Abstract
Alzheimer’s disease (AD) is a progressive, dementing, whole-body disorder that presents with decline in cognitive, behavioral, and emotional functions, as well as endocrine dysregulation. The etiology of AD is not fully understood but stress- and anxiety-related hormones may play a role in its development and trajectory. The glucocorticoid cascade hypothesis posits that levels of glucocorticoids increase with age, leading to dysregulated negative feedback, further elevated glucocorticoids, and resulting neuropathology. We examined the impact of age (from 2 to 10 months) and stressor exposure (predator odor) on hormone levels (corticosterone and ghrelin), anxiety-like behavior (open field and light dark tests), and memory-related behavior (novel object recognition; NOR), and whether these various measures correlated with neuropathology (hippocampus and cortex amyloid beta, Aβ) in male and female APPswe/PS1dE9 transgenic and non-transgenic mice. Additionally, we performed exploratory analyses to probe if the open field and light dark test as commonly used tasks to assess anxiety levels were correlated. Consistent with the glucocorticoid cascade hypothesis, baseline corticosterone increased with age. Predator odor exposure elevated corticosterone at each age, but in contrast to the glucocorticoid cascade hypothesis, the magnitude of stressor-induced elevations in corticosterone levels did not increase with age. Overall, transgenic mice had higher post-stressor, but not baseline, corticosterone than non-transgenic mice, and across both genotypes, females consistently had higher (baseline and post-stress) corticosterone than males. Behavior in the open field test primarily showed decreased locomotion with age, and this was pronounced in transgenic females. Anxiety-like behaviors in the light dark test were exacerbated following predator odor, and female transgenic mice were the most impacted. Compared to transgenic males, transgenic females had higher Aβ concentrations and showed more anxiety-like behavior. Performance on the NOR did not differ significantly between genotypes. Lastly, we did not find robust, statistically significant correlations among corticosterone, ghrelin, recognition memory, anxiety-like behaviors, or Aβ, suggesting outcomes are not strongly related on the individual level. Our data suggest that despite Aβ accumulation in the hippocampus and cortex, male and female APPswePS1dE9 transgenic mice do not differ robustly from their non-transgenic littermates in physiological, endocrine, and behavioral measures at the range of ages studied here.
Keywords: corticosterone, ghrelin, predator odor, anxiety, aging, between-task correlations, within-task correlations
1. INTRODUCTION
Alzheimer’s disease (AD) is a progressive dementing disorder which presents with decline in cognitive, behavioral, and emotional functions. The neuropathology is classically defined by the presence of amyloid beta (Aβ) plaques, neurofibrillary tau tangles, neuroinflammation, and widespread neuronal loss particularly in the hippocampus and neocortex (Jack et al., 2018). The etiology of this disease is not known, but several factors are associated with AD, including advanced age (Thies and Bleiler, 2012), female sex (Thies and Bleiler, 2012), previous diagnosis with depression or anxiety (Burton et al., 2013; Gao et al., 2013), and increased exposure to psychosocial stressors and accompanying elevated glucocorticoid levels (Caruso et al., 2018; Dong and Csernansky, 2019; Justice, 2018). Although the neuropathology associated with AD and the impact of AD on cognitive function are well established, recent data suggest AD is a whole-body disease, impacting multiple organ systems and many facets of organism physiology and behavior (Bu et al., 2018; Morris et al., 2014). Additionally, it is becoming increasingly clear that AD is not just associated with changes in cognitive function, but also with changes in emotionality, affective state, psychological stress response, and hypothalamic-pituitary-adrenal (HPA) axis function (Ismail et al., 2018). Importantly, neuropsychiatric symptoms (e.g., anxiety, depression, irritability, euphoria) may appear before the onset of cognitive deficits (Gallagher et al., 2017; Ismail et al., 2018), and when present with mild cognitive impairment, can increase odds of progressing from mild cognitive impairment to dementia (Ismail et al., 2016). These symptoms can be an expression of co-morbid states that increase the risk of development of AD or may be prodromal disease-related manifestations (Ismail et al., 2018). Therefore, understanding longitudinal, within-individual changes in behavior and neuropathology is important.
In humans, psychosocial stressor exposure is positively associated with increased AD diagnosis and pathogenesis (Dong and Csernansky, 2009; Hasegawa, 2007; Wilson et al., 2011, 2007, 2006, 2003), and higher self-reported stress is related to reduced hippocampal and prefrontal cortex volume, future cognitive decline, and dementia diagnosis (Gianaros et al., 2007; Johansson et al., 2012, 2010; Wang et al., 2012). Additionally, AD can present with dysregulation, generally overactivity, of the HPA axis (Carnes et al., 1983; Hartmann et al., 1997; Raskind et al., 1982; Weiner et al., 1997). In AD patients, higher cortisol levels correlate positively with increased dementia progression (Csernansky et al., 2006; Joshi and Praticò, 2013; Miller et al., 1998) and in subjects with probable AD, increased cortisol is related to higher severity of hippocampal volume loss (De Leon et al., 1988). These data align with the glucocorticoid cascade hypothesis of aging, which posits that age-associated increases in glucocorticoid levels lead to hippocampal changes, dysregulation of HPA axis function, decreased negative feedback leading to even greater increases in glucocorticoid levels, brain aging, and impaired cognition (Landfield et al., 2007; Sapolsky et al., 1986). This positive feedback cycle of stress and disease has recently been described as a “vicious cycle of stress” (Justice, 2018).
In AD, HPA axis dysregulation seems to be both a cause and a consequence of neuropathology and disease progression. As people age, HPA axis dysregulation leads to increased glucocorticoids which contribute to increased progression and severity of disease. As the disease progresses, increased neuropathology drives further HPA axis dysregulation and worsening of psychological stress (see Justice, 2018). Data from transgenic AD mouse models largely mirror the findings from humans in that AD pathophysiology is associated with and worsened by stressor exposure and elevated glucocorticoids (Dong et al., 2008; Green et al., 2006; Hendrickx et al., 2021; Rothman et al., 2012; Stuart et al., 2017). However, much less is known overall about how these interrelated changes in neuropathology and HPA axis function relate to changes in emotionality and other behaviors. Many studies focus on how stress and glucocorticoids drive brain changes and disease (e.g., Conrad, 2008), but fewer describe how disease progression impacts stress responsiveness and HPA axis function. To our knowledge no longitudinal corticosterone data from commonly used mouse models has been published and data on corticosterone in females from AD models are scarce, indicating that more holistic collection of variables for different ages and sexes would be desirable.
In addition to changes in HPA axis function, several mouse models of AD display elevated anxiety and altered affective state (see Janus and Westaway, 2001). For example, 12-month-old male 3xTgAD mice displayed higher anxiety (less distance traveled in open field) than non-transgenic mice under baseline and post-stress conditions (Rothman et al., 2012). These behavioral differences are in line with outcomes noted in human AD patients and suggest that AD-associated pathological changes can lead to affective symptoms, as emotional changes are often noted before cognitive deficits (Justice, 2018; Mahgoub and Alexopoulos, 2016).
It is currently unclear how amyloid beta, corticosterone, and other stress-responsive hormones, such as ghrelin, interact to influence anxiety-like behaviors, especially longitudinally, within individual animals. Ghrelin, administered into the cerebral ventricles, is anxiogenic in rats (Gahete et al., 2011). Acute stress is associated with increased ghrelin (Kristenssson et al., 2006) and increased anxiety-like behavior in rats (Carlini et al., 2002). Interestingly, ghrelin can also influence cognitive function, learning, memory, and can have neuroprotective effects (Albarran-Zeckler et al., 2011; Diano et al., 2006; Gahete et al., 2011). Moreover, ghrelin can prevent neurotoxic effects of Aβ oligomers in rat hippocampal neuronal cultures (Martins et al., 2013). In mice injected with Aβ oligomer in the hippocampus, peripheral ghrelin administration decreased Aβ-induced changes in the hippocampus, rescued memory, and prevented cholinergic fiber loss (Moon et al., 2011). Lastly, a recent study in 3xTgAD mice found that male, but not female, transgenic mice had higher baseline plasma ghrelin at 7 months of age compared to non-transgenic mice of the same sex (Robison et al., 2020). Thus, it seems that ghrelin may be an important variable for studies testing interactions of AD and stress (Reich and Hölscher, 2020).
