Background and objectives
Significant health inequities exist in screening uptake for certain types of cancer. The review question was to identify and describe interactive, tailored digital, computer, and web-based interventions to reduce health inequity in cancer screening and review the effectiveness of such interventions in increasing screening rates versus usual care.
Methods
We searched four medical literature databases for randomized control trials (RCTs) published until 12 January 2023 that evaluated interventions aimed at increasing the percentage of breast, prostate, cervical, or colorectal cancer screening uptake. Meta-analysis was not conducted due to heterogeneity among studies.
Results
After screening 4200 titles and abstracts, 17 studies were included. Studies focused on colorectal (n = 10), breast (n = 4), cervical (n = 2), and prostate (n = 1) cancer screening. All were based in the USA except two. Most studies focused on ethnicity/race, while some included low-income populations. Intervention types were heterogeneous and used computer programs, apps, or web-based methods to provide tailored or interactive information to participants about screening risks and options. Some studies found positive effects for increasing cancer screening uptake in the intervention groups compared to usual care, but results were heterogeneous.
Conclusion
Interventions that use individual and cultural tailoring of cancer screening educational material should be further developed and investigated outside of the USA. Designing effective digital intervention strategies, with components that can be adapted to remote delivery may be an important strategy for reducing health inequities in cancer screening during the coronavirus disease 2019 pandemic.
Keywords: apps, breast cancer, cancer screening, cervical cancer, colorectal cancer, digital health, health equity, health inequality, mammography, multimedia
Introduction
Health inequity has been defined by the WHO as ‘systematic differences in the health status of different population groups’ (who.int, 2018). In oncology, health inequity can occur across different stages, from risk factors, screening, diagnosis, and treatment, to mortality. From a public health perspective, screening is an important strategy for certain types of cancers such as cervical, prostate, colorectal, and breast cancers. The Centers for Disease Control and Prevention, the US Preventive Service Taskforce, and similar agencies in Europe support screening for breast, colorectal, and cervical cancers in specific age groups, whereas screening for other cancers is typically recommended for high-risk groups, for example, lung cancer screening is typically done in people who smoke or who have a history of heavy smoking and are between 50 and 80 years old (cdc.gov, 2022a; Centers for Disease Control and Prevention, 2022b; ec.europa.eu 2022; US pstf).
Some research suggests that mammography screening is associated with a reduction in breast cancer mortality (Nelson et al., 2016) and colorectal cancer mortality can be reduced with screening through guaiac fecal occult blood testing or flexible sigmoidoscopy (Fitzpatrick-Lewis et al., 2016). It has been shown that cervical cancer screening currently prevents 70% of cervical cancer deaths for all ages in the UK and it is estimated that if everyone attends screening regularly, 83% of deaths could be prevented (Landy et al., 2016). Studies on health inequity, mostly from the US, suggest that there are lower screening rates for these common cancer types for certain racial and ethnic groups (Johnson et al., 2008; Consedine et al., 2015; Jun and Nan, 2018), those with low-incomes or living in socio-economically deprived neighborhoods (Smith et al., 2019), people living in rural areas (Wang et al., 2019), vulnerable groups such as incarcerated women (Kelly et al., 2018), persons living with HIV (Spence et al., 2021), as well as lesbian, gay, bisexual, and transgender (LGBTQ+) persons (Ceres et al., 2018). A systematic review of cervical cancer screening reported numerous sociocultural factors influencing health-related beliefs and healthcare utilization among immigrant and ethnic minorities in the US (Johnson et al., 2008). Sociodemographic and cultural norms (Rogers et al., 2017; Lau et al., 2020), as well as perceived susceptibility, benefits, and barriers, can all contribute to screening intention or completion (Lau et al., 2020), as well as cancer stigma, which is significantly higher in men and those from ethnic minorities (Vrinten et al., 2019).
Health inequity in cancer screening can be addressed by designing specific interventions that focus on the barriers and motives underlying the lack of screening. A systematic review of cervical cancer screening recommends that culturally relevant screening strategies and programs which address such factors should be developed to address growing health disparities (Johnson et al., 2008). The literature provides evidence on a wide range of intervention types (West et al., 2004; Ahmed et al., 2010; Kreuter et al., 2010; Sadler et al., 2012; Horne et al., 2015; Murphy et al., 2015; Coronado et al., 2016; Cuaresma et al., 2018; Mehta et al., 2019, 2020, 2021) and focus group discussions on colorectal cancer screening revealed that different ways to spread knowledge and communicate about colorectal cancer and colorectal cancer-screening could be applied such as community-based information campaigns, decision aids, interactive questionnaires, chat-functions, and telephone support (Fritzell et al., 2017). In recent years digital technologies have been increasingly used to deliver interventions to increase cancer screening uptake that have interactive, tailored multimedia methods (e.g. web-based, apps, touchscreens, etc). The objective of the current review is to describe interactive or tailored digital interventions to reduce health inequity in cancer screening and review the effectiveness of such interventions in increasing screening rates. Specifically, we aim to assess whether participants using digital interventions have higher cancer screening over follow-up than people receiving standard screening programs (usual care). Due to heterogeneity in interventions and target populations, it was not feasible to conduct a meta-analysis.
Methods
The review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (Page et al., 2021).
Searches
We searched four databases for articles published until 12 January 2023: PubMed electronic database of the National Library of Medicine; Web of Science; Embase; and Cochrane Library. Medical Subject Headings (MeSH) terms and free words referring to health equity and cancer screening were used as keywords. Tailored searches were developed for each database as structured in blocks capturing cancer, screening, and inequities. The PubMed search string, for example, was
(‘neoplasms’[MeSH Terms] OR Neoplas* OR Tumor* Or Cancer* OR Malignan* OR ‘Malignant Neoplasm*’ OR ‘Neoplasm, Malignant’)
AND
(‘diagnosis’[Subheading] OR ‘mass screening’[MeSH Terms] OR ‘early detection of cancer’[MeSH Terms] OR Screening[Text Word])
AND
(‘health equity’ OR ‘health inequity’ OR ‘health inequality’ OR ‘health equality’ OR ‘health disparities’ OR inequity OR equity OR race OR racial OR socioeconomic OR SES OR income OR minority OR latin*)
References from the selected papers and other relevant articles were also screened for potential studies.
Study inclusion and exclusion criteria
We used a population, intervention, comparison, outcome (PICO) to identify studies. Population included people and groups that have been documented to have lower rates of cancer screening than others due to health inequity factors such as income, race, etc. We focused on cancers that are often screened at the general population level (e.g. colorectal, breast, prostate and cervical cancers) as a public health strategy for early detection for everyone of a certain age. We did not include, for example, screening for lung cancer as it is not routinely done in people unless they are in high-risk groups such as heavy smokers, etc. Thus, we included only colorectal, breast, prostate, and cervical cancer. Intervention included any intervention to increase cancer screening uptake that used a digital, computerized, or web-based method to provide information to a specific group of people (low socioeconomic status, ethnic groups with low health equity, etc). Comparison was usual care (i.e. usual screening invitation and process, which may differ depending on the cancer type, screening procedure, and type of healthcare system) or suitable low-attention control. Outcome was the percentage of cancer screening uptake during follow-up (self-reported or medical record documentation of screening completion). Any type of screening was included, such as pap test, human papillomavirus self-sampling test kits, mammography, clinical breast exam, fecal immunochemical test (FIT), flexible sigmoidoscopy, colonoscopy, etc. . Study design was limited exclusively to randomized control trials (RCTs) with a usual care or low-attention comparison group. Exclusion criteria were documents without peer review (e.g. congress abstract) or not written in English.
Study selection
Two assessors independently screened the titles and abstracts of the selected studies. The full texts of the articles selected by one or more of the assessors were retrieved for evaluation. Two assessors independently read the full texts and extracted the information from the selected studies. A third assessor reviewed the data extraction and any disagreement was resolved through consensus. The numbers of abstracts screened and studies assessed for eligibility with reasons for exclusions at each stage are presented in Fig. 1.
Fig. 1.
