Abstract
Background
We compared 6 new interferon-γ release assays (IGRAs; hereafter index tests: QFT-Plus, QFT-Plus CLIA, QIAreach, Wantai TB-IGRA, Standard E TB-Feron, and T-SPOT.TB/T-Cell Select) with World Health Organization (WHO)–endorsed tests for tuberculosis infection (hereafter reference tests).
Methods
Data sources (1 January 2007–18 August 2021) were Medline, Embase, Web of Science, Cochrane Database of Systematic Reviews, and manufacturers’ data. Cross-sectional and cohort studies comparing the diagnostic performance of index and reference tests were selected. The primary outcomes of interest were the pooled differences in sensitivity and specificity between index and reference tests. The certainty of evidence (CoE) was summarized using the GRADE approach.
Results
Eighty-seven studies were included (44 evaluated the QFT-Plus, 4 QFT-Plus CLIA, 3 QIAreach, 26 TB-IGRA, 10 TB-Feron [1 assessing the QFT-Plus], and 1 T-SPOT.TB/T-Cell Select). Compared to the QFT-GIT, QFT Plus’s sensitivity was 0.1 percentage points lower (95% confidence interval [CI], −2.8 to 2.6; CoE: moderate), and its specificity 0.9 percentage points lower (95% CI, −1.0 to −.9; CoE: moderate). Compared to QFT-GIT, TB-IGRA's sensitivity was 3.0 percentage points higher (95% CI, −.2 to 6.2; CoE: very low), and its specificity 2.6 percentage points lower (95% CI, −4.2 to −1.0; CoE: low). Agreement between the QFT-Plus CLIA and QIAreach with QFT-Plus was excellent (pooled κ statistics of 0.86 [95% CI, .78 to .94; CoE: low]; and 0.96 [95% CI, .92 to 1.00; CoE: low], respectively). The pooled κ statistic comparing the TB-Feron and the QFT-Plus or QFT-GIT was 0.85 (95% CI, .79 to .92; CoE: low).
Conclusions
The QFT-Plus and the TB-IGRA have very similar sensitivity and specificity as WHO-approved IGRAs.
Keywords: tuberculosis, IGRAs, tuberculin skin test, interferon-gamma, infection
We compared the diagnostic performance of the QFT-Plus, QIAreach, QFT-Plus CLIA, TB-IGRA, TB-Feron, and T-SPOT.TB/T-Cell Select with that of WHO-endorsed tests. The QFT-Plus and the Wantai TB-IGRA showed similar performance as their comparators; studies assessing other new tests are limited.
One of the most effective tuberculosis (TB) preventive strategies is the treatment of high-risk individuals with Mycobacterium tuberculosis (Mtb) infection (TBI) [1]. To this end, the World Health Organization (WHO) recommends performing either the tuberculin skin test (TST) or interferon-gamma (IFN-γ) release assays (IGRAs) [2]. IGRAs are blood-based tests that measure IFN-γ production by T lymphocytes after their in vitro exposure to Mtb antigens [3]. The first IGRAs endorsed by WHO included the QuantiFERON-Gold (QFT-G, Qiagen), the QuantiFERON-TB Gold In-Tube (QFT-GIT, Qiagen), and the T-SPOT.TB (Oxford Immunotec) assays. Early studies assessing their diagnostic performance confirmed a higher specificity relative to the TST, although their sensitivity was similar [4,5].
In 2015 Qiagen launched the QuantiFERON-TB Gold Plus (QFT-Plus), which added a new stimulation tube (TB2) using the same ESAT-6 and CFP-10 antigens but designed to induce a CD8+-specific response to increase its sensitivity [6]. In 2021, a chemiluminescence immunoassay analyzer (Liaison XL) was adapted to the QFT-Plus to fully automate IFN-γ quantification (QFT-Plus CLIA, Qiagen/Diasorin) [7]; furthermore, in 2021 Qiagen also released the QIAreach, which uses the same TB2 tube from the QFT-Plus but dropped positive and negative controls and includes digital fluorescence lateral flow nanoparticle technology to quantify IFN-γ [8,9]. The Wantai TB-IGRA (TB-IGRA, Beijing Wantai Biological Pharmacy Enterprise) and the Standard E TB-Feron (TB-Feron, SD Biosensor) were released in 2011 and 2018, respectively [10,11]; both include positive and negative test controls plus a tube with Mtb-specific antigens (ESAT-6 and CFP-10) [10–13]. Finally, in 2021, Oxford Immunotec released the T-Cell Select, a reagent kit that automatically isolates mononuclear cells from blood samples stored for up to 54 hours at room temperature using a magnetic bead–based cell separation system (the rest of the procedure being the same as with the T-SPOT.TB) [14].
To determine whether new or updated IGRAs could be included under current WHO recommendations for IGRA testing, we conducted a systematic review and meta-analysis to compare the diagnostic performance of the above-mentioned new tests (QFT-Plus, QIAreach, QFT-Plus CLIA, TB-IGRA, TB-Feron, and T-SPOT.TB with T-Cell Select) with the WHO-endorsed IGRAs (QFT-G, QFT-GIT, or T-SPOT.TB). Partial results from this review for QFT-Plus, QIAreach, TB-IGRA, TB-Feron, and T-SPOT.TB with T-Cell Select were presented to a WHO technical advisory group in October 2021.
METHODS
Data Sources and Searches
With the aid of a librarian (Dr Genevieve Gore), we searched Medline, Embase, Web of Science, the Cochrane Database of Systematic Reviews, and the International Clinical Trials Registry Platform from 1 January 2007 (3 years before the earliest release date of all index IGRAs) to 18 August 2021, using no language restrictions (Supplementary Table 1 [Supplementary Material part A]). We considered 4 additional data sources: (1) all references of included studies; (2) a hand search of the International Journal of Tuberculosis and Lung Disease (as this focuses on clinical and epidemiologic TB-related studies); (3) manufacturers’ data submitted to regulatory authorities, including published or unpublished studies; and (4) a public call for data coordinated by WHO on 23 August 2021. The last date for evaluating these additional sources was 27 October 2021.
Study Selection
We included cross-sectional and cohort studies, with any number of participants, both published and unpublished, conducted by independent investigators or the manufacturer, comparing any selected new IGRA with WHO-endorsed tests or with the QFT-Plus in the same subjects. Both tests were performed simultaneously, and technicians were blinded to the results of the other tests. Eligible studies assessed sensitivity in patients with newly diagnosed active TB, and specificity in healthy individuals, ideally at low risk of TBI, although because we estimated the difference in specificity between 2 tests in the same population, we included studies conducted in general population samples in countries with intermediate TB incidence rates (10–120 cases per 100 000/year), as well as low TB incidence rates (<10 cases per 100 000/year), as long as the participants did not have additional risk factors for exposure. Included studies estimated agreement in any population provided tests were simultaneous. Studies assessing the predictive ability for incident TB or reproducibility were also included. Four reviewers (L. A., S. L.-C., T. M., E. O.-B.) screened titles and abstracts independently and in duplicate. Discordance at this stage meant the study was included for full-text review. The same 4 reviewers screened full texts for eligibility; at this stage, discordance was solved by consensus or with the help of D. M. Full eligibility criteria are shown in Supplementary Table 2.
Data Extraction and Quality Assessment
Four reviewers (L. A., S. L.-C., T. M., E. O.-B.) extracted data independently and in duplicate using a standardized form designed for this study. Information retrieved included the characteristics of the study population, sampling methods, tests being compared, diagnostic outcomes, potential conflicts of interest, and results from contingency tables. If data were missing, the corresponding authors were contacted via email. Four reviewers (L. A., S. L.-C., T. M., E. O.-B.) assessed the risk of bias (RoB) of the included studies in duplicate for each diagnostic outcome using the Quality Assessment of Diagnostic Accuracy Studies–comparative tool (QUADAS-C) tailored to the needs of this review (Supplementary Tables 3–5) [15]. Overall study RoB was classified as follows: (1) low: if all comparison domains were considered at low RoB; (2) high: if ≥ 1 comparison domain was considered at high RoB; or (3) unknown: if ≥ 1 comparison domain was considered as at unknown RoB but none was considered at high RoB.
