Abstract
Objectives
The peritoneal regression grading score (PRGS) is a four-tied pathologic score measuring tumor regression in biopsies from patients with peritoneal metastasis (PM) receiving chemotherapy.
Methods
This retrospective analysis of a prospective registry (NCT03210298) analyses 97 patients with isolated PM under palliative chemotherapy. We examined the predictive value of the initial PRGS for overall survival (OS) and the prognostic value of PRGS in repeated peritoneal biopsies.
Results
The 36 (37.1 %) patients with an initial mean PRGS≤2 had a longer median OS (12.1 months, CI 95 % 7.8–16.4) vs. 8.0 months (CI 95 % 5.1–10.8 months) in 61 (62.9 %) patients with PRGS≥3 (p=0.02) After stratification, the initial PRGS was an independent predictor of OS (Cox-regression, p<0.05). Out of 62 patients receiving≥two chemotherapy cycles, 42 (67.7 %) had a histological response (defined as a lower or stable mean PRGS in successive therapy cycles), and 20 (32.3 %) progressed (defined as an increasing mean PRGS). PRGS response was associated with a longer median OS (14.6 months, CI 5–95 % 6.0–23.2) vs. 6.9 (CI 5–95 % 0.0–15.9) months. PRGS response was prognostic in the univariate analysis (p=0.017). Thus, PRGS had both a predictive and prognostic significance in patients with isolated PM receiving palliative chemotherapy in this patient cohort.
Conclusions
This is the first evidence for the independent predictive and prognostic significance of PRGS in PM. These encouraging results need validation in an adequately powered, prospective study.
Keywords: antineoplastic agents, metastasis, neoplasm, pathology
Introduction
Therapy response assessment in patients with peritoneal metastasis (PM) is a particular challenge in modern oncology. RECIST 1.1, as assessed by CT-scan, is the reference system for evaluating tumor response in oncology studies [1]. However, in patients with peritoneal metastasis, the accuracy of current imaging systems is limited. The healthy peritoneum is a single-layered membrane [2] that CT-scan can barely visualize. PM is often a small-volumetric disease with a diameter of lesions at the low detection limit of cross-sectional imaging methods such as CT [3]. Moreover, the patient’s breathing and bowel motility can cause a loss of image sharpness. Unfortunately, in the absence of target lesions, CT-based determination of progression-free survival or time-to-progression is impossible in patients with PM. Therefore, laparoscopy is increasingly used to determine the response of PM to chemotherapy [4, 5] and to indicate cytoreductive surgery,
Laparoscopy allows macroscopic staging of the extent of peritoneal disease and biopsies from tumor-suspect areas. A prognostic significance of tumor regression grading in peritoneal metastasis has already been documented in the neoadjuvant setting in colorectal and ovarian cancer [6]. In the palliative setting, a group of pathologists in Europe proposed in 2016 a novel regression grading for therapy response assessment in PM, the peritoneal regression grading score (PRGS) [7]. PRGS is a four-tied grading system considering several specific characteristics of PM, in particular their frequent mucinous character. Peritoneal biopsies are taken from all four abdominal quadrants to consider tumor heterogeneity. PRGS is determined as follows: Grade 1: complete response (absence of tumor cells), Grade 2: major response (major regression features, few residual tumor cells), Grade 3: minor response (some regressive features but predominance of residual tumor cells), Grade 4: no response (tumor cells without any regressive features). Acellular mucin and infarct-like necrosis are regarded as regression features. Then, the mean PRGS is calculated from all biopsies available.
Since 2016, PRGS is diffusing rapidly into clinical practice worldwide [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28] underscoring the high need for a uniform histological therapy response score in PM. In the meantime, extensive methodological and validation work has been done on PRGS. In a multicentric study on 331 peritoneal biopsies, the interobserver variability for PRGS of each biopsy and the mean PRGS per biopsy set was moderate to good/substantial. The intra-observer variability for PRGS of each biopsy and the mean PRGS per biopsy set was good to excellent/almost perfect [29]. The minimal number of biopsies required for determining PRGS with an accuracy superior to 80 % was found to be three, and grading “PRGSx” was recommended for the cases with two or fewer biopsies [30]. Finally, the value of additional staining in biopsies with no tumor cells visible was evaluated: Further staining reduced the instances of unclear PRGS to zero and changed the therapy response assessment in 21 % of patients [31].
Many patients selected for locoregional therapy with cytoreductive surgery, HIPEC and/or PIPAC receive preoperative systemic chemotherapy. In surgical, oncology, when it comes to the efficacy of metastasectomy, “Biology is King, and selection of cases is Queen” [32]. Intuitively, patients with chemoresistant tumors (highly vital tumors, PRGS 3 and 4) are expected to benefit less from aggressive surgery than patients with chemosensitive tumors. However, at the present time, conclusive evidence on the predictive and prognostic significance of PRGS in clinical practice is still largely missing. This study delivers solid evidence that PRGS allows to stratify patients with PM based on long-term outcomes, improving patient selection for surgical, potentially curative therapy, vs. medical, palliative therapy.
