salt, Cea, Vta, Ends, Vapor
Introduction
Electronic Nicotine Delivery Systems (ENDS) represent the most currently used and available form of nicotine system, and their popularity has rapidly grown with ~3.7% of young adults (18–24) using ENDS (9.1 million) [1]. With this growing popularity and the variability in ENDS devices, the availability and use of different nicotine formulations and concentrations has also increased [2]. Two types of nicotine formulations are predominant within the market, nicotine freebase and nicotine salt. Nicotine freebase is highly volatile and when vaporized it enters the bloodstream through the mouth/upper respiratory tract. This solution is often described as bitter and harsh. Nicotine salt, on the other hand, is made by adding organic acids into freebase nicotine which results in protonated nicotine salt. When vaporized, this solution travels further down the respiratory tract and is absorbed into the bloodstream by the alveoli, which is more similar to the absorption that occurs with cigarettes. The addition of organic acids into nicotine freebase is reported to increase its smoothness and reduce its bitterness [3,4]. The smoothness of the nicotine salt has made it more popular amongst first time smokers [3] but there is a relatively equal prevalence of nicotine salt and nicotine freebase amongst ENDS users [5]. Humans who have smoked nicotine salt showed higher nicotine serum levels than those who used nicotine freebase. At a higher concentration, nicotine salt was also able to closely resemble nicotine serum levels reached when smoking a cigarette [4] which suggests other underlying physiological differences might result from consuming nicotine salt and nicotine freebase.
Potential physiological differences between nicotine formulations remain unclear, as there are few studies that investigate the pharmacokinetic effects of nicotine salt and freebase in preclinical models. It has been previously shown in rats that a single subcutaneous injection of nicotine freebase reached and maintained higher serum nicotine levels than a single injection of nicotine salt [6]. Using an e-vape self administration paradigm, Henderson and Cooper et al. [7], found that mice exposed to nicotine salt sought more nicotine deliveries than their free-base counterparts. In addition, mice exposed to nicotine salt yielded higher plasma cotinine levels than mice exposed to freebase [7]. Thus suggesting that nicotine salt is metabolized at a faster rate and therefore more vapor puffs are required to maintain an effect. These serum results were similar to previous clinical studies [4] and when taken into consideration with the perceived smoothness of nicotine salt vapor, nicotine salts can potentially pose a higher likelihood of developing dependence than nicotine freebase in clinical populations.
Further, potential differences between nicotine salt and nicotine free-base and sex differences can arise in behavioral effects observed after vapor exposure. One preclinical study in rats found sex differences where male rats exposed to nicotine vapor showed higher serum cotinine levels than females, as well as hyperlocomotion following passive vape administration [8]. These effects could be due to differences in absorption or metabolism however, the possibility of sex differences in metabolic enzyme levels should also be considered. Previous studies have also shown that nicotine vapor exposure results in changes in thermoregulation and locomotor function [8–11]. A comprehensive review of preclinical and clinical studies found that male rats and mice showed increased anxiety following prenatal and postnatal nicotine exposure. A number of caveats such as variability in methodology and inconsistency in literature reporting prevented the authors from reaching any conclusions [12]. We have also previously shown that acute nicotine vapor exposure increases activity in the central amygdala (CeA), however that study only investigated a single nicotine formulation (freebase) and was only performed in male mice [11]. Few studies have examined the effects of formulation on behavior or brain region-specific activity in a preclinical model. The goal of the current study was to investigate the physiological and behavioral effects of different nicotine formulations and concentrations in male and female mice following a single session of vapor, to determine the changes after initial exposure to nicotine vapor. In order to further understand the effects of nicotine formulation on anxiety-like and reward-related behavior we specifically examined activity in the CeA due to its association with anxiety and substance use disorders [13]. We also targeted the ventral tegmental area (VTA) for its role in reward signaling and nicotine effects [14]. Additionally, we examined the impact of formulation and concentration on nicotine metabolism and anxiety-like and motivated behavior.
Materials and methods
Animals
Female and male adult (10– 12 weeks) C57/BL6J mice were obtained from Jackson Labs and group-housed in a temperature- and humidity-controlled 12hr light/dark (7am lights on) facility with ad libitum food and water access. All experimental procedures were approved by the UNC Institutional Animal Care and Use Committee.
Drugs
(−)- Nicotine free base (C10H14N2; Sigma Aldrich) was diluted to a 3% (30 mg/ml) nicotine concentration in 1:1 propylene glycol (Sigma Aldrich):vegetable glycerol (Fisher, PG/VG). Nicotine ditartrate dihydrate salt, 98% (C10H14N2·2C4H6O6·2H2O; Sigma Aldrich) was dissolved in 1:1 PG/VG to make 1% (10 mg/ml) and 3% (30 mg/ml) nicotine concentration, taking into account the difference in molecular weight of the salt to match the nicotine content of the nicotine freebase concentrations.
Electronic nicotine vapor exposure
Mice were exposed to electronic nicotine vapor in airtight vacuum-controlled chambers as previously described [11]. Briefly, PG/VG control, 3% nicotine freebase, 1% nicotine salt, or 3% nicotine salt solutions are added to electronic nicotine vape tanks (Baby Beast Brother, Smok) to be heated and vaporized (95 watt, 0.25Ω, 200 °C, SVS200, Scientific Vapor). The vacuum pressure allows the vaporized solution to be circulated through the chambers at ~1 L/min flow rate. Vapor delivery was triggered by e-vape controllers (SSV-1, La Jolla Alcohol Research) that are set to deliver vapor for 3 s every 10 min for a total of 3 h (Fig. 1, left).
Fig. 1.
