Abstract
Despite substantial advances in knowledge and understanding about Zika virus (ZIKV), limitations in surveillance for this mainly asymptomatic infection constrain attempts to characterize the epidemiological distribution of the virus. Monitoring of fecal waste streams including sewage offers opportunities to track the spread of arboviruses such as ZIKV, known to be present in fecal waste and urine. To demonstrate the feasibility of ZIKV RNA detection in sewage, we examined viral RNA decay in sewage from a local wastewater treatment plant. We added ZIKV (MEX 1–44) to unpasteurized sewage and stored the samples at 4°C, 25°C or 35°C for one month. We extracted nucleic acids from the mixture using a QiaAmp Minelute Virus Spin Kit and measured ZIKV RNA using a TaqPath Zika Virus Kit. We found no appreciable decline in ZIKV RNA detection at 4°C during the month. We estimate that 90% decay of detectable ZIKV RNA occurred after 21 days at 25°C and after 8.5 days at 35°C. Our preliminary work suggests ZIKV RNA can remain detectable in sewage over a range of temperatures and that sewage provides a cost-effective, community diagnostic tool that deserves further investigation as a novel epidemiologic surveillance approach.
Graphical Abstract
Introduction
Zika virus (ZIKV) was first isolated in Uganda in 19471 and identified as an infection in humans in 19542, but it was not until 2007 that a large-scale human outbreak of ZIKV was reported in Yap Island of the Federated States of Micronesia.3 The first detection of ZIKV in the Americas was not until March 2015 when Brazil reported a novel febrile illness outbreak to the World Health Organization.4 Initial reports indicated symptoms were mild and tests were negative for many pathogens.4 By May 2015, Brazil confirmed ZIKV was circulating in the country4 with the initial sites of the outbreak in Northeast Brazil.5 Within two years, ZIKV emerged as a cause of the serious birth defect microcephaly, and the rare neurological disorder Guillain-Barre syndrome.6
Since the initial outbreak of ZIKV in 2015, 87 countries have discovered evidence of local ZIKV transmission.7 More than 20 countries had increases in Guillain-Barre syndrome associated with ZIKV infections, and more than 30 countries had an increase in microcephaly which was likely associated to ZIKV infection.8 Recent evidence suggests that the ZIKV strain circulating in the Americas is responsible for an outbreak of microcephaly in Angola.9 Globally, 61 countries with established competent Aedes aegypti vector populations have not yet documented ZIKV transmission highlighting the potential for continuing transmission in immunologically naïve populations.10
Currently, surveillance efforts mandate that only individuals who display ZIKV-like symptoms undergo laboratory testing for ZIKV infection.11 Furthermore, it is estimated that between 60~80% of ZIKV infected patients are asymptomatic.3, 12 This results in an underestimation of the true prevalence of infection and related complications, leaving estimations biased towards those who seek care or develop symptoms.13 Developing a cost-effective method for detection of ZIKV would allow a more accurate evaluation of the extent of virus circulation and distribution of virus infections in resource-limited settings.
ZIKV is a lipid-enveloped flavivirus that has a structure similar to other mosquito-borne human pathogen flaviviruses including West Nile virus and Dengue virus.14 These flaviviruses have a single-stranded positive sense RNA genome. ZIKV is 50 nm in diameter and its genome is 11kb in length.14 ZIKV RNA is found in human saliva, semen, and urine,15 and recently was detected in feces.16 While mosquitoes are the primary vector for human infection with ZIKV, there is also evidence of human vertical and sexual transmission of this virus.17 Furthermore, there is some evidence to suggest that the structural stability of ZIKV may allow the virus to remain intact and infectious under harsher conditions than other flaviviruses and this is perhaps why it can be found in additional bodily fluids.18 More specifically, researchers demonstrated that ZIKV retained higher levels of infectivity when incubated in cells at 40°C than Dengue virus.18 In addition, researchers demonstrated that ZIKV could remain infectious on surfaces for up to three days in laboratory settings.19 However, due to the presence of the virion envelope, these viruses are not expected to be stable in water under non-physiological salt concentrations.
