Abstract
Apical periodontitis is an oral common inflammatory disease initiated by infection of pulp chamber and is characterized by destruction and resorption of the periapical bone. As a local infection, pathogens and their products in periapical tissues, as well as inflammatory cytokines produced in periapical lesions, enter the blood circulation, triggering systemic immune responses and leading to the pathogenesis of various types of systemic disease. Therefore, apical periodontitis might be associated with systemic disease rather than solely simple local oral disease. In addition, the existence of a hyperinflammatory state in certain patients with chronic inflammation-related disorder may affect the progression or prognosis of apical periodontitis. However, the association and potential mechanisms between apical periodontitis and systemic diseases remain unclear. An in-depth understanding of the association between apical periodontitis and systemic disease will be useful for both dentists and physicians to eliminate the possible risk factors and promote the healing of apical periodontitis and systemic disease. Thus, the aim of the present review is to introduce the potential relationship between apical periodontitis and systemic disease.
Keywords: apical periodontitis, systemic disease, inflammation, microbiota, association
1. Introduction
Apical periodontitis (AP) is a type of chronic oral inflammatory disease characterized by destruction and absorption of alveolar bone in the periapical tissue (1,2). It is usually caused by severe caries, pulp lesions or periodontal diseases. As a type of highly prevalent oral disease, 52% of the adult population worldwide was reported to have at least one tooth with AP according to a systematic review and meta-analysis in 2021 (3). Untreated teeth with AP can lead to tooth loss, jaw osteomyelitis and systemic disease associated with mortality (4-6).
In recent years, the association between AP and systemic disease has attracted attention, leading to the concept of endodontic medicine (7). As a local infection (8), previous studies have shown that microbes and toxins in periapical lesions have access to the bloodstream from the root canal system during or following endodontic therapy of teeth (9-11). Furthermore, AP modulates the systemic immune response by modifying the levels of inflammatory cytokines, such as C-reactive protein (CRP), tumor necrosis factor (TNF)-α, IL-6 and IL-1 (12-14). The aforementioned findings suggested that periapical inflammation is important for maintaining the health of the whole body. Furthermore, the long-term persistence of chronic inflammatory disease is implicated in systemic immune dysregulation and altered inflammatory factors in the circulation (15). Hence, chronic inflammation-associated disorder affects the status of AP, which is dependent on the antagonistic balance between the pathogenic microorganism and host immune response (16). To the best of our knowledge, however, evidence of the direct relationship between AP and systemic disease is lacking.
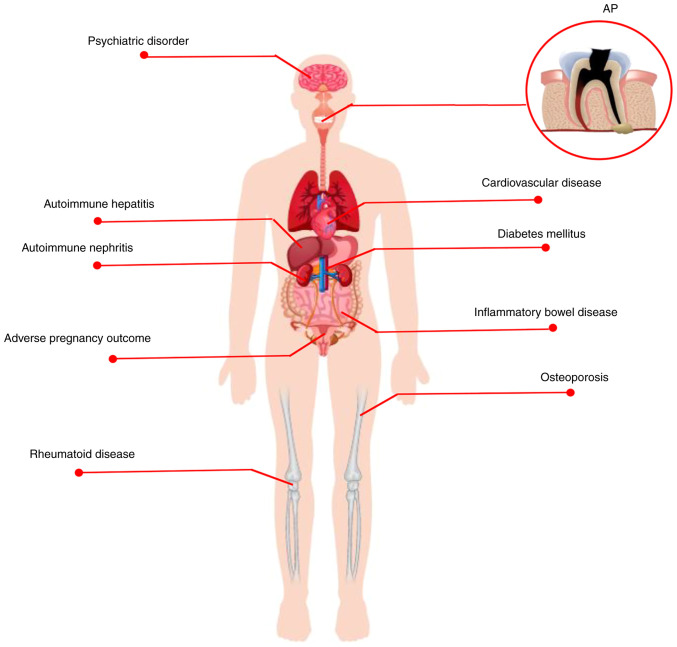
Over the past decades, there has been growing evidence on epidemiology (Table I) and mechanistic studies suggesting a potential link between periapical disease and systemic disease (17-19). However, the available studies (18-20) analyzing the relationship between AP and systemic disease are not comprehensive. The present review summarizes the association between AP and systemic diseases, including metabolic, autoimmune and cardiovascular disease (CVD), adverse pregnancy outcome (APO), as well as psychiatric disorder (Fig. 1). Further attention and understanding of the association between AP and systemic disease will help dentists and physicians develop innovative therapeutic proposals and promote effective healing of AP and systemic disease.
Table I.
Epidemiological evidence of the association between AP and systemic disease.
| Systemic disease | Study design | Main findings | Association reported | (Refs.) |
|---|---|---|---|---|
| DM | Cross-sectional | Patients with DM have a higher susceptibility to AP | Yes | (31,35,34,68) |
| Patients with long-term DM are predisposed to AP compared with short-term DM. | Yes | (36) | ||
| Periapical status/prevalence of AP is correlated positively with HbA1c levels in patients with T2DM | Yes | (32,33) | ||
| DM decreases the success of RCT in teeth with AP | Yes | (54,66) | ||
| T2DM does not influence the prognosis of AP | No | (70) | ||
| Case-control | DM does not increase the levels of bone resorption mediators in periapical lesions | No | (57) | |
| T2DM is independently associated with significantly greater prevalence of AP. Use of metformin or statins decreases the prevalence of AP in DM | Yes | (29) | ||
| DM has a negative effect on the prognosis of AP | Yes | (61-63) | ||
| Cohort | Metformin or statins promote healing of AP | Yes | (40) | |
| DM is a risk factor for tooth extraction after NSRCT | Yes | (58) | ||
| Patients with T2DM exhibit significantly less periapical healing compared with patients without DM | Yes | (67) | ||
| Pilot | AP does not affect the glycemic control or inflammatory levels in patients with T2DM | No | (83) | |
| Osteoporosis | Cross-sectional | Marginal association exists between bone mineral density and periapical radiolucency | Yes | (87) |
| Prevalence of AP is significantly higher in osteoporotic patients. The use of BPs decreases prevalence of AP | Yes | (92) | ||
| RD | Cross-sectional | Patients with RD are predisposed to AP compared with control group | Yes | (116) |
| RD does not increase the prevalence of RCT compared with control group | No | (119) | ||
| RD does not alter the periapical histological features or the incidence of AP | No | (121,122) | ||
| Amyloid protein is almost 5 times more frequent in periapical lesions of patients with RD than in controls | Yes | (125) | ||
| Autoimmune hepatitis/nephritis | Case-control | Patients with periapical radiolucency have higher prevalence of cirrhosis-associated complications | Yes | (138) |
| Cross-sectional | Liver transplant candidates have significantly higher prevalence of radiographic periapical lesions | Yes | (137) | |
| ESRD is significantly associated with AP | Yes | (146,147) | ||
| IBD | Retrospective clinical | Patients with IBD taking immunomodulators have a higher prevalence of AP. Patients with IBD and AP have larger periapical lesions than healthy subjects | Yes | (161) |
| Case-control | Patients with IBDs have higher prevalence of AP | Yes | (162) | |
| IBD is associated with higher prevalence of RFT and higher percentage of RFT with AP | Yes | (165) | ||
| CVD | Cross-sectional | AP and endodontic burden (AP and/or RCT) are associated with CAB | Yes | (195,205) |
| ALEO is associated with systemic inflammatory burden and cardiovascular risk | Yes | (196) | ||
| Endodontic lesions are independently associated with CAD and ACS | Yes | (200) | ||
| Patients with AP have a 2.79 times higher risk of developing CAD | Yes | (201) | ||
| There is no statistically significant positive correlation between AAP and CAD | No | (202) | ||
| Periapical lesions and root fillings are significantly associated with a first MI | Yes | (209) | ||
| CAP is moderately involved in plasma biomarker changes in hypertensive patients | Yes | (207) | ||
| AP has a potential association between endodontic infection and CVD | Yes | (216) | ||
| Cohort | In patients aged ≤40 years, ALEOs are significantly associated with the age at CHD diagnosis | Yes | (199) | |
| Young adults with AP are more likely to have early endothelial dysfunction | Yes | (215) | ||
| Adverse pregnancy outcome | Case-control | Pregnant people with AP are more likely to have LBWPB | Yes | (225,226) |
| Maternal AP is significantly associated with occurrence of PE | Yes | (227) | ||
| There is no association between maternal CAP and LBWPB | No | (231) |
HbA1c, glycated hemoglobin; T2DM, type 2 diabetes mellitus; NSRCT, non-surgical root canal treatment; ALEO, apical lesion of endodontic origin; CAD, coronary artery disease; CHD, coronary heart disease; ACS, acute coronary syndrome; AAP, asymptomatic apical periodontitis; MI, myocardial infarction; RFT, root-filled teeth; PE, preeclampsia; BP, bisphosphonate; ESRD, end-stage renal disease; LBWPB, low birth weight and preterm birth; RD, rheumatoid disease; CAP, chronic apical periodontitis.
Figure 1.
AP is associated with systemic disease. AP is a common oral disease associated with a number of systemic diseases, including metabolic (diabetes mellitus and osteoporosis), autoimmune (rheumatoid disease, autoimmune hepatitis/nephritis, inflammatory bowel disease) and cardiovascular diseases, adverse pregnancy outcomes, as well as psychiatric disorders. AP, apical periodontitis.
2. Metabolic disease
Diabetes mellitus (DM)
Globally, DM is a chronic metabolic disease characterized by hyperglycemia owing to insulin resistance and/or a deficiency in secretion of insulin or both (21). As one of the most common types of metabolic disease, it is estimated that the prevalence of DM for all age groups worldwide will rise from 2.8% in 2000 to 4.4% in 2030 and may affect ≥693 million people in 2045 (22,23). Diabetes is accompanied by various complications (24). It is considered that oral complications in patients with diabetes, such as AP, could affect quality of life (25). As the prevalence of diabetes increases, it may lead to increasing consequences of the complications of DM, such as enhancing the inflammatory response in apical tissue and accelerating alveolar bone loss (26). There have been numerous studies on the possible association between DM and AP (27-30). Notably, there may be a bidirectional association between DM and AP (Fig 2). DM may increase the prevalence of AP, accelerate the progression of AP and undermine the efficacy of root canal treatment (RCT). However, AP can affect insulin signaling pathways or reduce insulin sensitivity, leading to increased insulin requirements and blood glucose levels.
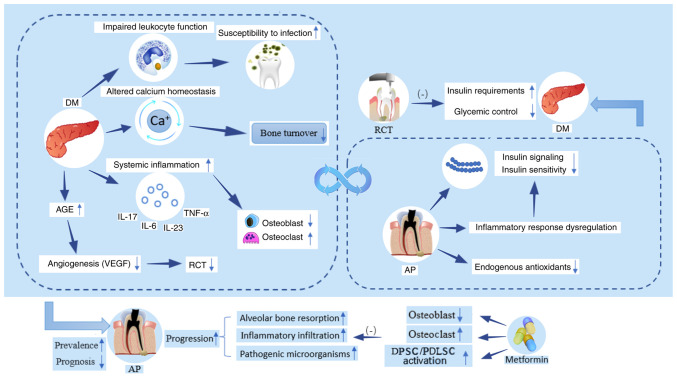
Figure 2.
Bidirectional association between AP and DM. DM may affect the prevalence, progression and prognostic course of AP by elevating the oral susceptibility due to impaired leukocyte function, increasing the systemic inflammatory response (increased secretion of IL-6, IL-17, IL-23 and TNF-α), decreasing bone turnover, altering osteoblast/osteoclast homeostasis, as well as decreasing levels of VEGF and angiogenesis by accumulation of advanced glycation end-products. However, use of metformin can inhibit the negative effects of DM on AP. AP can disrupt the inflammatory response, impair insulin signaling pathways or decrease insulin sensitivity and deplete endogenous antioxidants, leading to increased insulin requirements and blood glucose levels. RCT has been shown to attenuate this adverse effect. AP, apical periodontitis; DM, diabetes mellitus; RCT, root canal treatment; AGE, advanced glycation end-product; DPSC, dental pulp stem cell; PDLSC, periodontal ligament stem cell.
DM affects the prevalence, progression and prognosis of AP
Epidemiological studies (31-35) confirm that DM is positively associated with the prevalence of AP, especially in DM patients with poor glycemic control. Segura-Egea et al (31) performed a retrospective cohort study in which 70 participants were divided into patients with type (T)2 DM and control group. Patients with DM had a higher prevalence of AP compared with controls. In a hospital-based population, Saleh et al (34) indicated that patients with DM are ≥3 times more likely to exhibit AP compared with subjects without DM. Moreover, the association between AP and DM is more pronounced in patients with poor glycemic control and long-term diabetes (33,32,36) which is consistent with other investigations (35,30,47-39). Conversely, the use of metformin and statins decreases prevalence and promote the healing of AP in diabetic patients (29,40).
In addition to affecting the prevalence of AP, DM accelerates the progression of AP. In animal studies, high sugar intake and hyperglycemic states increase susceptibility to oral infection as a result of the disruption of leukocyte function, which is also supported by a greater and unbalanced presence of pathogenic microorganisms in root canals in periapical lesions in diabetes (41,42). In a rat model of combined AP and DM, increased inflammatory infiltration, higher levels of proinflammatory cytokines (IL-17, IL-23, IL-6 and TNF-α) and larger lesion size are observed in periapical tissue (41,43). Cintra et al (44) hypothesized that this might be partly due to enhanced systemic inflammation of DM, which contributes to a greater destruction of the alveolar bone in the periapical lesions. For example, IL-17, as a destructive inflammatory cytokine, serves a critical role in the resorption of periapical bone (45,46). Moreover, TNF-α could also promote osteoclast differentiation and maturation by activating the NF-κB signaling pathway to trigger a decrease in osteoblast function and apoptosis, resulting in an imbalance in osteoblast-osteolysis coupling (47). Moreover, the imbalance of calcium homeostasis associated with insulin deficiency leads to bone loss (48,49). Metformin, a classical diabetic prescription drug, improves hyperglycemic conditions and enhances osteogenesis by regulating glucose metabolism, promoting cell migration and increasing angiogenesis (50,51). In addition, metformin could activate the proliferation and osteogenic differentiation of dental pulp and periodontal ligament stem cells, which serve an important role in the repair and mineralization of oral-associated bone defects (52,53). In animal studies, intramuscular injection of metformin in rats with AP is effective in decreasing the size of periapical lesions via suppression of the NF-κB signaling pathway and diminishing the hypoxia-induced apoptosis of osteoblasts (54,55). In addition, intracanal metformin decreases expression of inducible nitric oxide synthase and nitric oxide to inhibit monocyte migration in periapical tissue (56). The aforementioned results confirm the positive effect of DM on the progression of AP. By contrast, Sarmento et al (57) found no significant differences in bone resorption mediators between patients with DM and normoglycemic patients with AP; this may be because the sample population was too small and the DM group comprised well-controlled individuals with a mean glycated hemoglobin (HbA1c) <7%.
