Abstract
BACKGROUND AND PURPOSE:
IV thrombolysis with alteplase before mechanical thrombectomy for emergent large-vessel-occlusion stroke is associated with access-site bleeding complications. However, the incidence of femoral access-site complications with tenecteplase before mechanical thrombectomy requires exploration. Here, femoral access-site complications with tenecteplase versus alteplase before mechanical thrombectomy for large-vessel-occlusion stroke were compared.
MATERIALS AND METHODS:
All patients receiving IV thrombolytics before mechanical thrombectomy for large-vessel-occlusion stroke who presented from January 2020 to August 2022 were reviewed. In May 2021, our health care system switched from alteplase to tenecteplase as the primary thrombolytic for all patients with stroke, facilitating the comparison of alteplase-versus-tenecteplase femoral access-site complication rates. Major (requiring surgery) and minor (managed conservatively) access-site complications were assessed.
RESULTS:
One hundred thirty-nine patients underwent transfemoral mechanical thrombectomy for large-vessel-occlusion stroke, of whom 46/139 (33.1%) received tenecteplase and 93/139 (66.9%) received alteplase. In all cases (n = 139), an 8F sheath was inserted without sonographic guidance, and vascular closure was obtained with an Angio-Seal. Baseline demographics, concomitant antithrombotic medications, and periprocedural coagulation lab findings were similar between groups. The incidence of conservatively managed groin hematomas (2.2% versus 4.3%), delayed access-site oozing requiring manual compression (6.5% versus 2.2%), and arterial occlusion requiring surgery (2.2% versus 1.1%) was similar between the tenecteplase and alteplase groups, respectively (P = not significant). No dissection, arteriovenous fistula, or retroperitoneal hematoma was observed.
CONCLUSIONS:
Tenecteplase compared with alteplase before mechanical thrombectomy for large-vessel-occlusion stroke is not associated with an alteration in femoral access-site complication rates.
Transfemoral access is the predominant route for mechanical thrombectomy (MT) of large-vessel-occlusion (LVO) acute ischemic stroke (AIS).1 Access-site complications are potentially underreported in the literature due to a lack of rigorous clinical assessment and charting.2,3 A set of prospective LVO MT landmark trials, with mostly (68%–73%)4,5 or exclusively (100%)6-9 concomitant alteplase (TPA) administration, provided access-site complication rates in the endovascular treatment arm. A pooled incidence of 1.7% (range, 1.2%–2.9%)4,6,7 for severe and 7.0% (range, 3.0%–11.7%)2,4,5,8,9 for overall femoral access-site complications provides a framework for TPA-associated access-site complications in LVO MT. More recent trials investigating the safety and efficacy of MT with IV thrombolysis versus MT alone also reported femoral access-site complications. Femoral hematomas10-13 and pseudoaneurysms10-12 were observed in 0.3%–7.9% and 1.2%–4.4% of the MT with TPA groups compared with 0.7%–4.2% and 0.5%–1.1% of the MT-alone groups, respectively.
The third-generation thrombolytic tenecteplase (TNK) is a bioengineered variant of TPA. It overcomes some of the major shortcomings of TPA, resulting in a decreased plasma clearance, higher fibrin specificity, and improved resistance against plasminogen activator inhibitor 1.14,15 Recently, TNK has received increasing attention as a thrombolytic medication in AIS treatment.16-24 On the basis of the mounting evidence and advantages of TNK over TPA, our health care system underwent a system-wide transition of thrombolytic medication from TPA to TNK. Recently, we reported our first-year experience with TNK compared with TPA.25 Compatible with the trial findings of Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK), significantly higher spontaneous recanalization rates with TNK versus TPA in patients with LVO AIS (about 20% versus 10%) were observed.26 Despite similar overall safety profiles (intracranial hemorrhage and mortality), the impact of TNK use on extra- and intracranial hemorrhagic complications in LVO AIS is yet to be explored. Here, we seek to compare the incidence of and analyze risk factors for access-site-related complications with TNK versus TPA before MT for LVO stroke.