Patients and methods
Study design
This is a registry study on a cohort of consecutive patients with histologically documented peritoneal metastasis first treated between 1.7.2016 and 1.5.2019 in a tertiary care center specialized in multimodal therapy or peritoneal metastasis. Data were entered prospectively into a patient registry, and the analysis was retrospective. No primary/secondary endpoint was defined in advance.
Ethical and regulatory framework
Clinical, pathology and follow-up data were entered into the international PIPAC patient registry (www.clinicaltrials.gov, NCT03210298). This registry was approved by the Ethics Committee, Ruhr-University Bochum, on January 11th, 2016 under the reference 15–5,280). Each patient gave their written informed consent for data storage management and analysis. The retrospective analysis was authorized by the Ethics Committee, University of Tübingen, on 18.3.2021, under the reference 073/2019BO.
Therapy
All patients received intraperitoneal chemotherapy as pressurized intraperitonel aerosol chemotherapy (PIPAC) with oxaliplatin 92 mg/m2 BSA or with a combination of cisplatin 7.5 mg/m2 BSA and doxorubicin 1.5 mg/m2 BSA. A majority of patients (64.9 %) received combined systemic chemotherapy and PIPAC. A total of 244 PIPAC procedures were performed on 97 patients. The mean number of PIPACs administered was 2.5 (minimum one, maximum seven). Sixty-two patients had at least 2 PIPAC. PIPAC cycles were repeated at 6-week intervals. In the case of major or complete histological regression, the time interval was extended to 3 months. In the cases of combined treatment (systemic and intraperitoneal chemotherapy), a minimum delay of two weeks (bevacizumab: 4 weeks) was observed between the last application of systemic chemotherapy and PIPAC.
Peritoneal biopsy specimens
Millimetric biopsies were taken with a 5-mm endoscopic instrument (ClickLine Biopsy forceps, Karl Storz, Tuttlingen, Germany) from macroscopically tumor suspect areas in all four abdominal quadrants. Peritoneal biopsies were fixed in 10 % buffered formalin for 24–48 h. Then, samples were embedded in paraffin using a controlled temperature. Two series of three 2.5 µm thick step sections from each biopsy were stained with hematoxylin and eosin (H&E).
Determination of the peritoneal regression grading score (PRGS)
Histopathological tumor regression was graded according to the 4-tied peritoneal regression grading score (PRGS), as described previously [31]. Shortly, PRGS 1 equals complete histological response and is defined as the absence of residual cancer and a large amount of regressive fibrosis (and/or acellular mucin pools and/or infarct-like necrosis). PRGS 2 is defined as a specimen where regressive changes are predominant over cancer cells. PRGS 3 is a specimen where regressive changes are present but where cancer cells are dominant over fibrosis. PRGS 4 is a specimen without histological features of regression. Infarct- or mucin-like necrosis is regarded as indicative of tumor regression. In contrast, unspecific tumor necrosis is indicative of a lack of response in the respective area, as described for colorectal liver metastases [33]. Various independent senior pathologists assessed PRGS by grading tumor biopsies taken during each laparoscopy, and no blinding was applied. The mean PRGS was determined in each patient, thus taking into account all information from available biopsies. PRGS was used for all tumor histologies.
Follow-up
The closing date was Feb 22nd, 2020, and the mean follow-up was 32.1 months (min 281 days, max 1,417 days). Patients or their general practitioners were contacted by phone or email.
Statistical analysis
Values are given as means or medians, where appropriate. Comparisons of clinicopathologic features at diagnosis and treatment details were analyzed using the Pearson 2 exact test and Kruskal-Wallis test, as appropriate. Overall survival was defined as the time elapsed between the first intraperitoneal (PIPAC) chemotherapy cycle and death or date of last censored. Overall survival was estimated by the Kaplan-Meier method and compared by the log-rank test. We performed a multivariable logistic regression model (Cox) with survival as the dependent variable and PCI, Karnofsky index, PRGS, timepoint of peritoneal metastasis (synchronous vs. metachronous), number of previous chemotherapy lines, and presence of ascites (yes/no) as independent variables. All p-values are two-tailed, and a p-value of less than 0.05 was considered statistically significant. We used SPSS 24 for Windows (SPSS Inc., Chicago, IL, USA) for statistical analysis and SigmaPlot 13 (Systat Software Inc., San José, CA, USA) for creating graphs.
