ABSTRACT
Introduction:
This review aims to systematically evaluate the available evidence on the different urodynamic diagnoses of lower urinary tract symptoms (LUTS) in young adult men aged 18–50 years and to summarize the various urodynamic parameters based on these diagnoses.
Methods:
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analysis statement and the search was performed in PubMed, Embase, and Cochrane library from inception till September 2021. A total of 295 records were identified using a combination of keywords such as LUTS, urodynamics (UDS), and young males. The review was registered in PROSPERO (CRD42021214045).
Results:
All the ten studies, which were included in this analysis, categorised the patients into either of the four primary diagnoses after the UDS – primary bladder neck obstruction (PBNO), dysfunctional voiding, detrusor underactivity (DU), or detrusor overactivity. Five of these studies used the conventional UDS, and in the other five a video UDS was performed. The most common abnormality on the conventional UDS was DU with a pooled estimate of 0.24 (95% confidence interval [CI] - 0.104–0.463, I2-95.35, (τ2-1.07). The most common abnormality on the video UDS was PBNO with a pooled estimate of 0.49 (95% CI - 0.413–0.580, I2-66.59, 2-0.09). The point estimates of various UDS parameters were also recorded.
Conclusion:
A urodynamic diagnosis was possible in 79% and 98% of the young men who underwent a conventional UDS or a video UDS, respectively. However, the men subjected to the conventional UDS and the video UDS had significant differences in their primary urodynamic diagnostic label. These results will help to plan future trials for the evaluation and management of LUTS in young men.
INTRODUCTION
According to the European epidemiological data, about one-fourth of the young men report lower urinary tract symptoms (LUTS) with an equal prevalence of storage, voiding, and post-micturition symptoms.[1] While a careful clinical evaluation is strongly recommended, it is seldom sufficient to make a working diagnosis, except in conditions such as phimosis, meatal stenosis, or acute prostatitis. Additional diagnostic evaluation recommended for all adult men, regardless of the age, includes a validated symptom questionnaire, a bladder diary, and a urinalysis (strong recommendation), as well as an estimation of the postvoid residual urine by ultrasonography and an uroflowmetry (both weak recommendations).[2] Most men are prescribed empirical treatment, including lifestyle changes, bladder training, and drug therapy for overactive bladder or voiding dysfunction based on these noninvasive investigations. However, a proportion of the young men have an unsatisfactory response to the treatment and undergo invasive urodynamics (UDS). In such men, the final urodynamic diagnosis might vary considerably from that determined by the noninvasive investigations.[3] A precise urodynamic diagnosis paves the way for additional therapeutic options in men with refractory bothersome LUTS.
Few researchers have evaluated the role of UDS in young men. Variations in the urodynamic technique and the inclusion of a relatively small number of patients has resulted in a lack of coherent data which could support the clinical decision-making. Hence, the authors embarked on this systematic review to critically appraise the available evidence regarding the UDS findings in young men with LUTS.
MATERIALS AND METHODS
Search strategy
This review was conducted and reported according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) and Meta-analysis of Observational Studies of Epidemiology guidelines.[4] After the initial scoping searches, a systematic search of the PubMed, Embase, and Cochrane Central Register of Controlled Trials databases was performed, including studies from the inception to September 2021 to identify the relevant studies published in the English language. Secondary sources such as the guidelines and the citations were examined in the relevant articles and additional hand searches were performed. The review was registered in PROSPERO (CRD42021214045). This study is a systematic review of the available evidence and is exempted from the Institutional Review Board. The various search terms used a combination of LUTS, young males, UDS, and age 18–50 years.
The detailed search strategy used for each database is given in Supplementary Table 1.
Supplementary Table 1.
Search strategy for selection of eligible studies to be included in the systematic review
| Database | Search terms | Number of studies identified |
|---|---|---|
| PubMed | (Lower urinary tract symptoms OR LUTS OR prostatism) AND (young males OR males less than 50 years OR aged 50 years and younger) AND (urodynamics OR video urodynamics OR ambulatory urodynamics) NOT prostate cancer NOT benign prostatic hyperplasia NOT BPH NOT women NOT children | 177 |
| Embase | (Lower urinary tract symptoms OR LUTS OR prostatism) AND (young males OR males less than 50 years OR aged 50 years and younger) AND (urodynamics OR video urodynamics OR ambulatory urodynamics) NOT prostate cancer NOT benign prostatic hyperplasia NOT BPH NOT women NOT children | 36 |
| Cochrane | (Lower urinary tract symptoms OR LUTS OR prostatism) AND (young males OR males less than 50 years OR aged 50 years and younger) AND (urodynamics OR video urodynamics OR ambulatory urodynamics) NOT prostate cancer NOT benign prostatic hyperplasia NOT BPH NOT women NOT children | 82 |
Evidence synthesis
A systematic review was conducted, and the relevant quantitative and qualitative data were extracted from the eligible studies based on the inclusion and the exclusion criteria. Males between 18–50 years of age with LUTS in whom an invasive UDS was performed were included. Patients with neurogenic LUTS, those with neoplasms of the urinary tract or with structural obstruction such as urethral stricture disease, and the studies reporting data for heterogeneous group of patients without separately reporting the outcomes for young men were excluded. After searching all the accessible databases, a total of 295 studies were identified. After removing the duplicates, 285 studies were selected for screening. The title and the abstracts of these studies were screened and 18 studies were identified for the full-text screening. After following the inclusion and the exclusion criteria, ten studies were eligible for the analysis. A manual search of the references of each of the included study was also performed. Finally, ten studies were included in this systematic review. A meta-analysis could not be carried out due to the high heterogeneity among the included studies.
Figure 1 shows the flow chart of the steps taken to select the eligible studies based on the PRISMA guidelines. The first reviewer performed the data extraction, and it was independently cross-checked by the second reviewer using a predesigned data extraction pro forma. The pro forma was modified and adapted throughout the extraction process, according to the characteristics mentioned in the included studies. The extracted data included, but was not limited to, the study design, sample size, year of publication, and the outcome measures.
Figure 1.