PRISMA flow chart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data extraction strategy
Data extraction was conducted by one researcher and checked by another. Information was extracted on study design, the number of participants (controls and intervention), participant demographics and baseline characteristics, type of cancer screening, type of intervention (including the description of the intervention), comparison group, and outcome (screening uptake). Data was recorded using RevMan.
Study quality assessment
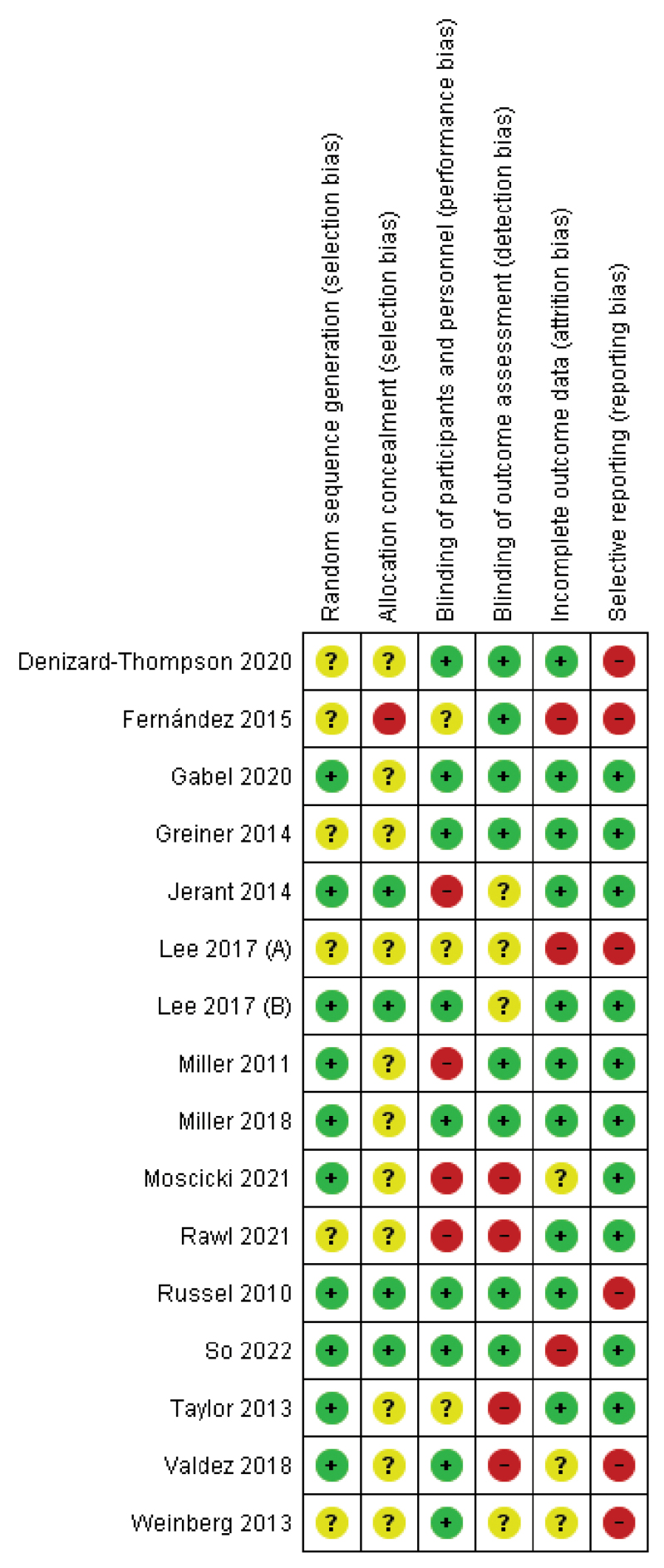
The Review Manager software and the Cochrane Risk of Bias Tool (Version 5.4) were used for a methodological quality assessment of the risk of bias in the included studies (Higgins et al., 2011) (Fig. 1 and Fig. 2). The following domains were evaluated: selection bias: sequence generation, allocation concealment; detection bias: blinding of outcome assessment; attrition bias: incomplete outcome data; and reporting bias: selective reporting. In the case of a low possibility of bias, the studies were categorized as ‘low risk’, in the case of a high possibility of bias ‘high risk’, and if the occurrence of risk of bias could not be indicated – ‘unclear risk’. An in-detail summary of the risk of bias assessment is included in Appendix 1, http://links.lww.com/EJCP/A386. Bias assessment was done independently by two authors and discussed to reach a consensus in case of disagreement.
Fig. 2.
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Results
Search results
As shown in the PRISMA flowchart in Fig. 1, 4200 papers were identified in the search after duplicates were removed. After screening the titles and abstracts, 190 were assessed as potentially eligible, but two reports could not be retrieved. We excluded 25 conference abstracts and 19 protocols. After reading the full text, 129 were excluded and 17 were included in the final review (Russell et al., 2010; Miller et al., 2011, 2018; Taylor et al., 2013; Weinberg et al., 2013; Greiner et al., 2014; Jerant et al., 2014; Fernández et al., 2015; Gathirua-Mwangi et al., 2016, Lee et al., 2017a,b, Valdez et al., 2018; Denizard-Thompson et al., 2020; Gabel et al., 2020; Moscicki et al., 2021; Rawl et al., 2021).
Study characteristics
The characteristics of the studies are shown in Tables 1–4. There was one study from Denmark (Gabel et al., 2020) and one from Hong Kong (So et al., 2022), but the remainder were from the USA. The interventions were targeted to populations with either low socioeconomic status (low household income, high rates of unemployment, not covered by medical insurance) or to specific cultural or ethnic/racial populations, including Latina/Hispanic (Fernández et al., 2015; Valdez et al., 2018; Moscicki et al., 2021), Korean-American (Lee et al., 2017), South Asian adults (So et al., 2022), and African American (Russell et al., 2010; Taylor et al., 2013; Denizard-Thompson et al., 2020; Rawl et al., 2021) populations, or a combination of these factors. The outcomes in the studies included screening completion for cervical cancer (n = 2) (Valdez et al., 2018; Moscicki et al., 2021), prostate cancer (n = 1) (Taylor et al., 2013), breast cancer (n = 4) (Russell et al., 2010; Gathirua-Mwangi et al., 2016, Lee et al., 2017), and colorectal cancer (n = 10) (Miller et al., 2011, 2018; Weinberg et al., 2013; Greiner et al., 2014; Jerant et al., 2014; Fernández et al., 2015; Denizard-Thompson et al., 2020; Gabel et al., 2020; So et al., 2022).
Table 1.
Cervical cancer screening: study characteristics and results of screening completion (n = 2)
| Author (year), country | Population N (intervention group, control group) Age range |
Intervention description | Results: screening completion | |
|---|---|---|---|---|
| Moscicki et al. (2021), USA | Latina 84% Intervention group 1: n = 3455 Control group 1: n = 5768 Intervention group 2: n = 1361 Control group 2: n = 3052 Age: 21–29 |
Intervention group 1: Healthcare provider mobile phone application only (ProvAPP) Intervention group 2: Healthcare provider mobile phone application + patient educational tool. Healthcare mobile phone applications gave information to the clinic regarding cervical cancer screening and the management of abnormal cytology. The patient educational tool accessed on a computer tablet was a patient-centered educational tool used in the waiting room before the clinical visit. Different content depending on whether a woman was coming in for routine care or abnormal screening follow-up. Spanish or English versions. Aimed to assist women in understanding cervical cancer screening guidelines, emphasize the role of human papillomavirus vaccination in preventing cervical cancer, encourage them to ask questions to their providers, and assist in evaluating the risks and benefits of screening intervals and of management choices for abnormal cytology (including details on colposcopic exams). The app included a combination of pictures, words, short videos, and interactive buttons designed to engage participants. Also assessed health literacy. |
Pap test completion and colposcopy Follow-up: 18 months Pap test rate and rate ratio Intervention group 1 : 44% Rate ratio = 1.1, CI = 0.7–1.5, P = 0.77 Intervention group 2 : 52% Rate ratio = 0.8, CI = 0.5–1.2, P = 0.23 Colposcopy rate and rate ratio Intervention group 1 : 0.7% Rate ratio = 0.9, CI = 0.4–2.0, P = 0.77 Intervention group 2 : 0.1% Rate ratio = 0.6, CI = 0.2–1.6, P = 0.23 |
|
| Valdez et al. (2018), USA | Low-income Latinas who had not had a Pap test for the last 2 years Intervention group: n = 480 Control group: n = 463 Age: 21–69 |
Intervention delivered through interactive, multimedia touchscreen kiosks that created an individualized, self-paced learning experience tailored via on-screen prompts to language preference (Spanish/English) and age group (18–24, 25–49, 50–69). Featured age-appropriate behavioral models and multimedia elements – text, voice, music, graphics, animation, and video – to overcome cultural, linguistic, literacy, and attention barriers. 8 interactive modules: What is cervical cancer? How is HPV transmitted? HPV screening and prevention methods. What increases or decreases the risk of developing cervical cancer? What is a Pap test and a Pap test walk-through to demystify the procedure. How to schedule a Pap test and follow up on the results, and what does an abnormal Pap test result mean. Questions for your doctor. What to do if you don’t have insurance or a regular doctor. |
Self-reported cervical cancer screening Follow-up: 6 months Intervention group: 51% vs control group: 48%, P = 0.35 |
|
CI, confidence interval; HPV, human papillomavirus.
