Abstract
BACKGROUND AND PURPOSE:
Cortical venous outflow has emerged as a robust measure of collateral blood flow in acute ischemic stroke. The addition of deep venous drainage to this assessment may provide valuable information to further guide the treatment of these patients.
MATERIALS AND METHODS:
We performed a multicenter retrospective cohort study of patients with acute ischemic stroke treated by thrombectomy between January 2013 and January 2021. The internal cerebral veins were scored on a scale of 0–2. This metric was combined with existing cortical vein opacification scores to create a comprehensive venous outflow score from 0 to 8 and stratify patients as having favorable-versus-unfavorable comprehensive venous outflow. Outcome analyses were primarily conducted using the Mann-Whitney U and χ2 tests.
RESULTS:
Six hundred seventy-eight patients met the inclusion criteria. Three hundred fifteen were stratified as having favorable comprehensive venous outflow (mean age, 73 years; range, 62–81 years; 170 men), and 363, as having unfavorable comprehensive venous outflow (mean age, 77 years; range, 67–85 years; 154 men). There were significantly higher rates of functional independence (mRS 0–2; 194/296 versus 37/352, 66% versus 11%, P < .001) and excellent reperfusion (TICI 2c/3; 166/313 versus 142/358, 53% versus 40%, P < .001) in patients with favorable comprehensive venous outflow. There was a significant increase in the association of mRS with the comprehensive venous outflow score compared with the cortical vein opacification score (−0.74 versus −0.67, P = .006).
CONCLUSIONS:
A favorable comprehensive venous profile is strongly associated with functional independence and excellent postthrombectomy reperfusion. Future studies should focus on patients with venous outflow status that is discrepant with the eventual outcome.
Collateral blood flow supplies critical blood flow to the penumbra and limits growth of the ischemic core in patients presenting with acute ischemic stroke (AIS).1-4 Robust collaterals are associated with higher rates of successful reperfusion and better clinical outcomes in patients with AIS with large-vessel occlusion (AIS-LVO) after endovascular thrombectomy.5,6 Although collateral blood flow is governed by both arterial inflow and venous outflow (VO), arterial collaterals have historically been used to measure collateral robustness. More recently, the importance of cortical VO as a measure of collateral blood flow through ischemic brain tissue and into the veins draining this tissue has been recognized.6-18
Currently VO is measured by the cortical vein opacification score (COVES), which characterizes opacification of the superficial middle cerebral vein, vein of Labbé, and sphenoparietal sinus, each on a scale of 0–2, with 0 representing zero opacification and 2 representing complete opacification and robust VO.10 Favorable cortical VO (dichotomized as COVES 3–6) is independently associated with excellent tissue reperfusion and favorable long-term functional outcomes.6,13,18 Although COVES has proved to be an excellent measure of ischemic brain perfusion and long-term outcomes, it measures only cortical venous drainage that represents just a portion of the venous egress from the MCA territory.
The basal ganglia and striatum derive their arterial supply from the lenticulostriate arteries that arise from the M1 segment of the MCA, and the venous drainage of this territory is predominately into the internal cerebral veins (ICVs).19,20 It is unclear whether assessment of venous opacification of the ICVs to the existing COVES score provides additional information to cortical venous outflow assessment.
In this study, we evaluated whether a comprehensive VO score that includes both cortical (COVES) and deep (ICV) veins that drain the MCA territory predicts favorable outcomes in patients with AIS-LVO who are treated by endovascular thrombectomy.
MATERIALS AND METHODS
Data generated or analyzed during the study are available from the corresponding author by request.
Study Design
We performed a multicenter retrospective cohort study of consecutive patients undergoing thrombectomy triage for AIS treatment at 2 comprehensive stroke centers between January 2013 and January 2021. The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines were followed to conduct this study.
Standard Protocol Approvals, Registrations, and Patient Consent
The study protocol was approved by the institution review boards of both study centers, complied with the Health Insurance Portability and Accountability Act, and followed the guidelines of the Declaration of Helsinki. Patient informed consent was waived by our review boards for this retrospective study.