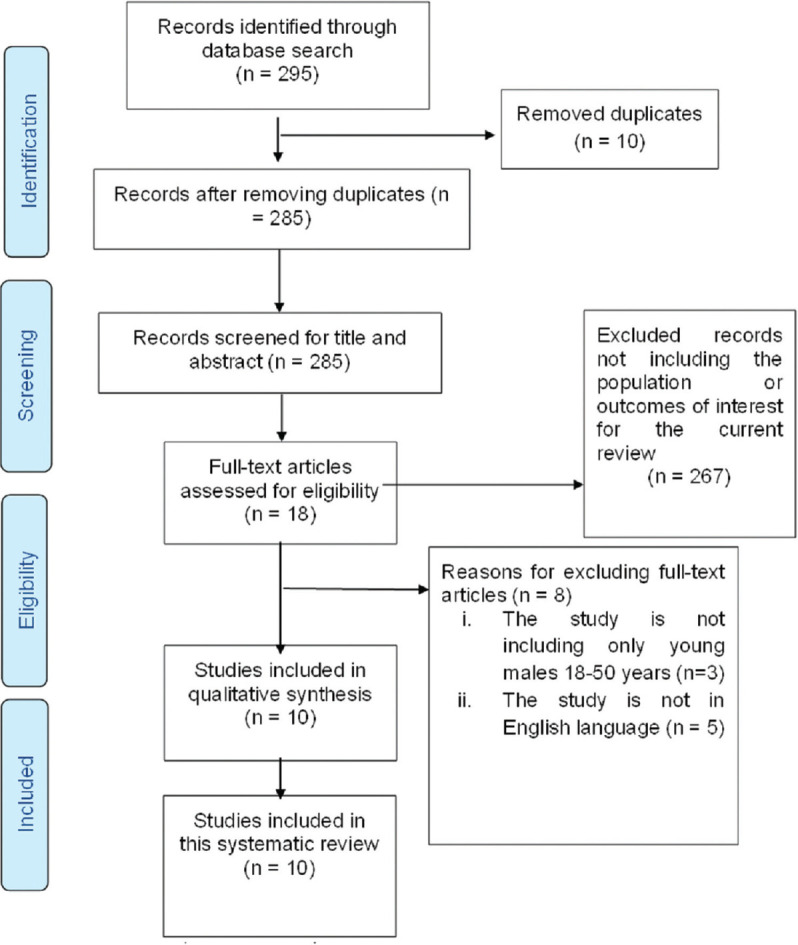
PRISMA flow diagram for selection of eligible studies. PRISMA = Preferred Reporting Items for Systematic Review and Meta-analysis
Risk of bias assessment
The methodological index for non-randomized studies (MINORS) was used to asses the risk of bias. It contains eight methodological items for the non-randomized non-comparative studies and an additional four items for the comparative non-randomized studies. The items are scored as 0 for the non-reported data, 1 for the reported but inadequate data, and 2 for the reported and adequate data. This index has an excellent inter-reviewer agreement, good internal consistency, and has high test–retest reliability. Studies with MINORS >50% were considered to be of high quality.[5] The studies included in this review were descriptive cohorts; therefore, the MINORS scale was modified as there was no data on the follow-up of the patients included in the studies. The risk of bias, as determined by MINORS is shown in Supplementary Table 2.
Supplementary Table 2.
Methodological index for nonrandomized studies bias assessment
| Author | Clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of study | Unbiased assessment of the study endpoints | Prospective calculation of study sample | Total MINORS score | Oxford level of evidence |
|---|---|---|---|---|---|---|---|---|
| Crisp, 1976[6] | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 4 |
| Abrams, 1981[7] | 0 | 0 | 2 | 2 | 2 | 0 | 6 | 4 |
| Kaplan, 1996[8] | 2 | 0 | 0 | 2 | 2 | 0 | 6 | 4 |
| Kaplan, 1997[9] | 2 | 0 | 0 | 2 | 2 | 0 | 6 | 4 |
| Nitti, 2002[3] | 2 | 2 | 2 | 2 | 2 | 0 | 10 | 2B |
| Wang, 2003[10] | 2 | 2 | 2 | 2 | 2 | 0 | 10 | 2B |
| Toh, 2006[11] | 2 | 0 | 0 | 2 | 2 | 0 | 6 | 4 |
| Karami, 2011[12] | 2 | 0 | 2 | 2 | 2 | 0 | 8 | 2B |
| Jamzadeh, 2014[13] | 2 | 0 | 0 | 2 | 2 | 0 | 6 | 4 |
| Jeong, 2014[14] | 2 | 0 | 0 | 2 | 2 | 0 | 6 | 4 |
|
| ||||||||
| MINORS Scale interpretation | ||||||||
|
| ||||||||
| Interpretation of individual factors | Score of individual factors | |||||||
|
| ||||||||
| Not reported | 0 | |||||||
| Reported but inadequate | 1 | |||||||
| Reported and adequate | 2 | |||||||
MINORS >50% is considered high-quality study. MINORS=Methodological index for nonrandomized studies
The MINORS scale was modified according to the studies in this review. The risk of bias was high in one study, intermediate in seven studies, and low in 2 studies.
The oxford level of evidence of the studies was also assessed. Three studies were level 2B studies, and seven were Level 4 studies.
Statistical analysis
The data management was performed using the Microsoft Excel for study characteristics, and the data was modified accordingly. A narrative synthesis of the data was undertaken for all the included studies. The studies were checked for the principle of homogeneity. I2 test was used to test for the statistical heterogeneity among the studies. Comprehensive Meta-Analysis version 3 software was used for creating the forest plots of the selected studies for different outcome measures using a random-effects model. The results of the forest plots were reported along with the values of the I2 test, and 95% confidence intervals (CIs) to depict the high heterogeneity among the studies. Different outcome measures were reported using appropriate statistics, such as mean and standard deviation or median and interquartile range for the continuous variables and proportions or percentages for the categorical variables and other appropriate statistical parameters based on the data available in the studies. For the studies that reported means with the standard error or CIs, the standard deviation was calculated to determine the pooled estimates.
Outcome measures
Primary outcome:
The role of UDS for the evaluation of LUTS in young males
Identification of different urodynamic diagnosis in young males with LUTS.