Results
A total of 97 consecutive patients with histologically proven, isolated peritoneal metastasis were available for analysis. The patients’ characteristics are summarized in Table 1. Primary tumors were gastric cancer (n=33), hepatobiliary/pancreatic cancer (n=20), colorectal cancer (n=18), ovarian cancer (n=8), appendiceal cancer (n=7), pseudomyxoma peritonei (n=2) and other tumors (n=9, cancer of unknown origin, malignant peritoneal mesothelioma, yolk sac tumor, breast, and prostate cancer). Most patients (n=86) received palliative systemic chemotherapy before the first PRGS determination. The minority (n=11) were chemo-naïve at this point.
Table 1:
Patient characteristics.
| Variable | Value |
|---|---|
| Number of patients | 97 |
| Sex (M:F) | 44:53 |
| Age, years (±SD) | 55.8 ± 11.8 |
| Organ of origin Gastric Colorectal/appendiceal Hepatobiliary-pancreatic Ovarian PMP Others: CUP, mesothelioma, yolk sac, prostate |
33 (34.0 %) 25 (25.8 %) 20 (20.6 %) 8 (8.2 %) 2 (2.0 %) 9 (9.4 %) |
| Peritoneal metastasis Synchronous Metachronous |
56 (57.7 %) 41 (42.3 %) |
| Extraperitoneal metastasis | 9 (9.3 %) |
| Peritoneal cancer index (PCI), mean ± SD | 20.0 ± 12.5 |
| Karnofsky index before first PIPAC, mean ± SD | 82.6 ± 13.3 |
| Ascites ≥300 mL | 21 (21.6 %) |
| Previous systemic chemotherapy None ≥1 line |
11 (11.3 %) 86 (88.7 %) |
| Therapy PIPAC Systemic chemotherapy |
97 (100 %) 63 (64.9 %) |
At first biopsy, the mean PRGS (CI 5–95 %) was higher in chemo-naive patients (2.24; 1.68–2.80) in 14 vs. 1.95 (1.76–2.13) in pre-treated patients, which probably reflects the therapy effect. However, this difference did not reach statistical significance, p=0.24. The proportion of PRGS 1 (no tumor cells detected in multiple peritoneal biopsies) and PRGS 2 (high-grade regression) at initial assessment was higher in HBP and gastric cancer than in other histologies (Table 2). This difference is due to the desmoplastic nature of biliopancreatic cancer [34, 35] and (diffuse) gastric cancer [36], with markedly increased cancer-associated fibroblasts and collagen deposition.
Table 2:
PRGS at initial assessment, depending on the organ of origin.
| Complete or high-grade regression, PRGS 1 and 2 | Vital tumor or minor regression, PRGS 3 and 4 | |
| Gastric cancer, n=33 | 27 (81.8 %) | 6 (8.2 %) |
| HBP cancer, n=20 | 15 (75.0 %) | 5 (25.0 %) |
| Other primaries, n=44 | 14 (31.8 %) | 30 (68.1 %) |
| All tumors, n=97 | 56 (57.7 %) | 41 (42.3 %) |
The proportion of PRGS 1 (no tumor cells detected in multiple peritoneal biopsies) and PRGS 2 (high-grade regression) at initial assessment was significantly higher in HBP and gastric cancer (mean PRGS 1.57, CI 5–95 % 1.42–1.72) than in other histologies (mean PRGS 2.48, CI 5–95 % 2.22–2.74, p<0.001).
Predictive value of PRGS for OS
At the end of the follow-up period, 71/97 patients had passed away, and 26 were still alive. For all organs of origin together, the median overall survival (OS) from the first PIPAC was 9.9 months (95 % CI: 6.4–13.4 months). From the time point of diagnosis of PM, the OS was 17.9 months (95 % CI: 14.2–21.7 months).
The 36 (37.1 %) patients with an initial mean PRGS ≤2 had a significantly longer median OS (12.1 months, CI 5–95 % 7.8–16.4) vs. 8.0 months (CI 5–95 % 5.1–10.8 months) in 61 (62.9 %) patients with mean PRGS >2 (Figure 1).
Figure 1:
Predictive value of PRGS in 97 patients with isolated peritoneal metastasis. The origin for overall survival. PRGS was grouped into two categories: 1) 36 patients with initial mean PRGS ≤2 with no tumor cell or high-grade regression, and 2) 61 patients with initial mean PRGS >2 with vital tumor or low-grade regression. Most patients (88.7 %) received previous systemic chemotherapy. In the multivariate analysis (Cox-regression model), PRGS (p<0.05) and organ of origin (p<0.001) are independent predictors of survival in this patient population.
