Abstract
Docetaxel (DTX) is the treatment of choice for metastatic castration-resistant prostate cancer. However, developing drug resistance is a significant challenge for achieving effective therapy. This study evaluated the anticancer and synergistic effects on DTX of four natural compounds (calebin A, 3′-hydroxypterostilbene, hispolon, and tetrahydrocurcumin) using PC-3 androgen-resistant human prostate cancer cells. We utilized the CellTiter-Glo® luminescent cell viability assay and human PC-3 androgen-independent prostate cancer cells to determine the antiproliferative effects of the four compounds alone and combined with DTX. Cytotoxicity to normal human prostate epithelial cells was tested in parallel using normal immortalized human prostate epithelial cells (RWPE-1). We used cell imaging and quantitative caspase-3 activity to determine whether these compounds induce apoptosis. We also measured the capacity of each drug to inhibit TNF-α-induced NF-kB using a colorimetric assay. Our results showed that all four natural compounds significantly augmented the toxicity of DTX to androgen-resistant PC-3 prostate cancer cells at IC50. Interestingly, when used alone, each of the four compounds had a higher cytotoxic activity to PC-3 than DTX. Mechanistically, these compounds induced apoptosis, which we confirmed by cell imaging and caspase-3 colorimetric assays. Further, when used either alone or combined with DTX, the four test compounds inhibited TNF-α-induced NF-kB production. More significantly, the cytotoxic effects on normal immortalized human prostate epithelial cells were minimal and non-significant, suggesting prostate cancer-specific effects. In conclusion, the combination of DTX with the four test compounds could effectively enhance the anti-prostate cancer activity of DTX. This combination has the added value of reducing the DTX effective concentration. We surmise that calebin A, 3′-hydroxypterostilbene, hispolon, and tetrahydrocurcumin were all excellent drug candidates that produced significant antiproliferative activity when used alone and synergistically enhanced the anticancer effect of DTX. Further in vivo studies using animal models of prostate cancer are needed to confirm our in vitro findings.
Keywords: docetaxel, calebin A, 3′-hydroxypterostilbene, tetrahydrocurcumin, hispolon, polyphenolic compounds
Introduction
Docetaxel (DTX) is a semisynthetic anticancer drug, the precursor of which was originally obtained from Taxus baccata leaf extract (a European tree).1 A combination of DTX plus prednisone is the primary regimen for treating metastatic castration-resistant prostate cancer (mCRPC).2,3 In addition to the treatment of mCRPC, DTX is also used in treating breast, head and neck, non-small cell lung, ovarian, and uterine cancers.1 Unfortunately, prostate tumor cells ultimately develop resistance to DTX. Overcoming chemoresistance is a significant challenge that requires the development of new treatment options.4 This study evaluated the anti-prostate cancer effects of DTX and four novel natural compounds (3′-hydroxypterostilbene, tetrahydrocurcumin, hispolon, and calebin A) using a well-characterized PC-3 androgen-resistant human prostate cancer cell line. All compounds were evaluated alone and in combination with DTX.
3′-Hydroxypterostilbene (HPSB)
3′-Hydroxypterostilbene (HPSB) is a natural compound isolated from the perennial herb Sphaerophysa salsula (alkali swainsonpea). The Chinese people use this herb to treat hyper-tension.5,6 Dietary HPSB has an anti-inflammatory effect on colitis-associated cancer mediated through suppression of the IL-6/STAT3 pathway in mice7 and antiproliferative and apoptosis-inducing ability in human colon cancer cells.8 It has also been reported to ameliorate obesity or high-fat diet-induced colitis.9 HPSB has antiproliferative effects on multidrug-resistant human leukemic HL60 and K562 cells.10 Other studies have also shown that topical treatment with HPSB prevents mouse skin tumorigenesis.11
Tetrahydrocurcumin (THC)
Tetrahydrocurcumin (THC) is a curcuminoid fraction of turmeric found in Curcuma zedoaria (white turmeric) roots.12 THC’s antioxidant and anti-neurodegenerative activities have been reported in Parkinson’s disease.13 As an antiproliferative agent, THC arrests the cell cycle at the glioma’s G0/G1 phase.14 It also inhibits the Wnt/β-catenin signaling pathway in colorectal cancer.13 Yang et al reported that THC induces apoptosis in mouse hepatocellular carcinoma H-22 cells and human breast cancer MCF-7 cells by activating caspases 3 and 9.15,16 Moreover, in human fibrosarcoma HT-1080 cells and osteosarcoma U-2OS cells, THC has an anti-metastatic effect in which matrix metalloproteinases (MMP) 2 and 9 are suppressed.16,17
Hispolon (HISP)
Hispolon (HISP) is a polyphenolic compound that can be isolated from different mushrooms, such as Phellinus linteus.18 HISP blocks the cell cycle at the G0/G1 phase and induces apoptosis in NB-4 human leukemia cells.19 In addition, it exerts anticancer effects on A-549 lung cancer cells.20 In human hepatocellular carcinoma Hep-3B cells, HISP induces apoptosis and cell arrest by modulating ERK phosphorylation.21 Similar effects have been reported in glioblastoma U87MG cells.22 The anti-metastatic results of HISP have been confirmed in breast cancer MCF-7 cells, which were said to be due to the inhibition of the ROS/ERK/Slug/E-cadherin signaling pathway,23 and in human hepatoma SK-Hep1 cells, where HISP downregulates MMP-2 and MMP-9.24 In human prostate cancer DU145 cells, HISP modulates apoptotic proteins of the Bcl-2 family, releasing cytochrome c and activating caspase production.25 In contrast, in leukemic NB-4 cells, HISP treatment enhances both the extrinsic (FasL/FasR) and intrinsic (cytochrome c) apoptotic pathways.19
Calebin A (CBA)
Calebin A (CBA) is a non-curcuminoid fraction of turmeric roots.26 CBA has an inhibitory effect on the NF-kB pathway, and it also modulates cell metastasis in human colorectal cancer HCT-116 cells.27 Moreover, CBA has been reported to induce apoptosis by enhancing caspase activity and blocking transmembrane drug transporter (P-gp) function in chemotherapeutic-resistant human gastric cancer cells.28
This study explored the anti-prostatic cancer and synergistic effects of CBA, HPSB, THC, and HISP when combined with DTX in androgen-resistant human PC-3 prostate cancer cells. The cytotoxicity of these natural compounds used either alone or combined with DTX was evaluated simultaneously in PC-3 prostate cancer cells and RWPE-1 noncancerous immortalized prostate epithelial cells. We also investigated the mechanism by which these four compounds augmented the chemotherapeutic effects of DTX in PC-3 cells.
