Objective:
The aim of this study was to investigate (a) the effects of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway inhibitor (baricitinib) on the multiple organ dysfunction syndrome (MODS) in a rat model of hemorrhagic shock (HS) and (b) whether treatment with baricitinib attenuates the activation of JAK/STAT, NF-κB, and NLRP3 caused by HS.
Background:
Posttraumatic MODS, which is in part due to excessive systemic inflammation, is associated with high morbidity and mortality. The JAK/STAT pathway is a regulator of numerous growth factor and cytokine receptors and, hence, is considered a potential master regulator of many inflammatory signaling processes. However, its role in trauma-hemorrhage is unknown.
Methods:
An acute HS rat model was performed to determine the effect of baricitinib on MODS. The activation of JAK/STAT, NF-κB, and NLRP3 pathways were analyzed by western blotting in the kidney and liver.
Results:
We demonstrate here for the first time that treatment with baricitinib (during resuscitation following severe hemorrhage) attenuates the organ injury and dysfunction and the activation of JAK/STAT, NF-κB, and NLRP3 pathways caused by HS in the rat.
Conclusions:
Our results point to a role of the JAK/STAT pathway in the pathophysiology of the organ injury and dysfunction caused by trauma/hemorrhage and indicate that JAK inhibitors, such as baricitinib, may be repurposed for the treatment of the MODS after trauma and/or hemorrhage.
Keywords: baricitinib, hemorrhagic shock, ischemia-reperfusion, janus kinase, multiorgan dysfunction syndrome, trauma
Trauma is one of the major causes of mortality in those aged under 44 and the number of patients dying from trauma surpasses those from tuberculosis, malaria, and human immunodeficiency syndrome/acquired immunodeficiency syndrome combined.1 Annually, there are ∼6 million trauma-associated mortalities worldwide. Trauma-associated hemorrhage accounts for almost 40% of all trauma deaths2–5 and is a significant driver of multiple organ dysfunction syndrome (MODS).6–11 There is good evidence that (a) uncontrolled systemic inflammation secondary to the release of damage-associated molecular patterns from substantial tissue damage and (b) ischemia-reperfusion injury contribute to the onset of MODS.12 However, there are no specific pharmacological interventions clinically used to prevent the onset of hemorrhagic shock (HS)-induced MODS.
Circulating proinflammatory mediators bind to cell-surface receptors and subsequently signal through activation of intracellular protein tyrosine kinases, including the Janus kinase (JAK) family. The JAK family is composed of JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), and these isoforms interact with the signal transducer and activator of transcription (STAT) class of proteins.13 JAK inhibitors affect the JAK-STAT protein interaction and disrupt the propagation of signals through the JAK/STAT pathway, thus modulating inflammatory signaling processes.
We have recently shown that JAK/STAT inhibition reduced both the diet-related metabolic derangements and the associated organ injury/dysfunction in a murine model of high-fat diet-induced type 2 diabetes.14 Specifically, treatment with baricitinib, a JAK1/JAK2 inhibitor, resulted in improvements of diet-induced myosteatosis, mesangial expansion, and associated proteinuria in addition to reducing blood cytokine levels, renal dysfunction, and skeletal muscle damage.
Driven by the coronavirus disease 2019 (COVID-19) pandemic, strategies focusing on drug repurposing which dampen the cytokine storm and pulmonary injury associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been examined. Recent investigations have shown that baricitinib, which is thought to possess antiviral properties through its affinity for adaptor-associated kinase-1 and the subsequent reduction in SARS-CoV-2 endocytosis, improves clinical status in patients with COVID-19.15–28 Thus, baricitinib appears to lower systemic inflammation, and several ongoing clinical trials are investigating the potential impact of this repurposing approach on outcome in COVID-19 patients (ClinicalTrials.gov Identifier: NCT04320277, NCT04321993, NCT04346147, NCT04381936, NCT04390464, NCT04399798, NCT04640168, NCT04693026, NCT04832880, NCT04890626, NCT04891133, NCT04970719, NCT05056558, NCT05082714, NCT05074420, NCT04393051).
Baricitinib is used in patients for the treatment of moderate-to-severe active rheumatoid arthritis and atopic eczema/dermatitis, with the latter use approved by the National Institute for Health and Care Excellence (NICE) in 2021.29 Given the evident beneficial effects of baricitinib administration in type 2 diabetes and COVID-19, we wished to explore the potential of repurposing baricitinib in trauma-hemorrhage. Currently, there is limited information about the role of JAK/STAT pathway in trauma.30–32
METHODS
JAK2 and STAT3 Gene Expression in Human Whole Blood
Original data were obtained under Gene Expression Omnibus (GEO) accession GSE36809, published by Xiao et al.33 RNA was extracted from whole blood leukocytes of severe blunt trauma patients (n=167) over the course of 28 days and healthy controls (n=37) and hybridized onto an HU133 Plus 2.0 GeneChip (Affymetrix) according to the manufacturer’s recommendations. The dataset was reanalyzed for JAK2 and STAT3 gene expression. A further reanalysis was performed dividing the trauma patients into uncomplicated (n=55) and complicated (n=41) groups.
Use of Experimental Animals—Ethics Statement
All animal procedures were approved by the Animal Welfare Ethics Review Board of Queen Mary University of London and by the Home Office (License number PC5F29685).
Experimental Design
Male Wistar rats (Charles River Laboratories Ltd, Kent, UK) weighing 260 to 310 g were kept under standard laboratory conditions and received a chow diet and water ad libitum. Baricitinib (Insight Biotechnology, UK) was diluted in 5% DMSO+95% Ringer lactate (vehicle) and rats were treated (intraperitoneally) upon resuscitation. A delayed application of baricitinib was performed to simulate delayed clinical interventions. Further information about baricitinib can be found in the Supplemental (Supplemental Digital Content 1, http://links.lww.com/SLA/E19).