MATERIALS AND METHODS
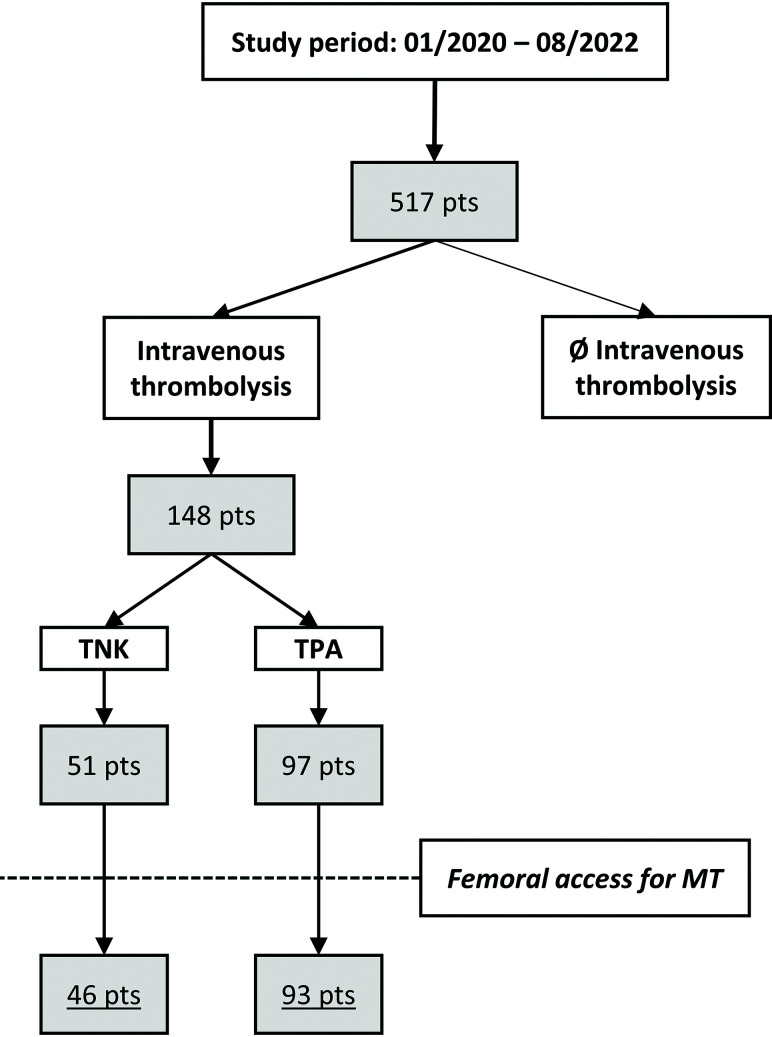
Consecutive patients with LVO AIS admitted to 2 North American comprehensive stroke centers (Geisinger Medical Center, and Geisinger Wyoming Valley Medical Center), part of the same health care system, were reviewed following local institutional review board approval (No. 2022–0571). Patient consent was waived for the retrospective analysis. All patients presenting between January 2020 and August 2022 who had received IV thrombolysis before MT were assessed in the analysis (Figure). In May 2021, our health care system switched from TPA to TNK as the primary thrombolytic for all patients with AIS, facilitating a comparison of TPA-versus-TNK femoral access complication rates.
FIGURE.
Patient flowchart. “Pts” indicates patients.
Technical Details on Femoral Access
In the hybrid operating room, femoral access was obtained via a 21-ga micropuncture or an 18-ga access needle at the physician’s discretion to insert an 8F short sheath. Sonography was used at the physician’s discretion in all upper extremity accesses (usually radial) but only in selected cases for femoral access. In this cohort, all femoral accesses were obtained without sonography. No patients underwent sonographically guided femoral access in the study period, and none were, therefore, excluded. Vascular closure for 8F sheath access is routinely performed with Angio-Seal (Terumo Medical), following a typical femoral artery angiogram to assess the sheath placement. An Angio-Seal was used in all cases.