Results
CBA Cytotoxicity on PC-3 and RWPE-1 Cells Alone and in Combination With DTX
The percent cell viability of PC-3 prostate cancer cells and RWPE-1 normal immortalized prostate epithelial cells grown in the presence of CBA, DTX, or CBA plus DTX was determined by the CellTiter-Glo assay using 48 h protocol. Comparing the three treatment protocols with the control (cells treated with vehicle only), DTX alone reduced the percentage of viable PC-3 cells to 57%. In contrast, the viability of PC-3 treated with CBA was only 25.3%. Combining DTX and CBA at 50% of the concentration used for single treatments resulted in 22% cell viability (Figure 1A). Interestingly, CBA alone and CBA plus DTX combination cytotoxicity on normal RWPE-1 immortalized prostate epithelial cells was low and statistically non-significant (Figure 1B). These results showed that CBA single treatments had a superior antiproliferative activity on prostate cancer cells that exceeded that of DTX when used alone. Most importantly, the CBA effect was additive when DTX and CBA were combined using half of the concentrations used in the single-drug treatments.
Figure 1.

(A) CBA at 40 μM IC50 concentration induced significant PC-3 cell death when used alone and augmented docetaxel (DTX) cytotoxicity compared to control untreated cells and PC-3 cells treated with DTX alone (112 nM). (B) No significant cell death was detected in RWPE-1 immortalized normal human prostate epithelial cells. Data is an average of three experimental repeats.
HPSB Cytotoxicity on PC-3 and RWPE-1 Cells Alone and in Combination With DTX
Like the CBA effect, HPSB significantly reduced the percentage of viable PC-3 cells to 17.6% compared to control cell cultures treated with vehicle alone. In contrast, the cell viability in the DTX-treated cultures was relatively high at 64.4% (Figure 2A). Interestingly, only 10% of PC-3 cells survived the robust toxic effect of the HPSB-DTX combination when each compound was used at half the concentration used for single treatments. Comparison of the viability of PC-3 cells treated with DTX alone with that of single HPBS or HPSB plus DTX combination treatments showed enhanced (P < 0.0001) antiproliferative effects of HPBS and HPBS/DTX combination that were superior to DTX alone (Figure 2A). Neither HPSB alone nor the HPSB plus DTX combination had significant toxic effects on normal immortalized RWPE-1 prostate epithelial cells, especially compared to DTX single-drug treatments (Figure 2B).
Figure 2.

(A) 3′-Hydroxypterostilbene (HPSB) at 40 μM IC50 induced a significant reduction of cell viability in human androgen resistance prostate PC-3 cancer cells and augmented docetaxel (DTX) anti-prostatic activity compared to untreated control and to PC-3 treated with DTX alone (112 nM). (B) No significant reduction in cell viability of RWPE-1 immortalized normal human prostate epithelial cells. Control cells were treated with vehicles. Data is an average of three experimental repeats.
THC Cytotoxicity on PC-3 and RWPE-1 Cells Alone and in Combination With DTX
Compared with vehicle treatments (set at 100% cell viability), THC single treatments reduced cell viability to 44.4% compared with 76.4% in cells treated with DTX. The combination of THC and DTX reduced cell viability to only 16.6%. The effects of THC alone and THC and DTX combination were significantly superior to the relatively higher 76.4% cell viability in cultures treated with DTX alone (Figure 3A). THC alone and in combination with DTX did not cause significant toxicity in normal immortalized RWPE-1 prostate epithelial cells, especially when compared with DTX (Figure 3B).
Figure 3.

(A) Tetrahydrocurcumin (THC) at 40 μM IC50 concentration induced a significant reduction of cell viability in human androgen resistance prostate PC-3 cancer cells and augmented docetaxel (DTX) anti-prostatic activity when compared to untreated control and to PC-3 cell treated with DTX alone (112 nM). (B) No significant reduction in cell viability of RWPE-1 immortalized normal human prostate cells. Control cells were treated with vehicles. Data is an average of three experimental repeats.
HISP Cytotoxicity on PC-3 and RWPE-1 Cells Alone and in Combination With DTX
Compared with vehicle treatments, HISP single-drug treatments showed significant inhibitory effects on PC-3 cell proliferation, with cell viability averaging 25.5%. In contrast, the combination of HISP and DTX, at 50% concentrations of their single-drug treatments, reduced PC-3 cell viability to only 6.3%. This drastic reduction in cell viability was a notable apoptotic effect, especially when compared with cell viability of 76.3% in PC-3 cells treated with DTX alone (Figure 4A). Like CBA, HPSB, and THC, HISP used either alone or in combination with DTX did not induce significant cell death in normal immortalized RWPE-1 cells, especially when we compare the HISP and DTX combination with DTX single-drug treatment (Figure 4B).
Figure 4.
