Abstract
A poor uterine environment causes changes in fetal development that affect the health of offspring long-term. Although there are multiple pathways that contribute to the development of cardiovascular and neurological disease, low birth weight or fetal growth restriction (FGR) predisposes offspring to these diseases. There is a link between fetal exposure to adverse influences and hypertension later in life. Many epidemiological studies support the link between fetal life and the risk of disease later in life. Experimental models have sought to provide mechanistic proof of this link while simultaneously investigating potential therapeutics or treatment pathways. Preeclampsia (PE), one of several hypertensive disorders in pregnancy, is a leading cause of morbidity and mortality for both the mother and fetus. Studies have shown that PE is a state of chronic inflammation and there is an imbalance between pro-inflammatory and regulatory immune cells and mediators. There is no cure for PE beyond the delivery of the fetal-placental unit, and many PE pregnancies result in FGR and preterm birth. Epidemiological data demonstrate that the sex of the offspring is correlated with the degree of cardiovascular disease that develops with the age of the offspring yet few studies examine the effect of sex on the development of neurological disorders. Even fewer studies examine the effects of therapeutics on offspring of different genders following a PE pregnancy. Moreover, there remain significant gaps in knowledge concerning the role the immune system plays in FGR offspring developing hypertension or neurovascular disorders later in life. Therefore, the purpose of this review is to highlight current research on sex differences in the developmental programming of hypertension and neurological disorders following a PE pregnancy.
Keywords: Offspring, Preeclampsia, Sex differences, Hypertension
Introduction
Preeclampsia (PE) is a hypertensive disorder of pregnancy typically defined as new-onset hypertension during the third trimester accompanied by end-organ dysfunction[1, 2]. PE affects 7-12% of pregnancies around the world each year and is the leading cause of maternal and fetal morbidity and mortality. Additionally, PE pregnancies are at much higher risk for premature birth, fetal growth restriction (FGR), and small for gestational age babies[3]. The only cure for PE is the delivery of the fetal-placental unit, and usually, therapeutics for PE treat symptoms that reduce maternal risk allowing pregnancies to continue which lowers the risks associated with prematurity. PE causes long-term adverse effects on maternal health including increased risk for hypertension, cardiovascular disease, metabolic disease, stroke, and cerebrovascular disease[4, 5]. Moreover, PE also can affect the fetus’s health after delivery and into adulthood.
A healthy intrauterine environment allows for optimal fetal growth and development. Pathological conditions that result in changes to the uterine environment have negative effects in utero that confer increased risk on the child’s health for years after the pregnancy. Negative conditions induce fetal distress through changes in nutrient and oxygen availability to the uterus. Placental dysfunction leads to disruptions in maternal-fetal oxygen exchange. Mechanisms such as low oxygen availability and placental dysfunction result in FGR and an increased risk of adult disease for the child. Many children born from a PE pregnancy are premature and small for their gestational age in addition to having a higher risk for hemorrhage, hypoxic-ischemic episodes, and perinatal death[2]. In adulthood, these children have an increased risk for cardiovascular disease and neurological disorders. These risks may be even more pronounced in the offspring of mothers with early-onset PE [6]. PE is characterized by the development of placental ischemia[1, 2]. This insult during fetal development may be a mechanism by which offspring of PE pregnancies have an increased risk for disease later in life. The complete pathophysiology by which these offspring have increased risk is not definitive, but a widely accepted theory is the Developmental Origins of Health and Disease (DOHaD) which was first proposed by David Barker[7]. This theory proposes that an adverse uterine environment will cause the fetus to adapt to the pathological condition and these adaptations increase the risk of disease later in life.
It is important to understand the adverse effects of preeclamptic offspring related to sex differences because of the potential to predict and offer intervention earlier in life, possibly perinatally, which may reduce the risk of disease in offspring from complicated pregnancies. This review examines the potential sex differences in the mechanisms contributing to the development of cardiovascular disease or neurological disorders following fetal exposure to PE. Additionally, this review aims to provide insight into how immune cells and mediators may play a role in offspring disease following PE. By summarizing studies that study sex differences in cardiovascular disease and neurological disorders alongside studies examining the effect of PE on offspring, we hope to contribute to the collection of research detailing the long-term pathophysiology of PE offspring as well as provide research gaps that are deserving of future study.