Each patient had received IV thrombolytic medication according to current guidelines, which included platelets, >100,000/mm³, international normalized ratios (INRs), ≤ 1.7, prothrombin times (PTs), ≤ 15, and activated partial thromboplastin times, ≤ 40. IV thrombolysis with TPA (0.9 mg/kg) and TNK (0.25 mg/kg) was administered according to contemporary guidelines. Eligible patients received TPA or TNK either at an outside facility with secondary transfer to the CSC for MT or in the emergency department of the CSC before transfer to the hybrid angio suite. At both CSCs, bridging thrombolysis was performed in each patient eligible for IV thrombolysis. Concomitant antithrombotic medication such as aspirin, clopidogrel, and warfarin (Coumadin), as well as their combinations, did not abrogate the decision to administer IV thrombolysis if coagulation profiles were in range. Following MT and with achievement of access-site hemostasis, patients were transferred to the intensive care unit. To assess potential lasting disruptions of the coagulation system due to the IV thrombolysis, we assessed INRs, PTs, and activated partial thromboplastin times in all patients. For the presented analysis, we considered labs drawn <8 hours since groin access to approximate coagulation profiles <12 hours since IV thrombolysis administration. However, labs were only available in a subset of patients because the timing of additional laboratory panels, including coagulation profiles after MT, was based on the discretion of the on-call critical care team.
Access-Site Complication Types
Minor access-site complications comprised the following: all conservatively managed groin hematomas, conservatively managed pseudoaneurysms, and delayed access-site oozing. Delayed oozing was defined as minor bleeding starting in the post-MT phase in the intensive care unit requiring additional manual compression to achieve hemostasis. Groin hematomas were diagnosed by bedside physical examination. Any bleeding complication was assessed as a composite variable of groin hematomas and delayed oozing.
Major access-site complications were defined as groin hematomas requiring transfusion, pseudoaneurysms requiring thrombin injection or surgical repair, dissections, arterial occlusions, and arteriovenous fistulas. Sonography in conjunction with additional contrast-enhanced CT was performed if indicated. Vascular surgery was consulted in all cases of suspected and diagnosed major access-site complications. Any groin complication was assessed as a composite variable of any minor or major access-site complication.
Statistical Analysis
The χ2, Fisher exact, and Mann-Whitney U tests were performed to compare TNK groups with TPA groups, with P < .05 considered statistically significant. SPSS, Version 25 (IBM), was used to analyze the data.
RESULTS
A total of 148 patients (TNK n = 51, TPA n = 97) were identified. Seven patients experienced rapid neurologic recovery averting emergent MT and thus femoral access. In addition, 2 patients underwent upper extremity access (radial ×1, brachial ×1) due to previous femoral artery operations. Finally, 139 patients underwent transfemoral access for LVO MT and were thus included in the analysis (Table 1). If one contrasted TNK (n = 46) and TPA (n = 93) cases, age, sex, and cardiovascular risk factors except for arterial hypertension were similar between groups. Arterial hypertension was numerically more frequent in the TNK group (84.8%) compared with the TPA group (69.6%) (P = .05). Significantly more patients primarily presented to the CSC in the TNK group compared with the TPA group (P = .004). Last known well to IV thrombolysis and IV thrombolysis to groin puncture times were similar in both groups (P = .28 and P = .07, respectively). The overall rate of concomitant antithrombotic medication was 50% in the TNK and 48.4% in the TPA group (P = .86).