Secondary outcomes:
The various urodynamic parameters in the different urodynamic diagnoses.
RESULTS
Overall study characteristics
This systematic review included ten studies with 1474 men between 18 and 50 years of age. Six of these studies were published between 2000 and 2020. The age group of the subjects varied in different studies [Supplementary Table 3]. The presenting symptoms were recorded in five studies but none mentioned whether the storage symptoms or the voiding symptoms were predominant. Five studies recorded the duration of the symptoms, and in 4 of these, the duration was over 24 months (mean 30.12 months). The conventional UDS was performed in five studies, while the remaining five used the video UDS.
Supplementary Table 3.
Overall characteristics of the included studies
| Characteristics of the studies | Number of studies, n (%) |
|---|---|
| Year of publication | |
| 1970-2000 | 4 (40) |
| 2001-2020 | 6 (60) |
| Lower age limit (years) | |
| 17 | 1 (10) |
| 18 | 4 (40) |
| 19 | 1 (10) |
| 20 | 1 (10) |
| 21 | 1 (10) |
| 23 | 1 (10) |
| 28 | 1 (10) |
| The upper limit (years) | |
| 40 | 2 (20) |
| 44 | 1 (10) |
| 45 | 2 (20) |
| 49 | 1 (10) |
| 50 | 4 (40) |
| Sample size (range) | |
| 0-100 | 6 (60) |
| 100-200 | 1 (10) |
| >200 | 3 (30) |
| Duration of symptoms (months) | |
| <24 | 1 (10) |
| >24 | 4 (40) |
| Data not available | 5 (50) |
| Mean duration of symptoms | 30.12 months |
| Urodynamic technique | |
| Conventional | 5 (50) |
| Video urodynamics | 5 (50) |
Characteristics of individual studies
The characteristics of the individual studies are summarised in Table 1 and Supplementary Table 4. Four of the studies were prospective and six were retrospective. The mean IPSS score was reported in four, and the pre-UDS treatment records of the patient population was reported only in three studies. Post UDS, all the studies classified patients in either of these four diagnoses – primary bladder neck obstruction (PBNO), dysfunctional voiding (DV), detrusor underactivity (DU), and detrusor overactivity (DO). Management options were also not properly evaluated in the studies and none reported the outcomes of the treatment which was advised based on the urodynamic diagnosis.[3,6,7,8,9,10,11,12,13,14] The study by Toh et al. was included in this systematic review, despite the lower age limit of inclusion in their study being 17 years, as the patient population, aims, and objectives of their study was similar to those required by this systematic review.[11]
Table 1.
Characteristics of individual studies
| Article reference | Age range | Number of patients | Mean duration of symptoms (months) | Mean IPSS | Pre-UDS treatment | Type of UDS | Post-UDS diagnosis | Post-UDS management | Follow-up | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| PBNO | DV | DU | DO | |||||||||
| Crisp et al., 1976[6] | 28-44 | 8 | NA | NA | NA | Conventional | 1 | 0 | 0 | 0 | NA | NA |
| Abrams et al., 1981[7] | 20-45 | 204 | NA | NA | NA | Conventional | 21 | 0 | 31 | 12 | NA | NA |
| Kaplan et al., 1996[8] | 21-50 | 137 | 37.6 | 17.6 | Antibiotics-137 (100%), alpha blockers-98 (72%) | Video | 74 | 33 | 7 | 0 | NA | NA |
| Kaplan et al., 1997[9] | 23-50 | 43 | 33.6 | 17.5 | Antibiotics-43 (100%) | Video | 30 | 0 | 0 | 17 | Behavioural therapy and feedback | 3 months |
| Nitti et al., 2002[3] | 18-45 | 85 | NA | 19.3 | NA | Video | 40 | 12 | 1 | 5 | NA | NA |
| Wang et al., 2003[10] | 18-50 | 90 | 28.3 | 19.8 | NA | Video | 37 | 39 | 0 | 0 | Multiple | NA |
| Toh and Ng, 2006[11] | 17-49 | 56 | NA | NA | NA | Conventional | 14 | 1 | 5 | 9 | NA | NA |
| Karami et al., 2011[12] | 18-40 | 456 | 12.3 | NA | Multiple | Conventional | 96 | 69 | 59 | 62 | NA | NA |
| Jamzadeh et al., 2014[13] | 19-40 | 87 | NA | NA | NA | Video | 37 | 25 | 10 | 7 | NA | NA |
| Jeong et al., 2014[14] | 18-50 | 308 | 38.8 | NA | NA | Conventional | 80 | 72 | 39 | 41 | NA | NA |
NA=Not available, PBNO=Primary bladder neck obstruction, DV=Dysfunctional voiding, DU=Detrusor underactivity, DO=Detrusor overactivity, IPSS=International prostate symptom score, UDS=Urodynamics
Supplementary Table 4.