Previous evidence has suggested that diabetes is a key preoperative factor in evaluating the prognosis of RCT and is a common cause for tooth extraction following non-surgical RCT (58-60). The accumulation of late glycosylated products in DM prolong the treatment time of RCT and increased the rate of infection in the oral cavity (60). In a retrospective case-control study, Uğur Aydın et al (61) assessed changes of fractal dimension and concluded that DM has a negative effect on the healing progress of periapical lesions after RCT. In the light of clinical and radiographic healing outcomes, the success rate of RCT is significantly lower in patients with DM or poor glycemic control due to impairment of the angiogenic process in DM (62,63), which is in accordance with other clinical studies (32,64-67). Similar results have been observed in patients with type 1 diabetes (68). Conversely, other studies suggest that DM does not affect the healing outcome of RCT (31,69-71). Different epidemiological methodologies may contribute to the different outcomes, including tooth type, radiographic method, assessment criteria and follow-up time. The healing of RCT in DM is a continuous process and could be affected by various factors, such as time to assess prognosis, status of general health, and control of oral reinfections. The outcomes of cross-sectional studies should be evaluated with care and more prospective studies with longer follow-up time, larger sample size and stricter control of confounding factors are required.
AP may affect insulin requirements and blood glucose status in DM
Inflammation is involved in the pathogenesis of DM, as inflammatory states could decrease insulin sensitivity (72). Chronic infection of AP could cause aggravated and dysregulated inflammatory response, which may result in poor glycemic control and increased insulin requirements (49,73,74). Similarly, animal experiments have showed that AP could alter insulin signaling and increase blood glucose concentrations by elevating serum inflammatory cytokines and activating the adaptive immune system (74-77). Maternal alterations in inflammation and insulin signaling pathways caused by AP may directly affect insulin resistance in adult offspring (78). In the rat model of DM, mean platelet count could be elevated in the presence of both AP and periodontitis (79). In addition, alterations in antioxidant status may be one of the potential mechanisms by which AP affects the pathogenesis of DM (80,81). AP could enhance systemic effects of DM, as shown by decreased serum albumin levels and increased uric acid concentrations in DM rats with AP (80).
The efficacy of RCT in diabetes is unknown. In a case report, a patient with DM noticed a rapid increase in insulin demand after an exacerbation of the endodontic-periodontic lesion of a tooth, while the requirement for insulin decreased suddenly within one day of the root canal preparation (82). By contrast, a prospective clinical study with a 1-year follow-up period by Arya et al (67) suggested that endodontic treatment does not improve the levels of HbA1c in patients with DM. Furthermore, a pilot investigation indicated that AP does not affect levels of inflammatory markers or glycemic control in patients with T2DM (83). The limited sample size and uncontrolled confounding variables may partly explain the findings of the pilot study. Moreover, the administration of metformin in some participants with combined DM and AP may be a confounding factor influence blood glucose levels, masking the contribution of AP to blood glucose. In addition, both periapical healing and HbA1c levels in diabetic patients vary over time. Hence, it is critical to determine a consistent and appropriate time to assess levels of HbA1c following endodontic therapy in DM patients with AP.
In summary, there is a two-way association between DM and AP (Fig. 2). DM affects the initiation, progression and prognosis after endodontic treatment of AP. AP might affect insulin sensitivity, increase blood glucose concentrations, as well as enhance the systemic effects of DM. However, it is unclear whether endodontic treatment helps to improve insulin resistance and HbA1c levels in patients with DM and more longitudinal studies are needed.
Osteoporosis may lead to increased AP incidence
Osteoporosis is a common metabolic bone disorder in post-menopausal patients with estrogen deficiency and manifests as increased bone fragility due to bone loss and microarchitectural deterioration of bone tissue (84,85). Osteoporosis decreases total skeletal mass, including jawbone (86-88). Bone changes in osteoporosis are correlated with decrease of alveolar bone density and height of the alveolar ridge and loss of teeth (89-91).
Similarly, López-López et al (87) observed a marginal correlation between osteoporosis and radiolucent periapical lesions in post-menopausal patients. In addition, emerging evidence indicates that the prevalence of AP is higher in osteoporotic patients (92). Bisphosphonates, a type of anti-resorptive medication, inhibit the progression of AP in osteoporotic patients (92-96).
Estrogen deficiency serves a key role in the development of AP in osteoporosis
Systemic factors of bone remodeling in osteoporosis, such as estrogen, modify the balance of periapical bone metabolism. In ovariectomized animal models, estrogen deficiency serves a key role in the pathogenesis of periapical lesion by upregulating expression of members of the NLRP3/caspase-1/IL-1β axis and RANKL (95,97,98), leading to increased periapical bone resorption. The disturbed systemic inflammatory state in patients with osteoporosis is hypothesized to be the main cause of the imbalance between periapical osteoclasts and osteoblasts, which induces osteoclast apoptosis (99,100). Lucisano et al (101) found oral microorganisms in the saliva as well as greater periapical bone loss in ovariectomized mice compared with a control group, which indicated that decreased estrogen aggravates development of periapical lesions by altering the microbiota in saliva. Similarly, Gomes-Filho et al (102) simulated the effects of estrogen by administering raloxifene (RLX) in ovariectomized mice and found that RLX was able to inhibit the production of local regulators of osteoclastogenesis and angiogenesis induced by estrogen deficiency during the development of AP.
Follicle-stimulating hormone (FSH), a hormone that increases with the decrease of estrogen levels, is an independent risk factor for periapical inflammation. FSH could exacerbate bone loss in periapical lesions via directly coupling FSH receptor on the surface of osteoclasts and elevated secretion of inflammatory cytokines (103). FSH inhibitor leuprorelin has a protective effect against periapical bone loss of the experimental periapical lesions in ovariectomized rats (104).
In general, osteoporosis is considered to show a unidirectional association with AP. Osteoporosis increases incidence of AP and estrogen linking these diseases. To the best of our knowledge, however, evidence for this conclusion is limited. More robust epidemiological evidence is required to corroborate this conclusion.
3. Autoimmune disease (AD)
AP is a chronic inflammatory disease involving a variety of immunocompetent cells, similar to the autoimmune system, such as such as T and B cells, macrophages and immunoglobulins synthesized by plasma cells (105,106). Given the similar immunocompetent cells, it is hypothesized that there is an associate the AP with AD. Previous studies have linked types of AD to AP, such as rheumatoid and inflammatory bowel disease (IBD) and autoimmune hepatitis/nephritis (107-109).
In addition, the use of immunosuppressive agents, a conventional option for the treatment of AD, is associated with AP. It is been hypothesized that the use of immunosuppressive agents leads to weakened resistance as a consequence of decreased systemic leukocyte count, thus increasing the risk of opportunistic oral infection and susceptibility to AP (110,111). Conversely, Yamasaki et al (112) conducted an animal study and suggested that the long-term use of immunosuppressive agents prior to pulpal exposure significantly inhibits inflammatory expansion of AP. By contrast, Waterman et al (113) and Teixeira et al (114) showed that use of immunosuppressive agents does not exacerbate or reduce the periapical inflammatory destruction.
The aforementioned contradictory views suggest a complex and uncertain role of the systemic immune response in AP. The pathological process of AP is determined by the balance between host immunity and virulence of the pathogen (111-113) but this dynamic balance is difficult to define. The differing findings may be related to the dose and duration of immunosuppressive agents used, as well as species or virulence of the invading bacteria.
Rheumatoid disease (RD) is associated with a higher prevalence of AP
Rheumatoid disease (RD) is a common autoimmune disease with a global prevalence of 0.24% in 2010 (115). A cross-sectional study conducted by Karataş et al (116) suggested that individuals with RD had at least two times higher risk of AP than controls. Rotstein and Katz (117) reported a similar tendency of patients with RD to develop AP based on the integrated data of hospital cases. Similarly, Oh et al (118) reported a clinical case of rapid destruction of periapical bone during the endodontic therapy in a patient with RD taking azathioprine. Conversely, Jalali et al (119) found no correlation between periapical rarefying osteitis and RD, which might be due to the restrictive diagnostic criteria of AP reducing the actual detection rate of periapical osteitis.
Periapical alveolar bone may be a bony target of RD
AP and RD are types of chronic inflammation involving similar inflammation markers, such as TNF-α, IL-6 and IL-17, which promote bone destruction by activating the NF-κB signaling pathway (120). In 1975, Malmström et al (121,122) performed a series of biopsies on periapical tissue from patients with RD and found no histological differences in periapical lesions between patients with RD and controls. However, they subsequently identified IgG, as well as other free rheumatoid factors, in periapical tissue from patients with RD (123,124). Furthermore, amyloid protein was almost five times more frequent in periapical lesions of patients with RD than in controls (125). Foxo3a, a representative protein that serves an osteolytic role in rheumatoid disease, is found in periapical tissue (126). Therefore, it is hypothesized that the periapical alveolar bone might be a bony target of RD.
Overall, most current studies (123-126) suggest a positive association between RD and AP and the osteolytic destruction of RD could also damage the periapical alveolar bone. To the best of our knowledge, however, there are few convincing studies focusing on the correlation between these diseases and the effect of AP on RD.
Autoimmune hepatitis/nephritis
Autoimmune hepatitis/nephritis is a chronic progressive inflammatory disease mediated by a dysfunctional immune response. Their etiology is obscure but higher susceptibility may be associated with genetics, medication and persistent infection (127-129). In the later stages of progression, severe autoimmune hepatitis/nephritis develops into end-stage liver/renal disease (ESRD), requiring liver/renal transplantation (130).
Autoimmune hepatitis/nephritis confers higher susceptibility to AP
Assessment of the oral health of patients with liver disease revealed that the patients with liver disease exhibit a higher frequency of oral infection (131-133), particularly in liver transplant candidates (134-136). Castellanos-Cosano et al (137) performed a descriptive cross-sectional study that found that the prevalence of AP in liver transplant candidates is higher than in controls, while the frequency of root-filled teeth (RFT) was lower. Furthermore, an epidemiological study by Grønkjær et al (138) confirmed that nearly half of patients with liver cirrhosis exhibited periapical radiolucency. These findings were confirmed by an animal study, in which increased periapical bone loss, inflammatory cell infiltration and expression of inflammatory factors in the periapical tissue were be observed in a rat model of liver fibrosis with AP (139).
Oral disease is also prominent in patients with kidney disease. Children with purpura nephritis are more likely to develop periapical infection (140,141). In patients with ESRD, oral hygiene status is worse than that of healthy individuals (142,143). Additionally, the prevalence of oral disease is associated with severity of the renal failure and worsens with length of dialysis treatment (144,145). A clinal study conducted by Buhlin et al (146) found that more than half of patients with ESRD have at least one tooth with periapical inflammation. In 2017, Khalighinejad et al (147) provided epidemiological evidence of the association between AP and ESRD and indicated that the incidence of AP is notably higher in patients with ESRD and number of AP teeth in patients with ESRD is significantly correlated with urea serum levels.
The aforementioned findings suggest that patients with autoimmune hepatitis/nephritis are at greater risk of AP compared with healthy individuals. The susceptibility of patients with autoimmune hepatitis/nephropathy to periapical inflammation may be partly attributable to liver/renal failure, loss of detoxification and accumulation of harmful metabolites, such as Urea, creatinine, uric acid, resulting in a systemic hyperinflammatory state and immunosuppression (130,148).
AP induces autoimmune hepatitis/nephritis
Bacterial colonies in AP (149) may be a source of infection in patients with autoimmune liver/nephropathy (140). To an extent, bacterial infections of oral origin may influence the morbidity and mortality of patients with autoimmune liver/nephropathy (150). The oral cavity is a well-known source of pathogens while the liver and kidney are key target organs for oral bacteria (151-153) Kojima et al (153) identified viable bacteria in hepatocytes of mice injected with Streptococcus pyogenes. In addition, Ogura et al (152) detected outer membrane antigens of Hemophilus influenzae (a common microorganism in AP) and specific antibodies in glomerular mesangium and serum from patients with nephritis. Moreover, there is a case report of root canal treatment of a child coincidentally inducing Henoch-Schönlein purpura nephritis (154).
The aforementioned data indicate that AP might serve a pathogenic role in autoimmune hepatitis/nephritis. The microorganisms within periapical tissue have the potential to colonize the liver/kidney either directly or by forming immune complexes, triggering a misdirected autoimmune response (152,154).
In brief, there is an interaction between AP and autoimmune hepatitis/nephritis. Extensive epidemiological evidence (137,138,146,147) suggests that patients with autoimmune hepatitis/nephritis are more likely to exhibit AP. Conversely, as an infectious source, periapical inflammation may trigger autoimmune hepatitis/nephritis. Therefore, early and thorough treatment of AP is important. In certain cases, endodontic treatment could be combined with antibiotics, especially in children who have a high propensity to develop purpura nephritis. In addition, it is essential to follow up children with Henoch Schönlein Purpura after endodontic treatment.
Inflammatory bowel disease (IBD)
IBDs are chronic recurrent autoimmune diseases characterized by diffuse inflammation of the intestinal mucosa and include ulcerative colitis (UC) and Crohn's disease (CD), which affect any section of digestive tract (155,156). The connections between IBD and oral health (157), particularly with respect to periodontitis and dental caries, are recognized (158-160). The relationship between AP and IBDs has also gained attention (109).
IBD is correlated with the onset and worsening of AP
In 2017, Piras et al (161) showed that patients with IBD are associated with a higher incidence of AP and higher periapical index (PAI) score, especially in female patients with IBD. Similar results were also observed by Poyato-Borrego et al (162), who confirmed that patients with IBD have nearly six times higher risk of developing AP than healthy individuals. In addition, a case-control study suggested that the number of RFT in patients with IBD is almost four times higher than in controls (163). This may explain why patients with IBD require more dental treatment (164). However, there is no significant difference in prevalence of AP between patients with IBD and controls (165), which might be attributed to the small sample size and uncontrolled confounding factors. The increased susceptibility of patients with IBD to AP might be partially attributable to an impaired immune system, which is the primary characteristic of autoimmune disease (109,166).
In addition to increased incidence, it is hypothesized that the impaired immune system of patients with IBD has a detrimental impact on the progression and prognosis of AP (107,167). CD is a T helper (Th) 1 type of immune disease, whereas UC is defined as a Th2 type of immune disease (168). Both types of immune response are associated with the pathological process of AP. For example, activation of osteoclasts in the Th1 lymphocyte response is implicated in the progression of periapical bone destruction, while the Th2 lymphocyte response is associated with the healing of AP following RCT (169-171). Accordingly, IBD may be associated with the onset and worsening of AP.
Microorganisms from the oral cavity link AP to IBD
From a microscopic perspective, microorganisms originating from the oral cavity may serve a role in the link between AP and IBD (151,172). The oral cavity is the start of the gastrointestinal tract (173). Oral bacteria that enter the gut during swallowing could stimulate intestinal pathogens (necrotrophy) and create novel phenotypical bacterial virulence genes by increasing bacterial virulence (151,174). On the other hand, oral bacteria might be directly linked with digestive disease by altering intestinal flora (175). An animal study in rats by Tavares et al (176) demonstrated that AP can elevate the intestinal leptin levels in metabolic disorders by modulating gut microbiota. Dysbiosis of the intestinal microbiota is a common feature of intestinal diseases, including IBD (177). Recently, Gan et al (178) established an AP model in rat infected by Porphyromonas gingivalis and found that AP changed the diversity of intestinal microbiota and P. gingivalis was not detected in the collected stools. The aforementioned results suggest that periapical inflammation might alter the intestinal flora through multiple pathways (178). Similarly, Kojima et al (153) found a significantly higher detection rate of Streptococcus mutans in patients with IBD compared with that in controls in a clinical study. In addition, it was observed that the injection of S. mutans into mice is more likely to cause IBD than oral administration (153).
