Abstract
Background
Most research on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants focuses on initial symptomatology with limited longer-term data. We characterized prevalences of prolonged symptoms 3 months post–SARS-CoV-2 infection across 3 variant time-periods (pre-Delta, Delta, and Omicron).
Methods
This multicenter prospective cohort study of adults with acute illness tested for SARS-CoV-2 compared fatigue severity, fatigue symptoms, organ system–based symptoms, and ≥3 symptoms across variants among participants with a positive (“COVID-positive”) or negative SARS-CoV-2 test (“COVID-negative”) at 3 months after SARS-CoV-2 testing. Variant periods were defined by dates with ≥50% dominant strain. We performed multivariable logistic regression modeling to estimate independent effects of variants adjusting for sociodemographics, baseline health, and vaccine status.
Results
The study included 2402 COVID-positive and 821 COVID-negative participants. Among COVID-positives, 463 (19.3%) were pre-Delta, 1198 (49.9%) Delta, and 741 (30.8%) Omicron. The pre-Delta COVID-positive cohort exhibited more prolonged severe fatigue (16.7% vs 11.5% vs 12.3%; P = .017) and presence of ≥3 prolonged symptoms (28.4% vs 21.7% vs 16.0%; P < .001) compared with the Delta and Omicron cohorts. No differences were seen in the COVID-negatives across time-periods. In multivariable models adjusted for vaccination, severe fatigue and odds of having ≥3 symptoms were no longer significant across variants.
Conclusions
Prolonged symptoms following SARS-CoV-2 infection were more common among participants infected during pre-Delta than with Delta and Omicron; however, these differences were no longer significant after adjusting for vaccination status, suggesting a beneficial effect of vaccination on risk of long-term symptoms.
Clinical Trials Registration. NCT04610515.
Keywords: COVID-19, SARS-CoV-2, Long COVID, Delta, Omicron
This prospective study including 2402 SARS-CoV-2–positive participants noted more severe fatigue and ≥3 symptoms at 3 months after acute illness in the pre-Delta cohort compared with Delta and Omicron. However, this was no longer significant after accounting for vaccination status.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been dynamic with numerous variants of concern (VOCs) throughout the coronavirus disease 2019 (COVID-19) pandemic, creating challenges for clinicians, patients, and researchers. VOCs are considered more severe due to increased transmissibility, more severe disease, reduced neutralization by antibodies, reduced treatment response, or diagnostic detection failures [1]. To date, the Centers for Disease Control and Prevention (CDC) has identified 6 major VOCs, of which Delta and Omicron (including subvariants) are generally considered the most important given ramifications for diagnosis, treatment, and public health efforts [2, 3].
Early research suggests that infections by different VOCs result in different disease presentations [4–6]. While most VOC research has focused on initial symptoms, the impact on longer-term symptomatology is less clear. The differential impact of VOCs is particularly important, given the increased recognition of long-term sequelae of COVID-19, often referred to as “long COVID.” Research suggests that up to half of patients infected with SARS-CoV-2 may experience symptoms lasting beyond 3 months postinfection [7–12]. Fatigue is one of the most reported symptoms and is particularly problematic, given its impact on quality of life and return to work [10, 13]. However, there is limited research examining differences in long COVID symptoms by viral strain. Given the evolving pathogenic properties of SARS-CoV-2 variants, we need to better elucidate the difference in long-term symptoms between variants to help patients, clinicians, researchers, and policy advisors plan for longer-term impacts of COVID-19.
The Innovative Support for Patients with SARS-CoV-2 Infections Registry (INSPIRE) is a multisite prospective study designed to assess long-term symptoms and outcomes of participants tested for SARS-CoV-2 who are symptomatic at time of testing, including both SARS-CoV-2–positive and SARS-CoV-2–negative participants. We sought to analyze the difference in post-COVID-19 symptoms measured at 3 months after first-time SARS-CoV-2 infection across 3 major variant time periods (pre-Delta, Delta, and Omicron).
METHODS
Study Design
INSPIRE is a national study across 8 major healthcare systems intentionally selected for diversity of geography and participant populations. All sites broadly recruited participants regardless of state of residence; there were no geographic or health system limitations. Study details have been previously published [14]. In brief, INSPIRE was developed to prospectively and longitudinally assess the symptoms and outcomes of participants who test positive for SARS-CoV-2 compared with symptomatic adults testing negative. Data are self-reported by participants using a standardized questionnaire. The study was funded by the CDC and approved by institutional review boards at all 8 institutions.
Participants were enrolled (virtually or in-person) if they met the following inclusion criteria: age ≥18 years, fluent in English or Spanish, self-reported symptoms suggestive of acute SARS-CoV-2 infection at time of testing (eg, fever, cough), and tested with a US Food and Drug Administration–approved/authorized molecular or antigen-based assay within the preceding 42 days. Both participants with a positive SARS-CoV-2 test (“COVID-positive”) and those with a negative SARS-CoV-2 test (“COVID-negative”) were eligible to enroll. We excluded participants with previous SARS-CoV-2 infection >42 days before enrollment and those without access to an internet-connected device (eg, smartphone, tablet, computer) needed for survey completion. Participants signed informed consent and completed a baseline survey and follow-up surveys every 3 months for up to 18 months postenrollment. Only baseline and 3-month follow-up surveys were examined in this study.
Surveys were sent via email or text (according to participant preference), and participants received nominal monetary reimbursement for each survey completion. Surveys included questions on sociodemographic characteristics; social determinants of health; baseline health status; testing site; common symptoms for SARS-CoV-2 infection; symptoms of postinfectious syndromes; reinfection or new infection with SARS-CoV-2; vaccination status before index illness; patient-reported outcomes on physical, mental, and social well-being; cognitive status; and return to work and daily activities [14]. Participants were also asked to share information from their electronic health record (EHR) via a secure digital platform (Hugo Health, LLC; Guilford, Connecticut). For this study, EHR data were only utilized to supplement vaccination data from the surveys and to determine COVID-19 status.
Participants were required to provide proof of their SARS-CoV-2 test result if not available in their EHR. If an initially COVID-negative participant had a subsequent SARS-CoV-2 infection, we retained them in their initial group for this analysis. Only 61 participants (2.5%) converted from COVID negative to COVID positive during the participants’ first 3 months in the study.
Survey Tools
We used the CDC Person Under Investigation symptom list to assess prevalence of symptoms commonly reported with SARS-CoV-2 infection. Research has suggested that systemic symptoms (eg, fatigue, sleep, cognition) are common with many postinfectious syndromes (eg, Epstein-Barr virus) [15]. Therefore, we also conducted detailed assessment of these symptoms using the CDC Short Symptoms Screener, a validated tool assessing 8 systemic symptom domains (fatigue, muscle aches, joint pain, unrefreshing sleep, problems going to/waking from sleep, forgetfulness, difficulty concentrating, and dizziness/fainting) [16]. The tool was modified to reduce participant burden by focusing on the most common symptoms, with an emphasis on fatigue-related components.
Outcomes
Our primary outcome was prolonged (≥3 months) severe fatigue, defined as a Fatigue Severity Score ≥25, which represents a clinically meaningful impact and is consistent with a previously established threshold for myalgic encephalomyelitis/chronic fatigue syndrome [17]. We focused on fatigue because of the impact on quality of life and daily activities [18]. Secondary outcomes included individual fatigue symptoms, individual and organ system–based symptoms, and multiple symptoms (defined as ≥3 total symptoms) at 3 months.