Definitions of urodynamic diagnoses used in different studies
| Article reference | PBNO | DV | DU | DO |
|---|---|---|---|---|
| Crisp et al., 1976[6] | NA | NA | NA | NA |
| Abrams et al., 1981[7] | An obstructed bladder was shown by maximum flow rates of <15 mL/s with a maximum detrusor pressure in excess of 70 cm H20 | NA | The diagnosis of an underactive detrusor was made when flow rates were below normal secondary to a maximum flow detrusor pressure of <30 cm H20 | An unstable bladder was defined as a bladder which, during filling, showed contractions of at least 15 cm. of water above resting bladder pressure, which occurred while the patient was trying to inhibit micturition |
| Kaplan et al., 1996[8] | BOO was defined as a sustained detrusor contraction of >45 cm H2O and a catheterized uroflow of <12 mL/s | Pseudodyssynergia (voluntary closure of the membranous urethra during voiding) was made based on a number of criteria, including electrical activity of the external sphincter during voiding in the absence of abdominal straining, and brief and intermittent closing of the membranous urethra during voiding. This was detected by both EMG and | Impaired detrusor contractility (IC) was defined by low detrusor contractions (<30 cm H2O) and a catheterized uroflow of <12 mL/s | DI was defined as a nonvolitional, phasic increase in detrusor pressure of at least 15 cm H20 or a rise in detrusor pressure associated with increased sensation of urgency |
| Kaplan et al., 1997[9] | BOO was defined as a sustained detrusor contraction of >45 cm and a catheterized uroflow of <12 mL/s | Pseudodyssynergia was diagnosed based on several criteria, including electrical activity of the external sphincter during voiding in the absence of abdominal straining and brief, intermittent closing of the membranous urethra during voiding detected on EMG and fluoroscopy | Impaired detrusor contractility was defined as low detrusor contractions (<30 cm water) and a catheterized urine flow rate of <12 mL/s | Detrusor instability was defined as a nonvolitional, phasic increase in detrusor pressure of at least 15 cm. water or an increase in detrusor pressure associated with increased sensation of urgency |
| Nitti et al., 2002[3] | NA | NA | Impaired contractility was diagnosed when BOO index was <20 and uroflow was <12 mL/s. Fluoroscopic images of the bladder outlet during voiding were taken with specific attention to opening (or nonopening) or focal narrowing of the bladder neck | Detrusor instability was considered present if there was an acute increase in detrusor pressure (involuntary contraction) associated with an urge regardless of pressure, or an increase in detrusor pressure of 15 cm. H2O or greater without an urge |
| Wang et al., 2003[10] | Primary bladder neck obstruction is defined as narrowing only at the vesical neck on fluoroscopic voiding cystourethrogram, sustained detrusor contraction during voiding, low peak flow rate, obstructive flow pattern and relaxed external sphincter EMG. The diagnostic criteria of benign prostatic obstruction are similar to those of primary bladder neck obstruction except the narrowing was noted at the prostatic urethra | Dysfunctional voiding is defined as obstruction at the external sphincter determined by intermittent increase in sphincter EMG and/or intermittent narrowing of membranous urethra on fluoroscopy | Impaired detrusor contractility is defined as detrusor pressure <30 cm H2O, Qmax <15 mL/s and no obstruction identified radiologically | Idiopathic detrusor overactivity is defined as an increase in detrusor pressure (involuntary contraction) associated with an urge regardless of pressure, or an increase in detrusor pressure of 15 cm H2O or greater without an urge |
| Toh et al., 2006[11] | NA | NA | NA | NA |
| Karami et al., 2011[12] | The diagnosis of bladder neck dysfunction was made indirectly by the urodynamic findings of outlet obstruction in a typical clinical situation in the absence of urethral stricture, prostatic enlargement, and striated sphincter dyssynergia | Dysfunctional voiding was defined as an intermittent and/or fluctuating flowrate due to involuntary intermittent contractions of the peri-urethral striated muscle during voiding, in neurologically normal individuals | Detrusor underactivity was defined as a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying within anormal time span | Detrusor over activity is a urodynamic observation characterized by involuntary contractions during the filling phase which may be spontaneous or provoked |
| Jamzadeh et al., 2014[13] | BOO was defined as a sustained detrusor contraction of >45 cm H2O and a catheterized uroflow of <12 mL/s | Dysfunctional voiding (voluntary closure of the membranous urethra during voiding) was made based on a number of criteria, including electrical activity of the external sphincter during voiding in the absence of abdominal straining, and brief and intermittent closing of the membranous urethra during voiding. In addition, to make the diagnosis of dysfunctional voiding, a uroflow measurement performed in a private setting showing intermittent increases and decreases in flow in an undulating fashion was required | Detrusor underactivity was defined by low detrusor contractions (<30 cm H2O) and a catheterized uroflow of <12 mL/s | DO was defined as a nonvolitional, phasic increase in detrusor pressure of at least 15 cm H2O or a rise in detrusor pressure associated with increased sensation of urgency |
| Jeong et al., 2014[14] | BOO, defined as an AG number of 40 or greater or 20-39.9 with a slope of the linear passive urethral resistance ratio of >2 cm H2O/mL/s, where the AG number was calculated as the PdetQmax-2 Qmax, was present concomitant with EMG evidence of external sphincter relaxation, and neither urethral stricture nor prostatic enlargement was observed | DV was diagnosed on the basis of the EMG activity of the external sphincter/pelvic floor during voiding in the absence of abdominal straining. If DV was diagnosed during a PFS, a free uroflow measurement was performed in a private setting to identify undulating intermittent increases and decreases in flow | DU was diagnosed when the AG number was <20 and the Qmax was <12 mL/s during a PFS and no obstruction was recognized in urethrocystoscopy or TRUS | Patients were regarded as positive for idiopathic DO if a spontaneous or provoked involuntary detrusor contraction was observed during the filling cystometry |
NA=Not available, PBNO=Primary bladder neck obstruction, DV=Dysfunctional voiding, DU=Detrusor underactivity, DO=Detrusor overactivity, BOO=Bladder outlet obstruction, EMG=Electromyography, AG=Abrams-Griffith, DI=Detrusor instability, PdetQmax=Detrusor pressure at maximum flow, PFS=Pressure flow study, TRUS=Transrectal Ultrasound, IC=Involuntary contraction, AG=Abrams-Griffith number
Synthesis of results
Diagnosis based on conventional urodynamics
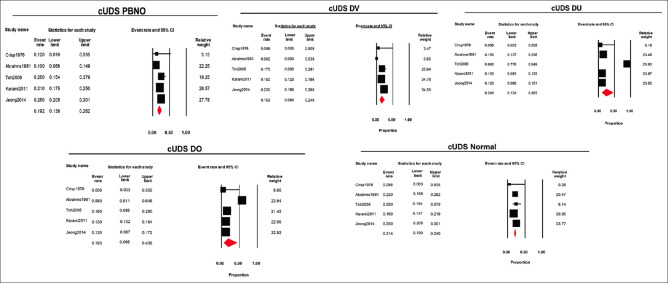
Five (50%) studies used the conventional UDS to diagnose LUTS in young adult men. The pooled estimate was determined using the diagnosis made after the UDS by a random-effects model. The pooled estimate (95% CI, I2, and 2) of various diagnoses obtained from the conventional UDS for PBNO, DV, DU, and DO was 0.19 (0.138–0.262, 77.57, 0.13), 0.15 (0.094–0.240, 78.82, 0.21), 0.24 (0.104–0.463, 95.35, 1.07), and 0.19 (0.068–0.438, 97.49, 1.56), respectively. Normal findings were reported with a pooled estimate (95% CI, I2, and 2) of 0.21 (0.190–0.240, 43.41, and 0.02). Therefore, the relative frequency of PBNO, DV, DU, DO, and normal findings was 19%, 15%, 24%, 19%, and 21%, respectively [Figure 2].