While the fate and persistence of non-enveloped, enteric viruses in municipal wastewater has been well documented,20–23 the envelopes of viruses (such as ZIKV) are expected to negatively impact their persistence outside of the body and their nucleic acids in environmental waters such as wastewater. However, there is growing evidence that human enveloped virus surrogates, such as Φ6 and murine hepatitis virus, may be stable in the environment and persist in the urban water cycle along with evidence that human pathogenic viruses, such as influenza and SARS, may be stable in water.24–27 Effective methods to detect ZIKV RNA in sewage and environmental waters contaminated by sewage would provide an additional tool for determining the burden of virus infection in a community, especially in communities challenged by limitations of resources for providing standard clinical testing. Furthermore, there is a potential for application of surveillance using wastewater-based epidemiology for other enveloped viruses, such as SARS-COV-2, in this setting.28–29
The goal of this study was to develop a system for analysis of the persistence of ZIKV RNA in environmental waters using commercially available RNA extraction and detection kits. In addition, we evaluated the stability of ZIKV RNA detection in sewage over three temperatures (4°C, 25°C, or 35°C) using this system. The results suggest that ZIKV RNA will remain detectable for weeks in sewage.
Materials and Methods
ZIKV culture: Vero 76 cells were obtained from ATCC and grown in high glucose Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 4 mM glutamine, 1 mM sodium pyruvate, 1X nonessential amino acids and penicillin (100 U/ml)/streptomycin (100 μg/ml) at 37°C in 5% CO2. An aliquot of ZIKV, strain MEX 1–44, was provided by Dr. Robert Tesh from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA), University of Texas Medical Branch, Galveston, TX. A stock of ZIKV was prepared by infecting confluent monolayers of Vero cells in T175 flasks at a MOI of ~ 0.1. The culture media was harvested and fresh media replaced at 48, 72 and 96 hours post infection. Clarified culture fluid was aliquoted and stored at −80°C. The infectivity titer was determined by plaque assay on Vero cells. The 48-hour virus harvest, which had a titer of 2.4 × 106 PFU/ml, was used for the experiments described.
Sewage: Sewage was collected from the influent to the primary clarifier of a wastewater treatment plant in Atlanta, GA. Data from the wastewater treatment plant indicated that the total suspended solids for that week averaged 402 mg/L, volatile suspended solids averaged 323 mg/L and biological oxygen demand averaged 253 mg/L in the influent to the primary clarifier. The sewage was stored at 4°C for no longer than 7 days prior to use.
Controlled laboratory experiments: We spiked ZIKV MEX 1–44 in triplicate to aliquots of sewage to achieve a concentration of 105 PFU/ml. The samples were stored in incubators or the refrigerator and we extracted nucleic acid from the samples on days 0, 7, 14, and 29. Aliquots of sewage that were not spiked with ZIKV were also incubated and extracted to serve as negative biological controls.