Table 4.
Colorectal cancer screening: study characteristics and results of colorectal cancer screening including colonoscopy and fecal occult blood test/fecal immunochemical test (n = 10)
| Author (year), country | Population N (intervention group, control group) Age range |
Intervention group description | Results: screening completion |
|---|---|---|---|
| Denizard-Thompson et al. (2020), USA | African American unemployed or annual income <$20 000, limited health literacy Intervention group: n = 223 Control group: n = 227 Age. 50–74 |
iPad patient decision aid called Mobile Patient Technology for Health-CRC (mPATH-CRC) colorectal cancer. Interventions administered to patients prior to healthcare visit to provide ‘just in time’ info that they could discuss with their provider. 3 primary components: brief decision aid about CRC screening reviewing FOBT and colonoscopy; patient self-ordering of screening tests which triggered the research assistant to enter a co-signature required order under the primary care provider’s name in the ERH; follow-up text or email messages to promote screening test completion. |
Receipt of CRC screening Follow-up: 24 weeks Intervention group: 30% vs control group: 15%, P < 0.001 OR = 2.7, CI = 1.7–4.4, P < 0.001 |
| Gabel et al., 2020, Denmark | Low education Intervention group: n = 830 Control group: n = 849 Age: 53–74 |
Self-administered web-based 16-step decision aid to citizens in the intervention group provided via a link in a separate digital mail, a few days after receiving the standard screening reminder. Decision aid was developed for lower educational attainment citizens. Steps included presented benefits and harms to both options along with basic information about CRC (incidence, mortality, development, symptoms, treatment, etc) and CRC screening (effectiveness, FIT-test, colonoscopy). In each step pop-ups with more details and text were available. Most pop-ups had ‘read more’ functions. By using figures and charts as well as values clarification questions, the decision aid encouraged citizens to consider the information they had just seen or read. On the last page of the decision aid, the citizens were presented with a ‘choice indicator’, summing up the results of the values clarification questions. Further discussion of the choice with family or a doctor was encouraged. | CRC screening uptake with FIT Follow-up:45 days Intervention group: 34.7% vs control group: 27.1%, P < 0.05 |
| Greiner et al. (2014), USA | 42% non-Hispanic African American, 28% non-Hispanic white, 27% Hispanic Intervention group: n = 234 Control group: n = 236 Age 45–70 |
Info and education on CRC screening. Participants responded to ‘implementations intentions’ planning questions specific to CRC screening. Interactive, multimedia touchscreen computer that used either English or Spanish text with narration through headphones, as well as pictures and video targeted to the race/ ethnicity of the participant. | Self-reported CRC screening Follow-up: 26 weeks Intervention group: 54% vs control group: 42% OR = 1.83, CI = 1.23–2.73, P ≤ 0.01 |
| Fernández et al. (2015), USA | Hispanics on the Texas–Mexico border TIMI intervention group: n = 236 SMPI intervention group: n = 236 Control group: n = 204 Age:50–70+ |
Tailored interactive multimedia intervention (TIMI) delivered using tablet computers with touch screen. Tailoring elements based on responses to questions about readiness (stage of change) to be screened, pros and cons, self-efficacy, perceived risk, and subjective norms. Intervention efforts focused on individuals in pre-contemplation (no CRCS and no intention), contemplation (no CRCS, but considering getting screened), and preparation (no CRCS and planning to get screened) stages of change. As participants proceeded through the interactive media, they were presented with various questions related to psychosocial factors. On the basis of responses, they were presented with information to address their particular concern or encourage screening based on current stage of readiness. Small Media intervention (SMPI): flipchart and DVD about CRC and CRCS. |
Any CRCS uptake 6 months TIMI intervention group:10.2% SMPI intervention group: 13.6% Control group: 10.8% Adjusted P = 0.46 No significant difference between groups |
| Jerant et al. (2014), USA | Multi-ethnic 49.3% non-Hispanic 27.2%Hispanic/English 23.4%Hispanic/Spanish Intervention group: n = 595 Control group: n = 569 Age: 50–75 |
Tailored interactive multimedia computer program (IMCP), with a computer algorithm for presenting tailored messages, including a specific CRC screening test recommendation based on expanded health belief model measure response. One module assessed and then provided tailored information to increase knowledge of CRC screening tests, next module aimed to increase knowledge of screening harms and inconveniences. Final module assessed self-efficacy, barriers, readiness, test preference, and screening history, and then provided tailored information to enhance them. The IMCP allowed patients to decide how much information to view. English and Spanish versions were developed. | Record-documented CRC screening over 12 months Intervention group: 23% vs control group: 22% Adjusted difference = 0.5 percentage points, 95% CI, (4.3–5.3). Effects did not differ by ethnicity or language. |
| Miller et al. (2011), USA | African American 74% Mixed literacy, socially disadvantaged population Intervention group: n = 264 Control group: n = 264 Age 50–74 |
A CRC web-based screening decision aid, called CHOICE (Communicating Health Options through Interactive Computer Education, version 6.0 W), based on a previously validated videotape decision aid. Includes a short introductory overview of CRC screening including CRC prevalence, the rationale for screening, and a description of common screening tests. Program is designed to be accessible to low-literacy patients by using easy-to-understand audio segments, video clips, graphics, and animations. Allows participants to choose to learn more about a specific test, view comparisons of the tests, or end the program. |
CRC screening (any) Follow-up: 24 weeks Intervention group: 19% vs control group: 14%, P = 0.25 OR = 1.7, CI = 0.9–3.2, P = 0.12 |
| Miller et al. (2018), USA | 53% Low-income persons (<25 000 dollars) Intervention group: n = 223 Control group: n = 227 Age 50–74 |
mPATH-CRC: an iPad app that displays a CRC screening decision aid, lets patients order their own screening tests, and sends automated follow-up electronic messages to support patients. | CRC screening (any) Follow-up: 24 weeks Intervention group: 30% vs control group: 15% OR = 2.5, CI = 1.6–4.0 accounting for the stratification factor (clinic) |
| Rawl et al. (2021), USA | African American Intervention group: n = 335 Control group: n = 358 Age intervention group: 56.8 Age control group: 57.8 |
A computer-tailored intervention assessing a participant’s perceived risk, benefits and barriers to CRC screening, age, sex, and family history in real time followed by tailored messages to support development of beliefs that would be most aligned with a decision to screen for CRC. The intervention was based on the Health Belief and Transtheoretical Models. Program was fully narrated to accommodate people with low literacy. Example of tailored message: if a person had a close family member with CRC a message would be delivered to inform them that they also have an increased risk of CRC. | Screening completion with stool blood test or colonoscopy Follow-up: 6 months Stool blood test Intervention group: 12.5% vs control group 7.3%, P = 0.02 OR = 1.8, CI = 1.1–3.0, P = 0.018 Colonoscopy Intervention group: 18.5% vs control group 14.0%, P = 0.14 OR = 1.4, CI = 0.9–2.2, P = 0.14 Any test Intervention group: 12.5% vs control group: 7.3%, P = 0.02 OR = 1.6, CI = 1.1–2.4, P = 0.02 |
| So et al. (2022), Hong Kong | South Asian older adults and their younger families 320 dyads Intervention group: n = 160 Control group: n = 160 Age range: 56–75 |
A multimedia health talk, conveying the importance CRC screening and support from younger family members in encouraging their older relatives to undergo screening. Instructor-led health talk with powerpoint presentation + health info booklets (Urdu, Nepali, and Punjabi language) + video clip. Materials culturally and linguistically relevant. Topics: general info about CRC; risk factors, signs, and symptoms; common myths and misconceptions on CRC and screening; CRC prevention and early detection; FIT procedure; Hong Kong CRC screening programs and providers; procedures to follow if screening returned a positive result. Site coordinators assisted participants in accessing FIT. Dyads included one older adult and one younger family member. Control group: waitlist control group. |
CRC screening (FIT) Intervention group: 71.8% vs control group: 6.8%, P ≤ 0.01 While all control participants returned the sample through their younger family members, a significant proportion (62.2%) of the intervention participants returned the sample by themselves. |