Figure 2.
Forest plots of different diagnosis obtained using conventional urodynamics (PBNO = Primary bladder neck obstruction, DV = Dysfunctional voiding, DO = Detrusor overactivity, DU = Detrusor Underactivity)
Diagnosis based on video urodynamics
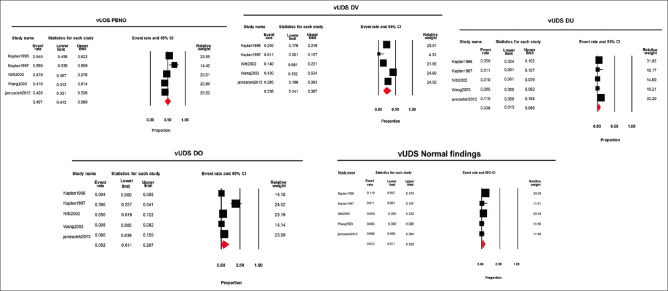
Five (50%) studies used the video UDS to diagnose LUTS in young adult males. The pooled estimate was determined using the diagnosis made after the video-UDS using a random-effects model. The pooled estimate (95% CI, I2, and 2) of various diagnoses obtained from the video UDS for PBNO, DV, DU, and DO was 0.49 (0.413–0.580, 66.59, 0.09), 0.23 (0.141–0.367, 83.76, 0.37), 0.03 (0.013–0.095, 64.34, 0.72), and 0.05 (0.011–0.207, 89.88, 2.50), respectively. Normal findings were reported with a pooled estimate (95% CI, I2, and 2) of 0.03 (0.011–0.100, 66.95, 0.95). Therefore, the relative frequency of PBNO, DV, DU, DO and normal findings was 49%, 23%, 3%, 5%, and 3%, respectively [Figure 3].
Figure 3.
Forest plots of different diagnosis obtained using video urodynamics (PBNO = Primary bladder neck obstruction, DV = Dysfunctional voiding, DO = Detrusor overactivity, DU = Detrusor Underactivity)
Urodynamic parameters
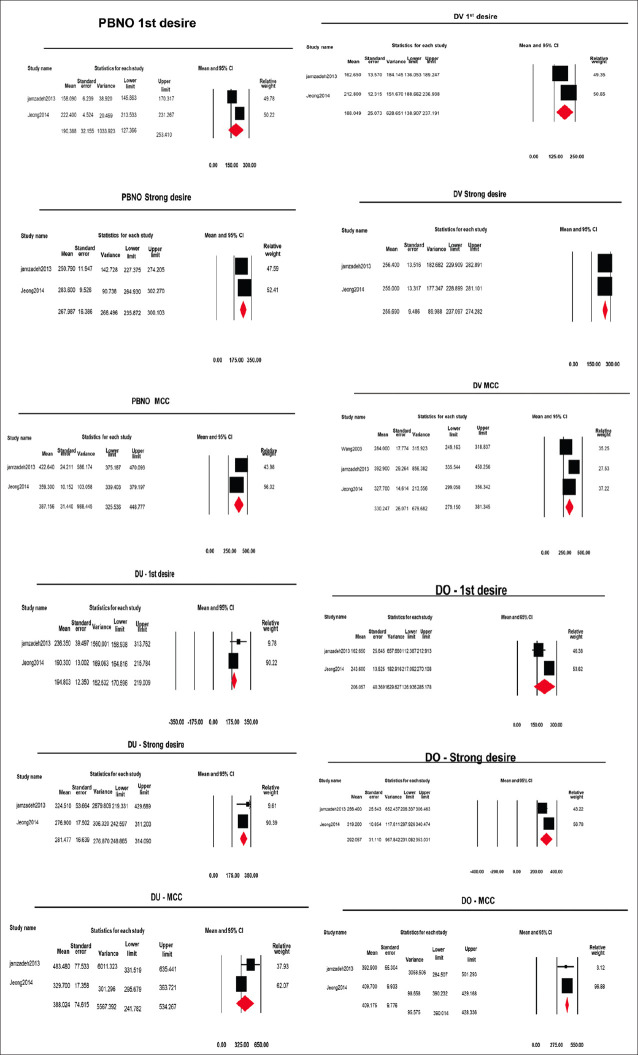
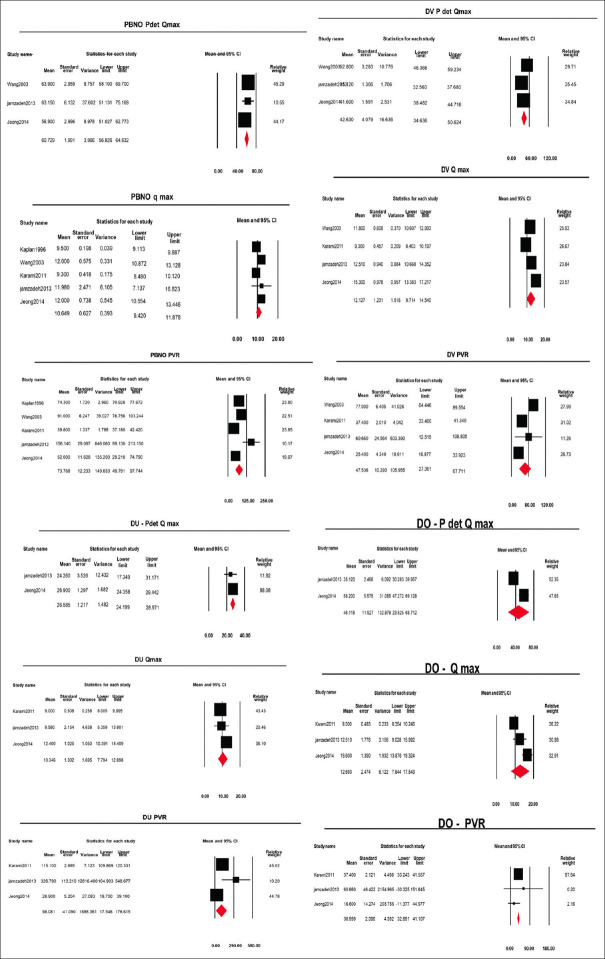
The urodynamic data were not reported in all of the studies. The forest plots were made according to the urodynamic data reported in the studies, as shown in Figures 4 and 5.
