Abstract
BACKGROUND:
Alcohol misuse is associated with externalizing behaviors, including rule breaking. Studies have implicated altered reward processing in externalizing behaviors and alcohol misuse. Here, we investigated whether reward or punishment reactivity more significantly influenced alcohol use severity and rule-breaking behavior in young adult drinkers.
METHODS:
We curated data from the Human Connectome Project and identified 181 binge (132 men) and 288 nonbinge (97 men) drinkers performing a gambling task during brain imaging. Alcohol use severity was quantified by the first principal component of principal-component analysis of all drinking measures. We analyzed the imaging data using published routines and evaluated the results at a corrected threshold. We examined the interrelationship between imaging and clinical metrics with mediation and path analyses.
RESULTS:
Compared with nonbingers, bingers showed more severe rule-breaking behavior and responded significantly faster during post-loss than during post-win trials. Compared with nonbingers, bingers demonstrated greater inferior/middle frontal gyrus and cerebellum activations in loss-predominating blocks but no differences in regional responses to win-predominating blocks, relative to an interblock baseline. The right caudate body showed loss reactivity that was positively correlated with the rule-breaking score. No regional responses to wins were significantly correlated with the rule-breaking score. Mediation and path analyses demonstrated significant models with inferior/middle frontal gyrus and caudate reactivity to loss interrelating rule breaking and alcohol use severity.
CONCLUSIONS:
Punishment rather than reward reactivity was associated with alcohol use severity and rule breaking in young adults. The findings highlight the roles of negative emotions in psychological models of externalizing behaviors and alcohol misuse.
Alcohol Misuse and Rule-Breaking Behavior
Externalizing behaviors, including rule-breaking, aggressive, and antisocial behaviors, are considered as a significant risk factor for binge drinking (1). Delinquency in early adolescence promotes problem drinking later in life (2,3). A genetic behavioral study showed that the GABAergic (gamma-aminobutyric acidergic) system may conduce to problem alcohol use via rule-breaking behavior in midadolescence (4). Conversely, problem drinkers are less able to control their impulsive behavior and are prone to making poor decisions and breaking rules (5,6). It is not surprising that rule breaking and alcohol use co-occur and relate in severity in both adolescents and young adults (7-9).
Alcohol Misuse and Motivational Reactivity
Individuals may engage in alcohol misuse because of dysfunctional positive and/or negative emotional reactivity (10). Alcohol consumption contributes to positive mood (11-15), social pleasure (16), and perceived friendship (17,18). Greater alcohol-elicited enhancement of positive mood predicted more drinking problems at 18 months (19). Indeed, difficulty in regulating positive emotions was implicated in alcohol misuse in adolescents and adults (20-23). On the other hand, alcohol misuse is associated with anxiety, depression, cognitive deficits, sleep disturbance, and a host of physical problems (24-29). Individuals may drink to manage depression and other negative emotions, and the negative reinforcement perpetuates alcohol misuse (30). Greater negative mood reduction from alcohol consumption predicted more drinking problems (19). Drinking relative to nondrinking individuals place higher bets after losses than after wins in gambling (31), suggesting heightened loss reactivity. Thus, alcohol misuse is also associated with dysfunctional reactivity to negative emotions.
Imaging research has provided evidence of the influences of both positive and negative emotional reactivity on alcohol misuse. People who wanted to consume more alcohol (32) and bingers relative to nonbingers (33) exhibited greater bilateral activation of the ventral striatum (VS) during monetary wins than during losses. Adolescents engaged in problem drinking and gambling showed a hyperactive VS and ventromedial prefrontal cortical responses to monetary rewards (34). Elevating the risk for alcohol use disorder (AUD) in early adulthood, adolescent alcohol use was associated with a greater VS response to reward anticipation (35). Adolescents with a family history of AUD showed higher reward-related brain activation (36), and a higher VS response to risky decisions predicted future binge drinking (37). Other studies have highlighted the role of negative emotions in problem drinking. For instance, individuals with alcohol dependency showed higher regional activations to negative than to positive emotional images and higher activations than control subjects to negative emotional images (38). An electrophysiological study also reported greater attentional responses, as evident in a larger P2, to salient images in drinkers than in nondrinkers, and the difference was particularly clear during exposure to negative images (39). The severity of problem drinking was associated with higher posterior cingulate response to punishment than to reward in the monetary incentive delay task (MIDT) in adolescents (40). Another study reported that reduced VS reactivity to monetary reward was associated with higher risk of anhedonia and problem drinking in individuals with early-life stress (41).
Thus, although the participant populations and behavioral paradigms varied across studies, the literature supports dysfunctional motivational reactivity in alcohol misuse.
Externalizing Traits and Motivational Reactivity
Risky behaviors most likely occur during intense emotions (23,42,43), accompanied by dysfunctional reward and punishment processing or behavioral activation and inhibition (44). Intense positive emotions promote distractibility (45), less discriminative use of information (46), actions for short- versus long-term goals (47), gambling (48), and risky sexual behavior (49,50). Individuals exhibiting aggressive responses to provocation showed greater VS activity during retaliation (51). Likewise, aggressive behavior during violent video gaming activated the striatal reward system (52). Over time, the externalizing behaviors become self-perpetuating (53). Individuals may also break rules, seeking external stimulation to alleviate negative emotions (54,55). Impulsive individuals showed higher physiological arousal to salient events, including performance errors—a negative outcome—in a stop signal task (56). Individuals with alcohol dependency showed reduced skin conductance response (SCR) to both positive and negative emotional stimuli, and blunted SCR to positive but not negative stimuli was associated with impulsivity (57). In imaging studies, an attenuated neural response to rewarding social–emotional signals was associated with rule breaking and other behavioral problems (58).
Thus, externalizing behaviors, including alcohol misuse and rule breaking, appear to implicate both positive and negative emotional reactivity. However, the great majority of these studies did not directly contrast their roles in alcohol misuse or rule breaking. Furthermore, no studies to our knowledge have investigated the neural processes interlinking alcohol misuse and rule breaking.
The Present Study
The current work aimed to address these issues using the dataset from the Human Connectome Project (HCP), which comprised imaging data of a gambling task from young adults. We hypothesized that binge drinkers would demonstrate more externalizing symptoms and rule-breaking behaviors as well as altered regional responses to both reward and punishment than nonbinge drinkers. We investigated how the neural processes were related to the severity of alcohol use and rule breaking and performed mediation and path analyses to examine the interrelationship between the neural correlates, rule-breaking behavior, and alcohol misuse.