Data Synthesis and Analysis
The primary outcomes of interest were the pooled differences in sensitivity and specificity between the new IGRAs and any WHO-endorsed tests. Because Qiagen replaced QFT-GIT with the QFT-Plus in 2019, some studies used the latter as a reference. The primary analysis was restricted to published independent studies fulfilling all our inclusion criteria and allowing the reconstruction of contingency tables with paired comparisons. In this context, ignoring the correlated nature of observations may lead to an overestimation of variability and wider confidence intervals (CIs) [16,17]. We conducted 3 secondary analyses in which we added studies to those included in the primary analysis: (1) adding unpublished reports while still making paired comparisons; (2) adding published studies that did not have contingency tables and, therefore, were analyzed making parallel (unpaired) comparisons; (3) making parallel comparisons of all data (published and unpublished studies). We pooled results when 2 or more studies were available; otherwise, we provided results from individual studies. For the agreement outcome, our primary analysis included only studies from peer-reviewed literature that fulfilled all our inclusion criteria; data from unpublished studies were incorporated in a secondary analysis.
We conducted all meta-analyses in R (version 4.1.0) using the following packages: meta, version 4.19-2 [18]; MKinfer, version 0.6 [19]; metafor, version 3.0–2 [20]; and psych, version 2.1.9 [21]. The sensitivity and specificity from individual studies were pooled using random-effects meta-analysis with generalized linear mixed-effects models and logit-transformed proportions [22]. When information from contingency tables was available, we estimated differences in sensitivity and specificity and their 95% CIs from individual studies using the Wilson method for paired comparisons with a continuity correction; otherwise, we used the Wilson method for independent binomial proportions [17]. Approximate standard errors for differences in sensitivity and specificity were obtained by dividing the absolute difference between the upper and lower limits of the 95% CIs by 3.92. We pooled these estimates via random-effects meta-analysis using the inverse variance method with the Sidik-Jonkman estimator [23]. We used Knapp-Hartung adjustments to estimate 95% CIs of pooled effects [24]. For the agreement outcome, we calculated the Cohen kappa (κ) statistic from individual studies [25]; 95% CIs were estimated using the Fleiss, Cohen, and Everitt method [26]. Then, approximate standard errors were obtained by dividing the absolute difference between the upper and lower limits of the 95% CI by 3.92. We pooled these estimates via random-effects meta-analysis using the inverse variance method with the DerSimonian-Laird estimator [27]. All results are presented in tables and using Forest plots; we also report the I2 statistic for all meta-analyses [28]. Finally, the certainty of evidence was summarized using the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) as recommended elsewhere [29].
Role of the Funding Source
This study was funded by the WHO Global TB Programme to inform its policy development activities. Two of its members (A. K. and N. I.) are included as co-authors of this publication because they contributed significantly to the project’s conception, assisted with the acquisition of data, provided critical revisions to the manuscript, and approved its final version. The review protocol was developed for and approved by the WHO in July 2021.
RESULTS
Studies Included in the Review
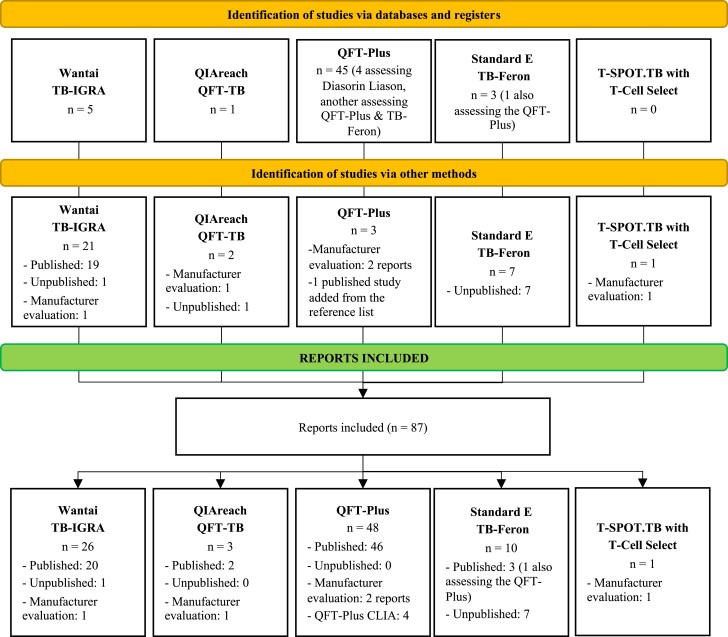
We identified 5895 unique titles from databases and registries; of these, 5475 and 367 were excluded after screening and full-text review, respectively (Supplementary Figure 1; for reasons of exclusion after full-text review, see Supplementary Table 6). We retrieved 147 additional reports from other sources; of these, we excluded 9 and 104 after screening and full-text review, respectively (Supplementary Figure 2). Among 87 reports included, 48 evaluated the QFT-Plus; of these, 44 assessed the QFT-Plus with enzyme-linked immunosorbent assay (ELISA) (12 of them assessing sensitivity [30–40] including 1 unpublished evaluation supplied by Qiagen; 9 specificity [31–37,41] including 1 unpublished evaluation supplied by Qiagen; 33 agreement [31,33,36–39,41–66] including 1 unpublished evaluation supplied by Qiagen; 3 predictive ability [67–69]; and 3 reproducibility [57,70,71]), and 4 evaluated the QFT-Plus with CLIA (all 4 agreement [7,72–74]); 3 reports evaluated the QIAreach (all 3 agreement [9,75] including 1 unpublished evaluation supplied by Qiagen); 26 reports evaluated the TB-IGRA (26 sensitivity [12,76–99] including 1 unpublished evaluation supplied by Beijing Wantai; 16 specificity [12,76–79,83–86,92,93,95–98] including 1 unpublished evaluation supplied by Beijing Wantai; and 8 agreement [12,78,79,87,89,98,99] including 1 unpublished evaluation supplied by Beijing Wantai); 10 reports evaluated the TB-Feron (3 sensitivity [100–102]; 2 specificity [100,101]; 10 agreement [13,66,100–102], 1 of them also assessing the QFT-Plus [66], and unpublished independent evaluations by the Duzen Lab [Turkey], the National Mycobacteria Reference Laboratory [Greece], the Korean National Tuberculosis Association [Korea; 2 separate evaluations], and the Université de Lille [France]); and 1 report evaluated the T-SPOT.TB with T-Cell Select (agreement [1 unpublished evaluation supplied by Oxford Immunotec]) (Figure 1). As part of our search, we identified 11 additional commercial IGRAs that have been developed and undergone some evaluation but were not included in this review since the review protocol, and especially the search strategy, did not consider them. These tests are listed in Supplementary Table 39 (Supplementary Material Part D).
Figure 1.
Flow diagram of the studies included according to their sources. The complete search is shown in Supplementary Figures 1 and 2.
QFT-Plus, QFT-Plus CLIA, and QIAreach
As seen in Supplementary Table 7 (Supplementary Material Part B), none of the studies assessing QFT-Plus sensitivity were considered to have low RoB. In our primary analysis (paired comparisons of published studies) including 505 subjects, the sensitivity of the QFT-Plus was 0.1 percentage points lower than that of the QFT-GIT (95% CI, −2.8 to 2.6) (Table 1). In our secondary analysis (parallel comparisons of published studies), including 252 subjects, the sensitivity of the QFT-Plus was 5.8 percentage points higher than that of the T-SPOT.TB (95% CI, −22.2 to 33.8); findings were similar in 1 study allowing paired comparisons of these tests [33]. As seen in Supplementary Table 8, 1 of 9 studies assessing QFT-Plus specificity was classified at low RoB. In parallel comparisons of published studies including 529 subjects, the specificity of the QFT-Plus was 0.9 percentage points lower than that of the QFT-GIT (95% CI, −1.0 to −.9); findings were similar in 1 study allowing paired comparisons of these tests’ specificities [41]. In parallel comparisons of all studies, including 156 subjects, the specificity of the QFT-Plus was 0.6 percentage points lower than that of the T-SPOT.TB (95% CI, −12.0 to 10.9); findings were similar in the 1 study allowing paired comparisons of these tests’ specificity (Table 1).
