Abstract
Pd(II)-catalyzed γ-C(sp3)−H (hetero)arylation of aliphatic ketones is developed using α-amino acid as transient directing groups (TDG). A variety of aliphatic ketones were (hetero)arylated at the γ-position via a 5,6-membered fused cyclopalladation intermediate to afford the remotely arylated products in up to 88% yield. The crucial ligand effect of 2-pyridone is further enhanced by reducing the loading of acid additives. Consequentially, the improved reactivity of this catalytic system has also made possible the cyclic γ-methylene C(sp3)−H arylation of ketones. Mechanistic investigtigation and comparison to the γ-C–H arylation of aldehydes revealed a structural insight for designing site selective TDG.
Keywords: C−H activation, Palladium, Ketones, transient directing group, arylation, pyridone ligands, palladacycle
Graphical Abstract
Ketones are ubiquitous functional groups in natural products and synthetic intermediates. Wide range of transformations of aliphatic ketones that rely on the reactivity of ipso- and α-carbon centers have been developed.1 From the viewpoint of developing new synthetic disconnections, development of C–H functionalization of inert C(sp3)–H bonds at β- or even γ-positions is of great importance. Covalent installation of imine based directing groups have been explored to achieve β-C(sp3)–H functionalizations.2,3 In 2016, our group realized Pd(II)-catalyzed β-C(sp3)–H functionalization of free aliphatic ketones and o-tolualdehydes using L,X-type transient directing groups.4 This strategy, based upon reversible imine linkage in similar fashion to that of organocatalysis, provided strong impetus for further development of directed C–H activation of ketones,5 aldehydes6 and amines7 by omitting the extra steps for installation and removal of covalent directing groups. Using this protocol, β-methylene4a,4b or even methine4j C(sp3)–H arylation of aliphatic ketones has been recently achieved (Scheme 1a).
Scheme 1.
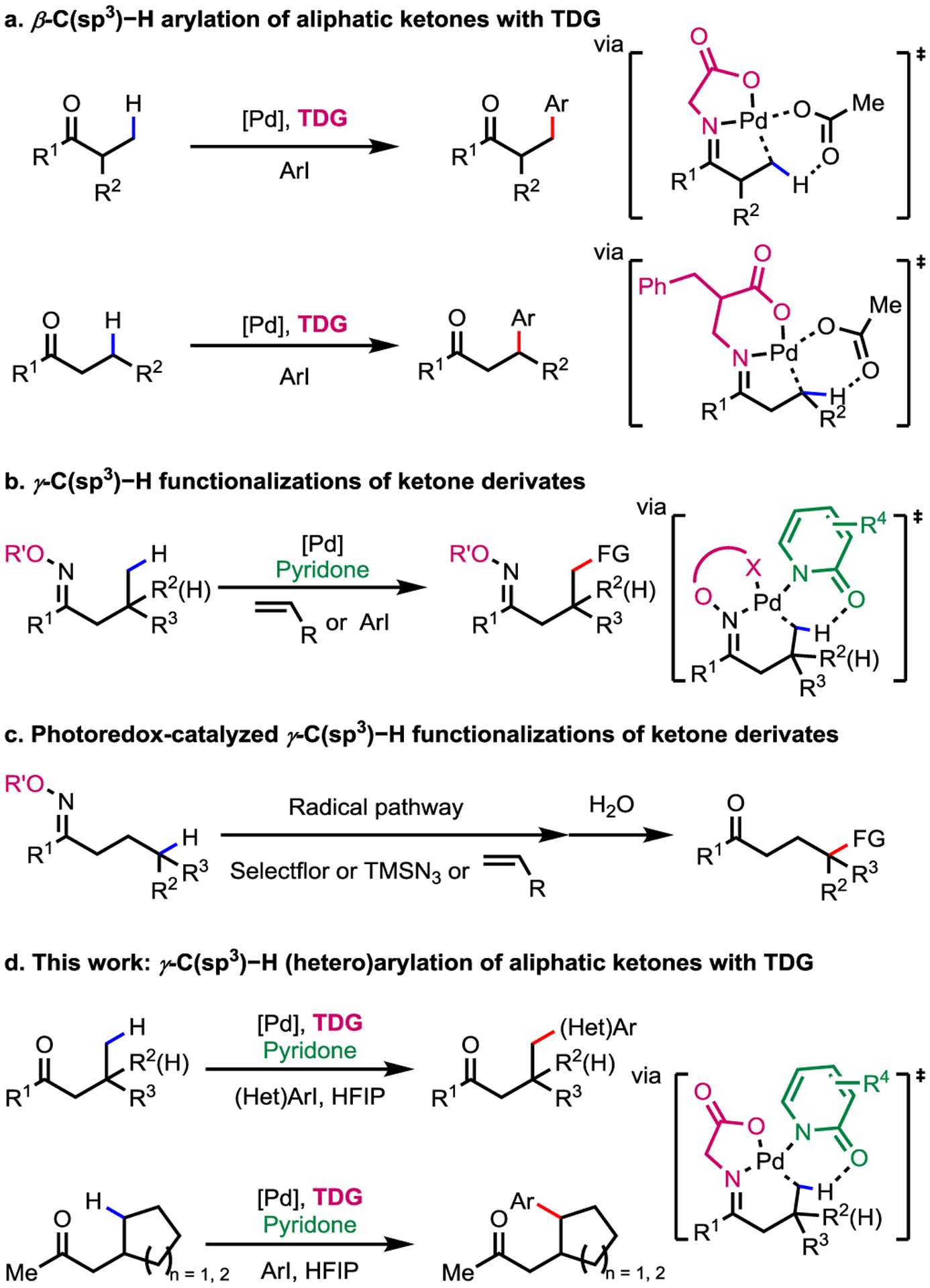
PdII-Catalyzed C(sp3)−H Functionalization of Ketones directed by TDG and Covalent Auxiliaries
Despite significant advances in Pd-catalyzed β-C(sp3)–H functionalizations of ketones using TDG, this approach has met limited success towards C–H activation at the relatively remote γ-position. For example, a single example of γ-C–H arylation requires the presence of tri-methyl groups at the β-position.4 To address this limitation, α-imino-oxy acids were developed as a covalent directing group in our laboratory. γ-C(sp3)–H arylation using this directing group were enabled by 2-pydrione ligands (Scheme 1b).8 Interestingly, iminyl-radical generated from α-imino-oxy acids or oxime esters effectively performed an intramolecular 1,5-HAT and forged new bonds at the γ-position (Scheme 1c).9 However, these methods have a common limitation: extra synthetic steps are needed for the covalent installation and removal of the directing groups. Therefore, we embarked on the development of γ-C(sp3)–H functionalization of free aliphatic ketones based upon TDG strategy.