Sex Differences in Preeclampsia Offspring and Cardiovascular Outcomes
Both male and female offspring of PE are at a higher risk for developing hypertension[8]. Many studies indicate that birth weight is inversely related to blood pressure later in an offspring’s life[9, 10]. PE is one of the leading causes of FGR, therefore there is a higher risk for PE offspring to be born with lower birth weight. Sympathetic tone, nephron number, diet, secondary insults, and sex hormones have been implicated as mechanisms contributing to hypertension following FGR[11, 12]. Studies also indicate that by 17 years of age, offspring born from PE pregnancies have higher BMI than those born from normal pregnancies[13, 14]. Also at 17 years of age offspring from PE pregnancies display concentric cardiac remodeling with greater cardiac relative wall thickness and decreased left ventricular end-diastolic volume compared to offspring of normal pregnancies[15]. Based on the DOHaD theory, developmental programming changes occur during pregnancy as a result of placental ischemia in PE causing the offspring’s increased risk for diseases later in life. However, the mechanisms during pregnancy that cause these changes are poorly understood, with many groups working trying to identify specific pathways leading to the increased risk for disease in PE offspring.
Some studies have shown differences in cardiovascular outcomes for male and female offspring of preeclamptic mothers. Male offspring of mothers with PE are more likely to exhibit elevation in their systolic blood pressure, and female offspring are more likely to exhibit elevation of their diastolic blood pressure [16]. Moreover, females have significant endothelial dysfunction while males exhibit increased vascular stiffness compared to females following a PE pregnancy.[17]. Obesity is the 6th most significant risk factor for worldwide disease burden including elevated risk for cardiovascular, metabolic, respiratory, and psychological disease[18, 19]. While offspring exposed to PE are at increased risk for low birth weight, there is evidence that they are also more likely to have a higher BMI later in life. This increase in BMI is more prevalent in males exposed to PE rather than females [20, 21]. Studies have found elevated low-density lipoprotein (LDL) and triglycerides and decreased high-density lipoprotein (HDL) in the offspring of preeclamptic mothers [22, 23]. However, no statistically significant difference was seen between males and females in these studies. The small sample size in these studies and their overall heterogeneity make it difficult to draw conclusions regarding long-term metabolic function in offspring exposed to PE.
The major mechanisms that control blood pressure play different roles in men and women. Within the general population, men have higher blood pressure than women do during early adulthood[24]. Aging reduces this trend and women are at significantly increased risk for cardiovascular disease in the postmenopausal period[25, 26]. One possible mechanism that results in these differences between men’s and women’s blood pressure is the renin-angiotensin-aldosterone system (RAAS), which is one of the most essential control systems for the regulation of blood pressure. The angiotensin II type 1 receptor (AT1R) is typically associated with increased sympathetic tone, blood pressure, vasoconstriction, and aldosterone secretion[27]. The angiotensin II type 2 receptor (AT2R) is typically associated with effects countering the AT1R: decreased blood pressure and increases in vasodilation and nitric oxide production[27]. Female hypertensive animals have lower activation of the AT1R and higher activation of the MAS receptor, which has opposite functions to the AT1R, than male rats[28]. The AT2R has a depressor influence on the response to angiotensin II (ANG II) infusion in female animals but not in males which correlates with higher blood pressure response in male animals compared to female animals following ANG II infusion[29]. While there are clear differences in blood pressure regulation via the RAAS and the development of hypertension in males and females in the general population, whether there are sex differences in the developmental programming that results from a PE pregnancy is also an important area to examine.
