Abstract
Background:
Cannabis plant extracts suppress gastric acid secretion and inflammation, and promote gastroduodenal ulcer healing, all of which are triggered by Helicobacter Pylori infection (HPI). Here, we evaluate the association between cannabis use and HPI among a representative community sample.
Materials and Methods:
We identified respondents who completed cannabis use questions and were tested for HPI (H. pylori IgG antibody seropositivity) from the National Health and Nutrition Examination Survey III dataset (n=4556). Cannabis usage was categorized as ever-use (ever, never), cumulative lifetime use (>10-times, 1–10-times, never), or recent use (>31-days-ago, within-31-days, never). We calculated the crude and adjusted risk (prevalence rate ratio, cPRR and aPRR) of having HPI with cannabis use using generalized Poisson models (SAS 9.4). The models were adjusted for demographics and risk factors for HPI.
Results:
The prevalence of HPI was lower among ever versus never cannabis users (18.6% vs. 33%, p<0.0001). Cannabis use was associated with a decreased risk of HPI (cPRR: 0.56 confidence interval [95% CI: 0.47–0.67]; p<0.0001), which persisted after adjusting for demographics (aPRR: 0.75 [95% CI: 0.63–0.90]; p=0.0016) and comorbidities (aPRR: 0.79 [95% CI: 0.66–0.95]; p=0.0145). Further, individuals with >10-times lifetime cannabis use had a decreased risk of HPI compared with those with 1–10-times lifetime use (aPRR: 0.70 [95% CI: 0.55–0.89]; p=0.0011) and never-users (aPRR: 0.65 [95% CI: 0.50–0.84]; p=0.0002).
Conclusion:
Recreational cannabis use is associated with diminished risk of HPI. These observations suggest the need for additional research assessing the effects of medical cannabis formulations on HPI.
Keywords: antibiotic resistance, cannabinoids, concomitant, peptic ulcer disease, prevalence
Introduction
Gastrointestinal infection caused by Helicobacter pylori (Hp) bacteria is the most common cause of peptic ulcer disease afflicting more than 80% of the world's population.1,2 Environmental factors such as food types, household pets, bad hygiene, socioeconomic status, tobacco, and alcohol use are associated with an increased risk of H. pylori infection (HPI).3,4
After stomach infection by Hp, host factors play a significant modulatory role in determining the patho-histological pattern, as well as the extent and severity of the chronic gastric inflammation induced (antral-predominant, corpus-predominant, or pan–gastritis).5–7 Without treatment, the characteristic sequelae of HPI include asymptomatic gastric colonization, duodenal inflammation, ulcerations (gastric and duodenal), pernicious anemia, and gastric adenocarcinoma.8–10
The standard clinical treatment for HPI involves the combination of antibiotics and anti-secretory medications (triple or quadruple regimens) for 10 to 14 days. However, treatment failures are being noticed due to antibiotic resistance.11,12 Cannabis plant extracts contain more than 600 active ingredients and cannabinoids,13 have been shown to suppress gastric acid secretion, acidic erosion of gastric mucosa, and gastritis, and promote gastric ulcer healing,14,15 all of which are associated with HPI. Reports have shown that cannabis use can ameliorate duodenal and gastric ulcerations via its anti-inflammatory actions.15–18 In addition, cannabinoids have demonstrated antibacterial activities against Clostridium difficile,19 Streptococcus pneumonia,20 and Staphylococcus aureus,21 and other pathogens.22 Finally, cannabis has been shown to modulate colonic microbiota.23 Taken together, these published observations led us to surmise that cannabis use might have a modulatory effect on gastric pH and microbiota, which can impact HPI. Notwithstanding, no studies have evaluated the effects of cannabis and HPI in humans.
Unlike other countries where initial HPI is primarily among children due to poor hygiene, the majority of HPI in the United States occurs in adults, increasing from 16.7% among the age group of 20–29 years, to 56.9% among the age group of ≥70 years. Since cannabis usage is primary among adults, there is a potential for interaction between cannabis use and HPI.24 Further, increased global legalization of recreational cannabis use means that a high proportion of individuals within communities are now using this drug, which can have public health implications for HPI. Delineating any potential associations between cannabis use and HPI will help direct scientific research, and it will shape health policy decisions and recommendations on recreational cannabis use. Therefore, we assessed the association between HPI (defined as positive H. pylori IgG antibody test) and the frequency, quantity, and duration of cannabis use among community dwellers in the United States.