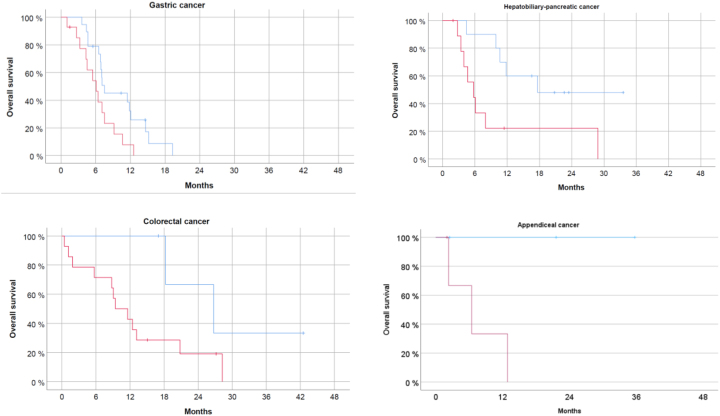
A multivariate analysis (Cox regression model) was then performed, including established prognostic factors in PM patients: general condition measured with the Karnofsky index, the extent of peritoneal spreading measured with the Peritoneal Cancer Index (PCI), the presence of ascites >300 mL and the organ of origin. Together with the organ of origin (p<0.001), PRGS remained an independent predictor of OS (p<0.05). Then, we calculated the survival curves for each organ of origin with >20 patients included (PM of gastric, colorectal, and hepatobiliary-pancreatic origin). Results are shown in Figure 2.
Figure 2:
PRGS predicts overall survival of patients with peritoneal metastasis from different primaries. For patients with gastric cancer, colorectal, hepatobiliary-pancreatic and appendiceal cancer, PRGS is a predictor of survival. Patients with a favorable histological score (PRGS ≤2) survived longer than patients with a PRGS >2. Due to the small sample size, the differences do not reach statistical significance in the subgroups.
Prognostic value of histological tumor regression for overall survival
In 42/62 patients (67.7 %) who received at least two cycles of PIPAC, repeated peritoneal biopsies unraveled an improved or stable PRGS. According to the histological response criteria defined above, this objective evidence of tumor regression or stable disease was considered a therapeutic response. A typical example of such a PRGS regression under intensified palliative chemotherapy is shown in Figure 3.
Figure 3:
Example of histological regression under palliative chemotherapy. Repeated biopsies and determination of the peritoneal regression grading score (PRGS) in a patient with isolated peritoneal metastasis from the colorectal origin with a progressive disease according to RECIST 1.1 under palliative chemotherapy (6 cycles FOLFOX). Left panel, cycle 1: vital tumor with no sign of regression after intravenous chemotherapy, PRGS 4. Middle panel, cycle 2: minor regression after intensified systemic and intraperitoneal chemotherapy; arrows: the direction of application of PIPAC with typical superficial sclerosis of the tumor node, PRGS 3. Right panel, cycle 3: complete histological regression with no vital tumor cells, PRGS 1. Hematoxyline-eosin (HE) staining.
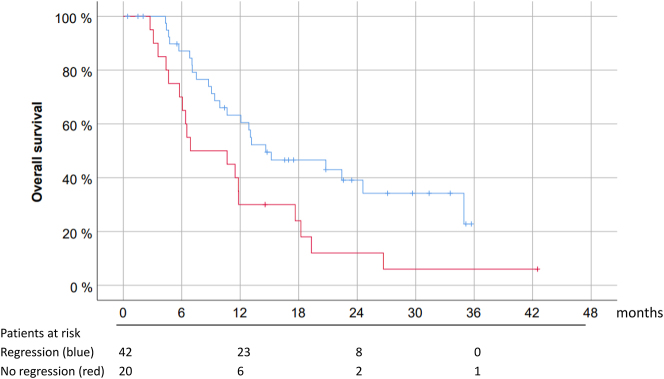
Out of the patients who received repeated intraperitoneal chemotherapy, 14/62 patients (22.6 %) had a complete histological regression (PRGS=1) in all peritoneal biopsies with no visible tumor cells. In the remaining 20 patients, PRGS deteriorated under therapy. As shown in Figure 4, patients with a PRGS regression (or stable) have better survival (median 14.6 months, CI 5–95 % 6.0–23.2 months) than patients with a progression (median 6.89 months, CI 5–95 % 0 – 15.9 months), and this difference was significant in the univariate analysis p<0.05. However, in this relatively small patient cohort, PRGS regression did not remain predictive for OS in the multivariate analysis.
Figure 4:
Histological therapy response (assessed as changes in the mean PRGS) is an independent predictor of survival in patients with isolated peritoneal metastasis. Prognostic value of PRGS for overall survival in 62 patients with PM from various primaries with at least 2 consecutive histological assessments in the course of palliative chemotherapy. Patients were grouped into two categories: 1) 42 patients with a histological response (the mean PRGS remained stable or decreased under therapy, blue line), and 2) 20 patients with a histological progression (the mean PRGS increased under therapy, red line). After stratification for organ of origin, the mean PRGS was an independent predictor of survival in this patient population (p<0.02).
