Abstract
Background
Current understanding of severe respiratory syncytial virus (RSV) infections in adults is limited by clinical underrecognition. We compared the prevalence, clinical characteristics, and outcomes of RSV infections vs influenza in adults hospitalized with acute respiratory illnesses (ARIs) in a prospective national surveillance network.
Methods
Hospitalized adults who met a standardized ARI case definition were prospectively enrolled across 3 respiratory seasons from hospitals participating across all sites of the US Hospitalized Adult Influenza Vaccine Effectiveness Network (2016–2019). All participants were tested for RSV and influenza using real-time reverse-transcription polymerase chain reaction assay. Multivariable logistic regression was used to test associations between laboratory-confirmed infection and characteristics and clinical outcomes.
Results
Among 10 311 hospitalized adults, 6% tested positive for RSV (n = 622), 18.8% for influenza (n = 1940), and 75.1% negative for RSV and influenza (n = 7749). Congestive heart failure (CHF) or chronic obstructive pulmonary disease (COPD) was more frequent with RSV than influenza (CHF: 37.3% vs 28.8%, P < .0001; COPD: 47.6% vs 35.8%, P < .0001). Patients with RSV more frequently had longer admissions (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.06–1.80) for stays >1 week) and mechanical ventilation (OR, 1.45; 95% CI, 1.09–1.93) compared with influenza but not compared with the influenza-negative group (OR, 1.03; 95% CI, .82–1.28 and OR, 1.17; 95% CI, .91–1.49, respectively).
Conclusions
The prevalence of RSV across 3 seasons was considerable. Our findings suggest that those with RSV have worse outcomes compared with influenza and frequently have cardiopulmonary conditions. This study informs future vaccination strategies and underscores a need for RSV surveillance among adults with severe ARI.
Keywords: respiratory syncytial virus, influenza, hospitalization, adults
In this study, we evaluated adults hospitalized with respiratory syncytial virus (RSV), influenza, or neither among participants of a multicenter surveillance network. Those with RSV were more likely to have cardiopulmonary comorbidities, experience a longer stay, and require mechanical ventilation.
Respiratory syncytial virus (RSV) is widely regarded as a disease of young children and is most severe in children aged <2 years [1, 2]. However, clinically significant RSV infection occurs at all ages, and adults present with illness that ranges from mild upper respiratory infections to severe respiratory distress [3, 4]. Prior research has identified the following patient risk factors for RSV-associated hospitalization: severe immunocompromising conditions, underlying cardiopulmonary comorbidities including congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD), and age with frailty [5–10]. Epidemiologic descriptions of RSV from retrospective studies are limited by undertesting, particularly among hospitalized adults. Results from such research have the potential to inform targeted vaccination strategies to protect high-risk adults from adverse health outcomes as safe and effective vaccines become available.
Hospitalizations and in-hospitalization outcomes, such as intensive care unit (ICU) admission and the need for mechanical ventilation, are widely accepted as markers of clinical severity with respect to acute respiratory illness (ARI). Moreover, hospitalization with influenza is well documented and well researched, making it a strong benchmark for comparing RSV hospitalization outcomes [11–14]. A prior analysis of 1 Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN) site (Michigan) concluded that patients hospitalized with RSV, compared with patients hospitalized with influenza, had more comorbidities and a longer symptomatic period prior to hospital admission (days) [13].
In this study, our aim was to characterize the frequency and clinical severity of RSV among hospitalized adults aged ≥18 years for 3 respiratory illness seasons (2016–2019) across surveillance sites in 4 states participating in the HAIVEN study. We had 2 objectives: to identify population characteristics and key differences among adults (aged ≥18 years) and to compare clinical outcomes among adults hospitalized with RSV, influenza, or neither from all sites participating in the HAIVEN study.
METHODS
Source Population
Data collected for this analysis came from the HAIVEN study, which is a prospective study of adults hospitalized, defined as having an admission order, with an ARI and with a respiratory specimen collected ≤10 days after illness onset and ≤72 hours from hospital admission. ARI was determined by daily review of chief complaints, admitting diagnoses, and clinical notes against a standardized list of conditions/syndromes associated with influenza-like illness or ARI. Eligible conditions also included exacerbations of cystic fibrosis, CHF, asthma, or COPD accompanied by at least 1 systemic sign or symptom of infection and altered mental status accompanied by new onset of a respiratory symptom [12, 13, 15]. The HAIVEN study was designed as a laboratory-confirmed case–test negative control study aimed at estimating vaccine effectiveness in the prevention of hospitalization associated with adult influenza cases [12, 13, 15]. Adults aged ≥18 years admitted to a participating HAIVEN site hospital were prospectively identified from September 2016 through May 2019 through chief complaint(s) and/or admission diagnosis of an ARI. HAIVEN was comprised of 8 hospitals in 2016–2017, 9 in 2017–2018, and 10 in 2018–2019, with locations in Michigan, Pennsylvania, Texas, and Tennessee [16]. Participating sites included both academic medical centers and community hospitals. HAIVEN study eligibility criteria have been described elsewhere [12, 13, 15, 16]. To participate in the study, written informed consent was provided by patients or a proxy/surrogate. The University of Michigan Institutional Review Board, the Vanderbilt University Medical Center Institutional Review Board, the University of Pittsburgh Human Research Protection Office, and the Baylor Scott & White Research Institute Institutional Review Board gave ethical approval for this work. The Centers for Disease Control and Prevention (CDC) Institutional Review Board issued a reliance on site-specific approvals.