Patient Inclusion and Exclusion Criteria
Patients were identified from prospectively maintained stroke databases at each center that had previously been processed for patients who underwent thrombectomy within 16 hours of stroke onset and had appropriate follow-up imaging. Demographic, imaging, and clinical data were obtained from the electronic medical records. Inclusion and exclusion criteria are detailed in the Online Supplemental Data.
Imaging Analysis
All CT and MR perfusion studies were automatically analyzed with RApid processing of PerfusIon and Diffusion (RAPID; iSchemaView). The ischemic core was defined as the volume of tissue with a 70% reduction in CBF relative to the contralateral cerebral hemisphere on CTP or the volume of tissue with restricted diffusion (ADC <620 × 10−6 mm2/s) on DWI.21
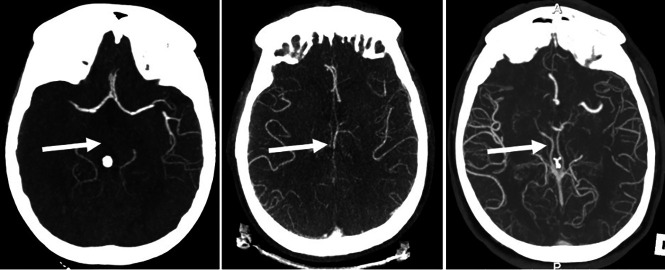
In all included patients, COVES had previously been scored by 2 neuroradiologists. The new ICV opacification metric was scored by 2 independent radiologists on both the ipsilateral and contralateral ICVs in alignment with the COVES scoring system: 0 = not visible, 1 = moderate opacification, and 2 = full opacification (Fig 1).
FIG 1.
Degrees of internal cerebral vein opacification. From left to right, zero opacification of the ICV, moderate opacification of the ICV, and full opacification of the ICV.
COVES and the ICV metric were consolidated into a new comprehensive VO (CVO) score that measures both the cortical and deep venous opacification score. The CVO ranged from 0 (no opacification of the 1 deep and 3 cortical venous pathways) to 8 (full opacification of the 1 deep and 3 cortical venous pathways). Analogous to existing VO thresholds, the new CVO was defined as favorable (CVO+) by a score of 4–8 and unfavorable (CVO–) by a score of 0–3.
Postthrombectomy TICI scores were previously interpreted on DSA images. Successful reperfusion status was defined as TICI ≥ 2b (50%–100% revascularization).22 Excellent reperfusion status was defined as TICI 2c/3 (95%–100% revascularization).23
Infarct volume was acquired by manual segmentation on noncontrast CT images or b = 1000 DWIs using the software package Horos Project (Version 3.3.6; Horos) by a single neuroradiologist. Final infarct volumes were acquired on noncontrast CT images 48–72 hours after thrombectomy.
Readers were blinded to the clinical and outcome information of each patient during the scoring process.
Outcome Measures
Our primary outcome was functional independence at 90-day follow-up, defined by an mRS score of 0–2.24 This score was previously obtained from each patient by a stroke neurologist or specialized study nurse at follow-up. Secondary outcomes included successful reperfusion status, excellent reperfusion status, excellent functional outcome (mRS 0–1), baseline ischemic core volume, final infarct volume, and change in infarct volume from the initial to follow-up examination.
Statistical Analyses
All statistical analyses were performed with SPSS Statistics, Version 29.0 (IBM). Patient demographics and imaging variables including infarct volume were compared between patients with CVO+ and CVO– by the Mann-Whitney U test; χ2 analysis was performed to determine statistically significant differences in mRS and TICI score distributions between the 2 groups. The Kendall τ bivariate correlations were used to assess the relationships of CVO scores with mRS, TICI, baseline ischemic core volume, final infarct volume, and change in infarct volume. Significant differences in correlations between COVES versus CVO against these variables were evaluated by converting the Kendall τ to the Pearson coefficient by the equation r = sin (0.5 × π × τ) and performing a Fisher Z-test for comparison. We set α at the .05 level for significance and reported 2-tailed results for all tests.