HS Model
The acute HS model was performed as previously described in this journal.34–37 Briefly, 40 rats were anesthetized with sodium thiopentone (120 mg/kg intraperitoneally initially and 10 mg/kg intravenously for maintenance as needed) and randomized into 4 groups: sham+vehicle (n=10); sham+baricitinib (1 mg/kg; n=9), HS+vehicle (n=11); HS+baricitinib (1 mg/kg; n=10). Blood was withdrawn to achieve a fall in mean arterial pressure (MAP) to 35±5 mm Hg, which was maintained for 90 minutes. At 90 minutes after initiation of hemorrhage (or when 25% of the shed blood had to be reinjected to sustain MAP), resuscitation was performed and either baricitinib (1 mg/kg) or vehicle was administered. At 4 hours postresuscitation, blood was collected for the measurement of biomarkers of organ injury/dysfunction (MRC Harwell Institute, Oxfordshire, UK). Sham-operated rats were used as control and underwent identical surgical procedures, but without hemorrhage or resuscitation. Detailed description of the model can be found in the Supplemental (Supplementary Fig. 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E19).
Western Blot Analysis
Semiquantitative immunoblot analysis was carried out in liver and kidney tissue samples as previously described.34,38 Detailed description of the method can be found in the Supplemental (Supplemental Digital Content 1, http://links.lww.com/SLA/E19).
CD68 Immunohistochemical Staining
Renal sections (2 µm) were deparaffinized and hydrated as previously described39 and stained for CD68. Detailed description of the method can be found in the Supplemental (Supplemental Digital Content 1, http://links.lww.com/SLA/E19).
Periodic Acid Schiff (PAS) Staining
PAS staining of renal sections (2 µm) was performed using a PAS staining kit (Carl Roth, Karlsruhe, Germany). Histomorphological changes were evaluated at ×20 magnification using a scoring system as previously described.39 Images were taken using a KEYENCE BZ-X800 microscope and BZ-X800 viewer after performing white balance and auto exposure.
Statistical Analysis
All data in text and figures are expressed as mean±SEM of n observations, where n represents the number of animals/experiments/subjects studied. Measurements obtained from the patient groups and vehicle and baricitinib treated animal groups were analyzed by 1-way/2-way analysis of variance followed by Bonferroni post hoc test or Kruskal-Wallis test followed by Dunn post hoc test on GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA). The distribution of the data was verified by Shapiro-Wilk normality test and the homogeneity of variances by Bartlett test. When necessary, values were transformed into logarithmic values to achieve normality and homogeneity of variances. P value <0.05 was considered statistically significant.
RESULTS
JAK2 and STAT3 Gene Expression Is Elevated in Trauma Patients
Xiao et al33 compared genome-wide gene expression in leukocytes from trauma patients against matched healthy controls. We reanalyzed this dataset for JAK2 and STAT3 expression, as STAT3 is the most commonly stimulated downstream STAT following JAK2 activation. When compared with healthy controls, JAK2 expression was significantly elevated in patients with trauma at all time points (P<0.05; Fig. 1A). An initial peak was noted at day 1 followed by a gradual decrease, however, JAK2 expression still remained elevated at day 28 after trauma. Similarly, STAT3 expression was significantly increased at all time points (P<0.05; Fig. 1B) and an initial peak was noted at 12 hours followed by a gradual decrease. However, STAT3 expression also still remained elevated at day 28 after trauma. When comparing trauma patients stratified into uncomplicated (recovery in <5 days) and complicated (recovery after 14 days, no recovery by day 28 or death) groups, JAK2 expression was significantly higher on days 4 and 7 (P<0.05; Fig. 1C) and STAT3 expression was significantly raised at 12 hours (P<0.05; Fig. 1D) in complicated patients when compared with uncomplicated trauma patients. These data indicate that the JAK2/STAT3 pathway may play a role in the pathophysiology of trauma. Please note the y-axis intervals for Figure 1C and D are 0.3 and 0.4, respectively which show the intergroup differences are small (but still statistically significant).
FIGURE 1.
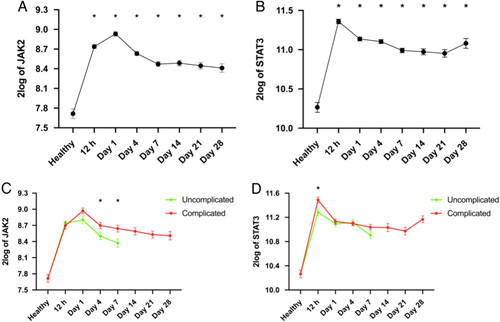
JAK2 and STAT3 gene expression is elevated in trauma patients. Original data was obtained from the Gene Expression Omnibus under dataset accession number GSE36809 which was published by Xiao et al.33 RNA was extracted from whole blood leukocytes over a 28-day time course from trauma patients (n=167) and matched healthy controls (n=37). Data were reanalyzed for JAK2 (A) and STAT3 (B) gene expression in trauma patients and uncomplicated versus complicated trauma patient groups (C, D). Data are expressed as mean±SEM. Statistical analysis was performed using 1-way or 2-way analysis of variance followed by a Bonferroni post hoc test. *P<0.05 denoted statistical significance.