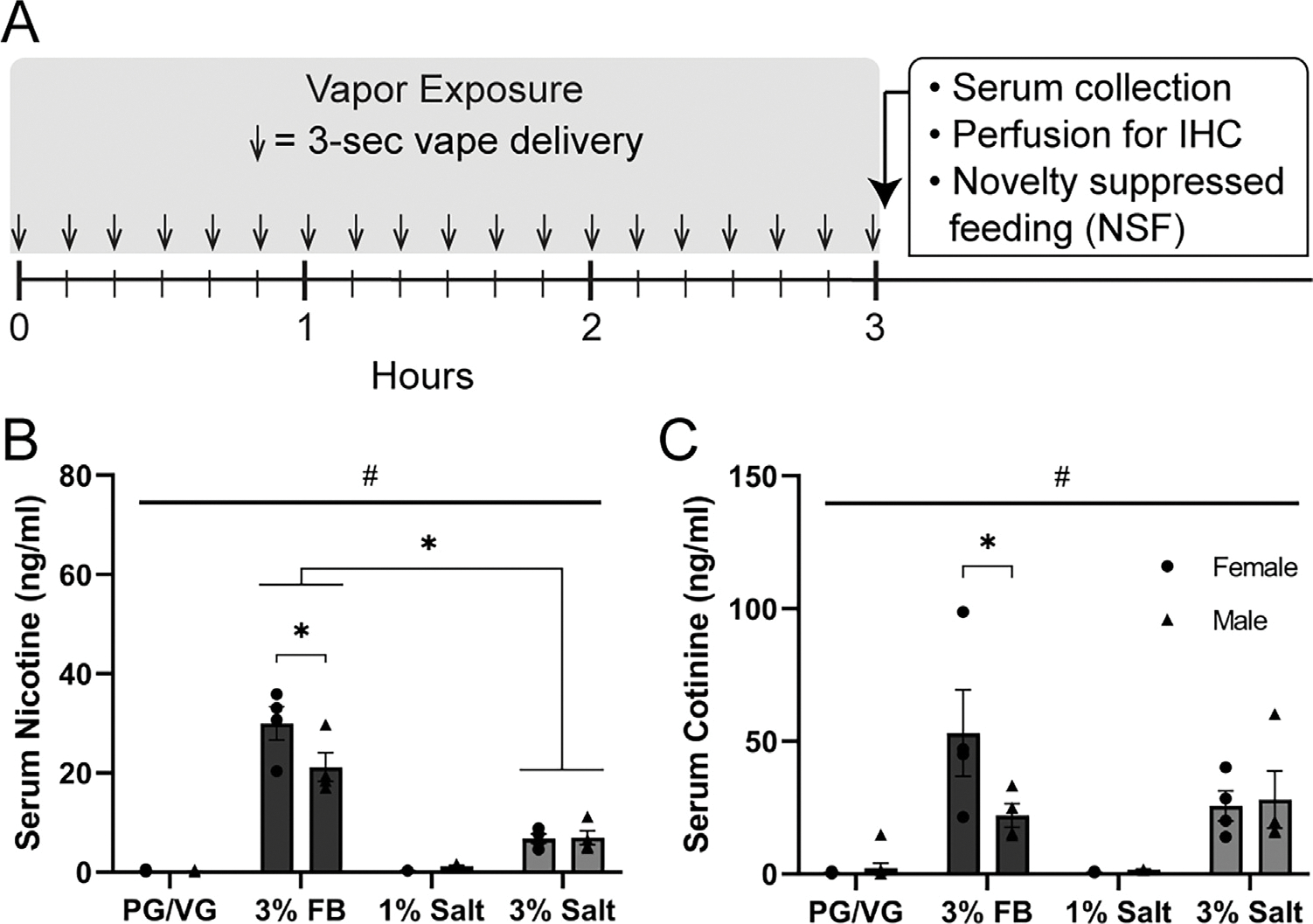
Experimental timeline and serum nicotine and cotinine following nicotine vapor exposure.
A) Experimental timeline of vapor exposure (3 second vapor every 10 min for 3 h), followed by serum collection and perfusion for immunohistochemistry or NSF behavioral assay.
B) Serum nicotine levels in females and males following PG/VG, 3% nicotine freebase (3% FB), 1% nicotine salt (1% salt), or 3% nicotine salt (3% salt) vapor exposure. Significant interaction (p = 0.0058) and #Main effect of vapor content (p < 0.0001); * Post-hoc Tukey’s: Female 3% FB vs Male 3% FB, Female and Male 3% FB vs 3% salt.
C) Serum cotinine levels in females and males following PG/VG, 3% nicotine freebase (FB), 1% nicotine salt, or 3% nicotine salt vapor exposure. Significant interaction (p = 0.0335) and #Main effect of vapor content (p < 0.0001); * Post-hoc Tukey’s: Female 3% FB vs Male 3% FB.
Nicotine serum analysis
Immediately following an acute vapor exposure session, trunk blood was collected from cardiac puncture. Nicotine and cotinine levels were analyzed through liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described previously [11,15].
Immunohistochemistry (IHC)
Immediately following an acute vapor exposure session, mice were anesthetized with isoflurane and perfused with 1x phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. Brains were left to postfix overnight in 4% PFA before transferring to 30% sucrose in PBS at 4 °C. Brains were then sectioned at 40 μm using a microtome (HM450, Thermo Fisher Scientific) and stored at 4 °C in 0.01% sodium azide in PBS. Slices containing the CeA and VTA were washed in PBS for 10 mins, incubated in 50% methanol in PBS for 30 mins, washed for 5 mins in 3% hydrogen peroxide and incubated in blocking solution (0.3% Triton X-100; Thermo Fisher), 1% bovine serum albumin (BSA; Sigma) for 1hr. They were then incubated for 48 hrs at 4 °C in rabbit anti-cFos primary antibody (1:3000, Millipore Sigma; ABE457) in blocking solution. Slices were washed with Tris, NaCl, Triton X-100 (TNT) buffer for 10 mins. Followed by a Tris, NaCl, blocking reagent (TNB; PerkinElmer) wash for 30 mins, a 30 min incubation in Horse Radish Peroxide (HRP; 1:200, Abcam ab6721) and a 5 min washes in TNT. Fluorescence signal was amplified by incubating the slices in Cy3 [1:50] TSA amplification diluent (Akoya Biosciences, NEL744001KT) for 10 min at room temperature. CeA slices were then washed in TNT buffer for 10 min and mounted onto slides with Vectashield DAPI with hard-set (Vector labs; H1500–10). In VTA slices, tyrosine hydroxylase expression was labeled by an additional 10 min washes in PBS, and a 60 min incubation in blocking solution (0.3% Triton X-100, 10% Normal Donkey Serum (NDS), and 1% Bovine serum (BSA)). VTA slices were then incubated overnight in primary antibody (Mouse anti-TH [1:1000], Sigma T1299) in blocking solution at 4 °C. Finally the slices were washed for 10 mins in PBS and incubated for 2 hrs in secondary antibody (Donkey anti mouse [1:700], Abcam ab150105) in PBS, washed in PBS and mounted onto slides using Vectashield DAPI (Vector labs; H1500). Fluorescence signals were detected and imaged on fluorescent microscopes (Nikon Eclipse 6600 for CeA and Keyence BZ-X800 for VTA).
Novelty suppressed feeding (NSF) test
To assess the effects of nicotine formulation on anxiety-like behavior following a single vapor session, the novelty suppressed feeding test was conducted. All mice received highly palatable food (Froot Loop, Kellogg’s) in their home cage 48 h prior to testing and were food deprived 24 h prior to testing. Testing was conducted in a sound-attenuated behavior cabinet with a clean, empty rat cage inside and equipped with a light source set at 200 lux. Following vapor exposure described above, each mouse was placed inside an empty cage with a Froot Loop to record their latency to feed time as a measure of anxiety-like behavior. Once the mouse engaged in feeding or reached the maximum time limit of 10 min, the time was recorded and the NSF post-test commenced where the mouse was transferred back to its home cage and allowed 10 min to feed on a pre-weighed amount of Froot Loops. The weight of Froot Loops consumed was calculated as a measure of motivation to feed.