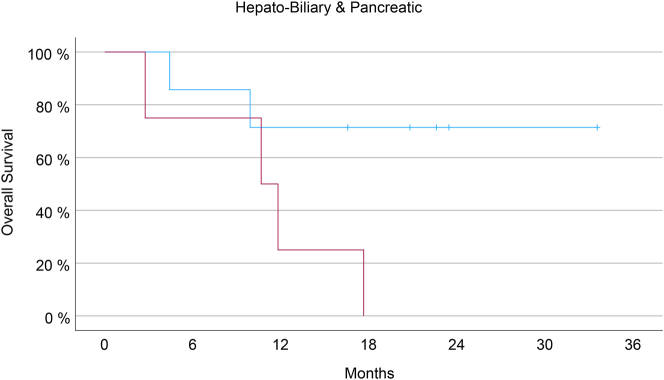
As shown in Figure 5, the survival difference between responders and non-responders was particularly striking in the 20 patients with HBP tumors. In this group, the PRGS sharply distinguished between the two groups with different outcomes (p=0.06).
Figure 5:
Survival of patients with isolated peritoneal metastasis of hepatobiliary-pancreatic (HBP) origin under palliative chemotherapy. All patients with PRGS progression (red curve) died within 18 months after intervention, which was expected in patients with peritoneal metastasis from HBP cancer. However, there are long-time survivors in the patients with an increase in the mean PRGS, reflecting an objective histological response to chemotherapy. The difference in overall survival between subgroups reaches statistical significance (Kaplan-Meyer, log-rank test, p<0.05).
Discussion
Histological regression scores have already demonstrated their clinical utility in liver metastasis of colorectal origin, in the neoadjuvant setting, such scores are commonly used in rectal cancer [37] and esophageal cancer [38].
Our research fits well into the current research landscape for defining new preoperative, predictive markers allowing better selection of patients with PM before major peritoneal surgery. For example, the preoperative PRGS after systemic therapy was predictive for OS in a cohort of 196 colorectal cancer patients treated with cytoreductive surgery and HIPEC for synchronous PM [8]. Similarly, PRGS after prior systemic chemotherapy was predictive for OS in a cohort of 47 patients with PM from gastrointestinal origin undergoing surgery or PIPAC. Taken together with our data, the evidence available strongly suggests that PRGS obtained from PM delivers crucial predictive information to distinguish between chemo-sensitive and-resistant tumors. This predictive information might be instrumental for improving therapeutic algorithms in patients with PM, in particular for selecting patients potentially benefitting from major peritoneal surgery [39].
The value of the PRGS for determining the response to chemotherapy was proven in a mouse model of PM from colorectal cancer. Previous clinical data evidenced the prognostic significance of PRGS in combination with peritoneal cytology [9]. PRGS was already used for objective therapeutic response in PM of colorectal [10, 11] gastric [13, 40] and various [41–43] origins. In our cohort of patients, repeated PRGS in the course of palliative chemotherapy had prognostic value. PRGS regression vs. progression was determined by comparing biopsies gathered just before the second vs. the first cycle of PIPAC, at a time interval of only six weeks. The PRGS improved in about two-thirds of patients, which is in line with previous reports [44]. The subgroup of responders to chemotherapy had a significantly better overall survival than those with a deteriorating mean PRGS. The short time between PIPAC is a methodological advantage enabling the identification of responders vs. non-responders at a very early point.
Peritoneal metastasis remains an unsolved challenge in modern oncology. Intratumoral heterogeneity is a fundamental property of cancer, in particular of PM. In the era of personalized cancer therapy, obtaining early prognostic and predictive information from tumor biopsies meets an urgent, unmet medical need. PRGS provides a solid methodological basis for reliable histological assessment of the therapeutic response in PM. However, further validation of PRGS in adequately powered studies is still needed before this score can be included in therapeutic algorithms and the WHO guidelines. A first step was the integration of PRGS into the international PIPAC registry to collect rapidly a large set of real-world data [45].
The next step will be to integrate meaningful molecular features that will strengthen PRGS’s prognostic and predictive accuracy and distinguish between subgroups of patients. For example, RAS/RAF mutations could be included since they impair survival after CRS and HIPEC in patients with PM of colorectal origin [46]. In gastric cancer, tumor heterogeneity was documented between the primary tumor and PM, between individual PM at different locations, and a 12-gene signature had prognostic significance [47]. In PM of ovarian origin also, changes in gene expression patterns during repeated intraperitoneal chemotherapy cycles were prognostic of overall survival [48]. Further development of PRGS, reflecting tumor biology, will stratify patients with PM based on long-term outcomes and improve patient selection for surgical, potentially curative therapy, vs. medical, palliative therapy. Moreover, profiling repeated PM biopsies under palliative chemotherapy (as PIPAC) gives new insights into the clonal evolution and the tumor escape mechanisms under palliative chemotherapy. This unique observational window offers new chances for unraveling tumor chemoresistance’s, molecular and immunological mechanisms in PM and beyond.
