Abstract
Background
Safer, better, and shorter treatments for multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) are an urgent global health need. The phase 3 clinical trial Nix-TB (NCT02333799) tested a 6-month treatment of MDR and XDR-TB consisting of high-dose linezolid, bedaquiline, and pretomanid (BPaL). In this study, we investigate the relationship between the pharmacokinetic characteristics of the drugs, patient characteristics and efficacy endpoints from Nix-TB.
Methods
Pharmacokinetic data were collected at weeks 2, 8, and 16. Efficacy endpoints including treatment outcomes, time to stable culture conversion, and longitudinal time to positivity in the mycobacterial growth indicator tube assay were each characterized using nonlinear mixed-effects modeling. Relationships between patient, treatment pharmacokinetics, and disease characteristics and efficacy endpoints were evaluated.
Results
Data from 93 (85% of the total) participants were analyzed. Higher body mass index was associated with a lower incidence of unfavorable treatment outcomes. Median time to stable culture conversion was 3 months in patients with lower baseline burden compared with 4.5 months in patients with high baseline burden. Participants with minimal disease had steeper time to positivity trajectories compared with participants with high-risk phenotypes. No relationship between any drugs’ pharmacokinetics (drug concentration or exposure metrics) and any efficacy outcomes was observed.
Conclusions
We have successfully described efficacy endpoints of a BPaL regimen from the Nix-TB trial. Participants with high-risk phenotypes significantly delayed time to culture conversion and bacterial clearance. The lack of a relationship between pharmacokinetic exposures and pharmacodynamic biomarkers opens the possibility to use lower, safer doses, particularly for toxicity-prone linezolid.
Clinical Trials Registration
Keywords: efficacy, NixTB, linezolid, bedaquiline, pretomanid
Baseline disease burden and high-risk group significantly delayed time to culture conversion and bacterial clearance in patients with multidrug- and extensively drug-resistant tuberculosis. A relationship between drug exposure and biomarkers could not be established.
The development of better and safer treatments for tuberculosis (TB) is an urgent global health need, especially for extensively drug-resistant (XDR) TB (pre-2021 change in XDR definition), and multidrug-resistant (MDR) TB [1]. At the time of the Nix-TB trial, XDR was defined as TB that is resistant to any fluoroquinolone and to at least 1 of 3 second-line injectable drugs (capreomycin, kanamycin, and amikacin) in addition to multidrug resistance. In Nix-TB [2], a phase 3 clinical trial (NCT02333799) that evaluated combination therapy with high-dose linezolid (1200 mg), bedaquiline, and pretomanid (BpaL regimen) for 6 months against highly resistant TB, a treatment success rate of 90% was recently reported. This success rate greatly exceeded the 38% reported in MDR and 14% in XDR-T B [2, 3]; however, the use of this regimen was associated with a high rate of anemia and peripheral neuropathy.
Based on the safety and efficacy data reported from this trial, the US Food and Drug Administration and the European Medicines Agency approved BpaL for the treatment of XDR-TB and MDR-TB (before the change in definition of MDR and XDR) [4–7]. From this treatment, both bedaquiline and pretomanid are newly developed anti-TB drugs, whereas linezolid is repurposed from its indication against gram-positive bacterial infections. Linezolid use is associated with drug concentration–related myelosuppression and neuropathy, which are often treatment limiting and related to dose reductions and interruptions [8–11], and have been observed in this trial, urging exploration of lower doses of linezolid.
In this study, we evaluated the relationships between bedaquiline, pretomanid, and linezolid pharmacokinetics (PK) and individual patient characteristics and efficacy endpoints.
METHODS
Patient Population
We evaluated adults with smear-positive pulmonary MDR- or XDR-TB enrolled in the Nix-TB trial (NCT02333799). The detailed clinical trial design and participant characteristics have been previously described [2].
Pharmacokinetic Data
All patients received 26 weeks of oral treatment consisting of bedaquiline at a dose of 400 mg once daily for 2 weeks followed by 200 mg 3 times weekly for 24 weeks, plus 200 mg of pretomanid daily for 26 weeks, and 1200 mg linezolid daily for 26 weeks. Dose adjustments for linezolid due to adverse events were made at the discretion of the investigators. Complete dosing histories were accounted for in the analysis. A predose sampling was collected for all patients at weeks 2, 8, and 16 to measure trough concentration of the 3 drugs.