Table 1:
Femoral access-site complications
| TNK (n = 46) | TPA (n = 93) | P Value | ||
|---|---|---|---|---|
| Baseline | ||||
| Age (IQR) | 73 (64–79) | 74 (62–83) | .62 | |
| Female | 24 (52.2%) | 44 (64.7%) | .59 | |
| Risk factors | ||||
| Arterial hypertensiona | 39 (84.8%) | 64 (69.6%) | .05 | |
| Type 2 diabetesa | 11 (23.9%) | 19 (20.7%) | .66 | |
| Dyslipidemiaa | 29 (63.0%) | 49 (53.3%) | .27 | |
| Coronary artery diseasea | 10 (21.7%) | 22 (23.9%) | .78 | |
| Atrial fibrillationa | 13 (28.3%) | 38 (41.3%) | .14 | |
| Chronic kidney diseasea | 8 (17.4%) | 13 (14.1%) | .62 | |
| Ischemic strokeb | 9 (19.6%) | 22 (23.7%) | .59 | |
| Smoking (ever)c | 26 (56.5%) | 40 (44.0%) | .16 | |
| Primary presentation at CSC | 26 (56.5%) | 29 (31.2%) | .004 | |
| LKW to IV thrombolytic (IQR) | 109 (90–149) | 119 (91–172) | .28 | |
| IV thrombolytic to groin puncture (IQR) | 74 (48–109) | 91 (66–136) | .07 | |
| Antithrombotics | 23 (50.0%) | 45 (48.4%) | .86 | |
| Aspirin | 18 (39.1%) | 31 (33.3%) | ||
| Aspirin and Clopidogrel | 3 (6.5%) | 5 (5.4%) | ||
| Clopidogrel | 0 (0.0%) | 1 (1.1%) | ||
| Coumadin | 1 (2.2%) | 4 (4.3%) | ||
| Aspirin and Coumadin | 0 (0.0%) | 1 (1.1%) | ||
| Clopidogrel and Coumadin | 1 (2.2%) | 3 (3.2%) | ||
| Periprocedural | ||||
| 8F femoral sheath | 46 (100.0%) | 93 (100.0%) | NA | |
| Sonographically guided access | 0 (0.0%) | 0 (0.0%) | NA | |
| Angio-Seal closure device | 46 (100.0%) | 93 (100.0%) | NA | |
| Coagulation labs < 12h | ||||
| INR (IQR)d | 1.07 (0.99–1.12) | 1.18 (1.09–1.31) | .001 | |
| PT (IQR)e | 14 (13–15) | 15 (14–16) | .001 | |
| aPTT (IQR)f | 30 (28–31) | 29 (28–32) | 1.00 | |
Note:—IQR indicates interquartile range; LKW, last known well; aPTT, activated partial thromboplastin times; NA, not available.
Data missing in 1 TPA.
Data missing in 3 TPAs.
Data missing in 5 TPAs.
Data available in 22 and 45 patients with TNK and TPA, respectively.
Data available in 22 and 46 patients with TNK and TPA, respectively.
Data available in 18 and 29 patients with TNK and TPA, respectively.
Coagulation labs within 12 hours of IV thrombolysis were available in a subset of patients (Table 1). The median INR and PT were higher in the TPA subgroups compared with TNK subgroups, compatible with 8/93 (8.6%) patients receiving TPA and 2/46 (4.3%) receiving TNK who had been on Coumadin on admission.
Femoral Access-Site Complications
Nonmajor groin hematomas were observed in 1/46 (2.2%) patients on TNK and 4/93 (4.3%) on TPA (P = 1.0) (Table 2). None of these hematomas caused a relevant hemoglobin drop, required transfusion, required surgery or intervention or a prolonged stay, or affected the clinical outcome. In 3/46 (6.5%) and 2/93 (2.2%) TNK and TPA cases, respectively, delayed access-site oozing occurred, hence, requiring additional manual compression at the bedside with subsequent hemostasis in all cases (P = .33). A pseudoaneurysm was identified in 1 patient with TPA who also presented with a groin hematoma (1/93, 1.1%). Conservative management was sufficient to treat the pseudoaneurysm. Among the major groin complications, an acute lower extremity ischemia due to arterial occlusion at the access-site occurred in 1/46 (2.2%) patients receiving TNK and 1/93 (1.1%) receiving TPA (P = 1.0). Both cases required emergent vascular surgery. If one contrasted the TNK-versus-TPA groups, the occurrence of any groin complication (P = .53) and any groin bleeding (0.73) was similar. No dissection, arteriovenous fistula, or retroperitoneal hematoma was observed.