Analytic Approach
We reported descriptive statistics (eg, sociodemographics, clinical characteristics, symptoms) and frequency of each symptom by VOC time period (pre-Delta, Delta, Omicron). The VOC time period was defined as the week in which ≥50% of new infections across the United States were attributed to each viral VOC based on the CDC's approach and genomic surveillance program [19, 20]. Time-period thresholds were based on last date of the corresponding week. Pre-Delta was defined as all participants tested between 11 December 2020 and 25 June 2021, Delta was defined as testing between 26 June 2021 and 24 December 2021, and Omicron was defined as testing between 25 December 2021 and 25 June 2022 (Supplementary Appendix 2). We performed a sensitivity analysis using time periods where ≥90% of new infections were attributed to a given VOC, defined as follows: pre-Delta (11 December 2020–4 June 2021), Delta (24 July 2021–17 December 2021), and Omicron (8 January 2022–25 June 2022).
We conducted χ2 testing to compare the risk of 8 individual fatigue symptoms, severe fatigue, 21 individual acute symptoms, other constitutional symptoms, 5 organ system–based symptoms (head/ears/eyes/nose/throat [HEENT], pulmonary, cardiovascular, gastrointestinal, and musculoskeletal), and presence of multiple symptom (≥3 total symptoms) across the 3 VOC periods within the COVID-positive and COVID-negative groups, separately. We performed multivariable regression to examine differences across the 3 VOC time periods in risk of severe fatigue, individual acute symptoms, organ system–based symptoms, and presence of multiple symptom with adjustment for potential confounders (model 1): age, preexisting comorbidities, hospitalization for COVID-19, and selected demographic information (gender, race, and ethnicity). In model 2, we additionally adjusted for vaccination status (≥1 dose before index SARS-CoV-2 test). We performed post hoc analyses among the 3 VOC time periods specifically as Delta versus pre-Delta, Omicron versus pre-Delta, and Omicron versus Delta, and reported the adjusted odds ratios with 95% confidence intervals (CIs). We also ran the same 2 models within the COVID-negative group enrolled during the same variant time periods as a validation exercise. All analyses were conducted using SAS version 9.4 software (SAS Inc, Cary, North Carolina).
RESULTS
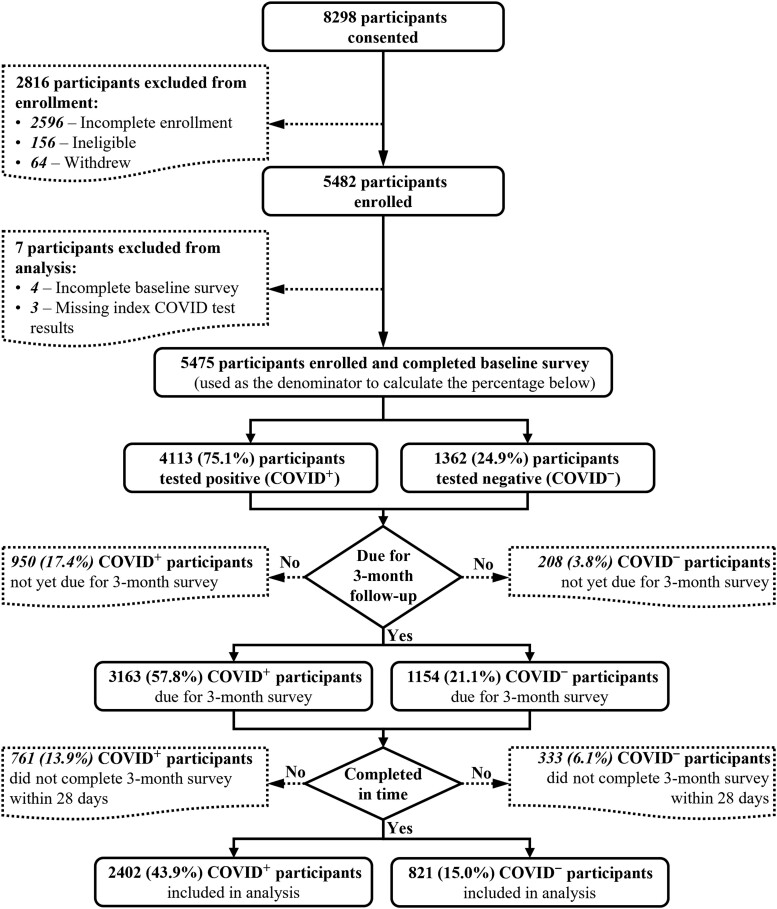
At the time of analysis, 8298 individuals were screened for participation, of whom 2596 were excluded due to incomplete enrollment (ie, initiated but did not finish enrollment), 156 were ineligible, and 64 withdrew from the study. Seven participants were excluded due to missing baseline survey or test result. A total of 5475 were enrolled and completed a baseline survey. Of these, 1158 participants (21.1%) were excluded because their 3-month survey was not yet due and 1094 (20.0%) had not completed the survey within 28 days, leaving 3223 total participants (58.9%) with complete baseline and 3-month data for analysis (Figure 1, Supplementary Appendix 3). Median time to 3-month survey completion was 90 days (interquartile range [IQR], 79–95 days) for the pre-Delta group, 78 days (IQR, 77–81 days) for Delta, and 78 days (IQR, 77–81 days) for Omicron.
Figure 1.
Patient flow diagram. Abbreviations: COVID–, negative test for severe acute respiratory syndrome coronavirus 2; COVID+, positive test for severe acute respiratory syndrome coronavirus 2.
Sociodemographics
Of these 3223 participants, 2402 (74.5%) were COVID positive and 821 (25.5%) were COVID negative. Within the COVID-positive group, participants were more commonly female (66.6%), non-Hispanic (86.0%), White (71.1%), married or partnered (58.4%), and had private health insurance (73.3%). Most participants were not hospitalized for COVID-19 (94.5%). Across time periods, we enrolled 463 (19.3%) COVID-positive participants during pre-Delta, 1198 (49.9%) during Delta, and 741 (30.8%) during Omicron periods.
Sociodemographics across variant time periods are included in Table 1. Pre-Delta COVID-positive participants were more commonly ≥50 years of age (32.2%) compared with Delta (26.7%) or Omicron (22.5%). Other notable differences among COVID-positive participants between groups for pre-Delta, Delta, and Omicron were race (Black: 18.0% vs 5.7% vs 5.3%), health insurance (private insurance: 64.8% vs 74.5% vs 76.7%), unemployment (unemployed: 20.3% vs 15.6% vs 17.9%), hospitalization (hospitalized: 13.5% vs 4.8% vs 2.5%), and vaccination status (vaccinated: 18.4% vs 74.1% vs 98.4%).
Table 1.