Animal Models Used to investigate Hypertension in offspring
To study the mechanistic effects of PE on offspring, many groups use animal models wherein some maternal insult results in FGR. FGR in animal models is associated with increased risks which are similar to those observed in human children born with growth restriction. In a recent systemic review of animal models for FGR where 202 studies were evaluated[30], rodents were the most common model for FGR followed by sheep, with the most common method to induce FGR being surgery. Out of those 202 studies, only 9% reported long-term outcomes following FGR, with most ending at or before pregnancy term[30]. In one surgically induced rodent model of FGR, the Reduced Uterine Perfusion Pressure (RUPP) model of PE, low birth weight animals develop increased blood pressure by 4 weeks of age, but at 12 weeks of age, only the male animals are hypertensive[31]. This difference is likely due to sex hormone production which occurs as the animals undergo adolescence[32]. Interestingly, estrogen in female growth-restricted offspring decreases the expression of Ang II and the angiotensin-converting enzyme, potentially protecting the female from large increases in blood pressure[33]. Soluble FMS like tyrosine kinase-1 (sFlt-1), a potent anti-angiogenic factor upregulated in PE, induces a PE phenotype in pregnant animals[34]. Only male offspring of these animals are shown to develop hypertension[35]. These data indicate a greater susceptibility of male embryos to developmental programming changes. Placental insufficiency affects the developmental programming of the RAAS with animal models showing increased sensitivity to ANG II[36-38]. Blocking the RAAS with angiotensin-converting enzyme inhibitors (ACE) abolished increased blood pressure associated with decreased birth weight[39, 40]. Moreover, FGR also results in a smaller nephron number at birth[41, 42]. This lower nephron number increases the risk for hypertension later in life [43] likely through compensatory hyperfiltration and hypertrophy as fewer nephrons try to meet normal filtration load. In fact, recipients of kidneys from low birth weight donors require more hypertensive therapy than those from normal birth weight donors[44]. These studies demonstrate the important effect of growth restriction on the development of hypertension and how animal models can be used to study how sex can modulate that risk in humans.
Sex Differences in Preeclampsia Offspring and Neurological Disorders
There is increased risk of the development of neurological disorders such as autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) in offspring born from PE women[45, 46]. There is also increased risk for impaired memory and cognition in offspring born from PE women[45]. Maternal hypertensive disorders are associated with lower cognitive ability in offspring than in offspring of normal pregnancies[47]. Additionally, PE offspring are at higher risk for intellectual disabilities[48]. There is an early-life neurodevelopmental vulnerability in males[49] that mimics the male vulnerability to hypertension following PE but the effects have not been demonstrated consistently[50]. PE is also associated with the risk of fetal brain injury that leads to adverse neurodevelopmental outcomes. Abnormal placental function, a classical feature of PE, is associated with abnormal fetal brain development[51, 52]. Poor placental function leading to impaired transfer of nutrients and oxygen are likely mechanisms that result in abnormal fetal brain development. Gestational hypoxia in pregnant rats results in impaired fetal brain development that affected female offspring more during puberty and male offspring more during adulthood[53].
Additionally, studies have examined whether in-utero exposure to PE is associated with mental and behavioral health problems later in life. A Nordic epidemiological study and systemic review of 23 studies found that children of hypertensive pregnancies have an increased risk to develop attention deficit hyperactivity disorder, autism spectrum disorder, schizophrenia, and intellectual disability [54, 55]. However, the literature appears to be inconsistent in that these associations may be stronger in the setting of PE with perinatal complications or with early onset PE [56]. While there is evidence showing that male children are more at risk than female children for ASD[57], there is no proven association between PE and this phenomenon. No evidence has been found to suggest that these risks differ between males and females exposed to PE in utero. However, further research in this area is needed. This collection of information further reinforces the idea that sex as a biological variable is necessary for studies examining the effects of a PE pregnancy on offspring health long-term, including neurological disorder risk.
Role of Immune Cells in Offspring Disease Following PE
Women typically display greater immune responses than men and must have a more potent regulatory immune response during pregnancy to protect the allogenic fetus[58]. Women are also more likely than men to develop inflammatory disorders and autoimmune diseases[59], and there is increasing evidence that associates inflammation with hypertension and cardiovascular disease[60, 61]. PE has been increasingly characterized as a disorder with chronic inflammation contributing to hypertension[62, 63]. Proinflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are elevated in pregnancies affected by PE and contribute to the development of neurological disorders[64]. The role of proinflammatory cytokines or immune cells on offspring cardiovascular function has not been studied through PE seems to induce neuroinflammation in neonatal brains[65]. Interestingly, immunomodulators have a beneficial effect on the birth weight of offspring over multiple animal models of FGR[30]. However, these studies did not follow offspring long-term to determine the effect of immunomodulators on offspring immune function or other physiological changes long-term. Whether offspring of PE mothers have chronic inflammation and what role immune cells and mediators may play in offspring disease is unclear and is an area deserving of study.