METHODS AND MATERIALS
Dataset and Demographics
With permission from the HCP (59), we used the 1200 Subjects Release (S1200) data, as in our previous work (18,60-63). Binge drinking was defined as having ≥4/5 drinks (women/men) on a single occasion (64). The binge drinking group comprised 181 adults who reported binge drinking at least once a week for the last 12 months (132 men, 72.9%) (65). A total of 314 adults reported no binge drinking in the prior year. However, 26 of the 314 met criteria for lifetime alcohol abuse or dependence and were excluded, leaving 288 adults (97 men, 33.7%) in the nonbinge drinking group. Thus, the data of a total of 469 adults (229 men, mean age ± SD = 27.9 ± 3.6 years; 240 women, mean age 29.8 ± 3.7 years) were included in this study, with more men than women in the binge drinking group (χ21 = 68.52, p < .001). In male (n = 132) and female (n = 49) bingers, 75 and 20, respectively, each met DSM-IV criteria for alcohol abuse or dependence. Analysis of variance (ANOVA) showed significant group (F468 = 5.76, p = .017) and sex (F468 = 13.63, p < .001) main effects as well as a group × sex interaction (F468 = 5.12, p = .024) effect of age. Age and sex were included as covariates in the analyses of all subjects, and age alone was included as a covariate in the analyses of men and women separately. All subjects were physically healthy with no severe neurodevelopmental, neuropsychiatric, or neurologic disorders. The HCP was approved by the Washington University Institutional Review Board (IRB #201204036).
Clinical Measures
The HCP data comprised 15 interrelated drinking metrics to assess the severity of alcohol use. We performed a principal component analysis on the 15 measures and identified one principal component (PC1) with an eigenvalue of >1 that accounted for 60.73% of the variance. Table S1 shows the mean ± SD of the drinking measures, PC1 (“drinking severity PC1,” henceforth), and the statistics of the group × sex ANOVA. All participants were assessed using the Achenbach Adult Self Report syndrome scales (66). The rule-breaking subscale (Supplemental Methods) comprises 14 items, each scored from 0 to 2, so the score sums from 0 to 28 (range: 0–20 for the current sample), with a higher score indicating more rule-breaking behavior. We performed a group × sex ANOVA of the score with age as a covariate and followed with simple effects analyses to examine sex differences in each drinking group (e.g., male vs. female bingers) and group differences in each sex (e.g., male bingers vs. nonbingers).
Imaging Protocol and Gambling Task for Functional Magnetic Resonance Imaging
Imaging protocols are described in Supplemental Methods (60-62). Participants completed 2 runs of a gambling task (~3 minutes and 12 seconds per run), each with 4 blocks—2 each of reward and punishment, with more win and loss trials, respectively—and a fixation period (baseline, 15 seconds) between blocks (67).
We performed a 4-way (trial × block × group × sex) repeated-measures ANOVA of the reaction time (RT) of trials following loss (post-loss) and win (post-win) in reward and punishment blocks to assess loss and win reactivity. We also performed a linear regression of post-loss RT and post-win RT against rule-breaking score and PC1 for all subjects with age and sex as covariates and for men and women separately with age as a covariate. Further, we examined sex differences in the regression with a slope test.
Imaging Data Modeling and Statistics
We followed published routines (68,69) in image data preprocessing and modeled the blood oxygen level–dependent signals to identify regional responses to reward and punishment blocks relative to the baseline (Supplemental Methods). In group analyses, we performed a group × sex full factorial with age as a covariate and evaluated the results with uncorrected voxel p < .001 in combination with familywise error (FWE)-corrected cluster p < .05.
Functional regions of interest were defined based on clusters obtained from whole-brain analyses. We used MarsBaR (http://marsbar.sourceforge.net/) to derive the activity (β contrast averaged across voxels) of the regions of interest for individual subjects.
Mediation and Path Analyses
We performed mediation analyses following published routines (70,71), as detailed earlier (68,72,73) (Supplemental Methods), to evaluate the relationships among neural markers, rule-breaking score, and drinking severity PC1 across all subjects (see Results).
Following up on mediation analysis and the findings of a significant correlation between inferior frontal gyrus/middle frontal gyrus (IFG/MFG) and caudate activity (see Results), we performed path analyses (Supplemental Methods) to examine the interrelationship between IFG/MFG and caudate activity and rule-breaking score and drinking severity PC1.
Note that the results of mediation or path analyses did not imply causality. Rather, the findings served to clarify the interrelationships of multiple, correlating variables.
RESULTS
Clinical Measures
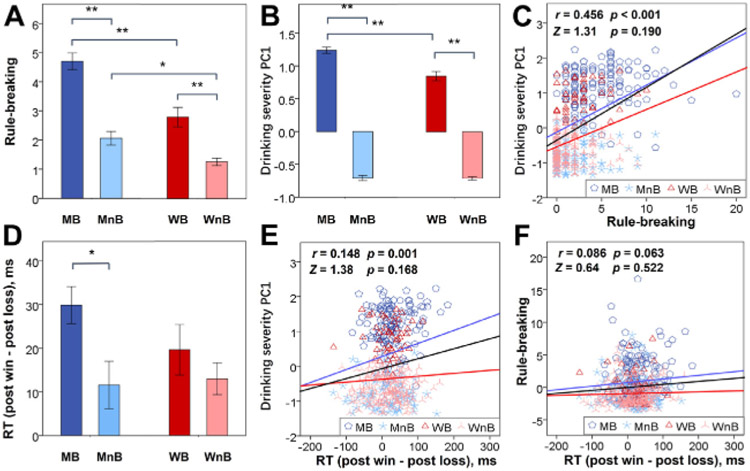
Rule-breaking score showed significant group main (F468 = 57.51, p < .001), sex main (F468 = 25.40, p < .001), and group × sex interaction (F468 = 9.09, p = .003) effects (Figure 1A). Post hoc analyses showed higher rule-breaking scores in men than in women and in both male and female bingers than in nonbingers. Male bingers compared with nonbingers showed a greater difference in the rule-breaking score than female bingers compared with nonbingers. Table S2 shows the results of an ANOVA on other Achenbach Adult Self Report measures.
Figure 1.
Clinical and behavioral measures of male binger (MB), male nonbinger (MnB), female binger (WB), and female nonbinger (WnB) subjects. (A) Rule-breaking score. (B) Severity of alcohol use as quantified by the weight of the first principal component (PC1) of a principal component analysis of all 15 drinking measures. (C) Correlation of drinking severity PC1 with rule-breaking score across all subjects (regression line in black) and men (blue line) and women (red line) separately. (D) Difference in post-win and post-loss reaction time (RT). All histograms show mean ± SE; **p ≤ .001 and *p ≤ .05 show the statistics of simple effects analyses. Correlation of (E) drinking severity PC1 and of (F) rule-breaking score with RT (post-win minus post-loss) are shown for all subjects (black line), men (blue line), and women (red line). Data points are represented by different symbols for MB, MnB, WB, and WnB. The r and p values reflect the correlation for all subjects, and the Z and p values reflect the slope tests of sex differences in the regression. We did not show the r and p values separately for men and women because the regressions did not show significant sex differences in the slope. Note that the residuals are plotted here with age accounted for in the regressions.
Severity of alcohol use, as indexed by the PC1, showed significant group (F468 = 1375.70, p < .001), sex (F468 = 16.47, p < .001), and group × sex interaction (F468 = 14.27, p < .001) effects, with a greater difference in male than in female bingers versus nonbingers (Figure 1B).