Baricitinib Attenuates HS-induced Organ Injury/Dysfunction in HS
Here, we explored whether pharmacological intervention with the JAK1/JAK2 inhibitor baricitinib administered upon resuscitation attenuates the MODS associated with HS in rats. When compared with sham-operated rats, rats subjected to HS displayed significant increases in serum urea (P<0.05; Fig. 2A) and creatinine (P<0.05; Fig. 2B); indicating the development of renal dysfunction. When compared with sham-operated rats, vehicle-treated HS rats exhibited significant increases in alanine transaminase (P<0.05; Fig. 2C) and aspartate transaminase (AST) (P<0.05; Fig. 2D) indicating the development of hepatic injury, while the increases in amylase (P<0.05; Fig. 2E) and creatine kinase (P<0.05; Fig. 2F) denote pancreatic and neuromuscular injury, respectively. The significant increase in lactate dehydrogenase (P<0.05; Fig. 2F) in vehicle-treated HS rats confirmed tissue injury. Treatment of HS rats with baricitinib significantly attenuated the renal dysfunction, hepatic injury, pancreatic injury, neuromuscular injury, and general tissue damage caused by HS as shown by the reduction in serum parameter values (all P<0.05; Fig. 2A–G). Administration of baricitinib to sham-operated rats had no significant effect on any of the parameters measured (P>0.05; Fig. 2).
FIGURE 2.
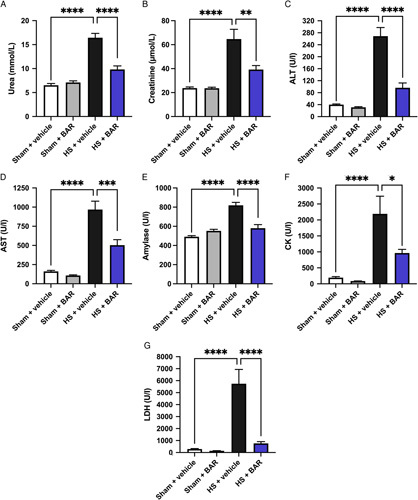
Baricitinib attenuates HS-induced organ injury/dysfunction in HS. Rats were subjected to HS and 4 hours after resuscitation, levels of serum urea (A), creatinine (B), alanine aminotransferase (ALT) (C), AST (D), amylase (E), creatine kinase (CK) (F), and lactate dehydrogenase (LDH) (G) were determined in vehicle and BAR (baricitinib) treated rats. Sham-operated rats were used as control. Data are expressed as mean±SEM of 9 to 11 animals per group. Statistical analysis was performed using one-way analysis of variance followed by a Bonferroni post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 denoted statistical significance.
Baricitinib Abolishes Hepatic and Renal JAK/STAT Activation in HS
Using western blot analysis, we examined whether HS leads to the activation of the JAK2/STAT3 pathway in the liver and kidney, given that treatment with baricitinib significantly attenuated HS-associated hepatic injury and renal dysfunction. When compared with sham-operated rats, vehicle-treated HS rats displayed significant increases in the phosphorylation of hepatic and renal Jak2 at Tyr1007-1008 (P<0.05; Fig. 3A, B) and Stat3 at Tyr705 (P<0.05; Fig. 3C, D), indicating that the JAK2/STAT3 pathway is activated in the injured liver and kidneys. Treatment of HS rats with baricitinib significantly abolished these increases in JAK2/STAT3 phosphorylation (P<0.05; Fig. 3A–D).
FIGURE 3.
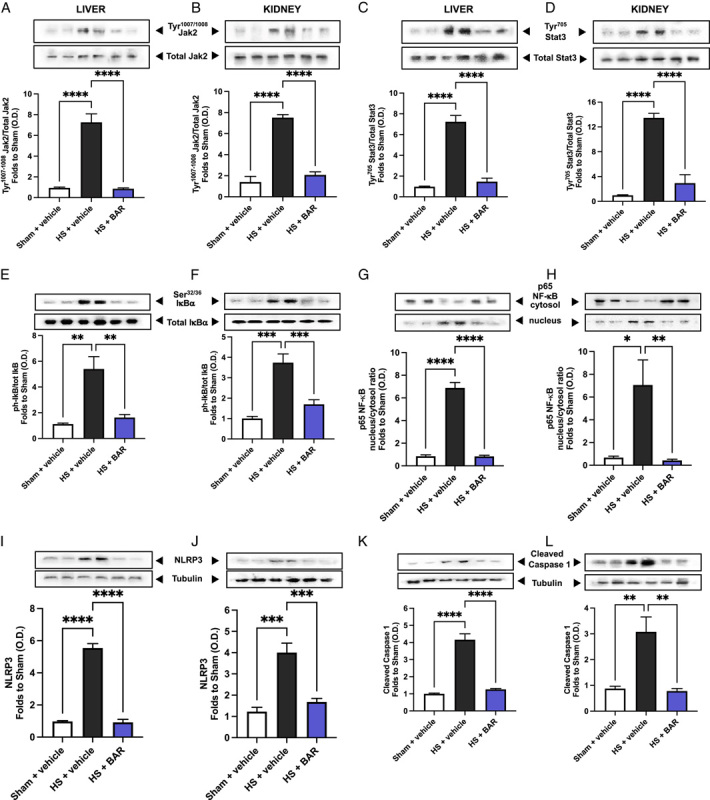
Baricitinib abolishes JAK/STAT, NF-κB, and NLRP3 activation in HS. The phosphorylation of Jak2 at Tyr1007-1008 (A, B), the phosphorylation of Stat3 at Tyr705 (C, D), the phosphorylation of IκBα at Ser32/36 (E, F), the nuclear translocation of p65 (G, H), the activation of NLRP3 (I, J), and the cleaved (activated) form of caspase 1 of vehicle and BAR (baricitinib) treated rats were determined by western blotting in the liver and kidney (K, L). Protein expression was measured as relative optical density (OD) and normalized to the sham band. Data are expressed as mean±SEM of 4 to 5 animals per group. Statistical analysis was performed using 1-way analysis of variance followed by a Bonferroni post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 denoted statistical significance.