Data Collection
Through structured enrollment interviews with HAIVEN research staff, consenting participants self-reported, or reported via proxy/surrogate where appropriate, demographic data, illness onset date, frailty score (0, 1, 2, 3, 4/5), and current and prior season influenza vaccination status (yes/no). At the time of the enrollment interview, research staff collected throat and nasal swabs that were combined in universal transport media, or clinical mid-turbinate swabs were tested with either singleplex or multiplex respiratory pathogen panel molecular assays per site protocol. Specimens were transported to HAIVEN site laboratories and tested for RSV and influenza using real-time reverse-transcription polymerase chain reaction assay with primers, probes, and protocols developed by the CDC Division of Viral Diseases and Influenza Division or with existing clinical laboratory assays following completion of CDC proficiency testing. Electronic medical records (EMRs) were reviewed to extract data for calculating Charlson comorbidity index (CCI) scores (0, 1–2, and ≥3), determining body mass index (BMI) as well as documented evidence of COPD, CHF, and asthma from corresponding International Classification of Diseases, Tenth Revision, Clinical Modification, codes obtained from patient health records. Obesity was defined as having a BMI ≥ 30 kg/m2. The outcomes of interest were also extracted from participant EMRs, including length of stay, ICU admission, need for invasive or noninvasive mechanical ventilation, and death prior to or 30 days after discharge. An extended length of stay was defined as longer than 1 week (≥8 days). Frailty was determined subjectively using 5 self-reported questions to assess ability to complete daily tasks and energy to engage in interests (eg walking 100 yards or lifting/carrying 10 lb) and unintended weight loss, and responses were dichotomized and summed to create a score ranging from 0 (not frail) to 5 (very frail).
Statistical Analyses
Descriptive statistics were calculated for the following variables across each comparison group of interest: age group (18–49, 50–64, and 65+ years), sex, race/ethnicity (White non-Hispanic, Black non-Hispanic, other non-Hispanic, and Hispanic), BMI (normal, overweight, and obese), CCI scores, asthma, CHF, COPD, frailty, site (Michigan, Texas, Pennsylvania, and Tennessee), season (2016–2017, 2017–2018, and 2018–2019), and influenza vaccination status. Descriptive statistics were calculated for in-hospital outcomes of interest across each comparison group of interest, including extended length of stay, ICU admission, mechanical ventilation (any), and death (pre-discharge and 30 days post-discharge). CCI scores, time from illness onset to hospital admission, and time from illness onset to laboratory specimen collection were described with median and interquartile range (IQR) statistics. CCI scores for individuals who tested positive for RSV were compared with those for individuals who tested positive for influenza using Wilcoxon rank sum tests, stratified by age group (18–49, 50–64, and 65+ years). Overall and age-stratified (18–49, 50–64, and 65+ years) proportions of CHF and COPD for RSV-positive participants were compared with proportions of CHF and COPD for influenza-positive participants using χ2 statistics. Age-adjusted, Firth logistic regression models were used to test the association between CHF/COPD and RSV detection compared with influenza detection.
We evaluated characteristics and clinical outcomes by comparing RSV-positive vs the following 2 comparison groups separately: influenza-positive and RSV-negative/influenza-negative cases. Multivariable logistic regression models were used to assess participant characteristics as risk factors, that is, age, sex, CCI score, BMI, site, season, time from illness onset to admission, and time from illness onset to specimen collection, associated with case detection status using the 2 comparison groups. Multivariable logistic regression models were used to test the association between clinical outcomes of interest, that is, extended length of stay, ICU admission, need for any mechanical ventilation, and death, and case detection status using the above comparison groups. Participant characteristics and clinical outcome multivariable models were adjusted for age, race/ethnicity, sex, BMI, CCI score, site, season, and time from illness onset to admission. To account for small cell counts resulting from stratification, all logistic models used Firth adjustment [17]. Profile-likelihood confidence intervals and Wald P values were used to determine statistical significance. A P value < .05 was considered statistically significant for all analyses, and all analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Population Characteristics and RSV–Influenza Epidemiology
A total of 11 369 adults hospitalized with an ARI were enrolled in HAIVEN across the 3 respiratory seasons included in this study. Subsequent enrollments within a given respiratory illness season (n = 602) and/or participants missing influenza or RSV laboratory results (n = 499) and those with an RSV–influenza coinfection (n = 21) were excluded from analysis. The final study population for this analysis was comprised of 10 311 inpatients, 26.0% (n = 2679) from 2016–2017, 37.7% (n = 3885) from 2017–2018, and 36.3% (n = 3747) from 2018–2019 (Table 1).