The rule-breaking score showed a significant correlation with PC1 (all: r = 0.456, p < .001; men: r = 0.411, p < .001; women: r = 0.305, p < .001) with age as a covariate; in slope tests, men and women did not differ in the slope of regression (Figure 1C).
Behavioral Measures of the Gambling Task
To characterize how individuals reacted to wins and losses, we computed individual RTs of trials following loss (post-loss RT) and win (post-win RT) each for reward and punishment blocks. The results of a 4-way (trial × block × group × sex) repeated-measures ANOVA are shown in Table S3. There were significant block (F468 = 60.90, p < .001) and group (F468 = 3.91, p = .049) main effects, with simple effects analyses showing shorter RT in punishment (434 ± 112 ms) than in reward (452 ± 111 ms) blocks (t468 = −8.38, p < .001, paired t test) and shorter RT in binging (426 ±101 ms) than in nonbinging (454 ± 112 ms) individuals (t467 = −2.75, p = .006, two-sample t test). There were also significant trial × block (F468 = 6.05, p = .014) and trial × group (F468 = 4.62, p = .032) interactions, each with higher post-win RT than post-loss RT in reward (11 ± 77 ms) than in punishment (−1 ± 76 ms) blocks (t468 = 2.50, p = .013, paired t test) and in binging (27 ± 47 ms) than in non-binging (13 ± 51 ms) individuals (t467 = 3.11, p = .002, two-sample t test). No other main or interaction effects, including those involving sex as a factor, were significant. However, in Figure 1D, we followed the format of Figure 1A and Figure1B and showed the difference between post-win and post-loss RT separately for men and women.
In linear regressions with age as a covariate, the difference in RT between post-win and post-loss trials was significantly correlated with drinking severity PC1 across all subjects (r = 0.148, p = .001) and in men (r = 0.183, p = .006) but not in women (r = 0.057, p = .377) alone. However, a slope test did not show a significant sex difference in the correlation (Figure 1E). The difference in RT was not correlated with the rule-breaking score across all (r = 0.086, p = .063), male (r = 0.089, p = .183), or female (r = 0.030, p = .646) subjects, and a slope test did not show a sex difference (Figure 1F). Thus, the extent of faster RT in post-loss compared with post-win trials was positively correlated with the severity of drinking but not rule breaking.
Binge Drinking and Win-/Loss-Related Regional Brain Activations
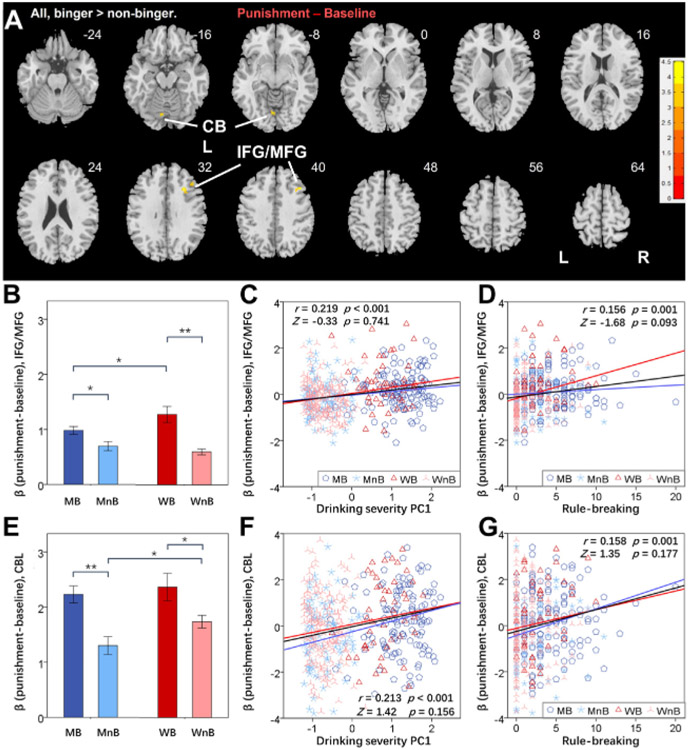
A group × sex full factorial of the contrast reward minus baseline did not reveal any significant clusters for group (binger vs. nonbinger) at uncorrected voxel p = .001 and FWE-corrected cluster p < .05. At the same threshold, a full factorial of the contrast punishment minus baseline showed higher activation in bingers than in nonbingers in the cerebellum (CBL) (x = −4, y = −74, z = −10; Z = 4.48, volume - 504 mm3) and a cluster comprising the right IFG/MFG (x = 28, y = 22, z = 32; Z = 4.44, volume = 1096 mm3) (Figure 2A). We extracted the βs of the IFG/MFG and CBL for individual subjects and visualized the group difference (binger vs. nonbingei) in IFG/MFG (men: t227 = 2.64, p = .009; women: t238 = 5.32, p < .001) (Figure 2B) and the CBL (men: t227 = 4.18, p < .001; women: t238 = 2.43, p = .016) (Figure 2E). The IFG/MFG but not the CBL showed a significant group × sex interaction (F468 = 5.99, p = .015 and F468 = 0.48, p = .491, respectively).
Figure 2.
(A) Regional brain activations to the contrast punishment minus baseline in bingers vs. nonbingers from the full factorial analysis; color bars show voxel t values, and warm indicates a positive value. Threshold: voxel p < .001 in combination with a cluster familywise error–corrected p < .05. Clusters are overlaid on a T1 structural image in neurologic orientation: right = right. The β contrast of punishment minus baseline (mean ± SE) of the (B) Inferior frontal gyrus/middle frontal gyrus (IFG/MFG) and (E) cerebellum (CBL) were computed for all subjects and are shown here separately for male binger (MB), male nonbinger (MnB), female binger (WB), and female nonbinger (WnB) subjects. *p < .05 and **p < .001 indicate the statistics of post hoc simple effects analyses. Linear regression of the β contrast of IFG/MFG against (C) drinking severity first principal component (PC1) and (D) rule-breaking score and of the β contrast of CBL against (F) drinking severity PC1 and (G) rule-breaking score are shown for all subjects (black line), men (blue line), and women (red line). Data points are represented by different symbols for MB, MnB, WB, and WnB. The r and p values reflect the correlation for all subjects, and the Z and p values reflect the slope tests. Note that residuals are plotted here with age accounted for in the regressions. L, left; R, right.
The β estimate of the IFG/MFG was significantly correlated with PC1 across all subjects (r = 0.219, p < .001) and in men (r = 0.198, p = .003) and women (r = 0.227, p < .001) alone. The β estimate of the CBL was significantly correlated with PC1 across all subjects (r = 0.213, p < .001) as well as in men (r = 0.277, p < .001) and women (r = 0.151, p = .019) alone. The slopes of regressions did not show sex differences (IFG/MFG: Z = −0.33, p = .741) (Figure 2C) (CBL: Z = 1.42, p = .156) (Figure 2F).