Characteristics of Severe Acute Respiratory Syndrome Coronavirus 2–Positive Study Participants by Variant of Concern Time Period
| Characteristic | Variant of Concern Time Period | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 2402) | Pre-Delta (n = 463) | Delta (n = 1198) | Omicron (n = 741) | ||||||
| Age, ya | <.001 | ||||||||
| 18–34 | 1000 | (42.0) | 166 | (35.9) | 500 | (41.8) | 334 | (46.0) | |
| 35–49 | 752 | (31.5) | 147 | (31.8) | 376 | (31.4) | 229 | (31.5) | |
| 50–64 | 446 | (18.7) | 116 | (25.1) | 217 | (18.1) | 113 | (15.6) | |
| ≥65 | 186 | (7.8) | 33 | (7.1) | 103 | (8.6) | 50 | (6.9) | |
| Genderb | .193 | ||||||||
| Female | 1554 | (66.6) | 297 | (66.3) | 767 | (65.3) | 490 | (68.9) | |
| Male | 750 | (32.2) | 149 | (33.3) | 388 | (33.1) | 213 | (30.0) | |
| Transgender/nonbinary/other | 29 | (1.2) | 2 | (0.5) | 19 | (1.6) | 8 | (1.1) | |
| Ethnicityc | .019 | ||||||||
| Hispanic | 330 | (14.0) | 77 | (16.9) | 143 | (12.1) | 110 | (15.4) | |
| Raced | <.001 | ||||||||
| American Indian/Alaskan Native | 16 | (0.7) | 4 | (0.9) | 9 | (0.8) | 3 | (0.4) | |
| Asian/Native Hawaiian/Pacific Islander | 275 | (11.8) | 24 | (5.3) | 141 | (12.0) | 110 | (15.4) | |
| Black | 186 | (8.0) | 81 | (18.0) | 67 | (5.7) | 38 | (5.3) | |
| Other | 199 | (8.5) | 36 | (8.0) | 93 | (7.9) | 70 | (9.8) | |
| White | 1665 | (71.1) | 305 | (67.8) | 867 | (73.7) | 493 | (69.1) | |
| Educatione | <.001 | ||||||||
| Less than high school | 26 | (1.1) | 10 | (2.2) | 12 | (1.0) | 4 | (0.6) | |
| High school graduate | 155 | (6.7) | 69 | (15.4) | 47 | (4.0) | 39 | (5.5) | |
| Some college | 347 | (14.9) | 78 | (17.4) | 176 | (15.1) | 93 | (13.0) | |
| 2-y degree | 176 | (7.6) | 44 | (9.8) | 88 | (7.6) | 44 | (6.2) | |
| 4-y degree | 814 | (35.0) | 125 | (27.9) | 434 | (37.2) | 255 | (35.7) | |
| More than 4-y degree | 810 | (34.8) | 122 | (27.2) | 409 | (35.1) | 279 | (39.1) | |
| Marital statusf | .012 | ||||||||
| Married/partner | 1389 | (58.4) | 240 | (53.6) | 716 | (60.1) | 433 | (58.6) | |
| Divorced/widowed/separated | 225 | (9.5) | 57 | (12.7) | 113 | (9.5) | 55 | (7.4) | |
| Never married | 764 | (32.1) | 151 | (33.7) | 362 | (30.4) | 251 | (34.0) | |
| Family income, USDg | <.001 | ||||||||
| <10 000 | 130 | (5.5) | 34 | (7.6) | 58 | (4.9) | 38 | (5.1) | |
| 10 000–34 999 | 265 | (11.1) | 69 | (15.4) | 110 | (9.2) | 86 | (11.6) | |
| 35 000–49 999 | 222 | (9.3) | 55 | (12.3) | 104 | (8.7) | 63 | (8.5) | |
| 50 000–74 999 | 327 | (13.8) | 67 | (14.9) | 164 | (13.8) | 96 | (13.0) | |
| ≥75 000 | 1296 | (54.5) | 218 | (48.6) | 677 | (56.8) | 401 | (54.3) | |
| Prefer not to answer | 139 | (5.8) | 6 | (1.3) | 78 | (6.6) | 55 | (7.4) | |
| Health insuranceh | <.001 | ||||||||
| Private | 1761 | (73.3) | 300 | (64.8) | 893 | (74.5) | 568 | (76.7) | |
| Public | 450 | (18.7) | 110 | (23.8) | 215 | (18.0) | 125 | (16.9) | |
| Private and public | 88 | (3.7) | 16 | (3.5) | 50 | (4.2) | 22 | (3.0) | |
| Self-insured | 103 | (4.3) | 37 | (8.0) | 40 | (3.3) | 26 | (3.5) | |
| Employmenti | <.001 | ||||||||
| Employed, essential | 1000 | (42.1) | 198 | (44.1) | 463 | (38.9) | 339 | (45.9) | |
| Employed, nonessential | 970 | (40.8) | 160 | (35.6) | 542 | (45.6) | 268 | (36.3) | |
| Not employed | 408 | (17.2) | 91 | (20.3) | 185 | (15.6) | 132 | (17.9) | |
| Tobacco usej | .199 | ||||||||
| Daily | 184 | (6.1) | 25 | (5.6) | 74 | (6.2) | 36 | (4.9) | |
| Weekly | 62 | (2.0) | 15 | (3.3) | 24 | (2.0) | 9 | (1.2) | |
| Monthly | 44 | (1.5) | 9 | (2.0) | 14 | (1.2) | 13 | (1.8) | |
| Less than monthly | 139 | (4.6) | 17 | (3.8) | 59 | (5.0) | 39 | (5.3) | |
| Not at all | 2605 | (85.9) | 384 | (85.3) | 1019 | (85.6) | 642 | (86.9) | |
| COVID-19 testing sitek | <.001 | ||||||||
| At home | 188 | (7.9) | 4 | (0.9) | 51 | (4.3) | 133 | (18.0) | |
| Clinic including urgent care | 357 | (14.9) | 69 | (15.1) | 190 | (15.9) | 98 | (13.2) | |
| Emergency department | 96 | (4.0) | 36 | (7.9) | 41 | (3.4) | 19 | (2.6) | |
| Hospital | 215 | (9.0) | 83 | (18.2) | 77 | (6.4) | 55 | (7.4) | |
| Other | 167 | (7.0) | 30 | (6.6) | 72 | (6.0) | 65 | (8.8) | |
| Tent/drive-up testing site | 1370 | (57.3) | 234 | (51.3) | 765 | (64.0) | 371 | (50.1) | |
| Preexisting conditionsl | |||||||||
| Asthma | 285 | (12.3) | 53 | (13.7) | 146 | (13.0) | 86 | (12.5) | .800 |
| Hypertension | 323 | (13.9) | 75 | (19.4) | 166 | (14.7) | 82 | (12.0) | .003 |
| Diabetes | 118 | (5.1) | 27 | (7.0) | 52 | (4.6) | 39 | (5.7) | .212 |
| Obesity | 631 | (27.2) | 125 | (32.3) | 316 | (28.0) | 190 | (27.7) | .206 |
| Emphysema/COPD | 19 | (0.8) | 9 | (2.3) | 7 | (0.6) | 3 | (0.4) | .003 |
| Heart conditions | 57 | (2.5) | 21 | (5.4) | 24 | (2.1) | 12 | (1.8) | <.001 |
| Smoking | 101 | (4.4) | 25 | (6.5) | 53 | (4.7) | 23 | (3.4) | .055 |
| Kidney disease | 31 | (1.3) | 6 | (1.6) | 16 | (1.4) | 9 | (1.3) | .936 |
| Liver disease | 16 | (0.7) | 8 | (2.1) | 5 | (0.4) | 3 | (0.4) | .003 |
| Other condition | 348 | (15.0) | 60 | (15.5) | 180 | (16.0) | 108 | (15.7) | .913 |
| None | 428 | (18.4) | 56 | (14.5) | 228 | (20.2) | 144 | (21.0) | .025 |
| Don’t know | 515 | (22.2) | 88 | (22.7) | 256 | (22.7) | 164 | (23.9) | .883 |
| Prefer not to answer | 123 | (5.3) | 22 | (5.4) | 50 | (4.3) | 51 | (6.9) | .040 |
| Hospitalized for COVID-19m | <.001 | ||||||||
| Hospitalized | 129 | (5.6) | 55 | (13.5) | 56 | (4.8) | 18 | (2.5) | |
| Vaccination statusn,o | <.001 | ||||||||
| Vaccinated before index COVID test | 1357 | (67.9) | 80 | (18.4) | 797 | (74.1) | 480 | (98.4) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; USD, United States dollars.