Biological medications (BMs) are advocated for treatment of patients with IBD and AP
Immunosuppressive agents are generally used to treat IBD in the clinic. However, use of immunosuppressive agents increases the risk of oral opportunistic infections and bone marrow suppression, thus increasing the susceptibility to AP or promoting further deterioration of periapical lesions (118,179). BMs are recombinant human proteins that target inflammatory factors, such as anti-TNF agents (180). Existing studies have shown that patients with IBDs and AP exhibit faster and better healing with few complications in endodontic treatment combined with anti-TNF therapy (109,181). Theoretically, anti-TNF agents block not only the direct stimulatory effect of TNF-α on osteoclasts, but also the negative effect on osteoblast activity and differentiation, which relieves destruction of periapical tissue and stimulates regeneration of supporting tissue (182). Accordingly, it is hypothesized that patients with IBD and severe treatment-resistant AP and with elevated levels of circulating TNF-α may benefit from anti-TNF treatment.
Current studies (107,161,163) suggest an association between IBD and AP. IBD shows a positive correlation with onset and progression of AP (161-163). In turn, microorganisms in AP lead to the dysbiosis and immune imbalance of the intestinal flora via multiple pathways (176,178). Conventional immunosuppressive agents may not be appropriate for patients with IBD and AP (179). Instead, targeted treatment with BMs to promote healing of systemic disease and AP is favored (180).
4. CVD
CVD, particularly cerebrovascular and ischemic heart disease, are primary causes of mortality and disability worldwide. An estimated 17.7 million deaths could be attributed to CVD, accounting for 31% of all deaths worldwide in 2019 (183), which is a major threat to human life (184,185). Therefore, it is key to determine the risk factors associated with CVD. The relationship between AP and CVD has been investigated for several decades and numerous reviews or meta-analyses have been published (186-189). Most studies have found a weak correlation between AP and CVD (186-191), with only a few disagree (192,193).
Patients with CVD are at higher risk of AP
A systematic review with meta-analysis in 2022 suggested a weak positive association between AP and CVD (194). Atherosclerosis is the main pathological basis of CVD and is highly correlated with the prevalence of AP, as shown by epidemiological studies (195,196). Additionally, Conti et al (197) confirmed that atherosclerosis increases the inflammatory reaction and size of periapical lesions. As one of the most common types of CVD, coronary artery disease (CAD) is associated with a higher prevalence of periapical inflammation (198-200). In comparison with non-CAD subjects, patients with CAD have a nearly threefold increase in the risk of AP (201), which is consistent with another study detected AP in 42.6% of patients with CAD and in 40.1% of non-CAD controls (202). Hypertension, a recognized primary risk factor for CVD, is also linked with a high prevalence and increased radiographic area of AP (203), and the prevalence of AP in hypertensive patients is notably decreased following treatment with angiotensin II receptor blockers (204).
AP contributes to progression of CVD
Gan et al (178) established a model of AP by inoculating P. gingivalis into the pulpal cavity of apolipoprotein E-deficient mice and found that AP exacerbated formation of atherosclerotic plaque in the aortic arch. Consistently, Conti et al (197) demonstrated that AP could promote the progression of atherosclerosis by increasing triglyceride levels and the thickness of the carotid artery intima tunic. Furthermore, endodontic therapy is hypothesized to be an effective approach to reverse the inflammatory effect of AP on atherosclerosis (205). In patients with hypertension combined with AP, AP increases levels of serum inflammatory markers such as IL-6, CRP and fibrinogen, disturbs the balance of the oxidative state and impairs cardiac function (203,206,207). Messing et al (208) found a positive association between AP and a single nucleotide polymorphism in potassium channel subfamily K member 3 (KCNK3), a gene that increases susceptibility to hypertension, which suggested that AP and hypertension may share a common genetic variation. AP is linked with an increased risk of first myocardial infarction (209).
Potential mechanisms linking AP and CVD
Numerous underlying mechanisms have been proposed for the association between AP and CVD (Fig. 3). Bacteria and/or their products may translocate from periapical tissue to various parts of the body via systemic circulation, especially areas with pre-existing cardiovascular lesions, and accelerate the progression of CVD. For example, DNA of P. endodontalis in monocytes (210) and elevated levels of anti-P. endodontalis IgG are found in peripheral circulation of patients with AP (211). In addition, Kazanci et al (212) reported an 8-year-old boy was admitted to hospital for acute cerebral infarct; cerebrospinal fluid cultures yielded Streptococcus oralis from alveolar abscess of the left maxillary first premolar. These findings are in accordance with a previous study that found bacteria and/or their products in circulation trigger low-grade systemic inflammation and contribute to the formation of plaques or thrombi (178).
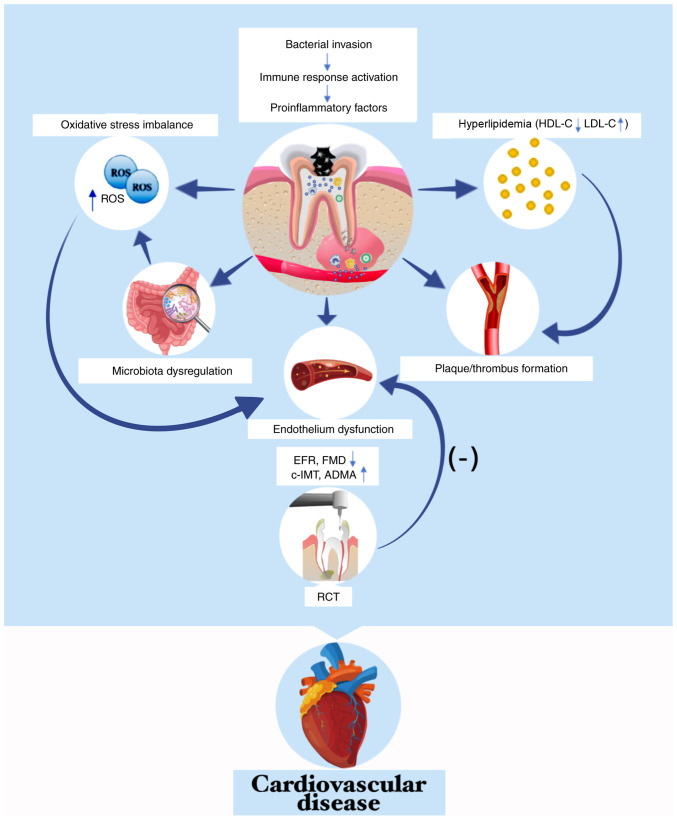
Figure 3.
Apical periodontitis accelerates progression of cardiovascular disease. Bacteria and/or their products in the periapical region are transferred to systemic circulation, which can activate the systemic immune response and promote the secretion of pro-inflammatory factors, eventually causing dysbiosis of the intestinal flora, aggregating overloads of ROS and/or developing into hyperlipidemia. Elevated serum inflammatory factors and lipids contribute to plaque/thrombus formation. Excessive ROS and inflammatory mediators directly damage vascular endothelium, impairing FMD and EFR and increase c-IMT and concentration of ADMA. Conversely, root canal treatment improves early endothelial dysfunction. EFR, endothelial flow reserve; ROS, reactive oxygen species; HDL-C, high density lipid-cholesterol; LDL-C, low density lipid-cholesterol; FMD, flow-mediated dilatation; c-IMT, carotid intima-media thickness; ADMA, asymmetrical dimethylarginine.
Inflammatory response (197), oxidative stress (203) and endothelial dysfunction (213) are potential mechanisms underlying the interaction between AP and CVD. Inflammatory mediators serve a crucial role in the initiation and progression of both AP and CVD (196). Systemic inflammation, which is amplified by CVDs, also acts on periapical tissues by intensifying the inflammatory reaction and increasing bone resorption in periapical lesions (197). In addition, inflammatory mediators mediate endothelial dysfunction (214). Previous studies have demonstrated that patients with AP exhibit increased lipid levels and serum inflammatory mediators (TNF-α, IL-1, IL-6, IL-2 and asymmetric dimethylarginine), which implies early endothelial dysfunction (196,197,213,215). Moreover, Chauhan et al (216) observed impaired flow-mediated dilatation and endothelial flow reserve, as well as greater carotid intima-media thickness, in individuals with AP compared with controls in a cross-sectional study. However, successful local endodontic therapy does not ameliorate early endothelial dysfunction (215).
In terms of oxidative stress, reactive oxygen species (ROS) are by-products of cellular metabolism involved in the pathological process of both AP and atherosclerosis (217). During pulp infection, bacterial motifs bind toll-like receptors (TLRs) on the surface of phagocytes and promote synthesis and release of ROS (218). Furthermore, the dysbiosis of intestinal flora due to AP may be a source of accumulated ROS in the body (96,176). Excess ROS production directly damages the vascular endothelium and activates multiple components of the immune system, such as increasing the inflammatory cytokine expression and amplifying the neutrophil recruitment (219), which was confirmed by a study demonstrating that AP could disrupt the oxidative status and impair cardiodynamics in hypertensive rats (203). Moreover, antioxidant agent tempol exerts a prevent effect on the establishment of AP in rats with doxorubicin-induced cardiomyopathy (220).
Previous studies (194,195,197) suggest an association between AP and CVD. To the best of our knowledge, however, detailed epidemiological studies on the mechanism underlying their interaction are lacking, especially for the effect of CVD on AP. CVD is a highly complex disease with various risk factors so more well-designed animal and epidemiological studies with strict control of confounding factors are essential to clarify the causal association between these two diseases.
5. APO
APOs are harmful to both pregnant people and newborn infants and related with early spontaneous abortion to an extent (221,222). In particular, the alteration of hormone levels during pregnancy increases vascular permeability and accelerates the spread of inflammation (223). Maternal infection is more likely to cross the placental barrier, leading to APOs (224).
Epidemiological evidence of the association between AP and APO
Harjunmaa et al (225) found a significant association between prevalence of AP and preterm delivery and fetal growth restriction in a cross-sectional study in Malawi. Leal et al (226) performed a case-control study involving 63 pregnant people and found that pregnant people with AP were nearly 5 times more likely to undergo preterm labor and to deliver a low-birth weight (LBW) newborn than those without AP. In addition to the adverse effects on fetal intrauterine growth, pregnant patients with AP exhibit greater risk of developing preeclampsia compared with those without AP (227). To an extent, the likelihood of APO varies with size of the periapical lesion (226). By contrast, Bain et al (228,229) hypothesized that AP contributes to a higher birthweight and a longer pregnancy, which might be related to gestational diabetes caused by insulin resistance (228-230).
Shah et al (231) recently conducted a cross-sectional study and found a protective association between AP and APOs, which is contrary to previous results (226,227). This result is biologically implausible, as the invasion of oral pathogens in periapical periodontitis is intrinsically harmful to the body and it can trigger a series of resistance behaviors of the body. This implausible outcome may be caused by sample selection bias, excessive loss to follow-up and uncontrolled confounding factors. Additionally, defining PAI≥3 as a diagnostic criterion for AP may underestimate the incidence of AP in the sample population (232).
Potential underlying mechanisms of AP and APO
To verify the relationship between AP and APO, Ao et al (233) created an AP model in rat infected with P. gingivalis and found that AP was associated with LBW and preterm birth (LBWPB). Immunohistochemistry and PCR showed P. gingivalis localization in placental tissue. Consistent with these results, Doyle et al (234) detected higher levels of Fusobacterium nucleatum, a representative specialized anaerobic bacteria in oral cavity, in amniotic fluid of premature infants.
The aforementioned results suggest that bacteria from periapical lesions could enter the circulation and induce temporary bacteremia, together with increased vascular permeability due to changes in hormone levels during pregnancy, which allow bacteria in the bloodstream to easily cross the placental barrier and induce maternal and fetal inflammatory reactions and immune responses. TNF-α, IL-1β and IL-6 induce premature labor, while IL-10 and vascular endothelial growth factor prevent LBW (235-238). Theoretically, maternal infection with P. gingivalis may inhibit expression of IL-10, thereby promoting intrauterine growth restriction; therefore, AP may induce LBWPB (239).
To the best of our knowledge, studies about the association between AP and APOs are lacking. The majority of studies show that AP is positively associated with APO. However, more studies are required to investigate the association between AP and APO and determine whether it is necessary to take preventive measures against AP in pregnant people.
6. Psychiatric disorder
With the rapid pace and increasing pressure of modern society, psychiatric disorders are becoming increasingly prominent, it is estimated the lifetime prevalence rates were 22.5% for any non-substance abuse mental disorder in the United States (240). Excessive stress, persistent negative emotion and severe sleep disorder affect psychiatric health to varying degrees. On one hand, psychiatric disorders affect the balance of endocrine hormone and immune response, which decreases host resistance and increases susceptibility to chronic disease (241). On the other hand, objective causes such as difficulties in maintaining oral hygiene confer susceptibility to AP. Negative mental state is involved in the underlying mechanism of various types of oral disease, such as periodontitis, oral mucosal ulcers and bruxism (241-243). For example, Maes (244) hypothesized that severe depression is accompanied by hypersecretion of adrenaline, disordered systemic immune response and activation of acute inflammatory markers, which lead to decreased host resistance and allow oral pathogens to trigger periapical lesions. Furthermore, in the dental clinic, excessive mental stress and disturbed sleep schedule are common in asymptomatic patients with AP who typically seek emergency dental treatment for acute toothache (245).
Wang et al (246) found that P. gingivalis induces a depressive phenotype. The potential mechanism may be that the free lipopolysaccharide (LPS) in the blood crosses the blood-brain barrier and downregulates expression of neurotrophic factor receptor p75 in astrocytes via the LPS/TLR4 signaling pathway (246). Furthermore, Martínez et al (247) detected the translocation of Fusobacterium nucleatum in in the frontal cortex and hypothesized that F. nucleatum might link oral disease to depression by inducing neuroinflammation (248). Transfer of oral bacteria across the blood-brain barrier may underlie the association between oral disease and psychiatric disorder.
Mental stress is associated with severity of AP
Mental stress is a state of physical or psychological tension caused by adverse mental or emotional stimuli that interferes with normal physiological functions of the body (249). Studies (250,251) have shown that the levels of catecholamines, cortisol hormones and inflammatory factors in the body are closely associated with mental stress Excessive stress stimulates secretion of catecholamines by exciting the sympathetic nervous system and activating the hypothalamic-pituitary-adrenocortical axis, promoting the release of cortisol (250,252). Cortisol regulates immunity by decreasing the number and activity of immune cells and inhibiting secretion of cytokines (253). In a survey, Haug and Marthinussen (245) found that a sudden onset of acute toothache in patients with chronic AP is typically associated with greater stress at home or work; higher levels of cortisol and inflammatory factors in saliva may be the direct cause of acute episodes of AP (254). Similarly, exogenous chronic stress exacerbates the pathological process of AP by increasing periapical bone resorption and release of inflammatory cytokines (255,256). In addition, the application of adrenergic blockers is effective in reducing the number of osteoclasts in the periapical region (257). Thus, mental stress induces imbalance between the secretion of cortisol and inflammatory factors in saliva, which is strongly correlated with the severity of AP (254).