Table 1.
Summary of Differences in Sensitivity and Specificity Between the QuantiFERON-TB Gold Plus and the QFT-GIT or the T-SPOT.TB Assays
| Comparator/Outcome | Analysis | Studies, No. | Subjects Tested, No. | Correctly Classified (QFT-Plus), No. | Correctly Classified (Comparator), No. | Pooled Estimate QFT-Plus, % (95% CI); I2 | Pooled Estimate Comparator, % (95% CI); I2 | Difference in Sensitivity or Specificitya (QFT-Plus – Comparator), % Points (95% CI); I2 | Certainty of the Evidence (GRADE) |
|---|---|---|---|---|---|---|---|---|---|
| QFT-GIT as comparator | |||||||||
| Sensitivity | Paired comparisons (published studies)b | 5 | 505 | 432 | 431 | 90.1 (76.6–96.2); 90% | 89.4 (76.7–95.6); 92% | −0.1 (−2.8 to 2.6); 11% | Moderatec ⊕⊕⊕○ |
| Paired comparisons (all studies) | 7 | 972 | 861 | 864 | 90.8 (82.4–95.4); 90% | 90.6 (82.4–95.2); 92% | −0.5 (−1.9 to 0.9); 10% | ||
| Parallel comparisons (published studies) | 9 | 903 | 792 | 787 | 90.3 (84.2–94.2); 86% | 89.3 (83.1–93.4); 88% | 0.5 (−1.6 to 2.6); 12% | ||
| Parallel comparisons (All studies) | 12 | 1397 | 1244 | 1244 | 90.4 (85.8–93.6); 84% | 90 (85.4–93.3); 87% | 0.0 (−1.5 to 1.5); 11% | ||
| Specificity | Paired comparisons (published studies)b | 1 | 211 | 207 | 209 | 98.1 (95.2–99.5); NA | 99.1 (96.6–99.9); NA | −0.9 (−3.5 to 1.1); NA | Moderatec ⊕⊕⊕○ |
| Paired comparisons (all studies) | 6 | 1137 | 1101 | 1113 | 97.1 (92.6–98.9); 75% | 98.6 (94.4–99.7); 72% | −1.0 (−2.0 to 0.1); 26% | ||
| Parallel comparisons (published studies) | 3 | 529 | 518 | 523 | 97.9 (96.3–98.8); 0% | 98.9 (97.5–99.5); 0% | −0.9 (−1.0 to −0.9); 0% | ||
| Parallel comparisons (all studies) | 9 | 1505 | 1461 | 1476 | 97.3 (94.9–98.6); 65% | 98.5 (96.5–99.4); 62% | −0.9 (−1.5 to −0.3); 11% | ||
| T-SPOT.TB as comparator | |||||||||
| Sensitivity | Paired comparisonsb (published studies) | 1 | 99 | 98 | 96 | 99.0 (94.5–100); NA | 97.0 (91.4–99.4); NA | 2.0 (−2.4 to 7.4); NA | Lowc,e ⊕⊕○○ |
| Paired comparisons (all studies)d |
1 | 99 | 98 | 96 | 99.0 (94.5–100); NA | 97.0 (91.4–99.4); NA | 2.0 (−2.4 to 7.4); NA | ||
| Parallel comparisons (published studies) | 3 | 252 | 238 | 223 | 95.1 (87.5–98.2); 63% | 90.8 (75.4–97.0); 89% | 5.8 (−22.2 to 33.8); 84% | ||
| Parallel comparisons (all studies)d | 3 | 252 | 238 | 223 | 95.1 (87.5–98.2); 63% | 90.8 (75.4–97.0); 89% | 5.8 (−22.2 to 33.8); 84% | ||
| Specificity | Paired comparisonsb (published studies) | 0 | … | … | … | … | … | … | Lowc,e ⊕⊕○○ |
| Paired comparisons (all studies) | 1 | 50 | 49 | 50 | 98.0 (89.4–99.9); NA | 99.0 (92.9–100); NA | −2.0 (−10.5 to 5.3); NA | ||
| Parallel comparisons (published studies) | 1 | 106 | 104 | 104 | 98.1 (93.4–99.8); NA | 98.1 (93.4–99.8); NA | 0.0 (−4.9 to 4.9); NA | ||
| Parallel comparisons (all studies) | 2 | 156 | 153 | 154 | 98.1 (94.2–99.4); 0% | 98.7 (95–99.7); 0% | −0.6 (−12.0 to 10.9); 1% | ||
A summary of each analysis, including information from individual studies, is shown in Supplementary Material Part B, Supplementary Tables 12–15 (sensitivity) and Supplementary Tables 16–19 (specificity). Forest plots of pooled sensitivities and specificities are shown in in Supplementary Material Part B, Supplementary Figures 10–12 and 13–14, respectively. For a summary of the risk of bias assessment, please refer to Supplementary Figures 3–6. In line with the GRADE approach, the certainty of evidence (CoE) is categorized into four levels: very low (⊕○○○), low (⊕⊕○○), moderate (⊕⊕⊕○), and high (⊕⊕⊕⊕).
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation; NA, not available.
Pooled differences in sensitivity or specificity do not match exactly to the differences in the pooled sensitivities or specificities since they correspond to different meta-analytical approaches.
Primary analysis.
Downgraded because most studies were considered at unclear risk of bias.
No unpublished studies were included in this analysis; results are repeated from the analysis including only published studies.
Downgraded because CIs are very wide and hence consistent with both an appreciable gain and appreciable loss in diagnostic accuracy.
Of the 33 studies assessing the agreement between QFT-Plus and the WHO-endorsed tests, 21 (63.6%) were classified at low RoB (Supplementary Table 9). In our primary analyses, the pooled κ statistics comparing the QFT-Plus against the QFT-GIT and the T-SPOT.TB were 0.82 (95% CI, .78 to .85; N = 6586 subjects) and 0.72 (95% CI, .57 to .86; N = 3139 subjects), respectively (Table 2). On the other hand, the pooled κ statistic comparing the QFT-Plus and the TST was 0.32 (95% CI, .20 to .44; N = 1312 subjects). Of the studies assessing the agreement of the QFT-Plus CLIA and the QIAreach with the QFT-Plus, 25% (1/4) and 33.3% (1/3) were classified at low RoB, respectively (Supplementary Tables 10 and 11). In our primary analyses, the pooled κ statistics comparing the QFT-Plus CLIA and the QIAreach with the QFT-Plus were 0.86 (95% CI, .78 to .94; N = 1173 samples) and 0.96 (95% CI, .92 to 1.00; N = 289 samples), respectively (Table 2). As summarized in Supplementary Tables 20 and 21, we found very limited information regarding QFT-Plus reproducibility or its predictive ability for incident TB.
Table 2.