Immune mediators play a key role in the pathophysiology of PE, with many immune mechanisms being linked to hypertension, FGR, proteinuria, and cerebrovascular dysfunction in the disease[66]. One immune cell type that has a critical role in normal pregnancies and PE pathophysiology is the T cell[67]. Additionally, there is growing evidence that T cells play a critical role in the pathophysiology of hypertension and that there are sex differences in the mechanisms through which T cells contribute to blood pressure control[68]. The Sullivan lab has shown that female blood pressure can be suppressed with immunosuppression similarly to male blood pressure and at baseline female animals have more regulatory T cells in the kidneys and males have more TH17s, a pro-inflammatory T cell subtype, in their kidneys[69]. Following nitric oxide synthase inhibition, there was a greater degree of dysregulation in the regulatory T cell-TH17 axis in females than in males[70]. Another study showed that increased regulatory T cells in adipose tissue in females but not males, protect females from metabolic changes[71]. Moreover, lower levels of T regulatory cells have been implicated as a causal factor in PE, and TH17s are implicated as a contributive mediator in PE[67]. Changes in T cell programming during fetal development may contribute to a predisposition toward hypertension in PE offspring later in life.
Additionally, B cells producing autoantibodies have also been implicated in the pathophysiology of PE[72, 73]. B cells from PE women produce agonistic autoantibodies to the angiotensin II type 1 receptor (AT1-AA)[74]. AT1-AA contributes to the PE phenotype through inflammatory cytokine production, elevation of circulating anti-angiogenic factors, activation of natural killer cells, and increased oxidative stress in the kidney and placenta[73]. While AT1-AA has been found in the cord blood of women with PE[75] and infant serum[76], indicating it can cross the maternal-fetal barrier, it has not been measured or examined in adult offspring of PE pregnancies. Moreover, AT1-AA has been found in maternal circulation up to 8 years postpartum indicating a memory mechanism in play [77] which could also occur in offspring of PE pregnancies and may play a role in the predisposition of offspring to hypertension and neurological complications. The effects of AT1-AA on offspring health and increased risk of neurological disorders and cardiovascular disease have not been examined. While these studies highlight the roles that immune mediators can play in the development of hypertension or neurological complications, the connection between maternal PE and offspring disease through immune mediators has not been made.
Summary
Uterine exposure to insult results in changes to developmental programming so that the fetus may survive, but predisposes offspring to disease later in life. The PE phenotype includes placental dysfunction and ischemia, compromising the health and viability of the fetus. Collectively, the review demonstrates that PE offspring are at much higher risk for hypertension, cardiovascular disease, and neurological disorders later in their life compared to the general population. Moreover, male offspring appear to be more susceptible to these developmental programming changes, at least in early adulthood. FGR causes changes to the RAAS which mediates some increased risk for hypertension in offspring. Sex steroids play a role in cardiovascular risk following a developmental insult and are likely a contributor to the sex differences in risk. Immune cells play a critical role in PE pathophysiology and also contribute to hypertension in the general population. Immune cells’ role in sex differences following a pregnancy complicated by PE is not clear. Studies investigating the role of specific maternal immune changes on offspring health are warranted. Studies investigating changes in offspring immune phenotypes following a complicated pregnancy are also warranted. These studies would help clarify mechanisms that contribute to the increased risk of offspring disease following a preeclamptic pregnancy. Studies focused on immune factors may also show whether immune cells and mediators contribute to sex differences in the risk of cardiovascular and neurological disorders in offspring. Moreover, such studies may also provide potential therapeutic pathways to treat these offspring early so that the increased risk may be alleviated before the development of disease. It is clear that offspring of PE pregnancies should be monitored closely as they are at higher risk for a range of cardiovascular and neurological disorders. Physicians and researchers should also recognize that the mechanisms that contribute to these risks are different in male and female children and they must be studied and prepared for accordingly.