| Weinberg et al. (2013), USA | Only women, non-adherent to screening Non-white 3.4% Only high school education or less = 34.7% 5 arm study Intervention group 1: n = 170 (high attentional); n = 172 (low attentional) Intervention group 2: n = 174 (high attentional); n = 173 (low attentional) Control group: n = 171 Age 50–94 |
Intervention group 1: visually appealing webpage and either high- or low-attentional-style information Intervention group 2: print interventions and either high- or low-attentional-style information The educational content was identical: essential info about CRC screening, rationale, description of different screening techniques, benefits, risk and timing, and info on additional resources. High monitoring version was lengthier and contained extensively detailed messages pertaining to CRC risk status. Screening descriptions were more substantial and the benefits of adherence to preventive behaviors emphasized. All messages were positively framed to underline the potential for gain. Low monitoring was briefer, less detailed, and included messages that were negatively framed to highlight the costs to health if recommended behaviors were not pursued Participants were identified as having high or low monitoring attentional styles from their baseline Cognitive Social Information Processing (C-SHIP) score. |
CRC screening (FOBT, colonoscopy, Barium enema) Follow-up: 4 and 12 months 4 months Intervention group 1 : 12.2% Intervention group 2 : 12.0% Control group 12.9% P = 0.95 4 months Intervention group 1 : 18.6% vs Intervention group 2 : 22.4% Control group: 23.4% P = 0.32 No difference in the high or low-attentional-styles |
CI, confidence interval; COL/FS, colonoscopy or flexible sigmoidoscopy; CRCS, colorectal cancer screening; FIT, fecal immunochemical test; FOBT, fecal occult blood test; ERH, electronic health records; OR, odds ratio: RR, relative risk; SBT, stool blood test; SMPI, small media intervention; TIMI, tailored interactive multimedia intervention.
Table 2.
Prostate cancer screening: study characteristics and results of screening completion (n = 1)
| Author (year), country | Population N (intervention group, control group) Age range |
Intervention description | Results: screening completion |
|---|---|---|---|
| Taylor et al. (2013), USA | African American 39.9% Intervention group 1: n = 628 Intervention group 2: n = 625 Control group: n = 626 Age: 40–75 |
Intervention group 1: print-based decision aid Intervention group 2: web-based interactive decision aid Both include introductory material about the prostate gland; description of screening tests and possible results; information about treatment options, risks, and adverse effects; review of risk factors, encouragement to discuss screening with a physician; a 10-item values clarification tool; and resources for more info. Intervention group 2, web-based also included a voice-over that presents most of the text, pop-up definitions of 77 terms, 8 video testimonials, an interactive values clarification tool, and figures, animation, and graphics. |
Self-reported screening Follow-up: 13 months Direct rectal examination Intervention group 1 : 46.8% vs control group 45.2%, P = 0.65 Intervention group 2 : 47.9% vs control group 45.2%, P = 0.74 PSA Intervention group 1 : 45.3% vs control group 44.7%, P = 0.77 Intervention group 2 : 47.6% vs control group 44.7%, P = 0.50 |
PSA, prostate-specific antigen testing.
Intervention types
A summary of the interventions is provided in Table 1. The types of interventions differed but most used interactive and tailored computer, smartphone, or tablet-based touch-screen programs. Many used belief models to design educational modules and most used a combination of materials aimed to provide participants with information about the importance of screening, screening methods, and risk of disease to increase knowledge. Some were culturally tailored with narrators of the same ethnicity as well as different language options. Many were designed to engage the user with a mixture of text, pictures, videos, and narration. A few had elements targeted both to the individual and system level, to overcome additional barriers within healthcare systems. For example, Denizard-Thompson et al. (2020) combined their iPad patient decision aid with options for the participant to self-order screening tests, which triggered the research assistant to enter a “co-signature required” order under the primary care provider’s name in the electronic health records. Other systems utilized follow-up messages to remind participants to complete screening (Miller et al., 2018; Denizard-Thompson et al., 2020). Computer algorithms were used for presenting tailored messages in numerous interventions (Russell et al., 2010; Rawl et al., 2012; Jerant et al., 2014), for example, if a person had a close family member with colorectal cancer a message would be delivered to inform them that they also have an increased risk of the disease (Rawl et al., 2012). Miller et al.’s (2011) web-based decision aid allowed participants to choose to learn more about a specific test of their choice and view comparisons of the tests. Many had specific designs aimed to increase usability in people with low literacy or low health literacy; for example, Miller et al.’s, (2011) web-based screening decision aid used easy-to-understand audio segments, video clips, graphics, and animations while Rawl et al.’s (2021) tailored computer program was fully narrated to accommodate people with low literacy. Some used emotive aspects, such as video messages from people sharing their personal experiences (Lee et al., 2017). Rewards and incentives were also used, for example, in a breast cancer intervention, (Lee et al., 2017) participants could earn and collect digital pink ribbons when responding to questions.
Fig. 3.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Cervical cancer screening
As shown in Table 1, two studies examined interventions to increase screening for cervical cancer (Valdez et al., 2018; Moscicki et al., 2021), both from the USA and predominantly with Latina women. Neither found significantly higher screening in the intervention group versus controls. Moscicki et al. (2021) used an intervention that targeted both the system (healthcare provider clinics were given a mobile phone application with information regarding cervical cancer screening and management of abnormal cytology) and the patient (tablet-based educational tool), but they concluded that providing both clinicians and patients with information on guidelines had no significant effect on 18-month Pap and colposcopy rates.
Prostate cancer screening
Only one study from the USA (Taylor et al., 2013) was found on prostate screening, which reported that a web-based interactive decision aid did not increase screening compared to usual care.
Breast cancer screening
There were four studies on breast cancer screening (Russell et al., 2010; Gathirua-Mwangi et al., 2016, Lee et al., 2017), all from the USA (Table 3). Two were from the same study group and targeted Korean-American women (Lee et al., 2017). First, Lee et al. (2017) found no significant effect of a web-based intervention on mammography completion, although the sample size was small. In another study, Lee et al. (2017) reported a significant effect on mammography completion with a culturally and personally tailored multilevel intervention with multimedia messages over 1 week through a mobile phone app along with a health navigator. They utilized reward and incentive systems, for example, for each response to a question or a prompt, participants could earn a digital pink ribbon and collect these ribbons throughout the intervention period. They also included culturally tailored and emotive aspects, for example, video messages featured Korean-American women who shared their personal experiences with mammogram screening, including how they have handled issues related to their cultural beliefs. Another study using a tailored, narrative DVD specifically found an effect of the intervention on women only with low incomes (≤$30 000) (Gathirua-Mwangi et al., 2016). This latter study was particularly interesting because it compared two intervention types with usual care, and the other intervention (a computer-tailored telephone counseling service) did not increase mammography completion after 6 months, despite having a much higher cost than the DVD intervention (cost $22.50/person vs $6.84/person). Russell et al. (2010) (USA) found a significant effect of a combined intervention of interactive, tailored computer instruction at baseline and 4-monthly lay health advisor counseling sessions on mammography screening over 6 months; however, as they combined digital and lay health advisors it is not possible to establish which of the two components had the largest effect on screening behaviors.