Summary of Agreement Between the New or Updated Interferon-γ Release Assays and the World Health Organization–Endorsed Tests or the Tuberculin Skin Test
| Index Test/Comparator | Studies Included | Studies, No. | Subjects Tested, No. | Total Concordant, No. | Agreement Pooled κ Statistic (95% CI); I2 | Certainty of the Evidence (GRADE) |
|---|---|---|---|---|---|---|
| QFT-Plus | ||||||
| QFT-GIT | Published studies | 22 | 6586 | 6204 | 0.82 (.78–.85); 67.4% | High ⊕⊕⊕⊕ |
| All studies | 23 | 7187 | 6799 | 0.82 (.79–.86); 75.4% | ||
| T-SPOT.TB | Published studiesa | 7 | 3139 | 2767 | 0.72 (.57–.86); 97.5% | Moderateb ⊕⊕⊕○ |
| TST | Published studiesa | 7 | 1312 | 914 | 0.32 (.20–.44); 81.2% | Moderateb ⊕⊕⊕○ |
| QFT-Plus CLIAc | ||||||
| QFT-Plus | Published studiesa,d | 4 | 1173 | 1039 | 0.86 (.78–.94); 74.8% | Lowe ⊕⊕○○ |
| QIAreachc | ||||||
| QFT-Plus | Published studiesd | 2 | 289 | 279 | 0.96 (.92–1.00); 24.9% | Lowe ⊕⊕○○ |
| All studiesd | 3 | 529 | 498 | 0.95 (.92–.98); 0.0% | ||
| Wantai TB-IGRA | ||||||
| QFT-GIT | Published studies | 3 | 1127 | 950 | 0.79 (.64–.94); 92.1% | Very lowb,f,g ⊕○○○ |
| All studies | 4 | 2355 | 2043 | 0.79 (.70–.88); 90.6% | ||
| T-SPOT.TB | Published studiesa | 3 | 340 | 320 | 0.87 (.81–.93); 0.0% | Lowf,g ⊕⊕○○ |
| TST | Published studiesa | 2 | 141 | 103 | 0.37 (.05–.69); 51.6% | Very lowd,f,g ⊕○○○ |
| TB-Feron ELISAc | ||||||
| QFT-Plus or QFT-GIT | Published studies | 4 | 1062 | 1001 | 0.85 (.79–.92); 62.0% | Lowf,g ⊕⊕○○ |
| QFT-Plus or QFT-GIT or QFT-Gold | All studies | 10 | 2454 | 2326 | 0.88 (.84–.93); 73.2% | Lowf,g ⊕⊕○○ |
Forest plots of each agreement analysis included in this table are shown in the following figures: Supplementary Material Part B: Supplementary Figures 15–18 (QFT-Plus), Supplementary Figure 19 (QFT-Plus CLIA), Supplementary Figures 20 and 21 (QIAreach); Supplementary Material Part C: Figures 39–42 (Wantai TB-IGRA); and Supplementary Material Part D: Supplementary Figures 48 and 49 (TB-Feron). Only the manufacturer evaluation of the T-SPOT.TB with T-Cell Select was identified/included in this study; results are summarized in Supplementary Material Part D, Supplementary Figure 50 and Supplementary Table 38. For a summary of the risk of bias assessment, please refer to Supplementary Figures 7–9 (QFT-Plus), 28–30 (TB-IGRA), and 43–45 (TB-Feron). In line with the GRADE approach, the certainty of evidence (CoE) is categorized into four levels: very low (⊕○○○), low (⊕⊕○○), moderate (⊕⊕⊕○), and high (⊕⊕⊕⊕).
Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GRADE, Grading of Recommendations Assessment, Development and Evaluation.
No unpublished studies assessing this comparison were identified/included.
Downgraded because of the wide CIs.
Insufficient studies were identified that assessed sensitivity or specificity of these tests. Hence, only measures of agreement could be pooled and are shown.
Three studies assessing QFT-Plus CLIA [73], [7], [74] and 1 study assessing the QIAreach [75] reported results by number of samples instead of per patient.
Downgraded because 3 of 4 QFT-Plus CLIA studies and 2 of 3 QIAreach studies were considered at high risk of bias.
Downgraded because most studies were considered at either unclear or high risk of bias.
Downgraded because of the risk of publication bias.
Wantai TB-IGRA
As seen in Supplementary Table 22 (Supplementary Material Part C), only 1 of 26 studies assessing the sensitivity of the TB-IGRA was considered to have low RoB. In parallel comparisons of published studies including 1600 subjects, the sensitivity of the TB-IGRA was 3.0 percentage points higher than that of the QFT-GIT (95% CI, −.2 to 6.2); (Table 3). In parallel comparisons of published studies including 1288 subjects, the sensitivity of the TB-IGRA was 1.6 percentage points lower than that of the T-SPOT.TB (95% CI, −4.2 to 1.0); we did not find any significant differences between these tests' sensitivities in 1 study allowing paired comparisons [99]. As seen in Supplementary Table 23, only 1 of 16 studies assessing TB-IGRA's specificity was classified as having low RoB. In parallel comparisons of published studies, the specificity of the TB-IGRA was 2.6 percentage points lower than that of the QFT-GIT (95% CI, −4.2 to −1.0; N = 818 subjects) and 10.3 percentage points lower than the T-SPOT.TB (95% CI, −17.2 to −3.4; N = 185 subjects) (Table 3). As seen in Supplementary Table 24, 1 of 8 studies assessing TB-IGRA agreement was considered to have low RoB. In our primary analyses, the pooled κ statistics comparing the TB-IGRA against the QFT-GIT and T-SPOT.TB were 0.79 (95% CI, .64 to .94; N = 1127 subjects) and 0.87 (95% CI, .81 to .93; N = 340 subjects), respectively (Table 2).
Table 3.
Summary of Pooled Differences in Sensitivity and Specificity Between the Wantai TB-IGRA and the QFT-GIT or the T-SPOT.TB
| Comparator/Outcome | Analysis | Studies, No. | Subjects Tested, No. | Correctly Classified (Wantai TB-IGRA), No. | Correctly Classified (Comparator), No. | Pooled Estimate Wantai TB-IGRA, % (95% CI); I2 | Pooled Estimate Comparator, % (95% CI); I2 | Difference in Sensitivity or Specificitya (Wantai TB-IGRA – Comparator), % Points (95% CI); I2 | Certainty of the Evidence (GRADE) |
|---|---|---|---|---|---|---|---|---|---|
| QFT-GIT as comparator | |||||||||
| Sensitivity | Paired comparisons (published studies)b | 0 | … | … | … | … | … | … | Very lowc,d,e ⊕○○○ |
| Paired comparisons (all studies) | 1 | 43 | 31 | 35 | 72.1 (56.3–84.7); NA | 81.4 (66.6–91.6); NA | −9.3 (−21.5 to 2.7); NA | ||
| Parallel comparisons (published studies) | 5 | 1600 | 1373 | 1321 | 86.4 (81.5–90.2); 87% | 83.2 (76.3–88.4); 93% | 3.0 (−.2 to 6.2); 32% | ||
| Parallel comparisons (all studies) | 7 | 2429 | 2041 | 1966 | 84.5 (79.8–88.4); 85% | 82.2 (76.8–86.6); 90% | 2.8 (−.3 to 5.9); 57% | ||
| Specificity | Paired comparisons (published studies)b | 0 | … | … | … | … | … | … | Lowc,e ⊕⊕○○ |
| Paired comparisons (all studies) | 0 | … | … | … | … | … | … | ||
| Parallel comparisons (published studies) | 4 | 818 | 714 | 733 | 85.9 (77.1–91.6); 90% | 88.7 (77.7–94.7); 91% | −2.6 (−4.2 to −1.0); 2% | ||
| Parallel comparisons (all studies) | 6 | 1093 | 927 | 958 | 84.3 (77.0–89.7); 87% | 86.8 (78.0–92.4); 87% | −2.8 (−4.7 to −.8); 17% | ||
| T-SPOT.TB as comparator | |||||||||
| Sensitivity | Paired comparisons (published studies)b | 1 | 68 | 66 | 66 | 97.1 (89.8–99.6); NA | 97.1 (89.8–99.6); NA | .0 (−7.0 to 7.0); NA | Very lowc,d,e ⊕○○○ |
| Paired comparisons (all studies)f | 1 | 68 | 66 | 66 | 97.1 (89.8–99.6); NA | 97.1 (89.8–99.6); NA | .0 (−7.0 to 7.0); NA | ||
| Parallel comparisons (published studies) | 6 | 1288 | 1065 | 1092 | 87.7 (80.9–92.3); 89% | 88.7 (83–92.7); 87% | −1.6 (−4.2 to 1.0); 16% | ||
| Parallel comparisons (all studies)f | 7 | 1332 | 1104 | 1132 | 87.8 (81.9–91.9); 88% | 88.9 (83.8–92.6); 85% | −1.6 (−3.9 to .6); 11% | ||
| Specificity | Paired comparisons (published studies)b | 0 | … | … | … | … | … | … | Very lowc,d,e ⊕○○○ |
| Paired comparisons (all studies) | 0 | … | … | … | … | … | … | ||
| Parallel comparisons (published studies) | 1 | 185 | 151 | 170 | 81.6 (75.3–86.9); NA | 91.9 (87–95.4); NA | −10.3 (−17.2 to −3.4); NA | ||
| Parallel comparisons (all studies) | 3 | 285 | 241 | 261 | 85.5 (77.3–91.1); 45% | 91.6 (87.7–94.3); 0% | −5.6 (−20 to 8.7); 33% | ||
A summary of each analysis, including information from individual studies, is shown in Supplementary Material Part C, Supplementary Tables 25–29 (sensitivity) and Supplementary Tables 30–32 (specificity). Forest plots of pooled sensitivities and specificities are shown in Supplementary Material Part C, Supplementary Figures 31–34 and 35–38, respectively. For a summary of the risk of bias assessment, please refer to Supplementary Figures 22–27. In line with the GRADE approach, the certainty of evidence (CoE) is categorized into four levels: very low (⊕○○○), low (⊕⊕○○), moderate (⊕⊕⊕○), and high (⊕⊕⊕⊕).