Table 2:
Femoral access-site complications
| Access-Site Complications | TNK (n = 46) | TPA (n = 93) | P Value |
|---|---|---|---|
| Minor | |||
| Groin hematoma | 1 (2.2%) | 4 (4.3%) | 1.00 |
| Delayed oozing | 3 (6.5%) | 2 (2.2%) | .33 |
| Pseudoaneurysm (conservative) | 0 (0.0%) | 1 (1.1%) | 1.00 |
| Major | |||
| Arterial occlusion | 1 (2.2%) | 1 (1.1%) |
A trend was observed for the associations of coronary artery disease with any groin bleeding complication (P = .05) and chronic kidney disease with any groin complication (P = .09). Additional analysis demonstrated no significant associations.
DISCUSSION
TNK use for AIS is rapidly increasing in the United States, yet it remains to be determined how it will affect the thrombectomy landscape of emergent LVO. This US 2-center study compares rates of femoral access-site complications with TNK versus TPA before MT. Notably, about half of the TNK and TPA cohorts had concomitant antithrombotic medication. The overall incidence of access-site complications was low, and no significant differences were observed between the TNK and TPA groups.
Thrombolytic medication is the cornerstone of AIS treatment in the 4.5-hour time window. Currently, TNK is only approved for acute ST-elevation myocardial infarction. However, recent studies point to TNK being a noninferior alternative to TPA in AIS treatment.16-24 Genetic modifications at 3 sites of the TPA molecule facilitate decreased clearance of TNK, higher fibrin specificity, and improved resistance against plasminogen activator inhibitor 1. Clinically, these features translate into a longer half-life of TNK paired with improved thrombolytic potency without the additional disruption of the coagulation cascade or bleeding complications.14,15 The ideal clot-dissolving agent preferentially acts on solid-phase fibrin bound by plasminogen. Eventually, the thrombolytic medication initiates fibrinolysis by activating plasminogen to plasmin. However, uncontrolled and excessive systemic lysis activates fluid-phase plasminogen, which eventually consumes fibrinogen, plasminogen, and α2-antiplasmin.14 Huang et al15 have demonstrated that TPA more than TNK affects coagulation, especially fibrinolytic cascades. In the present study, a subset of patients had coagulation labs within 12 hours of thrombolysis. We did not identify clinically relevant alterations or differences in coagulation lab findings in either group.
Unless rigorously followed, nonmajor access-site complications such as conservatively managed groin hematomas are likely underrecognized, reflected by only some studies containing detailed information. In the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN)-NO IV trial, 252/266 patients in the MT with TPA group eventually underwent femoral puncture. The authors reported groin hematomas in 20 patients (7.9%). In the MT alone group, 261/273 patients underwent femoral puncture, and groin hematomas were nonsignificantly lower (11/261; 4.2%).12 Catapano et al27 reported, in a recent retrospective study on patients receiving TPA undergoing MT, that groin hematomas after transfemoral access were observed in only 1.4% (4/293) of patients. Concomitant use of antithrombotic medication was not addressed. In the present study, half of the patients in both the TNK and TPA cohorts were on antithrombotics before admission. The groin hematoma rates were 2.2% and 4.3% in the TNK and TPA groups, respectively, thus in agreement with the literature. Delayed oozing from the access-site is a known clinical issue not yet dedicatedly reported in the stroke literature. The herein observed rates were low and similar to those observed in cardiology.28 Nevertheless, in the TNK group, delayed oozing was 3 times more frequent than in the TPA group (6.5% versus 2.2%). Whether this finding is related to not enough manual compression after closure device deployment, or disruption of the fibrinolytic cascade cannot be explained with this study.