Table 3.
Breast cancer screening: study characteristics and results of mammography completion (n = 3)
| Author (year), country | Population N (intervention group, control group) Age range |
Intervention description | Results: screening completion |
|---|---|---|---|
| Lee et al. (2017a), USA | Korean-American women and their spouses Intervention group: n = 57 Control group: n = 79 Age 40–79 |
Adapted a previous intervention to deliver it online. The original program consisted of (a) showing a project-team designed 30-min Korean-language film (in DVD format) to participants. This was split into 2 and uploaded to view online; (b) holding a brief group discussion session immediately after the film showing. This was replaced by posting the PowerPoint summary of the study for participants to view online after watching the DVD; (c) requiring each couple to complete a discussion activity together at home to enhance spousal support for the women within 24 h of watching the DVD. This was adapted by asking couples to complete a homework activity and then calling the study phone number to leave a message about what they discussed as proof of completing the homework activity. |
Mammogram completion Follow-up: 2 months Intervention group: 22% vs control group: 13%, P = 0.36 |
| Lee et al. (2017b), USA | Korean-American women Intervention group: n = 60 Control group: n = 60 Age 40–79 |
Culturally and personally tailored multilevel and multimedia messages over 1 week through a mobile phone app along with a health navigator. System had 5 components: web-based application to enroll participants, set user preferences, display area clinic information on GPS, and upload text and multimedia messages; a database to store participant records, rules, and messages sent and received; a program to establish the appropriate timing of messages, determine which messages to send, and process received replies; a text-message delivery or reception platform; and a health navigator for assistance navigating cancer screening information, addressing technical problems, and providing transportation and interpretation services. | Mammogram completion Follow-up: 6 months Intervention group: 75% vs control group: 30%, P < 0.001 |
| Russell et al. (2010), USA | Low-income African American women Intervention group: n = 89 Control group: n = 90 Age: 41–75 |
Interactive, tailored computer instruction at baseline and 4-monthly lay health advisor counseling sessions. Algorithm of tailored messages guided by behavior and belief models to identify the fear and fatalistic views of breast cancer to assess health beliefs, self-efficacy, barriers to screening, and stage of readiness for mammography. Incorporated African American narrators and storytellers and a video demonstration of the mammography screening procedure. | Medical records mammography completion Follow-up: 6 months Intervention group: 51% vs control group: 18%, P < 0.05 RR = 2.7, CI = 1.8–3.7, P < 0.0001 Adjusted for employment, disability, relative with breast cancer, health insurance, previous biopsies |
BCT, behavioral construct tailoring; CI, confidence interval; CRT, culturally relevant tailoring; OR, odds ratio; RR, relative risk.
Colorectal cancer screening
Ten studies examined colorectal cancer screening interventions (Table 4). All were from the USA except one study from Denmark and one from Hong Kong (Gabel et al, 2020; So et al, 2022). Results differed, with six studies reporting significant results and four finding no significant difference in the intervention versus the control for colorectal cancer screening. Two papers from the same RCT (Miller et al., 2018; Denizard-Thompson et al., 2020) reported higher colorectal cancer screening uptake in participants randomized to an iPad patient decision aid called Mobile Patient Technology for Health (mPATH-CRC)-colorectal cancer, compared to controls in an African American population with low income and limited health literacy. Miller et al. (2018) reported that the mPATH-CRC digital health program doubled the proportion of patients who completed colorectal cancer screening, primarily due to an increase in screening test orders. Interestingly, they also examined reasons why participants who had ordered screening tests did not complete them; the most cited reasons for failing to complete a fecal screening test were losing or not having the kit (20%), not wanting to complete the kit or finding it embarrassing/distasteful (13%), forgetting to do the kit (11%), and encountering difficulty adhering to required dietary or medication restrictions (11%). Of note, the intervention success may rely on the fact that it targets both patient-level mediators as well as system changes that target structural barriers; the authors highlighted that 76% of participants discussed screening with their healthcare provider, although they directly ordered screening themselves, which reduced time barriers and empowered patients in managing their own care. Miller et al. (2011) found that a web-based decision aid (Communicating Health Options through Interactive Computer Education) increased patients’ abilities to state a test preference and their readiness to receive screening regardless of literacy level because the tool was designed with easy-to-understand content aimed at a mixed-literacy audience; however, they did not observe a significant increase in screening but did find modest differences in that participants were more likely to order colorectal cancer screening tests immediately. The only study from Europe (Gabel et al., 2020) found that a web-based decision aid was effective at increasing screening uptake compared to usual care in Denmark. Greiner et al. (2014) also reported significant results from their interactive, multimedia touchscreen computer-intervention among a mixed-race population. The program included both English or Spanish text, as well as pictures and videos targeted to the race/ethnicity of the participant.
Although Fernández et al. (2015) (USA), did not find any significant differences between their complex tailored interactive multimedia intervention, a small media intervention, and no intervention on screening uptake, they hypothesized that it may have been due to the limited technology experience of the lay health workers who delivered the intervention. Jerant et al. (2014) also found no significant results on colorectal cancer screening with their interactive multimedia computer program, which had a computer algorithm for presenting tailored messages to a multi-ethnic population; however, of note, their intervention included sociopsychological tailoring but did not include any cultural tailoring, despite not having a multi-ethnic sample.
So et al.’s (2022) study in Hong Kong is noteworthy because it targeted dyads of older South Asian adults and younger family members and sought to tackle the importance of screening by involving the family members in the intervention. The proportion of older adults participating in fecal immunohistochemical testing was substantially and significantly higher among intervention dyads compared with controls.
In a study on African Americans, Rawl et al.’s (2021) computer-tailored intervention found significant associations for completion of any colon test or stool blood test but not for colonoscopy (although they observed a clinically important effect it did not reach statistical significance). Interestingly, they also found mediation effects; changes in scores measuring colorectal cancer knowledge, colonoscopy benefits, stool blood test barriers, and patient-provider discussion of stool blood tests all mediated the effect of the intervention. Finally, Weinberg et al. (2013) found no effect of a web-based intervention on 4- or 12-month screening compared to print educational content or usual screening care; however, it is noteworthy that only 24.4% of the women randomized to the web-based intervention logged onto the website and only 16.4% reported using the web intervention.
Risk of bias of the included studies
Random sequence generation (selection bias): 10 studies had a low risk of bias. The authors described in detail a random component of the sequence-generation process. Six studies were assessed as having an unclear risk of bias, as no information about the randomization process was provided (i.e. lack of random sequence generation).
Allocation concealment (selection bias): four studies were judged at low risk of bias, as the allocation methods used were appropriate. Eleven studies were assessed with an unclear risk of bias as they contained no information about allocation concealment procedures. One study had a high risk of bias because investigators enrolling participants could possibly foresee assignments.
Blinding of participants and personnel (performance bias): in nine studies it was unlikely that the blinding could have been broken, so the risk of bias was judged as low. Four studies were assessed with a high risk, due to lack of blinding or incomplete blinding. Three studies were judged with an unclear risk, due to a lack of information about the blinding of participants and providers.
Blinding of outcome assessment (detection bias): in eight studies the outcome assessment was blinded, so the risk of bias was judged as low. Four studies were assessed with a high risk, as the outcome assessment was not blinded. Four studies were judged with an unclear risk, due to a lack of information about the blinding of outcome assessor.
Incomplete outcome data (attrition bias): 10 studies were assessed with a low risk of bias because no missing data were found, or the purpose of participants’ exclusion was properly argued. Three studies had a high risk of bias related to the high number of drop-outs. Three studies were judged with an unclear risk, due to a lack of information about the reason for missing outcomes data.
Selective reporting (reporting bias): 10 studies were judged with a low risk of bias because the study protocol was registered with the study’s pre-specified outcomes. The study protocol was not available for six studies; thus they were judged with a high risk.