Understanding how changes in HPA axis function, glucocorticoid levels, anxiety-like behavior, and cognition are related over time is important for determining how these factors impact neuropathology and disease progression over the lifespan. Further, it is important to determine if observed relationships between HPA axis function, glucocorticoid levels, anxiety, and cognitive function are similar between sexes (Hodes and Epperson, 2019; Lee, 2018) and whether they are consistent over time. In our study we addressed several questions about the relationship between hormones and behavior, while considering the effects of genotype and sex in the APPswe/PS1dE9 mouse model of AD-associated amyloidosis from adolescence to middle adulthood (see Table 1). Mice were tested from 2 to 10 months of age and exposed to light dark, open field, and object recognition tests under both baseline (no pre-test manipulation) and post-stress (predator odor exposure) conditions. Baseline and post-stress corticosterone was also determined at 2, 4, and 10 months of age to assess effects of genotypes, sex and age, and ghrelin levels (post-stress), brain (cortex and hippocampus) amyloid beta concentrations, and organ mass (brain, adrenal glands) were determined at 10 months. Overall, we expected that baseline and post-stress corticosterone levels and anxiety-like behaviors would increase with age whereas baseline and post-stressor recognition memory would decrease with age and that these outcome measures would interact with genotype and sex (see Table 1 for details).
Table 1.
Summary of questions, predictions, and outcomes of 10-month, longitudinal study in transgenic (Tg) APPswePS1dE9 male and female mice, and their non-transgenic (non-Tg) littermates.
| Questions and predictions | Outcome |
|---|---|
| 1) Do baseline and post-stress corticosterone differ between transgenic and non-transgenic mice over time and by sex? | |
| Baseline and post-stress corticosterone will increase with age (glucocorticoid cascade hypothesis) | Partially supported (baseline only) |
| Predator odor stress will increase corticosterone; interaction with genotype (Tg > non-Tg) | Partially supported (odor only) |
| Tg > non-Tg overall (main effect of genotype) | Partially supported (post-stress only) |
| Female > male; interaction with genotype (Tg females > Tg males) | Partially supported (sex only) |
| 2) Does baseline and post-stress anxiety-like behavior differ between transgenic and non-transgenic mice over time & by sex? | |
| Baseline anxiety will increase w/ age; interaction w/ genotype (Tg > non-Tg; Tg increase earlier) & w/ sex (F > M) | Not supported |
| Post-stress anxiety will increase w/ age; interaction w/ genotype (Tg > non-Tg) & w/ sex (F > M) | Partially supported (light dark) |
| Tg > non-Tg overall | Not supported |
| 3) Does baseline and post-stress recognition memory differ between transgenic and non-transgenic mice over time & by sex? | |
| Baseline memory will decrease with age, interaction w/ genotype (Tg greater decline) & w/ sex (F worse than M) | Not supported |
| Post-odor memory will decrease with age, interaction w/ genotype (Tg greater decline) & w/ sex (F worse than M) | Not supported |
| Tg < non-Tg overall | Not supported |
| 4) Do stress-induced ghrelin levels differ between transgenic and non-transgenic mice & by sex? | |
| Tg > non-Tg; interaction with sex (males only) | Not supported |
| 5) Are corticosterone, ghrelin, anxiety-like behavior, recognition memory, and brain amyloid beta correlated? | |
| Higher (baseline and/or post-stress) corticosterone, higher anxiety | Not supported (opposite) |
| Higher corticosterone, lower recognition memory | Not supported |
| Higher anxiety, lower recognition memory | Partially supported (some measures) |
| Higher post-stress ghrelin, higher post-stress anxiety | Not supported |
| In Tg: higher corticosterone, higher amyloid beta | Not supported |
| In Tg: higher amyloid beta, lower recognition memory | Not supported |
| In Tg: higher ghrelin, lower amyloid beta | Not supported |
| 6) Do organ masses differ by genotype? | |
| Adrenals: Tg > non-Tg | Not supported |
| Thymus: Tg < non-Tg | Not supported |
| Spleen: Tg < non-Tg | Not supported |
We predicted that, 1) in line with the glucocorticoid cascade hypothesis, baseline and post-stress corticosterone would increase with age, that transgenic mice would have higher corticosterone than non-transgenic mice, and that females would have higher corticosterone than males; 2) transgenic mice would exhibit increased anxiety behavior under both baseline and post-stress conditions, with this outcome being more pronounced at older age and in females; 3) non-transgenic mice would outperform transgenic mice in the recognition memory task, and that differences would be more pronounced at older age, in females, and after stress; and 4) several dependent variables would be correlated. The evidence on the relationship among post-stress ghrelin, brain amyloid beta, and anxiety is not clear, making exact predictions difficult. Generally, we predict higher ghrelin to relate to higher anxiety and lower amyloid beta, but the relationship to genotype status is not clear. If results mirror those from 3xTgAD mice under baseline conditions we would predict male transgenic mice, vs. non-transgenics, will have higher ghrelin. Because stressor exposure increases ghrelin, and ghrelin is both neuro-protective and anxiogenic, we might expect that among transgenic mice, lower post-stress ghrelin will be associated with greater amyloid beta loads and lower anxiety. Alternatively, if ghrelin is upregulated in transgenic mice (or increases more in response to stress), we might find transgenic animals with high ghrelin, low amyloid beta, and high anxiety. For organ masses, if transgenic mice have sustained increased HPA axis activity, we would predict organ changes representative of classic chronic stress as described by Selye (Selye, 1936): increased adrenal mass and decreased splenic and thymic mass.
2. METHODS
2.1. Animals
A total of 32 mice, 14 hemizygous APPswe/PS1dE9 transgenic (7 male, 7 female) and 18 non-transgenic littermates (9 male, 9 female), completed the entirety of the experimental protocol. Colony-founding animals (non-carrier females and carrier, hemizygous males) were originally obtained from Jackson Laboratory (stock 005864, #34829-JAX | APP/PS1; Bar Harbor, ME, USA) and were bred. Mice for this experiment were offspring born at Texas Tech University, produced by pairing transgenic males with non-transgenic (non-carrier) females of the same strain background; all mice were black in color.
The APPswe/PS1dE9 line of transgenic mice, was deposited at Jackson Labs by Dr. David Borchelt, University of Florida, expresses chimeric mouse/human amyloid precursor protein (APP) and a mutant version of the human presenilin 1 (PS1) (Jankowsky et al., 2004; https://www.jax.org/strain/004462). Both transgenes are controlled by a mouse prion protein promoter, which drives the expression in multiple cell types with strong expression in neurons. Double Swedish mutation present in the APP transcript is associated with early-onset familial AD. These mice develop human beta amyloid (Aβ) deposits in the brain beginning around 4 months, with more deposition at 6–7 months of age and pronounced deposition in the hippocampus by 9 months of age (Savonenko et al., 2005). This line does not develop tau tangles but shows increased tau phosphorylation. Transgenic mice develop early deficits in episodic-like memory followed by later impairments in reference memory (Savonenko et al., 2005).
Roughly 30 days after birth (range: 29–33), pups were weaned from the dam, a small piece of ear tissue was collected for genotyping, and the pups were temporally housed individually. Animal genotype was determined by an independent company (Transnetyx; Cordova, TN, USA) using standard specific primers for one of the transgenes, which co-segregate. Once genotype status was known (roughly 3–7 days after weaning), animals were ear punched for ID and pair housed with a same-sex, opposite-genotype littermate for the remainder of the study, whenever possible. There were two exceptions: 1) in one female pair the individuals were opposite genotype but were not littermates and 2) in one male pair the individuals were same genotype littermates. A total of 18 females and 20 males initially entered the study, but due to fighting, 2 pairs of males had to be separated and could not complete the study; two transgenic females were euthanized due to dermatitis and thus did not finish the full protocol. Initial data from the 6 mice that did not complete the entire study is included in hormonal and behavioral results to which they contributed.
Mice were housed in 14” × 8” × 5.5” polysulfone cages (Blue Line 1285L) with corn cob bedding (Teklad, Envigo) and free access to food (Teklad 2020X Global Extruded Rodent Diet) and tap water in the Human Sciences Vivarium at Texas Tech University. Lights were on a 12:12 L:D cycle; lights-on at 0800 h. Cages were kept in ventilated racks. Animals were checked daily, water was changed weekly, and full cage changes occurred every 2 weeks. Animals were provided paper shavings, tissue, and plastic huts for nesting. Mice were weighed approximately once per week. TTU has AAALAC accreditation, and all procedures were approved by the TTU IACUC and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
2.2. Experimental Design
Data collection spanned roughly 10.5 months for each mouse. After weaning and pairing, mice entered the experimental protocol. Each mouse went through several behavioral assessments (Figure 1A) and was briefly anesthetized 6 times for collection of a blood sample. Behavioral assessments were conducted under baseline (no stressor immediately prior) conditions or immediately following a predator odor stress test (Figure 1B). Mice underwent 9 stressor exposures, 6 prior to behavioral tests to assess effects on behavioral performances and 3 prior to blood collection to assess effects on hormone levels. Mice underwent 3 baseline and 2 post-stress light dark tests, 3 baseline and 2 post-stress open field tests, and 2 baseline and 2 post-stress object recognition sessions. Six blood collections (3 baseline and 3 post-stress) were conducted with at least 1 week interval from behavioral tests. After all data were collected, mice were euthanized, and brain and tissue samples dissected. To minimize impacts of circadian rhythm, behavioral assessments and predator odor exposure occurred between 10:30 AM and 1:00 PM with most procedures occurring between 11:00 AM and Noon. Mice were only subjected to one behavioral test per day, with 2–4 days between tests that occurred in the same age range. For all behavioral assessments, sessions were video recorded for later scoring using Ethovision v13 (Noldus, Inc.).