Missing age information, n = 18.
Missing gender information, n = 69.
Missing ethnicity information, n = 48.
Missing race information, n = 61.
Missing education information, n = 74.
Missing marital status information, n = 24.
Missing family income information, n = 23.
Missing health insurance information, n = 0.
Missing employment information, n = 24.
Missing tobacco use information, n = 23.
Missing COVID-19 testing site information, n = 9.
Missing preexisting conditions information, n = 79.
Missing hospitalization for COVID-19 information, n = 79.
Missing vaccination status information, n = 404.
Vaccination status questions based on all available electronic health record and survey data; vaccine questions were added to the 3-month survey on 14 April 2021 with version change 11 March 2022 and were defined as at least 1 dose prior to the index severe acute respiratory syndrome coronavirus 2 test.
Prolonged Fatigue Severity
Within the COVID-positive group, the prevalence of severe fatigue at 3 months was significantly higher for pre-Delta compared with Delta and Omicron (16.7% vs 11.5% vs 12.3%; P = .017; Table 2). These remained consistent in the sensitivity analysis using 90% variant dominance threshold (Supplementary Appendix 4). However, after adjustment for sociodemographics, preexisting medical conditions, hospitalization, and vaccination status (model 2), the differences were no longer significant (Delta vs pre-Delta: 0.79 [95% CI, .52–1.20]; Omicron vs pre-Delta: 0.94 [95% CI, .56–1.60]; Omicron vs Delta: 1.20 [95% CI, .82–1.75]; Supplementary Appendix 5).
Table 2.
Proportion of Participants With Prolonged Fatigue Symptoms at 3 Months by Variant of Concern Time Period in Severe Acute Respiratory Syndrome Coronavirus 2–Positive Participants
| Fatigue Symptoms | Variant of Concern Time Period | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 2373)a | Pre-Delta (n = 456) | Delta (n = 1179) | Omicron (n = 738) | ||||||
| Fatigue, tiredness, or exhaustion | 838 | (35.3) | 161 | (35.3) | 407 | (34.5) | 270 | (36.6) | .655 |
| Muscle aches/muscle pains | 366 | (15.4) | 88 | (19.3) | 156 | (13.2) | 122 | (16.5) | .006 |
| Pain in joints | 312 | (13.2) | 66 | (14.5) | 141 | (12.0) | 105 | (14.2) | .233 |
| Unrefreshing sleep | 601 | (25.3) | 121 | (26.5) | 288 | (24.4) | 192 | (26.0) | .594 |
| Sleep disturbance | 652 | (27.5) | 129 | (28.3) | 304 | (25.8) | 219 | (29.7) | .163 |
| Forgetfulness/memory problems | 297 | (12.5) | 79 | (17.3) | 137 | (11.6) | 81 | (11.0) | .002 |
| Difficulty thinking or concentrating | 294 | (12.4) | 70 | (15.4) | 143 | (12.1) | 81 | (11.0) | .077 |
| Dizziness or fainting | 149 | (6.3) | 36 | (7.9) | 68 | (5.8) | 45 | (6.1) | .274 |
| Fatigue Severity Score ≥25 | 302 | (12.7) | 76 | (16.7) | 135 | (11.5) | 91 | (12.3) | .017 |
Data are presented as No. (%) unless otherwise indicated.
Twenty-nine patients were excluded due to missing data at 3 months.
Overall, more COVID-negative participants than COVID-positive participants reported severe fatigue (17.8% vs 12.7%, respectively). However, within the COVID-negative cohort, there was no significant difference in any individual fatigue elements or in severe fatigue across the VOC time periods (16.8% vs 19.8% vs 16.4%; P = .495; Supplementary Appendix 6).
Prolonged COVID-19 Symptoms
Among the COVID-positive cohort, significant differences were found in symptoms reported at 3 months between the VOC cohorts (Table 3). Significantly more participants in the pre-Delta group compared with Delta and Omicron reported prolonged symptoms at 3 months (52.6% vs 41.5% vs 41.5%; P < .001). Reports of ≥3 symptoms in pre-Delta were more common compared with Delta and Omicron (28.4% vs 21.7% vs 16.0%; P < .001).
Table 3.
Proportion of Severe Acute Respiratory Syndrome Coronavirus 2–Positive Study Participants With Symptoms at 3 Months by Variant of Concern Time Period
| Symptom Category | Prevalence of Symptoms by Variant of Concern Time Period No. (% of Period Total) |
P Value | |||
|---|---|---|---|---|---|
| Overalla (N = 2390) |
Pre-Delta (n = 462) |
Delta (n = 1190) |
Omicron (n = 738) |
||
| Constitutional | 587 (24.6) | 142 (30.7) | 278 (23.4) | 167 (22.6) | .003 |
| Tired | 546 (22.9) | 127 (27.5) | 263 (22.1) | 156 (21.1) | .027 |
| Chills | 147 (6.2) | 49 (10.6) | 84 (7.1) | 14 (1.9) | <.001 |
| Feeling hot | 131 (5.5) | 42 (9.1) | 76 (6.4) | 13 (1.8) | <.001 |
| Fever | 77 (3.2) | 30 (6.5) | 46 (3.9) | 1 (0.1) | <.001 |
| Shakes | 54 (2.3) | 22 (4.8) | 24 (2.0) | 8 (1.1) | <.001 |
| HEENT | 740 (31.0) | 180 (39.0) | 379 (31.9) | 181 (24.5) | <.001 |
| Headache | 340 (14.2) | 84 (18.2) | 166 (14.0) | 90 (12.2) | .014 |
| Runny nose | 231 (9.7) | 42 (9.1) | 132 (11.1) | 57 (7.7) | .047 |
| Loss of smell | 305 (12.8) | 89 (19.3) | 189 (15.9) | 27 (3.7) | <.001 |
| Loss of taste | 228 (9.5) | 70 (15.2) | 134 (11.3) | 24 (3.3) | <.001 |
| Sore throat | 186 (7.8) | 35 (7.6) | 109 (9.2) | 42 (5.7) | .022 |
| Loss of hair | 148 (6.2) | 36 (7.8) | 68 (5.7) | 44 (6.0) | .277 |
| Pulmonary | 346 (14.5) | 91 (19.7) | 168 (14.1) | 87 (11.8) | <.001 |
| Cough | 174 (7.3) | 34 (7.4) | 102 (8.6) | 38 (5.2) | .019 |
| Shortness of breath | 218 (9.1) | 67 (14.5) | 98 (8.2) | 53 (7.2) | <.001 |
| Wheezing | 63 (2.6) | 14 (3.0) | 32 (2.7) | 17 (2.3) | .737 |
| Cardiovascular | 187 (7.8) | 53 (11.5) | 86 (7.2) | 48 (6.5) | .004 |
| Chest pains | 127 (5.3) | 41 (8.9) | 59 (5.0) | 27 (3.7) | <.001 |
| Palpitations | 90 (3.8) | 25 (5.4) | 41 (3.5) | 24 (3.3) | .115 |
| Gastrointestinal | 153 (6.4) | 47 (10.2) | 70 (5.9) | 36 (4.9) | <.001 |
| Diarrhea | 70 (2.9) | 22 (4.8) | 31 (2.6) | 17 (2.3) | .032 |
| Nausea or vomiting | 71 (3.0) | 22 (4.8) | 37 (3.1) | 12 (1.6) | .007 |
| Abdominal pain | 51 (2.1) | 12 (2.6) | 27 (2.3) | 12 (1.6) | .475 |
| Musculoskeletal | 387 (16.2) | 104 (22.5) | 171 (14.4) | 112 (15.2) | <.001 |
| Aches | 303 (12.7) | 94 (20.4) | 131 (11.0) | 78 (10.6) | <.001 |
| Joint pain | 266 (11.1) | 71 (15.4) | 115 (9.7) | 80 (10.8) | <.001 |
| ≥3 symptoms (not including Other) | 507 (21.2) | 131 (28.4) | 258 (21.7) | 118 (16.0) | <.001 |
| Other symptoms | 105 (4.4) | 27 (5.8) | 50 (4.2) | 28 (3.8) | .218 |
| No symptoms | 1347 (56.4) | 219 (47.4) | 696 (58.5) | 432 (58.5) | <.001 |
Abbreviation: HEENT, head/ears/eyes/nose/throat.