Microbiologic Data
Two sputum samples were obtained for smear microscopy and culture by means of the mycobacterial growth indicator tube (MGIT) method [12] from all the patients at baseline; weeks 1, 2, 4, 6, and 8; and then monthly through week 26. The MGIT assay produces readouts of the time required to detect active oxygen consumption (time-to-positivity [TTP]) in sputum samples cultured in liquid medium. Higher TTP values are representative of lower bacterial burden in culture samples. TTP is censored at 42 days, representing the upper limit of quantification (ULOQ) of the assay and a negative culture result. All available data from sputum cultures that were found to be positive for MTB and not contaminated with other microorganisms were included in the analyses.
Individual Risk Assessment
Individual continuous risk scores were calculated using baseline predictors of TB-related outcomes that had been previously reported as significant [13, 14]. The characteristics included for the calculation of the individual risk were human immunodeficiency virus (HIV) status, sex, body mass index (BMI), and cavities on chest X-ray. Participants with risk scores within the first quartile of the risk-score distribution were defined as low risk, participants within the first and third quartile were defined as moderate risk, and participants within the fourth quartile were defined as high risk of poor TB outcomes.
Efficacy Endpoints
Primary Efficacy Endpoint: Treatment Failure, Disease Relapse, or Death
The primary endpoint was the incidence of an unfavorable outcome, defined as treatment failure, disease relapse, or death. Patients were considered to have a favorable outcome if their clinical TB disease had resolved, they had a negative culture status at 6 months after the end of the therapy, and they had not already been classified as having had an unfavorable outcome. Patient, treatment, and disease characteristics, including risk scores, were explored as predictors of the primary efficacy endpoint through logistic regression analysis.
Time to Stable Culture Conversion
Time to stable culture conversion (TSCC) was defined as 2 consecutive culture-negative samples without any positive culture afterwards and was described with parametric time-to-event models, allowing the identification of the hazard or probability of having a stable culture conversion over time.
Various hazard distributions were explored: exponential, Weibull, log-logistic, or Gompertz. Patient, treatment, and disease characteristics were evaluated as potential modulators of parameters in the best-fit hazard distribution function.
Time to Positivity in MGIT Assay
Nonlinear mixed-effects modeling was used to describe longitudinal TTP from baseline to 6 months. The model predicted the time-on-treatment patients required to remain culture negative until 42 days, when they were censored. Different models were tested and evaluated for fit, stability, and parsimony. The likelihood of censored observations being greater than the ULOQ was calculated [15, 16]. Due to the dynamics of this continuous biomarker, the correct description of the TTP data required the use of a nonparametric estimation of the parameters involved in the model. This allowed the identification of nonnormal parameter distributions and the decrease of bias and imprecision. Baseline disease burden was defined as baseline TTP.
Covariate Selection
The stepwise covariate model-building procedure implemented in Pearl Speaks NONMEM software [17] was used to build the covariate models for treatment failure, TTP, and TSCC. This procedure is based on a forward-inclusion followed by a backward-deletion approach, and during these, the levels of significance used to incorporate and keep the covariate in the model were set to .05 and .01, respectively. Age, sex, BMI, HIV status, presence of cavities on X-ray, and risk scores were tested as patient characteristics. Dynamic concentrations of linezolid, bedaquiline, and pretomanid; individual daily exposure; and cumulative area under the concentration-time curve (AUC) were tested as treatment characteristics, and baseline disease burden and culture conversion status at 2 months were tested as disease characteristics.
Model Selection Criteria and Model Evaluation
Selection among models was based on (1) significance based on log-likelihood testing, (2) precision of parameter estimates, and (3) results of model performance judged by visual exploration of the goodness-of-fit plots and visual predictive checks.
RESULTS
Patient Population
Of the 109 adults who were originally enrolled in the Nix-TB trial, 16 participants (15%) did not have positive baseline cultures and were therefore excluded from these analyses. Table 1 summarizes patient characteristics for the 93 participants included. Most participants (67%) had XDR-TB and the remaining had MDR-TB (33%).
Table 1.