The β estimate of the IFG/MFG was significantly correlated with the rule-breaking score across all subjects (r = 0.156, p = .001} as well as in women (r = 0.233, p < .001) but not in men (r = 0.083, p = .212) alone. However, the slope of regressions fell short of being a significant difference (Z = −1.66, p = .097) (Figure 2D). The β estimate of the CBL was significantly correlated with the rule-breaking score across all subjects (r = 0.158, p = .001) as well as in men (r = 0.214, p = .001) but not in women (r = 0.092, p = .157) alone. The slope of regressions did not differ significantly between men and women (Z = 1.35, p = .177) (Figure 2G).
The β estimate of the IFG/MFG was not correlated with post-loss RT (r = 0.025, p = .584) or post-loss minus post-win RT (r = 0.065, p = .157) in the punishment blocks across all subjects. Likewise, the β estimate of the CBL was not correlated with post-loss RT (r = 0.055, p = .237) or post-loss minus post-win RT (r = −0.005, p = .919) either.
Neural Correlates of Rule Breaking: Win and Loss Reactivity
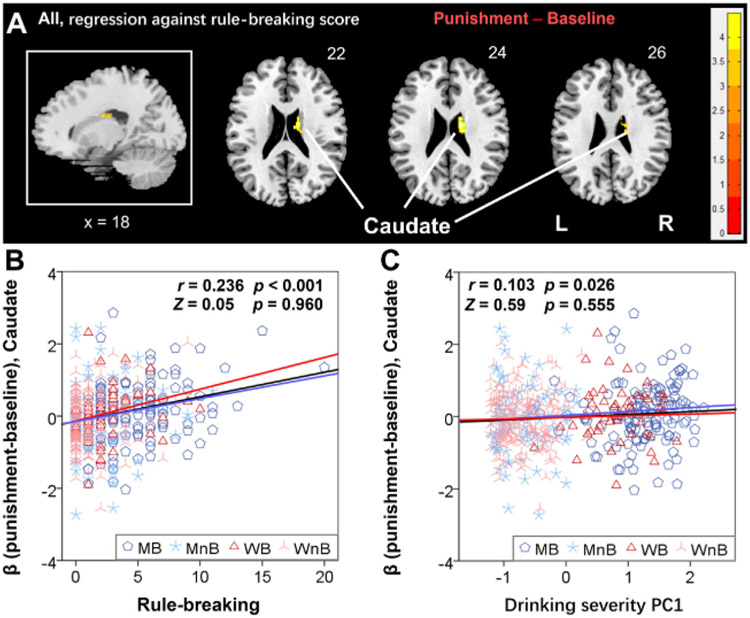
A whole-brain linear regression of the contrast reward minus baseline against the rule-breaking score for the entire sample did not reveal any clusters. The regression of the contrast punishment minus baseline showed a significant cluster in the right caudate (16, −16, 24, Z = 4.45, 520 mm3) (Figure 3A). The β estimate of the right caudate was significantly correlated with both the rule-breaking score (r = 0.236, p < .001) (Figure 3B) and drinking severity PC1 (r = 0.103, p = .026) (Figure 3C) in a linear regression with age as a covariate.
Figure 3.
(A) The linear regression of the β contrast punishment minus baseline against the rule-breaking score identified a cluster in the right caudate that was positively correlated for all subjects. Voxel p < .001 in combination with a cluster familywise error–corrected p < .05. Color bars show voxel t values; warm indicates a positive correlation. The cluster is overlaid on a T1 structural image in neurologic orientation: right = right. Linear regression of the β contrast of caudate against (B) rule-breaking score and (C) drinking severity first principal component (PC1). The r and p values reflect the correlation for all subjects, and the Z and p values reflect the slope tests. Note that residuals are plotted here with age accounted for in the regressions. L, left; MB, male binger; MnB, male nonbinger; R, right; WB, women binger; WnB, women nonbinger.
The β estimate of the right caudate was not correlated with post-loss RT (r = 0.047, p = .305) or post-loss minus post-win RT (r = 0.030, p = .518) of the punishment blocks.
The Interrelationship of Loss Reactivity, Rule-Breaking Behavior, and Severity of Alcohol Use
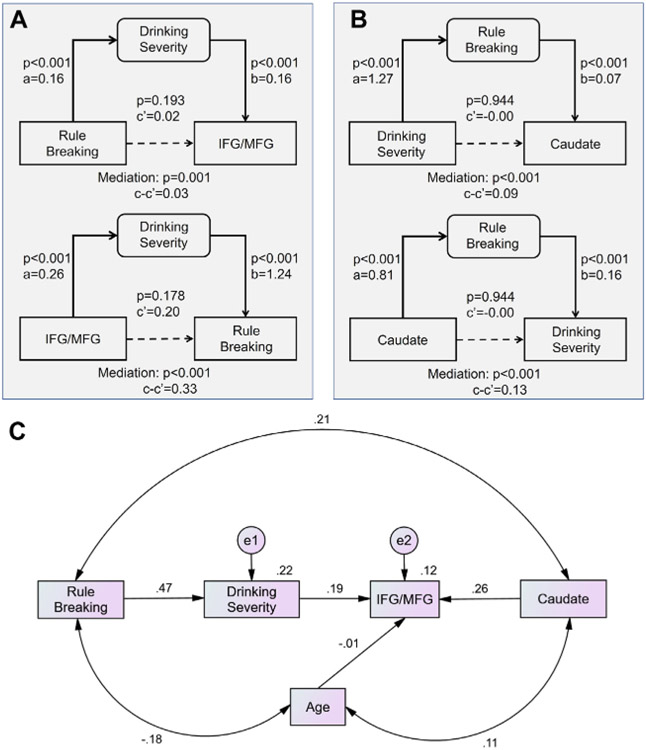
A principal component analysis of the 15 drinking measures identified a single factor (PC1) with an eigenvalue of >1 and that accounted for 60.73% of the variance (see Methods and Materials). Across subjects, the individual weight of PC1 was positively correlated with the rule-breaking score (r = 0.456, p < .001) and with the IFG/MFG β of punishment minus baseline (r = 0.219, p < .001) with age as a covariate. We performed a mediation analysis to examine the interrelationship between PC1, rule-breaking score, and IFG/MFG β, with age as a covariate. We considered all 6 models and used a corrected p value (.05/6 = .0083) to evaluate the mediation effects. Two models (rule-breaking score → PC1 → IFG/MFG β and IFG/MFG β → PC1 → rule-breaking score) showed significant (and complete) mediation (Figure 4A; statistics in Table S4). We also performed mediation analyses between PC1, rule-breaking score, and caudate β with age as a covariate, and 2 models (PC1 → rule-breaking score → caudate β and caudate β → rule-breaking score → PC1) showed significant (and complete) mediation (Figure 4B; Table S5).
Figure 4.