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation; NA, not available.
Pooled differences in sensitivity or specificity do not match exactly to the differences in the pooled sensitivities or specificities since they correspond to different meta-analytical approaches.
Primary analysis.
Downgraded because most studies were considered at either unclear or high risk of bias.
Downgraded because CIs are very wide, and consistent with both an appreciable gain and appreciable loss in diagnostic accuracy.
Downgraded because of the risk of publication bias.
No unpublished studies were included in this analysis; results are repeated from the analysis including only published studies.
TB-Feron ELISA
The characteristics of the studies assessing the sensitivity, specificity, and agreement of the TB-Feron are shown in Supplementary Tables 33–35 and Supplementary Figures 46–49 (Supplementary Material Part D). In paired comparisons of all studies including 139 subjects, the sensitivity of the TB-Feron was 3.7 percentage points higher (95% CI, −18.5 to 25.9) than that of the QFT-G or QFT-Plus (Supplementary Table 36). In our primary analysis including 327 subjects, the specificity of the TB-Feron was 5.4 percentage points lower than that of the QFT-Plus (95% CI, −15.3 to 4.4) (Supplementary Table 37). In our primary analyses including 1062 subjects, the pooled κ statistic comparing the TB-Feron and the QFT-Plus or QFT-GIT was 0.85 (95% CI, .79 to .92) (Table 2).
T-SPOT.TB With T-Cell Select
We only identified 1 report in which the manufacturer assessed the agreement of T-SPOT.TB when processing samples with T-Cell Select from 0 to 58 hours after blood collection (divided into 4 time points) versus without T-Cell Select within 8 hours. Overall agreement within 0–8 hours was 96.5% (95% CI, 94.7%–97.8%) and did not change significantly up to 48–55 hours (Supplementary Table 38 and Supplementary Figure 50).
DISCUSSION
In this review, we identified and summarized studies comparing the diagnostic performance of 6 new commercial IGRAs (ie, Qiagen's QFT-Plus, QIAreach, and QFT-Plus CLIA; Wantai's TB-IGRA; the Standard E TB-Feron; and Oxford Immunotec's T-SPOT.TB with T-Cell Select) against IGRAs that have previously been endorsed by the WHO. According to our results, the QFT-Plus and the TB-IGRA have similar diagnostic performance as their comparators; studies assessing other new tests are too limited to make valid conclusions.
Pooled differences in sensitivity and specificity between the QFT-Plus and its predecessor, the QFT-GIT, ranged within 1 percentage point, while their agreement was almost perfect. These results confirm findings from previous studies suggesting these tests are equivalent, with no apparent improvement in sensitivity [103]. Agreement between the QFT-Plus and the T-SPOT.TB was substantial; nevertheless, studies comparing their diagnostic accuracy were scarce. Notably, the studies assessing QFT-Plus sensitivity incorporated in this review included only 8 individuals with HIV, and none was focused on children. In both subgroups, CD8+-specific immune responses have a major role against Mtb [104–106]. Hence, studies focusing on these subpopulations with an appropriate study design are required. Although reports estimating the predictive ability for incident TB of QFT-Plus are limited, we would not expect major differences between them, given the excellent agreement of QFT-Plus with QFT-GIT.
We did not find clinically meaningful differences in sensitivity or specificity between the TB-IGRA and the QFT-GIT, and we found almost perfect agreement between the TB-IGRA and the T-SPOT.TB, although we found few studies comparing these tests. Inferences about the accuracy of the TB-IGRA are limited by the low quality of most reports, due to incomplete description of key methodologic aspects and missing data, precluding full assessment of their RoB. Most publications were identified by the manufacturer since they were not listed in the databases and registries included in our electronic search. We could not assess potential conflicts of interest for most studies due to a lack of information. Finally, the generalizability of results with TB-IGRA (and even availability of the test itself) to other settings and populations is uncertain as almost all studies were conducted in 1 country (China).
On the other hand, we found only fair agreement between the TST and the QFT-Plus or the TB-IGRA (Table 2), consistent with previous systematic reviews assessing the agreement of the TST with previous versions of the QFT or the T-SPOT.TB in healthcare workers (pooled κ = 0.28 [95% CI, .22 to .35]) [107]; people immigrating from high to low TB-incidence settings (individual κ values ranged from 0.32 to 0.56) [108]; and people with HIV (pooled κ = 0.37 [95% CI, .28 to .46]) [109].
The included studies assessing the remaining IGRAs (ie, QFT-Plus CLIA, QIAreach, TB-Feron, and T-SPOT.TB with T-Cell Select) were mainly limited to the evaluation of agreement with reference tests. The QIAreach and the TB-Feron are entirely new tests; therefore, independent evaluations of their sensitivity and specificity are needed before these can be adopted. On the other hand, the T-SPOT.TB with T-Cell Select and the QFT-Plus with CLIA are modifications of previously validated tests; nevertheless, independent evaluation of these tests would be desirable before widespread use. Finally, our search identified 11 additional commercial IGRAs for the diagnosis of TBI (Supplementary Table 39); however, most of these were described in a single publication, and our search was not designed specifically for these other tests. This would be needed to adequately assess their diagnostic accuracy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Edgar Ortiz-Brizuela, McGill International Tuberculosis Centre, Department of Medicine, McGill University, Montreal, Quebec, Canada; Department of Medicine, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
Lika Apriani, Tuberculosis Working Group, Research Centre for Care and Control of Infectious Diseases, Universitas Padjadjaran, Bandung, Indonesia; Department of Public Health, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
Tania Mukherjee, Faculty of Medicine and Health, University of Sydney, Sydney, New South Wales, Australia.
Sophie Lachapelle-Chisholm, McGill International Tuberculosis Centre, Department of Medicine, McGill University, Montreal, Quebec, Canada.
Michele Miedy, McGill University Health Center, Department of Intensive Care Unit, McGill University, Montreal, Quebec, Canada.
Zhiyi Lan, McGill International Tuberculosis Centre, Department of Medicine, McGill University, Montreal, Quebec, Canada.
Alexei Korobitsyn, Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland.
Nazir Ismail, Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland.
Dick Menzies, McGill International Tuberculosis Centre, Department of Medicine, McGill University, Montreal, Quebec, Canada; Respiratory Epidemiology and Clinical Research Unit, Research Institute of the McGill University Health Centre, Montreal Chest Institute, McGill University, Montreal, Quebec, Canada.
Notes
Acknowledgments. The authors acknowledge Dr Genevieve Gore, who provided invaluable help in the design of the search strategy for this review. E.O.-B. gratefully acknowledges the PhD scholarship from the Fundacion para la Salud y la Educacion Salvador Zubiran (FUNSaEd).
Financial support. This work was supported by the World Health Organization Global TB Programme.