Materials and Methods
Study population
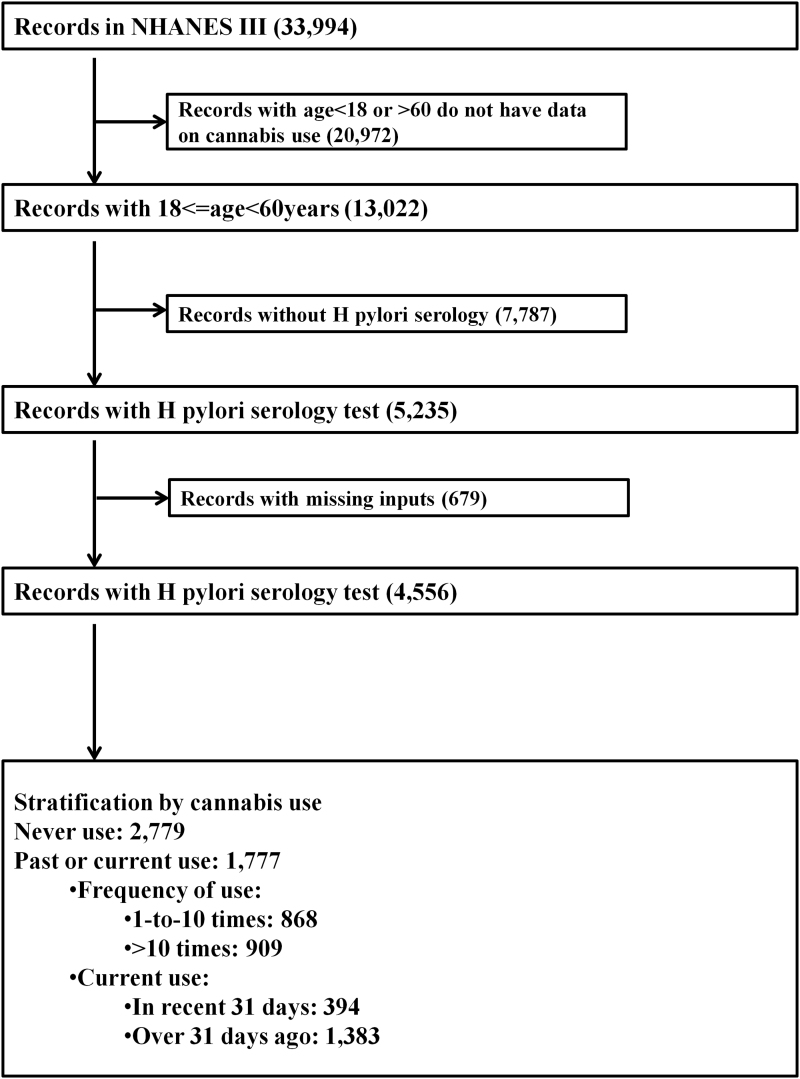
This study was performed using the National Health and Nutrition Examination Survey. Specifically, the Third National Health and Nutrition Examination Survey (NHANES III), which was conducted from 1988 through 1994, was used because these were the only NHANES data that contain information on cannabis use and serologic tests for HPI. Developed by the National Center for Health Statistics, the NHANES III is a nationally representative dataset of noninstitutionalized individuals residing in communities in the United States. The NHANES III is collected through a composite, stratified, and multi-staged methodology. Participating individuals were administered questionnaires on demographics, lifestyle choices, comorbidities, and drug use. Respondents also had physical and laboratory examinations at mobile examination centers. The NHANES III has been used by numerous studies to generate national estimates for various disorders, including the serological prevalence of HPI.24–28 All records with information on cannabis usage (only available for individuals aged 18 to 60 years) and those who had serologic tests for HPI were selected for the analysis before missing records were excluded (Fig. 1). Because the NHANES III is completely de-identified, secondary data analysis employed in this current study did not require any Institutional Review Board Approval.
FIG. 1.
Study population selection flow chart for statistical analyses. NHANES III, Third National Health and Nutrition Examination Survey.
Measurement of cannabis use and explanatory variables
The primary predictor was cannabis use, which was captured in the NHANES III dataset in three ways, and has been used in previous studies.29,30 The first is the question, “Have you ever used marijuana?” with the responses “Yes” or “No” encoded as “Ever-users” and “Never-users” of cannabis respectively. Respondents who had used marijuana were subsequently evaluated for frequency and quantity of use captured in the second question: “About how many times in your life have you used marijuana?” Responses were grouped into “>10-times” and “1-to-10-times.” Third, cannabis users were questioned on recent usage, “During the past month, on how many days did you use marijuana?” Responses were encoded as “past-31-days” and “>31-days-ago.” Our secondary predictors were the factors associated with HPI. These associated factors were identified from published literature24,25 and subsequently correlated with information from the NHANES III dataset. These factors include age, gender (male and female), body mass index (BMI), household size, Race (Whites, Blacks, Hispanics, and others), region of residence (Northeast, Midwest, South, and West), urbanization (metropolitan and nonmetropolitan), income status (≥$40,000, $20,000 to $40,000, and <$20,000), highest educational level (>12th grade, 12th grade, and <12th grade), household pets (yes, no), current smoking (yes, no), alcohol use (excessive use, modest use, and nonuse), use of tap water (yes, no), place of birth (outside the United Sates, within the United States), and marital status (married, separated, and single).1–4,24,25
Measurement of HPI
The outcome was HPI, which was quantified with Hp serologic testing, using commercial IgG ELISA (Wampole Laboratory, Cranbury, NJ). The analytic test details have been extensively described in other previous studies.24,25 Briefly, an immune status ratio (ISR) was calculated for each patient, by dividing the patient's sample optical density with the mean optical density of the cutoff controls. Samples with ISR between 0 and 0.90 were deemed negative, whereas those with ISR >0.9 were positive. Using optimal cutoffs, serologic IgG for HPI laboratory diagnosis has a 72.% sensitivity and 74.3% specificity for individuals aged ≥18 years.31
Statistical analysis
All statistical analysis was performed using the Statistical Analysis System (SAS V.9.4; SAS Institute Inc., Cary, NC). We plotted the estimated prevalence and confidence intervals (CI) using GraphPad Prism 8.1.2 (GraphPad Software, La Jolla, CA). The strata, clusters, and weights provided in the NHANES were used to generate national estimates. We summarized categorical variables with percentages and compared them with the chi-square test. Numerical variables were reported as means and compared with Student's T-tests. We estimated the prevalence rate and risk of HPI seropositivity with cannabis use by using generalized estimating equations with Poisson regression model and robust modification of the error variances.32 Three models were built to measure the risk of HPI with cannabis use. The base model was the crude model (model 1). Model 2 adjusted for demographic variables by including age, sex, race, BMI, household size, region, urbanization, income status, and education level in the base model. Model 3 additionally adjusted for risk factors of HPI, including household pets, current smoking, use of tap water, place of birth, alcohol use, and marital status in model 2. Each of these three statistical models was designed for each of the three different measurements of cannabis use (ever use, cumulative use, and recent use) as a predictor, to make nine models in total.