Statistical analysis
Statistical analysis and data visualization were performed using Prism 9.0 (GraphPad). Data were analyzed and compared using one-way or two-way ANOVAs with post hoc Tukey’s multiple comparisons as well as pearson’s correlation with p < 0.05 as the criterion for statistical significance. All data sets are expressed as mean ± SEM.
Results
Serum nicotine and cotinine analysis following exposure to nicotine freebase vs nicotine salt
To investigate the effects of nicotine formulation (freebase vs salt) and nicotine concentration (1% vs 3%), we separately exposed female and male mice to vapor composed of propylene glycol/vegetable glycerol (PG/VG, control), 3% nicotine freebase (3% FB), 1% nicotine salt (1% salt), or 3% nicotine salt (3% salt). Mice were exposed to a single vapor session comprised of 3 second vapor deliveries every 10 min for a total of 3 h. Immediately following exposure, serum was collected and mice were perfused for immunohistochemistry (Fig. 1A). Serum nicotine levels showed a significant sex × vapor content interaction (2-Way ANOVA, p = 0.0058, F (3, 31) = 5.039) and a main effect of vapor content (#p < 0.0001, F (3, 31) = 142.0, Fig. 1 B). Post-hoc Tukey’s multiple comparisons showed that mice of both sexes that were exposed to 3% FB displayed higher levels of serum nicotine than those exposed to 3% salt. Additionally, females exposed to 3% FB had higher serum nicotine levels than males exposed to 3% FB (Fig. 1B). Levels for serum cotinine, the main metabolite of nicotine, also showed a significant sex × vapor content interaction (2-Way ANOVA, p = 0.0335, F (3, 31) = 3.289) and a main effect of vapor content (#p < 0.0001, F (3, 31) = 18.18), and post-hoc Tukey’s showed that females exposed to 3% FB had higher serum cotinine levels than males exposed to 3% FB (Fig. 1C). Overall, these data indicate that nicotine vapor in free-base formulation produced higher serum nicotine levels than the salt formulation of the same concentration and within the freebase formulation group, females had higher serum nicotine and cotinine levels than males.
Neuronal activation in the central amygdala following exposure to nicotine freebase vs nicotine salt
To measure changes in neuronal activity in the central amygdala (CeA, Fig. 2A) we performed immunohistochemistry and labeled for cFos in female (Fig. 2B, top) and male (Fig. 2B, bottom) mice exposed to PG/VG, 3% FB, 1% salt, and 3% salt. In females, we observed no significant difference in average cFos expression between groups (1-Way ANOVA, p = 0.5118, F = 0.8153, Fig. 2C). In males however, we observed a significant increase in average cFos expression in the 3% FB, 1% salt, and 3% salt groups as compared to PG/VG controls (1-Way ANOVA, #p = 0.0007, F = 10.99, Fig. 2D). We then combined the female and male data to examine sex differences using 2-way ANOVA. We found a significant effect of vapor content (#p = 0.0415, F (3, 26) = 3.158, Fig. 2E), however, post hoc multiple comparisons between specific groups did not yield any significant differences. We also correlated CeA cFos expression with nicotine serum levels in females, males, and mice of both sexes and found no significant correlation (Female and Male r = −0.083, p = 0.73; Female r = −0.098, p = 0.79; Male r = −0.115, p = 0.75, Fig. 2F). Taken together these data suggest that in females, CeA activation is unaffected by vapor exposure, but in males CeA activity is increased following nicotine vapor exposure, regardless of concentration or formulation.
Fig. 2.
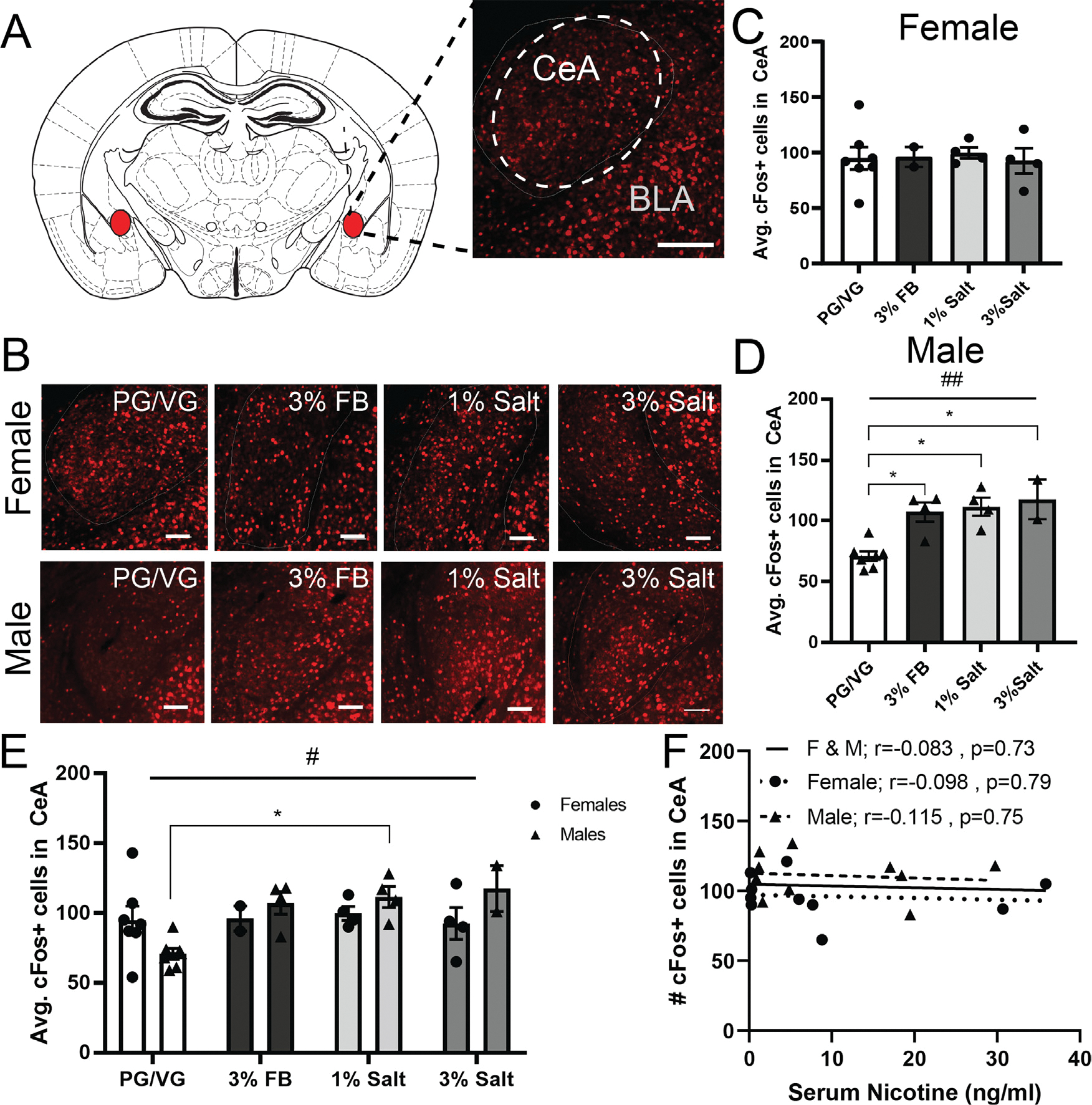
Neuronal activation in the central amygdala area following exposure to nicotine freebase vs. nicotine salt.