Baricitinib Abolishes Hepatic and Renal NF-κB Activation in HS
The effect of JAK/STAT inhibition on the activation of NF-κB were investigated in the liver and kidney. When compared with sham-operated rats, vehicle-treated HS rats had significant increases in the hepatic and renal phosphorylation of IκBα at Ser32/36 (P<0.05; Fig. 3E, F) and the translocation of p65 to the nucleus (P<0.05; Fig. 3G, H). Treatment of HS rats with baricitinib significantly abolished this activation of NF-κB (P<0.05; Fig. 3E–H).
Baricitinib Abolishes Hepatic and Renal NLRP3 and Caspase 1 Activation in HS
Having discovered that baricitinib significantly reduced NF-κB activation in the liver and kidney of HS rats, we next analyzed the potential involvement of the NLRP3 inflammasome complex. When compared with sham-operated rats, vehicle-treated HS rats exhibited significantly increased hepatic and renal expression of the NLRP3 inflammasome (P<0.05; Fig. 3I, J) and cleaved (activated) form of caspase 1 (P<0.05; Fig. 3K, L). Treatment of HS rats with baricitinib significantly inhibited these increases (P<0.05; Fig. 3I–L).
JAK2 Activation Correlates With Hepatic Injury, Renal Dysfunction, STAT3, NF-κB, and NLRP3 Activation in an Acute HS Model
Correlation analysis was performed to determine whether the degree of activation of JAK correlates with changes in liver condition, renal function, STAT3, NF-κB, and NLRP3 activation. Significant positive correlations were found between JAK2 activation and (with the exception of AST) all parameters investigated (Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E19).
Baricitinib Reduces Renal Macrophage Invasion and Tissue Injury in HS
Having shown that baricitinib significantly attenuated the HS-induced renal dysfunction, we investigated renal macrophage infiltration and tissue injury determined by CD68 and PAS staining, respectively. When compared with sham-operated rats, vehicle-treated HS rats exhibited a significantly increased expression of CD68 in the kidney (P<0.05; Fig. 4A). Treatment with baricitinib in HS rats significantly reduced CD68 expression, indicating an attenuation of renal macrophage invasion (P<0.05; Fig. 4A). When compared with sham-operated rats, vehicle-treated HS rats displayed a significantly raised renal PAS score (P<0.05; Fig. 4C). Treatment with baricitinib in HS rats reduced this rise in PAS score (albeit this change was not statistically significant), suggesting a tissue-protective effect of baricitinib in the kidney (Fig. 4C).
FIGURE 4.
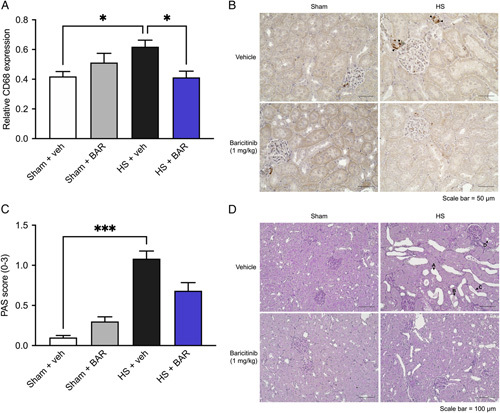
Baricitinib reduces renal macrophage invasion and tissue injury in HS. Rats were subjected to HS and 4 hours after resuscitation, relative CD68 expression (A) and PAS scores (C) (score 0: no damage, 1: <25% damaged, 2: 25%–50% damaged and 3: >50% damaged) were quantified in renal tissue of vehicle and BAR (baricitinib) treated rats. Representative images of (B) CD68 staining (×40 magnification) and (D) PAS staining (×20 magnification) are shown. Black arrows highlight (B) sites of positive CD68 staining and (D) tubule dilatation (arrow A), detached cell material (arrow B), flattened epithelial cells (arrow C), and beginning stages of tubular brush border loss in proximal tubules (arrow D). Data are expressed as mean±SEM of 8 animals per group. Statistical analysis was performed using a 1-way analysis of variance followed by a Bonferroni post hoc test (A) and Kruskal-Wallis test followed by a Dunn post hoc test (C). *P<0.05, ***P < 0.001 denoted statistical significance.
Effect of Baricitinib on HS-induced Circulatory Failure in HS
To investigate the effects of baricitinib on circulatory failure, MAP was measured from the completion of surgery to the termination of the experiment. Baseline MAP values were similar among all 4 groups. Rats subjected to HS demonstrated a decline in MAP which was ameliorated by resuscitation, but MAP remained lower than that of sham-operated rats during resuscitation (at the equivalent time points, Fig. 5). The MAP of baricitinib treated HS rats was similar to those of vehicle-treated HS rats at the end of the resuscitation period (Fig. 5). Administration of baricitinib to sham-operated rats had no significant effect on MAP (P>0.05; Fig. 5).
FIGURE 5.