Baseline Patient Characteristics
| Parameter | Cohort (N = 93) |
|---|---|
| Age, y | 35 (17–60) |
| Weight, kg | 55 (29–112) |
| BMI, kg/m2 | 19.70 (12.40–41.1) |
| Sex | |
| Male | 47 (51%) |
| Female | 46 (49%) |
| HIV status | |
| Negative | 49 (53%) |
| Positive | 44 (47%) |
| Tuberculosis form | |
| MDR | 31 (33%) |
| XDR | 62 (67%) |
| Cavities on chest X-ray | |
| No cavities | 14 (15%) |
| Unilateral cavities | 40 (43%) |
| Bilateral cavities | 39 (42%) |
| Karnofsky score | |
| 100 | 6 (6%) |
| 90 | 39 (42%) |
| 80 | 28 (30%) |
| 70 | 18 (19%) |
| 60 | 2 (2%) |
Data are presented as n (%) or median (range). Percentages may not total 100 because of rounding. BMI is the weight in kilograms divided by the square of the height in meters. The Karnofsky score ranges from 0 to 100, with lower scores indicating greater disability. Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; MDR, multidrug resistant; XDR, extensively drug resistant.
Pharmacokinetic Data
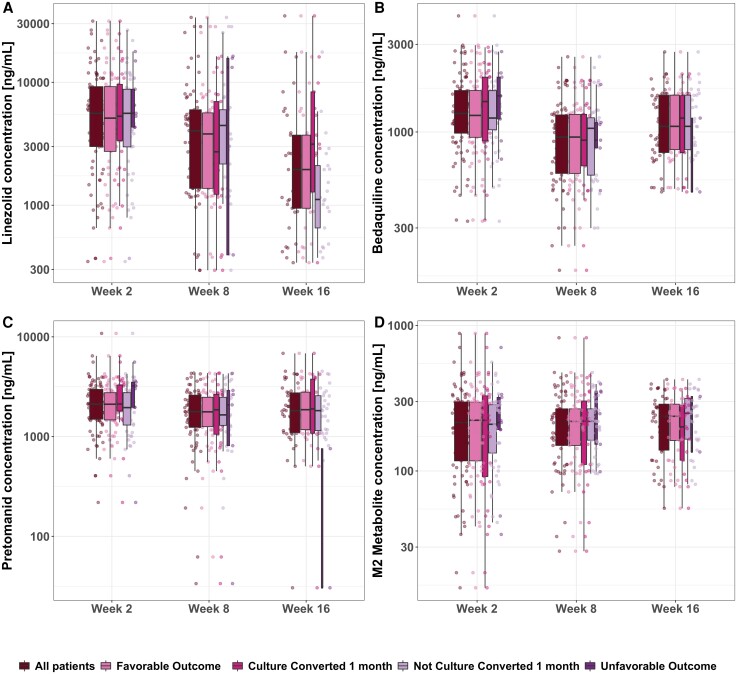
Trough concentration levels of bedaquiline and its metabolite M2, linezolid, and pretomanid at weeks 2, 8, and 16 were obtained for all subjects and are shown in Figure 1. For bedaquiline the mean and trough range concentrations were 1418.3 ng/mL (326.3–4288.4 ng/mL), 1008.1 ng/mL (176.4–2567.5 ng/mL), and 1209.4 ng/mL (314.6–4429.5 ng/mL) at weeks 2, 8, and 16, respectively. The reported linezolid trough means and ranges at weeks 2, 8, and 16 were 6955.6 ng/mL (358.4–31 489 ng/mL), 5792.3 ng/mL (295.6–33 716) ng/mL, and 4046.3 ng/mL (340.94–45 101) ng/mL, respectively. Pretomanid trough mean concentrations and ranges were 2359.3 ng/mL (218.6–6444.9 ng/mL), 1922.3 ng/mL (33.5–5388.7 ng/mL), and 2121.64 ng/mL (30.5–6831.6 ng/mL) at weeks 2, 8, and 16, respectively.
Figure 1.
No relationships between drug concentration and efficacy endpoints were identified in the data from the Nix-TB trial. Trough concentrations of linezolid (A), bedaquiline (B), pretomanid (C), and bedaquiline metabolite M2 (D) on the different visits that a sample was obtained. Overall data are shown in the first boxplot at all visits, and then data are split by outcome and by culture-conversion status at first month. Box width is proportional to sample size on each group.
Linezolid levels, in general, were higher at week 2, when it is unlikely that dose reductions or interruptions have occurred, in contrast to weeks 8 and 16. A drop in bedaquiline concentration can also be observed due to the intensive loading dose in the first 2 weeks of the treatment. This, combined with the fact that bedaquiline steady state is not reached because of its extremely long terminal half-life (6 months), explains the observed drop in bedaquiline trough concentrations. Trough levels were steady throughout the treatment for pretomanid and the bedaquiline metabolite M2. No correlation between individual-level troughs among all drugs was found (R2: 0.06 to 0.28).