A) Representative image of cFos (red) in the central amygdala. Scale bar = 100 μm.
B) Representative image of cFos (red) expression in females (top row) and males (bottom row) exposed to PG/VG, 3% nicotine freebase (3% FB), 1% nicotine salt (1% salt), or 3% nicotine salt (3% salt) vapor. Scale bars = 100 μm.
C) Average cFos expression in females following exposure to different vapor content. 1-way ANOVA, p = 0.5118.
D) Average cFos expression in males following exposure to different vapor content. 1-Way ANOVA, #p = 0.0007 * Post-hoc Tukey’s Male: PG/VG vs 3% FB, PG/VG vs 1% salt. PG/VG vs 3% salt.
E) Average cFos expression in females and males exposed to different vapor content. 2-way ANOVA, #p = 0.0415 *Post-hoc Tukey’s multiple comparison test Male: PG/VG vs 1% salt.
F) Correlation of averaged cFos expression in females,males, and both sexes combined with serum nicotine levels.
Neuronal activation in the ventral tegmental area following exposure to nicotine freebase vs nicotine salt
To investigate neuronal activation in the ventral tegmental area (VTA, Fig. 3A) and its colocalization with the dopaminergic population, we performed immunohistochemistry to label for cFos and tyrosine hydroxylase (TH) in female (Fig. 3B, top) and male (Fig. 3B, bottom) animals exposed to PG/VG, 3% FB, 1% salt, or 3% salt. In females, we found a significant difference in the average cFos expression in the VTA between vapor groups (1-Way ANOVA, #p = 0.0037, F = 7.214, Fig. 3C) and post hoc Tukey’s multiple comparisons show that cFos expression was higher in the 3% FB group compared to PG/VG (p = 0.0036), 1% salt (p = 0.0097), and 3% salt (p = 0.0306) groups. In males, there was no significant difference in the average cFos expression in the VTA between vapor groups (1-Way ANOVA, p = 0.1057, F = 2.402, Fig. 3D). When the data from the two sexes were combined to examine potential sex differences using 2-Way ANOVA, we found a main effect of vapor content (#p = 0.0001, F (3, 30) = 9.460) and specifically, that females exposed to 3% FB showed increased cFos expression as compared to female PG/VG (p = 0.0005), 1% salt (p = 0.0024), and 3% salt (p = 0.0157) vapor groups (post hoc Tukey’s, Fig. 3E). We next examined activity in dopaminergic neurons by quantifying colocalization of cFos and TH and again found a main effect of vapor content (2-Way ANOVA, p < 0.0001, F (3, 30) = 12.18). Specifically, females exposed to 3% FB showed increased cFos and TH colocalization as compared to female PG/VG (p = 0.0006) and 1% salt (p = 0.0013) vapor groups (post hoc Tukey’s, Fig. 3F). We correlated VTA cFos expression with nicotine serum levels in females, males, and mice of both sexes and found a significant positive correlation with female and male data combined (r = 0.63, p = 0.0011) and female only data (r = 0.69, p = 0.013) but not with male only data (Males r = 0.46, p = 0.13, Fig. 3G). The significant correlation in both sexes combined is likely driven by the females. We performed a similar correlation analysis to examine the relationship between cFos expression in the TH population of the VTA and serum nicotine and found a positive correlation with female and male data combined (r = 0.68, p = 0.0003), female only data (r = 0.69, p = 0.014), and male only data (r = 0.65, p = 0.022, Fig. 3H). Taken together, these data suggest that females exposed to nicotine vapor in 3% freebase formulation show significantly increased VTA neuronal activity as compared to exposure to either the 1% or 3% salt formulation, the increased activity is observed in both global VTA as well as specifically in the VTA dopaminergic population, and the increased activity is likely driven by the nicotine exposure as activity was correlated with serum nicotine levels.
Fig. 3.
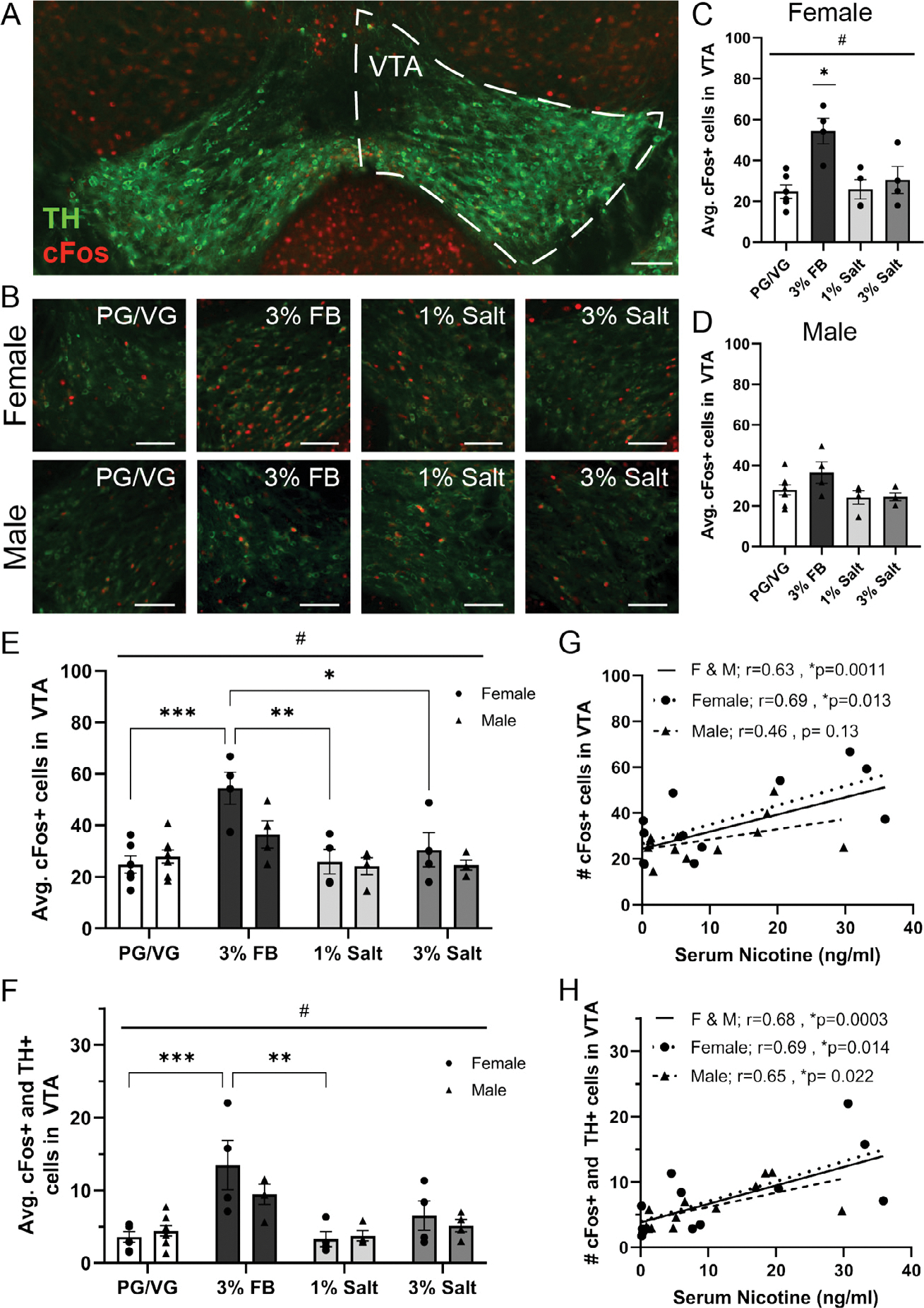
Neuronal activation in the ventral tegmental area following exposure to nicotine freebase vs. nicotine salt.