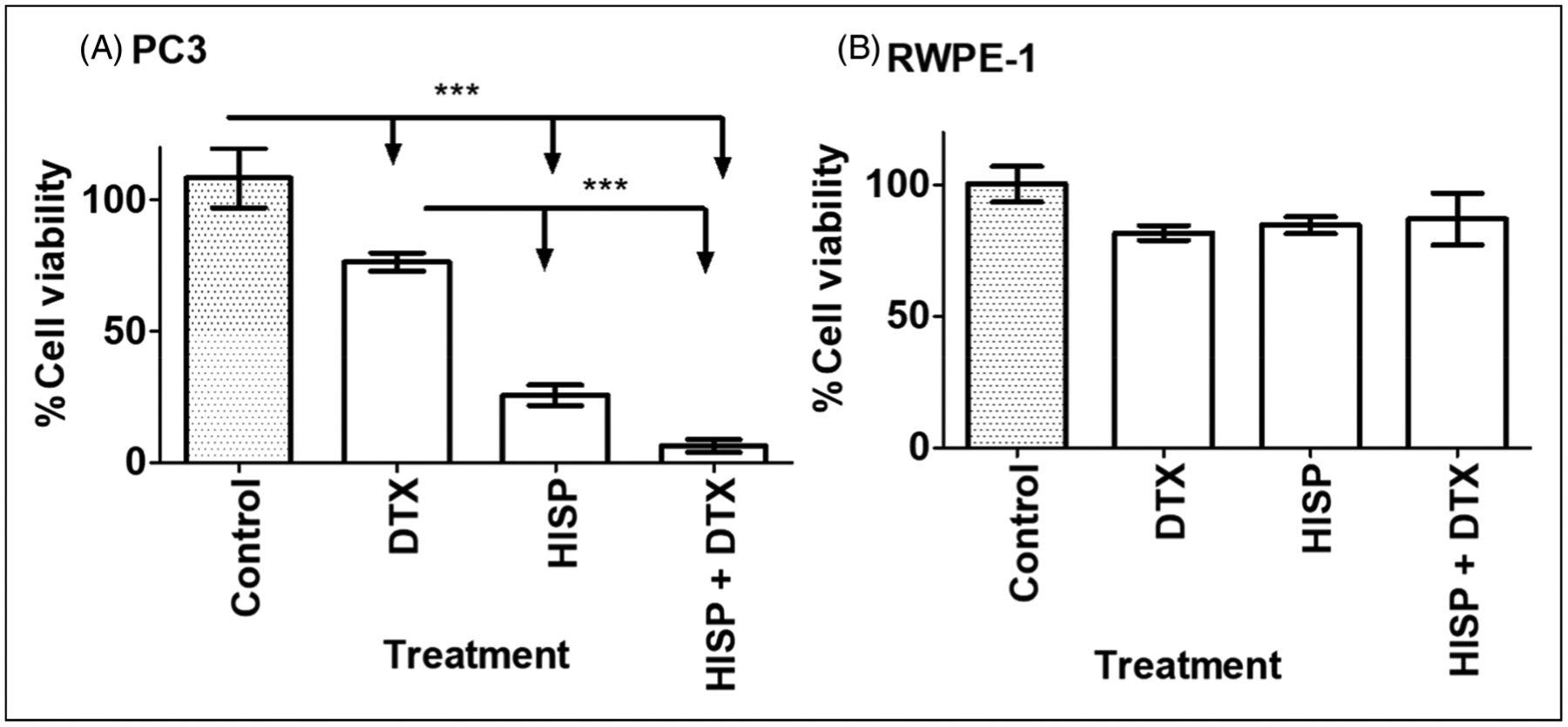
(A) Hispolon (HISP) at 250 μM IC50 concentration induced a significant reduction of cell viability in human androgen resistance PC-3 prostate cancer cells and augmented docetaxel (DTX) anti-prostatic activity when compared with untreated control cells and to PC-3 cells treated with DTX alone (112 nM). (B) No significant reduction in cell viability of RWPE-1 immortalized normal human prostate cells. Control cells were treated with vehicles. Data is an average of three experimental repeats.
CBA, HPSB, THC, and HISP Induce Apoptosis via Enhancing Caspase-3 Activity
To explore the induction of cell apoptosis as a possible mechanism of action for our test natural compounds, we investigated whether CBA, HPSB, THC, and HISP alone and combined with DTX induce cell death in PC-3 cells by measuring caspase-3 activity in cell culture. We treated PC-3 cells with the IC50 concentration of the above four test compounds alone and in combination with DTX. The caspase activity was determined 24 hours later using the caspase-3 colorimetric assay kit described in the section “Stimulation of the Executioner Caspase-3.” Staurosporine (ST), a commercially available caspase-3 inducer, was used as the positive control. Our results showed that PC-3 cells treated with CBA, THC, HISP and HPSB induced caspase-3 activity more significantly than the ST-positive control by 49%, 49%, 46%, and 16%, respectively. DTX, which is known to induce apoptosis via caspase-dependent and -independent pathways,2,3 enhanced caspase-3 activity by 37% compared to the positive ST control. The lowest effect over the ST control was recorded for HPSB, which increased the activity of caspase-3 by 16% over that of the ST control (Figure 5). Drug combinations caused 92%, 146%, 162%, and 148% caspase activity when DTX was combined with CBA, HPSB, THC, and HISP, respectively (Figure 5).
Figure 5.

Percent caspase-3 activity in androgen-resistant PC-3 human prostate cancer cells treated singly with IC50 concentrations for docetaxel (DTX), calebin A (CBA), 3′-hydroxypterostilbene (HPSB), tetrahydrocurcumin (THC) and Hispolon (HISP) or in combination with DTX for 24 hours. Staurosporine was used as a positive control set at 100%. Data is an average of three experimental repeats. All treatment induced significant caspase activity when compared with vehicle treatment (untreated −ve control). DTX combination with test compounds caused significant increase of caspase percent activity when compared with DTX alone. Vehicle treated PC-3 cells showed no significant caspase-3 activity.
Confirmation of the Apoptosis-Inducing Effect of CBA, HPSB, THC, and HISP by Cell Imaging
Compared to the ST-positive control, we observed the typical morphological features of apoptosis in CBA-, HPSB-, THC-, HISP-, and DTX-treated PC-3 cells. The treated PC-3 cells appeared shrunken, with a condensed cytoplasm. Nuclei showed pyknosis and karyorrhexis. Dark apoptotic bodies attached to the plasma membrane were observed (Figure 6).
Figure 6.