Twelve patients were excluded due to missing data at 3 months.
Among specific symptoms, pre-Delta cases had a higher rate of fever, chills, loss of taste/smell, chest pain, shortness of breath, nausea/vomiting, diarrhea, and muscle and joint aches compared with Delta and Omicron. These findings remained consistent in the sensitivity analysis with a 90% variant dominance threshold (Supplementary Appendix 7).
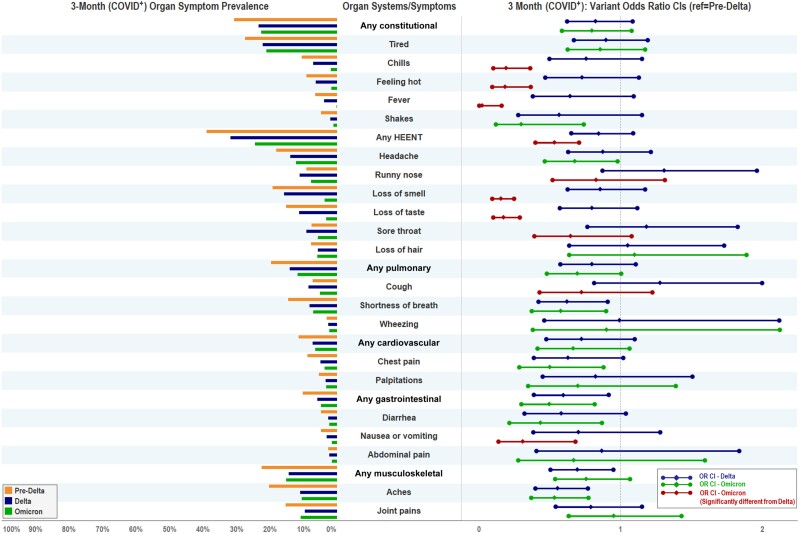
In the multivariable logistic regression of COVID-positive participants, we identified several differences in prolonged symptoms between VOCs (Figure 2). When compared with pre-Delta, Delta had lower odds of dyspnea, gastrointestinal symptoms, musculoskeletal symptoms, and myalgias (Table 4). When compared with pre-Delta, Omicron had lower odds of fever, chills, HEENT symptoms, headache, loss of taste/smell, dyspnea, chest pain, gastrointestinal symptoms, nausea/vomiting, diarrhea, and myalgias. When compared with Delta, Omicron had lower odds of fever, chills, HEENT symptoms, runny nose, loss of taste/smell, sore throat, cough, and nausea/vomiting. A sensitivity analysis using a 90% variant dominance threshold demonstrated no substantive difference in findings apart from the odds ratio for headache between Omicron versus pre-Delta no longer being statistically significant (Supplementary Appendix 8).
Figure 2.
Three-month symptom prevalence and adjusted odds ratios by variants at 3 months among study participants with a positive severe acute respiratory syndrome coronavirus 2 test (COVID+). Model 1 accounted for sociodemographics (age, gender, race, ethnicity), 9 preexisting conditions (specified in Table 1), and hospitalization for coronavirus disease 2019. Pre-Delta was considered as the reference variant period for Delta and Omicron. If the 95% confidence intervals (CIs) included 1, the difference between the paired variant periods was not statistically significant. If the 95% CIs excluded 1, then the risk of adverse outcome in the compared variant period was significantly lower or higher than the reference variant period. The statistically significant difference between Omicron and Delta was based on post hoc test results. Abbreviations: CI, confidence interval; COVID+, positive test for severe acute respiratory syndrome coronavirus 2; HEENT, head/ears/eyes/nose/throat; OR, odds ratio.
Table 4.
Adjusted Association Between Risk of Prolonged Symptoms Among the Variant Time Periods by Severe Acute Respiratory Syndrome Coronavirus 2 Test Status (Model 1)
| Outcome | aOR (95% CI)a | ||||||
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 Positive | SARS-CoV-2 Negative | ||||||
| Organ System | Organ Symptoms | Delta vs Pre-Delta | Omicron vs Pre-Delta | Omicron vs Delta | Delta vs Pre-Delta | Omicron vs Pre-Delta | Omicron vs Delta |
| Constitutional | Any constitutional | 0.82 (.62–1.09) | 0.80 (.59–1.08) | 0.97 (.77–1.22) | 1.35 (.82–2.22) | 1.06 (.64–1.77) | 0.79 (.52–1.19) |
| Tired | 0.89 (.67–1.19) | 0.86 (.63–1.17) | 0.96 (.76–1.22) | 1.23 (.75–2.03) | 0.97 (.58–1.63) | 0.79 (.52–1.20) | |
| Chills | 0.76 (.50–1.15) | 0.19 (.10–.36) | 0.25 (.14–.45) | 0.96 (.45–2.06) | 0.43 (.18–1.06) | 0.45 (.21–.99) | |
| Feeling hot | 0.73 (.47–1.13) | 0.19 (.09–.37) | 0.25 (.14–.47) | 1.26 (.54–2.98) | 0.36 (.12–1.06) | 0.28 (.11–.75) | |
| Fever | 0.64 (.38–1.09) | 0.02 (.00–.16) | 0.03 (.00–.25) | 1.03 (.39–2.70) | 0.24 (.06–.97) | 0.24 (.07–.85) | |
| Shakes | 0.56 (.28–1.15) | 0.30 (.12–.74) | 0.53 (.23–1.21) | 0.59 (.15–2.27) | 0.06 (.01–.68) | 0.11 (.01–1.06) | |
| HEENT | Any HEENT | 0.84 (.65–1.09) | 0.53 (.40–.71) | 0.63 (.50–.79) | 1.38 (.85–2.24) | 1.69 (1.04–2.73) | 1.22 (.83–1.79) |
| Headache | 0.87 (.63–1.21) | 0.68 (.47–.98) | 0.77 (.58–1.04) | 1.09 (.62–1.93) | 1.16 (.66–2.06) | 1.07 (.67–1.70) | |
| Runny nose | 1.31 (.87–1.96) | 0.83 (.52–1.31) | 0.63 (.45–.89) | 1.07 (.56–2.03) | 1.22 (.65–2.30) | 1.14 (.68–1.91) | |
| Loss of smell | 0.86 (.63–1.17) | 0.15 (.09–.25) | 0.18 (.12–.28) | 4.94 (1.03–23.7) | 1.25 (.21–7.51) | 0.25 (.08–.82) | |
| Loss of taste | 0.80 (.57–1.12) | 0.17 (.10–.29) | 0.22 (.14–.34) | 2.64 (.83–8.33) | 0.97 (.26–3.57) | 0.37 (.14–.98) | |
| Sore throat | 1.18 (.77–1.83) | 0.65 (.39–1.08) | 0.55 (.37–.81) | 1.43 (.75–2.76) | 0.64 (.31–1.32) | 0.44 (.24–.81) | |
| Loss of hair | 1.05 (.64–1.73) | 1.10 (.64–1.89) | 1.04 (.69–1.58) | 1.18 (.32–4.41) | 2.60 (.78–8.63) | 2.20 (.84–5.74) | |
| Pulmonary | Any pulmonary | 0.80 (.57–1.11) | 0.69 (.48–1.00) | 0.87 (.65–1.17) | 1.06 (.58–1.91) | 0.59 (.31–1.12) | 0.55 (.32–.97) |
| Cough | 1.28 (.81–2.00) | 0.72 (.43–1.23) | 0.57 (.38–.85) | 0.89 (.44–1.79) | 0.53 (.24–1.14) | 0.59 (.30–1.18) | |
| Shortness of breath | 0.62 (.42–.91) | 0.58 (.37–.90) | 0.93 (.64–1.35) | 1.28 (.58–2.85) | 0.55 (.22–1.37) | 0.43 (.20–.94) | |
| Wheezing | 0.99 (.46–2.12) | 0.90 (.38–2.12) | 0.91 (.48–1.74) | 0.65 (.19–2.18) | 0.41 (.11–1.56) | 0.64 (.18–2.26) | |
| Cardiovascular | Any cardiovascular | 0.72 (.48–1.10) | 0.66 (.41–1.06) | 0.92 (.62–1.36) | 0.61 (.27–1.37) | 0.67 (.30–1.50) | 1.11 (.52–2.37) |
| Chest pain | 0.63 (.39–1.02) | 0.50 (.28–.88) | 0.80 (.49–1.30) | 0.65 (.25–1.74) | 0.41 (.13–1.22) | 0.62 (.22–1.76) | |
| Palpitations | 0.82 (.45–1.51) | 0.70 (.35–1.39) | 0.85 (.48–1.49) | 0.31 (.10–.98) | 0.70 (.27–1.83) | 2.26 (.76–6.72) | |