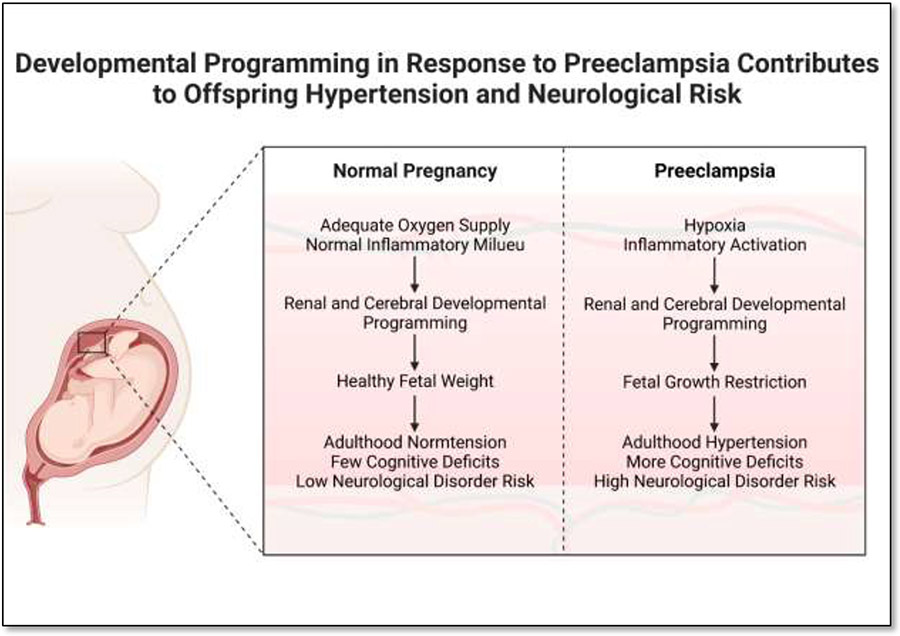
Figure 1:
Preeclampsia results in developmental programming that contributes to the higher risk for hypertension and cognitive deficits in offspring. Created with BioRender.com
Table 1:
Cardiovascular Outcomes following a Preeclamptic Pregnancy
| Preeclampsia | |||
|---|---|---|---|
| Risk Factor | Male | Female | Citations |
| Low Birth Weight | + | + | Lisonkova et al. [3] |
| High BM | ++ | + | Alsnes et al. [13], Fraser et al. [14], Seidman et al. [20], Wang et al. [21], Timpka et al. [15] |
| Cardiac Remodeling | + | + | Timpka et al. [15] |
| Hypertension | + | + | Lazdam et al. [6], Sacks et al. [8], Alsnes et al. [13], Fraser et al. [14], Timpka et al. [15] |
| Increased Systolic Blood Pressure | + | − | Davis et al. [16] |
| Increased Diastolic Pressure | − | + | Davis et al. [16] |
Table 2:
Risk for Neurological Disorders Following a Preeclamptic Pregnancy
| Preeclampsia | |||
|---|---|---|---|
| Complication | Male | Female | Citation |
| Autism Spectrum Disorder | + | + | Lu et al. [45], Maher et al. [46], Wang et al. [54], Kong et al. [56] |
| Attention Deficit Hyperactivity Disorder | + | + | Lu et al. [45], Maher et al. [46], Wang et al. [54], Dachew et al. [55], Kong et al. [56] |
| Impaired Memory | + | + | Lu et al. [45] |
| Intellectual Disabilities | + | + | Sun et al. [48], Wang et al. [54] |
| Neurodevelopmental Vulnerability | + | − | Nagy et al. [49] |
| Schizophrenia | + | + | Dachew et al. [55] |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
No disclosures to report.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Literature Cited
- 1.Brown MA, et al. , Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension, 2018. 72(1): p. 24–43. [DOI] [PubMed] [Google Scholar]
- 2.Obstetricians, A.C.o. and Gynecologists, ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol, 2019. 133(1): p. e1–e25. [DOI] [PubMed] [Google Scholar]
- 3.Lisonkova S and Joseph K, Incidence of preeclampsia: risk factors and outcomes associated with early-versus late-onset disease. American journal of obstetrics and gynecology, 2013. 209(6): p. 544. e1–544. e12. [DOI] [PubMed] [Google Scholar]
- 4.Sukmanee J and Liabsuetrakul T, Risk of future cardiovascular diseases in different years postpartum after hypertensive disorders of pregnancy: A systematic review and meta-analysis. Medicine, 2022. 101(30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham MW Jr and LaMarca B, Risk of cardiovascular disease, end-stage renal disease, and stroke in postpartum women and their fetuses after a hypertensive pregnancy. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2018. 315(3): p. R521–R528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazdam M, et al. , Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension, 2012. 60(5): p. 1338–45. [DOI] [PubMed] [Google Scholar]
- 7.Barker D, Developmental origins of adult health and disease. Journal of epidemiology and community health, 2004. 58(2): p. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks KN, et al. , Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy hypertension, 2018. 13: p. 181–186. [DOI] [PubMed] [Google Scholar]
- 9.Intapad S, et al. , Sex differences in the developmental origins of cardiovascular disease. Physiology, 2014. 29(2): p. 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knop MR, et al. , Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta-analysis of 7 646 267 participants from 135 studies. Journal of the American Heart Association, 2018. 7(23): p. e008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coats LE, et al. , Low birth weight, blood pressure and renal susceptibility. Current hypertension reports, 2019. 21(8): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasinger JH, et al. , Developmental programming of hypertension: physiological mechanisms. Hypertension, 2016. 68(4): p. 826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsnes IV, et al. , Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: prospective and sibling studies in the HUNT Study (Nord-Trøndelag Health Study) in Norway. Hypertension, 2017. 69(4): p. 591–598. [DOI] [PubMed] [Google Scholar]