RESULTS
Patient Inclusion and Exclusion
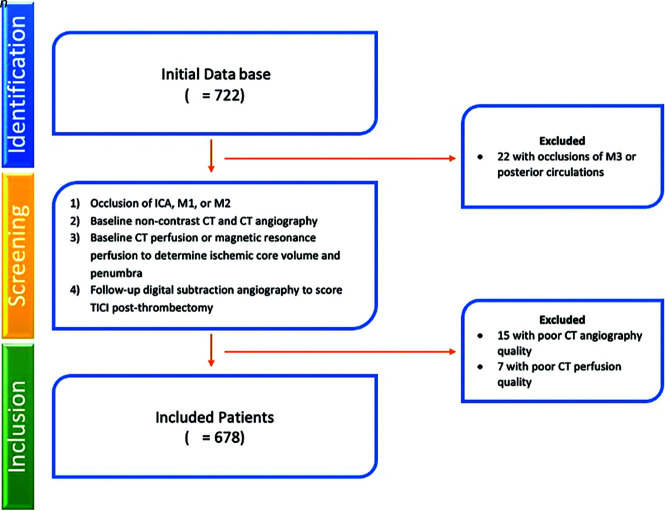
Of 722 patients who underwent thrombectomy and had appropriate follow-up imaging, 678 met the inclusion criteria (Fig 2). Twenty-two patients were excluded due to the occlusion being in the third segment of the MCA or in the posterior circulation. Fifteen patients were excluded due to poor CT angiogram quality, and 7 patients were excluded due to poor CTP image quality.
FIG 2.
Patient screening and inclusion flowchart.
COVES versus CVO for VO Grading
COVES and the ICV metric were combined to create a new CVO score, as detailed in the Materials and Methods. Per the original COVES scoring, there would be 261 patients in the VO+ group (38%) and 417 patients in the VO– group (62%). With the new CVO scoring, 315 patients were assigned to the favorable venous outflow group represented by CVO+ (46%), a significantly higher proportion than by the COVES scoring (P < .001). Accordingly, there were 363 patients in the CVO– group (54%). Interrater agreement for ICV opacification scoring on the affected side was substantial, κ = (0.77; 95% CI, 0.60–0.94). Interrater agreement for ICV opacification scoring on the contralateral side was almost perfect, κ = (0.90; 95% CI, 0.71–1.00).
Patient Demographics
Compared with patients with CVO–, those with CVO+ were younger (median age, 73 years; interquartile range [IQR], 62–81 years versus 77 years; IQR, 67–85 years; P < .001), presented with fewer baseline deficits (median NIHSS, 11; IQR, 7–17 versus 17; IQR, 12–20; P < .001), and were more likely to have received IV tPA (189/311 versus 148/355, 61% versus 42%; P < .001) (Table 1). There were fewer women in the CVO+ group than the CVO– group (145/315 versus 209/363, 46% versus 58%; P = .003).
Table 1:
Demographic profile of patients by VO status
| CVO+(n = 315) | CVO–(n = 363) | P Value | |
|---|---|---|---|
| Age (median) (IQR) (yr) | 73 (62–81) | 77 (67–85) | <.001 |
| Female (No.) (%) | 145 (46%) | 209 (58%) | .003 |
| Atrial fibrillation (No.) (%) (n = 311, 361) | 115 (37%) | 148 (41%) | .287 |
| Hypertension (No.) (%) (n = 313, 361) | 203 (65%) | 261 (72%) | .037 |
| Blood glucose level (median) (IQR) (mg/dL) (n = 272, 309) | 117 (103–144) | 123 (106–154) | .028 |
| Diabetes (No.) (%) (n = 313, 360) | 53 (17%) | 78 (22%) | .122 |
| Hyperlipidemia (No.) (%) (n = 276, 317) | 82 (30%) | 93 (29%) | .921 |
| Smoker (current/prior) (No.) (%) (n = 292, 342) | 85 (29%) | 79 (23%) | .085 |
| NIHSS score on presentation (median) (IQR) | 11 (7–17) | 17 (12–20) | <.001 |
| tPA administration (No.) (%) (n = 311, 355) | 189 (61%) | 148 (42%) | <.001 |
Imaging Characteristics
The median baseline ischemic core volume in the CVO+ group was significantly smaller than in the CVO– group (5 versus 18 mL; P < .001). The final infarct volume at 48–72 hours in the CVO+ group was also significantly smaller than in the CVO– group (12.4 versus 54.3 mL; P < .001). Concordantly, ischemic core volume growth occurred to a significantly less extent in the CVO+ group (+5.9 versus +32.1 mL; P < .001) (Table 2).