In the EXTEND-IA TNK study, groin hematomas were observed in 3/101 (3%) in the TPA before MT group and in 1/101 (1%) in the TNK before MT group. One patient in the TPA before MT group (1/101, 1%) had a pseudoaneurysm.26 Pseudoaneurysms in the TPA before MT groups compared with the MT-alone groups in the MR CLEAN-NO IV, Solitaire with the intention for thrombectomy plus IV t-PA versus direct Solitaire stent-retriever thrombectomy in acute anterior circulation stroke (SWIFT DIRECT), and Direct Endovascular Thrombectomy vs Combined IVT and Endovascular Thrombectomy for Patients With Acute Large Vessel Occlusion in the Anterior Circulation (DEVT) trials were observed in 1.2% versus 1.1%, 2.4% versus 0.5%, and 4.4% versus 0.9%, respectively.10-12 In the present study, 1 patient receiving TPA (1/93, 1.1%) with a groin hematoma was also found to have a small pseudoaneurysm that did not require any intervention and, thus, was considered minor. In summary, reported minor femoral access-site complication rates varied among studies, but differences between TPA and TNK were not apparent.
Major (serious) access-site complications are commonly defined as events causing a relevant hemoglobin drop, requiring blood transfusion, surgery, or an intervention; a prolonged stay; or death. In the prospective stroke trials Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE), EXTEND-IA, and Mechanical Thrombectomy After Intravenous Alteplase Versus Alteplase Alone After Stroke (THRACE), the serious access-site complication rates were 1.2%, 2.9%, and 2.1% in the endovascular treatment arm, respectively.4,6,7 In the Direct Intraarterial Thrombectomy in Order to Revascularize Acute Ischemic Stroke Patients with Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals: a Multicenter Randomized Clinical Trial (DIRECT MT) trial, major femoral bleeding was encountered in 1/312 (0.3%) patients in the MT with TPA group and in 2/299 (0.7%) in the MT without TPA group.13 Gerschenfeld et al29 analyzed a cohort of 588 patients with emergent LVO who received TNK. Major systemic bleeding from the groin puncture was reported in 1 patient. In EXTEND-IA TNK, 1 patient in the TNK before the MT group developed leg ischemia requiring an intervention for femoral artery occlusion.26 In the present study, 1 patient in the TNK (2.2%) and 1 patient in the TPA (1.1%) cohort experienced arterial occlusion and required emergent open vascular surgery. IV thrombolysis per se does not represent a risk factor for arterial occlusion. However, in the acute setting, the threshold to not place a closure device after insertion of an 8F sheath into the femoral artery is likely high, especially when considering that the patient received IV thrombolysis and is on concomitant antithrombotic medication.
Catapano et al27 observed the occurrence of retroperitoneal hematomas in 1.7% of transfemoral cases. Although not observed in the present study, retroperitoneal hematomas have been associated with common femoral artery punctures that are too high and represent a significant risk factor of femoral access-site-related morbidity and mortality.30 While closure devices appear to reduce the risk of pseudoaneurysm after femoral access, they do not appear to mitigate the risk of retroperitoneal hematomas.30,31 A shift toward other access sites, such as radial access, may prove beneficial.27,32 While radial access has gained significant attention for cerebral angiography and elective neurointervention, its adoption in emergent stroke care is not as uniformly present in the literature.1,33
Despite the high rate of concomitant antithrombotics in the present study, the overall femoral access-site complication rate was low and similar between the IV thrombolytics TNK and TPA. If TNK continues to prove valuable in stroke treatment, it might replace TPA due to lower cost, ease of administration, and clinical noninferiority. Hence, the data herein provide a framework for the safety of TNK compared with the current standard TPA regarding femoral access-site-related complications surrounding MT for emergent LVO stroke.
Strengths and Limitations
The study is among the first to report real-world data on femoral access-site complication rates with TNK compared with TPA from a recent treatment period in the United States. The femoral accesses analyzed here are homogeneous because, in all cases, sonography use was waived, an 8F sheath was inserted, and the arteriotomy was closed with the same vascular closure device (8F Angio-Seal). The study is predominantly limited by its nonrandomized, retrospective character; small sample size; and low event number. Adjustments for potential confounders were considered. Additional, larger, multicenter studies are required to further explore and compare the safety of TNK and TPA before transfemoral MT.