Figure 4.
Forest plots of urodynamic parameters in storage phase of different urodynamic diagnosis (PBNO = Primary bladder neck obstruction, DV = Dysfunctional voiding, DO = Detrusor overactivity, DU = Detrusor Underactivity)
Figure 5.
Forest plots of urodynamic parameters in voiding phase of different urodynamic diagnosis (PBNO = Primary bladder neck obstruction, DV = Dysfunctional voiding, DO = Detrusor overactivity, DU = Detrusor Underactivity)
Storage phase
Primary bladder neck obstruction
The point estimate (95% CI, I2, and 2) of volume at first desire, strong desire, and maximum cystometric capacity was 190.3 ml (127.3–253.4, 82.8, 1661.3), 267.9 ml (235.87–300.1, 78.3, 421.1), and 387.1 ml (325.5–448.7, 88.7, 2230.3), respectively [Forest plots in Figure 4].
Dysfunctional voiding
The point estimate (95% CI, I2, and 2) of volume at first desire, strong desire and maximum cystometric capacity was 188 ml (138.9–237.1, 86.65, 1089.6), 255.7 ml (237.0–274.2, 0.0, 0.0), and 330.2 ml (279.1–381.3,81.1, 1612.4), respectively.
Detrusor underactivity
The point estimate (95% CI, I2, and 2) of volume at first desire, strong desire, and maximum cystometric capacity was 194.8 (170.5–219.0, 18.4, 195.7), 281.4 (248.8–314.0, 0.0, 0.0), and 388 ml (241.7–534.2, 73.31, 8667.8), respectively.
Detrusor overactivity
The point estimate (95% CI, I2, and 2) of volume at first desire, strong desire and maximum cystometric capacity was 208 (126.9–285.1, 87.17, 2856.1), 292 (231.0–353.0, 80.47, 1586.8), and 409 ml (390.0–428.3, 0.0, 0.0), respectively.
Voiding phase
Primary bladder neck obstruction
The point estimate (95% CI, I2, and 2) of Pdet.Qmax was 60.7 cm H2O (56.82–64.63, 31.90, 6.31), Qmax was 10.4ml/min (9.42–11.87, 85.72, 1.40) and Post void residue (PVR) was 73.7 ml (49.79–97.74, 98.66, 652.72) [Forest plots in Figure 5].
Dysfunctional voiding
The point estimate (95% CI, I2, and 2) of Pdet.Qmax was 42.6 cm H2O (34.6–50.6, 93.1, 45.2), Qmax was 12.1ml/min (9.7–14.5, 91.9, 5.4), and PVR was 47.5 ml (27.3–67.7, 93.5, 337.5).
Detrusor underactivity
The point estimate (95% CI, I2, and 2) of Pdet. Qmax was 26.5 cm H2O (24.1–28.9, 0.0, 0.0), Qmax was 10.34 ml/min (7.7–12.8, 77.3, 3.64), and PVR was 98 ml (17.5–178.6, 99.1, 3743.0).
Detrusor overactivity
The point estimate (95% CI, I2, and 2) of Pdet.Qmax was 46.1 cm H2O (23.5–68.7, 93.0, 247.7), Qmax was 12.7 ml/min (7.8–17.5, 92.4, 16.7), and PVR was 36.9 ml (32.8–41.1, 14.4, 32.1).
DISCUSSION
This review suggests that UDS is as an effective tool for arriving at a diagnosis in young men with refractory non-neurogenic LUTS.[3,8,9,10,11,12,13] An abnormality was noted in 79% of the men undergoing a conventional UDS and 97% of those who underwent a video UDS, with the remaining being normal. Of note, there were no inconclusive reports. The commonest abnormality noted in men who underwent a conventional UDS was DU (24% of patients) whereas it was PBNO (49% of patients) in those subjected to the video UDS. However, these studies do not represent the real-world scenario as they are from the specialized centers.
Men under 50 years of age form a significant proportion of all the adult men who undergo an UDS evaluation, accounting for about a quarter of all the studies.[15] In the population based studies, up to 0.7% of the young men have been noted to need an alpha-adrenergic antagonist for the voiding symptoms.[16] Literature suggests that one-third to half of all the men with PBNO are refractory to alpha-adrenergic blockers.[17,18] Current guidelines, such as the European Association of Urology Guidelines, recommend UDS before invasive intervention in men under the age of 50 years with a weak strength rating.[2] In addition, overactive bladder affects about 1.3% of men under the age of 50 years.[19]
An unknown proportion of these men will ultimately be refractory to the medical management. While not universally recommended, some of these men might also be candidates for UDS evaluation.[20] Hence, the UDS can be useful in a wide spectrum of young men with LUTS, both with storage as well as voiding symptoms.[3,13]
The difference in the primary diagnostic label between the two urodynamic techniques is striking. About one-third of the men undergoing a conventional UDS were reported to be obstructed, against two-thirds of the men undergoing a video UDS. To determine whether this apparent difference might be attributable to the differences in the patient population, an evaluation of the clinical background of the cohorts is essential. Unfortunately, only four of the ten studies provided a standardized symptom score and all these studies performed the video UDS. Of note, the IPSS score of men in these four studies was very similar [Table 1]. It is uncertain whether one can assume that this consistent clinical presentation can be taken as representative for all the young men with refractory LUTS. Differences in the clinical spectrum of the other six studies might account for some of the observed differences in the final diagnostic label.