(A, B) Mediation analysis and (C) path model to show the interrelationship of caudate and IFG/MFG activity, severity of drinking, and rule-breaking score. Only significant models are shown. The statistics of all 6 mediation models are shown in Tables S4 and S5. Other path models and fit statistics are shown in Figure S2 and Table S6, respectively. e, measuring error; IFG, Inferior frontal gyrus; MFG, middle frontal gyrus.
Furthermore, because the caudate and IFG/MFG activities were correlated (r = 0.281, p < .001), we performed a path analysis to examine which path model of caudate β, IFG/MFG β, severity of alcohol use, and rule-breaking behavior demonstrated a significant and best statistical fit. With age as a covariate, one model demonstrated a significant and the best fit (Figure 4C) (root mean square error of approximation = 0.000 [90% CI, 0.000–0.072], χ20.800, standardized root mean square residual = 0.017, and comparative fit index = 1.000). The other models and fit statistics are shown in Figure S1 and Table S6.
DISCUSSION
Young adult binge drinkers demonstrated significantly higher rule-breaking scores than nonbinge drinkers as evaluated by the Achenbach Adult Self Report syndrome scales. Bingers relative to nonbingers demonstrated faster RT in post-loss than in post-win trials, suggesting loss-elicited impulsivity. Bingers relative to nonbingers did not demonstrate differences in regional activations during reward blocks but showed higher IFG/MFG and CBL responses in the punishment blocks, where they were exposed more frequently to monetary losses than wins, relative to baseline. Across subjects, the right caudate body response to punishment was correlated with a higher rule-breaking score. Furthermore, IFG/MFG and caudate responses showed a significant, positive correlation with both rule-breaking score and severity of alcohol use. Mediation and path analyses characterized the interrelationships of IFG/MFG and caudate reactivity to loss, rule-breaking behavior score, and the severity of alcohol use. Together, these findings support loss reactivity in association with both binge drinking and rule-breaking and highlight frontal striatal activities interlinking alcohol use severity and externalizing problems.
Alcohol Misuse, Externalizing Problems, and Loss Reactivity
Bingers as compared to nonbingers were significantly faster in responding during post-loss than during post-win trials, indicating loss-evoked impulsivity. Bingers as compared to nonbingers showed a higher CBL and IFG/MFG activation to punishment blocks in which loss trials predominated in the gambling task. These findings are consistent with studies showing a higher MFG response to monetary loss in individuals with alcohol dependence than in control subjects in the MIDT (74,75), and a recent review reports the same in AUD and gambling disorder (76). In a reward go/no-go task, higher physiological arousal, as reflected in SCR, in response to losses than to wins was positively correlated with intersubject variation in reward sensitivity (77). Furthermore, relative to control subjects, adolescent substance users had more externalizing problems and showed a higher MFG activation during failed than during successful inhibitions in a go/no-go task in association with the severity of externalizing problems (78). In our studies of the stop signal task, we also noted a positive correlation between impulsivity traits and SCR to stop errors in neurotypical adults (56). Thus, whether associated with a material reward or not, negative outcomes appear more salient and may elicit higher MFG reactivity in association with externalizing problems. Because the IFG/MFG activities were not correlated with RT differences between post-loss and post-win trials in punishment blocks, it remains to be seen how the IFG/MFG may signal downstream structures to influence behavior in the gambling task.
Notably, in a monetary risk-taking task in which participants placed a risky or safe bet, patients with alcohol dependence relative to control subjects showed lower right MFG activation in response to risky bets that resulted in loss as compared with safe bets with small wins (79). Anticipatory activities during risky bets—a decision involving greater conflict—may account for these contrasting findings. In support, overriding the conflicting, directional visual cue to make rewarded antisaccades, adolescents with more severe externalizing problems showed hypoactivation of the MFG (80). However, externalizing symptoms were positively associated with MFG activation during no-go responses to cues previously conditioned to a higher likelihood of reward in the MIDT (81). Thus, how the MFG responds during decision making in relation to alcohol misuse and/or externalizing behaviors may depend on which psychological processes—outcome, action, or inhibition of action to override conflict—are queried.
Alcohol Misuse, Externalizing Behaviors, and Caudate Reactivity
The caudate showed higher activities during punishment blocks in positive association with both the rule-breaking score and drinking severity. In an imaging study with half of the smokers allowed and the other half not allowed to smoke during a break, the group not allowed to smoke exhibited a greater right caudate response to monetary loss during a card guessing task than the group allowed to smoke (82). Cannabis users showed higher left caudate and bilateral IFG responses to monetary loss in the MIDT than nonusers (83). Boys with externalizing behavior problems showed greater caudate activation when offered less in return and incurring monetary loss in a trust task (84). Thus, as with the IFG/MFG, the caudate exhibited higher reactivity to negative outcomes in individuals with more externalizing problems. In accord, the caudate also responded intensely to negative emotional stimuli (85,86).
IFG/MFG and Caudate Loss Reactivity Interrelates Alcohol Use Severity and Rule Breaking
The findings of the mediation and path analyses interlink IFG/MFG and caudate loss reactivity with rule breaking and alcohol use severity. According to the most significant model (Figure 4C), rule-breaking behavior modulated the severity of alcohol misuse, and both alcohol misuse and, via caudate activity, rule-breaking modulated loss reactivity of the IFG/MFG. Thus, although the mediation analyses suggest bidirectional influences between alcohol misuse and rule breaking, the path analyses that incorporated caudate reactivity favored a model in which rule-breaking influenced alcohol misuse, but not the other way around. These results should be considered as specific to the HCP sample of young, neurotypical adults; how chronic alcohol exposure may impact rule breaking and other externalizing behaviors in AUD remains to be investigated. Furthermore, the mediation and path models do not imply causality, which can best be elucidated with longitudinal data and with respect to specific hypotheses. Also notable from the mediation model are the missing behavioral metrics of the gambling task, which we discuss below as a limitation.
Limitations, Other Considerations, and Conclusions
A few limitations need to be considered for this study. First, the sample comprised more bingers in males and more nonbingers in females. Although we have specifically examined and noted that there are largely no sex differences in the findings (see the Supplement), it remains unclear how this may have influenced the results. Second, behavioral reactivity (RT) was missing from the path models. As discussed earlier, neither IFG/MFG nor caudate responses in the punishment blocks were correlated with RT differences between post-loss and post-win trials. We were limited by the block design of the gambling task, which does not allow a clear dissection of regional brain responses to changes in RT trial by trial. New experiments with event-related paradigms are needed to elucidate the neural correlates specific to loss-related changes in RT. Third, the results of the mediation and path analyses did not inform causality. Rather, the results should be regarded as a way to conceptually organize the multiple, correlating variables in models that best describe their interrelationships. Finally, we used drinking severity PC1 rather than any of the measures that may more specifically reflect binge drinking because these measures (e.g., frequency of drinking 4+/5+ drinks in past 12 months, frequency drunk in past 12 months) (Table S1) were all highly correlated with PC1 across all subjects, in men, and in women (r = 0.721–0.905, ps < .001). Nonetheless, PC1 reflects the overall level of alcohol misuse but is not a unique feature of binge drinking.