CONCLUSIONS
Despite the high rate of concomitant antithrombotics in the present study, the overall femoral access-site complication rate was low and similar between the IV thrombolytic TNK and TPA.
ABBREVIATIONS:
- AIS
acute ischemic stroke
- CSC
comprehensive stroke center
- INR
international normalized ratio
- LVO
large-vessel-occlusion
- MT
mechanical thrombectomy
- PT
prothrombin time
- TPA
alteplase
- TNK
tenecteplase
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Siddiqui AH, Waqas M, Neumaier J, et al. Radial first or patient first: a case series and meta-analysis of transradial versus transfemoral access for acute ischemic stroke intervention. J Neurointerv Surg 2021;13:687–92 10.1136/neurintsurg-2020-017225 [DOI] [PubMed] [Google Scholar]
- 2.Oneissi M, Sweid A, Tjoumakaris S. Access-site complications in transfemoral neuroendovascular procedures: a systematic review of incidence rates and management strategies. Oper Neurosurg (Hagerstown) 2020;19:353–63 10.1093/ons/opaa096 [DOI] [PubMed] [Google Scholar]
- 3.Shapiro SZ, Sabacinski KA, Mantripragada K, et al. Access-site complications in mechanical thrombectomy for acute ischemic stroke: a review of prospective trials. AJNR Am J Neuroradiol 2020;41:477–81 10.3174/ajnr.A6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 6.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 7.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE Investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15:1138–47 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 9.Broderick JP, Palesch YY, Demchuk AM, et al. ; Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368:893–903 10.1056/NEJMoa1214300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer U, Kaesmacher J, Strbian D, et al. ; SWIFT DIRECT Collaborators. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. Lancet 2022;400:104–15 10.1016/S0140-6736(22)00537-2 [DOI] [PubMed] [Google Scholar]
- 11.Zi W, Qiu Z, Li F, et al. ; DEVT Trial Investigators. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA 2021;325:234–43 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeCouffe NE, Kappelhof M, Treurniet KM,et al. ; MR CLEAN–NO IV Investigators. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med 2021;385:1833–44 10.1056/NEJMoa2107727 [DOI] [PubMed] [Google Scholar]
- 13.Yang P, Zhang Y, Zhang L, et al. ; DIRECT-MT Investigators. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med 2020;382:1981–93 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 14.Tanswell P, Modi N, Combs D, et al. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet 2002;41:1229–45 10.2165/00003088-200241150-00001 [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke 2015;46:3543–46 10.1161/STROKEAHA.115.011290 [DOI] [PubMed] [Google Scholar]
- 16.Tsivgoulis G, Katsanos AH, Christogiannis C, et al. Intravenous thrombolysis with tenecteplase for the treatment of acute ischemic stroke. Ann Neurol 2022;92:349–57 10.1002/ana.26445 [DOI] [PubMed] [Google Scholar]
- 17.Mahawish K, Gommans J, Kleinig T, et al. Switching to tenecteplase for stroke thrombolysis: real-world experience and outcomes in a regional stroke network. Stroke 2021;52:e590–93 10.1161/STROKEAHA.121.035931 [DOI] [PubMed] [Google Scholar]
- 18.Zhong CS, Beharry J, Salazar D, et al. Routine use of tenecteplase for thrombolysis in acute ischemic stroke. Stroke 2021;52:1087–90 10.1161/STROKEAHA.120.030859 [DOI] [PubMed] [Google Scholar]