Table 1.
Epidemiologic Characteristics Among Participants Hospitalized With Acute Respiratory Illness
| Characteristic | Total (N = 10 311) |
RSV+ (n = 622; 6%) | Influenza+ (n = 1940; 18.8%) | RSV−/Influenza− (n = 7749; 75.2%) |
|---|---|---|---|---|
| Age group, n (%), y | ||||
| 18–49 | 2300 (22.3) | 109 (17.5) | 391 (20.2) | 1800 (23.2) |
| 50–64 | 3423 (33.2) | 187 (30.1) | 592 (30.5) | 2644 (34.1) |
| ≥65 | 4588 (44.5) | 326 (52.4) | 957 (49.3) | 3305 (42.7) |
| Sex | ||||
| Female | 5760 (55.9) | 395 (63.5) | 1086 (56.0) | 4279 (55.2) |
| Race/Ethnicity, n (%) | ||||
| White, Non-Hispanic | 6675 (64.7) | 444 (71.4) | 1225 (63.1) | 5006 (64.6) |
| Black, Non-Hispanic | 2852 (27.7) | 137 (22.0) | 569 (29.3) | 2146 (27.7) |
| Other, Non-Hispanic | 310 (3.0) | 15 (2.4) | 57 (2.9) | 238 (3.1) |
| Hispanic | 474 (4.6) | 26 (4.2) | 89 (4.6) | 359 (4.6) |
| Body mass index, n (%),a kg/m2 | ||||
| Normal (18.5–24.99) | 2090 (20.3) | 122 (19.6) | 403 (20.8) | 1565 (20.2) |
| Overweight (25–29.99) | 1771 (17.2) | 100 (16.1) | 380 (19.6) | 1291 (16.7) |
| Obese (≥30) | 3271 (31.7) | 223 (35.9) | 699 (36.0) | 2349 (30.3) |
| Charlson comorbidity index, n (%)b | ||||
| 0 | 1033 (10.0) | 50 (8.0) | 281 (14.5) | 702 (9.1) |
| 1–2 | 3361 (32.6) | 202 (32.5) | 658 (33.9) | 2501 (32.3) |
| ≥3 | 5917 (57.4) | 370 (59.5) | 1001 (51.6) | 4546 (58.7) |
| Asthma, n (%) | 2820 (27.3) | 177 (28.5) | 508 (26.2) | 2135 (27.6) |
| Congestive heart failure, n (%) | 3805 (36.9) | 232 (37.3) | 558 (28.8) | 3015 (38.9) |
| Chronic obstructive pulmonary disease, n (%) | 4506 (43.7) | 296 (47.6) | 694 (35.8) | 3516 (45.4) |
| Frailty score, n (%)c | ||||
| 0 | 1918 (18.6) | 110 (17.7) | 465 (24.0) | 1343 (17.3) |
| 1 | 2222 (21.6) | 161 (25.9) | 420 (21.7) | 1641 (21.2) |
| 2 | 2166 (21.0) | 117 (18.8) | 369 (19.0) | 1680 (21.7) |
| 3 | 1785 (17.3) | 102 (16.4) | 324 (16.7) | 1359 (17.5) |
| 4/5 | 2090 (20.3) | 123 (19.8) | 332 (17.1) | 1635 (21.1) |
| Site, n (%) | ||||
| Michigan | 2596 (25.2) | 137 (22.0) | 566 (29.2) | 1893 (24.4) |
| Texas | 2963 (28.7) | 175 (28.1) | 447 (23.0) | 2341 (30.2) |
| Pennsylvania | 2437 (23.6) | 180 (29.0) | 527 (27.2) | 1730 (22.3) |
| Tennessee | 2315 (22.5) | 130 (20.9) | 400 (20.6) | 1785 (23.1) |
| Season, n (%) | ||||
| 2016–2017 | 2679 (26.0) | 207 (33.3) | 457 (23.5) | 2015 (26.0) |
| 2017–2018 | 3885 (37.7) | 211 (33.9) | 948 (48.9) | 2726 (35.2) |
| 2018–2019 | 3747 (36.3) | 204 (32.8) | 535 (27.6) | 3008 (38.8) |
| Median time to admission, d (IQR) | … | 3 (1–5) | 3 (1–4) | 2 (1–5) |
| Median time to specimen collection, d (IQR) | … | 3 (2–5) | 3 (2–5) | 3 (1–5) |
| Influenza vaccination, n (%)d | 5381 (52.2) | 361 (58.0) | 981 (50.6) | 4039 (52.1) |
Abbreviations: IQR, interquartile range; RSV, respiratory syncytial virus.