Discussion
Main findings
This systematic review identified 17 studies that assessed interactive or tailored web-based, computer, or digital interventions to increase cancer screening and reduce health inequity. Results were heterogeneous as about half of the studies reported higher screening uptake in participants randomized to the interventions compared to control conditions, but this depended on the type of cancer; most of the studies on cervical and prostate cancer screening did not find significant intervention effects. Some studies found differences in intervention effects according to the individual characteristics of the participants, which may suggest that individual tailoring is needed, though more research is needed to confirm this. Our systematic search identified a lack of literature from worldwide settings, with all but two studies conducted in the USA, although we only included articles in English. Interventions that use individual and cultural tailoring of cancer screening educational material seem to be effective and need further development as well as adaptation to settings outside of the USA.
Differences according to patient characteristics and screening modalities
Some studies reported different success rates in improving cancer screening depending on the characteristics of the participants. A tailored, interactive, multimedia intervention improved mammography completion only in women with low household incomes (Gathirua-Mwangi et al., 2016). Most studies focused on colorectal cancer, and these interventions often differed from those for breast or cervical cancer, mainly because they sometimes included additional self-administered screening options (FIT/fecal occult blood test) that can be done at home and mailed to the healthcare provider. Thus, the interventions could include alerts, reminders, and components that could increase uptake by making testing easier and more accessible by mailing tests directly to participants. Such interventions may be of interest in the future for cervical cancer due to the increasing use of human papillomavirus self-sampling test kits, which may increase cervical cancer screening uptake compared with usual care; a meta-analysis reported a 2.1 (relative risk) greater screening uptake among human papillomavirus self-sampling participants compared with controls (Yeh et al., 2019). As conventional cervical screening relies on clinician-collected samples, the use of human papillomavirus self-sampling tests may be a relevant alternative that could overcome some of the barriers relevant to health inequity, relevant to health inequity barriers, such as emotional (e.g. embarrassment, fear) and practical (e.g. difficulties making appointments or lack of financial resources to get to clinics) barriers to cytological screening. As health beliefs about cervical cancer and barriers to screening differ according to ethnicity in immigrants (Johnson et al., 2008), human papillomavirus self-sampling may potentially be a relevant method of addressing a wide range of barriers affecting different populations, thus reducing health inequities.
Usability and design
Digital literacy and usability are important aspects to consider with multimedia interventions. Miller et al. (2017) reported that their mPATH-CRC iPad application had high usability (>90%) but this was lower for vulnerable participants (90.8%) compared to nonvulnerable participants (96.6%, P = 0.006). Only 6.9% of participants needed assistance in completing the program, and this was associated with older age, less use of text messaging, and lack of Internet use. It is noteworthy that mPATH-CRC was piloted and specifically created with features designed to reduce literacy barriers and enhance usability, (Miller et al., 2017) for example, each screen displays a single question with a large intuitive response button and a narrator reads each question and the answer that the user selects, etc. Another study investigated the feasibility of using computer-assisted instruction in patients of varying health literacy levels as well as their preferences (Duren-Winfield et al., 2015). Having health insurance and experience in using computers predicted the ability to complete the programs without assistance. Further, although patients with limited health literacy had less computer experience, the majority completed the programs without any assistance and felt that they learned more via the computer than they would have from a brochure. Mode of delivery can also help increase usability in certain types of individuals, for example, research suggests that touch-screen devices are better than computer mouses for older individuals because they can complete tasks more quickly and with fewer errors (Orphanides and Nam, 2017). Training of the healthcare workers or lay health advisors delivering the interventions is also of relevance. Fernández et al. (2015), for example, suggested that the low impact of their complex tailored interactive multimedia intervention on screening uptake was partially due to a lack of experience with technology of the lay health workers utilized in their study. This highlights the importance of designing suitable interventions that can be used both by participants and the people delivering them, and to provide appropriate training for healthcare workers and lay health advisors. Miller et al. (2011) (USA), specifically designed a web-based decision aid for a mixed-literacy audience, and by using easy-to-understand audio segments, video clips, graphics, and animations they reported that it was effective in informing and motivating both low- and adequate-literacy patients.
Advantages of interactive digital interventions
There are several advantages to delivering interventions via interactive digital formats, especially with regard to health inequity. First, delivery can be modified according to the characteristics of the individual, for example, in different languages or with different materials according to age or health literacy levels. Second, they can be personalized to the individual, through a preliminary evaluation of baseline health knowledge and beliefs. For example, Jerant et al. (2014) used a multimodule interactive multimedia computer program that assessed knowledge of colorectal cancer screening tests, screening harms and inconveniences, self-efficacy, barriers, readiness, test preference, and screening history, and subsequently provided tailored information to enhance them. From a research perspective, such tools can also be used to assess changes in users´ perceptions and beliefs and can identify factors associated with increased screening completion. Further, one of the useful design components of digital interventions is their ability to also address healthcare and system barriers, for example, by enabling participants to self-order screening and linking these decisions with the healthcare provider, as in Miller et al.’s (2018) mPATH system; however, it is important to note that these types of interventions may lead to further inequities because, for example, certain individuals may have less access to digital tools or have lower digital literacy than others. It is also worth considering how digital interventions can be designed without reducing the need for shared decision-making between patients and healthcare professionals (HCPs), and whether such interventions can specifically include elements to increase patient-HCP communication to encourage shared decision-making. A meta-analysis of people with an average risk for colorectal cancer demonstrated that digital interventions were more likely to promote colorectal cancer screening uptake than usual care, with an odds ratio of 1.13 (95% confidence interval, 1.1–1.6), especially with interventions that included interactive web- or tablet-based decision-making aids (Lau et al., 2022).
Methodological strengths and weaknesses
The major strength of the present study is the systematic, comprehensive literature search that, together with the careful study selection and quality assessment, provides a good overview of the evidence in this field. Further, all studies used a randomized control design; however, several limitations should be discussed. There was a lack of studies in the literature on different areas of health equity. as there was a lack of evidence in other groups, for example, cervical screening is often low in incarcerated women (Kelly et al., 2018) as well as LGBTQ+ persons (Ceres et al., 2018). All but one of the studies identified in our search were conducted in the USA. A scoping review in Europe reported that lack of information, lack of female healthcare providers, poor language skills, and emotional responses to the test (especially fear, embarrassment, and discomfort) were the most reported barriers to cervical cancer screening among migrants in EU/European Free Trade Association countries (Marques et al., 2020); however, it should be noted that we included only English-language articles and there may be some papers published in other languages they may be of interest. A further limitation is that there are problems in differentiating between health inequity and inequality. The terms are often confused but they are not interchangeable (globalhealtheurope.org, 2009); Global Health Europe describes inequity as ‘unfair, avoidable differences arising from poor governance, corruption or cultural exclusion’ while inequality refers to ‘the uneven distribution of health or health resources as a result of genetic or other factors or the lack of resources’. In the current systematic review, we acknowledge that we cannot distinguish between the two in terms of whether interventions were specifically designed to address inequity rather than equality. To ensure that we did not miss any publications of relevance, we included keywords covering both equity and equality in the search terms.
Relevance of the study and policy implications
It is important to find methods to increase cancer screening among groups with high health inequity and the results of the current review suggest that certain components such as cultural and individual tailoring should be incorporated in the development of future digital interventions. Health disparities in cancer screening may become even more relevant due to the coronavirus disease 2019 (COVID-19) pandemic and associated disruptions in routine medical services such as screening programs. Worldwide pooled incidence rate ratios (IRRs) were significantly lower for screening during the COVID-19 pandemic for breast cancer (IRR = 0.63), colon cancer (IRR = 0.11), and cervical cancer (IRR = 0.10) (Mayo et al., 2021). A systematic review reported a lower likelihood of returning for breast cancer screening after COVID-19-related closures were independently associated with residence in a higher poverty area, lack of health insurance, need for an interpreter, and longer travel time (Miller et al., 2021). Thus, the potential impact of COVID-19 to further increase health inequity is a relevant concern, and interventions to address these disparities are needed. It has been suggested that urgent policy interventions are needed to handle the backlog of routine cancer diagnostic services to reduce the negative effects of the COVID-19 pandemic (Alkatout et al., 2021). Digital interventions may help this as have the advantage that they can be implemented remotely, thus providing relevant alternatives for periods when public health restrictions are tightened and routine medical services are disrupted.