Conventional UDS can differentiate bladder outlet obstruction from impaired detrusor contractility and relies on the sphincter EMG and the clinical judgment to define the exact level of obstruction. Video UDS can localize the level of obstruction by simultaneous fluoroscopic imaging and provides a precise anatomical information about the lower urinary tract, including bladder morphology and reflux, which might influence the interpretation of the UDS findings.[10] As the management strategy would differ for each of the urodynamic diagnosis, an accurate diagnosis is vital for planning an invasive management.
Differences in the technique and philosophy of UDS are likely to be an important reason for some of the observed differences in the diagnosis. The diagnosis of DV and PBNO, on the conventional UDS, rests on the sphincteric activity during the voiding phase as detected by the EMG.[14] Bladder outflow obstruction by DV or PBNO can be differentiated by the abnormal, excessive EMG activity and the sphincteric silence during the voiding phase, respectively, on the conventional UDS. However, EMG remains the least reliable and the most subjective part of the UDS investigation. Some men might have received an erroneous diagnosis of PBNO or DV on the conventional UDS due to the erratic performance of the EMG during the test.[21] Karami et al. have made the diagnosis of bladder neck dysfunction based on the urodynamic observation of obstructive symptoms and an absence of urethral stricture, prostatic enlargement, and striated sphincter dyssynergia.[12] Centers offering video UDS tend to ignore the findings of sphincter EMG or altogether avoid its use and instead rely on the fluoroscopy data for making the diagnosis. Existing evidence is insufficient to draw conclusions regarding the accuracy of each of these approaches.
Further, a study of large cohorts of men undergoing UDS has shown that detrusor underactivity is common in young men. The median Bladder Contractility Index (BCI) has been reported to be about 100 in several studies.[22,23] This would imply that about half of all the men undergoing UDS technically have “underactivity.” However, many of these men might not get a primary label of underactivity if they have concomitant obstruction. A man with Pdet. Qmax 50 cm H20 and Qmax 5 ml/s, implying a BCI of 75 (underactive) and BOOI of 40 (obstructed), is very likely to be labeled as bladder outlet obstruction (and subsequently DV or PBNO depending on the outlet function). However, in men with equivocal obstruction, this is by no means so certain. Another man with Pdet. Qmax 40cm H20 and Qmax 5 ml/s, implying a BCI of 65 and BOOI of 30, might get labeled as “underactive” unless the diagnosis of (equivocal) obstruction can be made confidently. Video UDS might offer the additional confidence required to make the primary diagnosis in such a patient as PBNO or DV instead of just “underactive.” Overall, the video UDS appears to offer a more “actionable” primary diagnosis and enables the specific therapeutic pathways of management. An alternate approach might be to offer a standalone voiding cystourethrogram to these men and combine the findings with conventional UDS intuitively. This approach has been considered acceptable in some circumstances.[12,14] However, there is a lack of evidence regarding this combination in young men with refractory non-neurogenic LUTS.
Clinical symptoms are adequate to make a working diagnosis and initiate the noninvasive treatment. However, the predictive value of symptoms alone is inadequate to make the decisions concerning the invasive interventions, perhaps except for overactive bladder. Patients presenting with voiding symptoms may not demonstrate bladder outlet obstruction,[3] and in some young men with refractory storage symptoms, the symptoms might be associated with subclinical obstruction.[3,13] UDS offers the most accurate assessment for establishing an unequivocal diagnosis, however, the choice of the technique of the UDS remains an unresolved question. The urodynamic diagnosis has important implications in the patient care. Men with a diagnosis of DV can be confidently offered pelvic floor relaxation training sessions, intrasphincteric botulinum toxin, or neuromodulation, while those with PBNO could potentially undergo a curative bladder neck incision. Misdiagnosis can lead to the appropriate treatment being withheld or the delivery of an inappropriate treatment.
An important caveat in interpreting this review is that the analysis is based on the primary urodynamic diagnosis and disregards the importance of the ancillary findings. Such additional findings are typical and might influence the treatment decisions or the prognosis. Jeong et al. showed that half of the patients with voiding phase disorders had associated storage phase disorders on the urodynamic assessment, while two-thirds of those with storage phase disorders had associated voiding phase disorders.[14] Similarly, Nitti et al. showed that 85% of men with DO had concomitant voiding phase disorders.[3] Further studies are required to elucidate the appropriate treatment options in patients with such mixed disorders.
The long duration of symptoms before subjecting the men to UDS is noteworthy. On an average, men had symptoms for 2-and-a-½ years before being offered an UDS test. The lack of response to alpha-adrenergic blockers would have become apparent within days of the commencement of the therapy. Since tachyphylaxis is not a common finding in men on treatment with an alpha-adrenergic blocker, the long duration of waiting might reflect the deferment of the invasive testing until an invasive treatment was “on the table,” gradual progression of the disease, or a general reluctance to offer an invasive testing. In general practice, one would offer UDS if it has therapeutic implications. Of note, UDS can pave the way for alternative therapies such as pelvic floor relaxation training or intrasphincteric botulinum toxin injection when DV is identified.
An essential finding of this review is the lack of good-quality evidence regarding the role of UDS in young men. There is considerable heterogeneity in the studies evaluating young men with LUTS. Despite being a common clinical problem, there were only a handful of studies on the subject with a small number of patients. None of the studies compared the clinical and UDS-based management outcomes and none compared the conventional UDS with the video UDS. Centers appeared to offer the video UDS or the conventional UDS based on the availability rather than as an evidence-based choice. None of the studies examined the harms of UDS in this patient population. More data are required regarding the safety, tolerability, and acceptance of UDS and video UDS in young men. This would help to identify subgroups of men in whom the benefits of an invasive UDS evaluation outweighs the risks and will also clarify which young men (or whether all young men) undergoing UDS should be offered a video UDS.