We used both regional brain activities and RTs to reflect reactivity, as investigators typically do to quantify how participants behave in response to external events. For instance, investigators have used post-error slowing to identify the extent of behavioral adjustment and its neural correlates in the stop signal task (87-90). Other indices, including measures of physiological arousal, if available, can potentially be used to quantify reactivity on a trial-by-trial basis (56,77,91). We tested sex differences in the analytics of behavioral and neural metrics. Both male and female bingers showed higher rule-breaking scores and drinking severity than nonbingers. However, no correlations between clinical metrics and IFG/MFG or caudate reactivity showed a significant sex difference in regression slopes. Thus, it is possible that the neural processes of alcohol misuse and externalizing problems are related to loss reactivity similarly in men and women. Of interest is a recent study showing that compared with placebo, healthy participants receiving a selective serotonin reuptake inhibitor before scanning showed a diminished response of the right caudate to monetary loss in a similar card guessing task (92). The serotonergic system is implicated in the pathophysiology of mood and anxiety disorders; thus, the current findings provide clues for neuropharmacological research of loss reactivity.
To conclude, punishment rather than reward reactivity was associated with alcohol use severity and rule breaking in young adults. The findings highlight the roles of negative emotions in psychological models of externalizing behavior and alcohol misuse and of the prefrontal cortex and caudate in supporting the interrelationship between rule breaking and drinking severity.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This study is supported by the National Institutes of Drug Abuse (Grant No. DA051922 [to C-SRL]) and National Natural Science Foundation of China (Grant No. U20A20388 [to XT]). Data were provided by the Human Connectome Project, WU-Minn Consortium (Grant No. 1U54MH091657; principal investigators, David Van Essen and Kamil Ugurbil), which is funded by the 16 NIH institutes and centers that support the NIH Blueprint for Neuroscience Research and by the McDonnell Center for Systems Neuroscience at Washington University.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2022.06.001.
Contributor Information
Guangfei Li, Department of Biomedical Engineering, Faculty of Environment and Life Sciences, Beijing University of Technology, Beijing; Department of Biomedical Engineering, School of Life Sciences, Beijing Institute of Technology, Beijing, China.
Yu Chen, Department of Psychiatry, Yale University School of Medicine.
Shefali Chaudhary, Department of Psychiatry, Yale University School of Medicine.
Xiaoying Tang, Department of Biomedical Engineering, School of Life Sciences, Beijing Institute of Technology, Beijing, China.
Chiang-Shan R. Li, Department of Psychiatry; Department of Psychiatry, Interdepartmental Neuroscience Program, Yale University School of Medicine; Wu Tsai Institute, Yale University, New Haven, Connecticut
REFERENCES
- 1.Regan T, Tubman JG, Schwartz SJ (2020): Relations among externalizing behaviors, alcohol expectancies and alcohol use problems in a multi-ethnic sample of middle and high school students. Subst Abuse 14:1178221820928427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sitnick SL, Shaw DS, Hyde LW (2014): Precursors of adolescent substance use from early childhood and early adolescence: Testing a developmental cascade model. Dev Psychopathol 26:125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alati R, Baker P, Betts KS, Connor JP, Little K, Sanson A, Olsson CA (2014): The role of parental alcohol use, parental discipline and antisocial behaviour on adolescent drinking trajectories. Drug Alcohol Depend 134:178–184. [DOI] [PubMed] [Google Scholar]
- 4.Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, Zucker RA (2014): Rule breaking mediates the developmental association between GABRA2 and adolescent substance abuse. J Child Psychol Psychiatry 55:1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bø R, Billieux J, Gjerde LC, Eilertsen EM, Landrø NI (2017): Do executive functions predict binge-drinking patterns? Evidence from a longitudinal study in young adulthood. Front Psychol 8:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townshend JM, Kambouropoulos N, Griffin A, Hunt FJ, Milani RM (2014): Binge drinking, reflection impulsivity, and unplanned sexual behavior: Impaired decision-making in young social drinkers. Alcohol Clin Exp Res 38:1143–1150. [DOI] [PubMed] [Google Scholar]
- 7.van Nieuwenhuijzen M, Junger M, Velderman MK, Wiefferink KH, Paulussen TWGM, Hox J, Reijneveld SA (2009): Clustering of health-compromising behavior and delinquency in adolescents and adults in the Dutch population. Prev Med 48:572–578. [DOI] [PubMed] [Google Scholar]
- 8.Cordova D, Huang S, Arzon M, Freitas D, Malcolm S, Prado G (2011): The role of attitudes, family, peer and school on alcohol use, rule breaking and aggressive behavior in Hispanic delinquent adolescents. Open Fam Stud J 4:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reijneveld SA, van Nieuwenhuijzen M, Klein Velderman M, Paulussen TWGM, Junger M (2012): Clustering of health and risk behaviour in immigrant and indigenous Dutch residents aged 19–40 years. Int J Public Health 57:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss NH, Sullivan TP, Tull MT (2015): Explicating the role of emotion dysregulation in risky behaviors: A review and synthesis of the literature with directions for future research and clinical practice. Curr Opin Psychol 3:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang AR, Patrick CJ, Stritzke WGK (1999): Alcohol and emotional response: A multidimensional-multilevel analysis. In: Leonard KE, Blane HT, editors. Psychological Theories of Drinking and Alcoholism, 2nd ed. New York: The Guilford Press, 328–371. [Google Scholar]
- 12.Sayette MA (1993): An appraisal-disruption model of alcohol’s effects on stress responses in social drinkers. Psychol Bull 114:459–476. [DOI] [PubMed] [Google Scholar]
- 13.Orford J, Krishnan M, Balaam M, Everitt M, Van der Graaf K (2004): University student drinking: The role of motivational and social factors. Drugs: Educ Prev Policy 11:407–421. [Google Scholar]
- 14.Sayette MA, Creswell KG, Fairbairn CE, Dimoff JD, Bentley K, Lazerus T (2019): The effects of alcohol on positive emotion during a comedy routine: A facial coding analysis. Emotion 19:480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowles SKZ, Duka T (2004): Does alcohol affect memory for emotional and non-emotional experiences in different ways? Behav Pharmacol 15:111–121. [DOI] [PubMed] [Google Scholar]
- 16.Fairbairn CE, Sayette MA (2014): A social-attributional analysis of alcohol response. Psychol Bull 140:1361–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolder PC, Holze F, Liakoni E, Harder S, Schmid Y, Liechti ME (2017): Alcohol acutely enhances decoding of positive emotions and emotional concern for positive stimuli and facilitates the viewing of sexual images. Psychopharmacology 234:41–51. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Chen Y, Le TM, Zhornitsky S, Wang W, Dhingra I, et al. (2021): Perceived friendship and binge drinking in young adults: A study of the Human Connectome Project data. Drug Alcohol Depend 224:108731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venerable WJ, Fairbairn CE (2020): A multimodal, longitudinal investigation of alcohol’s emotional rewards and drinking over time in young adults. Psychol Addict Behav 34:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss NH, Forkus SR, Contractor AA, Schick MR (2018): Difficulties regulating positive emotions and alcohol and drug misuse: A path analysis. Addict Behav 84:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO (2015): Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychol Assess 27:1129–1146. [DOI] [PubMed] [Google Scholar]