Table 2:
Imaging metrics at baseline and follow-up by VO status
| CVO+(n = 315) | CVO−(n = 363) | P Value | |
|---|---|---|---|
| ASPECTS (median) (IQR) | 8 (7–10) | 7 (6–9) | <.001 |
| Baseline infarct volume (median) (IQR) (n = 309, 359) | 5 (0–17.5) | 18 (3–48) | <.001 |
| Follow-up infarct volume (median) (IQR) (n = 304, 343) | 12.4 (4.8–34.2) | 54.3 (22.1–120.3) | <.001 |
| Change in infarct volume (median) (IQR) (n = 298, 340) | +5.9 (−0.4–22.1) | +32.1 (4.1–82.7) | <.001 |
Cortical and Deep VO Associated with Functional Outcomes
A total of 648 patients presented for 90-day follow-up and had recorded mRS scores. The Kendall τ for the association of COVES with mRS was −0.47, which indicates a moderate negative correlation. The Kendall τ for the association of the ICV metric with mRS was −0.49, which denotes a similar relationship. With regard to the combined CVO score, the association with mRS was slightly stronger at −0.53. The values of τ for COVES and CVO were converted to Pearson r, resulting in −0.67 and −0.74, respectively. The increase in the association of mRS with CVO compared with COVES was significant at a Z-test statistic of 2.5 by the Fisher test (P = .006).
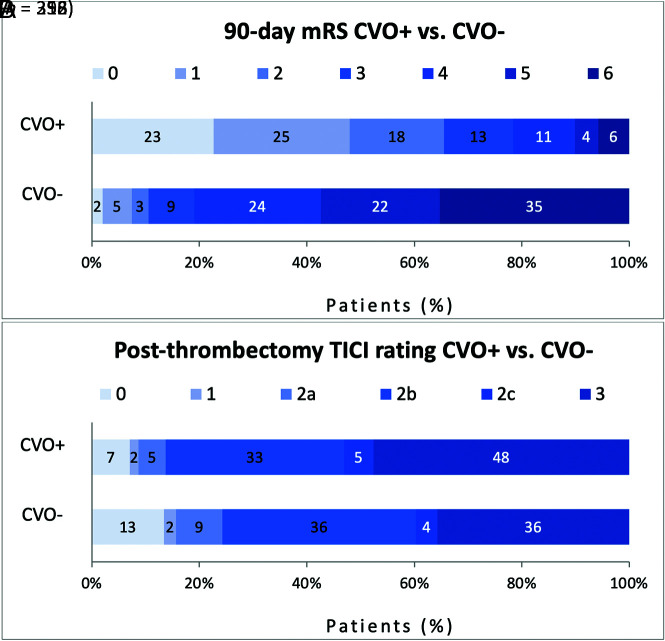
There were 296 patients in the CVO+ group and 352 patients in the CVO− group with recorded mRS scores. There was a significantly different distribution of mRS scores in the CVO+ group compared with the CVO– group (P < .001), with a greater proportion of favorable mRS scores observed in the CVO+ group. Concordantly, there were significantly higher rates of functional independence (mRS 0–2; 194/296 versus 37/352, 66% versus 11%; P < .001) and excellent functional outcome (mRS 0–1; 142/296 versus 26/352, 48% versus 7%; P < .001) in patients with CVO+ compared with those with CVO–. The mRS score distribution in patients with CVO+ versus those with CVO– is shown in Fig 3A. Level-by-level data may be found in the Online Supplemental Data.
FIG 3.
mRS score (A) and TICI score (B) distribution of patients with CVO+ versus CVO–. Within each mRS or TICI score category, the percentage of patients with that score is noted.
Cortical and Deep VO Associated with Angiographic Success
The TICI was scored on a total of 671 patients after thrombectomy. There were 313 patients in the CVO+ group and 358 patients in the CVO– group. There were significantly higher rates of successful reperfusion (TICI ≥ 2b; 270/313 versus 271/358, 86% versus 76%; P = .001) and excellent reperfusion (TICI 2c/3; 166/313 versus 142/358, 53% versus 40%; P < .001) in patients with CVO+ compared with those with CVO–. The TICI score distribution in patients with CVO+ versus those with CVO– is shown in Fig 3B. Level-by-level data may be found in the Online Supplemental Data.