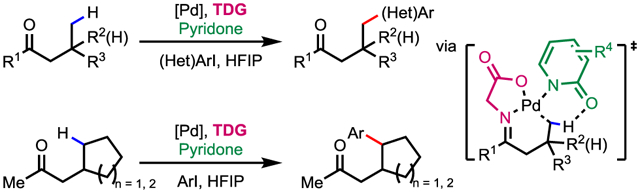
Herein, we report the first TDG-enabled γ-selective PdII-catalyzed C(sp3)–H (hetero)arylation of aliphatic ketones with electron-deficient 2-pyridone ligands (Scheme 1d). With the cheap and commonly available glycine (TDG1) as transient directing group, γ-C(sp3)–H (hetero)arylation and cyclic γ-methylene C(sp3)–H arylation could be achieved with up to 88% yield without the need of directing group installation and removal.
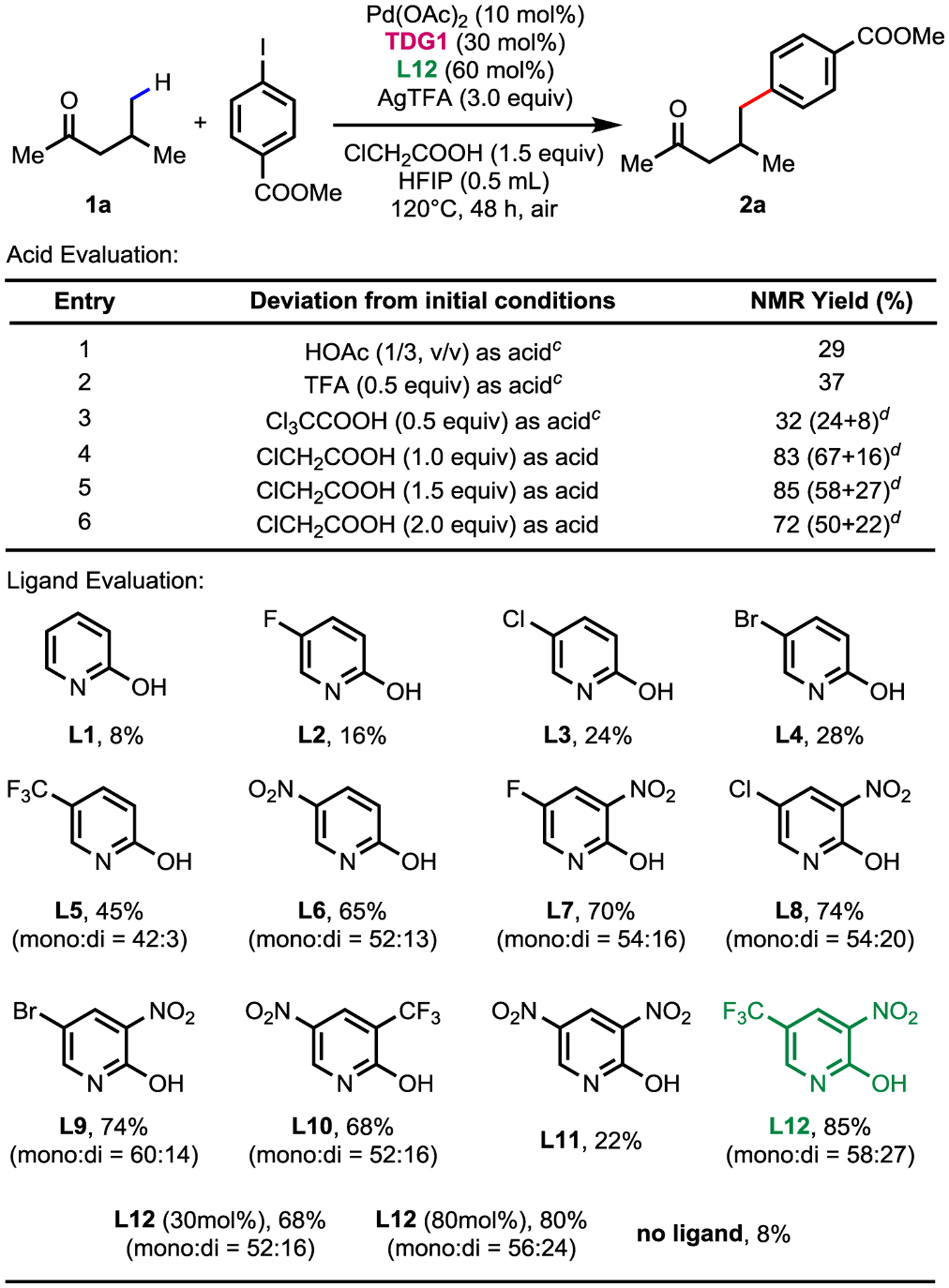
To address the challenge of the γ-C−H functionalization of aliphatic ketones, we began to search ligands and additives that can accelerate the C−H cleavage step. Guided by our previous findings on C(sp3)−H functionalization reactions that reducing the amount of chloroacetic acid can enhance the ligand effect of 2-pyridone by minimizing the competing carboxylates.10,11 We started testing different organic acids with lower pKa for γ-C−H arylation of 4-methyl-2-pentanone (1a) with 3-nitro-5-(trifluoromethyl)-2-pyridinone (L12) as ligand. To our delight, the NMR yield reached to its highest at 85% (mono:di = 58:27) when 1.5 equiv of chloroacetic acid was used (Table 1, entry 5). Other pyridone ligands were also tested. Unfunctionalized 2-pyridone (L1) and 5-substituted 2-pyridones (L2-L6) were examined first, which resulted in the highest yield of 65%. Substitution of electron withdrawing groups at the 3-position further improved the yield to 85% (L7-L10, L12), except for 3,5-dinitropyridione(L11). Not surprisingly, less than 10% arylation product was obtained in the absence of the ligand. Varied β-amino acids were also tested as TDG to substitute glycine (TDG1) which inhibited the reactivity (see the Supporting Information for detailed screening information), probably due to the preference of the 5,6-membered coordination.
Table 1.
|
Conditions: 1a (0.1 mmol, 1.0 equiv), methyl 4-iodobenzoate (2.0 equiv), Pd(OAc)2 (10 mol%), TDG1 (glycine, 30 mol%), L12 (60 mol%), AgTFA (3.0 equiv) and ClCH2COOH (1.5 equiv) in HFIP (0. 5 mL), 120°C, under air, 48 h.
Yield determined by 1H NMR; CH2Br2 as internal standard.
Loading that gave the highest yield within a serial of concentrations.
Ratio of mono:di.