Representatives bright filed microscopic images showing apoptosis in PC-3 prostate cancer cells. Micrographs of PC-3 cells treated with: (A) media, (B) staurosporine (positive control), (C) docetaxel (DTX), (D) tetrahydrocurcumin (THC), (E) hispolon (HISP), (F) 3′-hydroxypterostilbene (HPSB), and (G) calebin A (CBA). Drugs were used at IC50 concentrations. Scale bars = 200 μm.
Ability of CBA, HPSB, THC and HISP to Suppress TNF-α Induced NF-kB
Next, we examined the ability of IC50 concentrations of the four test compounds and DTX, both alone and in combination, to inhibit TNFα-induced NF-kB activity in cell cultures at 24 hours. Compared to the TNF-α positive control and lysate-positive control, we found that each of the four compounds and DTX-treated nuclear extracts significantly inhibited TNF-α induced NF-kB activity via suppression of p65 subunit DNA binding (P < 0.0001). Interestingly, the suppressive properties of the 4 test compounds on TNFα-induced NF-kB activity were far more robust than those of DTX when used either alone or when combined with DTX (Figure 7A and B).
Figure 7.

Docetaxel (DTX) and four natural compounds caused significant inhibition of TNFα–NF-kB activation (colorimetric assay) when used singly (A) or when used in combination (B) each at IC50 concentrations for 24 hours. Calebin A (CBA), 3′-hydroxypterostilbene (HPSB), tetrahydrocurcumin (THC) a hispolon (HISP), and DTX significantly inhibited TNFα-induced NF-kB production in PC-3 human prostate cancer cells when compared with positive controls (P < 0.0001). Cells treated with TNF-α alone were considered positive controls (first and second bars from the left). CBA, HPSB, THC, and HISP were superior to DTX in inhibiting NF-kB. Data are an average of three experimental repeats.
Discussion
This study showed that four compounds, curcumin analog calebin-A (CBA), curcumin metabolite THC, and polyphenols HPSB and HISP, have potent in vitro anti-prostatic cancer properties when used alone. Further, all four compounds strikingly augmented the cytotoxic effect of DTX, the mainstay chemotherapeutic treatment for mCRPC, when combined at the IC50 values of their effective concentrations in cell cultures. We found that the anticancer effects of the four test compounds and DTX are ostensibly mediated through the induction of apoptosis and inhibition of NF-kB. Our findings suggest that the pharmacological properties of these compounds make them attractive candidates for DTX combination therapy that could minimize the side effects of DTX and boost its anticancer effects.
Treatment of mCRPC with DTX chemotherapy and an androgen receptor pathways inhibitor such as abiraterone, enzalutamide, or apalutamide improves overall survival. However, a significant concern is developing undesirable chemoresistance leading to treatment failure.3 One of DTX’s most essential mechanisms of chemoresistance is the production of NF-KB, which suppresses apoptosis.4 NF-kB mediates the suppression of TP53 and the production of vascular endothelial growth factor (VEGF), interleukin 8 (IL-8), and MMP 2 and 9. These factors mediate the inhibition of apoptosis, augmentation of cancer cell proliferation, and tumor metastasis, resulting in chemoresistance.5–8
In the present study, we investigated the anti-prostatic cancer effects of CBA, HPSB, THC, and HISP, separately and in combination with DTX, using human androgen-resistant prostate cancer PC-3 cells. In parallel, we evaluated the cytotoxicity of these compounds, either independently or in combination with DTX, at the IC50 concentrations using normal immortalized prostate RWPE-1 epithelial cells. Employing 48 hours of cell culture protocols, CBA, HPSB, THC, and HISP had strong selective anticancer effects in PC-3 cells. The combination with DTX allowed for using only the IC50 concentration of each compound, resulting in a cytotoxic effect more significant than the sum of the individual drugs when used alone.
CBA, a curcumin analog, at 40 μM (IC50), enhanced the cytotoxic effect of DTX on PC-3 cells via its apoptotic and anti-inflammatory properties. Notably, the viability of normal prostate cells was not affected by this concentration either alone or in combination with DTX. Previous reports have indicated CBA’s apoptotic, anti-cell proliferation, and anti-inflammatory properties in colorectal cancer cells.9 Other reports have shown that CBA has a robust antiproliferative effect on NF-kB-mediated cell proliferation in three colorectal cancer cells (HCT-116, RKO, and SW480).10 Mechanistically, CBA was reported to suppress NF-kB induced by various stimuli in different cell lines by suppressing the direct binding of the NF-κB/p65 subunit essential for NF-kB transactivation and binding to DNA.11 Other studies reported induction of apoptosis, arrest of the S and G2/M phases of cell division, and modulation of the MAPK family activity in a vincristine resistant (SGC7901/VCR) human gastric adenocarcinoma cell line.12 Our CBA data suggest a similar mechanism of action in PC-3 androgen-resistant prostate cancer cells.
The antiproliferative effects of HPSB, a polyphenolic compound, on PC-3 androgen-resistant prostate cancer cells add further knowledge to the HPSB-reported anticancer effects in human colon cancer COLO-205, HCT-116, and HT-29 cells.13 In our study, HPSB consistently enhanced the antiproliferative effect of DTX when used at 40 μM concentration for 48 hours (4 × 105 cells per well). These findings are in line with the reported anti-tumorigenic properties of HPSB in colon cancer COLO-205 tumor xenografts,14 Fas-ligand resistant lymphoma cells, and drug-sensitive and immune leukemia cells (HL60-R and K562-ADR),15 hepatocellular carcinoma cells,16,17 and in MDA-MB-231 breast cancer cells.18
Several factors have been reported to contribute to the anti-cancer efficacy of HPSB.18 These include an extra 3-hydroxyl group in pterostilbene, which strengthens its antioxidant properties. Additionally, HPSB diminishes inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 levels, suppressing inflammatory responses.19,20 These findings suggest an inhibitory effect of HPSB on COX-2 and NF-kB transcription. HPSB has also been reported to induce apoptosis via intrinsic and extrinsic pathways. The extrinsic pathway includes the activation of FAS/CD95 and TRAIL receptors, leading to upregulating caspase-8 levels.21,22 Conspicuously, there was no significant HPSB toxicity to normal hematopoietic stem cells, normal peripheral blood mononuclear cells, or mice fed a high oral dose of HPSB in the studies above. These findings concur with our lack of significant cell death in normal prostate RWPE-1 cells. The little or no cytotoxic effects on normal RWPE-1 prostate epithelial cells indicate that this compound selectively targets prostate cancer cells.14,15
Two studies from other labs have confirmed the anti-prostatic effects of HPSB. The first study characterized for the first time the pharmacodynamics bioactivity of HPSB in several in vitro assays using several cell lines, including PC-3. The survey of PC-3 cells used dosages ranging between 3.6724 and 918.105 μM for three days, with reported IC50 values of 85.5 ± 31.14 mM (23.29 ± 8.48 μg/mL), much higher than those that we found.21 In the second study, Tsai et al22 reported that treatment with 20 μM HPSB for 48 hours was effective when the PC-3 cell concentration was low (5000 cells per well). Neither of these studies has investigated combination treatments with DTX.