- 14.Fraser A, et al. , Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension, 2013. 62(3): p. 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timpka S, et al. , Hypertensive disorders of pregnancy and offspring cardiac structure and function in adolescence. Journal of the American Heart Association, 2016. 5(11): p. e003906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis EF, et al. , Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics, 2012. 129(6): p. e1552–61. [DOI] [PubMed] [Google Scholar]
- 17.Plummer MD, et al. , Hypertensive disorders of pregnancy and later cardiovascular disease risk in mothers and children. J Dev Orig Health Dis, 2021. 12(4): p. 555–560. [DOI] [PubMed] [Google Scholar]
- 18.Singh GM, et al. , The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PloS one, 2013. 8(7): p. e65174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolley C, et al. , The effect of age upon the interrelationship of BMI and inpatient health outcomes. The journal of nutrition, health & aging, 2019. 23(6): p. 558–563. [DOI] [PubMed] [Google Scholar]
- 20.Seidman DS, et al. , Pre-eclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol, 1991. 98(10): p. 1009–14. [DOI] [PubMed] [Google Scholar]
- 21.Wang L.-b., et al. , Preeclampsia exposed offspring have greater body mass index than non-exposed offspring during peripubertal life: A meta-analysis. Pregnancy Hypertension, 2020. 19: p. 247–252. [DOI] [PubMed] [Google Scholar]
- 22.Catarino C, et al. , Fetal lipoprotein changes in pre-eclampsia. Acta Obstet Gynecol Scand, 2008. 87(6): p. 628–34. [DOI] [PubMed] [Google Scholar]
- 23.Howlader MZ, et al. , Oxidative stress and antioxidant status in neonates born to pre-eclamptic mother. J Trop Pediatr, 2009. 55(6): p. 363–7. [DOI] [PubMed] [Google Scholar]
- 24.Song J-J, et al. , Gender differences in hypertension. Journal of cardiovascular translational research, 2020. 13(1): p. 47–54. [DOI] [PubMed] [Google Scholar]
- 25.Kotsis V, et al. , Ambulatory blood pressure monitoring and target organ damage: effects of age and sex. Blood pressure monitoring, 2006. 11(1): p. 9–15. [DOI] [PubMed] [Google Scholar]
- 26.Kim J-M, et al. , Postmenopausal hypertension and sodium sensitivity. Journal of menopausal medicine, 2014. 20(1): p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AJ and Arnold AC, The renin–angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clinical Autonomic Research, 2019. 29(2): p. 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmerman MA and Sullivan JC, Hypertension: what's sex got to do with it? Physiology, 2013. 28(4): p. 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilliard LM, et al. , Gender differences in pressure-natriuresis and renal autoregulation: role of the angiotensin type 2 receptor. Hypertension, 2011. 57(2): p. 275–282. [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela I, et al. , Prenatal interventions for fetal growth restriction in animal models: A systematic review. Placenta, 2022. [DOI] [PubMed] [Google Scholar]
- 31.Alexander BT, Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension, 2003. 41(3): p. 457–462. [DOI] [PubMed] [Google Scholar]
- 32.Ojeda NB, et al. , Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension, 2007. 50(4): p. 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brosnihan KB, et al. , Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Levine RJ, et al. , Circulating angiogenic factors and the risk of preeclampsia. New England journal of medicine, 2004. 350(7): p. 672–683. [DOI] [PubMed] [Google Scholar]
- 35.Lu F, et al. , Gender-specific effect of overexpression of sFlt-1 in pregnant mice on fetal programming of blood pressure in the offspring later in life. American journal of obstetrics and gynecology, 2007. 197(4): p. 418. e1–418. e5. [DOI] [PubMed] [Google Scholar]