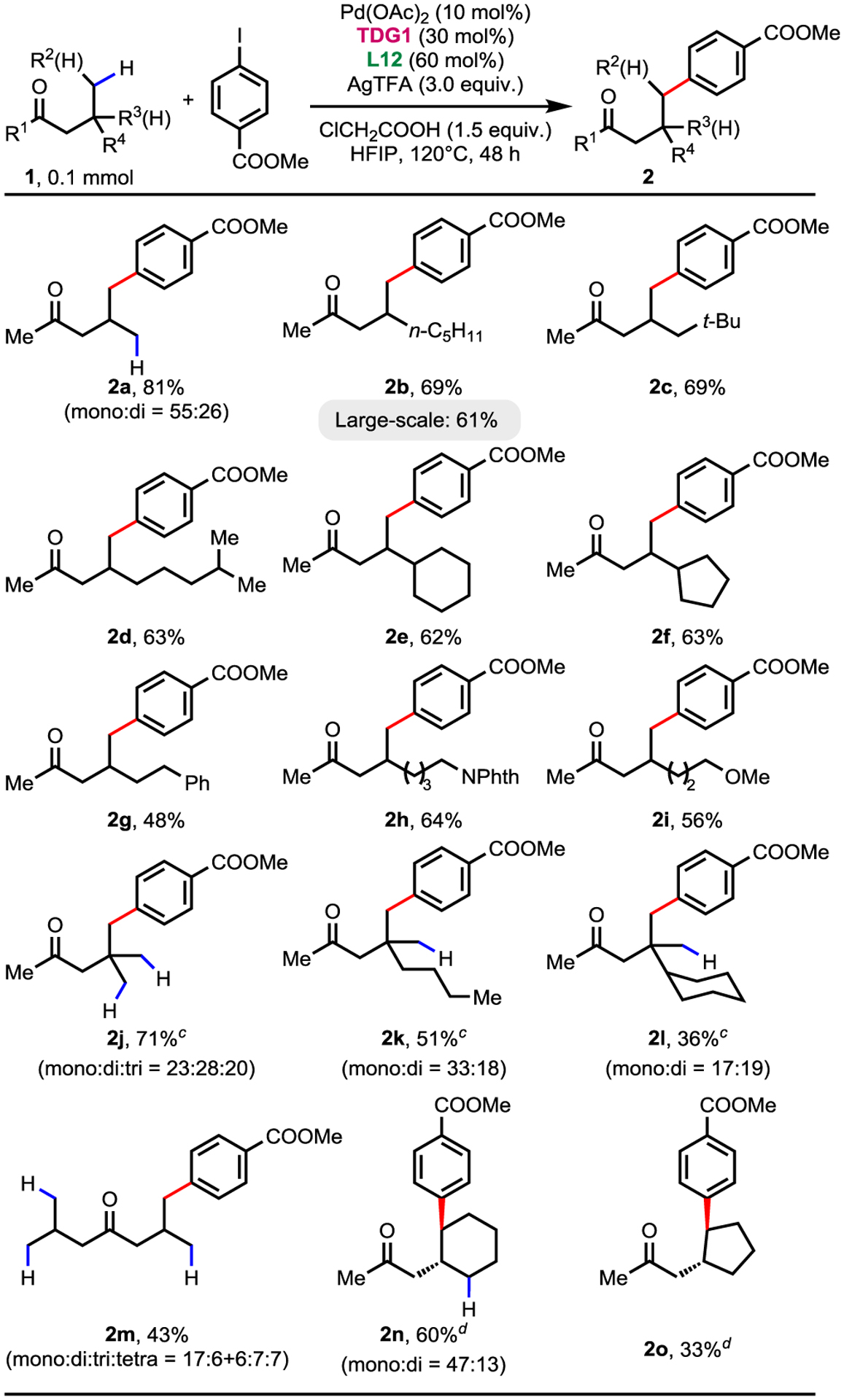
With the optimized conditions in hand, a variety of aliphatic ketones with methyl/cyclic methylene γ-C(sp3)−H bonds were tested using methyl 4-iodobenzoate as the coupling partner (Table 2). Substitution with methyl, pentyl, isohexyl and neopentyl groups at the β-position showed good reactivity (2a–2d). Larger substituents such as cyclohexyl or cyclopentyl groups were also compatible, providing 2e and 2f with good yields. Substrates containing phenyl, phthalimide and ether functionality were also tolerated with 48–64% yields (2g–2i). Ketones possessing β-quaternary centers could be well arylated at the γ-position with a lower loading of chloroacetic acid of 0.8 equiv, providing 2j–2l with good to moderated yields. Besides, the formation of tetra-functionated product of di-iso-butyl ketone further illustrates the powerful reactivity of this catalytic system (2m). Notably, two examples of cyclic γ-methylene-C(sp3)−H bonds could also be successfully arylated with good to moderate yields (2n–2o), providing a promising strategy for methylene C−H functionalization via 6-membered cyclopalladation. Furthermore, the reaction could be readily carried out on 3.0 mmol scale with 2b as standard substrate, affording the desired product in 61% yield.
Table 2.
|
Conditions: 1 (0.1 mmol, 1.0 equiv), methyl 4-iodobenzoate (2.0 equiv), Pd(OAc)2 (10 mol%), TDG1 (30 mol%), L12 (60 mol%), AgTFA (3.0 equiv) and ClCH2COOH (1.5 equiv) in HFIP (0. 5 mL), 120°C, under air, 48 h.
Isolated yields.
with ClCH2COOH (0.8 equiv) as additive
The reaction condition was modified with TDG1 (35 mol%), L10 (80 mol%) and ClCH2COOH (1.2 equiv) in HFIP (0.45 mL).