Our third drug, THC, a curcumin metabolite, has antiproliferative, apoptotic, and chemosensitizing effects on PC-3 cells. We found that THC blocked NF-kB pathway activation and augmented the anticancer effect of DTX.
Studies from other labs have suggested that the antiproliferative effect of THC is due to multiple mechanisms. For example, THC was found to induce G0/G1 cell cycle arrest in glioma cells by downregulating cyclin D1 and proliferating cell nuclear antigen (PCNA).23 Additionally, in colon cancer, THC prevents the expression of iNOS and COX-2, as well as suppressing the Wnt-1/ß-catenin proliferative signaling pathway.24 As an apoptosis inducer, THC was reported to disrupt mitochondrial membrane potential, activate apoptotic proteins, suppress anti-apoptotic factors, upregulate executioner caspase-3 activity in breast cancer MCF-7 cells and inhibit tumor growth in mice implanted with H-22 tumor xeno-grafts.24–27 Other studies have reported suppression of MMP 2 and 9, VEGF, VEGF receptor-2, NF-kB signaling, inhibition of metastasis, angiogenesis, and tumor progression in different types of cancers.25,28–32 Importantly, no significant THC cytotoxicity was detected in in vivo research studies. On the contrary, Kitani et al33 stated that prolonged intake of 300 mg/kg of THC increases the lifespan of mice. These findings align with our findings that showed no toxic effects of THC on normal RWPE-1 prostate epithelial cells.
Our fourth drug, HISP, a polyphenolic compound, induced significant cytotoxicity in PC-3 androgen-resistant prostate cells and, like the other three compounds, augmented the anticancer effect of DTX. Our findings concur with previous studies on HISP-induced apoptosis in hepatocellular carcinoma,34 skin cancer cells,35 nasopharyngeal carcinoma,36 acute myeloid leukemia,37 and human gastric cancer cells.38 HISP was reported to enhance the apoptotic executioner caspase-3, and to suppress NF-KB (a pivotal mediator of inflammation and cancer cell proliferation) by inhibiting NF-kB/p65 dimer phosphorylation, nuclear translocation, and nuclear DNA binding.39
HISP-induced apoptosis in prostate DU145 cancer cells via modulation of the mitochondrial and STAT3 pathways was reported in a study by another lab.40 Like our stated synergism between HISP and DTX, HISP was reported to act synergistically with doxorubicin on melanoma B16BL6 cells yielding higher cell death than doxorubicin alone.41 Further, the selectivity of HISP to gastric cancer SGC 7901 cells compared to minimal toxicity to normal gastric epithelial GES-1 cells was also reported.38 Collectively, these studies support our HISP-induced apoptosis in PC-3 and its synergism to DTX.
In conclusion, we surmise that activating caspase-dependent apoptosis and suppressing NF-kB inflammatory pathways are excellent strategies for overcoming DTX resistance.7 In the present study, we showed a robust anticancer effect of CBA, HPSB, THC, and HISP and their ability to augment the cytotoxic efficacy of DTX to androgen-resistant PC-3 prostate cancer cells. Our four test compounds could be promising drug candidates for preventing DTX resistance when used in optimal combination. Further in vivo studies using a mouse allograft model of prostate cancer are needed to confirm our in vitro findings.
Materials and Methods
Materials
Penicillin-streptomycin, dimethyl sulfoxide (DMSO), and hardware supplies for cell culture were bought from VWR International (Atlanta, GA, USA). Fetal bovine serum (FBS) and trypsin-EDTA solution (1X) were obtained from Atlanta Biologicals (Lawrenceville, GA, USA), DTX (BML-T129–0005) and HISP (ALX-350–384-M005) from Enzo (Farmingdale, NY, USA), calebin-A, 3′-hydroxyptersostilbene (HPSB), and THC from Sabinsa Corporation (East Windsor, NJ, USA), soybean trypsin inhibitor, trypan blue solution (0.4%), and ST (cat# S5921) from Sigma Aldrich (St. Louis, MO, USA), Bradford protein assay kit from Ameresco (Ohio, USA), and Caspase-3 Colorimetric Assay Kit (ab39401), nuclear extraction kit (ab113474), and NF-kB transcription factor assay kit (ab133112) from Abcam (Cambridge, MA, USA).
Cell Lines
The human prostate cancer cell line (PC-3) and human immortalized normal prostate epithelial cells (RWPE-1) were purchased from the American Type Culture Collection (Manassas, VA, USA). PC-3 cells were cultured in RPMI-1640 with HEPES and L-glutamine, supplemented with 10% FBS and 1% penicillin-streptomycin. RWPE-1 cells were maintained in keratinocyte-SFM supplemented with bovine pituitary extract, epidermal growth factor, insulin, hydrocortisone, epinephrine, transferrin, and CaCl2. Both types of cells were grown in T-75 vented-cap tissue culture flasks in a humidified incubator at 37°C and 5% CO2.