| Gastrointestinal | Any gastrointestinal | 0.60 (.39–.92) | 0.50 (.30–.82) | 0.83 (.54–1.27) | 1.03 (.51–2.09) | 1.19 (.59–2.39) | 1.15 (.64–2.06) |
| Diarrhea | 0.58 (.32–1.04) | 0.43 (.22–.87) | 0.75 (.41–1.38) | 1.11 (.45–2.73) | 1.13 (.46–2.78) | 1.02 (.48–2.15) | |
| Nausea or vomiting | 0.70 (.39–1.28) | 0.31 (.14–.68) | 0.44 (.22–.87) | 0.78 (.32–1.91) | 0.37 (.13–1.06) | 0.47 (.17–1.29) | |
| Abdominal pain | 0.87 (.41–1.84) | 0.67 (.28–1.60) | 0.77 (.38–1.56) | 0.46 (.14–1.49) | 1.01 (.36–2.82) | 2.20 (.77–6.31) | |
| Musculoskeletal | Any musculoskeletal | 0.69 (.51–.95) | 0.76 (.54–1.07) | 1.09 (.83–1.43) | 0.93 (.53–1.61) | 0.79 (.44–1.39) | 0.85 (.52–1.39) |
| Aches | 0.55 (.40–.77) | 0.54 (.37–.77) | 0.97 (.71–1.32) | 0.93 (.52–1.66) | 0.51 (.27–.95) | 0.55 (.31–.96) | |
| Joint pain | 0.79 (.54–1.15) | 0.95 (.64–1.43) | 1.21 (.88–1.66) | 1.02 (.52–2.00) | 0.97 (.49–1.92) | 0.95 (.54–1.70) | |
| Presence of ≥3 symptoms | 0.85 (.64–1.13) | 0.58 (.42–.80) | 0.68 (.52–.87) | 1.12 (.67–1.85) | 0.87 (.51–1.47) | 0.78 (.50–1.21) | |
| Fatigue Severity Score ≥25 | 0.71 (.49–1.02) | 0.81 (.55–1.19) | 1.13 (.83–1.54) | 1.24 (.73–2.11) | 0.87 (.50–1.52) | 0.70 (.44–1.12) | |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HEENT, head/ears/eyes/nose/throat; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Adjusted for age, gender, race, ethnicity, preexisting comorbidities, and hospitalization; bold font denotes statistical significance.
While adjustment for vaccination status (model 2) did not attenuate symptom differences for pre-Delta versus Delta, vaccination status did attenuate differences in Omicron versus pre-Delta for headache, chest pain, and diarrhea (Supplementary Appendix 5). Additionally, the presence of ≥3 symptoms no longer differed by VOCs among COVID-positive participants once vaccination status was included in the model.
In the multivariable logistic regression model of COVID-negative participants (adjusting for the same covariates except hospitalization), substantially fewer differences were found in symptoms across variant time periods with minimal areas of overlap (Table 4, Supplementary Appendix 9). When comparing COVID-positive with COVID-negative cohorts, none of the Delta versus pre-Delta differences in symptoms overlapped. In Omicron versus pre-Delta, only the differences in fevers, shakes, and myalgias overlapped. In Omicron versus Delta, differences in fever, loss of taste/smell, and sore throat had overlap.
DISCUSSION
In this large prospective cohort study of participants tested for SARS-CoV-2, we identified higher rates of prolonged severe fatigue and a greater number of prolonged symptoms at 3 months in participants infected during the pre-Delta timeframe compared with Delta or Omicron. Although these associations were significant in unadjusted analyses, they were not significant after adjusting for sociodemographics, clinical characteristics, and vaccination status, suggesting that differences in prolonged symptoms between variants might be a function of these factors in addition to or perhaps instead of characteristics of each variant. However, differences in the frequency of a few prolonged symptoms between the variants remained significant for chills, feeling hot, shakes, loss of taste/smell, cough, dyspnea, nausea/vomiting, and aches, which might be more VOC specific.
This study had several strengths compared with prior literature. We used a prospective cohort, rather than relying on retrospective data which can be subject to selection bias and limited symptom ascertainment from EHR data collected in routine care. We collected data directly using participant self-report at baseline and 3 months rather than relying on provider-documented symptoms. Therefore, our study captures a more complete symptom list and outcomes among patients who may commonly seek or receive care in settings without a single EHR. Our study is one of a limited number evaluating both symptoms and fatigue severity at 3 months postinfection across variants. Prior studies have focused on Delta and Omicron, with more limited data on pre-Delta for comparison. We performed sensitivity analyses using 90% thresholds, as well as using symptomatic COVID-negative cohorts to account for potential confounders. The inclusion of COVID-negative comparators is important to help differentiate the role of specific SARS-CoV-2 variants from the impact of other external factors (eg, societal impact of a pandemic, exacerbation of preexisting conditions with less access to healthcare) during each time period. Finally, we accounted for key variables, such as vaccination status, and identified an attenuation of both severe fatigue and presence of ≥3 symptoms, possibly due to vaccination.
Fatigue is a commonly reported initial and persistent symptom that has received special attention among patients with COVID-19 [13, 21, 22]. However, fatigue can vary from mild to debilitating and present with an array of symptoms; therefore, it is important to use validated tools to quantify presence and severity [23]. One large study comparing initial symptoms among Delta versus Omicron variants reported lower rates of fatigue early in the Omicron period [24]. We built upon this work by specifically quantifying fatigue severity and identifying differences in persistent fatigue 3 months postinfection, which was not performed in the prior study.