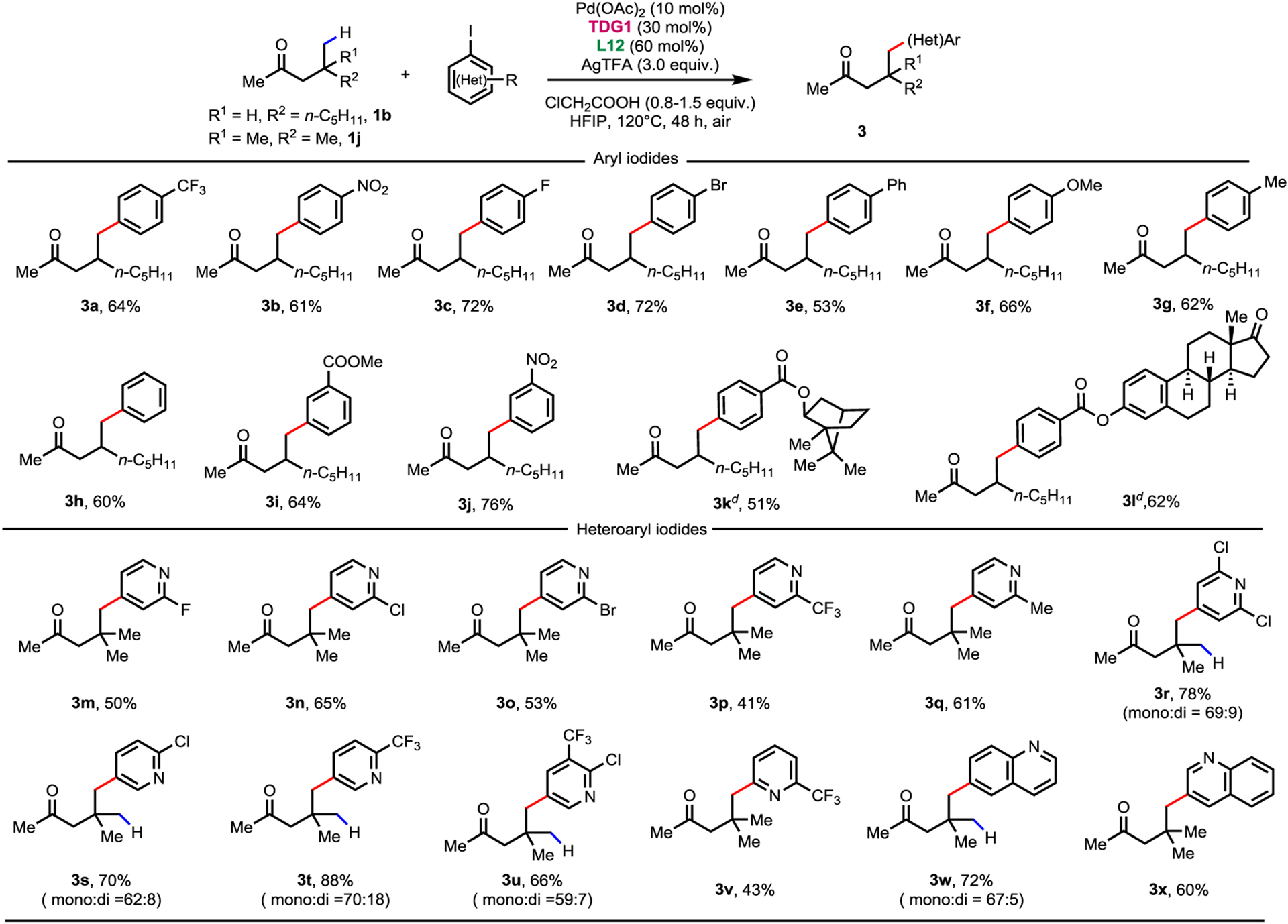
To broaden the synthetic application of this reaction, the scope of aryl and heteroaryl iodides was examined (Table 3). The model substrate 1b could be functionalized with excess of aryl iodides, exhibiting a good functional group compatibility (3a–3j). Aryl iodides derived from borneol and estrone were also tested, resulting in the desired products in 51% and 62% yields, respectively (3k–3l). More importantly, heterocycles could be installed through γ-C(sp3)−H functionalization with a modified condition. Various 4-iodopyridines derivates were tested as coupling partners. Good to moderate yields were acquired with 2-substituted 4-iodopyridines containing halogen, trifluoromethyl and methyl groups (3m–3q). For the less strongly coordinating 2,6-dichloro substituted pyridine, a total of 78% heteroarylated product was obtained (3r). Ketones could also be functionalized with 2 and 3-iodopyridines derivates at the γ-position, providing 3s–3v in good to moderate yields. Good yields could also be achieved with other heteroaryl iodides, such as 6-iodoqunoline and 3-iodoqunoline, which broadened the application of this protocol (3w–3x). However, non-substituted iodopyridines exhibited poor reactivity, with trace product observed, probably due to the strong coordinating ability which deactivated the catalyst.
Table 3.
|
Conditions: 1b (0.1 mmol, 1.0 equiv), Aryl Iodide (2.0 equiv), Pd(OAc)2 (10 mol%), TDG1 (30 mol%), L12 (60 mol%), AgTFA (3.0 equiv) and ClCH2COOH (1.5 equiv) in HFIP (0.5 mL), 120°C, under air, 48 h.
Conditions: 1j (0.1 mmol, 1.0 equiv), Heteroaryl Iodide (1.5 equiv), Pd(OAc)2 (10 mol%), TDG1 (30 mol%), L12 (60 mol%), AgTFA (3.0 equiv) and ClCH2COOH (0.8 equiv) in HFIP (0.4 mL), 120°C, under air, 48 h.
Isolated yields
dr =1:1.
A plausible catalytic cycle is outlined in Scheme 2. Glycine reacts with aliphatic ketones reversibly to form the transient imine A. Palladium species then coordinates to imine A to generate a chelating complex B, which, upon binding to a pyridone ligand, undergoes C−H bond activation process to form intermediate C. Complex D is generated via oxidative addition with aryl iodides. Finally, reductive elimination of the palladium complex D leads to arylated E.