Future research
Future research should focus on identifying effective intervention strategies within European settings and other countries worldwide to assess whether there are specific differences in screening barriers and uptake between countries, including a more diverse range of health inequity groups, for example, LGBTQ+ persons. A recent meta-analysis of digital interventions aiming to promote colorectal cancer screening uptake in people of average risk also noted that current evidence is based only on studies from Western settings and that more work was needed to verify if the same outcomes can be achieved in other populations, especially within Asia (Lau et al., 2022). More research is needed to identify how such interventions can increase screening uptake and through which mechanisms. For example, Rawl et al. (2021) identified that changes in scores measuring colorectal cancer knowledge, colonoscopy benefits, stool blood test barriers, and patient-provider discussion of stool blood tests all mediated the effect of their intervention on colorectal cancer screening uptake. Researchers should design studies that can identify the changes in knowledge that are associated with the potential increase in screening uptake that will help to develop future interventions with the most effective components. Also, although some interventions were effective at increasing screening orders, there are still numerous reasons why individuals do not actually complete screening. For example, some participants in Miller et al.’s (2018) (USA), the mPATH-CRC digital health program ordered colorectal cancer fecal screening tests but did not complete them due to reasons such as losing the kit, finding it embarrassing/distasteful, or having difficulty adhering to the required dietary or medication restrictions. We need additional studies such as this to identify a larger range of barriers to screening. In addition, it is imperative to assess the uptake of such interventions outside of a controlled study environment; Weinberg et al. (2013) reported that only a quarter of women randomized to a web-based colorectal cancer intervention actually logged onto the website and only 16.4% recalled and then reported using the web intervention. Thus, future trials need to include measures of usability, perhaps with quantitative techniques to better understand how often participants use the digital tools, reasons why they do not, and ways to improve the user experience to increase uptake.
Conclusion
In conclusion, interactive and tailored computer and web-based interventions to increase screening for breast, cervical, and colorectal cancer among populations with low screening uptake, including low-income groups and specific ethnic minorities, show some positive effects, though the results are mixed. Interventions that use individual and cultural tailoring of cancer screening educational material should be further developed and investigated outside of the USA to identify the most effective ones for use in other settings. In light of the COVID-19 pandemic, designing effective digital intervention strategies, with components that can be adapted to remote delivery may be an important strategy for reducing health inequities in cancer screening. A systematic and comprehensive approach to the identification, design, implementation, and assessment of the impact of cancer screening interventions is a key pillar to mitigate inequity in the way oncological diseases are detected. Strategies should be tailored around patients’ characteristics. This systematic review also highlights the importance of embedding cancer screenings into healthcare policies to ensure that individuals are not discriminated against based on their race, ethnicity, cultural heritage, socioeconomic features, or sex; in order to achieve this, policymakers and healthcare professionals should define a specific framework that takes into account the different dimensions of oncological patients.
Acknowledgements
The writing of this paper was commercially funded by Viatris in the form of payment for professional scientific writing services to Oliba S.r.l., Rome, Italy. No products or services of Viatris have been discussed or promoted within this manuscript.
Conflicts of interest
A.R.-P., S.D., S.W., and J.v.V. are employees of Viatris. For the remaining authors, there are no conflicts of interest.
Supplementary Material
References
- Ahmed NU, Haber G, Semenya KA, Hargreaves MK. (2010). Randomized controlled trial of mammography intervention in insured very low-income women. Cancer Epidemiol Biomark Prev 19:1790–1798. [DOI] [PubMed] [Google Scholar]
- Alkatout I, Biebl M, Momenimovahed Z, Giovannucci E, Hadavandsiri F, Salehiniya H, et al. (2021). Has COVID-19 affected cancer screening programs? A systematic review. Front Oncol 11:675038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and prevention (CDC) (2022a). How to Prevent Cancer or Find It Early: Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening.htm. [Accessed 24 October 2022].
- Centers for Disease Control and Prevention (2022b). U.S. Cancer Statistics Colorectal Cancer Stat Bite. US Department of Health and Human Services. https://www.cdc.gov/cancer/uscs/about/stat-bites/stat-bite-colorectal.htm. [Accessed 24 October 2022]. [Google Scholar]
- Ceres M, Quinn GP, Loscalzo M, Rice D. (2018). Cancer screening considerations and cancer screening uptake for lesbian, gay, bisexual, and transgender persons. Semin Oncol Nurs 34:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consedine NS, Tuck NL, Ragin CR, Spencer BA. (2015). Beyond the black box: a systematic review of breast, prostate, colorectal, and cervical screening among native and immigrant African-descent Caribbean populations. J Immigr Minor Health 17:905–924. [DOI] [PubMed] [Google Scholar]
- Coronado GD, Beresford SAA, McLerran D, Jimenez R, Patrick DL, Ornelas I, et al. (2016). Multilevel intervention raises Latina participation in mammography screening: findings from (sic)Fortaleza Latina!. Cancer Epidemiol Biomarkers Prev 25:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuaresma CF, Sy AU, Nguyen TT, Ho RCS, Gildengorin GL, Tsoh JY, et al. (2018). Results of a lay health education intervention to increase colorectal cancer screening among Filipino Americans: a cluster randomized controlled trial. Cancer 124:1535–1542. [DOI] [PubMed] [Google Scholar]
- Denizard-Thompson NM, Miller DP, Snavely AC, Spangler JG, Case LD, Weaver KE. (2020). Effect of a digital health intervention on decreasing barriers and increasing facilitators for colorectal cancer screening in vulnerable patients. Cancer Epidemiol Biomarkers Prev 29:1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duren-Winfield V, Onsomu EO, Case DL, Pignone M, Miller D., Jr. (2015). Health literacy and computer-assisted instruction: usability and patient preference. J Health Commun 20:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández ME, Savas LS, Carmack CC, Chan W, Lairson DR, Byrd TL, et al. (2015). A randomized controlled trial of two interventions to increase colorectal cancer screening among Hispanics on the Texas-Mexico border. Cancer Causes Control 26:1–10. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick-Lewis D, Ali MU, Warren R, Kenny M, Sherifali D, Raina P. (2016). Screening for colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer 15:298–313. [DOI] [PubMed] [Google Scholar]
- Fritzell K, Stake Nilsson K, Jervaeus A, Hultcrantz R, Wengström Y. (2017). The importance of people’s values and preferences for colorectal cancer screening participation. Eur J Public Health 27:1079–1084. [DOI] [PubMed] [Google Scholar]
- Gabel P, Edwards A, Kirkegaard P, Larsen MB, Andersen B. (2020). The LEAD trial-the effectiveness of a decision aid on decision making among citizens with lower educational attainment who have not participated in FIT-based colorectal cancer screening in Denmark: a randomised controlled trial. Patient Educ Couns 103:359–368. [DOI] [PubMed] [Google Scholar]
- Gathirua-Mwangi WG, Monahan PO, Stump T, Rawl SM, Skinner CS, Champion VL. (2016). Mammography adherence in African-American women: results of a randomized controlled trial. Ann Behav Med 50:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Health Europe (2009). Inequity and inequality in health. https://globalhealtheurope.org/values/inequity-and-inequality-in-health/. [Accessed 24 October 2022].
- Greiner KA, Daley CM, Epp A, James A, Yeh HW, Geana M, et al. (2014). Implementation intentions and colorectal screening: a randomized trial in safety-net clinics. Am J Prev Med 47:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al.; Cochrane Bias Methods Group (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne HN, Phelan DF, Pollack CE, Markakis D, Wenzel J, Ahmed S, et al. (2015). Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control 26:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Health Union: A new EU approach on cancer detection – screening more and screening better. (2022). https://ec.europa.eu/commission/presscorner/detail/en/ip_22_5562. [Accessed 24 October 2022].