- 36.Ojeda NB, et al. , Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2010. 298(5): p. R1421–R1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ojeda NB, et al. , Hypersensitivity to acute ANG II in female growth-restricted offspring is exacerbated by ovariectomy. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2011. 301(4): p. R1199–R1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasch R, Skriver E, and Woods L, The role of the RAS in programming of adult hypertension. Acta physiologica scandinavica, 2004. 181(4): p. 537–542. [DOI] [PubMed] [Google Scholar]
- 39.Ojeda NB, et al. , Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2007. 292(2): p. R758–R763. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno M, et al. , Enalapril attenuates the exaggerated sympathetic response to physical stress in prenatally programmed hypertensive rats. Hypertension, 2014. 63(2): p. 324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinchliffe S, et al. , The effect of intrauterine growth retardation on the development of renal nephrons. BJOG: An International Journal of Obstetrics & Gynaecology, 1992. 99(4): p. 296–301. [DOI] [PubMed] [Google Scholar]
- 42.Hughson M, et al. , Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney international, 2003. 63(6): p. 2113–2122. [DOI] [PubMed] [Google Scholar]
- 43.Luyckx VA and Brenner BM, Clinical consequences of developmental programming of low nephron number. The Anatomical Record, 2020. 303(10): p. 2613–2631. [DOI] [PubMed] [Google Scholar]
- 44.Schachtner T and Reinke P. Estimated nephron number of the donor kidney: impact on allograft kidney outcomes. in Transplantation Proceedings. 2017. Elsevier. [DOI] [PubMed] [Google Scholar]
- 45.Lu HQ and Hu R, Lasting effects of intrauterine exposure to preeclampsia on offspring and the underlying mechanism. American journal of perinatology reports, 2019. 9(03): p. e275–e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maher GM, et al. , Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA psychiatry, 2018. 75(8): p. 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuovinen S, et al. , Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. Journal of the American Society of Hypertension, 2014. 8(11): p. 832–847. e1. [DOI] [PubMed] [Google Scholar]
- 48.Sun BZ, et al. , Association of preeclampsia in term births with neurodevelopmental disorders in offspring. JAMA psychiatry, 2020. 77(8): p. 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagy E, et al. , Gender-related physiologic differences in human neonates and the greater vulnerability of males to developmental brain disorders. The journal of gender-specific medicine: JGSM: the official journal of the Partnership for Women's Health at Columbia, 2001. 4(1): p. 41–49. [PubMed] [Google Scholar]
- 50.Ojeda NB, et al. , Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension, 2012. 60(1): p. 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenfeld CS, The placenta-brain-axis. Journal of neuroscience research, 2021. 99(1): p. 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luna RL, et al. , Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. MHR: Basic science of reproductive medicine, 2016. 22(2): p. 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson EN, et al. , Gestational hypoxia in late pregnancy differentially programs subcortical brain maturation in male and female rat offspring. Biology of sex Differences, 2022. 13(1): p. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, et al. , Maternal hypertensive disorders and neurodevelopmental disorders in offspring: a population-based cohort in two Nordic countries. Eur J Epidemiol, 2021. 36(5): p. 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dachew BA, et al. , Association between hypertensive disorders of pregnancy and the development of offspring mental and behavioural problems: A systematic review and meta-analysis. Psychiatry Res, 2018. 260: p. 458–467. [DOI] [PubMed] [Google Scholar]