Figure 1.
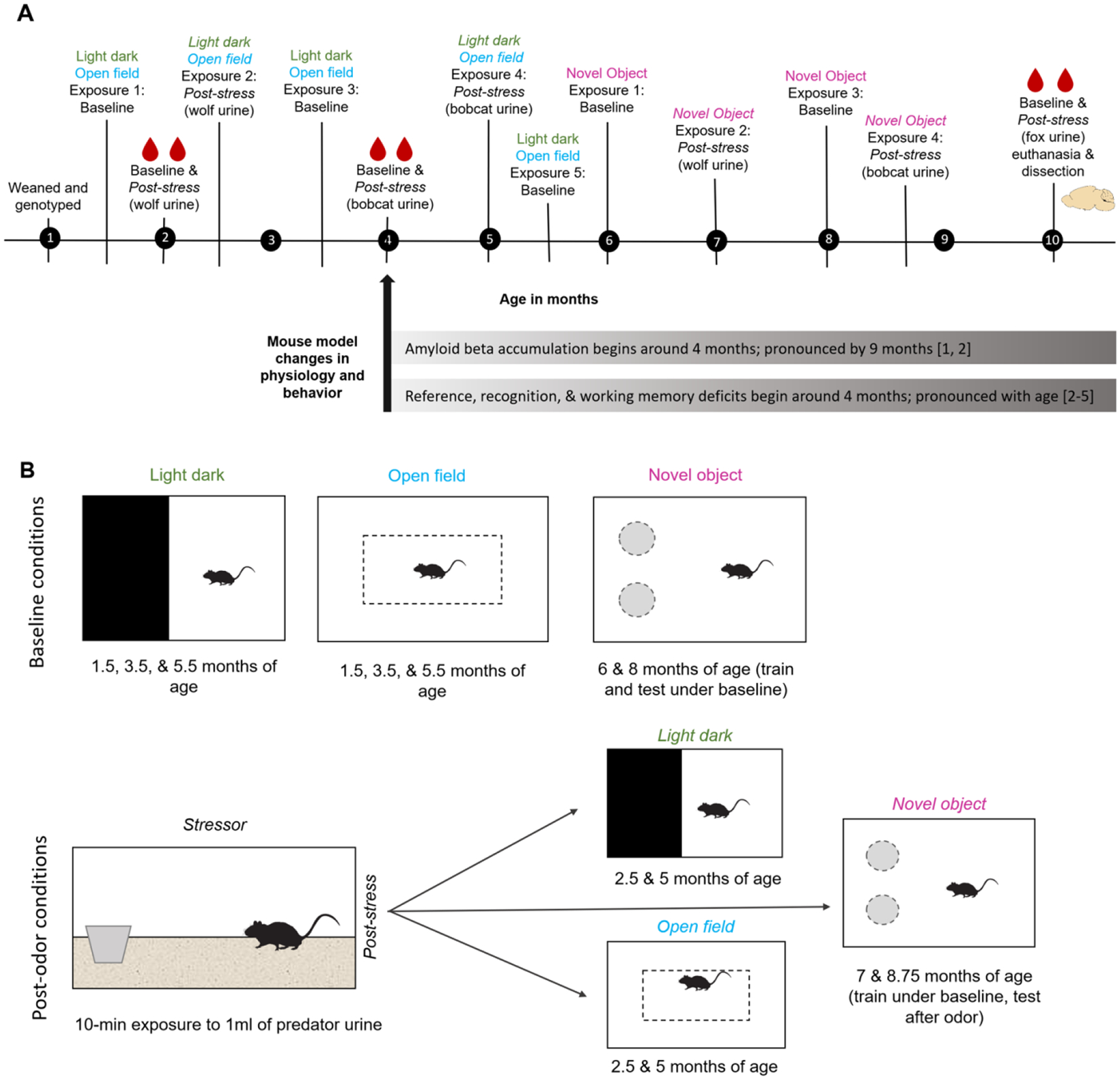
Experimental timeline (A) and behavioral tests (B) for all mice. A) Experimental timeline is shown in months. Stress sessions were exposure to predator odor for 10 min. Bottom bars display time course for amyloid neuropathology and behavioral deficits noted by other studies using this mouse model. B) Schematic of behavioral testing conditions. Baseline tests were conducted under undisturbed conditions and post-stress (odor) sessions were conducted immediately following a 10-min exposure to predator odor. Mice were only ever exposed to one behavioral test in a day. [References in A: 1 (Garcia-Alloza et al., 2006), 2 (Savonenko et al., 2005), 3 (Webster et al., 2014), 4 (Petrov et al., 2015), 5 (Pedrós et al., 2014)]
2.3. Predator Odor Exposure
For predator odor exposure, a cotton ball was wetted with 1 ml of predator urine (Maine Outdoor Solutions, Hermon, ME, USA) and placed in a plastic cup, cut down to ~2.5 cm tall, and put into a clean cage containing corn cob bedding but no food or water. Predator odor exposure was conducted in a separate testing room to avoid exposing all mice to the odor. A fresh cotton ball was used for each session and mice could interact with the urine and cup for 10 min. Predator odor elicits a robust corticosterone response in rodents (Harris and Carr, 2016) and we have used this stressor successfully in the past (Harris et al., 2012; Harris and Saltzman, 2013a).
Immediately following odor exposure, mice were either anesthetized with isoflurane for collection of a blood sample or subjected to individual behavioral testing (see below). Predator odor used differed by session but was consistent across mice (round 1 = wolf; round 2 = bobcat; round 3 = fox) and protocol of exposure was the same. Prior to blood sample collection, both mice from a pair were exposed to predator urine at the same time and then immediately taken for blood sampling. For behavioral sessions, mice were exposed to urine separately, spaced out by a few min, due to timing, apparatus availability, and recording options.
2.4. Light Dark Test
Light/dark tests were performed using custom-built choice boxes (37.1 × 29 × 17 cm; L × W × H) which consisted of a closed, dark area (14.5 × 29 cm; L × W; dark portion = ~39%) and an open, light area. The walls of the light area were white and opaque and open to the top; walls of the dark area were black and opaque, and this was an enclosed space. Testing was done in a room separate from the colony room and mice were allowed to acclimate to the testing room, in their home cage, for 10 min prior to testing. Mice were then placed in the center of the light arena and behavior was recorded for a duration of 5 min. Mice had the opportunity to freely move between the light and the dark portion of the cage throughout the testing period. The testing arena was cleaned with 70% ethanol between sessions and separate arenas were used for males and females. The total duration of time spent in the dark area (s), number of entries into the dark (transitions), and latency to first dark entry (s) were recorded for each light dark test. Increased time in the dark, fewer dark-light transitions (dark entries), and shorter latency to enter the dark are considered as markers of increased anxiety-like behavior (Bourin and Hascoët, 2003). Scoring was done in Ethovision using center point tracking and arena boundaries, and an experimenter blind to mouse sex, genotype, and testing condition watched the acquisition of each video scoring session to ensure Ethovision tracking was accurate.
2.5. Open Field Test
Open field tests were performed in a rectangular plastic arena (43.2 × 36.5 × 26 cm; L × W × H) which was further divided, via lines drawn on the bottom, into a center area (20.3 × 14.4 cm; ~18.5% of total area) and a border area. The arena walls were opaque colorless plastic, and the arena was open on top. Testing was done in a room separate from the colony room and mice were allowed to acclimate to the testing room, in their home cage, for 10 min. Mice were placed into the center area and allowed to explore freely for 5 min. The testing arena was cleaned with 70% ethanol between tests and separate arenas were used for males and females. The total distance traveled (cm), duration of time in the center (s), and duration of time spent moving (s), were recorded for each test. We then determined proportion of time in the center (total duration of time in the center (s)/300). Increased distance traveled and increased time moving are considered to be markers of increased exploratory behavior, and less time spent in the center as increased anxiety-like behavior (Prut and Belzung, 2003).
Scoring for the open field was done using Ethovision v13 (Noldus, Inc.). Duration of time spent moving was determined via Ethovision movement settings, set to center point tracking for mouse movement settings recommended for mice, with thresholds for start and stop velocity set at 2.0 and 1.75 cm/s, respectively.
Lastly, we correlated dependent variables from the light dark test and those from open field (time in dark, dark entries, duration of time in center, distance traveled) from the first baseline and first post-stress testing sessions to determine if variables represented similar and stable behavioral constructs.