A) Representative image of the ventral tegmental area (right hemisphere outlined with dotted line) labeled with tyrosine hydroxylase (TH, green) and cFos (red). Scale bar = 100 μm.
B) Representative image of double labeling of tyrosine hydroxylase (TH, green) and cFos (red) expression in females (top row) and males (bottom row) exposed to PG/VG, 3% Nicotine Freebase (3% FB), 1% Nicotine Salt (1% Salt), or 3% Nicotine Salt (3% Salt) vapor. Scale bars = 100 μm.
C) Average cFos expression in females following exposure to different vapor content. #1-way ANOVA, p = 0.0037 * Post-hoc Tukey’s Female: 3% FB vs PG/VG, 3% FB vs 1% Salt, 3% FB vs 3% Salt.
D) Average cFos expression in males following exposure to different vapor content. 1-Way ANOVA, p = 0.1057.
E) Average cFos expression in females and males exposed to different vapor content. #Main effect of vapor content, p = 0.0001 * Post-hoc Tukey’s Female: 3% FB vs PG/VG, 3% FB vs 1% Salt, and 3% FB vs 3% Salt.
F) Average cFos and TH colocalization in females and males exposed to different vapor content. #Main effect of vapor content, p < 0.0001 * Post-hoc Tukey’s Female: 3% FB vs PG/VG and 3%FB vs 1% Salt.
G) Correlation of averaged cFos expression in females, males, and both sexes combined with serum nicotine levels.
H) Correlation of averaged cFos and TH colocalization in females, males, and both sexes combined with serum nicotine levels.
Anxiety-like and motivated behavior following exposure to nicotine freebase vs nicotine salt
Following a single vapor session of either PG/VG, 3% FB, 1% salt, or 3% salt, anxiety-like behavior was assessed using the NSF test (Fig. 4A). Females displayed no significant difference in latency to feed time between the PG/VG, 3% FB, 1% salt, and 3% salt groups (1-way ANOVA, p = 0.6399, Fig. 4B) suggesting no difference in anxiety-like behavior following a single vapor session. In contrast, there was a main effect of vapor content when comparing male latency to feed times (1-way ANOVA, #p = 0.0453, F = 3.057, Fig. 4C) with trends of the 3% FB group displaying a longer latency to feed time and more anxiety-like behavior compared to the PG/VG group (p = 0.0680) and the 1% salt group (p = 0.0904). During post-test food consumption (Fig. 4D), females displayed no significant difference between the PG/VG, 3% FB, 1% salt, and 3% salt groups (1-way ANOVA, p = 0.3928, Fig. 4E) suggesting no group differences in overall motivation to feed. There was a main effect of vapor content in the male NSF post-test (1-way ANOVA, #p = 0.0192, F = 3.914, Fig. 4F) and post hoc Tukey’s multiple comparisons show that the male 3% FB group consumed significantly less food during the post-test compared to the PG/VG group (p = 0.0497) and the 3% salt group (p = 0.0264) but not the 1% salt group. Collectively, these findings show that vapor exposure did not alter anxiety-like behavior and motivation to feed in females. However, male mice exposed to 3% nicotine freebase vapor displayed lower motivation to feed and had trends toward an increase in anxiety-like behavior.
Fig. 4.
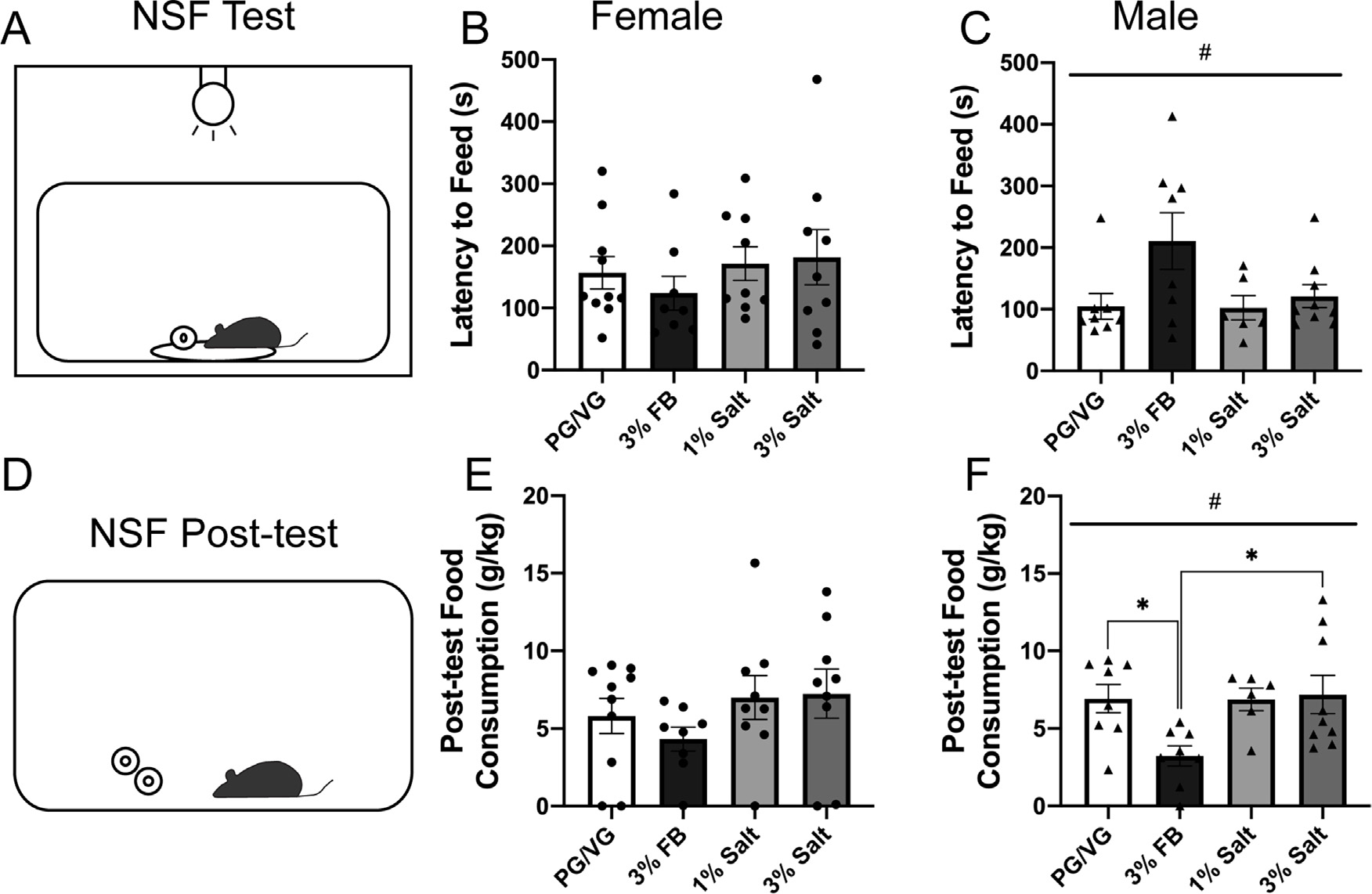
Anxiety-like and motivated behavior following exposure to nicotine freebase vs nicotine salt.