Scheme 2.
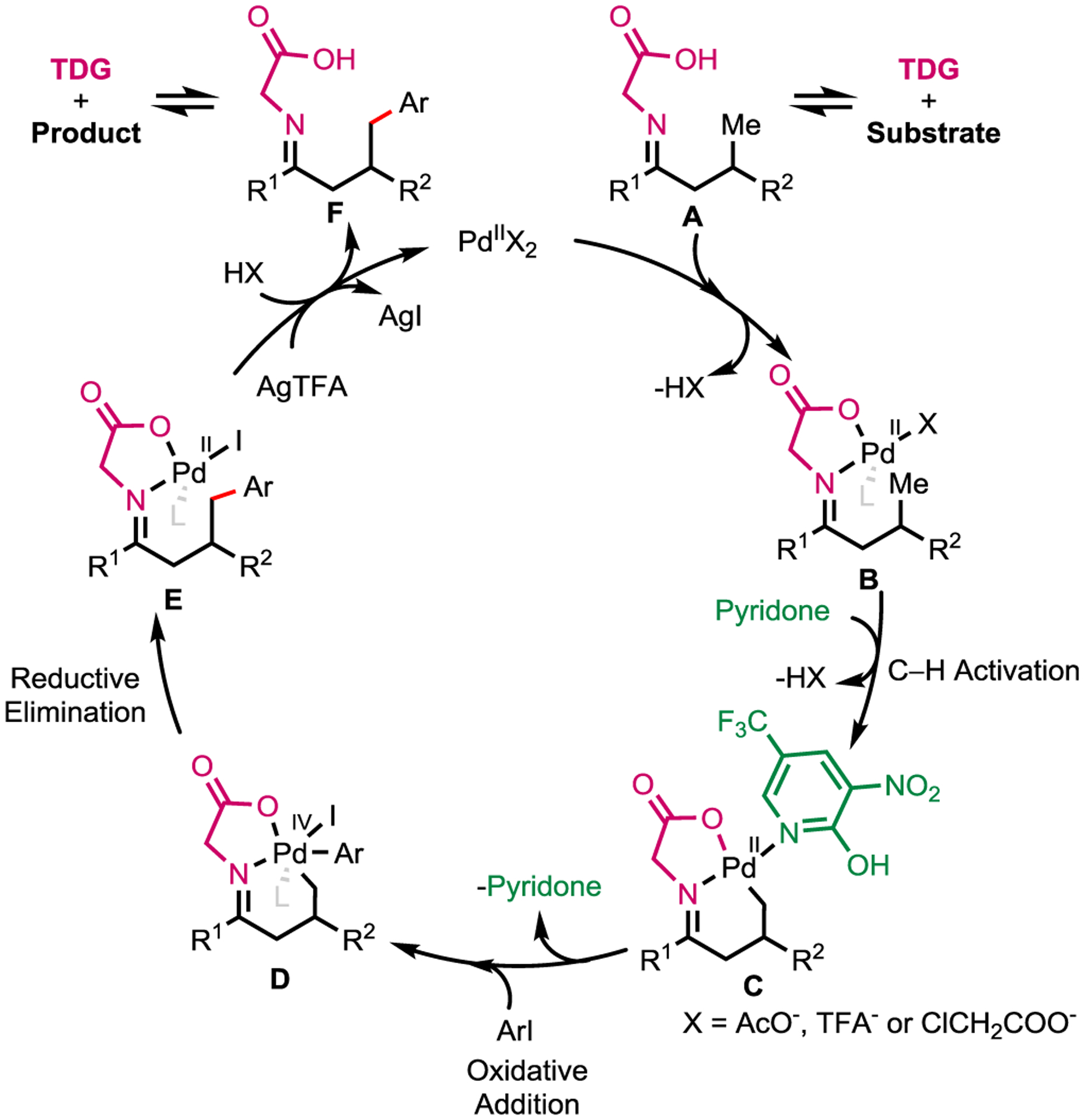
Proposed Catalytic Cycle
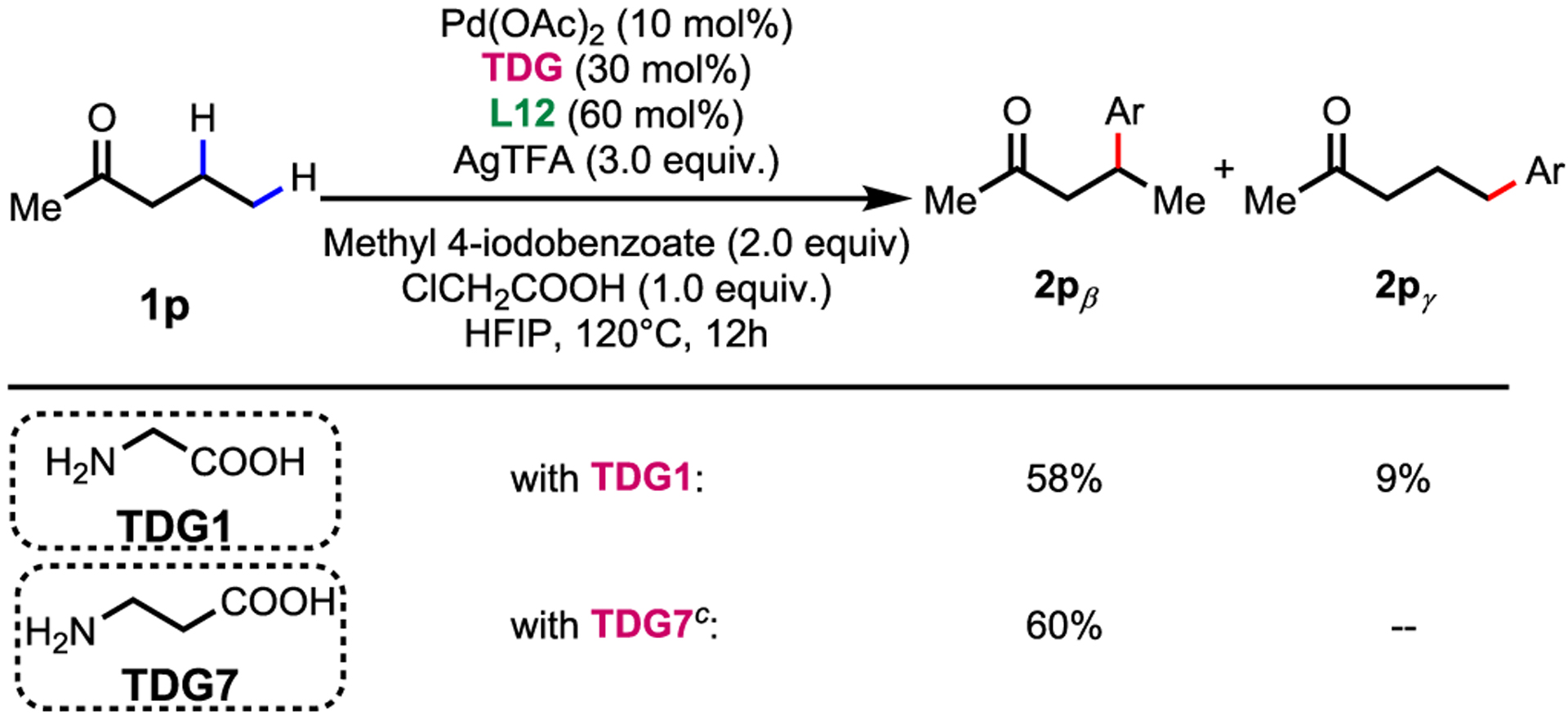
Finally, the distinct site-selectivity observed with the non-substituted acyclic ketones when compared to our recent report on corresponding aldehyde substrates11 are investigated. With aldehydes, the selectivity was switched to γ- with 5-membered chelating TDG. However, pentan-2-one (1p) containing both β-methylene and γ-methyl C(sp3)−H bonds afforded β-C−H arylation with either 5 or 6-membered TDG (Table 4). To obtain some mechanistic insight into the lack of γ-selectivity with 5-membered chelating TDG, we conducted deuterium incorporation experiments under the standard conditions in the presence of 2-chloroacetic acid-d and HFIP-OD (see the Supporting Information for details). The absence of deuterium incorporation at the β- and γ-position of the arylated products suggests that the C−H cleavage proceeds irreversibly, and is expected to serve as the rate-limiting step based on our previous studies.5e With this experimental evidence in hand, we pursued further DFT modeling and revealed that 5,5-membered coordination is favored over the 5,6-coordination due to increased 1,2 steric strain between the methyl group of the substrate and the TDG ring, evident from the difference in distance between them. Calculated regioselectivity is in excellent agreement with experimental observations. (Scheme 3). This mechanistic study provides a valuable structural insight into how to design site selective TDG for ketones and aldehydes.12
Table 4.
|
Conditions: 1 (0.1 mmol, 1.0 equiv), methyl 4-iodobenzoate (2.0 equiv), Pd(OAc)2 (10 mol%), TDG (30 mol%), L12 (60 mol%), AgTFA (3.0 equiv) and ClCH2COOH (1.0 equiv) in HFIP (0. 5 mL), 120°C, under air, 12 h.
Yield and ratio determined by 1H NMR of the crude mixture.
Reaction time: 48h.
Scheme 3.