- 19.Gerschenfeld G, Liegey JS, Laborne F-X, et al. Treatment times, functional outcome, and hemorrhage rates after switching to tenecteplase for stroke thrombolysis: insights from the TETRIS registry. EurStroke J 2022;7:358–64 10.1177/23969873221113729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon BK, Buck BH, Singh N, et al. ; AcT Trial Investigators. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 2022;400:161–69 10.1016/S0140-6736(22)01054-6 [DOI] [PubMed] [Google Scholar]
- 21.Li S, Pan Y, Wang Z, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol 2022;7:47–53 10.1136/svn-2021-000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma P, Zhang Y, Chang L, et al. Tenecteplase vs. alteplase for the treatment of patients with acute ischemic stroke: a systematic review and meta-analysis. J Neurol 2022;269:5262–71 10.1007/s00415-022-11242-4 [DOI] [PubMed] [Google Scholar]
- 23.Katsanos AH, Psychogios K, Turc G, et al. Off-label use of tenecteplase for the treatment of acute ischemic stroke: a systematic review and meta-analysis. JAMA Netw Open 2022;5:e224506 10.1001/jamanetworkopen.2022.4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yogendrakumar V, Beharry J, Churilov L, et al. Tenecteplase improves reperfusion across time in large vessel stroke. Ann Neurol 2023;93:489–99 10.1002/ana.26547 [DOI] [PubMed] [Google Scholar]
- 25.Hendrix P, Collins MK, Griessenauer CJ, et al. Tenecteplase versus alteplase before mechanical thrombectomy: experience from a US healthcare system undergoing a system-wide transition of primary thrombolytic. J Neurointerv Surg 2022. Nov 22. [Epub ahead of print] 10.1136/jnis-2022-019662 [DOI] [PubMed] [Google Scholar]
- 26.Campbell BC, Mitchell PJ, Churilov L, et al. ; EXTEND-IA TNK Investigators. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 2018;378:1573–82 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 27.Catapano JS, Rumalla K, Farhadi DS, et al. Safety and efficacy of radial versus femoral artery access for mechanical thrombectomy procedures following intravenous administration of tissue plasminogen activator. Stroke Vasc Interv Neurol 2022;2:e000238 10.1161/SVIN.121.000238 [DOI] [Google Scholar]
- 28.Wu PJ, Dai YT, Kao HL, et al. Access site complications following transfemoral coronary procedures: comparison between traditional compression and angioseal vascular closure devices for haemostasis. BMC Cardiovasc Disord 2015;15:34 10.1186/s12872-015-0022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerschenfeld G, Smadja D, Turc G; et al. ; TETRIS Study Group. Functional outcome, recanalization, and hemorrhage rates after large vessel occlusion stroke treated with tenecteplase before thrombectomy. Neurology 2021;97:e2173–84 10.1212/WNL.0000000000012915 [DOI] [PubMed] [Google Scholar]
- 30.Farouque HM, Tremmel JA, Raissi Shabari F, et al. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein IIb/IIIa inhibitors and vascular closure devices. J Am Coll Cardiol 2005;45:363–68 10.1016/j.jacc.2004.10.042 [DOI] [PubMed] [Google Scholar]
- 31.Naddaf A, Williams S, Hasanadka R, et al. Predictors of groin access pseudoaneurysm complication: a 10-year institutional experience. Vasc Endovascular Surg 2020;54:42–46 10.1177/1538574419879568 [DOI] [PubMed] [Google Scholar]
- 32.Kwok CS, Kontopantelis E, Kinnaird T, et al. Retroperitoneal hemorrhage after percutaneous coronary intervention: incidence, determinants, and outcomes as recorded by the British Cardiovascular Intervention Society. Circ Cardiovasc Interv 2018;11:e005866 10.1161/CIRCINTERVENTIONS.117.005866 [DOI] [PubMed] [Google Scholar]
- 33.Shaban S, Rastogi A, Phuyal S, et al. The association of transradial access and transfemoral access with procedural outcomes in acute ischemic stroke patients receiving endovascular thrombectomy: a meta-analysis. Clin Neurol Neurosurg 2022;215:107209 10.1016/j.clineuro.2022.107209 [DOI] [PubMed] [Google Scholar]