Footnotes
Research funding: Not applicable.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: The authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: The Ethics Committee, University of Tübingen, authorized the retrospective analysis on 18.3.2021 under reference 073/2019BO.
References
- 1.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solass W, Struller F, Horvath P, Königsrainer A, Sipos B, Weinreich FJ. Morphology of the peritoneal cavity and pathophysiological consequences. Pleura Peritoneum. 2016;1:193–201. doi: 10.1515/pp-2016-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tozzi R, Traill Z, Campanile RG, Kilic Y, Baysal A, Giannice R, et al. Diagnostic flow-chart to identify bowel involvement in patients with stage IIIC-IV ovarian cancer: can laparoscopy improve the accuracy of CT scan? Gynecol Oncol. 2019;155:207–12. doi: 10.1016/j.ygyno.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Melamed A, Nitecki R, Boruta DM, del Carmen MG, Clark RM, Growdon WB, et al. Laparoscopy compared with laparotomy for debulking ovarian cancer after neoadjuvant chemotherapy. Obstet Gynecol. 2017;129:861–9. doi: 10.1097/aog.0000000000001851. [DOI] [PubMed] [Google Scholar]
- 5.Fukagawa T. Role of staging laparoscopy for gastric cancer patients. Ann Gastroenterol Surg. 2019;3:496–505. doi: 10.1002/ags3.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zorzato PC, Zannoni GF, Tudisco R, Pasciuto T, Di Giorgio A, Franchi M, et al. External validation of a ’response score’ after neoadjuvant chemotherapy in patients with high-grade serous ovarian carcinoma with complete clinical response. Int J Gynecol Cancer. 2020;30:67–73. doi: 10.1136/ijgc-2019-000561. [DOI] [PubMed] [Google Scholar]
- 7.Solass W, Sempoux C, Detlefsen S, Carr NJ, Bibeau F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the peritoneal regression grading score (PRGS) Pleura Peritoneum. 2016;1:99–107. doi: 10.1515/pp-2016-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taibi A, Lo Dico R, Kaci R, Naneix AL, Mathonnet M, Pocard M. Impact of preoperative chemotherapy on the histological response of patients with peritoneal metastases from colorectal cancer according to peritoneal regression grading score (PRGS) and TRG. Surg Oncol. 2020;33:158–63. doi: 10.1016/j.suronc.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Benzerdjeb N, Durieux E, Tantot J, Isaac S, Fontaine J, Harou O, et al. Prognostic impact of combined progression index based on peritoneal grading regression score and peritoneal cytology in peritoneal metastasis. Histopathology. 2020;77:548–59. doi: 10.1111/his.14092. [DOI] [PubMed] [Google Scholar]
- 10.Tabchouri N, Buggisch J, Demtröder CR, Thiery J, Rezniczek G, Tempfer CB, et al. Pressurized intraperitoneal aerosol chemotherapy for colorectal peritoneal metastases. Ann Surg Oncol. 2021;28:5275–86. doi: 10.1245/s10434-020-09508-0. [DOI] [PubMed] [Google Scholar]
- 11.Ellebæk SB, Graversen M, Detlefsen S, Lundell L, Fristrup CW, Pfeiffer P, et al. Pressurized IntraPeritoneal aerosol chemotherapy (PIPAC)-directed treatment of peritoneal metastasis in end-stage colo-rectal cancer patients. Pleura Peritoneum. 2020;5:20200109. doi: 10.1515/pp-2020-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen M, Graversen M, Ellebæk SB, Kristensen TK, Fristrup C, Pfeiffer P, et al. Next-generation sequencing and histological response assessment in peritoneal metastasis from pancreatic cancer treated with PIPAC. J Clin Pathol. 2021;74:19–24. doi: 10.1136/jclinpath-2020-206607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellebæk SB, Graversen M, Detlefsen S, Lundell L, Fristrup CW, Pfeiffer P, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) of peritoneal metastasis from gastric cancer: a descriptive cohort study. Clin Exp Metastasis. 2020;37:325–32. doi: 10.1007/s10585-020-10023-5. [DOI] [PubMed] [Google Scholar]