A) Diagram of the novelty-suppressed feeding (NSF) test.
B) Latency to feed time measured in females following exposure to PG/VG, 3% nicotine freebase (3% FB), 1% nicotine salt (1% salt), or 3% nicotine salt (3% salt) vapor. 1-way ANOVA, p = 0.6399.
C) Latency to feed time measured in males following exposure to PG/VG, 3% FB, 1% salt, or 3% salt vapor. #1-way ANOVA, p = 0.0453.
D) Diagram of the NSF post-test.
E) Post-test food consumption measured in females following exposure to PG/VG, 3% FB, 1% salt, or 3% salt vapor. 1-way ANOVA, p = 0.3928
F) Post-test food consumption measured in males following exposure to PG/VG, 3% FB, 1% salt, or 3% salt vapor. #1-way ANOVA, p = 0.0192 * Post-hoc Tukey’s: PG/VG vs. 3% FB and 3% FB vs. 3% salt.
Discussion
The results of this study provide important insight into the role of formulation and concentration in the sex-specific effects of electronically vaporized nicotine on nicotine metabolism, region-specific neuronal activity, and anxiety-like and motivated behavior in female and male C57BL/6J mice. Notably, nicotine vapor composed of 3% nicotine freebase produced significantly higher serum nicotine levels as compared to 1% and 3% nicotine salt formulations in both males and females, and females in the 3% freebase group displayed higher serum nicotine and serum cotinine levels compared to males. Despite these sex-specific differences in serum nicotine levels by formulation and concentration, nicotine vapor exposure of any concentration or formulation significantly increased activity in the central amygdala (CeA), but only in males. CeA activity in females exposed to nicotine vapor was not significantly different from PG/VG controls, although females displayed higher basal activity. In contrast to the CeA, only the 3% free-base group displayed increased activity in the ventral tegmental area (VTA) and this was only observed in females in both the overall VTA neuronal population and specifically in the dopaminergic VTA population. In anxiety-like and motivated behavior, females were relatively unaffected by nicotine vapor exposure, however anxiety-like behavior in males was impacted by vapor exposure, an effect primarily driven by increased anxiety-like behavior in the 3% freebase group. Males in the 3% freebase group also exhibited parallel reductions in motivated behavior. Collectively this work demonstrates the differential (and sex-specific) impacts of nicotine vapor exposure of different formulations and concentrations. These findings have significant relevance for the metabolic, neuronal and behavioral consequences of vaping in both males and females.
When studying the physiological, metabolic and behavioral effects of drugs of abuse it is imperative to take into consideration sex differences as well as delivery methods [6,8]. Previous preclinical studies have noted a difference in nicotine and cotinine serum levels in male and female mice exposed to nicotine, with males showing higher nicotine serum levels than females [7,8]. However, they failed to further investigate the reason behind the nicotine metabolite differences such as enzyme levels. While previous clinical studies included both male and female participants [3,4], they did not look at sex differences that might arise from differences in nicotine metabolism or questionnaire responses. Thus resulting in a limited understanding of the sex-specific effects that nicotine salt and freebase have in a clinical population. One limitation of clinical research is the inability to directly examine the effect nicotine exposure might have on the activation of specific brain regions. Following immunohistochemical analysis in the CeA, we found that males displayed higher neuronal activation following exposure to nicotine vapor regardless of formulation or concentration, while females did not. When quantifying cFos expression in the VTA, which is known for its role in reward signaling, only females showed an effect of vapor content with the 3% freebase group demonstrating higher VTA activation compared to other nicotine vapor-exposed groups. Following the novelty suppressed feeding test, females were unaffected by nicotine exposure while males demonstrated an effect of vapor content with a trend toward increased anxiety-like behavior in the 3% freebase group. These results suggest that sex could potentially influence how nicotine freebase or salt alters reward-seeking and anxiety-like behaviors.
Nicotine metabolite levels are a good indication of the rate at which nicotine is absorbed and then metabolized in the body. Once ingested, nicotine is rapidly metabolized into the more stable metabolite, cotinine, which is often used as a marker of nicotine use. In a human population, vaping nicotine salt resulted in higher serum nicotine levels than vaping nicotine freebase [4], suggesting that nicotine salt is more effectively absorbed by the body. Clinical studies report that nicotine salt is associated with more positive vaping experiences [3], which raises the possibility of an altered puff topography (increased puff volume or number of puffs) based on preference that may promote increased absorption. Here, we observed that nicotine freebase vapor exposure produced higher nicotine serum levels, however this is not likely due to altered respiratory rates as the freebase vapor is generally considered more aversive. Preclinical studies on the effect of formulation on metabolism are contradictory. One study conducted in rats found that following a single sub-cutaneous dose of nicotine freebase, serum nicotine levels were higher than those in rats injected with nicotine salts [6], which are consistent with the results of the current study. However, in another study, mice exposed to nicotine salt vapor had significantly higher serum cotinine levels than mice exposed to nicotine freebase. Additionally, serum cotinine levels overall appear higher in males than in females [7]. These results differ from the current study where mice exposed to 3% nicotine freebase displayed significantly higher serum nicotine levels than those exposed to 3% nicotine salt, and females in the 3% freebase group displayed higher serum nicotine levels than males. These differences could be due to different concentrations of nicotine (30 mg/ml of nicotine freebase and 30 mg/ml of nicotine salt in our study as opposed to 6 mg/ml) [7]. Furthermore, the higher serum levels we found in females compared to males could suggest the need for more nicotine in order to maintain reinforcing effects. Clinical studies measuring urine and saliva Nicotine Metabolite Ratios (NMR) in both male and female smokers found that female smokers had higher NMR than males [16]. The higher serum nicotine levels observed in females could be due to increased absorption or diminished metabolism. The possibility of sex differences in nicotine metabolizing enzymes should also be considered. Further studies are required to determine the specific metabolic mechanisms driving this effect as well as any differences that may arise following chronic exposure as this paper focused on a single acute exposure.