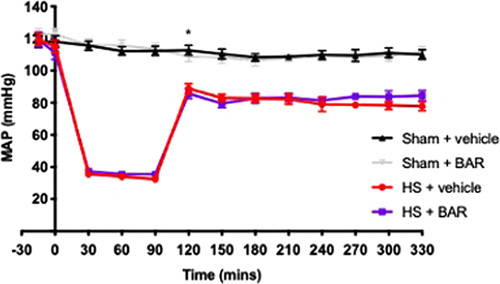
Effect of baricitinib on HS-induced circulatory failure in HS. MAP was measured from the completion of surgery to the termination of the experiment for vehicle and BAR (baricitinib) treated rats. Data are expressed as mean±SEM of 9 to 11 animals per group. Statistical analysis was performed using a 2-way analysis of variance followed by a Bonferroni post hoc test. *P<0.05 sham+vehicle versus HS+vehicle.
DISCUSSION
This study reports that inhibition of JAK/STAT activity attenuates the organ injury/dysfunction associated with HS (acute rat model; Fig. 2). Having shown that JAK2 and STAT3 gene expression is significantly elevated in leukocytes of trauma patients (Fig. 1A, B) and, most notably, that the expression of JAK2 and STAT3 is higher in trauma patients with a complicated recovery (Fig. 1C, D), we used a reverse translational approach to investigate whether pharmacological intervention with the JAK1/JAK2 inhibitor baricitinib ameliorates the MODS associated with HS in a well-established rat model.34–37
Baricitinib inhibits JAK1 and JAK2 to an equal degree and is currently used in patients with inflammatory diseases, including rheumatoid arthritis and atopic dermatitis.40 We report here for the first time that baricitinib significantly attenuated the renal dysfunction, hepatic injury, pancreatic injury, and neuromuscular injury caused by HS (Fig. 2). Similarly, JAK inhibition lowers disease severity in animal models of ischemia-reperfusion injury,41–46 sepsis,47–50 acute kidney injury,51 and endotoxemia.52
What, then, are the mechanisms by which baricitinib attenuates HS-associated organ injury/dysfunction? HS resulted in a significant increase in JAK2/STAT3 activity in the liver and kidneys (Fig. 3) which correlated with the rises in alanine aminotransferase, creatinine, and urea (Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E19). Indeed, inhibition of JAK2/STAT3 activity with baricitinib in the liver and kidneys of HS rats decreased the hepatic injury and renal dysfunction, suggesting that activation of the JAK2/STAT3 pathway plays a pivotal role in the pathophysiology of the MODS associated with HS. Despite not finding a significant correlation between JAK activation and AST in our study, genome-wide association study-prioritized genes and gene coexpression pattern analysis revealed JAK/STAT signaling was linked to AST-specific gene sets which supports the role of JAK/STAT in liver function.53
A synergistic interaction between STAT and NF-κB occur on many levels: these include physical interactions of the 2 molecules, cooperative binding at particular gene promoter subsets to induce target gene expression and cytokine feedback loops, whereby cytokines induced by STAT and NF-κB [eg, interleukin (IL)-6] can further prolong the activation of both molecules.54 Trauma leads to increased nuclear translocation of NF-κB.34–37,55 Inhibition of JAK2/STAT3 activity with baricitinib decreased NF-κB activation in both the liver and kidneys (Fig. 3). We also found a significant positive correlation between the activation of JAK2 and the phosphorylation of IκBα at Ser32/36 and translocation of p65 (Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E19). This may suggest that blocking the activation of NF-κB contributes to the observed effects of baricitinib in HS. Activation of NF-κB drives the production of multiple proinflammatory and anti-inflammatory mediators including enzymes, cytokines, and chemokines.56 As part of a positive feedback loop, these mediators can activate NF-κB and its upstream signaling components, further amplifying and sustaining the inflammatory responses mediated by NF-κB which can lead to greater permeability of the endothelium hypoperfused/hypoxic tissues, tissue injury, and eventually MODS.57
The JAK/STAT pathway has also been shown to be linked to the NLRP3 inflammasome, with pharmacological inhibition of JAK reducing the expression and activation of NLRP3 inflammasome components.58,59 NLRP3 inflammasome activation drives the formation of IL-1β which plays a key role in the systemic inflammation and/or organ dysfunction associated with trauma.34,55 Inhibition of JAK2/STAT3 activity with baricitinib decreased the assembly and successive activation of the NLRP3 inflammasome in the liver and kidneys (Fig. 3). We also discovered a significant positive correlation between JAK2 activation and the activation of NLRP3 and caspase 1 (Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E19). A recent phenotypic high-content, the high-throughput screen identified targeting JAK to inhibit NLRP3 inflammasome activation.60 This may suggest that blocking the activation of the NLRP3 inflammasome contributes to the observed protective effects of baricitinib in HS by reducing the proinflammatory effects of IL-1β and ensuing tissue inflammation.
The sterile inflammation caused by HS drives the recruitment of leukocytes to the tissues and is secondary to NF-κB and NLRP3 activation and their associated transcriptional regulation of proinflammatory cytokines.61–63 Furthermore, leukocytes and endothelial cells express adhesion molecules, and this is regulated by NF-κB to facilitate leukocyte extravasation from the circulation to the site of damage.64 It has been shown that experimental injury induces a transcriptomic shift in leukocytes 4 hours posttrauma and HS, and several pathways related to innate immunity were upregulated; including those involved in myeloid leukocyte activation and differentiation.65 We measured CD68 expression and PAS score in the kidney as markers of macrophage infiltration and overall tissue injury, respectively.66 HS resulted in a significant rise in relative CD68 expression and PAS score in the kidney which was attenuated by baricitinib treatment in HS rats (Fig. 4, see also the Supplement for an extended discussion, Supplemental Digital Content 1, http://links.lww.com/SLA/E19). A graphical abstract summarizing the protective effects of baricitinib following HS-induced multiple organ dysfunction is shown in Figure 6.