- 14.Di Giorgio A, Schena CA, El Halabieh MA, Abatini C, Vita E, Strippoli A, et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): a bidirectional approach for gastric cancer peritoneal metastasis. Surg Oncol. 2020;34:270–5. doi: 10.1016/j.suronc.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Graversen M, Detlefsen S, Asmussen J, Mahdi B, Fristrup C, Pfeiffer P, et al. Treatment of peritoneal carcinomatosis with pressurized IntraPeritoneal aerosol chemotherapy - PIPAC-OPC2. Pleura Peritoneum. 2018;3:20180108. doi: 10.1515/pp-2018-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath P, Yurttas C, Struller F, Bösmüller H, Lauer UM, Nadalin S, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastases in solid organ graft recipients: first experience. Ann Transplant. 2019;24:30–5. doi: 10.12659/aot.911905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graversen M, Detlefsen S, Bjerregaard JK, Fristrup CW, Pfeiffer P, Mortensen MB. Prospective, single-center implementation and response evaluation of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis. Ther Adv Med Oncol. 2018;10:1758835918777036. doi: 10.1177/1758835918777036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtz F, Struller F, Horvath P, Solass W, Bösmüller H, Königsrainer A, et al. Feasibility, safety, and efficacy of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis: a registry study. Gastroenterol Res Pract. 2018;2018:2743985. doi: 10.1155/2018/2743985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merlin JL. . Bonds between circulating tumoral ADN (ctDNA) and the development of peritoneal carcinomatosis for patients under PIPAC (PIPADN). NCT04766502; 2022.www.clinicaltrials.gov [Accessed 30 Aug 2022] [Google Scholar]
- 20.Kim G. . Pressurized intraperitoneal aerosol chemotherapy (PIPAC) and electrostatic PIPAC (ePIPAC) with paclitaxel in patients with peritoneal carcinomatosis. NCT05395910; 2022.www.clinicaltrials.gov [Accessed 30 Aug 2022] [Google Scholar]
- 21.Labidi-Galy I. . PIPAC with nab-paclitaxel and cisplatin in peritoneal carcinomatosis (Nab-PIPAC). NCT04000906; 2019.www.clinicaltrials.gov [Accessed 30 Aug 2022] [Google Scholar]
- 22.Di Giorgio A. . Combined nabpaclitaxel pressurized IntraPeritoneal aerosol chemotherapy with systemic nabpaclitaxel-gemcitabine chemotherapy for pancreatic cancer peritoneal metastases (Nab-PIPAC). NCT05371223; 2022.www.clinicaltrials.gov [Accessed 30 Aug 2022] [Google Scholar]
- 23.Ceelen WP. . Intraperitoneal aerosolized nanoliposomal irinotecan (Nal-IRI) peritoneal carcinomatosis from gastrointestinal cancer (PIPAC-NAL-IRI). NCT05277766; 2022.www.clinicaltrials.gov [Accessed 30 Aug 2022] [Google Scholar]
- 24.Kim HH. . Intraperitoneal paclitaxel plus systemic mFOLFOX6 (IPLUS). NCT03618758; 2018.www.clinicaltrials.gov [Accessed 30 Aug 2022] [Google Scholar]
- 25.So J. . 2017. Pressurized intraperitoneal Aerosol chemotherapy (PIPAC) with oxaliplatin in patients with peritoneal carcinomatosis (PIPAC). NCT03172416.www.clinicaltrials.gov [Accessed 30 Aug 2022] [Google Scholar]
- 26.Casella F. . PIPAC in multimodal therapy for patients with oligometastatic peritoneal gastric cancer (PIPAC_VEROne). NCT05303714; 2022.www.clinicaltrials.gov [Accessed 30 Aug 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chia C. . Aug. PIPAC for peritoneal metastases. NCT04956068; 2021.www.clinicaltrials.gov [Accessed 30. 2022] [Google Scholar]
- 28.de Hingh I. . 2017. PIPAC for peritoneal metastases of colorectal cancer (CRC-PIPAC). NCT03246321.www.clinicaltrials.gov [Accessed 30 Aug 2022] [Google Scholar]
- 29.Solass W, Sempoux C, Carr NJ, Bibeau F, Neureiter D, Jäger T, et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology. 2019;74:1014–24. doi: 10.1111/his.13829. [DOI] [PubMed] [Google Scholar]
- 30.Solass W, Meisner C, Kurtz F, Nadiradze G, Reymond MA, Bösmüller H. Peritoneal regression grading score (PRGS) in peritoneal metastasis: how many biopsies should be examined? Pleura Peritoneum. 2022;7:179–85. doi: 10.1515/pp-2022-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solass W, Nadiradze G, Reymond MA, Bösmüller H. The role of additional staining in the assessment of the Peritoneal Regression Grading Score (PRGS) in peritoneal metastasis of gastric origin. Appl Immunohistochem Mol Morphol. in press doi: 10.1097/PAI.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 32.Cady B. Basic principles in surgical oncology. Arch Surg. 1997;132:338–46. doi: 10.1001/archsurg.1997.01430280012001. [DOI] [PubMed] [Google Scholar]
- 33.Chang HH, Leeper WR, Chan G, Quan D, Driman DK. Infarct-like necrosis: a distinct form of necrosis seen in colorectal carcinoma liver metastases treated with perioperative chemotherapy. Am J Surg Pathol. 2012;36:570–6. doi: 10.1097/pas.0b013e31824057e7. [DOI] [PubMed] [Google Scholar]