Experimental Design
The selected IC50 concentrations of the four compounds and the treatment duration in this study were based on several initial optimization experiments. For the calculation of IC50 for CBA, HPSB, and THC, we tested the effect of a wide range of final concentrations using a 2-fold difference between the 1 and 80 μM final concentrations.
For HISP, we used a concentration range of 16–500 μM. All treatment concentrations were IC50 tested for 48 hours. The final IC50 drug concentrations were prepared using either RPMI-1640 or keratinocyte-SFM medium according to the type of cells tested. The negative controls were treated with vehicles. DTX was used at 117 nM IC50 final concentration based on a previous study in our lab,1 while ST, a positive control that induces cell apoptosis, was used at 1 μM final concentration. The selected IC50 effective concentrations were 40 μM for CBA, HPSB, and THC and 250 μM for HISP. The anticancer effects of the four compounds were tested individually and in combination with DTX in triplicate, as described below. Each experiment was repeated thrice.
Cell Viability Assay
The CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA) was used to evaluate the antiproliferative and cytotoxic effects of CBA, HPSB, HISP, and THC individually and in combination with DTX on PC-3 and RWPE-1 cells. For viability testing, cells were collected from stock cultures by trypsinization and counted using a TC20™ Automated Cell Counter (Bio-Rad, California, USA). Next, PC-3 and RWPE-1 cells (4 × 104 cells per well) were seeded in 96-well plates and incubated at 37°C and 5% CO2 overnight to allow the cells to adhere to the wells. The next day, the medium was aspirated and replaced with 100 μL containing 40 μM CBA, HPSB, THC, or vehicle. For HISP-treated cells, the wells received 100 μL containing a final concentration of 250 μM. DTX-treated cells received 117 nM final concentration in 100 μL. In the combination study, we added 100 μL/well combinations of each compound plus DTX at 50% of the concentrations used for individual treatments. All treatments were performed in triplicate, and all plates were incubated for 48 hours. Next, the CellTiter-Glo reagent was added to all wells, and the plates were incubated at room temperature for 15 minutes. The luminescent signals, which show the amount of ATP and reflect the proportion of viable cells, were detected using a FLUOstar OPTIMA microplate reader (BMG LABTECH, Offenburg, Germany).
Stimulation of the Executioner Caspase-3
We used the Caspase-3 Colorimetric Assay Kit (ab39401) to determine the apoptotic effects of the four test compounds used in the study. The assay allows for the spectrophotometric detection of the chromophore p-nitroaniline (p-NA) after cleavage from the labeled substrate DEVD-p-NA. Using a spectro-photometer, we measured the absorbance readings of p-NA light emission from treated and untreated control cells. Next, we calculated the fold increase in caspase activity and, hence, the ability of the test compounds alone and in combination to stimulate executioner caspases.
Briefly, 12-well plates were seeded with PC-3 (25 × 104 cells per well) and incubated overnight at 37°C and 5% CO2. In treated wells (duplicates for each treatment), the media were replaced with DTX (117 nM), CBA (40 μM), HPSB (40 μM), THC (40 μM), HISP (250 μM), ST (1 μM), or new media (untreated control samples). DTX and test compounds were combined at the IC50 concentration for each. ST is a bacterial-derived compound known to induce apoptosis via both the mitochondrial and caspase-dependent pathways11,38,39. Treated cell cultures were incubated for 24 hours under standard culture conditions. Next, cells were lysed using the kit’s cell lysis buffer (50 μL chilled lysis buffer per well), transferred into separate microcentrifuge tubes, incubated for 10 minutes on ice, and centrifuged at 10,000 × g for 1 minute. The super-natants (cytosolic extracts) were evaluated for their protein concentrations using the Bradford protein assay before being transferred into a new 96-well plate at 50 μL per well containing 100 μg total protein. Next, duplicate freshly prepared treated samples were thoroughly mixed with a 50-μL 2X reaction mix and DEVD-P-NA substrate (5 μL of 4 mM DEVD-p-NA substrate at a final concentration of 200 μM). Background wells received 50 μL of 2X reaction buffer and 50 μL of untreated samples. The plate was incubated at 37°C for 90 minutes before measuring the optical density at 400–405 nm using a FLUOstar OPTIMA microplate reader. Background readings from cell lysates and buffers were subtracted from the readings of both the treated and untreated cultures. The percent increase in caspase-3 activity was decided by comparing the activity in treated samples with the level of the vehicle treated negative control, ST-positive control, and with DTX.
Cell Imaging
PC-3 cells (4 × 104 cells per well) were seeded in a 12-well plate and allowed overnight incubation at 37°C and 5% CO2 to adhere. The media were replaced by CBA (40 μM), HPSB (40 μM), THC (40 μM), HISP (250 μM), DTX (117 nM), or ST (1 μM). Untreated wells were treated with fresh media. The plates were incubated for 48 hours. We used Motic Images Plus software (version 2.0) for imaging the cultures.
NF-kB Activity Assays
To investigate the anti-inflammatory properties of each of the four compounds and DTX, we followed the procedure described in the colorimetric NF-kB assay Kit (ab133112) to estimate the amount of activated p65 subunit in the nuclear extracts of treated and untreated cells. Briefly, a 96-well plate coated with a specific double-stranded DNA (dsDNA) sequence of the NF-kB response elements was provided with the kit. Nuclear extracts prepared from treated, untreated, and control samples containing NF-kB were added. The binding of free NF-kB in the assay samples was detected by a two-step process that included the addition of a specific primary antibody to NF-kB (subunit P65), followed by washing and adding a secondary antibody conjugated to horseradish peroxidase (HRP). The quantitative signal readout was measured at 450 nm. Assay buffers and antibody dilutions (1:100) were used according to the manufacturer’s recommendations.