FIGURE 6.
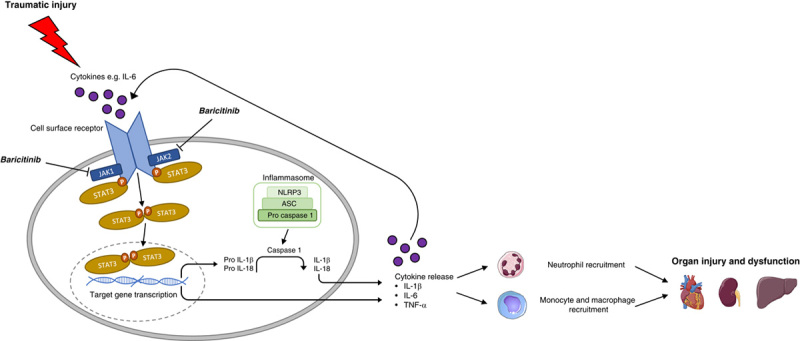
Graphical abstract highlighting the role of JAK/STAT in the pathophysiology of traumatic injury. Cytokines are released following trauma which trigger signaling pathways leading to JAK/STAT activation and target gene transcription. The activation of the NLRP3 inflammasome results in the cleavage of pro-IL-1β to IL-1β and further proinflammatory cytokine production. Leukocyte recruitment is also stimulated by cytokine release. Excessive systemic inflammation from the combination of cytokine release and innate immune cell recruitment contributes to the onset of MODS. Treatment with JAK inhibitors such as baricitinib can attenuate trauma-induced inflammation and thus MODS to improve clinical outcomes and patient prognosis. STAT indicates signal transducer and activator of transcription; TNF-α, tumor necrosis factor.
Our results and conclusions are supported by findings in COVID-19 patient cohorts where the JAK/STAT pathway has been proposed to play a role in disease pathogenesis. The beneficial effects of baricitinib in COVID-19 patients, as measured by the improved levels of oxygenation, decreased need for mechanical ventilation (invasive and noninvasive), reduced ICU admission, and mortality indicate that JAK/STAT activation contributes to the disease pathology.67 Parallels can be drawn between COVID-19 and trauma-associated MODS, with both displaying features of an excessive systemic inflammatory state.
LIMITATIONS OF THE STUDY
Although baricitinib demonstrated some striking, beneficial effects in the rat model of HS, there are limitations which should be taken into consideration. An acute model of HS with a single time point was used in this study, which results in systemic inflammation and MODS within a few hours following resuscitation. At 4 hours postresuscitation, there is organ injury and dysfunction and significant activation of JAK/STAT, NF-κB, and NLRP3. Despite the effectiveness of baricitinib in this acute setting at the 4-hour time point, we cannot conclude that the same protective effects will be observed in animal models with a longer follow-up period since there are delayed consequences/outcomes of trauma and severe hemorrhage on organ and immune function. Moreover, survival studies are needed to confirm that the observed early reduction in MODS does, indeed, translate to improved outcome and ultimately a decrease in mortality; all of which would strengthen the efficacy data of baricitinib in this study and provide more robust evidence in favor of designing a clinical trial. Organ injury and dysfunction were used as surrogate markers for mortality in our study (as the determination of mortality is not allowed by our ethics and Home Office license). Thus, caution should be taken when interpreting our preclinical results and extrapolating them to the clinical scenario. In this study, gender differences were not investigated, as only male rats were used to prevent any sex-dependent confounding effects (variations in female reproductive hormones and X chromosome) and to represent the population usually most affected by trauma (young males). Of note, the majority of trauma patients in our recent TOP-ART clinical trial were male and aged under 30, so the use of male rats is clinically appropriate.68 Nevertheless, we acknowledge a considerable number of trauma patients are female, and the efficacy of baricitinib in female rats should be assessed. Further studies in larger animals (eg, pigs) and/or higher species may be useful to increase understanding of both the mechanism of action (eg, blood gas analysis and microcirculatory effects) and verify efficacy of baricitinib in HS. We show that treatment with baricitinib upon resuscitation had no significant effect on arterial blood pressure, suggesting that the beneficial influence of baricitinib in HS may be independent of effects on blood pressure and, hence, microvascular perfusion of the tissues/organs. This could potentially limit the effectiveness of baricitinib treatment in HS, as alternative therapies may be required to counteract the vascular decompensation associated with HS. Nonetheless, clinical studies with large cohorts of trauma patients are required to robustly examine the relationship between JAK activity and clinical outcomes in humans.
CONCLUSIONS
We report here for the first time that baricitinib reduces the organ injury/dysfunction caused by severe hemorrhage in the rat, highlighting a role of the JAK2/STAT3 pathway in disease pathogenesis. In addition, experimental trauma-hemorrhage results in a significant activation of the JAK2/STAT3 pathway in the liver and kidneys. Administration of baricitinib during resuscitation after major hemorrhage abolished the activation of JAK2/STAT3 as well as the activation of NF-κB and the NLRP3 inflammasome (liver and kidney); both of which are major drivers of local and systemic inflammation. Therefore, we propose that JAK inhibitors, such as baricitinib, may be repurposed to reduce the organ injury and inflammation caused by severe hemorrhage and resuscitation in patients with trauma.
Supplementary Material
Footnotes
Author contributions: N.M.P. and C.T.: conception and design; drafting the manuscript for important intellectual content. N.M.P.: acquisition of data. N.M.P., D.C., E.A., G.F.A., S.K., S.M.C., M.C., and C.T: analysis and interpretation of data. All authors reviewed and approved the manuscript.