30.8% (n = 3179) were missing body mass index data.
1.3% (n = 134) were missing Charlson comorbidity index.
1.3% (n = 130) were missing frailty data.
1.9% (n = 195) were missing influenza vaccination status data.
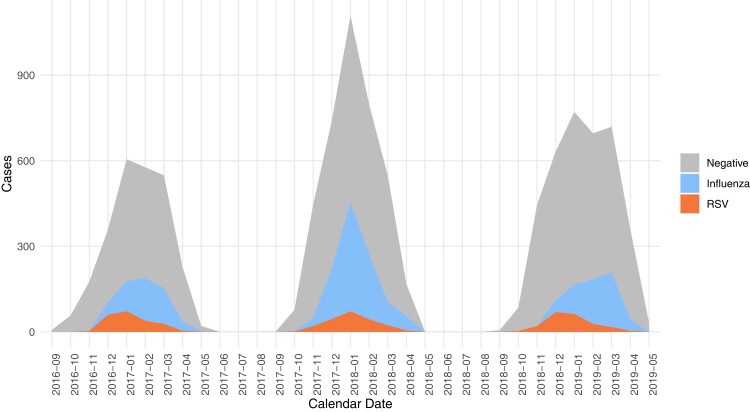
Overall, RSV was detected in 6.0% (n = 622) and influenza in 18.8% (n = 1940) of eligible participants. The remaining 75.2% (n = 7749) of included participants with an ARI had neither RSV nor influenza detected. RSV peaked in January for the 2016–2017 and 2017–2018 seasons but in December for 2018–2019 (Figure 1). On the other hand, the peak for influenza tended to vary, with peaks occurring in February, January, and March, respective to each season included in this analysis.
Figure 1.
Modified Hospitalized Adult Influenza Vaccine Effectiveness Network RSV influenza epidemic curve. Abbreviation: RSV, respiratory syncytial virus.
Just over half (52.4%) of all RSV cases in this study were among adults aged ≥65 years (Table 1). The age-specific proportion of RSV among adults hospitalized with an ARI that increased within each age group was 4.7% among those 18–49, 5.5% among those 50–64, and 7.1% among those ≥65 years. The age-specific prevalence of influenza was relatively consistent among those aged 18–49 (17%) and 50–64 (17.3%) years, with slightly higher (20.9%) detection among those aged ≥65 years. The median time from illness onset to hospital admission among RSV-positive patients was 3 days (IQR, 1–5) and similar to that among influenza-positive patients, which was 3 days (IQR, 1–4). Median time from illness onset to specimen collection for patients with influenza or RSV infection was 3 days (IQR, 2–5; Table 1).
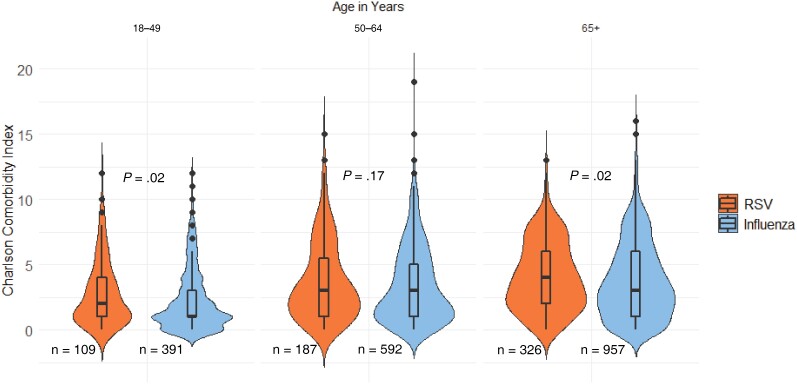
Overall, 90% (n = 9278) of included participants had a CCI score ≥1, indicating the presence of at least 1 underlying chronic comorbid condition (Table 1); this finding was expected given that all patients were hospitalized. The overall proportion of adults with CHF or COPD was significantly higher (CHF, P < .0001; COPD, P < .0001; X2 test) in those with RSV (37.3%, CHF; 47.6%, COPD) compared with those with influenza (28.8%, CHF; 35.8%, COPD; Table 1). When stratified by age group, among adults aged 50–64 years, the proportions of CHF and COPD between RSV-positive (31.0%, CHF; 47.1%, COPD) and influenza-positive (25.0%, CHF; 43.4%, COPD) patients were comparable (CHF, P = .10; COPD, P = .38; X2 test). Among adults aged 18–49 years, RSV-positive patients had higher proportions of CHF and COPD (26.6%, CHF; 29.4%, COPD) when compared with influenza-positive patients (14.3%, CHF; 13.6%, COPD; CHF, P = .003; COPD, P = .0001; X2 test). In adults aged ≥65 years, those with RSV had higher proportions of CHF and COPD (44.5%, CHF; 54.0%, COPD) when compared with those with influenza (37.0%, CHF; 40.1%, COPD; CHF, P = .02; COPD, P < .0001; X2 test). Adjusting for age, when compared with inpatients without CHF, those with CHF had significantly higher odds of having RSV compared with influenza (ORadj, 1.40; 95% CI, 1.15–1.70; P = .0007). Adjusting for age, when compared with inpatients without COPD, those with COPD had significantly higher odds of having RSV compared with influenza (ORadj, 1.57; 95% CI, 1.31–1.89; P < .0001). In the age groups of 18–49 years and ≥65 years, those with RSV had significantly higher median CCI scores of 2 (1–4) and 4 (2–6) when compared with those with influenza of 1 (1–3) and 3 (1–6), respectively (P = .02 each; Figure 2).