Sleep disorder affects progression of AP by altering hormone levels
Sleep disorder is a common key clinical feature of psychiatric disorder (258,259). Sleep disorder is a key risk factor for oral cancer, periodontitis and adolescent maxillofacial development (260-262). Sleep disorder affects rhythmic expression of various proinflammatory cytokines and endocrine hormones, including cortisol and melatonin (263). Intraperitoneal injection or sealing of cortisol in the root canal in an AP rat model causes damage to periapical tissue and increases resorption of periapical bone (254,264). Melatonin is an important hormone with anti-inflammatory and antioxidant activity that is secreted by the pineal gland during the night (265). Melatonin suppresses oral inflammation and decreases loss of alveolar bone (266). Tavares et al (267) found that melatonin is a feasible adjuvant to promote healing of periapical bone in AP by increasing the insulin sensitivity and decreasing plasma concentrations of inflammatory cytokines. In addition, Sarıtekin et al (268) demonstrated that systemic injection of melatonin could significantly inhibit the resorption of periapical alveolar bone and decrease plasma expression of inflammatory factors in rats with AP. Therefore, it is hypothesized that sleep disturbance may be a risk factor in exacerbating the inflammatory response of periapical tissue in AP.
Studies suggest (253,254) that the association between psychiatric disorder and AP is primarily mediated by disruption of hormone levels and the imbalance of host immunoreaction. In clinical practice, doctors should attach importance to the oral condition of patients with psychiatric disorder and provide necessary oral health education. However, due to the lack of epidemiological data and limited animal studies, the association between AP and psychiatric disorder is unclear. Therefore, more studies are needed to investigate the interaction between AP and psychiatric disorder.
7. Conclusion
An increasing number of studies (18-20) have investigated the association between AP and systemic disease, including animal experiments, clinical trials and epidemiological investigations. Therefore, in the diagnosis and treatment of AP, dentists should consider systemic disease and medications to assess patient condition, develop a therapeutic plan and evaluate prognosis. Physicians should be aware of the periapical status of patients with systemic disease to minimize the negative impact of AP. For example, physicians should raise awareness of oral health in patients with systemic disease associated with AP, focus on the possible adverse effects of specific drugs, such as immunosuppressants like methotrexate and cyclophosphamide, on the periapical tissue. Furthermore, physicians should collaborate with outpatient dentists for regular follow-up to better assess patient condition and oral status. As AP may be one of the etiologies or clinical manifestations of certain types of systemic disease, focusing on the interaction between AP and systemic disease will help dentists and physicians seek reasonable therapeutic options to promote the healing of AP and systemic disease as effectively as possible.
However, the majority of current studies (18,19,27) only provide the evidence of the association between AP and systemic diseases rather than a strict causal association. Associations between AP and systemic diseases are complex, which may be bidirectional or unidirectional. In addition, the association is susceptible to multiple confounding factors. It is difficult to exclude confounding factors to establish a strict causal association, as shown by a review by Segura-Egea et al (27). Establishment of strict causality should analyze and achieve the Hill's criteria (strength of the association, biological rationality, temporal relationship, dose-response gradient, consistency of the association). To the best of our knowledge, there are few studies (67,82) that have investigated whether RCT could decrease the epidemiological incidence or attenuate clinical symptoms of systemic disease. Therefore, clinical trials are needed to confirm AP as a potential risk factor for systemic disease. Furthermore, confounding factors and bias should be controlled as much as possible during the design process of the correlation studies, which will help to elucidate the correlation and the underlying mechanisms about the association between AP and systemic disease more convincingly.
Acknowledgments
Not applicable.
Abbreviations
- AP
apical periodontitis
- T2DM
type 2 diabetes mellitus
- RCT
root canal treatment
- CVD
cardiovascular disease
- HbA1c
glycated hemoglobin
- CAD
coronary artery disease
- CRP
C-reactive protein
- ROS
reactive oxygen species
- IBD
inflammatory bowel disease
- CD
Crohn's disease
- UC
ulcerative colitis
- PAI
periapical index
- RFT
root-filled teeth
- RD
rheumatoid disease
- RLX
raloxifene
- FSH
follicle-stimulating hormone
- ESRD
end-stage renal disease
- TLR
toll-like receptor
- LBWPB
low birth weight and preterm birth
- APO
adverse pregnancy outcome
Funding Statement
The present study was supported by National Natural Science Foundation of China (grant no. 82000500), Hubei Province Key Laboratory of Oral and Maxillofacial Development and Regeneration (grant no. 2021kqhm004) and Scientific Research Program of Hubei Provincial Health Commission (grant no. WJ2021Q059).
Availability of data and materials
Not applicable.
Authors' contributions
LY, LC, WS and CY wrote and revised the manuscript and constructed table and figures. QT and ZY conceived the study and revised the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yamasaki M, Kumazawa M, Kohsaka T, Nakamura H, Kameyama Y. Pulpal and periapical tissue reactions after experimental pulpal exposure in rats. J Endod. 1994;20:13–17. doi: 10.1016/S0099-2399(06)80020-8. [DOI] [PubMed] [Google Scholar]
- 2.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 3.Tibúrcio-Machado CS, Michelon C, Zanatta FB, Gomes MS, Marin JA, Bier CA. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int Endod J. 2021;54:712–735. doi: 10.1111/iej.13467. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami G, Vaeth M, Kirkevang LL, Wenzel A, Isidor F. Risk factors for tooth loss in an adult population: A radiographic study. J Clin Periodontol. 2008;35:1059–1065. doi: 10.1111/j.1600-051X.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 5.Gomes MS, Hugo FN, Hilgert JB, Sant'Ana Filho M, Padilha DM, Simonsick EM, Ferrucci L, Reynolds MA. Apical periodontitis and incident cardiovascular events in the baltimore longitudinal study of ageing. Int Endod J. 2016;49:334–342. doi: 10.1111/iej.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kierdorf U, Olsen MT, Kahle P, Ludolphy C, Kierdorf H. Dental pulp exposure, periapical inflammation and suppurative osteomyelitis of the jaws in juvenile Baltic grey seals (Halichoerus grypus grypus) from the late 19th century. PLoS One. 2019;14:e0215401. doi: 10.1371/journal.pone.0215401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cintra LTA, Gomes MS, da Silva CC, Faria FD, Benetti F, Cosme-Silva L, Samuel RO, Pinheiro TN, Estrela C, González AC, Segura-Egea JJ. Evolution of endodontic medicine: A critical narrative review of the interrelationship between endodontics and systemic pathological conditions. Odontology. 2021;109:741–769. doi: 10.1007/s10266-021-00636-x. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–558. doi: 10.1128/CMR.13.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savarrio L, Mackenzie D, Riggio M, Saunders WP, Bagg J. Detection of bacteraemias during non-surgicalroot canal treatment. J Dent. 2005;33:293–303. doi: 10.1016/j.jdent.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner JC, Heggers JP, Harrison JW. The incidence of bacteremias related to endodontic procedures. I. Nonsurgical endodontics. J Endod. 1976;2:135–140. doi: 10.1016/S0099-2399(76)80010-6. [DOI] [PubMed] [Google Scholar]
- 11.Debelian GJ, Olsen I, Tronstad L. Bacteremia in conjunction with endodontic therapy. Endod Dent Traumatol. 1995;11:142–149. doi: 10.1111/j.1600-9657.1995.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 12.Gomes MS, Blattner TC, Sant'Ana Filho M, Grecca FS, Hugo FN, Fouad AF, Reynolds MA. Can apical periodontitis modify systemic levels of inflammatory markers? A systematic review and meta-analysis. J Endod. 2013;39:1205–1217. doi: 10.1016/j.joen.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Samuel RO, Gomes-Filho JE, Azuma MM, Sumida DH, de Oliveira SHP, Chiba FY, Bomfim SRM, Ciarlini PC, Narciso LG, Cintra LTA. Endodontic infections increase leukocyte and lymphocyte levels in the blood. Clin Oral Investig. 2018;22:1395–1401. doi: 10.1007/s00784-017-2222-z. [DOI] [PubMed] [Google Scholar]
- 14.Poornima L, Ravishankar P, Abbott PV, Subbiya A, PradeepKumar AR. Impact of root canal treatment on high-sensitivity C-reactive protein levels in systemically healthy adults with apical periodontitis-a preliminary prospective, longitudinal interventional study. Int Endod J. 2021;54:501–508. doi: 10.1111/iej.13444. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Liu S, Leng SX. Chronic low-grade inflammatory phenotype (CLIP) and senescent immune dysregulation. Clin Ther. 2019;41:400–409. doi: 10.1016/j.clinthera.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Chan LT, Zhong S, Naqvi AR, Self-Fordham J, Nares S, Bair E, Khan AA. MicroRNAs: New insights into the pathogenesis of endodontic periapical disease. J Endod. 2013;39:1498–1503. doi: 10.1016/j.joen.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki H, Hirai K, Martins CM, Furusho H, Battaglino R, Hashimoto K. Interrelationship between periapical lesion and systemic metabolic disorders. Curr Pharm Des. 2016;22:2204–2215. doi: 10.2174/1381612822666160216145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segura-Egea JJ, Martín-González J, Castellanos-Cosano L. Endodontic medicine: Connections between apical periodontitis and systemic diseases. Int Endod J. 2015;48:933–951. doi: 10.1111/iej.12507. [DOI] [PubMed] [Google Scholar]
- 19.Khalighinejad N, Aminoshariae MR, Aminoshariae A, Kulild JC, Mickel A, Fouad AF. Association between systemic diseases and apical periodontitis. J Endod. 2016;42:1427–1434. doi: 10.1016/j.joen.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Cotti E, Mercuro G. Apical periodontitis and cardiovascular diseases: Previous findings and ongoing research. Int Endod J. 2015;48:926–932. doi: 10.1111/iej.12506. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 23.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Velasco-Ortega E, Delgado-Ruiz RA, López-López J. Dentistry and diabetes: The influence of diabetes in oral diseases and dental treatments. J Diabetes Res. 2016;2016:6073190. doi: 10.1155/2016/6073190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haag DG, Peres KG, Balasubramanian M, Brennan DS. Oral conditions and health-related quality of life: A systematic review. J Dent Res. 2017;96:864–874. doi: 10.1177/0022034517709737. [DOI] [PubMed] [Google Scholar]
- 26.Rohani B. Oral manifestations in patients with diabetes mellitus. World J Diabetes. 2019;10:485–489. doi: 10.4239/wjd.v10.i9.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segura-Egea JJ, Cabanillas-Balsera D, Jiménez-Sánchez MC, Martín-González J. Endodontics and diabetes: Association versus causation. Int Endod J. 2019;52:790–802. doi: 10.1111/iej.13079. [DOI] [PubMed] [Google Scholar]
- 28.Tibúrcio-Machado CDS, Bello MDC, Maier J, Wolle CFB, Bier CAS. Influence of diabetes in the development of apical periodontitis: A critical literature review of human studies. J Endod. 2017;43:370–376. doi: 10.1016/j.joen.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Yip N, Liu C, Wu D, Fouad AF. The association of apical periodontitis and type 2 diabetes mellitus: A large hospital network cross-sectional case-controlled study. J Am Dent Assoc. 2021;152:434–443. doi: 10.1016/j.adaj.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Losada FDL, Estrugo-Devesa A, Castellanos-Cosano L, Segura-Egea JJ, López-López J, Velasco-Ortega E. Apical periodontitis and diabetes mellitus type 2: A systematic review and meta-analysis. J Clin Med. 2020;9:540. doi: 10.3390/jcm9020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segura-Egea JJ, Jiménez-Pinzón A, Ríos-Santos JV, Velasco-Ortega E, Cisneros-Cabello R, Poyato-Ferrera M. High prevalence of apical periodontitis amongst type 2 diabetic patients. Int Endod J. 2005;38:564–569. doi: 10.1111/j.1365-2591.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- 32.Smadi L. Apical periodontitis and endodontic treatment in patients with type II diabetes mellitus: Comparative cross-sectional survey. J Contemp Dent Pract. 2017;18:358–362. doi: 10.5005/jp-journals-10024-2046. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Domínguez B, López-López J, Jané-Salas E, Castellanos-Cosano L, Velasco-Ortega E, Segura-Egea JJ. Glycated hemoglobin levels and prevalence of apical periodontitis in type 2 diabetic patients. J Endod. 2015;41:601–606. doi: 10.1016/j.joen.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Saleh W, Xue W, Katz J. Diabetes mellitus and periapical abscess: A cross-sectional study. J Endod. 2020;46:1605–1609. doi: 10.1016/j.joen.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Sisli SN. Evaluation of the relationship between type II diabetes mellitus and the prevalence of apical periodontitis in root-filled teeth using cone beam computed tomography: An observational cross-sectional study. Med Princ Pract. 2019;28:533–538. doi: 10.1159/000500472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mesgarani A, Haghanifar S, Eshkevari N, Ehsani M, Khafri S, Nafarzade S, Damankesh Z. Frequency of odontogenic periradicular lesions in diabetic patients. Caspian J Intern Med. 2014;5:22–25. [PMC free article] [PubMed] [Google Scholar]
- 37.Kohsaka T, Kumazawa M, Yamasaki M, Nakamura H. Periapical lesions in rats with streptozotocin-induced diabetes. J Endod. 1996;22:418–421. doi: 10.1016/S0099-2399(96)80243-3. [DOI] [PubMed] [Google Scholar]