N.M.P. was funded by the William Harvey Research Foundation.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Nikita M. Patel, Email: n.m.patel@qmul.ac.uk;nikita.patel.14@ucl.ac.uk.
Debora Collotta, Email: debora.collotta@unito.it.
Eleonora Aimaretti, Email: eleonora.aimaretti@unito.it.
Gustavo Ferreira Alves, Email: gustavo.ferreiraalves@unito.it.
Sarah Kröller, Email: sarah.kroeller@med.uni-jena.de.
Sina M. Coldewey, Email: sina.coldewey@med.uni-jena.de.
Massimo Collino, Email: massimo.collino@unito.it.
Christoph Thiemermann, Email: c.thiemermann@qmul.ac.uk.
REFERENCES
- 1. World Health Organization. Injuries and violence: the facts 2014; 2014. Accessed May 6, 2021. www.who.int/healthinfo/global_burden_disease/projections/en/
- 2. Curry N, Hopewell S, Dorée C, et al. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care. 2011;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dewar D, Moore FA, Moore EE, et al. Postinjury multiple organ failure. Injury. 2009;40:912–918. [DOI] [PubMed] [Google Scholar]
- 7. Denk S, Weckbach S, Eisele P, et al. Role of hemorrhagic shock in experimental polytrauma. Shock. 2018;49:154–163. [DOI] [PubMed] [Google Scholar]
- 8. Halbgebauer R, Braun CK, Denk S, et al. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J Crit Care. 2018;44:229–237. [DOI] [PubMed] [Google Scholar]
- 9. Kleinveld DJB, Simons DDG, Dekimpe C, et al. Plasma and rhADAMTS13 reduce trauma-induced organ failure by restoring the ADAMTS13-VWF axis. Blood Adv. 2021;5:3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cabrera CP, Manson J, Shepherd JM, et al. Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: a prospective cohort study. PLoS Med. 2017;14:e1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shepherd JM, Cole E, Brohi K. Contemporary patterns of multiple organ dysfunction in trauma. Shock. 2017;47:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lord JM, Midwinter MJ, Chen YF, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. [DOI] [PubMed] [Google Scholar]
- 14. Collotta D, Hull W, Mastrocola R, et al. Baricitinib counteracts metaflammation, thus protecting against diet-induced metabolic abnormalities in mice. Mol Metab. 2020;39:101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130:6409–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cantini F, Niccoli L, Matarrese D, et al. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81:318–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020;81:647–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez-Garcia JL, Sanchez-Nievas G, et al. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford). 2021;60:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Titanji BK, Farley MM, Mehta A, et al. Use of baricitinib in patients with moderate to severe coronavirus disease 2019. Clin Infect Dis. 2021;72:1247–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasan MJ, Rabbani R, Anam AM, et al. Additional baricitinib loading dose improves clinical outcome in COVID-19. Open Med (Wars). 2021;16:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosas J, Liaño FP, Cantó ML, et al. Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus COVID19: a real-world study. Reumatol Clin (Engl Ed). 2020;18:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pérez-Alba E, Nuzzolo-Shihadeh L, Aguirre-García GM, et al. Baricitinib plus dexamethasone compared to dexamethasone for the treatment of severe COVID-19 pneumonia: a retrospective analysis. J Microbiol Immunol Infect. 2021;54:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stebbing J, Nievas GS, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7:eabe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hasan MdJ, Rabbani R, Anam AM, et al. Impact of high dose of baricitinib in severe COVID-19 pneumonia: a prospective cohort study in Bangladesh. BMC Infect Dis. 2021;21:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tziolos N, Karofylakis E, Grigoropoulos I, et al. Real-life effectiveness and safety of baricitinib as adjunctive to standard-of-care treatment in hospitalized patients with severe COVID-19. Open Forum Infect Dis. 2021;9:ofab588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence (NICE). Overview. Baricitinib for treating moderate to severe atopic dermatitis. Guidance; 2021.
- 30. Zhao JB, Zhang Y, Li G, et al. Activation of JAK2/STAT pathway in cerebral cortex after experimental traumatic brain injury of rats. Neurosci Lett. 2011;498:147–152. [DOI] [PubMed] [Google Scholar]
- 31. Raible DJ, Frey LC, del Angel YC, et al. JAK/STAT pathway regulation of GABAA receptor expression after differing severities of experimental TBI. Exp Neurol. 2015;271:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang LJ, Ni SZ, Zhou XL, et al. Hemorrhagic shock sensitized the diaphragm to ventilator-induced dysfunction through the activation of IL-6/JAK/STAT signaling-mediated autophagy in rats. Mediators Inflamm. 2019;2019:3738409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel NM, Oliveira FRMB, Ramos HP, et al. Inhibition of Bruton’s tyrosine kinase activity attenuates hemorrhagic shock-induced multiple organ dysfunction in rats. Ann Surg. 2023;277:e624–e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sordi R, Chiazza F, Collotta D, et al. Resolvin D1 attenuates the organ injury associated with experimental hemorrhagic shock. Ann Surg. 2021;273:1012–1021. [DOI] [PubMed] [Google Scholar]
- 36. Sordi R, Nandra KK, Chiazza F, et al. Artesunate protects against the organ injury and dysfunction induced by severe hemorrhage and resuscitation. Ann Surg. 2017;265:408–417. [DOI] [PubMed] [Google Scholar]
- 37. Yamada N, Martin LB, Zechendorf E, et al. Novel synthetic, host-defense peptide protects against organ injury/dysfunction in a rat model of severe hemorrhagic shock. Ann Surg. 2018;268:348–356. [DOI] [PubMed] [Google Scholar]
- 38. Patel NM, Yamada N, Oliveira FRMB, et al. Inhibition of macrophage migration inhibitory factor activity attenuates haemorrhagic shock-induced multiple organ dysfunction in rats. Front Immunol. 2022;13:886421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dennhardt S, Pirschel W, Wissuwa B, et al. Modeling hemolytic-uremic syndrome: in-depth characterization of distinct murine models reflecting different features of human disease. Front Immunol. 2018;9:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chmp. Annex I summary of product characteristics. 2017.