Figure 2.
Violin plot comparing RSV and influenza participant Charlson comorbidity index stratified by age. Abbreviation: RSV, respiratory syncytial virus.
Clinical Outcomes
Among RSV-positive inpatients, 16.6% experienced a length of hospital stay ≥8 days, 12.4% were admitted to the ICU, and 15% required some type of mechanical ventilation, either invasive or noninvasive (Table 2). In contrast, among patients with influenza, 11.3% experienced a length of stay ≥8 days, 9.9% were admitted to the ICU, and 11.1% required some type of mechanical ventilation (Table 2). With respect to patient deaths captured by EHR, 9 (1.5%) occurred in RSV-positive patients, 25 (1.3%) in patients with influenza, and 105 (1.4%) in the RSV and influenza test-negative group (Table 2).
Table 2.
Factors Associated With Case Detection Using Adjusted Logistic Regression Models
| Characteristic | RSV+ vs Influenza+ a,b | P Valuec | RSV+ vs RSV−/Influenza− a,b | P Valuec |
|---|---|---|---|---|
| Charlson comorbidity index | (n = 2562) | … | (n = 8371) | … |
| 0 | Ref | … | Ref | … |
| 1–2 | 1.69 (1.20–2.40) | .003d | 1.01 (0.73–1.40) | .94 |
| ≥3 | 2.10 (1.50–2.93) | <.0001d | 0.93 (0.68–1.28) | .67 |
| Age group, y | (n = 2562) | … | (n = 8371) | … |
| 18–49 | Ref | … | Ref | … |
| 50–64 | 1.04 (0.79–1.37) | .80 | 1.18 (0.92–1.51) | .19 |
| ≥65 | 1.02 (0.78–1.32) | .89 | 1.65 (1.30–2.10) | <.0001d |
| Sex | (n = 2562) | … | (n = 8371) | … |
| Male | Ref | … | Ref | … |
| Female | 1.44 (1.19–1.75) | .0002d | 1.38 (1.16–1.63) | .0003d |
| Body mass index,e kg/m2 | (n = 1927) | … | (n = 5650) | … |
| Normal (18.5–24.99) | Ref | … | Ref | … |
| Overweight (25–29.99) | 0.90 (0.66–1.22) | .48 | 1.00 (0.76–1.32) | .99 |
| Obese (≥30) | 1.13 (0.87–1.47) | .35 | 1.29 (1.02–1.63) | .03d |
| Site | (n = 2562) | … | (n = 8371) | … |
| Michigan | Ref | … | Ref | … |
| Texas | 1.43 (1.07–1.92) | .02d | 0.90 (0.70–1.15) | .40 |
| Pennsylvania | 1.14 (0.86–1.53) | .36 | 1.35 (1.06–1.72) | .02d |
| Tennessee | 1.11 (0.82–1.49) | .51 | 0.90 (0.70–1.16) | .43 |
| Season | (n = 2562) | … | (n = 8371) | … |
| 2016–2017 | Ref | … | Ref | … |
| 2017–2018 | 0.47 (0.38–0.59) | <.0001d | 0.75 (0.61–0.92) | .006d |
| 2018–2019 | 0.80 (0.57–1.11) | .18 | 0.62 (0.50–0.76) | <.0001d |
| Time from symptom onset to hospital admission, d | (n = 2562) 1.05 (1.01–1.09) |
.01d | (n = 8371) 1.02 (0.99–1.05) |
.17 |
| Time from symptom onset to specimen collection, d | (n = 2562) 1.07 (0.99–1.15) |
.08 | (n = 8371) 1.00 (0.94–1.06) |
.94 |
Abbreviation: RSV, respiratory syncytial virus; n, indicates number of observations in regression model.