References
- 1. Collin SM, Wurie F, Muzyamba MC, et al. Effectiveness of interventions for reducing TB incidence in countries with low TB incidence: a systematic review of reviews. Eur Res Rev 2019; 28:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . WHO consolidated guidelines on tuberculosis: module 1: prevention: tuberculosis preventive treatment. Geneva, Switzerland: WHO, 2020. [PubMed] [Google Scholar]
- 3. Campbell JR, Pease C, Daley P, Pai M, Menzies D. Chapter 4: diagnosis of tuberculosis infection. Can J Respir Crit Care Sleep Med 2022; 6(Suppl 1):49–65. [Google Scholar]
- 4. Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–54. [DOI] [PubMed] [Google Scholar]
- 5. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiagen . QuantiFERON-TB Gold Plus ELISA package insert. Hilden, Germany: Qiagen, 2019. [Google Scholar]
- 7. De Maertelaere E, Vandendriessche S, Verhasselt B, et al. Evaluation of QuantiFERON-TB Gold Plus on Liaison XL in a low-tuberculosis-incidence setting. J Clin Microbiol 2020; 58:e00159–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qiagen . QIAreach QuantiFERON-TB test package insert. Hilden, Germany: Qiagen, 2021. [Google Scholar]
- 9. Fukushima K, Akagi K, Kondo A, Kubo T, Sakamoto N, Mukae H. First clinical evaluation of the QIAreach(TM) QuantiFERON-TB for tuberculosis infection and active pulmonary disease. Pulmonology 2022; 28:6–12. [DOI] [PubMed] [Google Scholar]
- 10. Beijing Wantai Biological Pharmacy Enterprise . Wantai TB-IGRA diagnostic kit for T cell infected with Mycobacterium tuberculosis (TB-IGRA) package insert. Beijing, China: Beijing Wantai Biological Pharmacy Enterprise, 2021. [Google Scholar]
- 11. Biosensor S. Standard E TB-Feron ELISA package insert. Gyeonggi-do, Republic of Korea: SD Biosensor, 2021. [Google Scholar]
- 12. Liu Y, Ou M, He S, et al. Evaluation of a domestic interferon-gamma release assay for detecting Mycobacterium tuberculosis infection in China. Tuberculosis (Edinb) 2015; 95:523–6. [DOI] [PubMed] [Google Scholar]
- 13. Kweon OJ, Lim YK, Kim HR, Kim TH, Lee MK. Evaluation of Standard E TB-Feron enzyme-linked immunosorbent assay for diagnosis of latent tuberculosis infection in health care workers. J Clin Microbiol 2019; 57:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oxford Immunotec . T-Cell Select package insert. Abingdon, United Kingdom: Oxford Immunotec, 2021. [Google Scholar]
- 15. Yang B, Mallett S, Takwoingi Y, et al. QUADAS-C: a tool for assessing risk of bias in comparative diagnostic accuracy studies. Ann Intern Med 2021; 174:1592–9. [DOI] [PubMed] [Google Scholar]
- 16. Sainani K. The importance of accounting for correlated observations. PM R 2010; 2:858–61. [DOI] [PubMed] [Google Scholar]
- 17. Altman D, Machin D, Bryant T. Statistics with confidence: confidence intervals and statistical guidelines. London:Wiley, 2000. [Google Scholar]
- 18. Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohl M. MKinfer: Inferential Statistics. R package version 0.9. Available at: https://www.stamats.de. 2020.
- 20. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36:1–48. [Google Scholar]
- 21. Revelle W. Psych: procedures for psychological, psychometric, and personality research. R package version 2.1.9 ed. Evanston, IL: Northwestern University, 2021. [Google Scholar]
- 22. Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010; 29:3046–67. [DOI] [PubMed] [Google Scholar]
- 23. Sidik K, Jonkman JN. A note on variance estimation in random effects meta-regression. J Biopharm Stat 2005; 15:823–38. [DOI] [PubMed] [Google Scholar]
- 24. Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22:2693–710. [DOI] [PubMed] [Google Scholar]
- 25. Cohen J. A coefficient of agreement for nominal scales. Edu Psychol Meas 1960; 20:37–46. [Google Scholar]
- 26. Fleiss JL, Cohen J, Everitt BS. Large sample standard errors of kappa and weighted kappa. Psychol Bull 1969; 72:323–7. [Google Scholar]
- 27. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schünemann HJ, Higgins JPT, Vist GE, et al. Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane handbook for systematic reviews of interventions version 6.3. 2022. Available at:www.training.cochrane.org/handbook. Accessed January 2023. [Google Scholar]
- 30. Petruccioli E, Chiacchio T, Pepponi I, et al. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect 2016; 73:588–97. [DOI] [PubMed] [Google Scholar]
- 31. Petruccioli E, Vanini V, Chiacchio T, et al. Analytical evaluation of QuantiFERON- Plus and QuantiFERON Gold In-Tube assays in subjects with or without tuberculosis. Tuberculosis 2017; 106:38–43. [DOI] [PubMed] [Google Scholar]
- 32. Suzukawa M, Takeda K, Akashi S, et al. Evaluation of cytokine levels using QuantiFERON-TB Gold Plus in patients with active tuberculosis. J Infect 2020; 80:547–53. [DOI] [PubMed] [Google Scholar]
- 33. Takasaki J, Manabe T, Morino E, et al. Sensitivity and specificity of QuantiFERON-TB Gold Plus compared with QuantiFERON-TB Gold In-Tube and T-SPOT.TB on active tuberculosis in Japan. J Infect Chemother 2018; 24:188–92. [DOI] [PubMed] [Google Scholar]
- 34. Takeda K, Nagai H, Suzukawa M, et al. Comparison of QuantiFERON-TB Gold Plus, QuantiFERON-TB Gold In-Tube, and T-SPOT.TB among patients with tuberculosis. J Infect Chemother 2020; 26:1205–12. [DOI] [PubMed] [Google Scholar]
- 35. Yi L, Sasaki Y, Nagai H, et al. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 2016; 6:30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoffmann H, Avsar K, Gores R, Mavi SC, Hofmann-Thiel S. Equal sensitivity of the new generation QuantiFERON-TB Gold Plus in direct comparison with the previous test version QuantiFERON-TB Gold IT. Clin Microbiol Infect 2016; 22:701–3. [DOI] [PubMed] [Google Scholar]
- 37. Hong JY, Park SY, Kim A, Cho SN, Hur YG. Comparison of QFT-Plus and QFT-GIT tests for diagnosis of M. tuberculosis infection in immunocompetent Korean subjects. J Thoracic Dis 2019; 11:5210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horne DJ, Jones BE, Kamada A, et al. Multicenter study of QuantiFERON-TB Gold Plus in patients with active tuberculosis. Int J Tuberc Lung Dis 2018; 22:617–21. [DOI] [PubMed] [Google Scholar]
- 39. Lee MR, Chang CH, Chang LY, et al. CD8 response measured by QuantiFERON-TB Gold Plus and tuberculosis disease status. J Infect 2019; 78:299–304. [DOI] [PubMed] [Google Scholar]
- 40. Fukushima K, Kubo T, Kaneko Y, et al. Comparison study of sensitivity of QuantiFERON TB Gold Plus with existing IGRAs in the patients with active pulmonary tuberculosis. Kekkaku 2018; 93:529–36. [Google Scholar]
- 41. Siegel SAR, Cavanaugh M, Ku JH, Kawamura LM, Winthrop KL. Specificity of QuantiFERON-TB plus, a new-generation interferon gamma release assay. J Clin Microbiol 2018; 56:e00629–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moon HW, Gaur RL, Tien SSH, Spangler M, Pai M, Banaei N. Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 2017; 55:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Catalan I P, Marti C R, Fortuno M G, et al. Concordance between the test of the tuberculin and interferon gamma release assay-IGRA in patients with immune-mediated inflammatory diseases [in Spanish]. Rev Esp Quimioter 2019; 32:445–50. [PMC free article] [PubMed] [Google Scholar]
- 44. Pieterman ED, Liqui Lung FG, Verbon A, et al. A multicentre verification study of the QuantiFERON-TB Gold Plus assay. Tuberculosis 2018; 108:136–42. [DOI] [PubMed] [Google Scholar]