- Jerant A, Kravitz RL, Sohler N, Fiscella K, Romero RL, Parnes B, et al. (2014). Sociopsychological tailoring to address colorectal cancer screening disparities: a randomized controlled trial. Ann Fam Med 12:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CE, Mues KE, Mayne SL, Kiblawi AN. (2008). Cervical cancer screening among immigrants and ethnic minorities: a systematic review using the Health Belief Model. J Low Genit Tract Dis 12:232–241. [DOI] [PubMed] [Google Scholar]
- Jun J, Nan X. (2018). Determinants of cancer screening disparities among Asian Americans: a systematic review of public health surveys. J Cancer Educ 33:757–768. [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Allison M, Ramaswamy M. (2018). Cervical cancer screening among incarcerated women. PLoS One 13:e0199220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter MW, Holmes K, Alcaraz K, Kalesan B, Rath S, Richert M, et al. (2010). Comparing narrative and informational videos to increase mammography in low-income African American women. Patient Educ Couns 81:S6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy R, Pesola F, Castañón A, Sasieni P. (2016). Impact of cervical screening on cervical cancer mortality: estimation using stage-specific results from a nested case-control study. Br J Cancer 115:1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J, Lim TZ, Jianlin Wong G, Tan KK. (2020). The health belief model and colorectal cancer screening in the general population: a systematic review. Prev Med Rep 20:101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J, Ng A, Wong GJ, Siew KY, Tan JKH, Pang Y, et al. (2022). How effective are digital technology-based interventions at promoting colorectal cancer screening uptake in average-risk populations? A systematic review and meta-analysis of randomized controlled trials. Prev Med 164:107343. . [DOI] [PubMed] [Google Scholar]
- Lee EE, Brecht ML, Park H, Lee J, Oh KM. (2017a). Web-based study for improving mammography among Korean American Women. J Cancer Educ 32:257–263. [DOI] [PubMed] [Google Scholar]
- Lee H, Ghebre R, Le C, Jang YJ, Sharratt M, Yee D. (2017b). Mobile phone multilevel and multimedia messaging intervention for breast cancer screening: pilot randomized controlled trial. JMIR Mhealth Uhealth 5:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques P, Nunes M, Antunes MDL, Heleno B, Dias S. (2020). Factors associated with cervical cancer screening participation among migrant women in Europe: a scoping review. Int J Equity Health 19:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo M, Potugari B, Bzeih R, Scheidel C, Carrera C, Shellenberger RA. (2021). Cancer screening during the COVID-19 pandemic: a systematic review and meta-analysis. Mayo Clin Proc Innov Qual Outcomes 5:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SJ, Pepe RS, Gabler NB, Kanneganti M, Reitz C, Saia C, et al. (2019). Effect of financial incentives on patient use of mailed colorectal cancer screening tests: a randomized clinical trial. JAMA Netw Open 2:e191156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SJ, Oyalowo A, Reitz C, Dean O, McAuliffe T, Asch DA, et al. (2020). Text messaging and lottery incentive to improve colorectal cancer screening outreach at a Community Health Center: a randomized controlled trial. Prev Med Rep 19:101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SJ, Reitz C, Niewood T, Volpp KG, Asch DA. (2021). Effect of behavioral economic incentives for colorectal cancer screening in a randomized trial. Clin Gastroenterol Hepatol 19:1635–1641.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DP, Jr, Spangler JG, Case LD, Goff DC, Jr, Singh S, Pignone MP. (2011). Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population. Am J Prev Med 40:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DP, Jr, Weaver KE, Case LD, Babcock D, Lawler D, Denizard-Thompson N, et al. (2017). Usability of a novel mobile health iPad app by vulnerable populations. JMIR Mhealth Uhealth 5:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DP, Jr, Denizard-Thompson N, Weaver KE, Case LD, Troyer JL, Spangler JG, et al. (2018). Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med 168:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, Meneveau MO, Rochman CM, Schroen AT, Lattimore CM, Gaspard PA, et al. (2021). Impact of the COVID-19 pandemic on breast cancer screening volumes and patient screening behaviors. Breast Cancer Res Treat 189:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicki AB, Chang C, Vangala S, Zhou X, Elashoff DA, Dehlendorf C, et al. (2021). Effect of 2 interventions on cervical cancer screening guideline adherence. Am J Prev Med 60:666–673. [DOI] [PubMed] [Google Scholar]
- Murphy ST, Frank LB, Chatterjee JS, Moran MB, Zhao N, Amezola de Herrera P, et al. (2015). Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health 105:2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. (2016). Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. preventive services task force recommendation. Ann Intern Med 164:244–255. [DOI] [PubMed] [Google Scholar]
- Orphanides AK, Nam CS. (2017). Touchscreen interfaces in context: a systematic review of research into touchscreens across settings, populations, and implementations. Appl Ergon 61:116–143. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawl SM, Skinner CS, Perkins SM, Springston J, Wang HL, Russell KM, et al. (2012). Computer-delivered tailored intervention improves colon cancer screening knowledge and health beliefs of African-Americans. Health Educ Res 27:868–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawl SM, Christy SM, Perkins SM, Tong Y, Krier C, Wang HL, et al. (2021). Computer-tailored intervention increases colorectal cancer screening among low-income African Americans in primary care: results of a randomized trial. Prev Med 145:106449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CR, Mitchell JA, Franta GJ, Foster MJ, Shires D. (2017). Masculinity, racism, social support, and colorectal cancer screening uptake among African American men: a systematic review. Am J Mens Health 11:1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell KM, Champion VL, Monahan PO, Millon-Underwood S, Zhao Q, Spacey N, et al. (2010). Randomized trial of a lay health advisor and computer intervention to increase mammography screening in African American women. Cancer Epidemiol Biomarkers Prev 19:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler GR, Beerman PR, Lee K, Hung J, Nguyen H, Cho J, et al. (2012). Promoting breast cancer screening among Asian American women: the Asian grocery store-based cancer education program. J Cancer Educ 27:612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Thomson K, Bambra C, Todd A. (2019). The breast cancer paradox: a systematic review of the association between area-level deprivation and breast cancer screening uptake in Europe. Cancer Epidemiol 60:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So WKW, Chan DNS, Law BMH, Choi KC, Krishnasamy M, Chan CWH. (2022). Effect of a family-based multimedia intervention on the uptake of faecal immunohistochemical test among South Asian older adults: a cluster-randomised controlled trial. Int J Nurs Stud 132:104254. [DOI] [PubMed] [Google Scholar]
- Spence AB, Levy ME, Monroe A, Castel A, Timpone J, Horberg M, et al. (2021). Cancer incidence and cancer screening practices among a cohort of persons receiving HIV care in Washington, DC. J Community Health 46:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KL, Williams RM, Davis K, Luta G, Penek S, Barry S, et al. (2013). Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med 173:1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S Preventive Services Task Force . https://www.uspreventiveservicestaskforce.org/uspstf/. [Accessed 24 October 2022].
- Valdez A, Napoles AM, Stewart SL, Garza A. (2018). A randomized controlled trial of a cervical cancer education intervention for Latinas delivered through interactive, multimedia kiosks. J Cancer Educ 33:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrinten C, Gallagher A, Waller J, Marlow LAV. (2019). Cancer stigma and cancer screening attendance: a population based survey in England. BMC Cancer 19:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Roy S, Kim J, Farazi PA, Siahpush M, Su D. (2019). Barriers of colorectal cancer screening in rural USA: a systematic review. Rural Remote Health 19:5181. [DOI] [PubMed] [Google Scholar]
- Weinberg DS, Keenan E, Ruth K, Devarajan K, Rodoletz M, Bieber EJ. (2013). A randomized comparison of print and web communication on colorectal cancer screening. JAMA Intern Med 173:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DS, Greene P, Pulley L, Kratt P, Gore S, Weiss H, et al. (2004). Stepped-care, community clinic interventions to promote mammography use among low-income rural African American women. Health Educ Behav 31(Suppl 4):29S–44S. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (2018). Health inequities and their causes. https://www.who.int/news-room/facts-in-pictures/detail/health-inequities-and-their-causes. [Accessed 24 October 2022].
- Yeh PT, Kennedy CE, de Vuyst H, Narasimhan M. (2019). Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health 4:e001351. [DOI] [PMC free article] [PubMed] [Google Scholar]