Nucleic acid extraction was performed using a QiaAMP Minelute Virus Spin kit. Briefly, 200 μL of virus-spiked sewage was removed from the sample at each time point. Nucleic acid was extracted according to the manufacturer’s recommendations and eluted to a final volume of 50 μL with Buffer AVE. Duplicate 25 μL aliquots of the eluates were stored at −20 °C. For each sample, we used 2 μL of the extract for analysis, equating to 8 μL of spiked wastewater in each reaction. Samples were tested for ZIKV RNA using a one-step real-time reverse transcription polymerase chain reaction (RT-PCR) assay using a TaqPath™ Zika Virus Kit (Fisher Scientific) in duplicate reactions. The primer and probe sequences of the TaqPath ZIKV kit are proprietary products of ThermoFisher but the assay targets the NS1 gene of the Asian lineage of ZIKV.30 The TaqPath kit contains lyophilized reagents that arrive in pre-loaded plates. No modifications to the primers, probe, Mg++, dNTP, polymerase, or additive concentrations were made, and the reactions were performed according to the manufacturers’ protocol. RT-qPCR was performed on an Applied Biosystems™ 7500 Real-Time PCR System Instrument. Cycling conditions were based on the recommendations of the manufacturer: Reverse-transcription at 50°C for 10 min, activation at 95°C for 2 min, PCR amplification for 40 cycles at 95°C for 3 sec and then 60°C for 30 sec. Each run included no-template controls consisting of UV-treated molecular grade water and a positive ZIKV RNA control. For quantification, standard curves were prepared by serially diluting the ATCC ZIKV RNA standard (ATCC® VR-1843DQ) in Buffer AVE to produce a six-point serial dilution. The slope of the two standard curves run on each RT-qPCR plate used in this study resulted in mean values of R2 and RT-qPCR efficiency of 0.98 and 109.15%. We consistently detected our lowest serial dilution at approximately 2 copies per reaction. The RT-qPCR efficiency was calculated with the online ThermoFisher Scientific qPCR Efficiency Calculator. In addition, we checked for RT-qPCR inhibition by testing 1:10 dilutions of samples from day 0 and day 29.31
Data analysis: Quantities of ZIKV RNA estimated from RT-qPCR were used to fit a general linear mixed model. RNA from each replicate (A, B, C) at each temperature condition (4°C, 25°C, 35°C) was extracted at four time points (day 0, 7, 14, 29) for a total of 36 repeated measures. Moderation of temperature in the temporal relationship was explored with testing of interaction effects. The treatment group at 4°C was used as the reference category in the model. In addition, the decay of ZIKV RNA as Ln (C/Co) reductions were analyzed over time and the first order decay constants were calculated.32 The analysis was completed using SAS 9.4 and Microsoft Excel Version 16.6.4 software.
Results and Discussion:
We estimated mean log10 RNA copies per RT-qPCR reaction as 4.91 (SD 0.13) for all ZIKV spiked samples at day 0 using the conservative assumption that our ATCC RNA concentration was 105 copies/μL. This suggests a ratio of ~100 RNA copies per plaque forming unit (pfu); a value approximately one order of magnitude lower than estimates from the literature.33 While the relative infectivity of the virus sample was lower, it did not impact our ability to measure the relative decay of the ZIKV RNA signal as ZIKV RNA was detectable in all spiked samples. Amplification was not observed in any of the biological or RT-qPCR no-template controls. We found no evidence of RT-qPCR inhibition in our analysis of diluted and undiluted samples.31
For the ZIKV RNA detection experiments (Figure 1a and 1b) we found limited decay in the detection of ZIKV RNA at 4°C over the length of the experiment with <1 log10 reduction (<90%) in detection of ZIKV RNA signal over 29 days. At 4°C, there appeared to be an increase in ZIKV RNA detection above baseline at day 7 and day 14 in all three replicates. Reduction in ZIKV RNA detection at 4°C did not occur until day 29. At 25°C, log10 ZIKV RNA detection demonstrated a linear decline from 105 copies per rxn on day 0 to 103 copies on day 29. At 35°C, there was a more variable and rapid decline in log10 ZIKV RNA detection with 105 copies on day 0, 102 copies on day 14, and 101 copies on day 29.
Figure 1a and b.