Odds ratios (OR) determined by Firth penalized logistic regression with profile-likelihood 95% confidence intervals.
Adjusted for age, race/ethnicity, sex, body mass index (BMI), Charlson comorbidity index, study site, season, and time from illness onset to admission.
Wald P values.
Statistically significant P value at α = .05.
2721 were with missing BMI data were excluded.
After adjusting for potential confounders, when compared with influenza-positive inpatients, RSV-positive patients had significantly higher odds of experiencing an extended length of stay ≥8 days (ORadj, 1.40; 95% CI, 1.08–1.82; P = .01) and a need for mechanical ventilation (ORadj, 1.46; 95% CI, 1.09–1.94; P = .01; Table 3). There were no significant death or other clinical outcome associations when inpatients with RSV were compared with the RSV-negative, influenza-negative group.
Table 3.
Frequencies and Odds Ratios of Clinical Outcomes Comparing Adults Hospitalized With Acute Respiratory Illness
| n (%) | ||||
|---|---|---|---|---|
| Frequency of Outcomes | Total (N = 10 311) |
RSV (n = 622) |
Influenza (n = 1940) |
RSV−/Influenza− (n = 7749) |
| Extended LOS (≥8 d) | 1616 (15.7) | 103 (16.6) | 220 (11.3) | 1293 (16.7) |
| ICU admissiona | 1340 (13.0) | 77 (12.4) | 192 (9.9) | 1071 (13.8) |
| Mechanical ventilation, anya | 1447 (14.0) | 93 (15.0) | 215 (11.1) | 1139 (14.7) |
| Death | ||||
| During hospitalizationb | 139 (1.4) | 9 (1.5) | 25 (1.3) | 105 (1.4) |
| 30 d post-dischargec | 222 (2.2) | 11 (1.8) | 23 (1.2) | 188 (2.4) |
| Adjusted logistic regression models odds ratio (95% confidence interval)d | ||||
| RSV vs Influenza |
P valuee | RSV vs RSV−/Influenza- | P valuee | |
| Extended LOS (≥8 d) | (n = 2562) 1.40 (1.08–1.82) |
.01f | (n = 8371) 1.03 (0.82–1.28) |
.83 |
| ICU admissiona | (n = 2558) 1.27 (0.95–1.69) |
.11 | (n = 8359) 0.95 (0.74–1.22) |
.70 |
| Mechanical ventilation, anya | (n = 2558) 1.46 (1.09–1.94) |
.01f | (n = 8359) 1.17 (0.91–1.49) |
.21 |
| In-hospital mortality | (n = 2537) 0.94 (0.46–1.94) |
.88 | (n = 8325) 1.19 (0.61–2.31) |
.61 |
| 30-d mortality | (n = 1863) 1.36 (0.65–2.84) |
.42 | (n = 6516) 0.72 (0.38–1.37) |
.32 |
Abbreviation: ICU, intensive care unit; LOS, length of stay; RSV, respiratory syncytial virus; n, number of observations overall, and by model.
0.14% (n = 14) were missing ICU and mechanical ventilation data.
0.6% (n = 63) were missing inpatient death data.
23.0% (n = 2376) were missing death after discharge data.
Adjusted for age, sex, race, Charlson comorbidity index, body mass index, site, season, and days to admission.
Wald P values.
Denotes statistical significance at α = .05.
Participant Characteristics as Risk Factors
Patients with laboratory-confirmed RSV had twice the odds of having a CCI score ≥ 3 compared with patients with influenza, which was statistically significant (ORadj, 2.10; 95% CI, 1.50–2.93; P <.0001; Table 3). Obesity (ORadj, 1.29; 95% CI, 1.02–1.63; P = .03) and age ≥65 years (ORadj, 1.65; 95% CI, 1.30–2.10; P <.0001) were significantly associated with RSV detection when compared with participants negative for both RSV and influenza. Female sex was significantly associated with RSV detection when compared with patients with influenza (ORadj, 1.44; 95% CI, 1.19–1.75; P = .0002) as well as RSV-negative, influenza-negative patients (ORadj, 1.38; 95% CI, 1.16–1.63; P = .0003).
DISCUSSION
Our goal in this study was to compare characteristics and clinical outcomes among adults hospitalized with an ARI between 2016 and 2019 from hospitals at 4 sites participating in the HAIVEN study with respect to laboratory confirmation of RSV, influenza, or neither virus. Hospitalized adults with RSV had a greater overall proportion of underlying cardiopulmonary comorbidities and higher CCI scores when compared with those with influenza. Specifically, adults aged 18–49 and 65+ years with RSV had significantly higher median CCI scores when compared with those with influenza, and the proportion of adults with CHF or COPD was significantly higher in those with RSV compared with those with influenza. With respect to clinical outcomes, hospitalized adults with RSV had higher odds of hospitalization exceeding 1 week and need for invasive or noninvasive mechanical ventilation when compared with those with influenza. Findings related to CCI score in our study are consistent with the findings of Malosh et al [13], and our findings corroborate studies that identified adults with a history of CHF and/or COPD as a high-risk group for infection with RSV [7, 10].