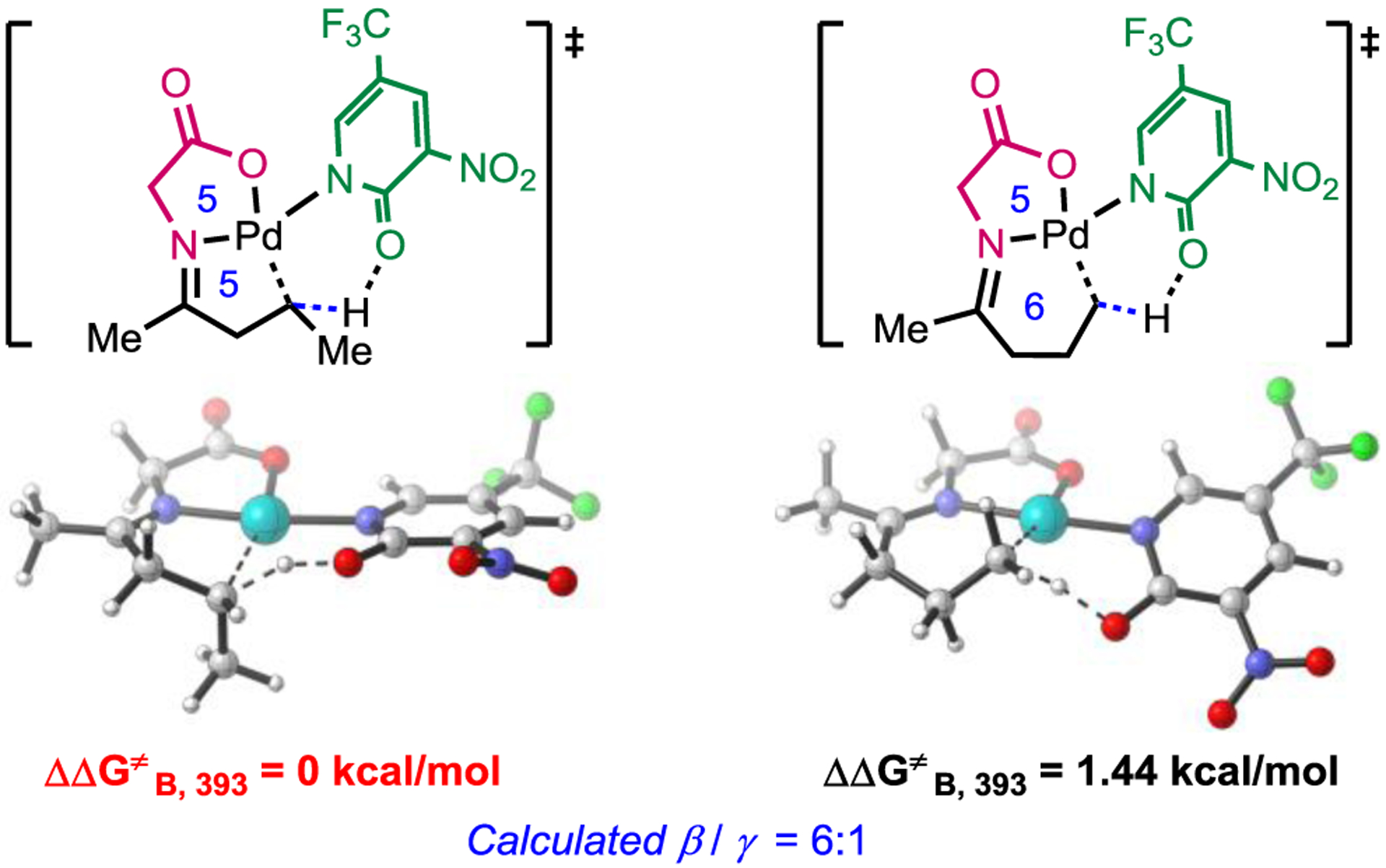
DFT Modeling of The TDG Influence on Site-selectivity of C(sp3)−H Cleavage
In summary, we have developed a protocol for PdII-catalyzed primary γ-C–H (hetero)arylation of aliphatic ketones enabled by a simple α-amino acid TDG and 2-pyridone ligands. This reaction features broad substrate scope without extra steps of installation and removal of directing groups. Functionalization of cyclic γ-methylene C(sp3)−H via a 5,6-membered coordination has also been demonstrated. Mechanistic study on the site selectivity of acyclic ketones and its comparison with corresponding aldehydes points to a valuable structural insight for designing site selective TDG.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS R01 GM084019) for financial support. We acknowledge Ghadiri lab and the Scripps Research High Performance Computing facility for providing computational re-sources.
Footnotes
Supporting Information
The Supporting Information is available free of charge at
Full experimental details, mechanistic studies, computational studies and characterization of new compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).a) Mukaiyama T Explorations into New Reaction Chemistry. Angew. Chem., Int. Ed 2004, 43, 5590–5614. [DOI] [PubMed] [Google Scholar]; (b) Evans DA; Vogel E; Nelson JV Stereoselective aldol condensations via boron enolates. J. Am. Chem. Soc 1979, 101, 6120–6123. [Google Scholar]; (c) Myers AG; Yang BH; Chen H; McKinstry L; Kopecky DJ; Gleason JL Pseudoephedrine as a Practical Chiral Auxiliary for the Synthesis of Highly Enantiomerically Enriched Carboxylic Acids, Alcohols, Aldehydes, and Ketones. J. Am. Chem. Soc 1997, 119, 6496–6511. [Google Scholar]; (d) Cowden CJ; Paterson I Asymmetric aldol reactions using boron enolates. Org. React 1997, 51, 1–200. [Google Scholar]; (e) Cano R; Zakarian A; McGlacken GP Direct Asymmetric Alkylation of Ketones: Still Unconquered. Angew. Chem., Int. Ed 2017, 56, 9278–9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).For selected examples of oxime-directed β-C(sp3)−H functionalization of ketones:; (a) Desai LV; Hull KL; Sanford MS Palladium-catalyzed oxygenation of unactivated sp3 C–H bonds. J. Am. Chem. Soc 2004, 126, 9542–9543. [DOI] [PubMed] [Google Scholar]; b) Thu H-Y; Yu W-Y; Che C-M Intermolecular Amidation of Unactivated sp2 and sp3 C–H Bonds via Palladium-Catalyzed Cascade C–H Activation/Nitrene Insertion. J. Am. Chem. Soc 2006, 128, 9048–9049. [DOI] [PubMed] [Google Scholar]; (c) Kang T; Kim Y; Lee D; Wang Z; Chang S Iridium-Catalyzed Intermolecular Amidation of sp3 C–H Bonds: Late-Stage Functionalization of an Unactivated Methyl Group. J. Am. Chem. Soc 2014, 136, 4141–4144. [DOI] [PubMed] [Google Scholar]; (d) Gao P; Guo W; Xue J; Zhao Y; Yuan Y; Xia Y; Shi Z Iridium(III)-catalyzed direct arylation of C-H bonds with diaryliodonium salts. J. Am. Chem. Soc 2015, 137, 12231–12240. [DOI] [PubMed] [Google Scholar]; (e) Mu YC; Tan XD; Zhang YM; Jing XB; Shi ZZ Pd(II)-Catalyzed β-C–H Arylation of O-Methyl Ketoximes with Iodoarenes. Org. Chem. Front 2016, 3, 380–384. [Google Scholar]; (f) Peng J; Chen C; Xi CJ β-Arylation of Oxime Ethers Using Diaryliodonium Salts through Activation of Inert C(sp3)–H Bonds Using a Palladium Catalyst. Chem. Sci 2016, 7, 1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For selected examples of α-imino-oxy acids-directed β-C(sp3)−H functionalization of ketones:; (a) Zhu RY; Liu LY; Yu J-Q Highly Versatile β-C(sp3)–H Iodination of Ketones Using a Practical Auxiliary. J. Am. Chem. Soc 2017, 139, 12394–12397. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu R-Y; Liu L-Y; Park HS; Hong K; Wu Y; Senanayake CH; Yu J-Q Versatile Alkylation of (Hetero) Aryl Iodides with Ketones via β-C(sp3)–H Activation. J. Am. Chem. Soc 2017, 139, 16080–16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Zhang F-L; Hong K; Li T-J; Park H; Yu J-Q Functionalization of C(sp3)−H Bonds Using a Transient Directing Group. Science 2016, 351, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).For selected C(sp3)−H arylations of aliphatic ketone, see:; (a) Hong K; Park H; Yu J-Q. Methylene C(sp3)–H Arylation of Aliphatic Ketones Using a Transient Directing Group. ACS Catal. 