Primary Efficacy Endpoint: Treatment Failure, Disease Relapse, or Death
At 6 months after the end of treatment, the number of patients classified as having a favorable outcome was 85 (91%). Of the 8 patients (9%) who had an unfavorable outcome, 6 died during treatment and 2 experienced relapses during follow-up.
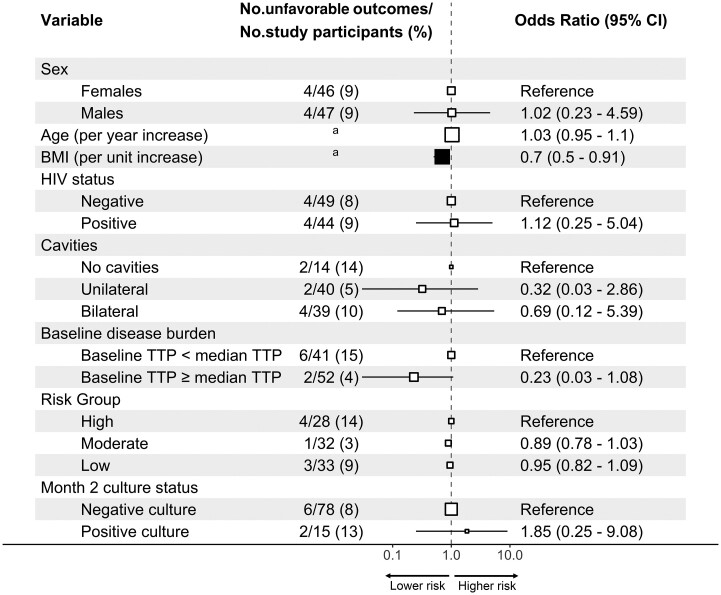
No relationship between any of the regimens’ drug exposure and treatment outcome was found (Figure 1), since PK levels in people with unfavorable outcomes were indistinguishable from the PK levels in people with favorable outcomes. Univariate analysis of the relationship between outcomes and patient- and disease-related factors is shown in Figure 2. Higher baseline BMI was associated with a lower incidence of unfavorable outcomes (P < .05). Higher TTP at baseline, an indication of lower baseline disease burden, showed a trend to lower incidence of unfavorable outcomes (P = .05).
Figure 2.
BMI univariately predicts unfavorable outcomes. Univariate analysis for the patients included in the analysis is shown. Odds ratios with 95% Wald CIs are reported. The size of the square denotes the relative sample size according to variable. Filled squares differentiate statistically significant variables. aAge < 35 years, 3 of 46 (7%) unfavorable outcomes, and age ≥35 y, 5 of 47 (11%) unfavorable outcomes; BMI <19.7 kg/m2, 7 of 47 (9%) unfavorable outcomes, and BMI ≥19.7 kg/m2, 1 of 46 (2%) unfavorable outcomes. Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; TTP, time to positivity.
Time to Stable Culture Conversion
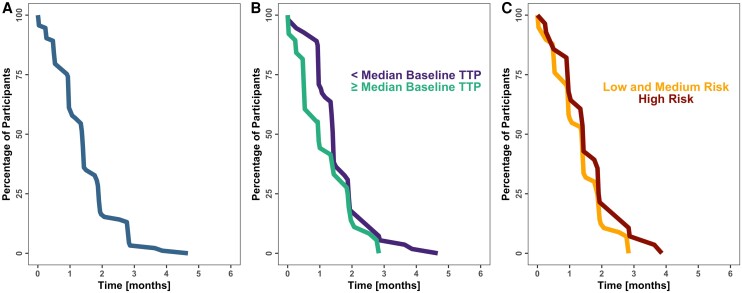
Figure 3 shows TSCC for the entire population (Figure 3A) and stratified by baseline disease burden (Figure 3B) or risk group (Figure 3C). Observed and model-based analysis showed that participants with a high baseline disease burden (TTP ≤ the median of 16 days) reached stable culture conversion at 4.5 months, whereas participants with a low baseline disease burden (TTP > 16 days) reached stable culture conversion by 3 months of treatment. The hazard of TSCC was best described by a Weibull distribution, and baseline disease burden showed statistically significant effects on the baseline hazard (P < .01) and shape parameters. Risk groups were also tested in the model but were not significant.
Figure 3.