Results
Study characteristics
From the NHANES III dataset, we evaluated records from 4556 individuals, among whom 1777 had a history of cannabis use (current or past) (Fig. 1). The frequency of HPI was about half among individuals with a history of cannabis use (18.62% vs. 32.95%; p<0.0001) compared with noncannabis users. Cannabis users were more likely to be among individuals who were young, of the male sex, of a white racial group, who reside in the Northeastern region of the United States, but less likely to reside in the Southern region of the United States (Table 1). In addition, cannabis users more often had income earnings of less than $20,000 per year, and paradoxically were more likely to have attained an education level at or above the 12th grade. Further, cannabis users were more frequently from households with pets, to be born within the United States, to have excessive alcohol use and current tobacco use, and to be single (vs. married or separated).
Table 1.
Baseline Characteristics of Study Participant by Cannabis Use, National Health and Nutrition Examination Survey III
| N | Cannabis use |
p | |
|---|---|---|---|
| Never used 2779 (∼32,253,074) | Ever used 1777 (26,483,091) | ||
| Frequency of H elicobacter pylori infection, % | 32.9748 | 18.6201 | <0.0001 |
| Age (SD), years | 38.44 (12.45) | 32.07 (8.92) | <0.0001 |
| Gender, % | |||
| Male | 43.46 | 56.60 | <0.0001 |
| Female | 56.54 | 43.40 | |
| BMI, median (IQR) | 25.9 [22.8–29.9] | 25 [22.2–28.9] | <0.0001 |
| Household size, median (IQR) | 4 [2–5] | 3 [2–5] | <0.0001 |
| Race, % | |||
| Whites | 73.94 | 81.45 | <0.0001 |
| Blacks | 10.26 | 10.84 | |
| Hispanics | 6.35 | 3.59 | |
| Others | 9.44 | 4.12 | |
| Hospital region, % | |||
| Northeast | 19.01 | 23.76 | <0.0001 |
| Midwest | 24.90 | 24.84 | |
| South | 37.76 | 29.07 | |
| West | 18.33 | 22.33 | |
| Urbanization, % | |||
| Metropolitan | 46.30 | 52.29 | <0.0001 |
| Non-Metropolitan | 53.70 | 47.71 | |
| Income status, % | |||
| ≥$40,000 | 39.06 | 35.19 | <0.0001 |
| $20,000–40,000 | 34.82 | 34.15 | |
| Less than $20,000 | 26.12 | 30.66 | |
| Educational level, % | |||
| Above grade 12 | 44.41 | 47.39 | <0.0001 |
| Grade 12 | 31.30 | 34.13 | |
| Below grade 12 | 24.29 | 18.48 | |
| Household pets, % | 45.26 | 52.55 | <0.0001 |
| Current smoking, % | 37.94 | 52.07 | <0.0001 |
| Tap water, % | 89.19 | 89.91 | 0.3897 |
| Place of birth, % | |||
| Outside the United States | 17.57 | 4.81 | <0.0001 |
| Within United States | 82.43 | 95.19 | |
| Alcohol use, % | |||
| Excessive use | 7.14 | 18.49 | <0.0001 |
| Modest use | 75.19 | 80.04 | |
| Nonuse | 17.67 | 1.47 | |
| Marital status, % | |||
| Married | 68.06 | 59.74 | <0.0001 |
| Separated | 12.80 | 11.83 | |
| Single | 19.15 | 28.43 | |
BMI, body mass index.