2.6. Novel Object Recognition (NOR) Task
The novel object recognition task is commonly used to assess recognition memory in AD mouse models (Grayson et al., 2015). We conducted this task in a plastic arena (47.3 × 25.4 × 37.8 cm; L × W × H) which contained two objects placed 7.5 cm from the arena walls. The arena was opaque blue plastic and was open on top. Testing was done in a room separate from the colony room and mice were allowed to acclimate to the testing room, in their home cage, for 5–10 min. Mice were each tested four times, twice under baseline conditions (at 6 and 8 months of age) and twice under post-stress conditions (at 7 and 8.75 months of age). For the post-stress sessions, the mouse was trained under unmanipulated conditions and was exposed to predator odor immediately before the novel item testing session as stressor exposure has been reported to decrease memory retrieval (Het et al., 2005). Baseline testing consisted of a training period followed by a testing period 24 h later. Post-stress testing consisted of a training period followed 24 h later by predator odor and immediate testing. For each training period, the mouse was placed into the arena for 10 min, and the arena contained two identical items (training sessions at 6 and 7 months of age = two square stainless steel whiskey ice cubes; training sessions at 8 and 8.75 months of age = two glass aquarium shells). After the 10 min training session, the mouse was placed back into its home cage. The object recognition testing took place 24 h after training and during this session the mouse was exposed to one familiar object from training (steel cube, 6 and 7 months of age; glass shell, 8 and 8.75 months of age) and one novel object (flat, glass aquarium marble, 6 and 7 months of age; Duplo toy block, 8 and 8.75 months of age). In all tests, the mouse was allowed to freely explore the arena and the objects during the 10 min testing period.
The location of the novel object (right or left side) was balanced across sessions to control for placement location. The testing arena was cleaned with 70% ethanol between sessions and separate arenas were used for males and females. Videos were scored in Ethovision for duration of time the mouse nose point was in direct contract with or within 2 cm of each item. During Ethovision acquisition, all videos were watched by an observer blind to treatment condition, genotype, and sex to ensure that tracking was accurate, and traces were edited when necessary (e.g., if nose and tail marker flipped). In a few instances the mouse moved the object out of the defined object area, in these cases the videos were hand scored within Ethovision. A recognition index (100*time with novel object / (time with novel + time with familiar)) was calculated for each testing session and used as the dependent variable for analysis. Recognition index values above 50 suggest a preference for interacting with the novel object and can be interpreted as better memory for the familiar object, values below 50 suggest preference for familiar object and values equal to 50 indicate no preference.
2.7. Blood Sample Collection
Mice were anesthetized via isoflurane inhalation and a blood sample (70–140 μl) was collected from the retro-orbital sinus with glass microhematocrit tubes. Following blood collection, the eye was blotted with sterile gauze, making sure not to scratch the cornea, and the mouse was allowed to recover (generally less than 1 min) before being returned to the colony room. Samples were centrifuged for 5 min, hematocrit was determined, to monitor health and blood volume, and plasma was frozen until analysis (see below). Samples from 5 mice (1 post-stress, 4 baseline) were not available due to centrifugation loss.
A total of 6 blood samples (3 baseline and 3 post-stressor) were collected from each mouse. Baseline samples were collected immediately following anesthesia from mice that were undisturbed other than being removed from their home cage. In general, samples were collected within 3 min of cage disruption (for baseline measures) or from the end of stress test with an average time of 2 min (minimum 0 min 46 sec, maximum 4 min 56 sec). Post-stress blood samples were collected 2–3 days after baseline samples at the same time of day ± 45 min. To minimize possible effects of various levels of satiety on blood variables, on days of blood sample collection, food was removed from cages between 9:00 and 9:45 AM and blood was collected between 11:00 AM and Noon. The average duration of time without food prior to blood sample collection was approximately 2.5 hrs.
2.8. Hormone Analysis
2.8.1. Plasma corticosterone
Plasma corticosterone concentration was determined using the commercially available enzyme immunoassay kit (DetectX EIA, #KH014–01; Arbor Assays, Ann Arbor, MI, USA) as per the manufacturer’s instructions. The standard curve ranged from 0.039 to 10 ng/ml. Plasma samples (5 μL) were diluted 1:100 prior to assay and were run in duplicate. Plasma corticosterone concentration is expressed ng/ml. Plasma corticosterone levels were log-10 transformed for analysis to reduce skew, but untransformed values are presented for ease of interpretation.
2.8.2. Acyl ghrelin
Due to limitations of mouse body size and total blood volume, only the final, post-stress blood sample was used for analysis of acyl (active) ghrelin. Immediately after collection, plasma samples (50 μl) were treated with HCl to achieve a final sample concentration of 0.5 N and with 1 mg/ml protease inhibitor cocktail (AEBSP), as recommended by the kit manufacturer and similar to published methods (Meyer et al., 2014). Plasma concentration of acylated ghrelin was determined using the commercially available active ghrelin enzyme immunoassay kit (EZRGRA −90K; Millipore Sigma, Burlington, MA, USA) according to the manufacturer’s instructions. The standard curve used ranged from roughly 55 to 2000 pg/ml and samples were run in duplicate. A total of 3 samples (2 non-tg and 1 tg) were below the range of detection of the assay and were assigned 55 pg/ml, which was the lowest detectable value. Ghrelin concentration is expressed in pg/ml of plasma. Plasma ghrelin levels were log-10 transformed for analysis.
2.9. Euthanasia and Organ Collection
Following completion of final stress test and blood sample collection, mice were weighed and then euthanized by carbon dioxide inhalation followed by cervical dislocation and decapitation. Immediately after death, brains were removed and put on wet ice. The whole left and right hippocampus and cortex were dissected out and stored individually for amyloid beta analysis; remaining brain tissues were stored separately. Brain samples were frozen at −80°C. The adrenals (right and left; 24 mice), spleen (18 mice), and thymus gland (18 mice) were dissected out, placed in 0.9% saline, trimmed of fat and connective tissue, blotted 3x, and weighed to the nearest 0.0001g, as we have done before (De Jong et al., 2013). Terminal procedures occurred after the final stress test and blood sample collection and were performed between Noon and 2:30 PM.
2.10. Amyloid Beta (Aβ) Analysis
Brain sections were assayed for concentration of human Aβ40 and Aβ42 levels with widely used, commercially available ELISA kits (KHB3481 and KHB2441, Biosource International/Invitrogen, Camarillo, CA) following the manufacturer’s instructions and previously published methods (Melnikova et al., 2016; Savonenko et al., 2005). Briefly, the right cortex and right hippocampus samples were weighed and homogenized in 8x mass of cold 5M guanidine HCL / 50 mM Tris HCL with a hand-held motor (Fisher k749540–0000) and then mixed at room temperature for 3–4 hours. Samples were then placed back at −80°C until dilution and assay. Brain homogenates were thawed and diluted 1:20 with reaction buffer (Dulbecco’s phosphate buffered saline with 5% BSA and 0.03% Tween-20) with 1 mM concentration of protease inhibitor (AEBSF; Calbiochem item 539131). The diluted brain samples were aliquoted and refrozen for analysis of amyloid beta. ELISA kits were used to determine concentration of Aβ40 and Aβ42, expressed as ug/g of brain tissue. Samples for analysis of Aβ40 were assayed at a final dilution of 1:1000 – 1:6000 and samples for Aβ42 at a final dilution of 1:2000 – 1:4000. A subset of non-transgenic mouse brains was run at a dilution of 1:50. Each assay was run at a single dilution and the assay curve was treated with the same dilution of guanidine HCL, as per the manufacturer’s instructions. Samples were all run in duplicate.
2.11. Statistical Analysis
All statistical analyses were conducted in SPSS v24 (IBM, Inc.) with alpha set at 0.05. Effect size estimates are reported for each analysis. Behavioral data were analyzed with repeated-measures ANOVA (baseline over time; post-stress over time) with sex and genotype as fixed factors. Corticosterone and acyl ghrelin data were log10 transformed to increase normality and analyzed using repeated-measures ANOVA with corticosterone concentration as a dependent variable and genotype and sex as fixed factors. Amyloid beta 40 and 42 concentration data from transgenic animal cortex and hippocampus samples were analyzed via MANOVA with sex as a fixed factor and the four brain amyloid beta measures as dependent variables. Organ data were initially analyzed via ANOVA and then MANCOVA (see below for details).
3. RESULTS
A descriptive summary of behavioral data (Supplemental Table 1) and physiological data (Supplemental Table 2) can be found in the supplementary material; statistics for all analyses can be found below. Full raw data tables for original and exploratory analysis can be found in the supplemental material (Supplemental File 1 and Data).