- 38.Al-Nazhan SA, Alsaeed SA, Al-Attas HA, Dohaithem AJ, Al-Serhan MS, Al-Maflehi NS. Prevalence of apical periodontitis and quality of root canal treatment in an adult Saudi population. Saudi Med J. 2017;38:413–421. doi: 10.15537/smj.2017.4.16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falk H, Hugoson A, Thorstensson H. Number of teeth, prevalence of caries and periapical lesions in insulin-dependent diabetics. Scand J Dent Res. 1989;97:198–206. doi: 10.1111/j.1600-0722.1989.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 40.Alghofaily M, Tordik P, Romberg E, Martinho F, Fouad AF. Healing of apical periodontitis after nonsurgical root canal treatment: The role of statin intake. J Endod. 2018;44:1355–1360. doi: 10.1016/j.joen.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 41.De la Torre-Luna R, Domínguez-Pérez RA, Guillén-Nepita AL, Ayala-Herrera JL, Martínez-Martínez RE, Romero-Ayala ME, Pérez-Serrano RM, Vázquez-Garcidueñas MS. Prevalence of Candida albicans in primary endodontic infections associated with a higher frequency of apical periodontitis in type two diabetes mellitus patients. Eur J Clin Microbiol Infect Dis. 2020;39:131–138. doi: 10.1007/s10096-019-03702-z. [DOI] [PubMed] [Google Scholar]
- 42.Graves DT, Corrêa JD, Silva TA. The oral microbiota is modified by systemic diseases. J Dent Res. 2019;98:148–156. doi: 10.1177/0022034518805739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuel RO, Ervolino E, de Azevedo Queiroz ÍO, Azuma MM, Ferreira GT, Cintra LTA. Th1/Th2/Th17/Treg balance in apical periodontitis of normoglycemic and diabetic rats. J Endod. 2019;45:1009–1015. doi: 10.1016/j.joen.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Cintra LTA, da Silva Facundo AC, Prieto AKC, Sumida DH, Narciso LG, Mogami Bomfim SR, Oliveira e Silva C, Dezan-Júnior E, Gomes-Filho JE. Blood profile and histology in oral infections associated with diabetes. J Endod. 2014;40:1139–1144. doi: 10.1016/j.joen.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Azuma MM, Gomes-Filho JE, Prieto AKC, Samuel RO, de Lima VMF, Sumida DH, Ervolino E, Cintra LTA. Diabetes increases interleukin-17 levels in periapical, hepatic, and renal tissues in rats. Arch Oral Biol. 2017;83:230–235. doi: 10.1016/j.archoralbio.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Hasiakos S, Gwack Y, Kang M, Nishimura I. Calcium signaling in T cells and chronic inflammatory disorders of the oral cavity. J Dent Res. 2021;100:693–699. doi: 10.1177/0022034521990652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Yang Y, Wang L, Chen Y, Han X, Sun L, Chen H, Chen Q. Effect of Bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front Endocrinol (Lausanne) 2023;14:1109296. doi: 10.3389/fendo.2023.1109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwama A, Nishigaki N, Nakamura K, Imaizumi I, Shibata N, Yamasaki M, Nakamura H, Kameyama Y, Kapila Y. The effect of high sugar intake on the development of periradicular lesions in rats with type 2 diabetes. J Dent Res. 2003;82:322–325. doi: 10.1177/154405910308200416. [DOI] [PubMed] [Google Scholar]
- 49.Cintra LTA, Samuel RO, Azuma MM, Ribeiro CP, Narciso LG, de Lima VM, Sumida DH, Coclete GA, Dezan-Júnior E, Gomes-Filho JE. Apical periodontitis and periodontal disease increase serum IL-17 levels in normoglycemic and diabetic rats. Clin Oral Investig. 2014;18:2123–2128. doi: 10.1007/s00784-014-1192-7. [DOI] [PubMed] [Google Scholar]
- 50.Bahrambeigi S, Yousefi B, Rahimi M, Shafiei-Irannejad V. Metformin; an old antidiabetic drug with new potentials in bone disorders. Biomed Pharmacother. 2019;109:1593–1601. doi: 10.1016/j.biopha.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 51.Zhu M, Zhao Z, Xu HHK, Dai Z, Yu K, Xiao L, Schneider A, Weir MD, Oates TW, Bai Y, Zhang K. Effects of metformin delivery via biomaterials on bone and dental tissue engineering. Int J Mol Sci. 2022;23:15905. doi: 10.3390/ijms232415905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Xia Y, Ma T, Weir MD, Ren K, Reynolds MA, Shu Y, Cheng L, Schneider A, Xu HHK. Novel metformin-containing resin promotes odontogenic differentiation and mineral synthesis of dental pulp stem cells. Drug Deliv Transl Res. 2019;9:85–96. doi: 10.1007/s13346-018-00600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang R, Liang Q, Kang W, Ge S. Metformin facilitates the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells in vitro. Cell Biol Int. 2020;44:70–79. doi: 10.1002/cbin.11202. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Zhang C, Hu Y, Peng B. Protective effect of metformin on periapical lesions in rats by decreasing the ratio of receptor activator of nuclear factor kappa B ligand/osteoprotegerin. J Endod. 2012;38:943–947. doi: 10.1016/j.joen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Lai EHH, Yang CN, Lin SK, Wang HW, Kok SH, Hong CY, Su IH, Yang H, Chang JZC. Metformin ameliorates periapical lesions through suppression of hypoxia-induced apoptosis of osteoblasts. J Endod. 2018;44:1817–1825. doi: 10.1016/j.joen.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Wang HW, Lai EHH, Yang CN, Lin SK, Hong CY, Yang H, Chang JZC, Kok SH. Intracanal metformin promotes healing of apical periodontitis via suppressing inducible nitric oxide synthase expression and monocyte recruitment. J Endod. 2020;46:65–73. doi: 10.1016/j.joen.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Sarmento EB, Gomes CC, Pires FR, Pinto LC, Antunes LAA, Armada L. Immunoexpression of bone resorption biomarkers in apical periodontitis in diabetics and normoglycaemics. Int Endod J. 2020;53:1025–1032. doi: 10.1111/iej.13305. [DOI] [PubMed] [Google Scholar]
- 58.Wang CH, Chueh LH, Chen SC, Feng YC, Hsiao CK, Chiang CP. Impact of diabetes mellitus, hypertension, and coronary artery disease on tooth extraction after nonsurgical endodontic treatment. J Endod. 2011;37:1–5. doi: 10.1016/j.joen.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 59.Mindiola MJ, Mickel AK, Sami C, Jones JJ, Lalumandier JA, Nelson SS. Endodontic treatment in an American Indian population: A 10-year retrospective study. J Endod. 2006;32:828–832. doi: 10.1016/j.joen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Lima SMF, Grisi DC, Kogawa EM, Franco OL, Peixoto VC, Gonçalves-Júnior JF, Arruda MP, Rezende TM. Diabetes mellitus and inflammatory pulpal and periapical disease: A review. Int Endod J. 2013;46:700–709. doi: 10.1111/iej.12072. [DOI] [PubMed] [Google Scholar]
- 61.Uğur Aydın Z, Ocak MG, Bayrak S, Göller Bulut D, Orhan K. The effect of type 2 diabetes mellitus on changes in the fractal dimension of periapical lesion in teeth after root canal treatment: A fractal analysis study. Int Endod J. 2021;54:181–189. doi: 10.1111/iej.13409. [DOI] [PubMed] [Google Scholar]
- 62.Rudranaik S, Nayak M, Babshet M. Periapical healing outcome following single visit endodontic treatment in patients with type 2 diabetes mellitus. J Clin Exp Dent. 2016;8:e498–e504. doi: 10.4317/jced.52859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinho JP, Coelho A, Oliveiros B, Pires S, Abrantes AM, Paulo S, Carvalho AC, Carrilho E, Paula A, Carvalho L, et al. Impairment of the angiogenic process may contribute to lower success rate of root canal treatments in diabetes mellitus. Int Endod J. 2021;54:1687–1698. doi: 10.1111/iej.13572. [DOI] [PubMed] [Google Scholar]
- 64.Britto LR, Katz J, Guelmann M, Heft M. Periradicular radiographic assessment in diabetic and control individuals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:449–452. doi: 10.1016/S1079-2104(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 65.Fouad AF, Burleson J. The effect of diabetes mellitus on endodontic treatment outcome: Data from an electronic patient record. J Am Dent Assoc. 2003;134(43-51):117–118. doi: 10.14219/jada.archive.2003.0016. [DOI] [PubMed] [Google Scholar]
- 66.Laukkanen E, Vehkalahti MM, Kotiranta AK. Impact of systemic diseases and tooth-based factors on outcome of root canal treatment. Int Endod J. 2019;52:1417–1426. doi: 10.1111/iej.13143. [DOI] [PubMed] [Google Scholar]
- 67.Arya S, Duhan J, Tewari S, Sangwan P, Ghalaut V, Aggarwal S. Healing of apical periodontitis after nonsurgical treatment in patients with type 2 diabetes. J Endod. 2017;43:1623–1627. doi: 10.1016/j.joen.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Limeira FIR, Arantes DC, de Souza Oliveira C, de Melo DP, Magalhães CS, Bento PM. Root canal treatment and apical periodontitis in a brazilian population with type 1 diabetes mellitus: A cross-sectional paired study. J Endod. 2020;46:756–762. doi: 10.1016/j.joen.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Doyle SL, Hodges JS, Pesun IJ, Baisden MK, Bowles WR. Factors affecting outcomes for single-tooth implants and endodontic restorations. J Endod. 2007;33:399–402. doi: 10.1016/j.joen.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 70.Marotta PS, Fontes TV, Armada L, Lima KC, Rôças IN, Siqueira JF., Jr Type 2 diabetes mellitus and the prevalence of apical periodontitis and endodontic treatment in an adult Brazilian population. J Endod. 2012;38:297–300. doi: 10.1016/j.joen.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 71.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. Int Endod J. 2011;44:583–609. doi: 10.1111/j.1365-2591.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- 72.Bloomgarden ZT. Inflammation and insulin resistance. Diabetes Care. 2003;26:1619–1623. doi: 10.2337/diacare.26.5.1619. [DOI] [PubMed] [Google Scholar]
- 73.Cintra LTA, Samuel RO, Facundo ACS, Prieto AK, Sumida DH, Bomfim SR, Souza JC, Dezan-Júnior E, Gomes-Filho JE. Relationships between oral infections and blood glucose concentrations or HbA1c levels in normal and diabetic rats. Int Endod J. 2014;47:228–237. doi: 10.1111/iej.12136. [DOI] [PubMed] [Google Scholar]
- 74.Astolphi RD, Curbete MM, Colombo NH, Shirakashi DJ, Chiba FY, Prieto AK, Cintra LT, Bomfim SR, Ervolino E, Sumida DH. Periapical lesions decrease insulin signal and cause insulin resistance. J Endod. 2013;39:648–652. doi: 10.1016/j.joen.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 75.Astolphi RD, Curbete MM, Chiba FY, Cintra LT, Ervolino E, da Mota MS, Antoniali C, Garbin CA, Sumida DH. Periapical lesions decrease insulin signaling in rat skeletal muscle. J Endod. 2015;41:1305–1310. doi: 10.1016/j.joen.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Pereira RF, de Oliveira da Mota MS, de Lima Coutinho Mattera MS, Tsosura TV, Chiba FY, Garbin CA, Ervolino E, Cintra LT, Okamoto MM, Machado UF, Sumida DH. Periapical lesions decrease Akt serine phosphorylation and plasma membrane GLUT4 content in rat skeletal muscle. Clin Oral Investig. 2016;20:1625–1630. doi: 10.1007/s00784-015-1664-4. [DOI] [PubMed] [Google Scholar]
- 77.Felipe Pereira R, Willian Lattari Tessarin G, Yamamoto Chiba F, Sara de Lima Coutinho Mattera M, Gomes Pereira A, Verônica Saori Tsosura T, Gustavo Balera Brito V, Akira Fujii, de Oliveira R, Ervolino E, Helena Penha, de Oliveira S, et al. Apical periodontitis promotes insulin resistance and alters adaptive immunity markers in rats. Saudi Dent J. 2021;33:979–986. doi: 10.1016/j.sdentj.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsosura TVS, Chiba FY, Mattera MSLC, Pereira RF, Cintra LTA, Conti LC, Santos RMD, Mateus JHP, Garbin CAS, Sumida DH. Maternal apical periodontitis is associated with insulin resistance in adult offspring. Int Endod J. 2019;52:1040–1050. doi: 10.1111/iej.13096. [DOI] [PubMed] [Google Scholar]
- 79.Ferreira LL, editor. Diabetic rats present high mean platelet count in the presence of oral infections. Braz Dent J. 2017;28:548–551. doi: 10.1590/0103-6440201701386. [DOI] [PubMed] [Google Scholar]
- 80.Prieto AKC, Gomes-Filho JE, Azuma MM, Sivieri-Araújo G, Narciso LG, Souza JC, Ciarlini PC, Cintra LTA. Influence of apical periodontitis on stress oxidative parameters in diabetic rats. J Endod. 2017;43:1651–1656. doi: 10.1016/j.joen.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 81.Barcelos RCS, Rosa HZ, Roversi K, Tibúrcio-Machado CDS, Inchaki PT, Burger ME, Bier CAS. Apical periodontitis induces changes on oxidative stress parameters and increases Na+/K+-ATPase activity in adult rats. Arch Oral Biol. 2020;118:104849. doi: 10.1016/j.archoralbio.2020.104849. [DOI] [PubMed] [Google Scholar]
- 82.Schulze A, Schönauer M, Busse M. Sudden improvement of insulin sensitivity related to an endodontic treatment. J Periodontol. 2007;78:2380–2384. doi: 10.1902/jop.2007.070033. [DOI] [PubMed] [Google Scholar]
- 83.Stys LPA, Böttcher DE, Scarparo RK, Gonçalves Waltrick SB, de Figueiredo JAP, Gomes MS, Campos MM. Serum levels of inflammatory markers and HbA1c in patients with type 2 diabetes and apical periodontitis: Preliminary findings. Aust Endod J. 2022;48:105–115. doi: 10.1111/aej.12569. [DOI] [PubMed] [Google Scholar]
- 84.Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-E. No authors listed. [DOI] [PubMed] [Google Scholar]
- 85.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 86.Gilles JA, Carnes DL, Dallas MR, Holt SC, Bonewald LF. Oral bone loss is increased in ovariectomized rats. J Endod. 1997;23:419–422. doi: 10.1016/S0099-2399(97)80294-4. [DOI] [PubMed] [Google Scholar]
- 87.López-López J, Castellanos-Cosano L, Estrugo-Devesa A, Gómez-Vaquero C, Velasco-Ortega E, Segura-Egea JJ. Radiolucent periapical lesions and bone mineral density in post-menopausal women. Gerodontology. 2015;32:195–201. doi: 10.1111/ger.12076. [DOI] [PubMed] [Google Scholar]
- 88.Tounta TS. Diagnosis of osteoporosis in dental patients. J Frailty Sarcopenia Falls. 2017;2:21–27. doi: 10.22540/JFSF-02-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 90.Brennan RM, Genco RJ, Hovey KM, Trevisan M, Wactawski-Wende J. Clinical attachment loss, systemic bone density, and subgingival calculus in postmenopausal women. J Periodontol. 2007;78:2104–2111. doi: 10.1902/jop.2007.070155. [DOI] [PubMed] [Google Scholar]
- 91.Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:349–356. doi: 10.1016/j.tripleo.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 92.Katz J, Rotstein I. Prevalence of periapical lesions in patients with osteoporosis. J Endod. 2021;47:234–238. doi: 10.1016/j.joen.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 93.Wayama MT, Yoshimura H, Ohba S, Yoshida H, Matsuda S, Kobayashi J, Kobayashi M, Gomes Filho JE, Sano K. Diminished progression of periapical lesions with zoledronic acid in ovariectomized rats. J Endod. 2015;41:2002–2007. doi: 10.1016/j.joen.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 94.Ikeda M, Karakawa A, Takizawa H, Azetsu Y, Sakai N, Chatani M, Suzuki N, Takami M. Effects of anti-receptor activator of nuclear factor kappa B ligand antibody and zoledronic acid on periapical lesion development in mice. J Endod. 2022;48:632–640. doi: 10.1016/j.joen.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 95.Xiong H, Peng B, Wei L, Zhang X, Wang L. Effect of an estrogen-deficient state and alendronate therapy on bone loss resulting from experimental periapical lesions in rats. J Endod. 2007;33:1304–1308. doi: 10.1016/j.joen.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 96.Silva RAB, Sousa-Pereira AP, Lucisano MP, Romualdo PC, Paula-Silva FWG, Consolaro A, Silva LAB, Nelson-Filho P. Alendronate inhibits osteocyte apoptosis and inflammation via IL-6, inhibiting bone resorption in periapical lesions of ovariectomized rats. Int Endod J. 2020;53:84–96. doi: 10.1111/iej.13206. [DOI] [PubMed] [Google Scholar]