CONCLUSION
This systematic review shows a paucity of evidence regarding the clinical role and technique of UDS in young men with refractory LUTS. Available studies are limited by the lack of adequate data on the clinical presentation as well as the treatment. A urodynamic diagnosis is possible in most of the young men who undergo the study. However, men subjected to the conventional UDS and the video UDS appear to demonstrate significant differences in their primary urodynamic diagnostic label, the reasons for which remain uncertain. While the video UDS might provide greater precision and a more actionable primary diagnosis, it remains uncertain whether this apparent difference is due to the differences in the patient characteristics. There is also a lack of data regarding the harms of UDS in this patient population.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Irwin DE, Milsom I, Kopp Z, Abrams P, Artibani W, Herschorn S. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: Impact of overactive bladder. Eur Urol. 2009;56:14–20. doi: 10.1016/j.eururo.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 2.EAU Guidelines: Management of Non-Neurogenic Male LUTS. Uroweb. [Last accessedon 2021 Jun 12]. Available from: https://uroweb.org/guideline/treatment-of-non-neurogenic-male-luts.
- 3.Nitti VW, Lefkowitz G, Ficazzola M, Dixon CM. Lower urinary tract symptoms in young men: Videourodynamic findings and correlation with noninvasive measures. J Urol. 2002;168:135–8. [PubMed] [Google Scholar]
- 4.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 6.Crisp JC, Green NA, Ashken MH. Urodynamic studies in the district general hospital. Br J Urol. 1976;48:383–7. doi: 10.1111/j.1464-410x.1976.tb06659.x. [DOI] [PubMed] [Google Scholar]
- 7.Abrams PH, Shah PJ, Feneley RC. Voiding disorders in young male adult. Urology. 1981;18:107–11. doi: 10.1016/0090-4295(81)90512-4. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan SA, Ikeguchi EF, Santarosa RP, D’Alisera PM, Hendricks J, Te AE, et al. Etiology of voiding dysfunction in men less than 50 years of age. Urology. 1996;47:836–9. doi: 10.1016/S0090-4295(96)00038-6. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan SA, Santarosa RP, D’Alisera PM, Fay BJ, Ikeguchi EF, Hendricks J, et al. Pseudodyssynergia (contraction of the external sphincter during voiding) misdiagnosed as chronic nonbacterial prostatitis and the role of biofeedback as a therapeutic option. J Urol. 1997;157:2234–7. [PubMed] [Google Scholar]
- 10.Wang CC, Yang SS, Chen YT, Hsieh JH. Videourodynamics identifies the causes of young men with lower urinary tract symptoms and low uroflow. Eur Urol. 2003;43:386–90. doi: 10.1016/s0302-2838(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 11.Toh KL, Ng CK. Urodynamic studies in the evaluation of young men presenting with lower urinary tract symptoms. Int J Urol. 2006;13:520–3. doi: 10.1111/j.1442-2042.2006.01347.x. [DOI] [PubMed] [Google Scholar]
- 12.Karami H, Valipour R, Lotfi B, Mokhtarpour H, Razi A. Urodynamic findings in young men with chronic lower urinary tract symptoms. Neurourol Urodyn. 2011;30:1580–5. doi: 10.1002/nau.21095. [DOI] [PubMed] [Google Scholar]
- 13.Jamzadeh AE, Xie D, Laudano M, Seklehner S, Elterman DS, Shtromvaser L, et al. Urodynamic characterization of lower urinary tract symptoms in men less than 40 years of age. World J Urol. 2014;32:469–73. doi: 10.1007/s00345-013-1134-z. [DOI] [PubMed] [Google Scholar]
- 14.Jeong SJ, Yeon JS, Lee JK, Jeong JW, Lee BK, Park YH, et al. Chronic lower urinary tract symptoms in young men without symptoms of chronic prostatitis: Urodynamic analyses in 308 men aged 50 years or younger. Korean J Urol. 2014;55:341–8. doi: 10.4111/kju.2014.55.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosier PF, Ten Donkelaar CS, de Kort LM. Clinical epidemiology: Detrusor voiding contraction maximum power, related to ageing. Urology. 2019;124:72–7. doi: 10.1016/j.urology.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Pöyhönen A, Åkerla J, Häkkinen JT, Koskimäki J, Tammela TL, Auvinen A. Severity and bother of lower urinary tract symptoms among men aged 30-80 years: Tampere ageing male urological study (TAMUS) Scand J Urol. 2018;52:296–301. doi: 10.1080/21681805.2018.1505944. [DOI] [PubMed] [Google Scholar]
- 17.Yang SS, Wang CC, Hsieh CH, Chen YT. Alpha1-Adrenergic blockers in young men with primary bladder neck obstruction. J Urol. 2002;168:571–4. [PubMed] [Google Scholar]
- 18.Li B, Gao W, Dong C, Han X, Li S, Jia R, et al. Long-term safety, tolerability, and efficacy of α1-adrenergic blocker in young men with primary bladder neck obstruction: Results from a single Centre in China. Int Urol Nephrol. 2012;44:711–6. doi: 10.1007/s11255-011-0096-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Bang W, Choi HG. Analysis of the prevalence and associated factors of overactive bladder in adult Korean men. PLoS One. 2017;12:e0175641. doi: 10.1371/journal.pone.0175641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha S, Agarwal MM, Vasudeva P, Khattar N, Madduri VK, Yande S, et al. The urological society of India guidelines for the evaluation and management of nonneurogenic urinary incontinence in adults (Executive Summary) Indian J Urol. 2019;35:185–8. doi: 10.4103/iju.IJU_125_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby AC, Nager CW, Litman HJ, Fitzgerald MP, Kraus S, Norton P, et al. Perineal surface electromyography does not typically demonstrate expected relaxation during normal voiding. Neurourol Urodyn. 2011;30:1591–6. doi: 10.1002/nau.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha S, Matai L. Is isolated bladder outlet obstruction associated with hydronephrosis? A database analysis. Neurourol Urodyn. 2020;39:2361–7. doi: 10.1002/nau.24495. [DOI] [PubMed] [Google Scholar]
- 23.Oelke M, Rademakers KL, van Koeveringe GA. Detrusor contraction power parameters (BCI and W max) rise with increasing bladder outlet obstruction grade in men with lower urinary tract symptoms: results from a urodynamic database analysis. World J Urol. 2014;32:1177–83. doi: 10.1007/s00345-014-1358-6. [DOI] [PubMed] [Google Scholar]