3.1. Light Dark Test
3.1.1. Baseline light dark tests with age
To determine if behavior in the light-dark test changed with age (1.5, 3.5, and 5.5 months) we ran a repeated-measures ANOVA for each dependent variable (time spent in the dark arena, number of transitions, and latency to enter dark) with genotype and sex as fixed factors and age as a within-subject factor. The duration of time spent in the dark portion of the arena did not change with age (F2,60 = 2.750, P = 0.072, partial eta squared = 0.084; Figure 2A), nor was there an effect of genotype (P = 0.669) or sex (P =0.734); no interaction terms were significant. The number of transitions did change with age (F1,60 = 4.088, P = 0.22, partial eta squared = 0.120; Figure 2B), with mice entering the dark box more times during the 3.5-month than during the 1.5-month session (t = 2.51, P = 0.018), but no other time points differed. There was no effect of genotype (P = 0.686) or sex (P = 0.635), nor were there any significant interaction terms. The latency to enter the dark portion of the arena did not change over time (F 2,60 = 1.341, P = 0.269, partial eta squared = 0.043; Figure 2C), nor were there any impacts of genotype (P = 0.223), sex (P = 0.657), or any interactions.
Figure 2.
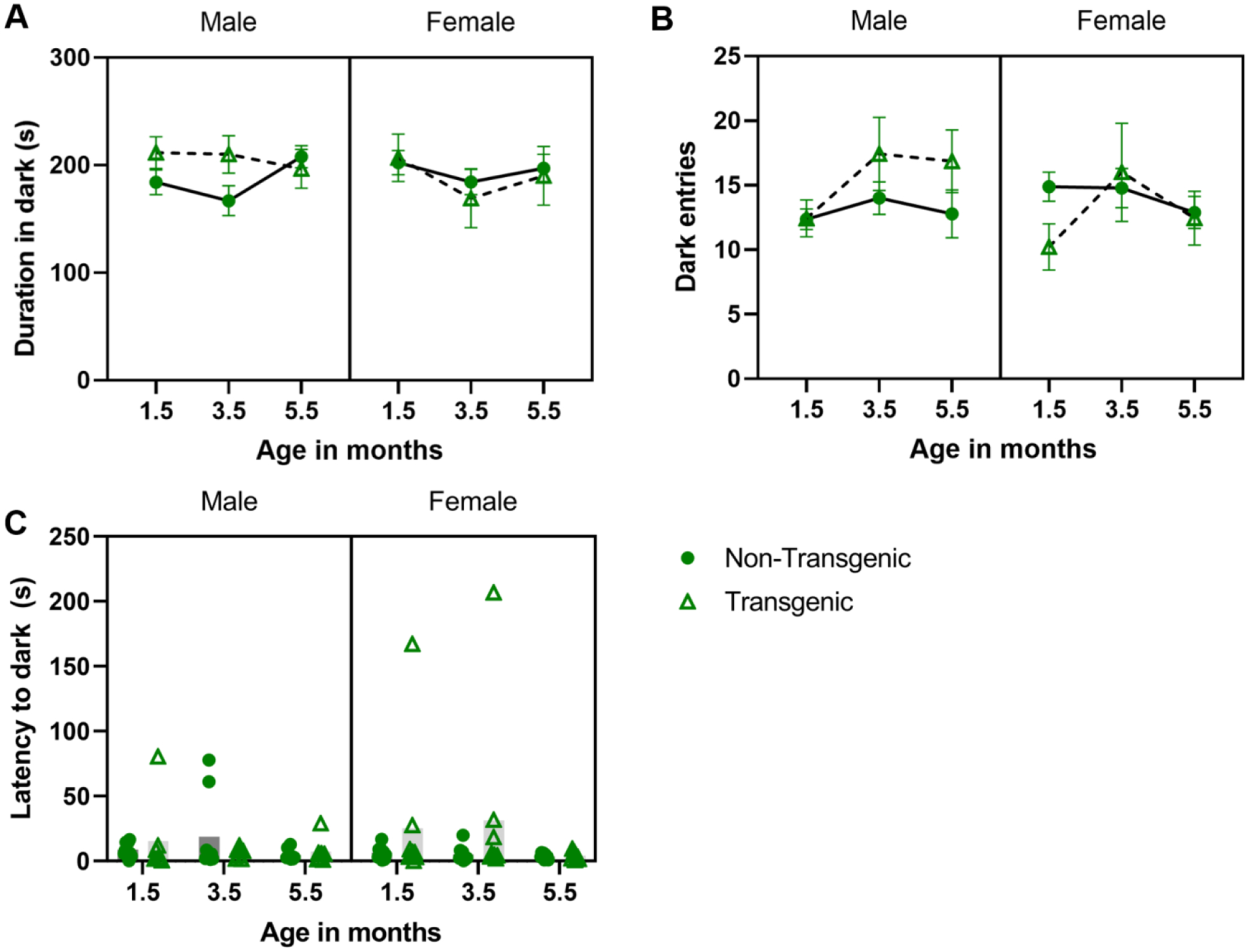
Effect of aging/repeated testing in the light dark test under baseline conditions. Mean ± SEM duration of time in the dark side of the arena (A), number of transitions (dark entries) (B), and latency to enter dark (C; plotted as individual data points with mean) across ages. Overall, mice made more dark entries at 3.5 months vs. 1.5 months (B; main effect of age), no other ages differed.
3.1.2. Post-stress light dark tests with age
At 2.5 months of age (range: 70–80 days), mice underwent their first post-stressor light-dark test following exposure to wolf urine. At 5 months of age (range: 140–150 days), mice underwent a second post-stressor light-dark test following exposure to bobcat urine. To determine if post-stress behavior in the light-dark test changed with age (2.5 and 5 months) we ran a repeated-measures ANOVA for each dependent variable (time spent in the dark arena, number of transitions, and latency to enter dark) with genotype and sex as fixed factors and age as a within-subject factor.
Mice spent more time in the dark portion of the arena at 5 months of age vs. 2.5 months of age (F 1,30 = 4.314, P = 0.046, partial eta squared = 0.126), but neither genotype (P = 0.318) nor sex (P =0.740) independently impacted duration (Figure 3A); no interaction terms were significant. Mice made more dark box transitions at 2.5 months of age vs. 5 months (F 1,30 = 11.165, P = 0.002, partial eta squared = 0.271; Figure 3B) and there was a sex*genotype interaction (F 1, 30 = 4.868, P = 0.035, partial eta squared = 0.140), within transgenic mice, males made more transitions that females (P = 0.013). Neither genotype (P = 0.814) nor sex (P = 0.141) impacted transitions and no other interaction terms were significant. The latency to enter the dark portion of the arena (Figure 3C) did not change with age (P = 0.067), but there was a sex*genotype interaction (F 1,30 = 7.653, P = 0.01, partial eta squared = 0.203); within transgenic mice, males took longer than females to enter dark (P = 0.049), and within males, transgenic males took longer than non-transgenic males to enter dark (P= 0.003). Neither genotype (P = 0.077) nor sex (P = 0.964) impacted latency; there were no other significant interactions.
Figure 3.
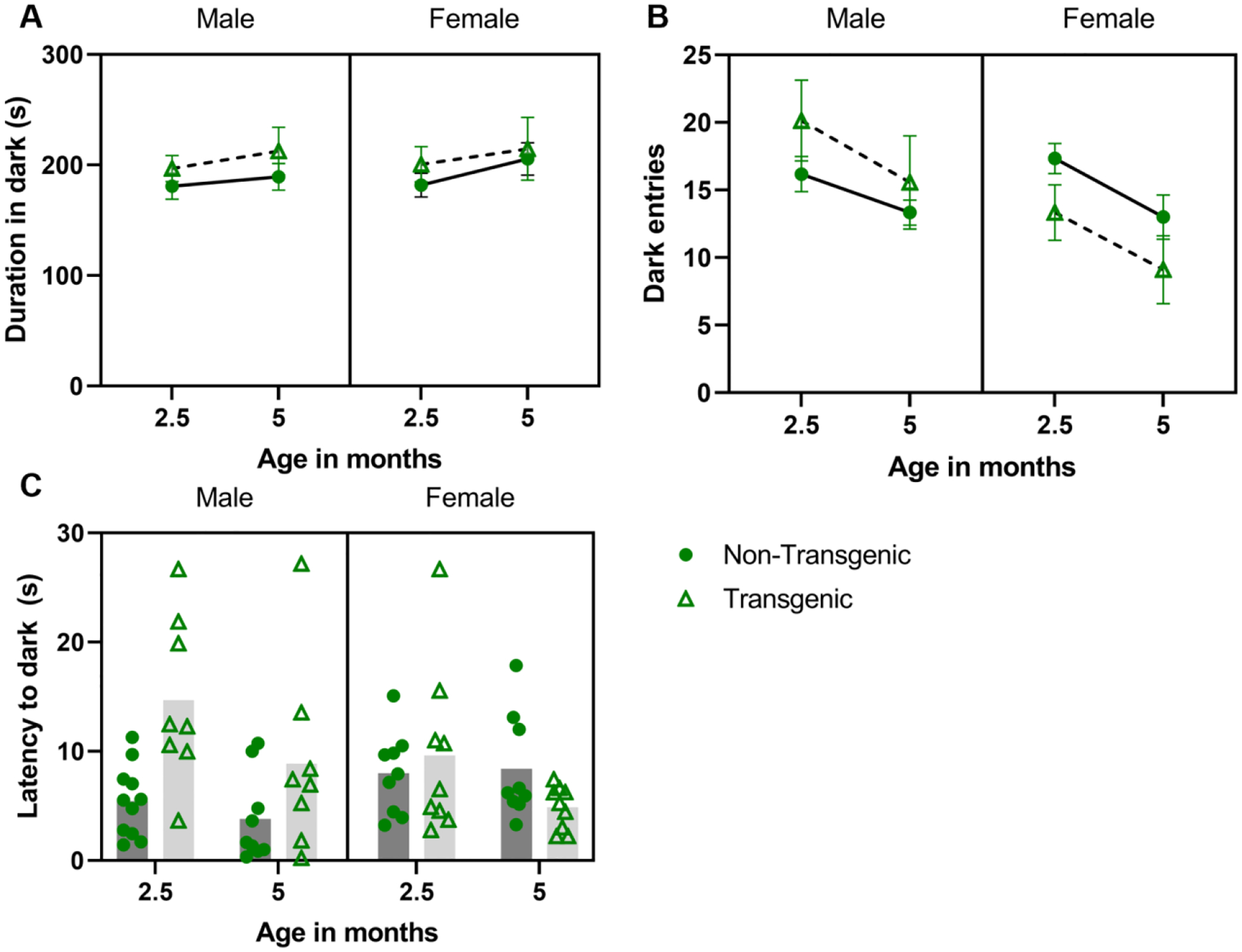
Effect of aging/repeated testing in the light dark test under post-stress (predator urine) conditions. Mean ± SEM duration of time in the dark side of the arena (A), number of transitions (dark entries) (B), and latency to enter dark (C; plotted as individual data points with mean) across ages. Mice spent more time in the dark and made more entries into the dark at 2.5 vs 5 months (A, main effect of age; B, main effect of age). Transgenic males made more transitions (B; sex*genotype) and took longer to enter the dark (C; sex*genotype) than did transgenic female; transgenic males took longer to enter the dark than transgenic females (C; sex*genotype).