Prevalence of HPI with cannabis use
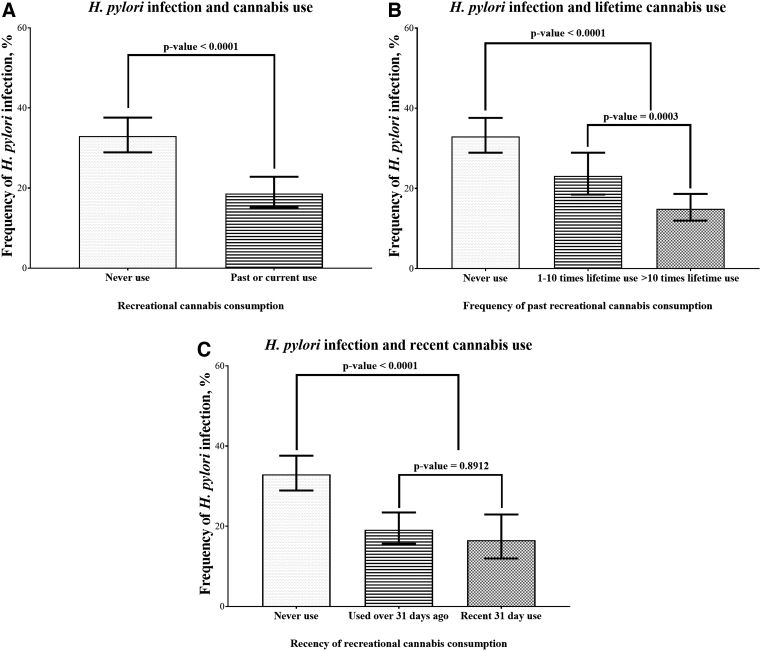
We observed reduced prevalence of HPI among cannabis users both before (Fig. 2A) and after stratification by the quantity of lifetime use (Fig. 2B) but not recency of use (Fig. 2C). Individuals with >10-times lifetime use had a lower prevalence of HPI compared with those with 1–10-times lifetime cannabis use (14.95% vs. 23.14%; p<0.0001) (Fig. 2B). However, the prevalence of HPI was similar among individuals with recent 31-day cannabis use compared with those who had taken cannabis over 31 days in the past (16.57% vs. 19.15%; p=0.8912) (Fig. 2C).
FIG. 2.
Cannabis use is associated with a decreased prevalence of Helicobacter pylori infection. There was a decreased prevalence of H. pylori infection with past/current cannabis use (A), with increasing lifetime usage (B), but not with recency of use (C).
Association between cannabis use and HPI
There was a 44% lower risk of HPI among individuals who had previously used cannabis compared with never users (adjusted risk prevalence rate ratio [aPRR]: 0.56 [95% CI: 0.47–0.67]; p<0.0001) (“A” in Table 2). This lower risk of HPI was diminished, respectively, to 25% and 21% after adjusting for demographic (aPPR: 0.75 [95% CI: 0.63–0.90]; p=0.0016) and other risk factors of HPI (aPPR: 0.79 [95% CI: 0.66–0.95]; p=0.0145) (“B” and “C” in Table 2).
Table 2.
Crude and Adjusted Risk (Prevalence Rate Ratio) of Helicobacter pylori Infection with Cannabis Use, National Health and Nutrition Examination Survey III
| Crude prevalence rate ratio | Lower confidence limit | Upper confidence limit | p | |
|---|---|---|---|---|
| A: Crude model | ||||
| Ever cannabis use | ||||
| Ever vs. never | 0.56 | 0.47 | 0.67 | <0.0001 |
| Cumulative lifetime cannabis use | ||||
| Greater than 10 times vs. never | 0.45 | 0.37 | 0.55 | <0.0001 |
| One to ten times vs. never | 0.70 | 0.58 | 0.86 | 0.0013 |
| Greater than 10 times vs. 1 to 10 times | 0.65 | 0.52 | 0.80 | 0.0003 |
| Recent cannabis use | ||||
| Used within the past 31 days vs. never | 0.50 | 0.37 | 0.68 | <0.0001 |
| Used over 31 days ago vs. never | 0.58 | 0.49 | 0.69 | <0.0001 |
| Used over 31 days ago vs. used over 31 days ago | 0.87 | 0.66 | 1.14 | 0.8912 |
| Adjusted prevalence rate ratio | Lower confidence limit | Upper confidence limit | p | |
|---|---|---|---|---|
| B: Adjusted for demographic factors | ||||
| Ever cannabis use | ||||
| Ever vs. never |
0.75 |
0.63 |
0.90 |
0.0016 |
| Cumulative lifetime cannabis use | ||||
| Greater than 10 times vs. never |
0.62 |
0.48 |
0.79 |
<0.0001 |
| One to 10 times vs. never |
0.90 |
0.70 |
1.14 |
0.8248 |
| Greater than 10 times vs. 1 to 10 times |
0.69 |
0.54 |
0.88 |
0.0006 |
| Recent cannabis use | ||||
| Used within the past 31 days vs. never |
0.61 |
0.42 |
0.89 |
0.0048 |
| Used over 31 days ago vs. never |
0.79 |
0.64 |
0.97 |
0.0181 |
| Used over 31 days ago vs. used over 31 days ago | 0.78 | 0.58 | 1.06 | 0.1544 |
| Adjusted prevalence rate ratio | Lower confidence limit | Upper confidence limit | p | |
|---|---|---|---|---|
| C: Adjusted for demographic and risk factors for H. pylori infection | ||||
| Ever cannabis use | ||||
| Ever vs. never |
0.79 |
0.66 |
0.95 |
0.0145 |
| Cumulative lifetime cannabis use | ||||
| Greater than 10 times vs. never |
0.65 |
0.50 |
0.84 |
0.0002 |
| One to 10 times vs. never |
0.93 |
0.72 |
1.20 |
1 |
| Greater than 10 times vs. 1 to 10 times |
0.70 |
0.55 |
0.89 |
0.0011 |
| Recent cannabis use | ||||
| Used within the past 31 days vs. never |
0.67 |
0.46 |
0.98 |
0.0369 |
| Used over 31 days ago vs. never |
0.82 |
0.65 |
1.02 |
0.0916 |
| Used over 31 days ago vs. used over 31 days ago | 0.82 | 0.61 | 1.12 | 0.3782 |
A, crude model; B, model adjusted for age, sex, race, BMI, house-hold size, income status, geographic region, rural versus urban location, and education level; C, model adjusted for model B and other risk factors for H. pylori infection including household pets, smoking, alcohol, tap water use, country of birth, and marital status.