- 97.Guan X, Guan Y, Shi C, Zhu X, He Y, Wei Z, Yang J, Hou T. Estrogen deficiency aggravates apical periodontitis by regulating NLRP3/caspase-1/IL-1β axis. Am J Transl Res. 2020;12:660–671. [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang X, Peng B, Fan M, Bian Z, Chen Z. The effect of estrogen deficiency on receptor activator of nuclear factor kappa B ligand and osteoprotegerin synthesis in periapical lesions induced in rats. J Endod. 2007;33:1053–1056. doi: 10.1016/j.joen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Romualdo PC, Lucisano MP, Paula-Silva FWG, Leoni GB, Sousa-Neto MD, Silva RAB, Silva LAB, Nelson-Filho P. Ovariectomy exacerbates apical periodontitis in rats with an increase in expression of proinflammatory cytokines and matrix metalloproteinases. J Endod. 2018;44:780–785. doi: 10.1016/j.joen.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Brasil SC, Santos RMM, Fernandes A, Alves FR, Pires FR, Siqueira JF, Jr, Armada L. Influence of oestrogen deficiency on the development of apical periodontitis. Int Endod J. 2017;50:161–166. doi: 10.1111/iej.12612. [DOI] [PubMed] [Google Scholar]
- 101.Lucisano MP, da Silva RAB, de Sousa Pereira AP, Romualdo PC, Feres M, de Queiroz AM, Nelson-Filho P, da Silva LAB. Alteration of the oral microbiota may be a responsible factor, along with estrogen deficiency, by the development of larger periapical lesions. Clin Oral Investig. 2021;25:3651–3662. doi: 10.1007/s00784-020-03688-5. [DOI] [PubMed] [Google Scholar]
- 102.Gomes-Filho JE, Wayama MT, Dornelles RCM, Ervolino E, Yamanari GH, Lodi CS, Sivieri-Araújo G, Dezan-Júnior E, Cintra LT. Raloxifene modulates regulators of osteoclastogenesis and angiogenesis in an oestrogen deficiency periapical lesion model. Int Endod J. 2015;48:1059–1068. doi: 10.1111/iej.12403. [DOI] [PubMed] [Google Scholar]
- 103.Qian H, Guan X, Bian Z. FSH aggravates bone loss in ovariectomised rats with experimental periapical periodontitis. Mol Med Rep. 2016;14:2997–3006. doi: 10.3892/mmr.2016.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu S, Cheng Y, Xu W, Bian Z. Protective effects of follicle-stimulating hormone inhibitor on alveolar bone loss resulting from experimental periapical lesions in ovariectomized rats. J Endod. 2010;36:658–663. doi: 10.1016/j.joen.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 105.Braz-Silva PH, Bergamini ML, Mardegan AP, De Rosa CS, Hasseus B, Jonasson P. Inflammatory profile of chronic apical periodontitis: A literature review. Acta Odontol Scand. 2019;77:173–180. doi: 10.1080/00016357.2018.1521005. [DOI] [PubMed] [Google Scholar]
- 106.Matsuo T, Ebisu S, Shimabukuro Y, Ohtake T, Okada H. Quantitative analysis of immunocompetent cells in human periapical lesions: Correlations with clinical findings of the involved teeth. J Endod. 1992;18:497–500. doi: 10.1016/S0099-2399(06)81350-6. [DOI] [PubMed] [Google Scholar]
- 107.Guerrero-Gironés J, Ros-Valverde A, Pecci-Lloret MP, Rodríguez-Lozano FJ, Pecci-Lloret MR. Association between pulpal-periapical pathology and autoimmune diseases: A systematic review. J Clin Med. 2021;10:4886. doi: 10.3390/jcm10214886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karataş E, Kul A, Tepecik E. Association of ankylosing spondylitis with radiographically and clinically diagnosed apical periodontitis: A cross-sectional study. Dent Med Probl. 2020;57:171–175. doi: 10.17219/dmp/114463. [DOI] [PubMed] [Google Scholar]
- 109.Barta Z. Apical periodontitis in patients with inflammatory bowel disease: A puppet master? Inflamm Bowel Dis. 2020;26:280–282. doi: 10.1093/ibd/izz129. [DOI] [PubMed] [Google Scholar]
- 110.Nakamura K, Yamasaki M, Nishigaki N, Iwama A, Imaizumi I, Nakamura H, Kameyama Y. Effect of methotrexate-induced neutropenia on pulpal inflammation in rats. J Endod. 2002;28:287–290. doi: 10.1097/00004770-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 111.Yoshinari N, Kameyama Y, Aoyama Y, Nishiyama H, Noguchi T. Effect of long-term methotrexate-induced neutropenia on experimental periodontal lesion in rats. J Periodontal Res. 1994;29:393–400. doi: 10.1111/j.1600-0765.1994.tb01240.x. [DOI] [PubMed] [Google Scholar]
- 112.Yamasaki M, Kumazawa M, Kohsaka T, Nakamura H. Effect of methotrexate-induced neutropenia on rat periapical lesion. Oral Surg Oral Med Oral Pathol. 1994;77:655–661. doi: 10.1016/0030-4220(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 113.Waterman PA, Jr, Torabinejad M, McMillan PJ, Kettering JD. Development of periradicular lesions in immunosuppressed rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:720–725. doi: 10.1016/S1079-2104(98)90041-5. [DOI] [PubMed] [Google Scholar]
- 114.Teixeira FB, Gomes BP, Ferraz CC, Souza-Filho FJ, Zaia AA. Radiographic analysis of the development of periapical lesions in normal rats, sialoadenectomized rats and sialoadenectomized-immunosuppressed rats. Endod Dent Traumatol. 2000;16:154–157. doi: 10.1034/j.1600-9657.2000.016004154.x. [DOI] [PubMed] [Google Scholar]
- 115.Wasserman A. Rheumatoid arthritis: Common questions about diagnosis and management. Am Fam Physician. 2018;97:455–462. [PubMed] [Google Scholar]
- 116.Karataş E, Kul A, Tepecik E. Association between rheumatoid arthritis and apical periodontitis: A cross-sectional study. Eur Endod J. 2020;5:155–158. doi: 10.14744/eej.2019.52824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rotstein I, Katz J. Prevalence of periapical abscesses in patients with rheumatoid arthritis. A cross sectional study. Am J Dent. 2021;34:211–214. [PubMed] [Google Scholar]
- 118.Oh WM, Hwang IN, Son HH, Hwang YC. Rapid periapical bone destruction during endodontic treatment of a patient with rheumatoid arthritis. J Endod. 2008;34:1261–1263. doi: 10.1016/j.joen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 119.Jalali P, Glickman GN, Schneiderman ED, Schweitzer JL. Prevalence of periapical rarefying osteitis in patients with rheumatoid arthritis. J Endod. 2017;43:1093–1096. doi: 10.1016/j.joen.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 120.Cotti E, Schirru E, Acquas E, Usai P. An overview on biologic medications and their possible role in apical periodontitis. J Endod. 2014;40:1902–1911. doi: 10.1016/j.joen.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 121.Malmström M. Immunoglobulin clases IgG, IgM, IgA and complement component C3 in dental periapical lesions of patients with rheumatoid disease. Scand J Rheumatol. 1975;4:57–64. doi: 10.3109/03009747509095616. [DOI] [PubMed] [Google Scholar]
- 122.Malmström M, Calonius PE. Teeth loss and the inflammation of teeth-supporting tissues in rheumatoid disease. Scand J Rheumatol. 1975;4:49–55. doi: 10.3109/03009747509095615. [DOI] [PubMed] [Google Scholar]
- 123.Malmström M, Jokinen EJ. Free rheumatoid factor in dental periapical lesions and gingivae of patients with rheumatoid disease. Scand J Rheumatol. 1975;4:121–124. doi: 10.3109/03009747509165440. [DOI] [PubMed] [Google Scholar]
- 124.Malmström M, Natvig JB. IgG rheumatoid factor in dental periapical lesions of patients with rheumatoid disease. Scand J Rheumatol. 1975;4:177–185. doi: 10.3109/03009747509165253. [DOI] [PubMed] [Google Scholar]
- 125.Malmström M, Husby G. Occurrence of amyloid in the teeth-supporting tissues of patients with rheumatoid diseases. An immunohistochemical study. Scand J Rheumatol. 1975;4:186–192. doi: 10.3109/03009747509165254. [DOI] [PubMed] [Google Scholar]
- 126.Ishii K, Hatori K, Takeichi O, Makino K, Himi K, Komiya H, Ogiso B. Expression of the Forkhead box transcription factor Foxo3a in human periapical granulomas. J Oral Sci. 2018;60:479–483. doi: 10.2334/josnusd.17-0439. [DOI] [PubMed] [Google Scholar]
- 127.Vergani D, Mieli-Vergani G. Autoimmune manifestations in viral hepatitis. Semin Immunopathol. 2013;35:73–85. doi: 10.1007/s00281-012-0328-6. [DOI] [PubMed] [Google Scholar]
- 128.Bjørklund G, Pivin M, Hangan T, Yurkovskaya O, Pivina L. Autoimmune polyendocrine syndrome type 1: Clinical manifestations, pathogenetic features, and management approach. Autoimmun Rev. 2022;21:103135. doi: 10.1016/j.autrev.2022.103135. [DOI] [PubMed] [Google Scholar]
- 129.Joyce E, Glasner P, Ranganathan S, Swiatecka-Urban A. Tubulointerstitial nephritis: Diagnosis, treatment, and monitoring. Pediatr Nephrol. 2017;32:577–587. doi: 10.1007/s00467-016-3394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cohen G. Immune dysfunction in Uremia 2020. Toxins (Basel) 2020;12:439. doi: 10.3390/toxins12070439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grønkjær LL, Vilstrup H. Oral health in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2015;27:834–839. doi: 10.1097/MEG.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 132.Coates EA, Brennan D, Logan RM, Goss AN, Scopacasa B, Spencer AJ, Gorkic E. Hepatitis C infection and associated oral health problems. Aust Dent J. 2000;45:108–114. doi: 10.1111/j.1834-7819.2000.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 133.Lins L, Bittencourt PL, Evangelista MA, Lins R, Codes L, Cavalcanti AR, Paraná R, Bastos J. Oral health profile of cirrhotic patients awaiting liver transplantation in the Brazilian Northeast. Transplant Proc. 2011;43:1319–1321. doi: 10.1016/j.transproceed.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 134.Barbero P, Garzino Demo MG, Milanesio M, Ottobrelli A. The dental assessment of the patient waiting for a liver transplant. Minerva Stomatol. 1996;45:431–439. In Italian. [PubMed] [Google Scholar]
- 135.Guggenheimer J, Eghtesad B, Stock DJ. Dental management of the (solid) organ transplant patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:383–389. doi: 10.1067/moe.2003.150. [DOI] [PubMed] [Google Scholar]
- 136.Helenius-Hietala J, Meurman JH, Höckerstedt K, Lindqvist C, Isoniemi H. Effect of the aetiology and severity of liver disease on oral health and dental treatment prior to transplantation. Transpl Int. 2012;25:158–165. doi: 10.1111/j.1432-2277.2011.01381.x. [DOI] [PubMed] [Google Scholar]
- 137.Castellanos-Cosano L, Machuca-Portillo G, Segura-Sampedro JJ, Torres-Lagares D, López-López J, Velasco-Ortega E, Segura-Egea JJ. Prevalence of apical periodontitis and frequency of root canal treatments in liver transplant candidates. Med Oral Patol Oral Cir Bucal. 2013;18:e773–e779. doi: 10.4317/medoral.19148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grønkjær LL, Holmstrup P, Schou S, Schwartz K, Kongstad J, Jepsen P, Vilstrup H. Presence and consequence of tooth periapical radiolucency in patients with cirrhosis. Hepat Med. 2016;8:97–103. doi: 10.2147/HMER.S113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cantiga-Silva C, Estrela C, Segura-Egea JJ, Azevedo JP, de Oliveira PHC, Cardoso CBM, Pinheiro TN, Ervolino E, Sivieri-Araújo G, Cintra LTA. Inflammatory profile of apical periodontitis associated with liver fibrosis in rats: Histological and immunohistochemical analysis. Int Endod J. 2021;54:1353–1361. doi: 10.1111/iej.13519. [DOI] [PubMed] [Google Scholar]
- 140.Echavarría-García AC, Pozos-Guillén A, Tejeda-Nava F, Flores Arriaga JC, Garrocho-Rangel A. Oral management of children with Henoch-Schönlein purpura and associated glomerulonephritis: A scoping review. Eur J Paediatr Dent. 2018;19:134–138. doi: 10.23804/ejpd.2018.19.02.07. [DOI] [PubMed] [Google Scholar]
- 141.Inoue CN, Nagasaka T, Matsutani S, Ishidoya M, Homma R, Chiba Y. Efficacy of early dental and ENT therapy in preventing nephropathy in pediatric Henoch-Schönlein purpura. Clin Rheumatol. 2008;27:1489–1496. doi: 10.1007/s10067-008-0954-5. [DOI] [PubMed] [Google Scholar]
- 142.Al-Wahadni A, Al-Omari MA. Dental diseases in a Jordanian population on renal dialysis. Quintessence Int. 2003;34:343–347. [PubMed] [Google Scholar]
- 143.Souza CM, Braosi APR, Luczyszyn SM, Casagrande RW, Pecoits-Filho R, Riella MC, Ignácio SA, Trevilatto PC. Oral health in Brazilian patients with chronic renal disease. Rev Med Chil. 2008;136:741–746. doi: 10.4067/S0034-98872008000600008. [DOI] [PubMed] [Google Scholar]
- 144.Bayraktar G, Kurtulus I, Duraduryan A, Cintan S, Kazancioglu R, Yildiz A, Bural C, Bozfakioglu S, Besler M, Trablus S, Issever H. Dental and periodontal findings in hemodialysis patients. Oral Dis. 2007;13:393–397. doi: 10.1111/j.1601-0825.2006.01297.x. [DOI] [PubMed] [Google Scholar]
- 145.Sobrado Marinho JS, Tomás Carmona I, Loureiro A, Limeres Posse J, García Caballero L, Diz Dios P. Oral health status in patients with moderate-severe and terminal renal failure. Med Oral Patol Oral Cir Bucal. 2007;12:E305–E310. [PubMed] [Google Scholar]
- 146.Buhlin K, Bárány P, Heimbürger O, Stenvinkel P, Gustafsson A. Oral health and pro-inflammatory status in end-stage renal disease patients. Oral Health Prev Dent. 2007;5:235–244. [PubMed] [Google Scholar]
- 147.Khalighinejad N, Aminoshariae A, Kulild JC, Sahly K, Mickel A. Association of end-stage renal disease with radiographically and clinically diagnosed apical periodontitis: A hospital-based study. J Endod. 2017;43:1438–1441. doi: 10.1016/j.joen.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 148.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/S0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 149.Nair PN. Apical periodontitis: A dynamic encounter between root canal infection and host response. Periodontol. 1997;2000(13):121–148. doi: 10.1111/j.1600-0757.1997.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 150.Mizutani K, Mikami R, Gohda T, Gotoh H, Aoyama N, Matsuura T, Kido D, Takeda K, Izumi Y, Sasaki Y, Iwata T. Poor oral hygiene and dental caries predict high mortality rate in hemodialysis: A 3-year cohort study. Sci Rep. 2020;10:21872. doi: 10.1038/s41598-020-78724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Olsen I, Yamazaki K. Can oral bacteria affect the microbiome of the gut? J Oral Microbiol. 2019;11:1586422. doi: 10.1080/20002297.2019.1586422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ogura Y, Suzuki S, Shirakawa T, Masuda M, Nakamura H, Iijima K, Yoshikawa N. Haemophilus parainfluenzae antigen and antibody in children with IgA nephropathy and Henoch-Schönlein nephritis. Am J Kidney Dis. 2000;36:47–52. doi: 10.1053/ajkd.2000.8264. [DOI] [PubMed] [Google Scholar]