For sample preparation, PC-3 cells were grown in six-well 100 mm sterile culture plates to 80% confluency. Positive controls included cell lysate in the kit and TNF-α-treated nuclear cell extract (10 ng per mL for 1 hour). TNF-α was used in combination with the four test compounds. Briefly, the media over cultured PC-3 cells were replaced with TNF-α (10 ng/mL), CBA (40 μM) + TNF-α, HPSB (40 μM) + TNFα, THC (40 μM) + TNF-α, HISP (250 μM) + TNF-α, or DTX (117 nM) + TNF-α. Next, all the plates were incubated at 37°C and 5% CO2 for 48 hours. A nuclear extraction kit (ab113474) was used according to the manufacturer’s protocol to extract nuclear material.
Treated, untreated, and control samples of nuclear extracts (10 μL per well) plus 90 μL of transcription assay binding buffer were added in duplicate to the 96-well assay plate. Background wells received 100 μL 1X transcription assay binding buffer. The sealed plate was incubated at room temperature for 1 hour on an orbital shaker. Next, wells were washed five times using 200 μL per well 1X washing buffer, followed by adding 100 μL per well primary NF-kB p65 antibody and incubation for 1 hour at room temperature on an orbital shaker. Next, all the wells were aspirated, washed five times with 1X wash buffer, and treated with secondary anti-rabbit HRP-conjugated antibody for 1 hour at room temperature on an orbital shaker. Next, the assay wells were washed by adding 100 μL per well from room temperature equilibrated developing solution for 30 minutes on an orbital shaker. After developing a dark blue color in the medium, 100 μL per well of stop solution was added for 5 minutes.
Absorbance was measured at an optical density of 450 nm by a FLUOstar OPTIMA microplate reader.
Statistical Analysis
We used GraphPad prism 5 software for data analysis. Data were expressed as means ± SE. Luminescence, caspase-3, and NF-kB assay data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. A P < 0.05 was considered statistically significant.
Acknowledgements
The authors would like to thank Sabinsa Corporation (East Windsor, NJ 08520 USA) for supplying the calebin A, 3-hydroxyptersostilbene, and tetrahydrocurcumin used in this study. The authors thank Dr Mahmoud Afifi and Julia Salamat for their technical help.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded partially by the Egyptian Ministry of Higher Education, Cultural Affairs, and Missions Sector to support Inass Ahmed and NIH award 1R61NS126618-01A1 to Drs. Raj Amin and Satyanarayana R. Pondugula.
Abbreviations
- DTX
docetaxel
- CBA
calebin A
- HPSB
3′-hydroxypterostilbene
- THC
tetrahydrocurcumin
- HISP
hispolon
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mansour M, van Ginkel S, Dennis JC, et al. The combination of omega-3 stearidonic acid and docetaxel enhances cell death over docetaxel alone in human prostate cancer cells. J Cancer. 2018;9(23):4536–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mediavilla-Varela M, Pacheco FJ, Almaguel F, et al. Docetaxel-induced prostate cancer cell death involves concomitant activation of caspase and lysosomal pathways and is attenuated by LEDGF/p75. Mol Cancer. 2009;8:68(15 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang C Overcoming docetaxel resistance in prostate cancer: a perspective review. Ther Adv Med Oncol. 2012;4(6):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godwin P, Baird AM, Heavey S, Barr MP, O’Byrne KJ, Gately K. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front Oncol. 2013;3:120(10 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ismail H, Lessard L, Mes-Masson AM, Saad FJTP. Expression of NF-κB in prostate cancer lymph node metastases. Prostate. 2004;58(3):308–313. [DOI] [PubMed] [Google Scholar]
- 6.McEleny K, Coffey R, Morrissey C, Fitzpatrick JM, Watson RW. Caffeic acid phenethyl ester-induced PC-3 cell apoptosis is caspase-dependent and mediated through the loss of inhibitors of apoptosis proteins. BJU Int. 2004;94(3):402–406. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill AJ, Prencipe M, Dowling C, et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol Cancer. 2011;10:126 (13 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6(3):1769–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhrmann C, Kunnumakkara AB, Kumar A, et al. Multitargeting effects of calebin A on malignancy of CRC cells in multicellular tumor microenvironment. Front Oncol. 2021;11 (13 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buhrmann C, Kunnumakkara AB, Popper B, Majeed M, Aggarwal BB, Shakibaei M. Calebin A potentiates the effect of 5-FU and TNF-β (lymphotoxin α) against human colorectal cancer cells: potential role of NF-κB. Int J Mol Sci. 2020;21(7):2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyagi AK, Prasad S, Majeed M, Aggarwal BB. Calebin A, a novel component of turmeric, suppresses NF-kappaB regulated cell survival and inflammatory gene products leading to inhibition of cell growth and chemosensitization. Phytomedicine. 2017;34:171–181. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Li S, Han Y, et al. Calebin-A induces apoptosis and modulates MAPK family activity in drug resistant human gastric cancer cells. Eur J Pharmacol. 2008;591(1–3):252–258. [DOI] [PubMed] [Google Scholar]
- 13.Tsai HY, Ho CT, Chen YK. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J Food Drug Anal. 2017;25(1):134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng TC, Lai CS, Chung MC, et al. Potent anti-cancer effect of 3′-hydroxypterostilbene in human colon xenograft tumors. PLoS One. 2014;9(11):e111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolomeo M, Grimaudo S, Di Cristina A, et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol. 2005;37(8):1709–1726. [DOI] [PubMed] [Google Scholar]