- 22.Coskunpinar A, Dir AL, Cyders MA (2013): Multidimensionality in impulsivity and alcohol use: A meta-analysis using the UPPS model of impulsivity. Alcohol Clin Exp Res 37:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cyders MA, Smith GT (2008): Emotion-based dispositions to rash action: Positive and negative urgency. Psychol Bull 134:807–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koob GF, Colrain IM (2020): Alcohol use disorder and sleep disturbances: A feed-forward allostatic framework. Neuropsychopharmacology 45:141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Chen Y, Tang X, Li C-SR (2021): Alcohol use severity and the neural correlates of the effects of sleep disturbance on sustained visual attention. J Psychiatr Res 142:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakkar MM, Sharma R, Sahota P (2015): Alcohol disrupts sleep homeostasis. Alcohol 49:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Berre AP (2019): Emotional processing and social cognition in alcohol use disorder. Neuropsychology 33:808–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosco FM, Capozzi F, Colle L, Marostica P, Tirassa M (2014): Theory of mind deficit in subjects with alcohol use disorder: An analysis of mindreading processes. Alcohol Alcohol 49:299–307. [DOI] [PubMed] [Google Scholar]
- 29.Onuoha RC, Quintana DS, Lyvers M, Guastella AJ (2016): A meta-analysis of theory of mind in alcohol use disorders. Alcohol Alcohol 51:410–415. [DOI] [PubMed] [Google Scholar]
- 30.Brown TA, Chorpita BF, Barlow DH (1998): Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol 107:179–192. [DOI] [PubMed] [Google Scholar]
- 31.Tobias-Webb J, Limbrick-Oldfield EH, Vearncombe S, Duka T, Clark L (2020): The effects of alcohol on sequential decision-making biases during gambling. Psychopharmacology (Berl) 237:395–407. [DOI] [PubMed] [Google Scholar]
- 32.Radoman M, Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, Phan KL (2021): Striatal activation to monetary reward is associated with alcohol reward sensitivity. Neuropsychopharmacology 46:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, Phan KL (2017): Preliminary evidence for disrupted nucleus accumbens reactivity and connectivity to reward in binge drinkers. Alcohol Alcohol 52:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noël X (2014): Why adolescents are at risk of misusing alcohol and gambling. Alcohol Alcohol 49:165–172. [DOI] [PubMed] [Google Scholar]
- 35.Waller R, Murray L, Shaw DS, Forbes EE, Hyde LW (2019): Accelerated alcohol use across adolescence predicts early adult symptoms of alcohol use disorder via reward-related neural function. Psychol Med 49:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stice E, Yokum S (2014): Brain reward region responsivity of adolescents with and without parental substance use disorders. Psychol Addict Behav 28:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morales AM, Jones SA, Ehlers A, Lavine JB, Nagel BJ (2018): Ventral striatal response during decision making involving risk and reward is associated with future binge drinking in adolescents. Neuropsychopharmacology 43:1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilman JM, Hommer DW (2008): Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol 13:423–434. [DOI] [PubMed] [Google Scholar]
- 39.Banz BC, Davalos DB (2018): Attentional and neural processing of affective and alcohol-related images in university-attending emerging adults. Emerg Adulthood 6:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aloi J, Meffert H, White SF, Blair KS, Hwang S, Tyler PM, et al. (2019): Differential dysfunctions related to alcohol and cannabis use disorder symptoms in reward and error-processing neuro-circuitries in adolescents. Dev Cogn Neurosci 36:100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corral-Frías NS, Nikolova YS, Michalski LJ, Baranger DAA, Hariri AR, Bogdan R (2015): Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med 45:2605–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leith KP, Baumeister RF (1996): Why do bad moods increase self-defeating behavior? Emotion, risk taking, and self-regulation. J Pers Soc Psychol 71:1250–1267. [DOI] [PubMed] [Google Scholar]
- 43.Baskin-Sommers AR, Newman JP (2013): Differentiating the cognition-emotion interactions that characterize psychopathy versus externalizing. In: Robinson MD, Watkins E, Harmon-Jones E, editors. Handbook of Cognition and Emotion. New York: The Guilford Press, 501–520. [Google Scholar]
- 44.Byrd AL, Loeber R, Pardini DA (2014): Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clin Child Fam Psychol Rev 17:125–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dreisbach G, Goschke T (2004): How positive affect modulates cognitive control: Reduced perseveration at the cost of increased distractibility. J Exp Psychol Learn Mem Cogn 30:343–353. [DOI] [PubMed] [Google Scholar]
- 46.Forgas JP (1992): Mood and the perception of unusual people: Affective asymmetry in memory and social judgments. Eur J Soc Psychol 22:531–547. [Google Scholar]
- 47.Slovic P, Finucane ML, Peters E, MacGregor DG (2004): Risk as analysis and risk as feelings: Some thoughts about affect, reason, risk, and rationality. Risk Anal 24:311–322. [DOI] [PubMed] [Google Scholar]
- 48.Cyders MA, Smith GT (2008): Clarifying the role of personality dispositions in risk for increased gambling behavior. Pers Individ Dif 45:503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C (2007): Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychol Assess 19:107–118. [DOI] [PubMed] [Google Scholar]
- 50.Zapolski TCB, Cyders MA, Smith GT (2009): Positive urgency predicts illegal drug use and risky sexual behavior. Psychol Addict Behav 23:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chester DS, DeWall CN (2016): The pleasure of revenge: Retaliatory aggression arises from a neural imbalance toward reward. Soc Cogn Affect Neurosci 11:1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klasen M, Mathiak KA, Zvyagintsev M, Sarkheil P, Weber R, Mathiak K (2020): Selective reward responses to violent success events during video games. Brain Struct Funct 225:57–69. [DOI] [PubMed] [Google Scholar]
- 53.Sommer J, Hinsberger M, Elbert T, Holtzhausen L, Kaminer D, Seedat S, et al. (2017): The interplay between trauma, substance abuse and appetitive aggression and its relation to criminal activity among high-risk males in South Africa. Addict Behav 64:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crepaz N, Marks G (2001): Are negative affective states associated with HIV sexual risk behaviors? A meta-analytic review. Health Psychol 20:291–299. [DOI] [PubMed] [Google Scholar]
- 55.Matthys W, Vanderschuren LJMJ, Schutter DJLG (2013): The neurobiology of oppositional defiant disorder and conduct disorder: Altered functioning in three mental domains. Dev Psychopathol 25:193–207. [DOI] [PubMed] [Google Scholar]
- 56.Zhang S, Hu S, Hu J, Wu PL, Chao HH, Li CS (2015): Barratt Impulsivity and Neural Regulation of Physiological Arousal. PLoS One 10:e0129139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carmona-Perera M, Sumarroca-Hernández X, Santolaria-Rossell A, Pérez-García M, Reyes del Paso GA (2019): Blunted autonomic responses to emotional stimuli in alcoholism: Relevance of impulsivity. Adicciones 31:221–232. [DOI] [PubMed] [Google Scholar]