- 153.Kojima A, Nakano K, Wada K, Takahashi H, Katayama K, Yoneda M, Higurashi T, Nomura R, Hokamura K, Muranaka Y, et al. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci Rep. 2012;2:332. doi: 10.1038/srep00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tahmassebi JF, Paterson SA. Development of acute Henoch-Schönlein purpura subsequent to endodontic treatment. Int J Paediatr Dent. 2007;17:217–222. doi: 10.1111/j.1365-263X.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 155.Papageorgiou SN, Hagner M, Nogueira AVB, Franke A, Jäger A, Deschner J. Inflammatory bowel disease and oral health: Systematic review and a meta-analysis. J Clin Periodontol. 2017;44:382–393. doi: 10.1111/jcpe.12698. [DOI] [PubMed] [Google Scholar]
- 156.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 157.Kalmar JR. Crohn's disease: Orofacial considerations and disease pathogenesis. Periodontol. 1994;2000(6):101–115. doi: 10.1111/j.1600-0757.1994.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 158.She YY, Kong XB, Ge YP, Liu ZY, Chen JY, Jiang JW, Jiang HB, Fang SL. Periodontitis and inflammatory bowel disease: A meta-analysis. BMC Oral Health. 2020;20:67. doi: 10.1186/s12903-020-1053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Y, Qiao D, Chen R, Zhu F, Gong J, Yan F. The association between periodontitis and inflammatory bowel disease: A systematic review and meta-analysis. Biomed Res Int. 2021;2021:6692420. doi: 10.1155/2021/6692420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tan CXW, Brand HS, Kalender B, De Boer NKH, Forouzanfar T, de Visscher JGAM. Dental and periodontal disease in patients with inflammatory bowel disease. Clin Oral Investig. 2021;25:5273–5280. doi: 10.1007/s00784-021-03835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Piras V, Usai P, Mezzena S, Susnik M, Ideo F, Schirru E, Cotti E. Prevalence of apical periodontitis in patients with inflammatory bowel diseases: A retrospective clinical study. J Endod. 2017;43:389–394. doi: 10.1016/j.joen.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 162.Poyato-Borrego M, Segura-Egea JJ, Martín-González J, Jiménez-Sánchez MC, Cabanillas-Balsera D, Areal-Quecuty V, Segura-Sampedro JJ. Prevalence of endodontic infection in patients with Crohn’s disease and ulcerative colitis. Med Oral Patol Oral Cir Bucal. 2021;26:e208–e215. doi: 10.4317/medoral.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Poyato-Borrego M, Segura-Sampedro JJ, Martín-González J, Torres-Domínguez Y, Velasco-Ortega E, Segura-Egea JJ. High prevalence of apical periodontitis in patients with inflammatory bowel disease: An age- and gender-matched case-control study. Inflamm Bowel Dis. 2020;26:273–279. doi: 10.1093/ibd/izz128. [DOI] [PubMed] [Google Scholar]
- 164.Johannsen A, Fored MC, Håkansson J, Ekbom A, Gustafsson A. Consumption of dental treatment in patients with inflammatory bowel disease, a register study. PLoS One. 2015;10:e0134001. doi: 10.1371/journal.pone.0134001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Segura-Sampedro JJ, Jiménez-Giménez C, Jane-Salas E, Cabanillas-Balsera D, Martín-González J, Segura-Egea JJ, López-López J. Periapical and endodontic status of patients with inflammatory bowel disease: Age- and sex-matched case-control study. Int Endod J. 2022;55:748–757. doi: 10.1111/iej.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lu Y, Li X, Liu S, Zhang Y, Zhang D. Toll-like receptors and inflammatory bowel disease. Front Immunol. 2018;9:72. doi: 10.3389/fimmu.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature-part 2. Influence of clinical factors. Int Endod J. 2008;41:6–31. doi: 10.1111/j.1365-2591.2008.01484.x. [DOI] [PubMed] [Google Scholar]
- 168.Bamias G, Martin C, Mishina M, Ross WG, Rivera-Nieves J, Marini M, Cominelli F. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 169.Fukada SY, Silva TA, Garlet GP, Rosa AL, da Silva JS, Cunha FQ. Factors involved in the T helper type 1 and type 2 cell commitment and osteoclast regulation in inflammatory apical diseases. Oral Microbiol Immunol. 2009;24:25–31. doi: 10.1111/j.1399-302X.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- 170.Stashenko P, Wang CY, Tani-Ishii N, Yu SM. Pathogenesis of induced rat periapical lesions. Oral Surg Oral Med Oral Pathol. 1994;78:494–502. doi: 10.1016/0030-4220(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 171.Wei L, Xu M, Xiong H. An update of knowledge on the regulatory role of Treg cells in apical periodontitis. Oral Dis. 2021;27:1356–1365. doi: 10.1111/odi.13450. [DOI] [PubMed] [Google Scholar]
- 172.Van Dyke TE, Dowell VR, Jr, Offenbacher S, Snyder W, Hersh T. Potential role of microorganisms isolated from periodontal lesions in the pathogenesis of inflammatory bowel disease. Infect Immun. 1986;53:671–677. doi: 10.1128/iai.53.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chi AC, Neville BW, Krayer JW, Gonsalves WC. Oral manifestations of systemic disease. Am Fam Physician. 2010;82:1381–1388. [PubMed] [Google Scholar]
- 174.Rodriguez Herrero E, Boon N, Pauwels M, Bernaerts K, Slomka V, Quirynen M, Teughels W. Necrotrophic growth of periodontopathogens is a novel virulence factor in oral biofilms. Sci Rep. 2017;7:1107. doi: 10.1038/s41598-017-01239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Read E, Curtis MA, Neves JF. The role of oral bacteria in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:731–742. doi: 10.1038/s41575-021-00488-4. [DOI] [PubMed] [Google Scholar]
- 176.Tavares CO, Rost FL, Silva RBM, Dagnino AP, Adami B, Schirmer H, de Figueiredo JAP, Souto AA, Maito FDM, Campos MM. Cross talk between apical periodontitis and metabolic disorders: Experimental evidence on the role of intestinal adipokines and akkermansia muciniphila. J Endod. 2019;45:174–180. doi: 10.1016/j.joen.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 177.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gan G, Lu B, Zhang R, Luo Y, Chen S, Lei H, Li Y, Cai Z, Huang X. Chronic apical periodontitis exacerbates atherosclerosis in apolipoprotein E-deficient mice and leads to changes in the diversity of gut microbiota. Int Endod J. 2022;55:152–163. doi: 10.1111/iej.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ideo F, Niazi S, Mezzena S, Mannocci F, Cotti E. Prevalence of apical periodontitis in patients with autoimmune diseases under immunomodulators: A retrospective cohort study. J Endod. 2022;48:722–729. doi: 10.1016/j.joen.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 180.Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs. 2005;65:2253–2286. doi: 10.2165/00003495-200565160-00002. [DOI] [PubMed] [Google Scholar]
- 181.Cotti E, Mezzena S, Schirru E, Ottonello O, Mura M, Ideo F, Susnik M, Usai P. Healing of apical periodontitis in patients with inflammatory bowel diseases and under anti-tumor necrosis factor alpha therapy. J Endod. 2018;44:1777–1782. doi: 10.1016/j.joen.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 182.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–831. doi: 10.1172/JCI200316069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, Mente A, Yusuf S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ Res. 2017;121:677–694. doi: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- 184.Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. doi: 10.1038/nrcardio.2017.154. [DOI] [PubMed] [Google Scholar]
- 185.Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in india: current epidemiology and future directions. Circulation. 2016;133:1605–1620. doi: 10.1161/CIRCULATIONAHA.114.008729. [DOI] [PubMed] [Google Scholar]
- 186.Cotti E, Dessì C, Piras A, Mercuro G. Can a chronic dental infection be considered a cause of cardiovascular disease? A review of the literature. Int J Cardiol. 2011;148:4–10. doi: 10.1016/j.ijcard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 187.Berlin-Broner Y, Febbraio M, Levin L. Association between apical periodontitis and cardiovascular diseases: A systematic review of the literature. Int Endod J. 2017;50:847–859. doi: 10.1111/iej.12710. [DOI] [PubMed] [Google Scholar]
- 188.González Navarro B, Pintó Sala X, Jané Salas E. Relationship between cardiovascular disease and dental pathology. Systematic review. Med Clin (Barc) 2017;149:211–216. doi: 10.1016/j.medcli.2017.05.010. In English, Spanish. [DOI] [PubMed] [Google Scholar]
- 189.Koletsi D, Iliadi A, Tzanetakis GN, Vavuranakis M, Eliades T. Cardiovascular disease and chronic endodontic infection. Is there an association? A systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:9111. doi: 10.3390/ijerph18179111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Garg P, Chaman C. Apical periodontitis-is it accountable for cardiovascular diseases? J Clin Diagn Res. 2016;10:ZE08–ZE12. doi: 10.7860/JCDR/2016/19863.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jakovljevic A, Duncan HF, Nagendrababu V, Jacimovic J, Milasin J, Dummer PMH. Association between cardiovascular diseases and apical periodontitis: An umbrella review. Int Endod J. 2020;53:1374–1386. doi: 10.1111/iej.13364. [DOI] [PubMed] [Google Scholar]
- 192.Aloutaibi YA, Alkarim AS, Qumri EM, Almansour LA, Alghamdi FT. Chronic endodontic infections and cardiovascular diseases: Does the evidence support an independent association? Cureus. 2021;13:e19864. doi: 10.7759/cureus.19864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jiménez-Sánchez MC, Cabanillas-Balsera D, Areal-Quecuty V, Velasco-Ortega E, Martín-González J, Segura-Egea JJ. Cardiovascular diseases and apical periodontitis: Association not always implies causality. Med Oral Patol Oral Cir Bucal. 2020;25:e652–e659. doi: 10.4317/medoral.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Noites R, Teixeira M, Cavero-Redondo I, Alvarez-Bueno C, Ribeiro F. Apical periodontitis and cardiovascular disease in adults: A systematic review with meta-analysis. Rev Cardiovasc Med. 2022;23:100. doi: 10.31083/j.rcm2303100. [DOI] [PubMed] [Google Scholar]
- 195.Leão TSS, Tomasi GH, Conzatti LP, Marrone LCP, Reynolds MA, Gomes MS. Oral inflammatory burden and carotid atherosclerosis among stroke patients. J Endod. 2022;48:597–605. doi: 10.1016/j.joen.2022.01.019. [DOI] [PubMed] [Google Scholar]
- 196.Garrido M, Cárdenas AM, Astorga J, Quinlan F, Valdés M, Chaparro A, Carvajal P, Pussinen P, Huamán-Chipana P, Jalil JE, Hernández M. Elevated systemic inflammatory burden and cardiovascular risk in young adults with endodontic apical lesions. J Endod. 2019;45:111–115. doi: 10.1016/j.joen.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 197.Conti LC, Segura-Egea JJ, Cardoso CBM, Benetti F, Azuma MM, Oliveira PHC, Bomfim SRM, Cintra LTA. Relationship between apical periodontitis and atherosclerosis in rats: Lipid profile and histological study. Int Endod J. 2020;53:1387–1397. doi: 10.1111/iej.13350. [DOI] [PubMed] [Google Scholar]
- 198.Caplan DJ. Chronic apical periodontitis is more common in subjects with coronary artery disease. J Evid Based Dent Pract. 2014;14:149–150. doi: 10.1016/j.jebdp.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 199.Caplan DJ, Chasen JB, Krall EA, Cai J, Kang S, Garcia RI, Offenbacher S, Beck JD. Lesions of endodontic origin and risk of coronary heart disease. J Dent Res. 2006;85:996. doi: 10.1177/154405910608501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liljestrand JM, Mäntylä P, Paju S, Buhlin K, Kopra KA, Persson GR, Hernandez M, Nieminen MS, Sinisalo J, Tjäderhane L, Pussinen PJ. Association of endodontic lesions with coronary artery disease. J Dent Res. 2016;95:1358–1365. doi: 10.1177/0022034516660509. [DOI] [PubMed] [Google Scholar]
- 201.Costa THR, de Figueiredo Neto JA, de Oliveira AE, Lopes e Maia Mde F, de Almeida AL. Association between chronic apical periodontitis and coronary artery disease. J Endod. 2014;40:164–167. doi: 10.1016/j.joen.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 202.Paloma de Oliveira B, Câmara AC, Aguiar CM. Prevalence of asymptomatic apical periodontitis and its association with coronary artery disease in a Brazilian subpopulation. Acta Stomatol Croat. 2017;51:106–112. doi: 10.15644/asc51/2/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Milojevic Samanovic A, Jakovljevic V, Vasovic M, Mitrovic S, Rankovic M, Mihajlovic K, Bolevich S, Zivkovic V. Cardiac, biochemical and histopathological analysis reveals impaired heart function in hypertensive rats with apical periodontitis. Int Endod J. 2021;54:1581–1596. doi: 10.1111/iej.13562. [DOI] [PubMed] [Google Scholar]
- 204.Katz J, Rotstein I. Prevalence of periapical abscesses in patients with hypertension: A cross-sectional study of a large hospital population. J Endod. 2021;47:1070–1074. doi: 10.1016/j.joen.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 205.Petersen J, Glaßl EM, Nasseri P, Crismani A, Luger AK, Schoenherr E, Bertl K, Glodny B. The association of chronic apical periodontitis and endodontic therapy with atherosclerosis. Clin Oral Investig. 2014;18:1813–1823. doi: 10.1007/s00784-013-1156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vidal F, Fontes TV, Marques TVF, Gonçalves LS. Association between apical periodontitis lesions and plasmatic levels of C-reactive protein, interleukin 6 and fibrinogen in hypertensive patients. Int Endod J. 2016;49:1107–1115. doi: 10.1111/iej.12567. [DOI] [PubMed] [Google Scholar]
- 207.Rashmi N, Galhotra V, Goel P, Rajguru JP, Jha SK, Kulkarni K. Assessment of C-reactive proteins, cytokines, and plasma protein levels in hypertensive patients with apical periodontitis. J Contemp Dent Pract. 2017;18:516–521. doi: 10.5005/jp-journals-10024-2076. [DOI] [PubMed] [Google Scholar]
- 208.Messing M, Souza LCD, Cavalla F, Kookal KK, Rizzo G, Walji M, Silva R, Letra A. Investigating potential correlations between endodontic pathology and cardiovascular diseases using epidemiological and genetic approaches. J Endod. 2019;45:104–110. doi: 10.1016/j.joen.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 209.Sebring D, Buhlin K, Norhammar A, Rydén L, Jonasson P, EndoReCo. Lund H, Kvist T. Endodontic inflammatory disease: A risk indicator for a first myocardial infarction. Int Endod J. 2022;55:6–17. doi: 10.1111/iej.13634. [DOI] [PubMed] [Google Scholar]