Results from our clinical outcomes analysis indicate that RSV-associated ARI may be more severe than influenza-associated ARI in some instances. Fifty percent of adults with influenza detected had received an influenza vaccine, which may offer some protection against more severe influenza-associated outcomes. Additionally, increased illness severity experienced by patients with RSV may be explained in part by the presence of increased baseline cardiopulmonary diseases in these patients. Furthermore, many adults hospitalized with influenza receive antiviral treatments that are not available for RSV.
There were no differences in clinical outcomes when we compared the RSV-positive group with the RSV-negative/influenza-negative ARI group. This double-negative group likely consisted of a combination of individuals with an ARI due to other untested pathogens and individuals with no known infectious etiology but a high level of chronic respiratory disease. Individuals with RSV were notably similar to individuals in this group with regard to proportions of CHF and COPD detected and contrasted the influenza group, which also had lower comorbidity measured by CCI score and were less likely to report high frailty, despite having a larger proportion of older adults compared with the RSV-negative/influenza-negative group. Overall, this comparison emphasizes the increased commonality of RSV among adults with higher-risk chronic conditions, similar to those hospitalized for non-RSV and noninfluenza illnesses, whereas influenza was more frequently seen in the relatively healthier subgroups.
When compared with each of those with influenza detected as well as RSV-negative, influenza-negative ARI participants, female sex was significantly associated with RSV detection, contrasting a previous study of 2225 adults aged ≥50 years with medically attended an ARI [18]. Our finding could be in part due to a larger sample size as well as the inclusion of adults aged ≥18 years. It is possible that women are at higher risk of contracting RSV due to increased time spent among children, both personally and professionally. Alternatively, prior research suggests that biologic sex, through various mechanisms that include environmental factors such as close contact with young children in the household as well as the presence of underlying chronic conditions, plays a key role in differential incidence, immune response, and differences in severity of ARI [19–22].
The analysis represents a large sample size with considerable geographic diversity due to the inclusion of all sites as well as more recent seasons of the HAIVEN study. Compared with a 6-year retrospective study of adults hospitalized with RSV that was conducted in 1 US city, we captured more cases of RSV from the 3 seasons included in our analysis [23]. A strength of using the HAIVEN study is the implementation of prospective, active participant enrollment that does not solely depend on clinical test orders for case status determination [12]; however, study-prompted testing did not include RSV subtype. Missing data were not a restrictive analytic issue due to data collection from participant EMRs. However, death post-discharge was missing for a considerable number of observations, which is expected given the potential for loss to follow-up in large, hospital-based studies, limiting our analysis of that outcome. Of greater concern may be the impact of missing enrollments, either through declination of eligible participants or for admissions that may have been missed during times of reduced staffing, despite efforts to enroll previous admissions following holidays and weekends. We were not able to systematically assess this element of missingness and were unable to adjust for this. Generalizability of this study is limited, and precaution should be taken when interpreting these results overall and for populations that are not predominantly non-Hispanic.
Multiple RSV vaccine candidates in various stages of clinical trials have demonstrated promising results of safety and efficacy. Determining which populations to focus on during initial vaccine rollout is a high-priority discussion that should happen prior to availability of a vaccine. Yamin et al posit that targeting children aged <5 years as a vaccine strategy would be the most efficient method of reducing RSV cases in children as well as adults and the elderly through indirect protection [24]. In a review by Stephens and Varga, the authors emphasize the need for a vaccine tailored to elderly populations that will elicit a high immune response to overcome immune dysfunction often associated with aging [25].
In this prospective, multisite study, we have confirmed the importance of RSV as a cause of a significant proportion of hospitalization in adults. We found those with underlying cardiopulmonary conditions to be a critical target for future campaigns as vaccines become available. Our data highlight the need for RSV prevention and testing for adults, including adults aged <65 years.
Contributor Information
Katherine M Begley, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Arnold S Monto, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Lois E Lamerato, Department of Public Health Sciences, Henry Ford Health, Detroit, Michigan, USA.
Anurag N Malani, Department of Medicine, Section of Infectious Diseases, Trinity Health St Joseph Mercy Hospital, Ann Arbor, Michigan, USA; Department of Infection Prevention and Control, Trinity Health St Joseph Mercy Hospital, Ann Arbor, Michigan, USA.
Adam S Lauring, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan, USA.
H Keipp Talbot, Department of Medicine and Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Manjusha Gaglani, Department of Pediatrics, Section of Pediatric Infectious Diseases, Baylor Scott & White Health, Temple, Texas, USA; Department of Medical Education at Texas A&M University, College of Medicine, Temple, Texas, USA.