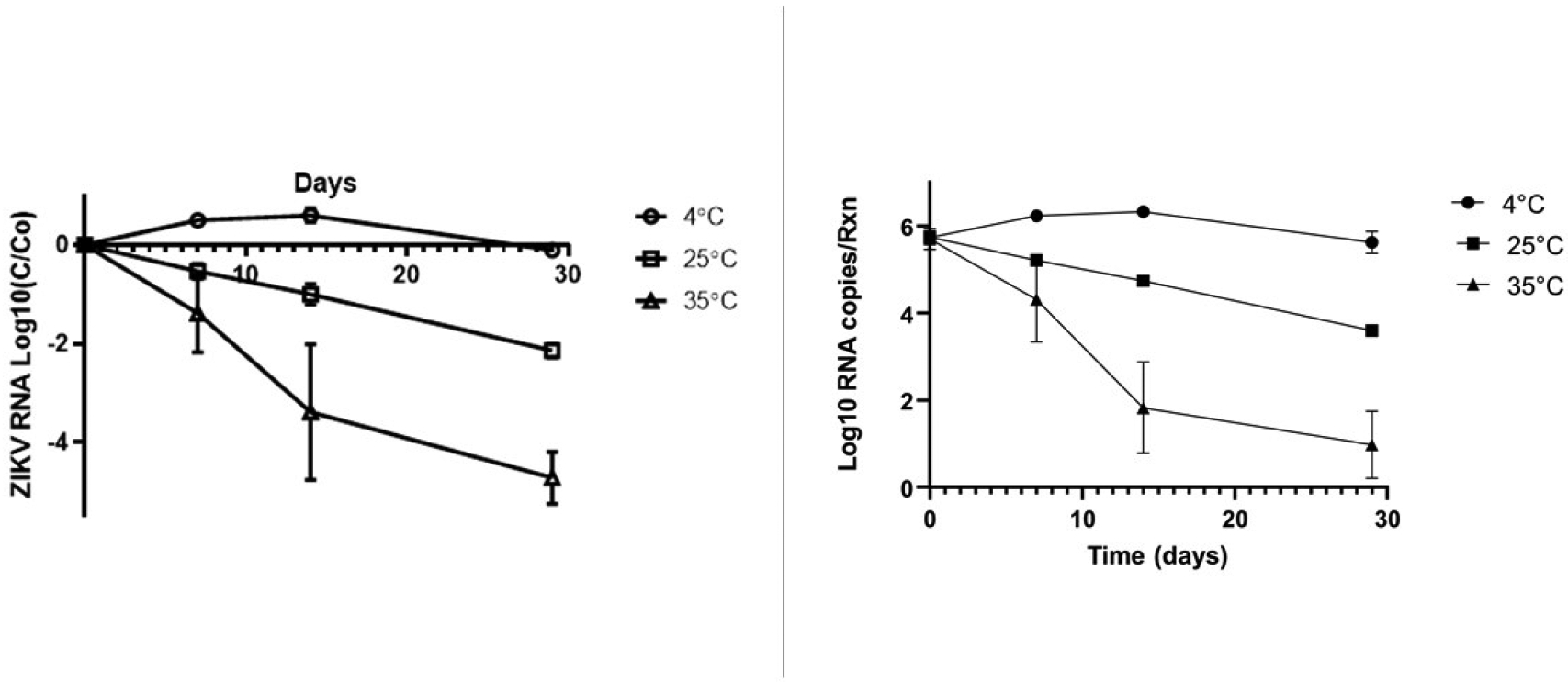
Log 10 (C/Co) ZIKV RNA decay over time in sewage and ZIKV RNA copies per rxn detected in sewage over time at 4°C, 25°C, and 35°C (bars show standard deviation of the mean of the biological replicates)
Using the average (across biological replicates) ZIKV RNA concentration for each temperature, we examined the decay of ZIKV RNA signal over time using a first order decay equation [Log10 (C)/Log10(Co)=−k*t/Ln(10)]. As shown in Table 1, the decay constant of the ZIKV RNA signal at 4°C was 0.01(d−1), at 25°C, it was 0.11(d−1), and at 35°C, it was the most rapid at 0.27(d−1). For 4°C, the R2 value was < 0.01 suggesting a poor fit for the model. A better fit was achieved for 25°C and 35°C (R2 >0.85). Using the first order decay equation, we estimate a 90% reduction in detectable ZIKV RNA would take 230 days at 4°C, 21 days at 25°C and 8.5 days at 35°C.
Table 1.