The CeA has been implicated in anxiety disorders and substance use such as the central effects of nicotine [13]. However the extent of CeA involvement and specific actions of nicotine in the CeA remain controversial and are potentially dependent on the method, amount, and time-course of nicotine exposure. One study found that acute nicotine exposure of a single intraperitoneal injection increased CeA phosphorylated extracellular regulated kinase (pERK) expression 20 min after injection [17], however a study of voluntary nicotine drinking found no increase in amygdala pERK after an acute (1.3 h) drinking session and only saw a significant increase in amygdala pERK after chronic (28–30 day) drinking [18]. Studies of the immediate early gene cFos are also contradictory. One study found that a single subcutaneous injection of nicotine increased cFos in the CeA, that there was no change in cFos after chronic nicotine exposure, but that cFos was increased with acute nicotine administration after chronic exposure [19]. We previously reported increased cFos and neuronal firing in the CeA after a single session of electronic nicotine vapor exposure, but no change in cFos or firing after five days of repeated sessions of nicotine vapor [11]. Another report observed increased CeA cFos after withdrawal from chronic nicotine self-administration [20]. However, exposure to different formulations and/or concentrations of nicotine may differentially impact CeA neuronal activity and subsequent affective behavior in a sex-specific manner. In females, there was no significant difference in CeA activity between groups which could be associated with the lack of nicotine effects on anxiety-like or motivated behavior observed for these groups in the NSF test. In males, CeA activity was significantly increased in the 3% freebase, 1% salt, and 3% salt groups compared to the PG/VG control group. However, only the male 3% freebase group displayed increased anxiety-like behavior and decreased motivated behavior. The use of the NSF test to examine anxiety-like behavior was first developed in studies using only males; however, this test has since been validated in females. Conditions such as anxiogenic stimuli should be considered when analyzing results from the NSF test and other behavioral assays. A study assessing how various factors (e.g. body weight, social isolation) affected food consumption and anxiety-like behavior in male and female C57Bl/6j mice in the NSF test found that estrous cyclicity was not the main source of variability in stress-induced feeding response but rather sex-specific effects of social isolation duration. All other variables tested resulted in similar anxiety-like responses from males and females [21]. In our study the group-specific differences in behavior could be associated with the higher levels of serum nicotine observed in the 3% freebase groups. As acute nicotine exposure has been associated with increased anxiety [22], the higher serum nicotine levels in males could contribute to the increased anxiety-like behavior and decreased motivated behavior in this group compared to other nicotine vapor-exposed groups. How-ever, females in the 3% freebase group also had significantly elevated serum nicotine levels, even higher levels than males, but did not display increased anxiety-like behaviors, potentially due to elevated basal CeA activity. In addition, as nicotine has appetite-suppressing properties [23], it is possible that the elevated serum nicotine levels in the male 3% freebase group contributed to the decreased motivated behavior seen in NSF, however this effect was not observed in females, suggesting that it is not a global suppression. Some limitations of using the NSF test, specifically in our study, include the stimulant effects of nicotine on locomotion and appetite. In future studies, it may be worthwhile to use an additional behavioral assay to assess anxiety-like behavior to support and validate the behavioral responses measured in the NSF test. They should also employ different (or multiple) tests of anxiety-like behavior to further validate the lack of effects of nicotine vapor on anxiety-like behavior in female mice.
The VTA and its dopaminergic activity is thought to underlie reward signaling in the brain and is a major target of drugs of abuse, including nicotine [24]. Nicotine has previously been shown to increase cell firing [25,26] and cFos expression [27] in the VTA. Additionally, studies have found that both rats [8] and mice [7] will self-administer nicotine vapor. In our study, we found that expression of the neuronal activity marker cFos in the VTA is significantly increased in females, but not males, exposed to 3% freebase vapor as compared to PG/VG controls and both 1% and 3% nicotine salt vapor. This effect is preserved in the dopaminergic population in the VTA which indicates that nicotine freebase specifically activates the dopaminergic population in the VTA more than nicotine salt, suggestive of increased reward signaling. The increased cFos expression was correlated with the serum nicotine levels indicating that these effects are likely driven by elevated levels of nicotine itself. These results may appear to contradict a previous study which found that in an electronic nicotine vapor self-administration model, female and male mice nosepoke more for nicotine salt vapor than nicotine freebase vapor [7]. However, we found that serum nicotine levels were significantly lower in the 3% nicotine salt groups as compared to the 3% nicotine freebase group which potentially could explain the higher reward-seeking behavior in the nicotine salt group in that study. If nicotine salt produces less serum nicotine than freebase nicotine, then the animals will nosepoke more to reach a level of serum nicotine that is similar to freebase nicotine exposure. Overall, our current findings indicate that nicotine formulation (freebase vs salt) can produce different serum nicotine and cotinine levels as well as differentially activate the dopaminergic VTA population which may underlie differences observed in the nicotine vapor self-administration studies. Additional experiments are required to further understand the mechanistic differences of nicotine freebase vs salt vapor exposure and how chronic exposure affects nicotine metabolism, CeA and VTA neuronal activation, and anxiety-like and motivated behaviors. As ENDS use increases in prevalence and popularity, it is important that preclinical studies investigate nicotine vapor exposure that models human use in an array of aspects such as frequency and volume of inhalation as well as emerging biochemical and technological adaptations to ENDS devices. The results of the present study highlight important sex differences in the effects of nicotine vapor exposure by formulation and concentration. These differences could have significant consequences in the acute effects of vaping as well as the development of nicotine dependence through ENDS usage in men and women. It is important to note that the changes here only reflect immediate effects following acute exposure, while clinical populations likely reflect a more prolonged chronic use. Future studies will investigate the role of formulation and concentration on long-term ENDS usage and on withdrawal or cessation of use.
Acknowledgements
This work was funded by: R01-AA-026858 (MAH), P60-AA-011605 (MAH), F31-DA-053064 (MZ), T32-NS-007431 (MZ), P30-ES10126 (CRE), P01-HL-108808 (CRE), P30-DK-065988 (CRE).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Maria Echeveste Sanchez: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft, Writing – review & editing. ManHua Zhu: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft, Writing – review & editing. Sarah Magee: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft, Writing – review & editing. Shyenne Grady: Investigation, Data curation. Hayley Guerry: Investigation, Data curation. Tara N. Guhr-Lee: Investigation, Data curation. Charles R. Esther Jr: Investigation, Data curation. Melissa A Herman: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition.