- 45. Primaturia C, Reniarti L, Nataprawira HMN. Comparison between the interferon gamma release assay-QuantiFERON Gold Plus (QFT-Plus) and tuberculin skin test (TST) in the detection of tuberculosis infection in immunocompromised children. Pulm Med 2020; 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ryu MR, Park MS, Cho EH, et al. Comparative evaluation of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus in diagnosis of latent tuberculosis infection in immunocompromised patients. J Clin Microbiol 2018; 56:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sellami M, Fazaa A, Cheikh M, et al. Screening for latent tuberculosis infection prior to biologic therapy in patients with chronic immune-mediated inflammatory diseases (IMID): interferon-gamma release assay (IGRA) versus tuberculin skin test (TST). Egypt Rheumatol 2019; 41:225–30. [Google Scholar]
- 48. Surucuoglu S, Ermertcan AT, Cetinarslan T, Ozkutuk N. The reliability of tuberculin skin test in the diagnosis of latent tuberculosis infection in psoriasis patients: a case-control study. Dermatol Ther 2020; 33:1–7. [DOI] [PubMed] [Google Scholar]
- 49. Theel ES, Hilgart H, Breen-Lyles M, et al. Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol 2018; 56:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsuyuzaki M, Igari H, Okada N, Suzuki K. Variation in interferon-gamma production between QFT-Plus and QFT-GIT assays in TB contact investigation. Res Invest 2019; 57:561–5. [DOI] [PubMed] [Google Scholar]
- 51. Venkatappa TK, Punnoose R, Katz DJ, et al. Comparing QuantiFERON-TB Gold Plus with other tests to diagnose Mycobacterium tuberculosis infection. J Clin Microbiol 2019; 57:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang PH, Lin SY, Lee SSJ, et al. CD4 Response of QuantiFERON-TB Gold Plus for positive consistency of latent tuberculosis infection in patients on dialysis. Sci Rep 2020; 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Won D, Park JY, Kim HS, Park Y. Comparative results of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus assays for detection of tuberculosis infection in clinical samples. J Clin Microbiol 2020; 58:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang HR, Xin HN, Wang DK, et al. Serial testing of Mycobacterium tuberculosis infection in Chinese village doctors by QuantiFERON-TB Gold Plus, QuantiFERON-TB Gold in-Tube and T-SPOT.TB. J Infect 2019; 78:305–10. [DOI] [PubMed] [Google Scholar]
- 55. Agarwal S, Nguyen DT, Lew JD, Graviss EA. Performance and variability of QuantiFERON Gold Plus assay associated with phlebotomy type. PLoS One 2018; 13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barcellini L, Borroni E, Brown J, et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Res J 2016; 48:1411–9. [DOI] [PubMed] [Google Scholar]
- 57. Chien JY, Chiang HT, Lu MC, et al. QuantiFERON-TB Gold Plus is a more sensitive screening tool than QuantiFERON-TB Gold in-Tube for latent tuberculosis infection among older adults in long-term care facilities. J Clin Microbiol 2018; 56:e00427–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fernandez-Blazquez A, Arguelles Menendez P, Sabater-Cabrera C, Garcia-Garcia JM, Asensi Alvarez V, Palacios Gutierrez JJ. Diagnosis of tuberculous infection in immunosuppressed patients and/or candidates for biologics using a combination of 2 IGRA tests: T-SPOT.TB/QuantiFERON TB Gold In-Tube vs. T-SPOT.TB/QuantiFERON TB Gold Plus. Arch Bronconeumol 2021; 58:305–10. [DOI] [PubMed] [Google Scholar]
- 59. Morales EN G, Knierer J, Schablon A, Nienhaus A, Kersten JF. Prevalence of latent tuberculosis infection among foreign students in Lubeck, Germany tested with QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus. J Occup Med Toxicol 2017; 12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gatechompol S, Harnpariphan W, Supanan R, et al. Prevalence of latent tuberculosis infection and feasibility of TB preventive therapy among Thai prisoners: a cross-sectional study. BMC Public Health 2021; 21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Igari H, Akutsu N, Ishikawa S, et al. Positivity rate of interferon-gamma release assays for estimating the prevalence of latent tuberculosis infection in renal transplant recipients in Japan. J Infect Chemother 2019; 25:537–42. [DOI] [PubMed] [Google Scholar]
- 62. Igari H, Ishikawa S, Nakazawa T, et al. Lymphocyte subset analysis in QuantiFERON-TB Gold Plus and T-Spot.TB for latent tuberculosis infection in rheumatoid arthritis. J Infect Chemother 2018; 24:83–7. [DOI] [PubMed] [Google Scholar]
- 63. Igari H, Takayanagi S, Yahaba M, Tsuyuzaki M, Taniguchi T, Suzuki K. Prevalence of positive IGRAs and innate immune system in HIV-infected individuals in Japan. J Infect Chemother 2021; 27:592–7. [DOI] [PubMed] [Google Scholar]
- 64. Kay AW, DiNardo AR, Dlamini Q, et al. Evaluation of the QuantiFERON-Tuberculosis Gold Plus assay in children with tuberculosis disease or following household exposure to tuberculosis. Am J Trop Med Hyg 2019; 100:540–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim SH, Jo KW, Shim TS. QuantiFERON-TB Gold PLUS versus QuantiFERON-TB Gold In-Tube test for diagnosing tuberculosis infection. Korean J Intern Med 2020; 35:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Benachinmardi K, Sampath S, Rao M. Evaluation of a new interferon gamma release assay, in comparison to tuberculin skin tests and QuantiFERON tuberculosis Gold Plus for the detection of latent tuberculosis infection in children from a high tuberculosis burden setting. Int J Mycobacteriol 2021; 10:142–8. [DOI] [PubMed] [Google Scholar]
- 67. Mendelsohn SC, Fiore-Gartland A, Penn-Nicholson A, et al. Validation of a host blood transcriptomic biomarker for pulmonary tuberculosis in people living with HIV: a prospective diagnostic and prognostic accuracy study. Lancet Glob Health 2021; 9:e841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scriba TJ, Fiore-Gartland A, Penn-Nicholson A, et al. Biomarker-guided tuberculosis preventive therapy (CORTIS): a randomised controlled trial. Lancet Infect Dis 2021; 21:354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gupta RK, Kunst H, Lipman M, et al. Evaluation of QuantiFERON-TB Gold Plus for predicting incident tuberculosis among recent contacts: a prospective cohort study. Ann Am Thorac Soc 2020; 17:646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Uwamino Y, Sakai A, Nishimura T, et al. Effect of refrigeration of blood samples in lithium-heparin tubes on QuantiFERON TB Gold Plus test result. J Infect Chemother 2020; 26:312–4. [DOI] [PubMed] [Google Scholar]
- 71. Knierer J, Gallegos Morales EN, Schablon A, Nienhaus A, Kersten JF. QFT-Plus: a plus in variability? Evaluation of new generation IGRA in serial testing of students with a migration background in Germany. J Occup Med Toxicol 2017; 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Altawallbeh G, Gabrielson D, Peters JM, Killeen AA. Performance of an advanced interferon-gamma release assay for Mycobacterium tuberculosis detection. J Appl Lab Med 2021; 6:1287–92. [DOI] [PubMed] [Google Scholar]
- 73. Bisognin F, Lombardi G, Re MC, Dal Monte P. QuantiFERON-TB Gold Plus with chemiluminescence immunoassay: do we need a higher cutoff? J Clin Microbiol 2020; 58:e00780–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fernandez-Huerta M, Moreto C, Vila-Olmo N, et al. Evaluation of the fully automated chemiluminescence analyzer liaison XL for the performance of the QuantiFERON-TB Gold Plus assay in an area with a low incidence of tuberculosis. J Clin Microbiol 2021; 59:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stieber F, Howard J, Manissero D, et al. Evaluation of a lateral-flow nanoparticle fluorescence assay for TB infection diagnosis. Int J Tuberc Lung Dis 2021; 25:917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Duochi W, Shoufeng H, Aizhen G. Clinical significance of interferon gamma release assay in the diagnosis of Mycobacterium tuberculosis infection. Guangdong Med J 2011; 10:1317–9. [Google Scholar]