When cannabis use was measured by cumulative lifetime usage, individuals with greater than 10-times lifetime use were associated with a significantly lower risk of HPI when compared with those who had taken cannabis 1 to 10 times or never-users (aPPR: 0.65 [95% CI: 0.52–0.8] and 0.45 [95% CI: 0.37–0.55]; p<0.001) (“A” in Table 2). These relationships were moderately diminished but remained significant after adjustment for demographics and other risk factors for HPI (“B” and “C” in Table 2). However, respondents with 1-to-10-times lifetime cannabis use were only associated with a decreased risk of HPI when compared with never-users in the crude model (aPPR: 0.70 [95% CI: 0.58–0.86], p=0.0013) (“A” in Table 2), but not after adjustments for demographics (“B” in Table 2) and other risk factors for HPI (“C” in Table 2).
Finally, when cannabis use was categorized by the timing of use, recent 31-day cannabis use was associated with a 50% decreased risk of HPI when compared with never-users (aPPR: 0.50 [95% CI: 0.37–0.68]; p<0.0001), which persisted after adjustments for demographics (“B” in Table 2) and other risk factors for HPI (“C” in Table 2). However, there was no difference in HPI among recent 31-day use versus cannabis use over 31 days in the past before or after adjustments (“A–C” in Table 2).
In the fully adjusted model, other factors associated with HPI were increasing age, male sex, increasing household size, race (any other race compared with Whites), lower-income status (less than $20,000 vs. ≥$40,000), lower educational level, and being born outside the United States (Table 3).
Table 3.
Factors Associated with Helicobacter Pylori Infection in a Multivariate Model, National Health and Nutrition Examination Survey III
| Characteristics associated with H. pylori infection | Adjusted prevalence rate ratio | Lower confidence limit | Upper confidence limit | p |
|---|---|---|---|---|
| Cannabis ever use vs. never use | 0.79 | 0.66 | 0.95 | 0.0145 |
| Age, per 10 year increase | 1.26 | 1.20 | 1.33 | <0.0001 |
| Sex, female vs. male | 0.86 | 0.77 | 0.96 | 0.0082 |
| BMI, per 5 increase | 1.00 | 1.00 | 1.00 | 0.4126 |
| Household size, per 2 increase | 1.08 | 1.02 | 1.14 | 0.0108 |
| Race | ||||
| Black vs. White | 2.26 | 1.83 | 2.79 | <0.0001 |
| Hispanics vs. Whites | 1.73 | 1.20 | 2.51 | 0.0005 |
| Others vs. Whites | 1.72 | 1.08 | 2.75 | 0.0134 |
| Hospital region | ||||
| Midwest vs. Northeast | 1.07 | 0.75 | 1.52 | 1 |
| South vs. Northeast | 1.29 | 0.92 | 1.82 | 0.2623 |
| West vs. Northeast | 1.13 | 0.77 | 1.67 | 1 |
| Rural vs. urban location | 1.02 | 0.83 | 1.25 | 0.88 |
| Income status | ||||
| $20,000–40,000 vs. less than $40,000 | 1.16 | 0.96 | 1.40 | 0.1959 |
| Less than $20,000 vs. $40,000 | 1.26 | 1.01 | 1.57 | 0.0319 |
| Educational level | ||||
| Below grade 12 vs. above grade 12 | 1.79 | 1.42 | 2.26 | <0.0001 |
| Grade 12 vs. above grade 12 | 1.59 | 1.21 | 2.09 | 0.0001 |
| Household pets | 0.96 | 0.85 | 1.07 | 0.4456 |
| Current smoking | 0.97 | 0.87 | 1.09 | 0.6505 |
| Tap water | 1.07 | 0.83 | 1.38 | 0.6188 |
| Place of birth, outside the United States vs. within United States | 1.62 | 1.20 | 2.18 | 0.0015 |
| Alcohol use | ||||
| Modest use vs. nonuse | 1.05 | 0.85 | 1.29 | 1 |
| Excessive use vs. nonuse | 0.96 | 0.69 | 1.33 | 1 |
| Marital status | ||||
| Married vs. single | 1.09 | 0.88 | 1.35 | 0.985 |
| Separated vs. single | 1.19 | 0.98 | 1.46 | 0.0951 |
Discussion
Using a well-characterized dataset of community dwellers in the United States, we reveal for the first time, to the best of our knowledge, that recreational cannabis use is associated with a reduced risk of HPI. Notably, our results unveiled that longer-term usage of cannabis was associated with the lowest risk for HPI. The reasons for these observations remain ill-defined.