First order decay constants for ZIKV RNA detection in sewage at three temperatures
| Temperature | First order rate constant k(d−1) (Standard Error) | R2 | T90(d) |
|---|---|---|---|
| 4°C | 0.01 (0.03) | <0.01 | 230 |
| 25°C | 0.11 (0.02) | 0.85 | 21 |
| 35°C | 0.27 (0.04) | 0.92 | 8.5 |
This is the first controlled laboratory experiment examining the persistence of the detection of an arbovirus genome RNA signal in spiked sewage. The TaqPath kit was originally designed to work with serum or urine and we were able to extract ZIKV RNA from sewage and detect it using this commercial test kit. This result suggests that these test kits may be used successfully for testing extracted environmental samples containing ZIKV RNA or the nucleic acid of other enveloped viruses of epidemic or pandemic concern.34–35 While the initial concentration of ZIKV added to the samples was high, the results support further investigation into concentration and recovery methods for detection of ZIKV RNA in sewage and environmental waters.
We found that detection of ZIKV RNA in sewage was consistent at 4°C for one month. At higher temperatures, the ability to detect ZIKV RNA decreased with time but ZIKV RNA detection was still possible for weeks even at 35°C. Wastewater collection, storage and treatment varies greatly across the world. In urban Brazil, more than half of all sewage produced is collected in seweraged networks and only 70% is treated; often in ponds (anaerobic and facultative).36 In these settings, sewage may be conveyed to ponds via open canals. The ability of ZIKV RNA to persist over time under tropical temperatures is an important finding.
Ye et al. (2016)25 examined the stability of two enveloped viruses, murine hepatitis virus and Pseudomonas phage Φ6, spiked into sewage at 25°C and 10°C and found that they remained infectious over weeks. A review of the stability and persistence of more than a dozen enveloped viruses in environmental waters documented that 90% inactivation of virus infectivity occurred from hours to weeks depending on the conditions studied.24 Our study documented a relatively low k-value (suggesting weeks for decay at 4°C). This may be the result of the use of a nucleic acid detection method. Our method detects only a small fragment of the ZIKV RNA (~100 base pairs). In their meta-analysis of decay constants of waterborne mammalian viruses, Boehm et al. (2019)37 documented smaller k-values when nucleic acid detection methods were compared to infectivity assays indicating greater persistence of molecular signals than of infectious viruses. The segment of ZIKV RNA detected in our assay is small and does not indicate that the virion was intact. However, the greater variability and more rapid decay of the ZIKV RNA signal at 35°C suggests more biologic and enzymatic activity that degraded the ZIKV RNA at this higher temperature. Notwithstanding, the ability to detect the ZIKV RNA over weeks in sewage even at 35°C suggests that some portion of the viral RNA may be more protected than originally thought; possibly due to the continued association of viral RNA fragments with capsid dimers.38
There is growing evidence that ZIKV may also be present in human feces as researchers detected ZIKV RNA in a metagenomic analysis of wastewater in Uganda39 and in rectal swabs from ZIKV infected patients.16 Environmental surveillance of sewage for the nucleic acids of ZIKV, or of those of other viruses of pandemic concern, may be advantageous partly because widespread, community-level surveillance of viruses causing mostly asymptomatic infections may not be logistically or economically feasible. Furthermore, although the worldwide spread of ZIKV has subsided, more than 2 billion people live in areas at risk of transmission and re-emergence.40–41
Our study adds support for the ability of an enveloped virus RNA to remain detectable in sewage for weeks. The results highlight the potential for examining ZIKV RNA stability in environmental waters as an epidemiologic screening tool, especially in those waters that receive human waste. The use of PCR technologies to surveil for a circulating virus could provide a method that has a more rapid turn-around time than viral infectivity assays and allow real-time tracking during an outbreak. Furthermore, the study results suggest that archived samples of wastewater collected during environmental surveillance for other pathogens, such as poliovirus, would be possible sources for exploration of ZIKV prevalence during the recent ZIKV outbreak, especially in high burden countries such as Brazil.
This study also adds knowledge to the growing field of wastewater epidemiology for infectious disease surveillance.42–43 The assumption that the genome RNAs of enveloped viruses rapidly degrade below detectable levels in water is in contrast to our findings. Utilizing wastewater samples to detect the circulation of an arbovirus or other non-enteric viruses within a community may be a viable alternative to or complement for clinical surveillance data especially with infectious diseases that may be primarily asymptomatic.