2017, 7, 6938–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pan L; Yang K; Li G; Ge H. Palladium-catalyzed site-selective arylation of aliphatic ketones enabled by a transient ligand. Chem. Commun 2018, 54, 2759–2762. [DOI] [PubMed] [Google Scholar]; (c) Wang J; Dong C; Wu L; Xu M; Lin J; Wei K. Palladium-Catalyzed β-C–H Arylation of Ketones Using Amino Amide as a Transient Directing Group: Applications to Synthesis of Phenanthridinone Alkaloids. Adv. Synth. Catal 2018, 360, 3709–3715. [Google Scholar]; (d) Dong C; Wu L; Yao J; Wei K. Palladium-Catalyzed β-C−H Arylation of Aliphatic Aldehydes and Ketones Using Amino Amide as a Transient Directing Group. Org. Lett 2019, 21, 2085–2089. [DOI] [PubMed] [Google Scholar]; (e) Xiao L-J; Hong K; Luo F; Hu L; Ewing WR; Yeung K-S; Yu J-Q. PdII-Catalyzed Enantioselective C(sp3)–H Arylation of Cyclobutyl Ketones Using a Chiral Transient Directing Group. Angew. Chem., Int. Ed 2020, 59, 9594–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Provencher PA; Bay KL; Hoskin JF; Houk KN; Yu J-Q; Sorensen EJ. Cyclization by C(sp3)–H Arylation with a Transient Directing Group for the Diastereoselective Preparation of Indanes. ACS Catal. 2021, 11, 3115–3127. [Google Scholar]; (g) Provencher PA; Hoskin JF; Wong JJ; Chen X; Yu J-Q; Houk KN; Sorensen EJ. Pd(II)-Catalyzed Synthesis of Benzocyclobutenes by β-Methylene-Selective C(sp3)–H Arylation with a Transient Directing Group. J. Am. Chem. Soc 2021, 143, 20035–20041. [DOI] [PubMed] [Google Scholar]; (h) Wang Y; Wu G; Xu X; Pang B; Liao S; Ji Y. Palladium-Catalyzed β-C(sp3)−H Arylation of Aliphatic Ketones Enabled by a Transient Directing Group. J. Org. Chem 2021, 86, 7296–7303; [DOI] [PubMed] [Google Scholar]; (i) Wu L-F; Yao J-W; Zhang X; Liu S-Y; Zhuang Z-N; Wei K. Pd-Catalyzed β-C–H Arylation of Aldehydes and Ketones Based on a Transient Directing Group. Org. Lett 2021, 23, 6237–6241. [DOI] [PubMed] [Google Scholar]; (j) Cheng J; Xiao L; Qian S; Zhuang Z; Liu A; Yu J-Q. Palladium(II)-Catalyzed Selective Arylation of Tertiary C−H Bonds of Cyclobutylmethyl Ketones Using Transient Directing Groups. Angew. Chem., Int. Ed 2022, 61, e202117233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).For selected C(sp3)−H arylations of aliphatic aldehydes using transient directing groups, see:; (a) Yang K; Li Q; Liu Y; Li G; Ge H Catalytic C−H Arylation of Aliphatic Aldehydes Enabled by a Transient Ligand. J. Am. Chem. Soc 2016, 138, 12775–12778. [DOI] [PubMed] [Google Scholar]; (b) St John-Campbell S; White AJP; Bull JA Single Operation Palladium Catalysed C(sp3)−H Functionalisation of Tertiary Aldehydes: Investigations into Transient Imine Directing Groups. Chem. Sci 2017, 8, 4840–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) St John-Campbell S; Bull JA Intramolecular Palladium(II/IV) Catalysed C(sp3)−H Arylation of Tertiary Aldehydes Using a Transient Imine Directing Group. Chem. Commun 2019, 55, 9172–9175. [DOI] [PubMed] [Google Scholar]; (d) Gou B-B; Liu H-F; Chen J; Zhou L Palladium-Catalyzed Site-Selective C(sp3)–H Arylation of Phenylacetaldehydes. Org. Lett 2019, 21, 7084–7088. [DOI] [PubMed] [Google Scholar]; (e) Li B; Lawrence B; Li G; Ge H Ligand-Controlled Direct γ-C−H Arylation of Aldehydes. Angew. Chem., Int. Ed 2020, 59, 3078–3082. [DOI] [PubMed] [Google Scholar]; (f) St John-Campbell S; White AJP; Bull JA Methylene C(sp3)−H β, β’-Diarylation of Cyclohexanecarbaldehydes Promoted by a Transient Directing Group and Pyridone Ligand. Org. Lett 2020, 22, 1807–1812. [DOI] [PubMed] [Google Scholar]
- (7).For selected aliphatic amine C(sp3)−H functionalization, see:; (a) Xu Y; Young MC; Wang C; Magness DM; Dong G. Catalytic C(sp3)−H Arylation of Free Primary Amines with an exo Directing Group Generated In Situ. Angew. Chem., Int. Ed 2016, 55, 9084–9087. [DOI] [PubMed] [Google Scholar]; (b) Wu Y; Chen Y-Q; Liu T; Eastgate MD; Yu J-Q Pd-Catalyzed γ-C(sp3)−H Arylation of Free Amines Using a Transient Directing Group. J. Am. Chem. Soc 2016, 138, 14554–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu Y; Ge H. Site-selective C–H arylation of primary aliphatic amines enabled by a catalytic transient directing group. Nat. Chem 2016, 9, 26–32. [Google Scholar]; (d) Chen Y-Q; Wang Z; Wu Y; Wisniewski SR; Qiao JX; Ewing WR; Eastgate MD; Yu J-Q Overcoming the Limitations of γ- and δ-C−H Arylation of Amines through Ligand Development. J. Am. Chem. Soc 2018, 140, 17884–17894. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chen YQ; Wu YW; Wang Z; Qiao JX; Yu JQ Transient Directing Group Enabled Pd-Catalyzed Gamma-C(sp3)-H Oxygenation of Alkyl Amines. ACS Catal. 2020, 10, 5657–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen Y-Q; Singh S; Wu Y; Wang Z; Hao W; Verma P; Qiao JX; Sunoj RB; Yu J-Q Pd-Catalyzed γ-C(sp3)-H Fluorination of Free Amines. J. Am. Chem. Soc 2020, 142, 9966–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]; For CO2-mediated C(sp3)−H arylation of aliphatic amine, see:; (g) Kapoor M; Liu D; Young MC Carbon Dioxide-Mediated C(sp3)–H Arylation of Amine Substrates. J. Am. Chem. Soc 2018, 140, 6818–6822. [DOI] [PubMed] [Google Scholar]