- 77. Xiaofei L, Junhui X, Yaling W, Lili Z, Songqin LV, Jun Z. Application value of in vitro release test of Mycobacterium tuberculosis associated interferon gamma in diagnosis of tuberculosis. Chinese J Clin Lab Sci 2011; 5:342–3. [Google Scholar]
- 78. Kan-kan G, Jun J, Zhong-xiang T. Comparison of detection performances between two kits for Mycobacterium tuberculosis infection. J Shanghai Jiaotong University 2011; 10:1440–3. [Google Scholar]
- 79. Jia-wen L, Li-jun K, Sheng-feng W, Xin-ying Z. Diagnostic value of quantitative diagnostic kit for Mycobacterium tuberculosis-IFN-gamma; release assay. Chin J Antitubercul 2011; 33:600–3. [Google Scholar]
- 80. Xiao-fei L, Ming-wu L, Jun-hua S, et al. Value of in vitro IFN-γ release assay in detection of Mycobacterium tuberculosis. Chinese J Nosocomiol 2012; 13:2952–4. [Google Scholar]
- 81. Lahong Z, Liquan H, Xian L, Shengxiang G, Liqun X, Zhaojun C. Application of Mycobacterium tuberculosis specific protein interferon-gamma release assay in tuberculosis diagnosis and therapeutic monitoring. J Radioimmunol 2013; 6:760–4. [Google Scholar]
- 82. Jianzhou Z, Yazuo W, Xiaowei Y. Comparison of the performance of two interferon gamma release tests in the diagnosis of tuberculosis. Chinese J Clin Lab Sci 2013; 7:556–7. [Google Scholar]
- 83. Qing Z. Clinical study of tuberculosis specific antigen interferon gamma release assay in the diagnosis of Mycobacterium tuberculosis infection. China Health Care Nutr 2013; 7:208–9. [Google Scholar]
- 84. Xiaoyan W, Hui L, Jun Z, Zheng Z, Haixia D, Fangling X. Value of TB-IGRA test in the diagnosis of tuberculosis. J Clin Pulm Med 2014; 5:944–5. [Google Scholar]
- 85. Chun-ling Z, Yuan-pei H, Su-zhen P, Cheng-yong L. Evaluation of IFN-γ release assay in the diagnosis of tuberculosis. J Clin Pulm Med 2014; 10:1833–5. [Google Scholar]
- 86. Yuan-hua Y, Yong-hui W, Xiao-hong X, Yu H. Clinical application of interferon gamma release assays for diagnosis of latent tuberculosis infection in children. Chinese J Biochem Pharmaceut 2014; 3:103–7. [Google Scholar]
- 87. Yong H, Tangbin Y. Comparison of two T-cell detection tests for tuberculosis infection. Chin J Lab Diagnos 2014; 12:2058–9. [Google Scholar]
- 88. Jian W. Value of interferon gamma release test in diagnosis of active tuberculosis infection of the chest. Harbin Med J 2015; 35:65–6. [Google Scholar]
- 89. Xiao WW, Li H, Hu H, et al. Comparison of the value of two tuberculosis-IFN-γ release assay kits in the diagnosis of tuberculosis. Chin J Antitubercul 2015; 37:768–73. [Google Scholar]
- 90. Yingdi C. Diagnostic value of interferon in vitro release enzyme-linked immunoassay for tuberculosis. Exp Lab Med 2016; 2:215–8. [Google Scholar]
- 91. Jinlan C, Yi Q, Yanlan Z, Shuran H. Sensitivity and specificity of interferon gamma release assay in diagnosis of tuberculosis and extrapulmonary tuberculosis. J Lab Med Clin Sci 2016; 21:3021–5. [Google Scholar]
- 92. Zheng-cai X, Jin Z, Mei-xia Z, Xiao-xiang L. Research on the clinical application of the domestic TB-IGRA kit in the rapid diagnosis of smear-negative pulmonary TB. Chin J Health Lab Technol 2016; 12:1680–2. [Google Scholar]
- 93. Chun-xian P, Xiao-xiang L, Wei-li L, Shun W, Zhi-yu W, Ai-hua S. Comparative research on the application value of different IGRA reagants in the diagnosis of tuberculosis. Chin J Health Lab Technol 2016; 12:1699–701. [Google Scholar]
- 94. Na L, Shan Q, Hui-ling L, De-bing M. Application and diagnostic significance of interferon in vitro release enzyme linked immunosorbent assay in clinical diagnosis of tuberculosis. Chin J Health Lab Technol 2017; 12:1770–2. [Google Scholar]
- 95. Weiguang F. Study of gamma-interferon release test for the diagnosis of Mycobacterium tuberculosis infection [thesis]. Shijiazhuang, China: Hebei Medical University,2015:1–41.
- 96. Qian F, Wang W, Qiu Z, et al. Evaluation of a new tuberculosis-related interferon gamma release assay for tuberculosis infection diagnosis in Huzhou, eastern China. Indian J Pathol Microbiol 2013; 56:125–8. [DOI] [PubMed] [Google Scholar]
- 97. Wan K, Qiu Y, Wang Y, et al. Multicenter clinical evaluation of three commercial reagent kits based on the interferon-gamma release assay for the rapid diagnosis of tuberculosis in China. Int J Infect Dis 2015; 40:108–12. [DOI] [PubMed] [Google Scholar]
- 98. Zhou J, Kong C, Shi Y, Zhang Z, Yuan Z. Comparison of the interferon-gamma release assay with the traditional methods for detecting Mycobacterium tuberculosis infection in children. Medicine (Baltimore) 2014; 93:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhu M, Zhu Z, Yang J, Hu K. Performance evaluation of IGRA-ELISA and T-SPOT.TB for diagnosing tuberculosis infection. Clin Lab 2019; 65. [DOI] [PubMed] [Google Scholar]
- 100. Jung J, Jhun BW, Jeong M, et al. Is the new interferon-gamma releasing assay beneficial for the diagnosis of latent and active Mycobacterium tuberculosis infections in tertiary care setting? J Clin Med 2021; 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yoo IY, Lee J, Choi AR, et al. Comparative evaluation of standard E TB-Feron ELISA and QuantiFERON-TB Gold Plus assays in patients with tuberculosis and healthcare workers. Diagnostics (Basel) 2021; 11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kothari N. Clinical performance of STANDARD E TB-Feron ELISA in culture-confirmed samples. Medical Buyer 2018; 37:1–2. [Google Scholar]
- 103. Oh CE, Ortiz-Brizuela E, Bastos ML, Menzies D. Comparing the diagnostic performance of QuantiFERON-TB Gold Plus to other tests of latent tuberculosis infection: a systematic review and meta-analysis. Clin Infect Dis 2021; 73:e1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chiacchio T, Petruccioli E, Vanini V, et al. Polyfunctional T-cells and effector memory phenotype are associated with active TB in HIV-infected patients. J Infect 2014; 69:533–45. [DOI] [PubMed] [Google Scholar]
- 105. Lancioni C, Nyendak M, Kiguli S, et al. CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am J Respir Crit Care Med 2012; 185:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rozot V, Vigano S, Mazza-Stalder J, et al. Mycobacterium tuberculosis–specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol 2013; 43:1568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lamberti M, Uccello R, Monaco MGL, et al. Tuberculin skin test and QuantiFERON test agreement and influencing factors in tuberculosis screening of healthcare workers: a systematic review and meta-analysis. J Occup Med Toxicol 2015; 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Campbell JR, Krot J, Elwood K, Cook V, Marra F. A systematic review on TST and IGRA tests used for diagnosis of LTBI in immigrants. Mol Diagn Ther 2015; 19:9–24. [DOI] [PubMed] [Google Scholar]
- 109. Ayubi E, Doosti-Irani A, Sanjari Moghaddam A, Sani M, Nazarzadeh M, Mostafavi E. The clinical usefulness of tuberculin skin test versus interferon-gamma release assays for diagnosis of latent tuberculosis in HIV patients: a meta-analysis. PLoS One 2016; 11:e0161983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.