Given our novel observations, the potential mechanisms by which cannabis can impact HPI need to be examined. Consequently, from published reports, we surmise that cannabis use may impact HPI through: (1) direct antibacterial action of cannabis on Hp; (2) suppression of gastric acidity; (3) anti-inflammatory and healing properties of cannabis in the gut; (4) modulation of a diversity of gastric microbiome; (5) modulation of the immune system; (6) and avoidance of cannabis consumption by individuals with HPI due to symptom exacerbation.14–18,20,22,23,33–35
First, cannabis may have direct antibacterial effects on Hp. Although studies on the direct action of cannabis on Hp are not available, cannabis has been reported to have antibacterial activities against S. aureus21 and Clostridoides difficile.19 Second, cannabis suppresses gastric acid production and increases gastric pH,15 thereby improving the healing of duodenal ulcers.16 These direct actions of cannabis might decrease duodenal Hp colonization and infection that may account for our observed decrease in the prevalence of HPI among recreational cannabis users.
Third, cannabis plant extracts have demonstrated anti-inflammatory and ulcer healing properties.14–18 Many studies have shown that intestinal mucosal CB2 receptor agonists can decrease gastric acidity, gastritis, and gastric ulcerations,15,17 The anti-inflammatory properties of cannabis suppress gastritis and gastric ulcer formation.16,18 Specifically, murine models have shown that CB2 agonists can promote the healing of gastric and duodenal ulcers, protecting mice from adverse gastric effects of nonsteroidal anti-inflammatory agents and alcohol.17,18 By promoting healing during/after infection, cannabis may decrease the severity and duration of HPI. As such, cannabis can reduce the intensity of HPI-induced inflammation characterized by increased inflammatory cytokines and Hp antibodies, which are revealed in our study. Fourth, infection by Hp can severely alter the gastric microbiome. Hp stomach colonization can severely decimate the population of three (Firmicutes, Actinobacteria, and Bacteroides) of the four most abundant gut bacteria flora, with concomitant amplification of the quantity of Proteobacteria from <20% to more than 90%.33 The ensuing dysbiosis with HPI correlates strongly with adverse disease progression in the stomach, increased gastric metaplasia, and adenocarcinoma.33 Although there are no studies of the impact of cannabis on the gastric microbiome, cannabis had been reported to maintain a healthy balance of the gut microbiome and prevent obesity when administered together with a fatty diet to mice.23 Cannabis might exert a similar effect on the stomach, countering the dysbiosis from HPI33 and limiting disease progression.
Alternative mechanisms may additionally explain our novel findings. A fifth possible explanation may be that cannabis use suppresses immune system functions34 and its production of optimal levels of IgG against HPI. This is especially possible, because our results reveal that after full adjustments when compared with never users, individuals with recent 31-day cannabis use had a diminished risk of HPI when compared with individuals with a history of last cannabis use being more than 31 days (“C” in Table 2). It may be that such individuals with recent cannabis use have HPI but do not produce adequate IgG and, therefore, tested negative to the serologic test; however, those with past (>31 days ago) cannabis use have recovered from cannabis suppression of IgG production against HPI and, therefore, were similar to individuals who never took cannabis. However, such patients will be expected to test positive for stool Hp antigen test and/or to show more symptoms. Unfortunately, the stool antigen tests were not available in the NHANES III dataset, and we are unable to assess this potential mechanism. Finally (sixth), it may be that cannabis use exacerbated symptoms of HPI, resulting in patients with HPI, avoiding cannabis use. By inhibiting gastric acid production, a major gastrointestinal antimicrobial defense mechanism, cannabis may predispose to some bacterial infections, including HPI. For example, heavy cannabis use has been associated with more voluminous diarrhea due to Vibro and Escherichia spp. infections.35 However, this possibility is less likely given that preclinical studies have reported the suppressive actions of cannabis on gastroduodenal acidity, inflammation, and ulcerations.15–18
Our findings on the risk of HPI with demographic factors are consistent with other studies in the literature,1–4,24,25 including increasing age, male sex, household size, non-White races, insufficient education (at or below 12th grade), and place of birth outside the United States. However, unlike some of the previous studies, we did not find any association of alcohol and tobacco use, tap water, marital status, and household pets with HPI.4 Also, the average BMI was lower among subjects with cannabis use in our frequency table, which is similar to recent findings.36 The consistency of our findings with most other studies,1–4,24,25 the large sample size of our study subjects, and multiple adjustments modeling employed by our study offer strong reliability of our novel findings.
The NHANES III datasets are, however, limited by their cross-sectional study design37 Therefore, direct causal and temporal relationships cannot be ascertained by just statistical analysis. In addition, errors in recalling the frequency or quantity of cannabis use; imperfect accuracies for the serologic marker of HPI, paucity of data on the specific type, or mode of consuming cannabis; and the possibility of residual confounding can all potentially affect the validity of our study. However, these errors are likely to be nondifferential across exposure and outcome groups and are more likely to diminish our measured effect. These shortcomings of the NHANES dataset are outweighed by the fact that our analysis incorporates multiple confounding factors in the statistical models. Also, the NHANES III (collected from 1988 to 1996) is quite old, and the HPI serologic tests performed during this period might be less accurate compared with more recent tests that have increased sensitivity and specificity. These drawbacks might potentially impact the validity of our results using the indicated dataset. Unfortunately, the NHANES III is the only cycle of NHANES to contain both cannabis use and HPI serologic tests. There are currently no recent NHANES datasets that capture cannabis use and HPI. Given our observations, it will be helpful if the NHANES and other datasets capture new information to strengthen similar future research on this important subject. Further, the NHANES III only samples noninstitutionalized members of the population and excludes homeless and incarcerated individuals, who may have a high risk of both HPI and cannabis use. Also, only about 40% (5235 of 13,022, Fig. 1) of eligible respondents had a serologic test for HPI, which may potentially result in sampling bias. However, we incorporated the recommended weighing methodologies in our statistical analysis to account for non-response. The NHANES III dataset does not have information on the strains/formulation of cannabis used or mode of consumption (oral vs. vaping vs. inhalation), which might impact interactions with Hp in the stomach. Finally, our measurement of HPI in the community assesses for evidence of infection (past or present) and does not specify whether the individual has active HPI.