Acknowledgements:
We would like to thank Sandie Wold and Fred Ede for their assistance in culturing ZIKV. This research was supported in part by a Faculty International Partnership Engagement grant from Georgia State University, a graduate research assistantship from the School of Public Health at Georgia State University, and a National Institute of Allergy and Infectious Diseases grant 1R21AI138206-01A1. In addition, Fisher donated three TaqPath kits.
References
- 1.Dick GW; Kitchen SF; Haddow AJ, Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952, 46 (5), 509–20. [DOI] [PubMed] [Google Scholar]
- 2.Macnamara FN, Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954, 48 (2), 139–45. [DOI] [PubMed] [Google Scholar]
- 3.Duffy MR; Chen TH; Hancock WT; Powers AM; Kool JL; Lanciotti RS; Pretrick M; Marfel M; Holzbauer S; Dubray C; Guillaumot L; Griggs A; Bel M; Lambert AJ; Laven J; Kosoy O; Panella A; Biggerstaff BJ; Fischer M; Hayes EB, Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009, 360 (24), 2536–43. [DOI] [PubMed] [Google Scholar]
- 4.The History of Zika Virus. https://www.who.int/emergencies/zika-virus/timeline/en/.
- 5.Campos GS; Bandeira AC; Sardi SI, Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis 2015, 21 (10), 1885–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Araujo TVBX, R. AA; Miranda-Filho DB; Souza WV; Montarroyos UR; de Melo APL; Valongueiro S; de Albuquerque M; Braga C; Filho SPB; Cordeiro MT; Vazquez E; Cruz D; Henriques CMP; Bezerra LCA; Castanha P; Dhalia R; Marques-Junior ETA; Martelli CMT; Rodrigues LC; investigators from the Microcephaly Epidemic Research, G.; Brazilian Ministry of, H.; Pan American Health, O.; Instituto de Medicina Integral Professor Fernando, F.; State Health Department of, P., Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case- control study. The Lancet Infectious Disease 2018, 18 (3), 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zika Epidemiology Update. https://www.who.int/emergencies/diseases/zika/zika-epidemiology-update-july-2019.pdf?ua=1.
- 8.Situation report: Zika virus, microcephaly, Guillain-Barré syndrome. http://www.who.int/iris/handle/10665/251462.
- 9.Hill SC; Vasconcelos J; Neto Z; Jandondo D; Ze-Ze L; Aguiar RS; Xavier J; Theze J; Mirandela M; Micolo Candido AL; Vaz F; Sebastiao CDS; Wu CH; Kraemer MUG; Melo A; Schamber-Reis BLF; de Azevedo GS; Tanuri A; Higa LM; Clemente C; da Silva SP; da Silva Candido D; Claro IM; Quibuco D; Domingos C; Pocongo B; Watts AG; Khan K; Alcantara LCJ; Sabino EC; Lackritz E; Pybus OG; Alves MJ; Afonso J; Faria NR, Emergence of the Asian lineage of Zika virus in Angola: an outbreak investigation. Lancet Infect Dis 2019, 19 (10), 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Countries and territories with current or previous Zika virus transmission. https://www.who.int/emergencies/diseases/zika/countries-with-zika-and-vectors-table.pdf.
- 11.Guidelines for surveillance of Zika virus disease and its complications. http://iris.paho.org/xmlui/handle/123456789/28405.