- 210.Bordagaray MJ, Fernández A, Garrido M, Astorga J, Hoare A, Hernández M. Systemic and extraradicular bacterial translocation in apical periodontitis. Front Cell Infect Microbiol. 2021;11:649925. doi: 10.3389/fcimb.2021.649925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jiménez C, Garrido M, Pussinen P, Bordagaray MJ, Fernández A, Vega C, Chaparro A, Hoare A, Hernández M. Systemic burden and cardiovascular risk to Porphyromonas species in apical periodontitis. Clin Oral Investig. 2022;26:993–1001. doi: 10.1007/s00784-021-04083-4. [DOI] [PubMed] [Google Scholar]
- 212.Kazanci E, Oguz KK, Gurgey A, Topçu M. Streptococcus oralis as a risk factor for middle cerebral artery thrombosis. J Child Neurol. 2005;20:611–613. doi: 10.1177/08830738050200071401. [DOI] [PubMed] [Google Scholar]
- 213.Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9:781. doi: 10.3390/biomedicines9070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cotti E, Dessì C, Piras A, Flore G, Deidda M, Madeddu C, Zedda A, Longu G, Mercuro G. Association of endodontic infection with detection of an initial lesion to the cardiovascular system. J Endod. 2011;37:1624–1629. doi: 10.1016/j.joen.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 215.Bergandi L, Giuggia B, Alovisi M, Comba A, Silvagno F, Maule M, Aldieri E, Scotti N, Scacciatella P, Conrotto F, et al. Endothelial dysfunction marker variation in young adults with chronic apical periodontitis before and after endodontic treatment. J Endod. 2019;45:500–506. doi: 10.1016/j.joen.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 216.Chauhan N, Mittal S, Tewari S, Sen J, Laller K. Association of apical periodontitis with cardiovascular disease via noninvasive assessment of endothelial function and subclinical atherosclerosis. J Endod. 2019;45:681–690. doi: 10.1016/j.joen.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 217.Hernández-Ríos P, Pussinen PJ, Vernal R, Hernández M. Oxidative stress in the local and systemic events of apical periodontitis. Front Physiol. 2017;8:869. doi: 10.3389/fphys.2017.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–296. doi: 10.1111/j.1600-051X.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 219.Tucker MA, Fox BM, Seigler N, Rodriguez-Miguelez P, Looney J, Thomas J, McKie KT, Forseen C, Davison GW, Harris RA. Endothelial dysfunction in cystic fibrosis: Role of oxidative stress. Oxid Med Cell Longev. 2019;2019:1629638. doi: 10.1155/2019/1629638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brilhante Wolle CF, de Aguiar Zollmann L, Etges A, Vitalis GS, Leite CE, Campos MM. Effects of the antioxidant agent tempol on periapical lesions in rats with doxorubicin-induced cardiomyopathy. J Endod. 2012;38:191–195. doi: 10.1016/j.joen.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 221.Vailhé B, Dietl J, Kapp M, Toth B, Arck P. Increased blood vessel density in decidua parietalis is associated with spontaneous human first trimester abortion. Hum Reprod. 1999;14:1628–1634. doi: 10.1093/humrep/14.6.1628. [DOI] [PubMed] [Google Scholar]
- 222.Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S10–S18. doi: 10.1016/S0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 223.Plaisier M. Decidualisation and angiogenesis. Best Pract Res Clin Obstet Gynaecol. 2011;25:259–271. doi: 10.1016/j.bpobgyn.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 224.Bobetsis YA, Graziani F, Gürsoy M, Madianos PN. Periodontal disease and adverse pregnancy outcomes. Periodontol. 2020;2000(83):154–174. doi: 10.1111/prd.12294. [DOI] [PubMed] [Google Scholar]
- 225.Harjunmaa U, Järnstedt J, Alho L, Dewey KG, Cheung YB, Deitchler M, Ashorn U, Maleta K, Klein NJ, Ashorn P. Association between maternal dental periapical infections and pregnancy outcomes: results from a cross-sectional study in Malawi. Trop Med Int Health. 2015;20:1549–1558. doi: 10.1111/tmi.12579. [DOI] [PubMed] [Google Scholar]
- 226.Leal ASM, de Oliveira AEF, Brito LMO, Lopes FF, Rodrigues VP, Lima KF, de Araújo Martins IC. Association between chronic apical periodontitis and low-birth-weight preterm births. J Endod. 2015;41:353–357. doi: 10.1016/j.joen.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 227.Khalighinejad N, Aminoshariae A, Kulild JC, Mickel A. Apical periodontitis, a predictor variable for preeclampsia: A case-control study. J Endod. 2017;43:1611–1614. doi: 10.1016/j.joen.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 228.Bain JL, Lester SR, Henry WD, Naftel JP, Johnson RB. Effects of induced periapical abscesses on rat pregnancy outcomes. Arch Oral Biol. 2009;54:162–171. doi: 10.1016/j.archoralbio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 229.Bain JL, Lester SR, Henry WD, Pongetti JL, Blackman ME, Johnson RB. Association between maternal periapical lesions and brain inflammation in rat pups. Arch Oral Biol. 2013;58:266–271. doi: 10.1016/j.archoralbio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 230.Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: A literature review. Ann Nutr Metab. 2015;66(Suppl 2):S14–S20. doi: 10.1159/000371628. [DOI] [PubMed] [Google Scholar]
- 231.Shah H, Nisar N, Hassan A, Butt S. Association between maternal chronic apical periodontitis (CAP) and low birth weight preterm birth (LBWPT) J Pak Med Assoc. 2022;72:436–439. doi: 10.47391/JPMA.0921. [DOI] [PubMed] [Google Scholar]
- 232.Orstavik D, Kerekes K, Eriksen HM. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Endod Dent Traumatol. 1986;2:20–34. doi: 10.1111/j.1600-9657.1986.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 233.Ao M, Miyauchi M, Furusho H, Inubushi T, Kitagawa M, Nagasaki A, Sakamoto S, Kozai K, Takata T. Dental Infection of Porphyromonas gingivalis induces preterm birth in mice. PLoS One. 2015;10:e0137249. doi: 10.1371/journal.pone.0137249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Doyle RM, Alber DG, Jones HE, Harris K, Fitzgerald F, Peebles D, Klein N. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta. 2014;35:1099–1101. doi: 10.1016/j.placenta.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 235.Lin D, Smith MA, Champagne C, Elter J, Beck J, Offenbacher S. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect Immun. 2003;71:5156–5162. doi: 10.1128/IAI.71.9.5156-5162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod. 2007;76:738–748. doi: 10.1095/biolreprod.106.056143. [DOI] [PubMed] [Google Scholar]
- 237.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 238.Awasthi S, Pandey M. Association of TLR4 and TNF-a gene polymorphisms and TLR4 mRNA levels in preterm birth in a Northern Indian population. Indian Pediatr. 2019;56:202–204. doi: 10.1007/s13312-019-1500-z. [DOI] [PubMed] [Google Scholar]
- 239.Jakovljevic A, Sljivancanin Jakovljevic T, Duncan HF, Nagendrababu V, Jacimovic J, Aminoshariae A, Milasin J, Dummer PMH. The association between apical periodontitis and adverse pregnancy outcomes: A systematic review. Int Endod J. 2021;54:1527–1537. doi: 10.1111/iej.13538. [DOI] [PubMed] [Google Scholar]
- 240.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264:2511–2518. doi: 10.1001/jama.1990.03450190043026. [DOI] [PubMed] [Google Scholar]
- 241.Sundararajan S, Muthukumar S, Rao SR. Relationship between depression and chronic periodontitis. J Indian Soc Periodontol. 2015;19:294–296. doi: 10.4103/0972-124X.153479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chen P, Yao H, Su W, He Y, Cheng K, Wang Y, Peng W, Li P. Sleep deprivation worsened oral ulcers and delayed healing process in an experimental rat model. Life Sci. 2019;232:116594. doi: 10.1016/j.lfs.2019.116594. [DOI] [PubMed] [Google Scholar]
- 243.Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: An overview for clinicians. J Oral Rehabil. 2008;35:476–494. doi: 10.1111/j.1365-2842.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 244.Maes M. Evidence for an immune response in major depression: A review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-M. [DOI] [PubMed] [Google Scholar]
- 245.Haug SR, Marthinussen MC. Acute dental pain and salivary biomarkers for stress and inflammation in patients with pulpal or periapical inflammation. J Oral Facial Pain Headache. 2019;33:227–233. doi: 10.11607/ofph.2007. [DOI] [PubMed] [Google Scholar]
- 246.Wang YX, Kang XN, Cao Y, Zheng DX, Lu YM, Pang CF, Wang Z, Cheng B, Peng Y. Porphyromonas gingivalis induces depression via downregulating p75NTR-mediated BDNF maturation in astrocytes. Brain Behav Immun. 2019;81:523–534. doi: 10.1016/j.bbi.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 247.Martínez M, Martín-Hernández D, Virto L, MacDowell KS, Montero E, González-Bris Á, Marín MJ, Ambrosio N, Herrera D, Leza JC, et al. Periodontal diseases and depression: A pre-clinical in vivo study. J Clin Periodontol. 2021;48:503–527. doi: 10.1111/jcpe.13420. [DOI] [PubMed] [Google Scholar]
- 248.Martín-Hernández D, Martínez M, Robledo-Montaña J, Muñoz-López M, Virto L, Ambrosio N, Marín MJ, Montero E, Herrera D, Sanz M, et al. Neuroinflammation related to the blood-brain barrier and sphingosine-1-phosphate in a pre-clinical model of periodontal diseases and depression in rats. J Clin Periodontol. 2023;50:642–656. doi: 10.1111/jcpe.13780. [DOI] [PubMed] [Google Scholar]
- 249.Saei Ghare Naz M, Ramezani Tehrani F, Behroozi Lak T, Mohammadzadeh F, Nasiri M, Kholosi Badr F, Ozgoli G. Quality of life and emotional states of depression, anxiety and stress in adolescents with polycystic ovary syndrome: A cross-sectional study. Psychol Res Behav Manag. 2020;13:203–209. doi: 10.2147/PRBM.S241192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Montoro J, Mullol J, Jáuregui I, Dávila I, Ferrer M, Bartra J, del Cuvillo A, Sastre J, Valero A. Stress and allergy. J Investig Allergol Clin Immunol. 2009;19(Suppl 1):S40–S47. [PubMed] [Google Scholar]
- 251.Eisenhofer G, Lambie DG, Johnson RH. Beta-adrenoceptor responsiveness and plasma catecholamines as determinants of cardiovascular reactivity to mental stress. Clin Sci (Lond) 1985;69:483–492. doi: 10.1042/cs0690483. [DOI] [PubMed] [Google Scholar]
- 252.Haug SR, Heyeraas KJ. Modulation of dental inflammation by the sympathetic nervous system. J Dent Res. 2006;85:488–495. doi: 10.1177/154405910608500602. [DOI] [PubMed] [Google Scholar]
- 253.Esteban MA, Rodríguez A, Ayala AG, Meseguer J. Effects of high doses of cortisol on innate cellular immune response of seabream (Sparus aurata L.) Gen Comp Endocrinol. 2004;137:89–98. doi: 10.1016/j.ygcen.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 254.do Nascimento IV, Rodrigues MIDQ, Isaias PHC, Barros-Silva PG, Sousa FB, Nunes Alves APN, Mota MRL. Chronic systemic corticosteroid therapy influences the development of pulp necrosis and experimental apical periodontitis, exacerbating the inflammatory process and bone resorption in rats. Int Endod J. 2022;55:646–659. doi: 10.1111/iej.13724. [DOI] [PubMed] [Google Scholar]
- 255.Gomes ESB, Farias LC, Silveira LH, Jesus CÍ, Rocha RGD, Ramos GV, Magalhães HTAT, Brito-Júnior M, Santos SHS, Jham BC, et al. Conditioned fear stress increases bone resorption in apical periodontitislesions in Wistar male rats. Arch Oral Biol. 2019;97:35–41. doi: 10.1016/j.archoralbio.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 256.Minhoto GB, Khoury RD, Orozco EIF, Prado RF, Valera MC. Effect of chronic unpredictable stress on the progression of experimental apical periodontitis in rats. Int Endod J. 2021;54:1342–1352. doi: 10.1111/iej.13515. [DOI] [PubMed] [Google Scholar]
- 257.Khoury RD, Prado RFD, Matos FDS, Meireles BR, Cardoso FGDR, Oliveira LD, Carvalho CAT, Valera MC. The influence of adrenergic blockade in rats with apical periodontitis under chronic stress conditions. Arch Oral Biol. 2020;110:104590. doi: 10.1016/j.archoralbio.2019.104590. [DOI] [PubMed] [Google Scholar]
- 258.Van Someren EJW. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol Rev. 2021;101:995–1046. doi: 10.1152/physrev.00046.2019. [DOI] [PubMed] [Google Scholar]
- 259.Scott J, Kallestad H, Vedaa O, Sivertsen B, Etain B. Sleep disturbances and first onset of major mental disorders in adolescence and early adulthood: A systematic review and meta-analysis. Sleep Med Rev. 2021;57:101429. doi: 10.1016/j.smrv.2021.101429. [DOI] [PubMed] [Google Scholar]
- 260.Park JS, Jeong Y, Jung J, Ryu JJ, Lim HK, Jung SK, Song IS. Shift work sleep disorder is closely associated with an increased risk for periodontal disease. J Clin Periodontol. 2021;48:1066–1075. doi: 10.1111/jcpe.13508. [DOI] [PubMed] [Google Scholar]
- 261.Fang HF, Miao NF, Chen CD, Sithole T, Chung MH. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: A nationwide nested case-control study. J Cancer. 2015;6:1140–1147. doi: 10.7150/jca.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Chen LL. Advances in circadian rhythms in oral maxillofacial tissues and oral-related diseases. Zhonghua Kou Qiang Yi Xue Za Zhi. 2022;57:481–489. doi: 10.3760/cma.j.cn112144-20220228-00082. In Chinese. [DOI] [PubMed] [Google Scholar]
- 263.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 264.Chu FCS, Leung WK, Tsang PCS, Chow TW, Samaranayake LP. Identification of cultivable microorganisms from root canals with apical periodontitis following two-visit endodontic treatment with antibiotics/steroid or calcium hydroxide dressings. J Endod. 2006;32:17–23. doi: 10.1016/j.joen.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 265.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 266.Reiter RJ, Rosales-Corral SA, Liu XY, Acuna-Castroviejo D, Escames G, Tan DX. Melatonin in the oral cavity: Physiological and pathological implications. J Periodontal Res. 2015;50:9–17. doi: 10.1111/jre.12176. [DOI] [PubMed] [Google Scholar]
- 267.Tavares BS, Tsosura TVS, Mattera MSLC, Santelli JO, Belardi BE, Chiba FY, Cintra LTA, Silva CC, Matsushita DH. Effects of melatonin on insulin signaling and inflammatory pathways of rats with apical periodontitis. Int Endod J. 2021;54:926–940. doi: 10.1111/iej.13474. [DOI] [PubMed] [Google Scholar]
- 268.Sarıtekin E, Üreyen Kaya B, Aşcı H, Özmen Ö. Anti-inflammatory and antiresorptive functions of melatonin on experimentally induced periapical lesions. Int Endod J. 2019;52:1466–1478. doi: 10.1111/iej.13138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.