DISCUSSION
In this study, we describe a new comprehensive venous outflow score (CVO) for the assessment of venous outflow in patients with anterior circulation AIS-LVO. We found that CVO was strongly associated with functional outcomes to a significantly greater degree than the superficial cortical venous outflow (COVES) scale, and patients with favorable CVO (CVO+) were more likely to achieve functional independence and excellent functional outcome by 90-day follow-up compared with those with unfavorable CVO (CVO–).
Recently, the importance of considering collateral blood flow as a more complete cascade of blood flow15,16 or as a collaterome unit7,9 rather than as a dichotomous assessment of pial arteries has been appreciated. For example, the cerebral collateral cascade provides a complete assessment of collateral blood flow through the pial arteries, tissue-level collaterals, and VO. Each of these 3 components of collateral blood flow may be assessed through specific imaging parameters to determine the overall robustness of collateral blood flow through ischemic brain tissue. Work on the cerebral collateral cascade ultimately showed that patients with a favorable metric in at least one of these components had significantly better functional outcomes than patients with a comprehensively unfavorable cerebral collateral cascade. However, these prior studies have not considered a more comprehensive assessment of VO from the anterior circulation that includes both deep venous and superficial cortical venous egress from this vascular territory.
Our finding that CVO+ has such a strong correlation with favorable functional outcomes further underscores VO as an important measure of collateral blood flow in patients with AIS-LVO. The association of CVO+ with favorable functional outcomes was stronger than that of superficial venous outflow (COVES) alone.6,10,13,18 These results parallel the evolution in thinking about collateral blood flow through the ischemic brain more comprehensively, and the robustness of the correlation with CVO and outcome suggests that VO may be the most important component of collateral blood flow. Validation of these findings with prospective studies is warranted.
We also found that patients with CVO+ were significantly more likely to achieve successful (TICI 2b/2c/3) and excellent reperfusion (TICI 2c/3) after thrombectomy. This association of CVO with reperfusion success is a major finding because several prior studies have failed to demonstrate reperfusion differences between patients with favorable-versus-unfavorable collateral scores. Work on Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) showed that good pial arterial collaterals alone were not predictive of reperfusion success or functional outcomes in patients with AIS.25 The Tan score on pial arterial collaterals and the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) score of collaterals on DSA similarly were not able to distinguish reperfusion differences.26,27 Favorable cortical VO, however, has been independently associated with excellent vessel reperfusion after thrombectomy, regardless of arterial collateral status. Venous biomarkers of CBF may indeed be more sensitive for the assessment of tissue perfusion and collateral robustness because they reflect blood flow after ischemic tissue has been permeated.6 Our CVO scale increases the robustness of venous profile evaluation and contributes further to this association.
We also found baseline ischemic core volume, final infarct volume, and change in the infarct volume to be significantly lower in patients with CVO+. Although favorable cortical VO has already been associated with slower progression of infarct edema and lower rates of reperfusion hemorrhage, there is key information missing in cortical VO scoring.12,14 Cerebral tissue drained predominantly by deep veins, such as the striatocapsular region, often has a dearth of collateral supply and may be more susceptible to edema and reperfusion hemorrhage after mechanical thrombectomy. Robust deep venous outflow through the ICVs may, thus, play a key role in limiting infarct edema, decreasing the risk of reperfusion hemorrhage, and ultimately minimizing the evolution of the ischemic core in patients with stroke affecting those regions.19,20 Deep VO may, thus in part, explain the significantly better functional status of patients with favorable CVO at 90-day follow-up.
An additional interesting finding must be noted; patients with favorable and unfavorable CVO had similar rates of technical success of 50%–95% vessel reperfusion. The uncoupling of reperfusion success with functional outcomes at 90 days in this group comparison suggests the potential futility of sub-100% reperfusion in patients with poor CVO. This result warrants additional study because CVO may prove crucial in defining a population of high interest for neuroprotective trials.