- 12.Rodriguez-Barraquer I; Costa F; Nascimento EJM; Nery NJ; Castanha PMS; Sacramento GA; Cruz J; Carvalho M; De Olivera D; Hagan JE; Adhikarla H; Wunder EA Jr.; Coelho DF; Azar SR; Rossi SL; Vasilakis N; Weaver SC; Ribeiro GS; Balmaseda A; Harris E; Nogueira ML; Reis MG; Marques ETA; Cummings DAT; Ko AI, Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019, 363 (6427), 607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozier M; Adams L; Febo MF; Torres-Aponte J; Bello-Pagan M; Ryff KR; Munoz-Jordan J; Garcia M; Rivera A; Read JS; Waterman SH; Sharp TM; Rivera-Garcia B, Incidence of Zika Virus Disease by Age and Sex - Puerto Rico, November 1, 2015-October 20, 2016. MMWR Morb Mortal Wkly Rep 2016, 65 (44), 1219–1223. [DOI] [PubMed] [Google Scholar]
- 14.Hasan SS; Sevvana M; Kuhn RJ; Rossmann MG, Structural biology of Zika virus and other flaviviruses. Nat Struct Mol Biol 2018, 25 (1), 13–20. [DOI] [PubMed] [Google Scholar]
- 15.Bingham AM; Cone M; Mock V; Heberlein-Larson L; Stanek D; Blackmore C; Likos A, Comparison of Test Results for Zika Virus RNA in Urine, Serum, and Saliva Specimens from Persons with Travel-Associated Zika Virus Disease - Florida, 2016. MMWR Morb Mortal Wkly Rep 2016, 65 (18), 475–8. [DOI] [PubMed] [Google Scholar]
- 16.Botto-Menezes CHA; Neto AM; Calvet GA; Kara EO; Lacerda MVG; Castilho MDC; Stroher U; Antunes de Brito CA; Modjarrad K; Broutet N; Brasil P; Bispo de Filippis AM; Franca RFO; Team ZS, Zika Virus in Rectal Swab Samples. Emerg Infect Dis 2019, 25 (5), 951–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zika Virus Fact Sheet. https://www.who.int/en/news-room/fact-sheets/detail/zika-virus.
- 18.Kostyuchenko VA; Lim EX; Zhang S; Fibriansah G; Ng TS; Ooi JS; Shi J; Lok SM, Structure of the thermally stable Zika virus. Nature 2016, 533 (7603), 425–8. [DOI] [PubMed] [Google Scholar]
- 19.Muller JA; Harms M; Schubert A; Jansen S; Michel D; Mertens T; Schmidt-Chanasit J; Munch J, Inactivation and Environmental Stability of Zika Virus. Emerg Infect Dis 2016, 22 (9), 1685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puig M; Jofre J; Lucena F; Allard A; Wadell G; Girones R, Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl Environ Microbiol 1994, 60 (8), 2963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama H; Haramoto E; Oguma K; Yamashita H; Tajima A; Nakajima H; Ohgaki S, One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res 2008, 42 (6–7), 1441–8. [DOI] [PubMed] [Google Scholar]
- 22.Fumian TM; Leite JP; Castello AA; Gaggero A; Caillou MS; Miagostovich MP, Detection of rotavirus A in sewage samples using multiplex qPCR and an evaluation of the ultracentrifugation and adsorption-elution methods for virus concentration. J Virol Methods 2010, 170 (1–2), 42–6. [DOI] [PubMed] [Google Scholar]
- 23.Tambini G; Andrus JK; Marques E; Boshell J; Pallansch M; de Quadros CA; Kew O, Direct detection of wild poliovirus circulation by stool surveys of healthy children and analysis of community wastewater. J Infect Dis 1993, 168 (6), 1510–4. [DOI] [PubMed] [Google Scholar]
- 24.Aquino de Carvalho N, et al. , Evaluation of Phi6 Persistence and Suitability as an Enveloped Virus Surrogate. Environmental Science & Technology Aug 2017, 51, 8692–8700. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y; Ellenberg RM; Graham KE; Wigginton KR, Survivability, Partitioning, and Recovery of Enveloped Viruses in Untreated Municipal Wastewater. Environ Sci Technol 2016, 50 (10), 5077–85. [DOI] [PubMed] [Google Scholar]