Whereas in the unadjusted models, prolonged severe fatigue was greatest among COVID-positive patients in the pre-Delta period, these differences were absent after accounting for potentially confounding variables including sociodemographics and vaccination status. This might be explained by baseline differences between participants contributing to higher rates of postevent symptoms, including prior hospitalization and preexisting comorbidities. Prior research has identified an association between sociodemographics and preexisting comorbidities and development of long COVID [25]. Additionally, while Omicron has milder acute symptoms, post-COVID severe fatigue did not appear to be significantly lessened between variants after factoring in sociodemographics and vaccination, suggesting that the prolonged course might differ from acute symptoms. Vaccination might have mitigated the difference across variants and reduced the severity of fatigue experienced. Prior research has demonstrated a reduction in long COVID among vaccinated patients (without known variant status) [26–32]. However, there was a significantly elevated rate of vaccination in our Omicron patient population (98% of participants) compared to the other variants and some evidence of collinearity between vaccination status and variant time period. This reflects the challenges of real-world pandemic data (including vaccine release timing), and we cannot fully disentangle the impact of vaccination from other variant-related factors.
Interestingly, we identified a higher rate of prolonged fatigue in COVID-negative participants compared with COVID positive. While the reason for this finding is not fully apparent, this may reflect recognition of longer-term fatigue from other infectious conditions prompting these participants to get testing [15]. It is also possible that this may be influenced by the pandemic itself, including the psychosocial impact, isolation, and physical inactivity leading to deconditioning. Despite this, we did not identify a significant difference in fatigue symptoms or severity across variant time periods in the COVID-negative group, suggesting that specific time periods did not appear to influence this.
We identified differences in the number and range of prolonged symptoms at 3 months between variants. Even after adjusting for baseline differences, lower rates of symptoms involving ≥3 organ systems in the Omicron cohort compared with pre-Delta or Delta persisted. Notably, this differed from prolonged fatigue, which was more common in the pre-Delta group. However, these differences were absent after adjustment for vaccination, suggesting a potential benefit on prolonged symptoms. The equalization of symptoms across time periods was similar in the COVID-negative cohort, suggesting that these symptoms may be a pandemic footprint and COVID-positive symptoms that remained unique may be the true long COVID footprint. Prior studies have reported differences in initial symptoms (particularly loss of taste/smell) with Omicron [24, 33]. Our study builds upon this by demonstrating lower frequency at 3 months of several other symptoms, including persistent fever, chills, and multiple HEENT symptoms (beyond taste/smell). Antonelli and colleagues [34] reported lower rates of long COVID (defined as any symptom at 4 weeks). Our study adds to this by providing data on specific symptoms, controlling for more confounders, and extending follow-up to 3 months, allowing better characterization of prolonged symptoms across variants.
There were several limitations to this study. Most participants did not receive viral strain testing, which might have led some to be misclassified with the incorrect variant. However, we believe this risk is low given the short time from emergence to dominance of VOCs. We also conducted a sensitivity analysis looking at only those time periods with near-exclusive VOC dominance, with no significant difference in our findings. False-negative SARS-CoV-2 test results might also have led to misclassification. This study required participants have access to an internet-capable device, which might have led to selection bias. It is possible that the findings may reflect differences in populations enrolled across time periods. To lessen this risk, we accounted for many epidemiologic, sociodemographic, and clinical variables in our adjusted analyses. We also had differential responder rates to the follow-up survey at 3 months, with a higher proportion of nonresponders having been impacted by social determinants of health; therefore, these results may be less generalizable to that population. Nearly one-third of potential participants did not complete enrollment, and it is possible this group may differ from those who chose to complete enrollment. The rates of symptoms identified in our study are higher than some other studies, which may reflect differences in population, symptom resolution timeframe (eg, some symptoms may resolve after the 3-month timeframe), and varied definitions of long COVID. Further data are needed on longer-term outcomes to ascertain symptom distribution and course over extended timeframes. Finally, data on vaccination timing, doses, and specific vaccine type were incomplete, so we utilized a more limited definition for vaccination status (≥1 dose), which might not capture subtle differences between vaccine regimens.
CONCLUSIONS
Our study demonstrated that post-COVID conditions differed across SARS-CoV-2 variants. While higher rates of prolonged severe fatigue and multiple symptoms during pre-Delta compared with Delta and Omicron were found, these differences disappeared after accounting for sociodemographics and vaccination status. We also identified distinct differences in some prolonged symptoms between variants, which remained relatively consistent even after accounting for covariates and comparing with symptomatic COVID-negative participants who had stable rates of symptoms across the time periods. Our study suggests that newer variants such as Delta and Omicron might have a different distribution of symptoms postillness, but no significant difference in fatigue severity or symptom quantity after accounting for vaccination status. Despite this, persistent symptoms remain common at 3 months post–COVID-19 among our COVID-positive and COVID-negative cohorts, with 1 in 8 participants reporting severe fatigue.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Michael Gottlieb, Department of Emergency Medicine, Rush University Medical Center, Chicago, Illinois, USA.
Ralph C Wang, Department of Emergency Medicine, University of California, San Francisco, California, USA.
Huihui Yu, Center for Outcomes Research and Evaluation, Yale School of Medicine, New Haven, Connecticut, USA; Section of Cardiovascular Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Erica S Spatz, Center for Outcomes Research and Evaluation, Yale School of Medicine, New Haven, Connecticut, USA; Department of Epidemiology, Yale School of Public Health, New Haven, Connecticut, USA.
Juan Carlos C Montoy, Department of Emergency Medicine, University of California, San Francisco, California, USA.
Robert M Rodriguez, Department of Emergency Medicine, University of California – San Francisco School of Medicine, San Francisco, California, USA.
Anna Marie Chang, Department of Emergency Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Joann G Elmore, Division of General Internal Medicine and Health Services Research, David Geffen School of Medicine, University of California – Los Angeles, Los Angeles, California, USA.
Paavali A Hannikainen, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Mandy Hill, Department of Emergency Medicine, UTHealth Houston, Houston, Texas, USA.
Ryan M Huebinger, Department of Emergency Medicine, UTHealth Houston, Houston, Texas, USA.
Ahamed H Idris, Department of Emergency Medicine, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Zhenqiu Lin, Center for Outcomes Research and Evaluation, Yale School of Medicine, New Haven, Connecticut, USA; Section of Cardiovascular Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Katherine Koo, Department of Medicine, Division of Infectious Diseases, Rush University Medical Center, Chicago, Illinois, USA.
Samuel McDonald, Department of Emergency Medicine, University of Texas Southwestern Medical Center, Dallas, Texas, USA; Clinical Informatics Center, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Kelli N O’Laughlin, Department of Global Health, University of Washington, Seattle, Washington, USA.
Ian D Plumb, National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Michelle Santangelo, Department of Medicine, Division of Infectious Diseases, Rush University Medical Center, Chicago, Illinois, USA.
Sharon Saydah, National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Michael Willis, Department of Global Health, University of Washington, Seattle, Washington, USA.
Lauren E Wisk, Division of General Internal Medicine and Health Services Research, David Geffen School of Medicine, University of California – Los Angeles, Los Angeles, California, USA.
Arjun Venkatesh, Center for Outcomes Research and Evaluation, Yale School of Medicine, New Haven, Connecticut, USA; Department of Emergency Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Kari A Stephens, Department of Biomedical Informatics and Medical Education, University of Washington, Seattle, Washington, USA; Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, Washington, USA.