- (8).(a) Zhu R-Y; Li ZQ; Park HS; Senanayake CH; Yu J-Q Ligand-Enabled γ-C(sp3)−H Activation of Ketones. J. Am. Chem. Soc 2018, 140, 3564–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Park HS; Fan Z; Zhu R-Y; Yu J-Q, Distal γ-C(sp3)−H Olefination of Ketone Derivatives and Free Carboxylic Acids. Angew. Chem. Int. Ed 2020, 59, 12853–12859. [DOI] [PMC free article] [PubMed] [Google Scholar]; For selected examples and reviews of γ-C(sp3)−H activation with other covalent directing groups, please see:; (c) Cabrera PJ; Lee M; Sanford MS Second-Generation Palladium Catalyst System for Transannular C–H Functionalization of Azabicycloalkanes. J. Am. Chem. Soc 2018, 140, 5599–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Alemán-Ponce de León D; Sánchez-Chávez AC; Polindara-García LA Pd-Mediated γ-C(sp3)–H Bond Activation in Ammonia–Ugi 4-CR Adducts by Using Picolinamide as Directing Group. J. Org. Chem 2019, 84, 12809–12834. [DOI] [PubMed] [Google Scholar]; (e) Liu B; Romine AM; Rubel CZ; Engle KM; Shi BF Transition-Metal-Catalyzed, Coordination-Assisted Functionalization of Nonactivated C(sp3)–H Bonds. Chem. Rev 2021, 121, 14957–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Li B; Elsaid M; Ge H Transition-metal-catalyzed site-selective γ- and δ-C(sp3)–H functionalization reactions. Chem 2022, 8, 1254–1360. [Google Scholar]
- (9).(a) Dauncey EM; Morcillo SP; Douglas JJ; Sheikh NS; Leonori D Photoinduced Remote Functionalisations by Iminyl Radical Promoted C–C and C–H Bond Cleavage Cascades. Angew. Chem., Int. Ed 2018, 57, 744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiang H; Studer A α-Aminoxy Acid-Auxiliary-Enabled Intermolecular Radical γ-C(sp3)–H Functionalization of Ketones. Angew. Chem., Int. Ed 2018, 57, 1692–1696. [DOI] [PubMed] [Google Scholar]; (c) Torres-Ochoa RO; Leclair A; Wang Q; Zhu J Iron-Catalysed Remote C(sp3)–H Azidation of O-Acyl Oximes and N-Acyloxy Imidates Enabled by 1,5-Hydrogen Atom Transfer of Iminyl and Imidate Radicals: Synthesis of γ-Azido Ketones and β-Azido Alcohols. Chem. Eur. J 2019, 25, 9477–9484 [DOI] [PubMed] [Google Scholar]; (d) Li Z; Torres-Ochoa RO; Wang Q; Zhu J Functionalization of remote C(sp3)-H bonds enabled by copper-catalyzed coupling of O-acyloximes with terminal alkynes. Nat. Commun 2020, 11, 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wu D; Cui S-S; Bian F; Yu W. Visible Light Driven and Copper-Catalyzed C(sp3)–H Functionalization of O-Pentafluorobenzoyl Ketone Oximes. Org. Lett 2021, 23, 6057–6061. [DOI] [PubMed] [Google Scholar]
- (10). For selected examples on 2-pyridone accelerated TDG mediated C(sp3)−H activation reactions, see: 5e, 5f, 5g, 5j, 6f, 6g, 7d, 7e and 7f. For selected examples on 2-pyridone accelerated non TDG mediated C(sp3)−H activation reactions, see:; (a) Xia G; Weng J; Liu L; Verma P; Li Z; Yu J-Q, Reversing Conventional Site-selectivity in C(sp3)–H Bond Activation. Nat. Chem 2019, 11, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Park H; Yu J-Q Palladium-Catalyzed [3 + 2] Cycloaddition via Twofold 1,3-C(sp3)–H Activation. J. Am. Chem. Soc 2020, 142, 16552–16556. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xia G; Zhuang Z; Liu L-Y; Schreiber SL; Melillo B; Yu J-Q Ligand-Enabled β-Methylene C(sp3)-H Arylation of Masked Aliphatic Alcohols. Angew. Chem. Int. Ed 2020, 59, 7783–7787. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li Y; Zhang P; Liu Y; Yu Z; Shi B Remote γ-C(sp3)−H Alkylation of Aliphatic Carboxamides via an Unexpected Regiodetermining Pd Migration Process: Reaction Development and Mechanistic Study. ACS Catal. 2020, 10, 8212–8222. [Google Scholar]; (e) Liu S; Zhuang Z; Qiao JX; Yeung K-S; Su S; Cherney EC; Ruan Z; Ewing WR; Poss MA; Yu J-Q Ligand Enabled Pd(II)-Catalyzed γ-C(sp3)–H Lactamization of Native Amides. J. Am. Chem. Soc 2021, 143, 21657–21666. [DOI] [PMC free article] [PubMed] [Google Scholar]; Also see: 8.
- (11).Li Y-H; Ouyang Y; Chekshin N; Yu J-Q PdII–Catalyzed Site-selective β- and γ-C(sp3)–H Arylation of Primary Aldehydes Controlled by Transient Directing Groups. J. Am. Chem. Soc 2022, 144, 4727–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).For other selected examples of site-selective aryl C−H functionalization reactions, please see:; (a) Bisht R; Chattopadhyay B Formal Ir-Catalyzed Ligand-Enabled Ortho and Meta Borylation of Aromatic Aldehydes via in Situ-Generated Imines. J. Am. Chem. Soc 2016, 138, 84–87. [DOI] [PubMed] [Google Scholar]; (b) Das SK; Roy S; Khatua H; Chattopadhyay B Iron-Catalyzed Amination of Strong Aliphatic C(sp3)–H Bonds. J. Am. Chem. Soc 2020, 142, 16211–16217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