- 26.Dublineau A; Batejat C; Pinon A; Burguiere AM; Leclercq I; Manuguerra JC, Persistence of the 2009 pandemic influenza A (H1N1) virus in water and on non-porous surface. PLoS One 2011, 6 (11), e28043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabenau HF; Cinatl J; Morgenstern B; Bauer G; Preiser W; Doerr HW, Stability and inactivation of SARS coronavirus. Med Microbiol Immunol 2005, 194 (1–2), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed W; Angel N; Edson J; Bibby K; Bivins A; O’Brien JW; Choi PM; Kitajima M; Simpson SL; Li J; Tscharke B; Verhagen R; Smith WJM; Zaugg J; Dierens L; Hugenholtz P; Thomas KV; Mueller JF, First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ 2020, 728, 138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodder W; de Roda Husman AM, SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol 2020, 5 (6), 533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pyke AT; Daly MT; Cameron JN; Moore PR; Taylor CT; Hewitson GR; Humphreys JL; Gair R, Imported zika virus infection from the cook islands into australia, 2014. PLoS Curr 2014, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehm AB; Van De Werfhorst LC; Griffith JF; Holden PA; Jay JA; Shanks OC; Wang D; Weisberg SB, Performance of forty-one microbial source tracking methods: a twenty-seven lab evaluation study. Water Res 2013, 47 (18), 6812–28. [DOI] [PubMed] [Google Scholar]
- 32.Geeraerd AH; Valdramidis VP; Van Impe JF, GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int J Food Microbiol 2005, 102 (1), 95–105. [DOI] [PubMed] [Google Scholar]
- 33.Priye A; Bird SW; Light YK; Ball CS; Negrete OA; Meagher RJ, A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci Rep 2017, 7, 44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu F; Xiao A; Zhang J; Gu X; Lee WL; Kauffman K; Hanage W; Matus M; Ghaeli N; Endo N; Duvallet C; Moniz K; Erickson T; Chai P; Thompson J; Alm E, SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv 2020, 2020.04.05.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medema G; Heijnen L; Elsinga G; Italiaander R; Brouwer A, Presence of SARS-Coronavirus-2 in sewage. medRxiv 2020, 2020.03.29.20045880. [DOI] [PubMed] [Google Scholar]
- 36.von Sperling M Urban wastewater treatment in Brazil; 2016.
- 37.Boehm AB; Silverman AI; Schriewer A; Goodwin K, Systematic review and meta-analysis of decay rates of waterborne mammalian viruses and coliphages in surface waters. Water Res 2019, 164, 114898. [DOI] [PubMed] [Google Scholar]
- 38.Tan TY; Fibriansah G; Kostyuchenko VA; Ng TS; Lim XX; Zhang S; Lim XN; Wang J; Shi J; Morais MC; Corti D; Lok SM, Capsid protein structure in Zika virus reveals the flavivirus assembly process. Nat Commun 2020, 11 (1), 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien E; Nakyazze J; Wu H; Kiwanuka N; Cunningham W; Kaneene JB; Xagoraraki I, Viral diversity and abundance in polluted waters in Kampala, Uganda. Water Res 2017, 127, 41–49. [DOI] [PubMed] [Google Scholar]
- 40.Musso D; Ko AI; Baud D, Zika Virus Infection - After the Pandemic. N Engl J Med 2019, 381 (15), 1444–1457. [DOI] [PubMed] [Google Scholar]
- 41.Kasprzykowski JI; Fukutani KF; Fabio H; Fukutani ER; Costa LC; Andrade BB; Queiroz ATL, A recursive sub-typing screening surveillance system detects the arising of the ZIKV African lineage in Brazil: Is there risk of a new epidemic? Int J Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 42.Hendriksen RS; Lukjancenko O; Munk P; Hjelmso MH; Verani JR; Ng’eno E; Bigogo G; Kiplangat S; Oumar T; Bergmark L; Roder T; Neatherlin JC; Clayton O; Hald T; Karlsmose S; Pamp SJ; Fields B; Montgomery JM; Aarestrup FM, Pathogen surveillance in the informal settlement, Kibera, Kenya, using a metagenomics approach. PLoS One 2019, 14 (10), e0222531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sims N; Kasprzyk-Hordern B, Future perspectives of wastewater-based epidemiology: Monitoring infectious disease spread and resistance to the community level. Environ Int 2020, 139, 105689. [DOI] [PMC free article] [PubMed] [Google Scholar]