Baseline bacterial burden predicts time to stable culture conversion. (A) Time to stable culture conversion. (B) Time to stable culture conversion stratified by the value of the disease burden at the baseline visit. Median baseline TTP is equal to 16 days. (C) Time to stable culture conversion stratified by risk subgroups. Abbreviation: TTP, time to positivity.
Drug-exposure measures, such as individual daily exposure and cumulative AUC up to 2 months for all of the drugs, were also tested. The inclusion of markers of drug exposure did not improve the model fit (P > .05) and were not included in the final model.
Parameter estimates of the TSCC model are listed in Table 2, and model equations are shown in the Supplementary Material. Results shown in Supplementary Figure 1 indicate that the model describes well the overall and stratified TSCC data.
Table 2.
Parameter Estimates With Uncertainty of the Final Models
| Parameter | Estimate (%RSE) [95% CI] | IIV [%RSE] [95% CI] |
|---|---|---|
| Time-to-culture-negative-status model | ||
| Base | .031 (5.04) [.029–.035] | … |
| Shape | 1.61 (7.18) [1.48–1.90] | … |
| Baseline TTP effect on shapea | −.018 (26.13) [−.019–.004] | … |
| Baseline TTP effect on baseb | .0095 (24.89) [.0012–.019] | … |
| TTP model | ||
| Baseline low risk, days | 25.81 (23.87) [16.44–41.35] | 79.79 (23.81) [61.73–100.10] |
| Baseline moderate risk, days | 15.53 (14.15) [11.73–20.63] | 79.79 (23.81) [61.73–100.10] |
| Baseline high risk, days | 11.11 (19.58) [7.90–16.10] | 79.79 (23.81) [61.73–100.10] |
| Slope low risk, days−1 | 1.75 (23.18) [1.18–2.78] | 63.61 (36.11) [42.79–85.44] |
| Slope moderate risk, days−1 | 1.13 (13.81) [.89–1.53] | 63.61 (36.11) [42.79–85.44] |
| Slope high risk, days−1 | 1.00 (18.92) [.69–1.45] | 63.61 (36.11) [42.79–85.44] |
| Correlation baseline—slope | 67.85 (24.06) [52.04–89.49] | … |
| RUV | .25 (7.19) [.21–.28] | … |
Interindividual variability measured as % coefficient of variation (CV%). “95% CI” indicates 95% CI after 1000 bootstraps. Parameters “TTP model”: baseline, TTP at asymptotic baseline (pre-study); slope, rate of change; RUV, residual unexplained variability modeled as proportional error. Parameters “Time-to-culture-negative-status model”: base, baseline hazard; shape, rate of change of hazard. Abbreviations: CI, confidence interval; IIV, inter individual variability; RSE, residual standard error; TTP, time-to-positivity.
Baseline TTP effect on shape: (1 + baseline TTP effect on shape) × (individual baseline −16.38).
Baseline TTP effect on base: (1 + baseline TTP effect on base) × (individual baseline −16.38).
Time to Positivity in MGIT Assay
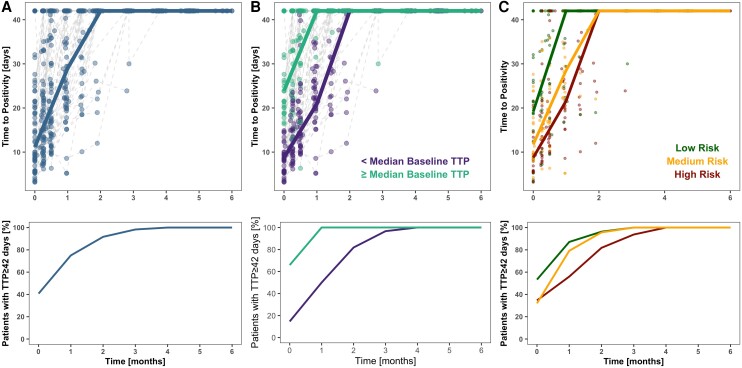
The TTP trajectories were best described by a mixed-effects linear model (Figure 4). All of the parameter estimates are listed in Table 2. The inclusion of risk group as a covariate on both baseline and slope parameters significantly improved the model fit (P < .05). Baseline TTP was highest in the low-risk group (26 days; residual standard error [RSE]: 24%) compared with the moderate-risk (16 days; RSE: 14%) or high-risk (11 days; RSE: 20%) groups. Similarly, slopes varied among risk groups, with higher slopes in the low-risk group (1.75 days−1) versus the moderate-risk (1.13 days−1) or high-risk (1.00 days−1) groups. Baseline TTP value had large interindividual variability with a coefficient of variation of 80%. A significant positive correlation of 68% between individual baseline TTP and slope of the TTP trajectory was identified. This suggests that participants with lower baseline burden also have a faster rate of bacterial clearance. Other disease and patient characteristics tested as predictors in the model were not significant and therefore were not included in the model.