Our current study has many strengths. First, the use of a large, nationally representative database, with detailed racial and sociodemographic information, allowed us to study the relationship between cannabis and HPI at a population level. Second, cannabis consumption was captured in different ways, providing us separate perspectives and deeper understanding. Third, our analysis built on previous studies on the NHANES,24 where a majority of HPI occurred in adulthood, coinciding most likely during periods of cannabis use, allowing for an opportunity to study the effect of cannabis on HPI. Finally, cannabis use history was collected using a confidentially completed questionnaire, providing reliable estimates.
In the past few years, there has been increased legalization of cannabis use for recreational purposes, especially in North America.38,39 Canada has legalized cannabis use for recreational use for individuals ≥18 years.39 Our current article analyzed data, which are several years old when cannabis was illegal in North America. As such, our observations from the data might not currently reflect the situation. This is because more individuals are now more regularly using a wide range of full-spectrum cannabis formulations. Cannabis is legal in Canada and an increasing number of states in the United States40; as such, most individuals do readily disclose their cannabis use status without fear of adverse legal implications. Therefore, it will be very beneficial for future research if population datasets that incorporate cannabis use habits additionally capture more extensive/comprehensive clinical and laboratory information on all disease conditions, including HPI. This is because cannabis contains more than 400 active chemicals that are shown to have numerous and diverse anti-pathogenic and physiological chemical regulatory properties that will most certainly affect numerous disease prevalence, progression, and clinical outcomes.41–43
In conclusion, our novel findings reveal that cannabis use was associated with a lower prevalence of HPI among noninstitutionalized residents of the United States. Further, individuals with recent use and those with larger lifetime cannabis use were less likely to test positive for HPI. Our interesting observations suggest the need for additional molecular studies to delineate the direct impact of cannabis use and HPI. Specifically, future studies evaluating how the active ingredients in cannabis exert potential antibacterial effects on Hp and immunomodulatory activities in humans are highly needed.
Abbreviations Used
- aPRR
adjusted risk prevalence rate ratio
- cPRR
crude risk prevalence rate ratio
- BMI
body mass index
- CI
confidence intervals
- Hp
Helicobacter pylori
- HPI
Helicobacter pylori infection
- ISR
immune status ratio
- NHANES III
Third National Health and Nutrition Examination Survey
Authors' Contributions
A.C.A., P.L., and T.N.B. conceived and designed the study; acquired, analyzed, and interpreted the data; and wrote the article. T.N.B. supervised the project.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by a Start-up Grant from the INRS-CAFSB to T.N.B. A.C.A. is currently supported by an NIH T32DK063922 grant. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the article.
Cite this article as: Adejumo AC, Labonte P, Bukong TN (2023) Relationship between recreational cannabis use and Helicobacter pylori infection, Cannabis and Cannabinoid Research 8:3, 537–546, DOI: 10.1089/can.2021.0139.
References
- 1. Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 2. Leontiadis GI, Nyrén O. Epidemiology of Helicobacter Pylori infection, peptic ulcer disease and gastric cancer. In: Talley NJ, Locke GR, Moayyedi P, West J, Ford AC & Saito YA (eds). GI epidemiology. John Wiley & Sons, Ltd, 2014. p. 135–157. [Google Scholar]
- 3. Mhaskar RS, Ricardo I, Azliyati A, et al. Assessment of risk factors of Helicobacter Pylori infection and peptic ulcer disease. J Glob Infect Dis. 2013;5:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogihara A, Kikuchi S, Hasegawa A, et al. Relationship between Helicobacter pylori infection and smoking and drinking habits. J Gastroenterol Hepatol. 2000;15:271–276. [DOI] [PubMed] [Google Scholar]
- 5. Malaty HM. Helicobacter pylori infection: genetic and environmental influences: a study of twins. Ann Intern Med. 1994;120:982. [DOI] [PubMed] [Google Scholar]
- 6. Israel DA, Salama N, Arnold CN, et al. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J Clin Invest. 2001;107:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calam J, Gibbons A, Healey ZV, et al. How does Helicobacter pylori cause mucosal damage? Its effect on acid and gastrin physiology. Gastroenterology. 1997;113(6 Suppl):S43–S49; discussion S50. [DOI] [PubMed] [Google Scholar]
- 8. Waldum HL, Kleveland PM, Sørdal ØF. Helicobacter pylori and gastric acid: an intimate and reciprocal relationship. Ther Adv Gastroenterol. 2016;9:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. [DOI] [PubMed] [Google Scholar]