Robert A Weinstein, Department of Medicine, Division of Infectious Diseases, Rush University Medical Center, Chicago, Illinois, USA; Department of Medicine, Division of Infectious Diseases, Cook County Hospital, Chicago, Illinois, USA.
for the Innovative Support for Patients with SARS-CoV-2 Infections Registry (INSPIRE) Group:
Robert A Weinstein, Michael Gottlieb, Michelle Santangelo, Katherine Koo, Antonia Derden, Michael Gottlieb, Kristyn Gatling, Diego Guzman, Geoffrey Yang, Marshall Kaadan, Minna Hassaballa, Ryan Jerger, Zohaib Ahmed, Michael Choi, Arjun Venkatesh, Erica Spatz, Zhenqiu Lin, Shu-Xia Li, Huihui Yu, Imtiaz Ebna Mannan, Zimo Yang, Arjun Venkatesh, Erica Spatz, Andrew Ulrich, Jeremiah Kinsman, Jocelyn Dorney, Senyte Pierce, Xavier Puente, Graham Nichol, Kari Stephens, Jill Anderson, Dana Morse, Karen Adams, Zenoura Maat, Tracy Stober, Kelli N O'Laughlin, Nikki Gentile, Rachel E Geyer, Michael Willis, Luis Ruiz, Kerry Malone, Jasmine Park, Kristin Rising, Efrat Kean, Morgan Kelly, Kevin Schaeffer, Paavali Hannikainen, Lindsey Shughart, Hailey Shughart, Nicole Renzi, Grace Amadio, Dylan Grau, Phillip Watts, David Cheng, Jessica Miao, Carly Shutty, Alex Charlton, Mandy Hill, Ryan Huebinger Site, Summer Chavez, Arun Kane, Peter Nikonowicz, Ahamed H Idris, Samuel McDonald, David Gallegos, Riley Martin, Joann G Elmore, Lauren E Wisk, Michelle L'Hommedieu, Christopher W Chandler, Megan Eguchi, Kate Diaz Roldan, Raul Moreno, Robert M Rodriguez, Ralph C Wang, Juan Carlos C Montoy, Robin Kemball, Virginia Chan, Cecilia Lara Chavez, Angela Wong, Mireya Arreguin, Ian D Plumb, Aron J Hall, Sharon Saydah, and Melissa Briggs-Hagen
Notes
Acknowledgments. The authors thank the California Department of Public Health for assistance with participant recruitment for this study. The authors also thank the Clinical and Translational Science Institute (CTSI) COVID Clinical Research Steering Committee and the CTSI Office of Clinical Research Patient Navigation Team and Bioinformatics Program for assistance with study recruitment.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the National Center for Immunization and Respiratory Diseases, CDC (contract number 75D30120C08008; principal investigator: R. A. W.). Partners from the CDC (I. D. P. and S. S.) assisted with study design and the preparation of this manuscript.
References
- 1. Centers for Disease Control and Prevention . SARS-CoV-2 variant classifications and definitions. Available at:https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html#concern. Accessed 28 August 2022.
- 2. Taylor CA, Patel K, Pham H, et al. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance—COVID-NET, 14 states, January–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods—United States, December 2020–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nyberg T, Twohig KA, Harris RJ, et al. Risk of hospital admission for patients with SARS-CoV-2 variant B.1.1.7: cohort analysis. BMJ 2021; 373:n1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Houhamdi L, Gautret P, Hoang VT, Fournier P-E, Colson P, Raoult D. Characteristics of the first 1119 SARS-CoV-2 Omicron variant cases, in Marseille, France, November–December 2021. J Med Virol 2022; 94:2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA 2022; 327:583–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 2021; 4:e2128568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernandez-Romieu AC, Leung S, Mbanya A, et al. Health care utilization and clinical characteristics of nonhospitalized adults in an integrated health care system 28–180 days after COVID-19 diagnosis—Georgia, May 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021; 70:644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirschtick JL, Titus AR, Slocum E, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis 2021; 73:2055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021; 4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wisk LE, Gottlieb M, Spatz ES, et al. Association of initial SARS-CoV-2 test positivity with patient-reported well-being 3 months after a symptomatic illness. JAMA Netw Open 2022; 5:e2244486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spatz ES, Gottlieb M, Wisk LE, et al. Three-month symptom profiles among symptomatic adults with positive and negative severe acute respiratory syndrome coronavirus 2 tests: a prospective cohort study from the INSPIRE group. Clin Infect Dis 2023; 76:1559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen AK, Wang X, McCluskey LP, et al. Neuropsychiatric sequelae of long COVID-19: pilot results from the COVID-19 neurological and molecular prospective cohort study in Georgia, USA. Brain Behav Immun Health 2022; 24:100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Laughlin KN, Thompson M, Hota B, et al. Study protocol for the Innovative Support for Patients with SARS-COV-2 Infections Registry (INSPIRE): a longitudinal study of the medium and long-term sequelae of SARS-CoV-2 infection. PLoS One 2022; 17:e0264260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandler CX, Wyller VBB, Moss-Morris R, et al. Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect Dis 2021; 8:ofab440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagner D, Nisenbaum R, Heim C, Jones JF, Unger ER, Reeves WC. Psychometric properties of the CDC symptom inventory for assessment of chronic fatigue syndrome. Popul Health Metr 2005; 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reeves WC, Wagner D, Nisenbaum R, et al. Chronic fatigue syndrome—a clinically empirical approach to its definition and study. BMC Med 2005; 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spudich S, Nath A. Nervous system consequences of COVID-19. Science 2022; 375:267–9. [DOI] [PubMed] [Google Scholar]
- 19. Taylor CA, Whitaker M, Anglin O, et al. COVID-19-associated hospitalizations among adults during SARS-CoV-2 Delta and Omicron variant predominance, by race/ethnicity and vaccination status—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention . COVID data tracker. Available at:https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed 28 August 2022.
- 21. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One 2020; 15:e0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 2021; 4:e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008; 31:1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vihta KD, Pouwels KB, Peto TE, et al. Omicron-associated changes in SARS-CoV-2 symptoms in the United Kingdom. Clin Infect Dis 2022; 76:e133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 2022; 28:1706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ 2022; 377:e069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nehme M, Braillard O, Salamun J, et al. Symptoms after COVID-19 vaccination in patients with post-acute sequelae of SARS-CoV-2. J Gen Intern Med 2022; 37:1585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tran V-T, Perrodeau E, Saldanha J, Pane I, Ravaud P. Efficacy of COVID-19 vaccination on the symptoms of patients with long COVID: a target trial emulation using data from the ComPaRe e-cohort in France. Res Square [Preprint]. February 17, 2022 [cited 2022 Aug 28]. Available from: https://www.researchsquare.com/article/rs-1350429/v1.
- 29. Senjam SS, Balhara YPS, Kumar P, et al. Assessment of post COVID-19 health problems and its determinants in North India: a descriptive cross section study. medRxiv [Preprint] . October 7, 2021 [cited 2022 Aug 28]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.10.03.21264490.
- 30. Zisis SN, Durieux JC, Mouchati C, Perez JA, McComsey GA. The protective effect of coronavirus disease 2019 (COVID-19) vaccination on postacute sequelae of COVID-19: a multicenter study from a large national health research network. Open Forum Infect Dis 2022; 9:ofac228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA 2022; 328:676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022; 28:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet 2022; 399:1618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with Delta versus Omicron variants of SARS-CoV-2. Lancet 2022; 399:2263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.