- 41. Yang N, Luo M, Li R, et al. Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial Transplant. 2008;23:91–100. [DOI] [PubMed] [Google Scholar]
- 42. Zhao X, Zhang E, Ren X, et al. Edaravone alleviates cell apoptosis and mitochondrial injury in ischemia-reperfusion-induced kidney injury via the JAK/STAT pathway. Biol Res. 2020;53:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mascareno E, El-Shafei M, Maulik N, et al. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation. 2001;104:325–329. [DOI] [PubMed] [Google Scholar]
- 44. Wen SH, Li Y, Li C, et al. Ischemic postconditioning during reperfusion attenuates intestinal injury and mucosal cell apoptosis by inhibiting JAK/STAT signaling activation. Shock. 2012;38:411–419. [DOI] [PubMed] [Google Scholar]
- 45. Freitas MCS, Uchida Y, Zhao D, et al. Blockade of Janus kinase-2 signaling ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transpl. 2010;16:600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Si Y, Bao H, Han L, et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J Transl Med. 2013;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hui L, Yao Y, Wang S, et al. Inhibition of Janus kinase 2 and signal transduction and activator of transcription 3 protect against cecal ligation and puncture-induced multiple organ damage and mortality. J Trauma. 2009;66:859–865. [DOI] [PubMed] [Google Scholar]
- 48. Peña G, Cai B, Deitch EA, et al. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J Mol Med. 2010;88:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsirigotis P, Papanikolaou N, Elefanti A, et al. Treatment of experimental Candida sepsis with a janus kinase inhibitor controls inflammation and prolongs survival. Antimicrob Agents Chemother. 2015;59:7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jarneborn A, Mohammad M, Engdahl C, et al. Tofacitinib treatment aggravates Staphylococcus aureus septic arthritis, but attenuates sepsis and enterotoxin induced shock in mice. Sci Rep. 2020;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yun Y, Chen J, Wang X, et al. Tofacitinib ameliorates lipopolysaccharide-induced acute kidney injury by blocking the JAK-STAT1/STAT3 signaling pathway. Biomed Res Int. 2021;2021:8877056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruetten H, Thiemermann C. Effects of tyrphostins and genistein on the circulatory failure and organ dysfunction caused by endotoxin in the rat: a possible role for protein tyrosine kinase. Br J Pharmacol. 1997;122:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen VL, Du X, Chen Y, et al. Genome-wide association study of serum liver enzymes implicates diverse metabolic and liver pathology. Nat Commun. 2021;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Martin L, Patel NM, Yamada N, et al. The inhibition of macrophage migration inhibitory factor by ISO-1 attenuates trauma-induced multi organ dysfunction in rats. medRxiv; 2021.
- 56. Senftleben U, Karin M. The IKK/NF-kappa B pathway. Crit Care Med. 2002;30:S18–S26. [PubMed] [Google Scholar]
- 57. Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. [DOI] [PubMed] [Google Scholar]
- 58. Zhu H, Jian Z, Zhong Y, et al. Janus kinase inhibition ameliorates ischemic stroke injury and neuroinflammation through reducing NLRP3 inflammasome activation via JAK2/STAT3 pathway inhibition. Front Immunol. 2021;0:2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Furuya MY, Asano T, Sumichika Y, et al. Tofacitinib inhibits granulocyte–macrophage colony-stimulating factor-induced NLRP3 inflammasome activation in human neutrophils. Arthritis Res Ther. 2018;20:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nizami S, Millar V, Arunasalam K, et al. A phenotypic high-content, high-throughput screen identifies inhibitors of NLRP3 inflammasome activation. Sci Rep. 2021;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen CC, Manning AM. Transcriptional regulation of endothelial cell adhesion molecules: a dominant role for NF-κB. Agents Actions Suppl. 1995;47:135–141. [DOI] [PubMed] [Google Scholar]
- 62. Collins T, Read MA, Neish AS, et al. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 63. Campbell SJ, Anthony DC, Oakley F, et al. Hepatic nuclear factor κB regulates neutrophil recruitment to the injured brain. J Neuropathol Exp Neurol. 2008;67:223–230. [DOI] [PubMed] [Google Scholar]
- 64. Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21:223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Debler L, Palmer A, Braumüller S, et al. Hemorrhagic shock induces a rapid transcriptomic shift of the immune balance in leukocytes after experimental multiple injury. Mediators Inflamm. 2021;2021:6654318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chistiakov DA, Killingsworth MC, Myasoedova VA, et al. CD68/macrosialin: not just a histochemical marker. Lab Invest. 2016;97:4–13. [DOI] [PubMed] [Google Scholar]
- 67. Lin Z, Niu J, Xu Y, et al. Clinical efficacy and adverse events of baricitinib treatment for coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis. J Med Virol. 2022;94:1523–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. ISRCTNregistry. The TOP-ART Study: Trauma Organ Protection—Artesunate. ISRCTN15731357. 2015. Accessed November 15, 2021. https://www.isrctn.com/ISRCTN15731357