3.2. Open Field Test
3.2.1. Baseline open field tests with age
To determine if behavior in the open field test changed with age (1.5, 3.5, and 5.5 months of age)/repeated testing, we ran a repeated-measures ANOVA for each dependent variable (time spent in the center of the arena, distance traveled, and time spent moving) with genotype and sex as fixed factors and age as a within-subject factor.
The distance traveled did not change with age (F 1.41,58 = 2.107, P = 0.147, partial eta squared = 0.068; Figure 4A), nor was there an effect of genotype (P = 0.269, partial eta squared = 0.042) or sex (P =0.497, partial eta squared = 0.016); however, there was a three-way interaction among sex, genotype, and age (F 1.41,58 = 3.759, P = 0.046, partial eta squared = 0.115) and a sex by genotype interaction (F 1,29 = 4.325, P = 0.046, partial eta squared = 0.130); no other interaction terms were significant (this analysis did not pass Mauchly’s test of sphericity so GreenhouseGeisser outcomes are reported where applicable). The three-way and two-way interactions were followed up with simple main effects tests; significant outcomes from those analyses are described below. Within transgenic females only, mice traveled farther at 1.5 months of age vs 3.5 months of age (P < 0.001) and vs. 5.5 months of age (P = 0.028), distance traveled in 3.5 and 5.5 months did not differ (P = 0.794). Among transgenic animals, males traveled farther than females at 3.5 months of age (P = 0.046) and 5.5 months of age (P = 0.042), but not at 1.5 months of age (0.666). Among males, overall, transgenic mice traveled farther than did non-transgenic mice (= 0.038). The duration of time moving changed with age (F2, 58 = 22.714, P < 0.001, partial eta squared = 0.439; Figure 4B); mice spent more time moving at 1.5 months of age vs 3.5 months of age (t = 5.06, P < 0.001) and vs. 5.5 months of age (t = 6.32, P < 0.001; 3.5 and 5.5 months did not differ, P = 0.076). Neither genotype (P = 0.365) nor sex (P =0.360) impacted duration of time moving; no interaction terms were significant. The proportion of time spent in the center of the arena did not change with age (F 2,58 = 1.69, P = 0.193, partial eta squared = 0.055; Figure 4C), nor was there an effect of genotype (P = 0.978) or sex (P =0.276); no interaction terms were significant.
Figure 4.
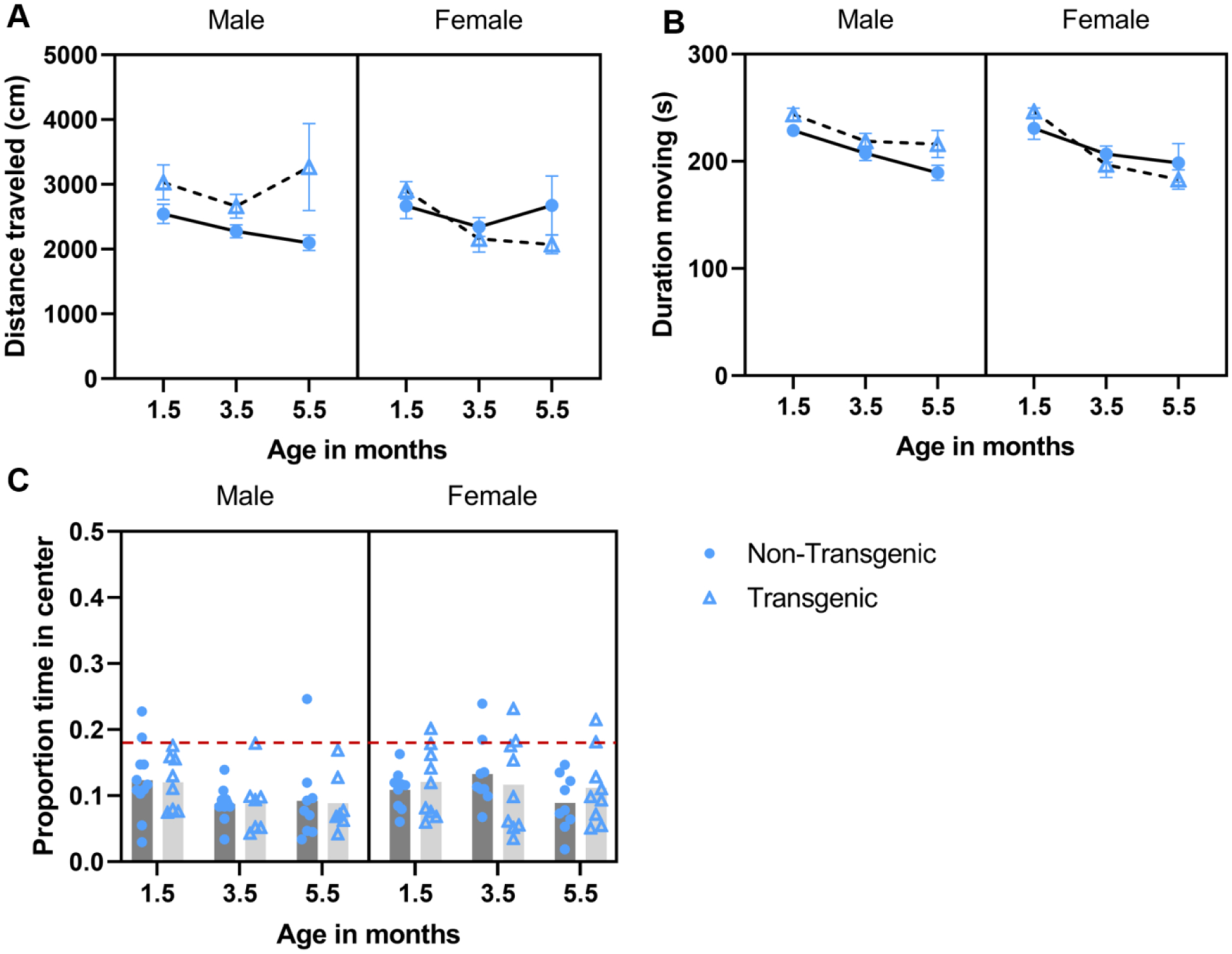
Effect of aging/repeated testing in the open field test under baseline conditions. Mean ± SEM distance traveled (A),duration of time moving (B), and mean proportion of time spent in the center arena (C) during 5-min baseline open field assessments conducted at 1.5, 3.5, and 5.5 months of age. A) Transgenic female mice traveled farther at 1.5 vs. 3.5 or 5.5 months of age, and transgenic male mice traveled farther than transgenic females at 3.5 and 5.5 months of age (sex × age × genotype interaction). Overall, transgenic male mice traveled farther than did non-transgenic male mice (sex × genotype interaction). B) Mice spent more time moving at 1.5 vs 3.5 and 5.5 months of age (main effect of age). C) Proportion of time spent in the center did not differ across time or between groups. Red dashed line corresponds to chance level as the center is 18.5% of the arena.