- 16.Guo L, Tan K, Wang H, Zhang X. Pterostilbene inhibits hepato-cellular carcinoma through p53/SOD2/ROS-mediated mitochondrial apoptosis. Oncol Rep. 2016;36(6):3233–3240. [DOI] [PubMed] [Google Scholar]
- 17.Wang R, Xu Z, Tian J, et al. Pterostilbene inhibits hepatocellular carcinoma proliferation and HBV replication by targeting ribonucleo-tide reductase M2 protein. Am J Cancer Res. 2021;11(6):2975–2989. [PMC free article] [PubMed] [Google Scholar]
- 18.Alosi JA, McDonald DE, Schneider JS, Privette AR, McFadden DW. Pterostilbene inhibits breast cancer in vitro through mitochondrial depolarization and induction of caspase-dependent apoptosis. J Surg Res. 2010;161(2):195–201. [DOI] [PubMed] [Google Scholar]
- 19.Lai CS, Yang G, Li S, et al. 3′-Hydroxypterostilbene Suppresses colitis-associated tumorigenesis by inhibition of IL-6/STAT3 signaling in mice. J Agric Food Chem. 2017;65(44):9655–9664. [DOI] [PubMed] [Google Scholar]
- 20.Lim JW, Kim H, Kim KH. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab Invest. 2001;81(3):349–360. [DOI] [PubMed] [Google Scholar]
- 21.Takemoto JK, Remsberg CM, Davies NM. Pharmacologic activities of 3′-hydroxypterostilbene: cytotoxic, anti-oxidant, anti-adipogenic, anti-inflammatory, histone deacetylase and sirtuin 1 inhibitory activity. J Pharm Pharm Sci. 2015;18(4):713–727. [DOI] [PubMed] [Google Scholar]
- 22.Tsai H-Y. Growth inhibitory effects of 3′-hydroxypterostilbene in human prostate cancer cells and xenograft mice. 2016;
- 23.Zhang X, Peng L, Liu A, Ji J, Zhao L, Zhai G. The enhanced effect of tetrahydrocurcumin on radiosensitivity of glioma cells. J Pharm Pharmacol. 2018;70(6):749–759. [DOI] [PubMed] [Google Scholar]
- 24.Lai CS, Wu JC, Yu SF, et al. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol Nutr Food Res. 2011;55(12):1819–1828. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Zhang Z, Lin G, et al. Tetrahydrocurcumin is more effective than curcumin in inducing the apoptosis of H22 cells via regulation of a mitochondrial apoptosis pathway in ascites tumor-bearing mice. 10.1039/C7FO00484B. Food Funct. 2017;8(9):3120–3129. [DOI] [PubMed] [Google Scholar]
- 26.Kang N, Wang MM, Wang YH, et al. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem Toxicol. 2014;67:193–200. [DOI] [PubMed] [Google Scholar]
- 27.Han X, Deng S, Wang N, Liu Y, Yang X. Inhibitory effects and molecular mechanisms of tetrahydrocurcumin against human breast cancer MCF-7 cells. Food Nutr Res. 2016;60:30616(11 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Y, Zhang Y, Yuan J, et al. Tetrahydrocurcumin mitigates acute hypobaric hypoxia-induced cerebral oedema and inflammation through the NF-κB/VEGF/MMP-9 pathway. Phytother Res. 2020;34(11):2963–2977. [DOI] [PubMed] [Google Scholar]
- 29.Yoysungnoen P, Wirachwong P, Changtam C, Suksamrarn A, Patumraj S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J Gastroenterol. 2008;14(13):2003–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoysungnoen B, Bhattarakosol P, Patumraj S, Changtam C. Effects of tetrahydrocurcumin on hypoxia-inducible factor-1α and vascular endothelial growth factor expression in cervical cancer cell-induced angiogenesis in nude mice. Biomed Res Int. 2015;2015:391748(11 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Liu Y, Zou J, et al. Tetrahydrocurcumin induces mesenchymal-epithelial transition and suppresses angiogenesis by targeting HIF-1α and autophagy in human osteosarcoma. Oncotarget. 2017;8(53):91134–91149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao F, Gong Y, Hu Y, et al. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: translocation of nuclear factor-κB as potential target. Mol Med Rep. 2015;11(4):3087–3093. [DOI] [PubMed] [Google Scholar]
- 33.Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007;8(5):567–573. [DOI] [PubMed] [Google Scholar]
- 34.Huang GJ, Deng JS, Huang SS, Hu ML. Hispolon induces apoptosis and cell cycle arrest of human hepatocellular carcinoma Hep3B cells by modulating ERK phosphorylation. J Agric Food Chem. 2011;59(13):7104–7113. [DOI] [PubMed] [Google Scholar]
- 35.Huang GJ, Yang CM, Chang YS, et al. Hispolon suppresses SK-Hep1 human hepatoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen activator through the PI3 K/akt and ERK signaling pathways. J Agric Food Chem. 2010;58(17):9468–9475. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh MJ, Chien SY, Chou YE, Chen CJ, Chen J, Chen MK. Hispolon from Phellinus linteus possesses mediate caspases activation and induces human nasopharyngeal carcinomas cells apoptosis through ERK1/2, JNK1/2 and p38 MAPK pathway. Phytomedicine. 2014;21(12):1746–1752. [DOI] [PubMed] [Google Scholar]
- 37.Hsiao PC, Hsieh YH, Chow JM, et al. Hispolon induces apoptosis through JNK1/2-mediated activation of a caspase-8, −9, and −3-dependent pathway in acute myeloid leukemia (AML) cells and inhibits AML xenograft tumor growth in vivo. J Agric Food Chem. 2013;61(42):10063–10073. [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Zhao Z, Li L, et al. Hispolon induces apoptosis in human gastric cancer cells through a ROS-mediated mitochondrial pathway. Free Radic Biol Med. 2008;45(1):60–72. [DOI] [PubMed] [Google Scholar]
- 39.Sun YS, Zhao Z, Zhu HP. Hispolon inhibits TPA-induced invasion by reducing MMP-9 expression through the NF-κB signaling pathway in MDA-MB-231 human breast cancer cells. Oncol Lett. 2015;10(1):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masood M, Rasul A, Sarfraz I, et al. Hispolon induces apoptosis against prostate DU145 cancer cells via modulation of mitochondrial and STAT3 pathways. Pak J Pharm Sci. 2019;32(5, Supplementary):2237–2243. [PubMed] [Google Scholar]
- 41.Al Saqr A, Aldawsari MF, Alrbyawi H, et al. Co-delivery of hispolon and doxorubicin liposomes improves efficacy against melanoma cells. AAPS PharmSciTech. 2020/11/04 2020;21(8):304. [DOI] [PubMed] [Google Scholar]