Several limitations of our study must be noted. The retrospective design inherently introduces bias and may not be optimal at determining thresholds on scoring systems such as COVES or CVO.28 Future prospective studies evaluating cortical and deep VO may be important in testing the thresholds we have discussed in this article. There may also be confounding variables that were not accounted for in the initial data collection, such as prior carotid stenosis and endarterectomy, characteristics of atrial fibrillation in patients with this condition, prior history of venous thrombosis, or a history of cerebral endovascular or open procedures. This limitation cannot be corrected in a retrospective analysis of this nature. The imaging protocols at 2 separate comprehensive stroke centers were standardized, but given the variability of venous opacification with subtle changes in the timing of the contrast injection, this limitation must also be noted. Finally, single-phase CTA was used in this study, which may limit the evaluation of the entire venous phase. A recent study evaluated ICV opacification at different phases to stratify the degree of venous outflow, and this would be a valuable addition to our scoring system with the availability of multiphase imaging in future studies.29
Considering the findings of our study and the poor collaterals of deep venous outflow regions, future work focusing on deep VO alone as a predictor of safety and functional outcomes would be valuable. Patients with reperfusion success but unfavorable VO should also be stratified further to determine what may, nonetheless, predict a good functional outcome at 90 days.
CONCLUSIONS
The addition of deep VO scoring to the COVES resulted in the new CVO score, which demonstrated a significantly greater association with functional outcomes at 90 days than COVES alone. Patients with favorable CVO had significantly higher rates of long-term functional independence and excellent reperfusion and significantly less baseline ischemic core burden and evolution by 48–72 hours. Our findings suggest that CVO is the most predictive marker of collateral blood flow and patient success after mechanical thrombectomy to date. Future work should study the uncoupling of reperfusion success with functional outcomes in patients with poor VO and evaluate deep VO by itself as a predictor of functional and reperfusion outcomes in patients with AIS.
ABBREVIATIONS:
- AIS
acute ischemic stroke
- COVES
cortical vein opacification score
- CVO
comprehensive venous outflow
- CVO+
favorable comprehensive venous outflow
- CVO–
unfavorable comprehensive venous outflow
- ICV
internal cerebral vein; IQR = interquartile range
- LVO
large-vessel occlusion
- VO
venous outflow
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Bang OY, Saver JL, Buck BH, et al. ; UCLA Collateral Investigators. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008;79:625–29 10.1136/jnnp.2007.132100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 2011;42:693–99 10.1161/STROKEAHA.110.595256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke. Stroke 2015;46:3302–09 10.1161/STROKEAHA.115.010508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasawa E, Ichijo M, Ishibashi S, et al. Acute development of collateral circulation and therapeutic prospects in ischemic stroke. Neural Regen Res 2016;11:368–71 10.4103/1673-5374.179033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks MP, Lansberg MG, Mlynash M, et al. ; Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 Investigators. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke 2014;45:1035–39 10.1161/STROKEAHA.113.004085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faizy TD, Kabiri R, Christensen S, et al. Association of venous outflow profiles and successful vessel reperfusion after thrombectomy. Neurology 2021. May 5. [Epub ahead of print] 10.1212/WNL.0000000000012106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebeskind DS. Mapping the collaterome for precision cerebrovascular health: theranostics in the continuum of stroke and dementia. J Cereb Blood Flow Metab 2018;38:1449–60 10.1177/0271678X17711625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Ding J, Leng X, et al. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc Neurol 2018;3:117–30 10.1136/svn-2017-000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebeskind DS. Imaging the collaterome: a stroke renaissance. Curr Opin Neurol 2015;28:1–3 10.1097/WCO.