3.2.2. Post-stress open field tests with age
Distance traveled in the open field following predator urine exposure did not differ by age (P = 0.965), or by genotype (P = 0.473) or sex (P = 0.914). There was a significant sex*age interaction (F 1,30 = 4.612, P = 0.040, partial eta squared = 0.134), but upon post-hoc analysis no groups differed significantly (Figure 5A). There were no other significant interactions. Duration of time moving in the arena following predator urine exposure was greater at 2.5 months (1st exposure) vs. 5 months (2nd exposure; F 1,30 = 6.025, P = 0.020, partial eta squared = 0.167; Figure 5B). Neither genotype (P = 0.788) or sex (P = 0.648) impacted duration moving; no interactions were significant. Proportion of time spent in the center of the arena was not impacted by age (P = 0.393), genotype (P = 0.561), sex (P = 0.227), or a sex*genotype interaction (P = 0.922). There was an age*genotype interaction (F 1,30 = 8.175, P = 0.008, partial eta squared = 0.214), within transgenic mice, proportion in the center following urine exposure was greater at 5 months than at 2.5 months of age (P = 0.011; Figure 5C).
Figure 5.
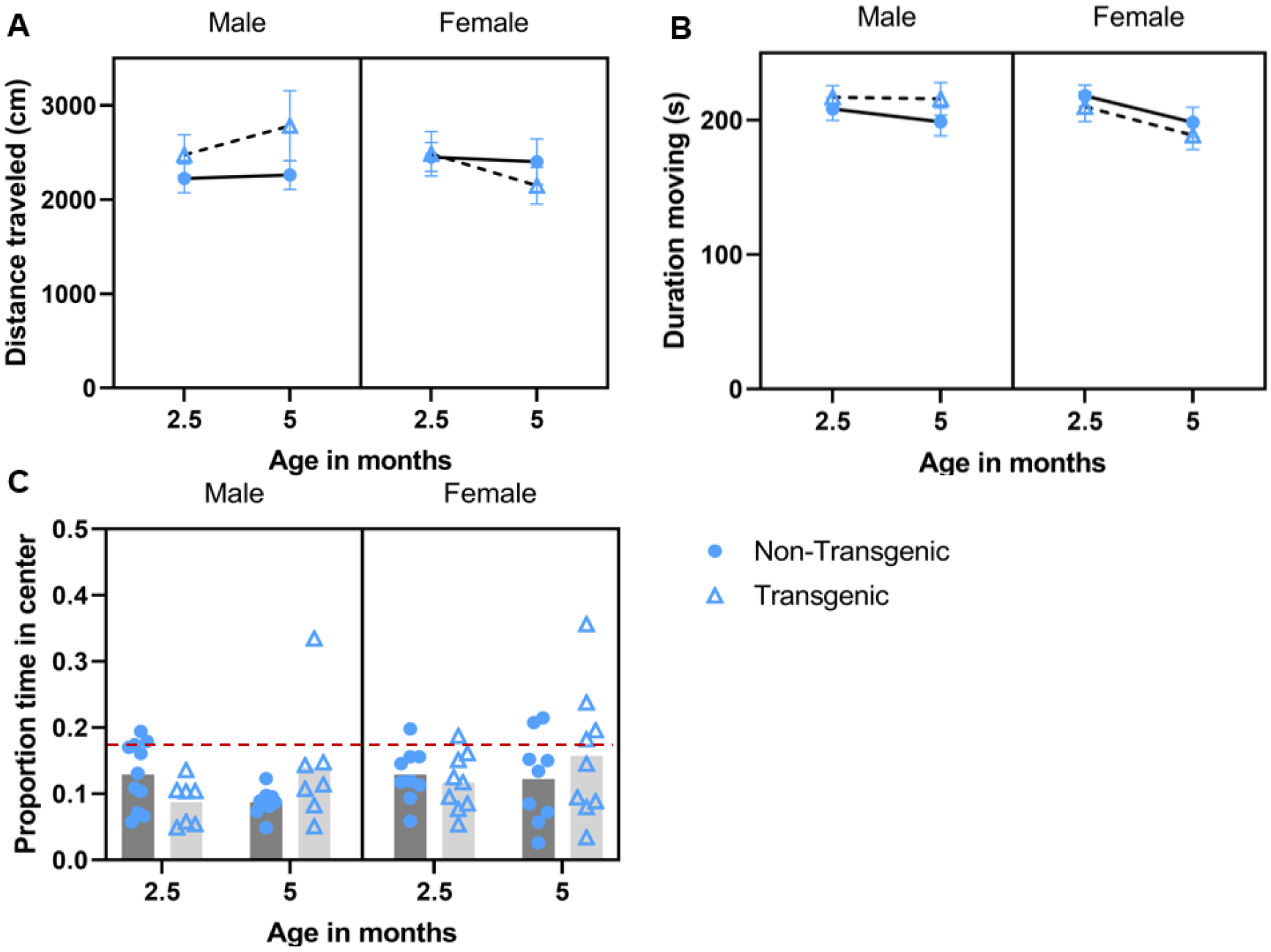
Effect of aging/repeated testing in the open field test under post-stress (predator urine) conditions. Mean ± SEM distance traveled (A), duration of time moving (B), and mean proportion of time spent in the center arena (C) during 5-min post-stressopen field assessments conducted at 2.5 and 5 months of age. Mice spent more time moving following the predator odor exposure at 2.5 vs 5 mnths (B; main effect of age). Transgenic mice spent more time in the center of the arena at 5 months vs. 2.5 months (C; age*genotpye). Red dashed line corresponds to chance level as the center is 18.5% of the arena.
3.3. Object Recognition Task
3.3.1. Baseline memory with age
Under baseline conditions, recognition index scores improved with age (6 to 8 months; F 1,30 = 56.86, P < 0.001, partial eta squared = 0.655; Figure 6A). There was also an age*sex interaction (F 1, 30 = 6.56, P = 0.016, partial eta squared = 0.180); at 6 months of age, males outperformed females (t = 2.17, P = 0.039). However, genotype (P = 0.074) and sex (P = 0.455) did not impact performance. No other interaction terms were significant.
Figure 6.
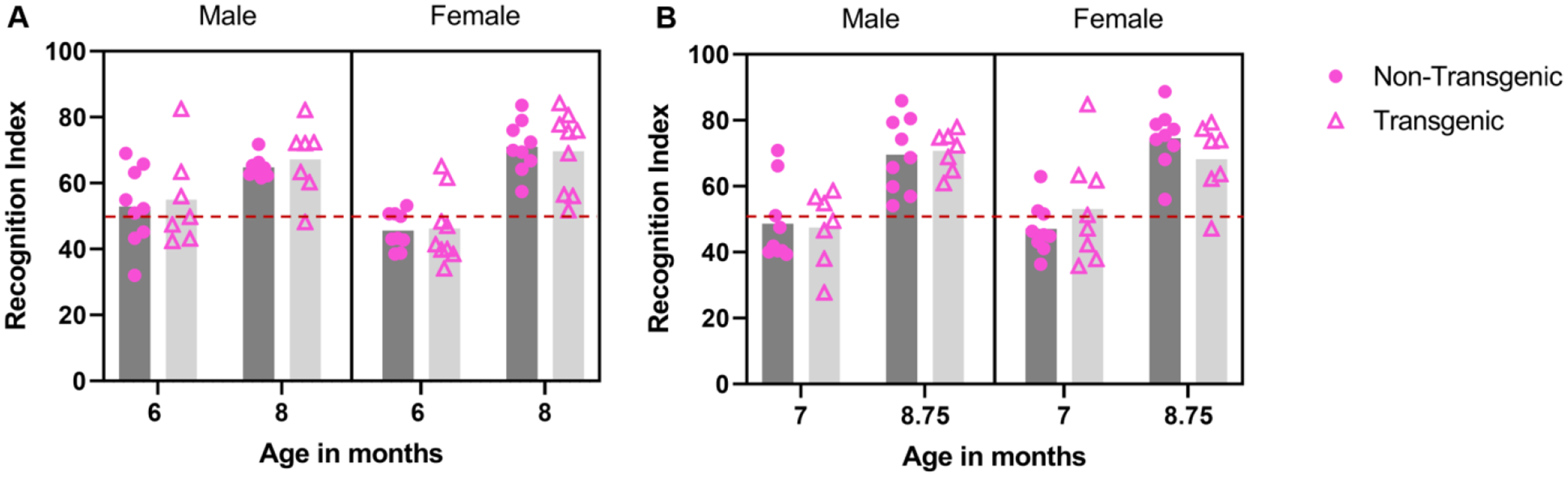
Object recognition performance under baseline (A) and post-stress (B) conditions. Recognition index improved from 6 months to 8 months of age (A; main effect of age), and at 6 months, males outperformed females (A; sex*age interaction). Post-stress recognition index improved from 7 months to 8.75 months of age (B; main effect of age). Recognition index scores above 50 suggest preference for the novel object, scores below 50 suggest preference for familiar object, score of 50 suggests no preference; 50 is shown on figure with a dashed red line.