Data availability
Data will be made available on request.
References
- [1].Cornelius ME, Loretan CG, Wang TW, Jamal A, Homa DM, Tobacco product use among adults - United States, 2020, MMWR Morb. Mortal. Wkly. Rep. 71 (11) (2022) 397–405, doi: 10.15585/mmwr.mm7111a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ozga JE, Felicione NJ, Douglas A, Childers M, Blank MD, Electronic cigarette terminology: where does one generation end and the next begin? Nicotine Tob. Res. 24 (3) (2022) 421–424, doi: 10.1093/ntr/ntab164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Leventhal AM, Madden DR, Peraza N, Schiff SJ, Lebovitz L, Whitted L, . . . Tackett AP, Effect of Exposure to e-cigarettes with salt vs free-base nicotine on the appeal and sensory experience of vaping: a Randomized Clinical Trial, JAMA Netw. Open 4 (1) (2021) e2032757, doi: 10.1001/jamanetworkopen.2020.32757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].O’Connell G, Pritchard JD, Prue C, Thompson J, Verron T, Graff D, Walele T, A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers, Intern. Emerg. Med. 14 (6) (2019) 853–861, doi: 10.1007/s11739-019-02025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cohen JE, Hardesty JJ, Nian Q, Crespi E, Sinamo JK, Kennedy RD, . . . Breland AB, Combinations of electronic nicotine delivery system device and liquid characteristics among U.S. adults, Addict. Behav. 135 (2022) 107441, doi: 10.1016/j.addbeh.2022.107441. [DOI] [PubMed] [Google Scholar]
- [6].Han S, Liu C, Chen H, Fu Y, Zhang Y, Miao R, . . . Hu Q, Pharmacokinetics of freebase nicotine and nicotine salts following subcutaneous administration in male rats, Drug Test. Anal. (2022), doi: 10.1002/dta.3363. [DOI] [PubMed] [Google Scholar]
- [7].Henderson BJ, Cooper SY, Nicotine formulations impact reinforcement-related behaviors in a mouse model of vapor self-administration, Drug Alcohol Depend. 224 (2021) 108732, doi: 10.1016/j.drugalcdep.2021.108732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lallai V, Chen YC, Roybal MM, Kotha ER, Fowler JP, Staben A, . . . Fowler CD, Nicotine e-cigarette vapor inhalation and self-administration in a rodent model: sex- and nicotine delivery-specific effects on metabolism and behavior, Addict. Biol. 26 (6) (2021) e13024, doi: 10.1111/adb.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Honeycutt SC, Garrett PI, Barraza AG, Maloy AN, Hillhouse TM, Repeated nicotine vapor inhalation induces behavioral sensitization in male and female C57BL/6 mice, Behav. Pharmacol. 31 (6) (2020) 583–590, doi: 10.1097/FBP.0000000000000562. [DOI] [PubMed] [Google Scholar]
- [10].Javadi-Paydar M, Kerr TM, Harvey EL, Cole M, Taffe MA, Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats, Drug Alcohol Depend. 198 (2019) 54–62, doi: 10.1016/j.drugalcdep.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhu M, Echeveste Sanchez M, Douglass EA, Jahad JV, Hanback TD, Guhr Lee TN, . . . Herman MA, Electronic nicotine vapor exposure produces differential changes in central amygdala neuronal activity, thermoregulation and locomotor behavior in male mice, eNeuro 8 (4) (2021), doi: 10.1523/ENEURO.0189-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sikic A, Frie JA, Khokhar JY, Murray JE, Sex differences in the behavioural outcomes of prenatal nicotine and tobacco exposure, Front. Neurosci. 16 (2022) 921429, doi: 10.3389/fnins.2022.921429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gilpin NW, Herman MA, Roberto M, The central amygdala as an integrative hub for anxiety and alcohol use disorders, Biol. Psychiatry 77 (10) (2015) 859–869, doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Markou A, Review. Neurobiology of nicotine dependence, Philos. Trans. R. Soc. Lond. B Biol. Sci. 363 (1507) (2008) 3159–3168, doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR Jr., Alexis NE, Tarran R, Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung, Am. J. Respir. Crit. Care Med. 200 (11) (2019) 1392–1401, doi: 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jain RB, Nicotine metabolite ratios in serum and urine among US adults: variations across smoking status, gender and race/ethnicity, Biomarkers 25 (1) (2020) 27–33, doi: 10.1080/1354750X.2019.1688866. [DOI] [PubMed] [Google Scholar]
- [17].Valjent E, Pages C, Herve D, Girault JA, Caboche J, Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain, Eur. J. Neurosci. 19 (7) (2004) 1826–1836, doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- [18].Brunzell DH, Russell DS, Picciotto MR, In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57BL/6J mice, J. Neurochem. 84 (6) (2003) 1431–1441, doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- [19].Salminen O, Seppa T, Gaddnas H, Ahtee L, The effects of acute nicotine on the metabolism of dopamine and the expression of Fos protein in striatal and limbic brain areas of rats during chronic nicotine infusion and its withdrawal, J. Neurosci. 19 (18) (1999) 8145–8151, doi: 10.1523/JNEUROSCI.19-18-08145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Le AD, Role of central amygdala neuronal ensembles in incubation of nicotine craving, J. Neurosci. 36 (33) (2016) 8612–8623, doi: 10.1523/JNEUROSCI.1505-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Francois M, Canal Delgado I, Shargorodsky N, Leu CS, Zeltser L, Assessing the effects of stress on feeding behaviors in laboratory mice, Elife (2022) 11, doi: 10.7554/eLife.70271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Biala G, Budzynska B, Effects of acute and chronic nicotine on elevated plus maze in mice: involvement of calcium channels, Life Sci. 79 (1) (2006) 81–88, doi: 10.1016/j.lfs.2005.12.043. [DOI] [PubMed] [Google Scholar]
- [23].Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, . . . Picciotto MR, Nicotine decreases food intake through activation of POMC neurons, Science 332 (6035) (2011) 1330–1332, doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Picciotto MR, Kenny PJ, Mechanisms of Nicotine Addiction, Cold Spring Harb. Perspect. Med. 11 (5) (2021), doi: 10.1101/cshperspect.a039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, . . . Changeux JP, Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine, Nature 391 (6663) (1998) 173–177, doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- [26].Yin R, French ED, A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: an in vitro electrophysiological study, Brain Res. Bull. 51 (6) (2000) 507–514, doi: 10.1016/s0361-9230(00)00237-9. [DOI] [PubMed] [Google Scholar]
- [27].Baur K, Hach A, Bernardi RE, Spanagel R, Bading H, Bengtson CP, c-Fos marking of identified midbrain neurons coactive after nicotine administration in-vivo, J. Comp. Neurol. 526 (13) (2018) 2019–2031, doi: 10.1002/cne.24471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