Figure 4.
Baseline bacterial burden and risk groups predict individual trajectory of TTP. (A) Raw TTP longitudinal profiles over 6 months of treatment. (B) Raw TTP longitudinal profiles over 6 months of treatment by baseline disease burden. Median baseline TTP is equal to 16 days. (C) Raw TTP longitudinal profiles over 6 months of treatment stratified by risk group. The upper panels show the actual values of the MGIT assay over time, with lines representing the first quartile of the data, and lower panels show the percentage of patients with a censored measurement (TTP value ≥ 42). Abbreviations: MGIT, mycobacterial growth indicator tube; TTP, time to positivity.
Visual predictive checks in Supplementary Figure 2 indicate that the model fit the TTP data accurately, both the longitudinal trajectories of TTP and the percentage of censored values over time.
Dynamic concentrations of linezolid, bedaquiline, and pretomanid; individual daily exposure; and cumulative AUC up to 2 months of treatment were tested as predictors of TTP trajectories. Interestingly, none of the PK measures included in the analysis were identified as significant. A difference attributable to drug exposure could not be identified on the first day at which there was a TTP value of 42 days or in the longitudinal TTP profiles over time or in the percentage of patients with a TTP value over 42.
DISCUSSION
In this study, our objective was to evaluate the relationship between PK of each drug in the BPaL regimen, patient and disease characteristics, and efficacy endpoints in the Nix-TB trial. Although relationships between PK and efficacy endpoints were not identified, we found that higher baseline disease burden was related to longer time to culture conversion as described by TSCC and TTP trajectories.
The identification of baseline disease burden, measured as baseline TTP, was consistent in both TSCC and TTP models. In the first case, baseline TTP was included in the model as a categorical covariate, as the patients were split into 2 groups depending on their baseline TTP value. Being in the high- or in the low-burden group modified the underlying hazard of having a stable culture conversion. In contrast, baseline TTP value in a continuous manner was found to be positively correlated to the rate of TTP change. Lower values of baseline disease burden were associated with faster achievement of the censored value at TTP and faster TSCC. This falls in line with previous studies that identified patients with higher pretreatment bacillary burdens to be less likely to convert-to-negative than those with a low burden, irrespective of treatment regimen [13, 14, 18, 19].
Previous studies have developed risk algorithms and identified risk groups that are predictors of long-term, TB-related unfavorable outcomes [13, 14]. Using these previously developed algorithms and models, we identified that the same risk groups predicted the individual trajectories of TTP. Faster time to culture conversion was reached in patients in the low-risk group as compared with high-risk patients. Even though the low-risk group of patients was identified to have almost 2.5 times lower baseline TTP and 1.8 times the rate of TTP change as compared with the high-risk group, an impact of the risk group on TSCC could not be identified. This highlights the usefulness of TTP as a biomarker, and the fact that using richer data allows the identification of the same or even more predictors of response earlier. This is supported by previous models of TTP obtained by means of the MGIT assay relating longitudinal TTP measurements and bacterial load [20–24].
Given the wide variation in trough levels that was present in this study (Figure 1), the lack of a PK-pharmacodynamic (PK-PD) relationship might indicate that we are at the maximum of the concentration-effect Emax curve. Using a different methodology and average pretomanid concentration at steady state as the exposure metric, Nedelman et al [25] found a significant PK-PD relationship. However, this relationship was mainly flat in the range of most exposure values, which supports the assumption of having reached the maximum of the concentration-effect curve. The relevance of being at the maximum of the concentration-effect Emax curve is that we are not able to characterize the full concentration-response relationship, since we only see concentrations that are already producing a maximum effect.