- 58.Bunford N, Kujawa A, Swain JE, Fitzgerald KD, Monk CS, Phan KL (2017): Attenuated neural reactivity to happy faces is associated with rule breaking and social problems in anxious youth. Eur Child Adolesc Psychiatry 26:215–230. [DOI] [PubMed] [Google Scholar]
- 59.Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, et al. (2012): The Human connectome Project: A data acquisition perspective. NeuroImage 62:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li G, Zhang S, Le TM, Tang X, Li CSR (2020): Neural responses to negative facial emotions: Sex differences in the correlates of individual anger and fear traits. NeuroImage 221:117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li G, Zhang S, Le TM, Tang X, Li CSR (2020): Neural responses to reward in a gambling task: Sex differences and individual variation in reward-driven impulsivity. Cereb Cortex Commun 1:tgaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li G, Chen Y, Wang W, Dhingra I, Zhornitsky S, Tang X, Li CR (2020): Sex differences in neural responses to the perception of social interactions. Front Hum Neurosci 14:565132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li G, Le TM, Wang W, Zhornitsky S, Chen Y, Chaudhary S, et al. (2021): Perceived stress, self-efficacy, and the cerebral morphometric markers in binge-drinking young adults. Neuroimage Clin 32:102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S (1994): Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. JAMA 272:1672–1677. [PubMed] [Google Scholar]
- 65.Gowin JL, Manza P, Ramchandani VA, Volkow ND (2021): Neuropsychosocial markers of binge drinking in young adults. Mol Psychiatry 26:4931–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Achenbach TM (2009): The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families. [Google Scholar]
- 67.Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. (2013): Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage 80:169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhornitsky S, Zhang S, Ide JS, Chao HH, Wang W, Le TM, et al. (2019): Alcohol expectancy and cerebral responses to cue-elicited craving in adult nondependent drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 4:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang S, Zhornitsky S, Le TM, Li CR (2019): Hypothalamic responses to cocaine and food cues in individuals with cocaine dependence. Int J Neuropsychopharmacol 22:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacKinnon DP, Fairchild AJ, Fritz MS (2007): Mediation analysis. Annu Rev Psychol 58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu S, Ide JS, Chao HH, Castagna B, Fischer KA, Zhang S, Li CR (2018): Structural and functional cerebral bases of diminished inhibitory control during healthy aging. Hum Brain Mapp 39:5085–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ide JS, Zhornitsky S, Hu S, Zhang S, Krystal JH, Li CSR (2017): Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. NeuroImage Clin 14:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, et al. (2007): Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35:787–794. [DOI] [PubMed] [Google Scholar]
- 75.Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, et al. (2009): Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry 66:734–742. [DOI] [PubMed] [Google Scholar]
- 76.Quaglieri A, Mari E, Boccia M, Piccardi L, Guariglia C, Giannini AM (2020): Brain network underlying executive functions in gambling and alcohol use disorders: An activation likelihood estimation meta-analysis of fMRI studies. Brain Sci 10:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le TM, Wang W, Zhornitsky S, Dhingra I, Zhang S, Li CSR (2019): Reward sensitivity and electrodermal responses to actions and outcomes in a go/no-go task. PLoS One 14:e0219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, Zucker RA (2014): Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend 141:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilman JM, Smith AR, Bjork JM, Ramchandani VA, Momenan R, Hommer DW (2015): Cumulative gains enhance striatal response to reward opportunities in alcohol-dependent patients. Addict Biol 20:580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quach A, Tervo-Clemmens B, Foran W, Calabro FJ, Chung T, Clark DB, Luna B (2020): Adolescent development of inhibitory control and substance use vulnerability: A longitudinal neuroimaging study. Dev Cogn Neurosci 42:100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodriguez-Thompson AM, Meyer KM, Davidow JY, Van Dijk KRA, Santillana RM, Snyder J, et al. (2020): Examining cognitive control and reward interactions in adolescent externalizing symptoms. Dev Cogn Neurosci 45:100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson SJ, Sayette MA, Delgado MR, Fiez JA (2008): Effect of smoking opportunity on responses to monetary gain and loss in the caudate nucleus. J Abnorm Psychol 117:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Enzi B, Lissek S, Edel MA, Tegenthoff M, Nicolas V, Scherbaum N, et al. (2015): Alterations of monetary reward and punishment processing in chronic cannabis users: An FMRI study. PLoS One 10:e0119150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharp C, Burton PC, Ha C (2011): “Better the devil you know”: A preliminary study of the differential modulating effects of reputation on reward processing for boys with and without externalizing behavior problems. Eur Child Adolesc Psychiatry 20:581–592. [DOI] [PubMed] [Google Scholar]
- 85.Canretié L, Ríos M, de la Gándara BS, Tapia M, Albert J, López-Martín S, Alvarez-Linera J (2009): The striatum beyond reward: Caudate responds intensely to unpleasant pictures. Neuroscience 164:1615–1622. [DOI] [PubMed] [Google Scholar]
- 86.Kim SH, Hamann S (2007): Neural correlates of positive and negative emotion regulation. J Cogn Neurosci 19:776–798. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Ide JS, Zhang S, Hu S, Valchev NS, Tang X, Li CR (2017): Distinct neural processes support post-success and post-error slowing in the stop signal task. Neuroscience 357:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang W, Hu S, Ide JS, Zhornitsky S, Zhang S, Yu AJ, Li CR (2018): Motor preparation disrupts proactive control in the stop signal task. Front Hum Neurosci 12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ide JS, Zhornitsky S, Chao HH, Zhang S, Hu S, Wang W, et al. (2018): Thalamic cortical error-related responses in adult social drinkers: Sex differences and problem alcohol use. Biol Psychiatry Cogn Neurosci Neuroimaging 3:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma SS, Li C-SR, Zhang S, Worhunsky PD, Zhou N, Zhang JT, et al. (2021): Altered functional network activities for behavioral adjustments and Bayesian learning in young men with Internet gaming disorder. J Behav Addict 10:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W, Zhornitsky S, Le TM, Dhingra I, Zhang S, Krystal JH, Li CR (2019): Cue-elicited craving, thalamic activity, and physiological arousal in adult non-dependent drinkers. J Psychiatr Res 116:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lewis CA, Mueller K, Zsido RG, Reinelt J, Regenthal R, Okon-Singer H, et al. (2021): A single dose of escitalopram blunts the neural response in the thalamus and caudate during monetary loss. J Psychiatry Neurosci 46:E319–E327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.