- 34.Fabris L, Perugorria MJ, Mertens J, Björkström NK, Cramer T, Lleo A, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39:63–78. doi: 10.1111/liv.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nizri E, Bar-David S, Aizic A, Sternbach N, Lahat G, Wolf I, et al. Desmoplasia in lymph node metastasis of pancreatic adenocarcinoma reveals activation of cancer-associated fibroblasts pattern and T-helper 2 immune cell infiltration. Pancreas. 2019;48:367–73. doi: 10.1097/mpa.0000000000001261. [DOI] [PubMed] [Google Scholar]
- 36.Lee D, Ham IH, Son SY, Han SU, Kim YB, Hur H. Intratumor stromal proportion predicts aggressive phenotype of gastric signet ring cell carcinomas. Gastric Cancer. 2017;20:591–601. doi: 10.1007/s10120-016-0669-2. [DOI] [PubMed] [Google Scholar]
- 37.Chetty R, Gill P, Govender D, Bateman A, Chang HJ, Deshpande V, et al. International study group on rectal cancer regression grading: interobserver variability with commonly used regression grading systems. Hum Pathol. 2012;43:1917–23. doi: 10.1016/j.humpath.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 38.Karamitopoulou E, Thies S, Zlobec I, Ott K, Feith M, Slotta-Huspenina J, et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. Am J Surg Pathol. 2014;38:1551–6. doi: 10.1097/pas.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 39.Toussaint L, Teixeira Farinha H, Barras JL, Demartines N, Sempoux C, Hübner M. Histological regression of gastrointestinal peritoneal metastases after systemic chemotherapy. Pleura Peritoneum. 2021;6:113–9. doi: 10.1515/pp-2021-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sindayigaya R, Dogan C, Demtröder CR, Fischer B, Karam E, Buggisch JR, et al. Clinical outcome for patients managed with low-dose cisplatin and doxorubicin delivered as pressurized intraperitoneal aerosol chemotherapy for unresectable peritoneal metastases of gastric cancer. Ann Surg Oncol. 2022;29:112–3. doi: 10.1245/s10434-021-10860-y. [DOI] [PubMed] [Google Scholar]
- 41.Graversen M, Detlefsen S, Ellebaek SB, Fristrup C, Pfeiffer P, Mortensen MB. Pressurized IntraPeritoneal Aerosol Chemotherapy with one minute of electrostatic precipitation (ePIPAC) is feasible, but the histological tumor response in peritoneal metastasis is insufficient. Eur J Surg Oncol. 2020;46:155–9. doi: 10.1016/j.ejso.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Ceribelli C, Debs T, Chevallier A, Piche MA, Bereder JM. Initial experience of pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a French hyperthermic intraperitoneal chemotherapy (HIPEC) expert center. Surg Endosc. 2020;34:2803–6. doi: 10.1007/s00464-020-07488-6. [DOI] [PubMed] [Google Scholar]
- 43.Alyami M, Hübner M, Grass F, Bakrin N, Villeneuve L, Laplace N, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20:e368–77. doi: 10.1016/s1470-2045(19)30318-3. [DOI] [PubMed] [Google Scholar]
- 44.de Bree E, Michelakis D, Stamatiou D, Romanos J, Zoras O. Pharmacological principles of intraperitoneal and bidirectional chemotherapy. Pleura Peritoneum. 2017;2:47–62. doi: 10.1515/pp-2017-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mortensen MB, Glehen O, Horvath P, Hübner M, Hyung-Ho K, Königsrainer A, et al. The ISSPP PIPAC database: design, process, access, and first interim analysis. Pleura Peritoneum. 2021;6:91–7. doi: 10.1515/pp-2021-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider MA, Eden J, Pache B, Laminger F, Lopez-Lopez V, Steffen T, et al. Mutations of RAS/RAF proto-oncogenes impair survival after cytoreductive surgery and HIPEC for peritoneal metastasis of colorectal origin. Ann Surg. 2018;268:845–53. doi: 10.1097/sla.0000000000002899. [DOI] [PubMed] [Google Scholar]
- 47.Wang R, Dang M, Harada K, Han G, Wang F, Pool Pizzi M, et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat Med. 2021;27:141–51. doi: 10.1038/s41591-020-1125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rezniczek GA, Jüngst F, Jütte H, Tannapfel A, Hilal Z, Hefler LA, et al. Dynamic changes of tumor gene expression during repeated pressurized intraperitoneal aerosol chemotherapy (PIPAC) in women with peritoneal cancer. BMC Cancer. 2016;16:654. doi: 10.1186/s12885-016-2668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]