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 10.Hoffman H, Ziechmann R, Swarnkar A, et al. Cortical vein opacification for risk stratification in anterior circulation endovascular thrombectomy. J Stroke Cerebrovasc Dis 2019;28:1710–17 10.1016/j.jstrokecerebrovasdis.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 11.Bhaskar S, Bivard A, Parsons M, et al. Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: associations with collateral status. J Cereb Blood Flow Metab 2017;37:671–82 10.1177/0271678X16637611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkelmeier L, Heit JJ, Adusumilli G, et al. Poor venous outflow profiles increase the risk of reperfusion hemorrhage after endovascular treatment. J Cereb Blood Flow Metab 2023;43:72–83 10.1177/0271678X221127089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faizy TD, Kabiri R, Christensen S, et al. Favorable venous outflow profiles correlate with favorable tissue-level collaterals and clinical outcome. Stroke 2021;52:1761–67 10.1161/STROKEAHA.120.032242 [DOI] [PubMed] [Google Scholar]
- 14.Faizy TD, Kabiri R, Christensen S, et al. Venous outflow profiles are linked to cerebral edema formation at noncontrast head CT after treatment in acute ischemic stroke regardless of collateral vessel status at CT angiography. Radiology 2021;299:682–90 10.1148/radiol.2021203651 [DOI] [PubMed] [Google Scholar]
- 15.Faizy TD, Mlynash M, Kabiri R, et al. The cerebral collateral cascade: comprehensive blood flow in ischemic stroke. Neurology 2022. April 28. [Epub ahead of print] 10.1212/WNL.0000000000200340 [DOI] [PubMed] [Google Scholar]
- 16.Faizy TD, Heit JJ. Rethinking the collateral vasculature assessment in acute ischemic stroke: the comprehensive collateral cascade. Top Magn Reson Imaging 2021;30:181–86 10.1097/RMR.0000000000000274 [DOI] [PubMed] [Google Scholar]
- 17.Broocks G, Kemmling A, Faizy T, et al. Effect of thrombectomy on oedema progression and clinical outcome in patients with a poor collateral profile. Stroke Vasc Neurol 2021;6:222–29 10.1136/svn-2020-000570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen IGH, van Vuuren AB, van Zwam WH, et al. ; MR CLEAN Trial Investigators. Absence of cortical vein opacification is associated with lack of intra-arterial therapy benefit in stroke. Radiology 2018;286:731 10.1148/radiol.2017174043 [DOI] [PubMed] [Google Scholar]
- 19.Shukir Muhammed Amin O, Aziz Abdullah A, Xaznadar A, et al. Striatocapsular infarction; a single institutional experience. Acta Inform Med 2012;20:106–12 10.5455/aim.2012.20.106-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin OS, Zangana HM, Ameen NA. The striatocapsular infarction and its aftermaths. BMJ Case Rep 2010;2010:bcr0220102703 10.1136/bcr.02.2010.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Han Q, Ding X, et al. Defining core and penumbra in ischemic stroke: a voxel- and volume-based analysis of whole brain CT perfusion. Sci Rep 2016;6:20932 10.1038/srep20932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fugate JE, Klunder AM, Kallmes DF. What is meant by “TICI”? AJNR Am J Neuroradiol 2013;34:1792–97 10.3174/ajnr.A3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal M, Fargen KM, Turk AS, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg 2014;6:83–86 10.1136/neurintsurg-2013-010665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38:1091–96 10.1161/01.STR.0000258355.23810.c6 [DOI] [PubMed] [Google Scholar]
- 25.de Havenon A, Mlynash M, Kim-Tenser MA, et al. ; DEFUSE 3 Investigators. Results from DEFUSE 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke 2019;50:632–38 10.1161/STROKEAHA.118.023407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baydemir R, Aykaç Ö, Acar BA, et al. Role of modified TAN score in predicting prognosis in patients with acute ischemic stroke undergoing endovascular therapy. Clin Neurol Neurosurg 2021;210:106978 10.1016/j.clineuro.2021.106978 [DOI] [PubMed] [Google Scholar]
- 27.Guenego A, Fahed R, Albers GW, et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol 2020;27:864–70 10.1111/ene.14181 [DOI] [PubMed] [Google Scholar]
- 28.Norvell DC. Study types and bias: don’t judge a study by the abstract's conclusion alone. Evid Based Spine Care J 2010;1:7–10 10.1055/s-0028-1100908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myint MZ, Yeo LL, Tan BY, et al. Internal cerebral vein asymmetry is an independent predictor of poor functional outcome in endovascular thrombectomy. J Neurointerv Surg 2022;14:683–87 10.1136/neurintsurg-2021-017684 [DOI] [PubMed] [Google Scholar]