Substantial side effects associated with this treatment have been reported, such as myelosuppression, peripheral neuropathy, optic neuritis (attributed to linezolid), QTc prolongation (due to bedaquiline and possibly pretomanid), and hepatotoxicity (caused by bedaquiline and pretomanid) [26, 27], and have been analyzed in depth elsewhere [11]. Unlike bedaquiline and pretomanid, linezolid was reported to produce dose-limiting toxicities throughout the duration of the trial, therefore causing numerous linezolid dose reductions and treatment interruptions. In this regard, the use of more informative concentration-time profiles accounting for dosage adjustments at different time points with respect to the time of initiation of the trial would be useful and might help predict the occurrence of toxic events at the individual level. Regardless, monitoring markers of toxicity throughout treatment can accurately inform and predict toxicities and might be more practical than therapeutic drug monitoring in clinical settings [11]. Therefore, the optimal dosing strategy for the BPaL regimen, especially for linezolid, that balances efficacy and toxicity is still unknown, and optimization of this regimen is still needed. The lack of an exposure–response relationship suggests that lower doses of linezolid can minimize toxicities while retaining comparable efficacy.
The results obtained from the analyses shown in this work are in line with what the ZeNix trial has reported (NCT03086486), a phase 3 clinical trial that has evaluated whether the efficacy of the BPaL drug regimen as shown in the Nix-TB trial can be maintained while reducing toxicity, through lower doses and shorter duration of linezolid. The results of this trial have revealed that the BPaL regimen remains effective against highly drug-resistant strains of TB with either reduced linezolid dosage or duration, limiting its toxicity [28]. In terms of efficacy, favorable outcomes at a linezolid dose of 1200 mg for 26 weeks or 9 weeks or 600 mg for 26 weeks or 9 weeks were reported to be 93%, 89%, 91%, and 84%, respectively. Even though the ZeNix trial was never powered to show efficacy, this has provided evidence that the BPaL regimen could be optimized.
In this work, we analyzed clinical outcome (categorical variable) and 2 biomarkers: TSCC (time-to-event variable) and the TTP biomarker (continuous longitudinal variable). These 3 variables differ in their data informativeness, with continuous TTP trajectory being the richest in information. This was indeed confirmed by the knowledge gained in the analysis of TTP data. The analysis of both TTP and TSCC revealed important risk factors for slower bacterial clearance and delayed culture conversion, which both can put patients at risk of TB treatment failure, in addition to lower BMI, as identified in the primary outcome analysis. However, more data are needed to recommend the use of TTP as a surrogate marker of efficacy.
The most important limitation of this analysis is the small sample size available. Trials involving a greater number of individuals and more informative concentration-time profiles would be useful to establish an optimal use of this regimen for patients with MDR- and XDR-TB.
In this study we have shown how the modeling of continuous longitudinal biomarkers might facilitate the identification of patient, disease, or treatment characteristics associated with delayed efficacious response at baseline than otherwise feasible. In addition, we have discussed that there is still room for establishing an optimal BPaL regimen that balances efficacy and safety.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Belén P Solans, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco Schools of Pharmacy and Medicine, San Francisco, California, USA; UCSF Center for Tuberculosis, University of California San Francisco, San Francisco, California, USA.
Marjorie Z Imperial, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco Schools of Pharmacy and Medicine, San Francisco, California, USA; UCSF Center for Tuberculosis, University of California San Francisco, San Francisco, California, USA.
Morounfolu Olugbosi, TB Alliance New York, New York, New York, USA.
Rada M Savic, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco Schools of Pharmacy and Medicine, San Francisco, California, USA; UCSF Center for Tuberculosis, University of California San Francisco, San Francisco, California, USA.
Notes
Author Contributions. B. P. S., M. Z. I., and R. M. S. contributed to the analysis plan. B. P. S. prepared the data for analysis. B. P. S. performed the modeling and simulation with support from M. Z. I. and R. M. S. B. P. S. drafted the figures, tables, and manuscript, with critical editorial support from M. Z. I., M. O., and R. M. S.
Acknowledgments. The authors thank Jerry Nedelman from TB Alliance for all his help and support during this project.
Financial support. This work was sponsored by TB Alliance.
References
- 1. Centers for Disease Control and Prevention . Tuberculosis data and statistics. Available at: http://www.cdc.gov/tb/statistics/default.htm. Accessed 18 July 2021.
- 2. Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 2020; 382:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Global tuberculosis report 2020. Geneva, Switzerland: World Health Organization, 2020. [Google Scholar]
- 4. Food and Drug Administration . FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-resistant-forms-tuberculosis-affects-lungs. Accessed July 2022.
- 5. World Health Organization . Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). 2019; 9. Available at: https://www.who.int/publications/i/item/WHO-UCN-TB-2022-2. Accessed July 2022.