- 10. Kusters JG, van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiota S, Reddy R, Alsarraj A, et al. Antibiotic resistance of Helicobacter pylori among male United States Veterans. Clin Gastroenterol Hepatol. 2015;13:1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andre CM, Hausman J-F, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adami M, Frati P, Bertini S, et al. Gastric antisecretory role and immunohistochemical localization of cannabinoid receptors in the rat stomach. Br J Pharmacol. 2002;135:1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdel-Salam O. Gastric acid inhibitory and gastric protective effects of Cannabis and cannabinoids. Asian Pac J Trop Med. 2016;9:413–419. [DOI] [PubMed] [Google Scholar]
- 16. Sofia D, Diamantis W, Harrison JE, et al. Evaluation of antiulcer activity of Δ9-tetrahydrocannabinol in the Shay Rat Test. Pharmacology. 1978;17:173–177. [DOI] [PubMed] [Google Scholar]
- 17. Abdel-Salam OME, Salama RAA, El-Denshary E-E, et al. Effect of Cannabis sativa extract on gastric acid secretion, oxidative stress and gastric mucosal integrity in rats. Comp Clin Pathol. 2015;24:1417–1434. [Google Scholar]
- 18. Kinsey SG, Cole EC. Acute Δ(9)-tetrahydrocannabinol blocks gastric hemorrhages induced by the nonsteroidal anti-inflammatory drug diclofenac sodium in mice. Eur J Pharmacol. 2013;715:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nissen L, Zatta A, Stefanini I, et al. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia. 2010;81:413–419. [DOI] [PubMed] [Google Scholar]
- 20. Bass R, Engelhard D, Trembovler V, et al. A novel nonpsychotropic cannabinoid, HU-211, in the treatment of experimental pneumococcal meningitis. J Infect Dis. 1996;173:735–738. [DOI] [PubMed] [Google Scholar]
- 21. Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008;71:1427–1430. [DOI] [PubMed] [Google Scholar]
- 22. Hernández-Cervantes R, Méndez-Díaz M, Prospéro-García Ó, et al. Immunoregulatory role of cannabinoids during infectious disease. Neuroimmunomodulation. 2017;24:183–199. [DOI] [PubMed] [Google Scholar]
- 23. Cluny NL, Keenan CM, Reimer RA, et al. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PLoS One. 2015;10:e0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Everhart JE, Kruszon-Moran D, Perez-Perez GI, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359–1363. [DOI] [PubMed] [Google Scholar]
- 25. Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaturvedi AK, Graubard BI, Broutian T, et al. NHANES 2009–2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res. 2015;75:2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cave M, Appana S, Patel M, et al. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect. 2010;118:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mitsuhashi S, Ballou S, Jiang ZG, et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2018;113:115–123. [DOI] [PubMed] [Google Scholar]
- 29. Adejumo AC, Flanagan R, Kuo B, et al. Relationship between recreational marijuana use and bowel function in a nationwide cohort study. Am J Gastroenterol. 2019;114:1894–1903. [DOI] [PubMed] [Google Scholar]
- 30. Alshaarawy O, Anthony JC. Cannabis smoking and serum C-reactive protein: a quantile regressions approach based on NHANES 2005–2010. Drug Alcohol Depend. 2015;147:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. She RC, Wilson AR, Litwin CM. Evaluation of Helicobacter pylori immunoglobulin G (IgG), IgA, and IgM serologic testing compared to stool antigen testing. Clin Vaccine Immunol CVI. 2009;16:1253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noto JM, Peek RM Jr. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathog. 2017;13:e1006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jan T-R, Su S-T, Wu H-Y, et al. Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice. Int Immunopharmacol. 2007;7:773–780. [DOI] [PubMed] [Google Scholar]
- 35. Nalin DR, Levine MM, Rhead J, et al. Cannabis, hypochlorhydria, and cholera. Lancet Lond Engl. 1978;2:859–862. [DOI] [PubMed] [Google Scholar]
- 36. Alshaarawy O, Anthony JC. Are cannabis users less likely to gain weight? Results from a national 3-year prospective study. Int J Epidemiol 2019;48:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levin KA. Study design III: cross-sectional studies. Evid Based Dent. 2006;7:24–25. [DOI] [PubMed] [Google Scholar]
- 38. Hall W, Lynskey M. Assessing the public health impacts of legalizing recreational cannabis use: the US experience. World Psychiatry. 2020;19:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cox C. The Canadian Cannabis Act legalizes and regulates recreational cannabis use in 2018. Health Policy Amst Neth. 2018;122:205–209. [DOI] [PubMed] [Google Scholar]
- 40. Lancione S, Wade K, Windle SB, et al. Non-medical cannabis in North America: an overview of regulatory approaches. Public Health. 2020;178:7–14. [DOI] [PubMed] [Google Scholar]
- 41. Farha MA, El-Halfawy OM, Gale RT, et al. Uncovering the hidden antibiotic potential of cannabis. ACS Infect Dis. 2020;6:338–346. [DOI] [PubMed] [Google Scholar]
- 42. Cohen K, Weizman A, Weinstein A. Positive and negative effects of cannabis and cannabinoids on health. Clin Pharmacol Ther. 2019;105:1139–1147. [DOI] [PubMed] [Google Scholar]
- 43. National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research [Internet]. Washington (DC): National Academies Press (US); 2017. (The National Academies Collection: Reports funded by National Institutes of Health). Available from: http://www.ncbi.nlm.nih.gov/books/NBK423845 Accessed October 8, 2021. [Google Scholar]