- 56.Kong L, et al. , Association of Preeclampsia and Perinatal Complications With Offspring Neurodevelopmental and Psychiatric Disorders. JAMA Netw Open, 2022. 5(1): p. e2145719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loomes R, Hull L, and Mandy WPL, What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 2017. 56(6): p. 466–474. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell E, et al. , On maternity and the stronger immune response in women. Nature communications, 2022. 13(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitacre CC, Sex differences in autoimmune disease. Nature immunology, 2001. 2(9): p. 777–780. [DOI] [PubMed] [Google Scholar]
- 60.Norlander AE, Madhur MS, and Harrison DG, The immunology of hypertension. Journal of Experimental Medicine, 2018. 215(1): p. 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drummond GR, et al. , Immune mechanisms of hypertension. Nature Reviews Immunology, 2019. 19(8): p. 517–532. [DOI] [PubMed] [Google Scholar]
- 62.Saito S, et al. , The role of the immune system in preeclampsia. Mol Aspects Med, 2007. 28(2): p. 192–209. [DOI] [PubMed] [Google Scholar]
- 63.Michalczyk M, et al. , The role of inflammation in the pathogenesis of preeclampsia. Mediators of Inflammation, 2020. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paquin V, et al. , Early environmental upheaval and the risk for schizophrenia. Annu Rev Clin Psychol, 2021. 17: p. 285–311. [DOI] [PubMed] [Google Scholar]
- 65.Katoh Y, et al. , Increased production of inflammatory cytokines and activation of microglia in the fetal brain of preeclamptic mice induced by angiotensin II. Journal of Reproductive Immunology, 2022: p. 103752. [DOI] [PubMed] [Google Scholar]
- 66.Campbell N, et al. , The Role of Different Lymphoid Cell Populations in Preeclampsia Pathophysiology. Kidney 360, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eghbal-Fard S, et al. , The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. Journal of Cellular Physiology, 2019. 234(4): p. 5106–5116. [DOI] [PubMed] [Google Scholar]
- 68.Tipton AJ and Sullivan JC, Sex differences in T cells in hypertension. Clinical therapeutics, 2014. 36(12): p. 1882–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tipton AJ, Baban B, and Sullivan JC, Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2012. 303(4): p. R359–R367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brinson KN, et al. , Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2013. 305(7): p. R701–R710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pettersson US, et al. , Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallukat G, et al. , Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT 1 receptor. The Journal of clinical investigation, 1999. 103(7): p. 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campbell N, LaMarca B, and Cunningham MW Jr, The role of agonistic autoantibodies to the angiotensin II type 1 receptor (AT1-AA) in pathophysiology of preeclampsia. Current pharmaceutical biotechnology, 2018. 19(10): p. 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jensen F, et al. , Peripheral CD19+ CD5+ B-1a B cells are increased in patients with preeclampsia and secrete AT1-AA antibodies. Journal of Reproductive Immunology, 2012. 1(94): p. 110–111. [Google Scholar]
- 75.Zhao X, Liu Z, and Liu C, Expression and significance of AT1-AA and ET1 in materal peripheral blood, umbilical cord blood and placenta in preeclampsia. Zhonghua fu Chan ke za zhi, 2012. 47(10): p. 721–725. [PubMed] [Google Scholar]
- 76.Herse F, et al. , Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension, 2007. 49(3): p. 604–611. [DOI] [PubMed] [Google Scholar]
- 77.Rieber-Mohn AB, et al. , Auto-antibodies against the angiotensin II type I receptor in women with uteroplacental acute atherosis and preeclampsia at delivery and several years postpartum. Journal of Reproductive Immunology, 2018. 128: p. 23–29. [DOI] [PubMed] [Google Scholar]