- 6. European Medicines Agency . Pretomanid FGK. Available at: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/pretomanid-fgk. Accessed July 2022.
- 7. World Health Organization . Meeting report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis, 27–29 October 2020. Geneva, Switzerland: World Health Organization, 2021. Available at: https://apps.who.int/iris/handle/10665/338776. Accessed 13 September 2022. [Google Scholar]
- 8. Wasserman S, Denti P, Brust JCM, et al. Linezolid pharmacokinetics in South African patients with drug-resistant tuberculosis and a high prevalence of HIV coinfection. Antimicrob Agents Chemother 2019; 63:e02164–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wasserman S, Meintjes G, Maartens G. Linezolid in the treatment of drug-resistant tuberculosis: the challenge of its narrow therapeutic index. Expert Rev Anti Infect Ther 2016; 14:901–15. [DOI] [PubMed] [Google Scholar]
- 10. Zhang X, Falagas ME, Vardakas KZ, et al. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis 2015; 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imperial MZ, Nedelman JR, Conradie F, Savic RM. Proposed linezolid dosing strategies to minimize adverse events for treatment of extensively drug-resistant Tuberculosis. Clin Infect Dis 2022; 74:7136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colangeli R, Jedrey H, Kim S, et al. Bacterial factors that predict relapse after tuberculosis therapy. N Engl J Med 2018; 379:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imperial MZ, Nahid P, Phillips PPJ, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med 2018; 24:1708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imperial MZ, Phillips PPJ, Nahid P, Savic RM. Precision-Enhancing risk stratification tools for selecting optimal treatment durations in Tuberculosis clinical trials. Am J Respir Crit Care Med 2021; 204:1086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28:481–504. [DOI] [PubMed] [Google Scholar]
- 16. Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn 2008; 35:401–21. [DOI] [PubMed] [Google Scholar]
- 17. Jonsson E, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res 1998; 15:1463–8. [DOI] [PubMed] [Google Scholar]
- 18. Sabiiti W, Azam K, Farmer ECW, et al. Tuberculosis bacillary load, an early marker of disease severity: the utility of tuberculosis molecular bacterial load assay. Thorax 2020; 75:606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Namugenyi J, Musaazi J, Katamba A, et al. Baseline Xpert MTB/RIF ct values predict sputum conversion during the intensive phase of anti-TB treatment in HIV infected patients in Kampala, Uganda: a retrospective study. BMC Infect Dis 2021; 21:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jayakumar A, Savic RM, Everett CK, et al. Xpert MTB/RIF assay shows faster clearance of Mycobacterium tuberculosis DNA with higher levels of rifapentine exposure. J Clin Microbiol 2016; 54:3028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shenai S, Ronacher K, Malherbe S, et al. Bacterial loads measured by the Xpert MTB/RIF assay as markers of culture conversion and bacteriological cure in pulmonary TB. PLoS One 2016; 11:e0160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diacon AH, Maritz JS, Venter A, van Helden PD, Dawson R, Donald PR. Time to liquid culture positivity can substitute for colony counting on agar plates in early bactericidal activity studies of antituberculosis agents. Clin Microbiol Infect 2012; 18:711–7. [DOI] [PubMed] [Google Scholar]
- 23. Bark CM, Okwera A, Joloba ML, et al. Time to detection of Mycobacterium tuberculosis as an alternative to quantitative cultures. Tuberculosis 2011; 91:257–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chigutsa E, Patel K, Denti P, et al. A time-to-event pharmacodynamic model describing treatment response in patients with pulmonary tuberculosis using days to positivity in automated liquid mycobacterial culture. Antimicrob Agents Chemother 2013; 57:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nedelman JR, Salinger DH, Subramoney V, et al. An exposure-response perspective on the clinical dose of pretomanid. Antimicrob Agents Chemother 2020; 65:e01121–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H, Salinger DH, Everitt D, et al. Long-term effects on QT prolongation of pretomanid alone and in combinations in patients with tuberculosis. Antimicrob Agents Chemother 2019; 63:e00445–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van de Berg SEJ, Pelzer PT, van der Land AJ, et al. Acceptability, feasibility, and likelihood of stakeholders implementing the novel BPaL regimen to treat extensively drug-resistant tuberculosis patients. BMC Public Health 2021; 21:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conradie F, Bagdasaryan TR, Borisov S, et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med 2022; 387:810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.