Tresa McNeal, Department of Medical Education at Texas A&M University, College of Medicine, Temple, Texas, USA; Department of Internal Medicine, Section of Hospital Medicine, Baylor Scott & White Health, Temple, Texas, USA.
Fernanda P Silveira, University of Pittsburgh and University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Richard K Zimmerman, University of Pittsburgh and University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Donald B Middleton, University of Pittsburgh and University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Shekhar Ghamande, Department of Medical Education at Texas A&M University, College of Medicine, Temple, Texas, USA; Department of Internal Medicine, Section of Critical Care and Pulmonary Medicine, Baylor Scott & White Health, Temple, Texas, USA.
Kempapura Murthy, Data/Biostatistics Research Core, Baylor Scott & White Health, Temple, Texas, USA.
Lindsay Kim, Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; US Public Health Service, Rockville, Maryland, USA.
Jill M Ferdinands, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Manish M Patel, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Emily T Martin, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Notes
Disclaimer. The findings and conclusions presented here are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC), the US Public Health Service, or the funding agency.
Financial support. This work was supported by the CDC (cooperative agreement IP15-002; grants U01IP000979 to the Tennessee site, U01IP000974 to the Michigan site, U01IP000972 to the Texas site, and U01IP000969 to the Pennsylvania site) and, in part, at the Pennsylvania site by the National Institutes of Health (NIH) Clinical and Translational Science Award Program (grant UL1TR001857). A. N. M. reports a research grant from the CDC for the US Hospital Vaccine Effectiveness Network (5 U01IP000974-05). S. G. reports support for this work from the CDC Hospitalized Adult Influenza Vaccine Effectiveness Network.
References
- 1. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012; 54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 2000; 13:371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walsh EE, Peterson DR, Falsey AR. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis 2007; 195:1046–51. [DOI] [PubMed] [Google Scholar]
- 5. Englund JA, Sullivan CJ, Jordan MC, Dehner LP, Vercellotti GM, Balfour HH. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med 1988; 109:203–8. [DOI] [PubMed] [Google Scholar]
- 6. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 7. Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173:639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis 1999; 179:25–30. [DOI] [PubMed] [Google Scholar]
- 9. Kujawski SA, Whitaker M, Ritchey MD, et al. Rates of respiratory syncytial virus (RSV)-associated hospitalization among adults with congestive heart failure—United States, 2015–2017. PLoS One 2022; 17:e0264890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walsh EE, Falsey AR, Hennessey PA. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am J Respir Crit Care Med 1999; 160:791–5. [DOI] [PubMed] [Google Scholar]
- 11. Falsey AR, Cunningham CK, Barker WH, et al. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis 1995; 172:389–94. [DOI] [PubMed] [Google Scholar]
- 12. Ferdinands JM, Gaglani M, Martin ET, et al. Prevention of influenza hospitalization among adults in the United States, 2015–2016: results from the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J Infect Dis 2019; 220:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malosh RE, Martin ET, Callear AP, et al. Respiratory syncytial virus hospitalization in middle-aged and older adults. J Clin Virol 2017; 96:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Kerkhove MD, Vandemaele KAH, Shinde V, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 2011; 8:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrie JG, Ohmit SE, Cheng CK, et al. Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin Infect Dis 2016; 63:1017–25. [DOI] [PubMed] [Google Scholar]
- 16. Ghamande S, Shaver C, Murthy K, et al. Vaccine effectiveness against acute respiratory illness hospitalizations for influenza-associated pneumonia during the 2015–2016 to 2017–2018 seasons: US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). Clin Infect Dis 2022; 74:1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80:27–38. [Google Scholar]
- 18. Sundaram ME, Meece JK, Sifakis F, Gasser RA Jr, Belongia EA. Medically attended respiratory syncytial virus infections in adults aged ≥50 years: clinical characteristics and outcomes. Clin Infect Dis 2014; 58:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respir Med 2007; 101:1845–63. [DOI] [PubMed] [Google Scholar]
- 20. Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. BioEssays 2012; 34:1050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klein SL, Hodgson A, Robinson DP. Mechanisms of sex disparities in influenza pathogenesis. J Leukoc Biol 2012; 92:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt H, Das A, Nam H, Yang A, Ison MG. Epidemiology and outcomes of hospitalized adults with respiratory syncytial virus: a 6-year retrospective study. Influenza Other Respir Viruses 2019; 13:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamin D, Jones FK, DeVincenzo JP, et al. Vaccination strategies against respiratory syncytial virus. Proc Natl Acad Sci U S A 2016; 113:13239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stephens LM, Varga SM. Considerations for a respiratory syncytial virus vaccine targeting an elderly population. Vaccines 2021; 9:624. [DOI] [PMC free article] [PubMed] [Google Scholar]