3.3.2. Post-stress memory with age
At 7 months of age (range: 197–204 days) mice underwent training and testing (24 h after training) with (wolf) urine exposure occurring immediately before testing. This was repeated at 8.75 months of age (range: 257–264 days) but bobcat urine was used. Under post-stress conditions, recognition index scores improved with age (7 to 8.75 months; F 1,27 = 91.808, P < 0.001, partial eta squared = 0.773; Figure 6B). However, genotype (P = 0.557) and sex (P = 0.886) did not impact performance. No interaction terms were significant.
3.4. Hormone Analysis
3.4.1. Corticosterone
3.4.1.1. HPA axis responsiveness: Baseline and Post-stress 1 – Wolf Odor
When mice were approximately 2 months old (range: 55–65 days), exposure to predator odor (wolf urine) significantly increased plasma corticosterone (F1,31 = 326.79, P < 0.001, partial eta squared = 0.913; Figure 7A). However, neither genotype (F1,31 = 0.504, P = 0.483, partial eta = 0.016) nor sex (F1,31 = 1.748, P = 0.196, partial eta = 0.053) influenced results. No interactions (condition*sex: P = 0.660; condition*genotype: P = 0.870; sex*condition*genotype P = 0.840) were significant.
Figure 7.
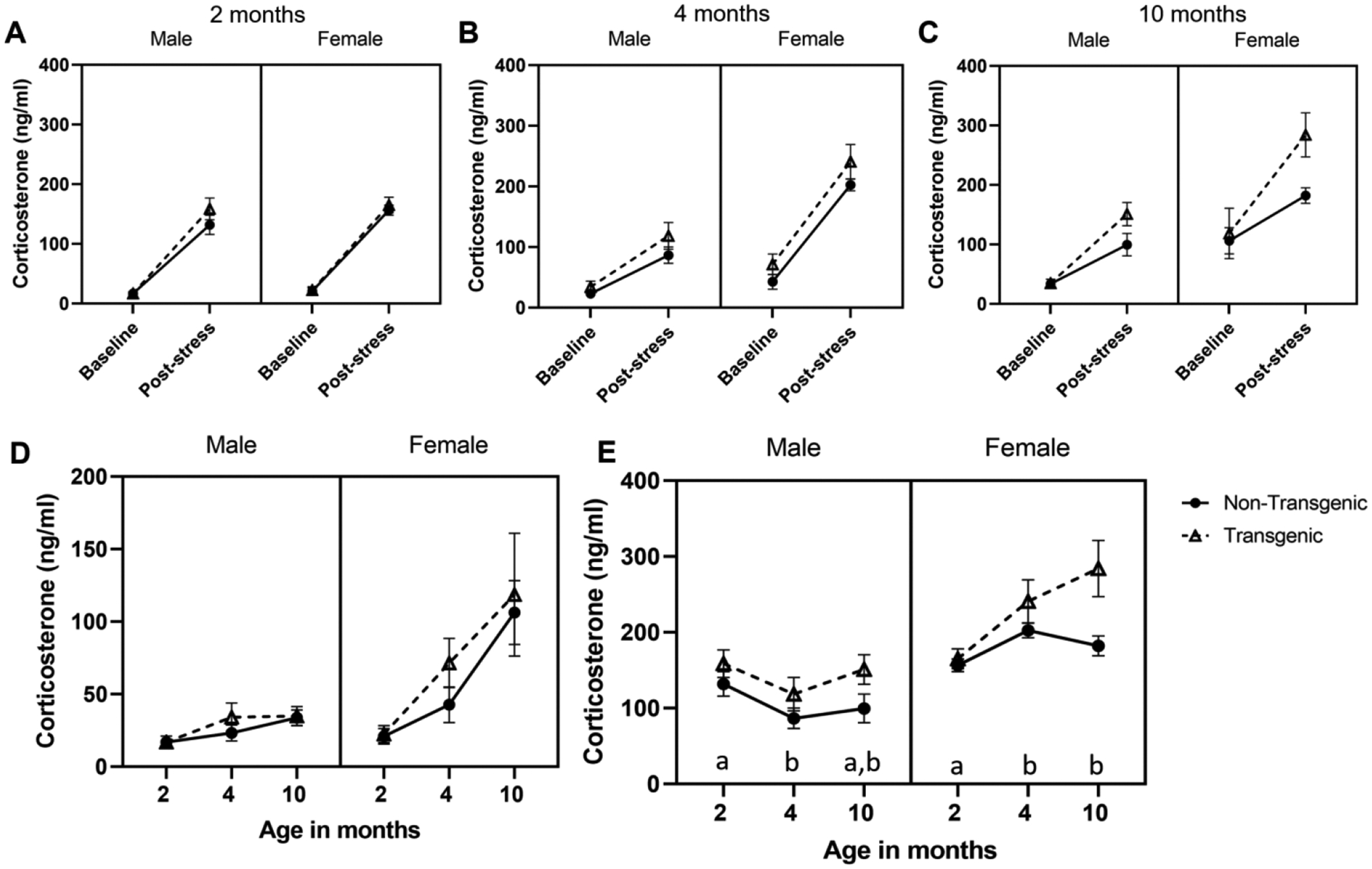
Effect of predator odor stress on levels of corticosterone. Mean ± SEM are shown for baseline and post-stress corticosterone in male and female transgenic APPswe/PS1dE9 mice and their non-transgenic littermates. A) Exposure to wolf odor at 2 months of age, B) bobcat odor at 4 months of age, and C) fox odor at 10 months of age (C) significantly increased corticosterone in all mice (main effect of condition). D) Baseline corticosterone increased with age (main effect of age) and females had higher corticosterone than males (main effect of sex). E) Post-stress corticosterone levels did not change with age, but overall, transgenic mice had higher post-stress corticosterone than non-transgenic mice (main effect of genotype; not highlighted on graph). Females had higher values than did males at each time point, and pattern of post-stress corticosterone over time differed by sex (sex*age interaction). *P< 0.05; in panel E, letters correspond to sex*age interaction; for each sex, ages that share a letter do not differ.
3.4.1.2. HPA axis responsiveness: Baseline and Post-stress 2 – Bobcat Odor
When mice were approximately 4 months old (range: 120–130 days), exposure to predator odor (bobcat urine) significantly increased plasma corticosterone (F1,28 = 174.11, P < 0.001, partial eta squared = 0.861; Figure 7B), and females had higher corticosterone than did males (main effect of sex: F1,28 = 18.66, P < 0.001, partial eta squared = 0.40). However, genotype (F1,28 = 2.598, P = 0.118, partial eta = 0.085), and interactions (condition*sex: P = 0.348; condition*genotype: P = 0.223; sex*condition*genotype: 0.541) were not significant.
3.4.1.3. HPA axis responsiveness: Baseline and Post-stress 3 – Fox Odor
When mice were approximately 10 months old (range: 295–306 days), exposure to predator odor (fox urine) significantly increased plasma corticosterone (F1,27 = 56.29, P < 0.001, partial eta squared = 0.676; Figure 7C). Females had higher corticosterone than did males (main effect of sex: F1,27 = 27.24, P < 0.001, partial eta squared = 0.503), but genotype did not impact corticosterone (main effect of genotype: F1,27 = 1.708, P = 0.202, partial eta = 0.059). None of the interactions were significant (condition*sex: P = 0.311; condition*genotype: P = 0.123; sex*condition*genotype: P = 0.931).
3.4.1.4. Baseline corticosterone with age
Baseline hormone levels increased significantly with age (F2,48 = 13.37, P < 0.001, partial eta squared = 0.358): baseline at 2 months of age was lower than baseline at 4 months of age (t = 4.25, P < 0.001) and baseline at 10 months of age (t = 4.50, P < 0.001), but baseline corticosterone at 4 months of age and 10 months of age did not differ (P = 0.085). Genotype did not impact baseline corticosterone (F 1,24 = 0.798, P = 0.381, partial eta squared = 0.032) but females had higher baseline than did males (F1,24 = 17.056, P < 0.001, partial eta squared = 0.415). Interactions did not influence corticosterone patterns over time (age*sex: P = 0.284; age*genotype: P = 0.353; age*genotype*sex: P = 0.849). Corticosterone data over time shown in Figure 7D.
3.4.1.5. Post-stress corticosterone with age
Transgenic mice had higher post-stress corticosterone than did non-transgenic mice (F 1, 27 = 15.53, P = 0.001, partial eta squared = 0.365) and females had higher values than did males (F 1, 27 = 62.5, P < 0.001, partial eta squared = 0.698). Post-stress corticosterone concentration did not change significantly with age (F 2, 54 = 1.93, P= 0.156, partial eta squared = 0.067), but sexes differed in their pattern of post-stress corticosterone with age (age*sex; F 2, 54 = 11.07, P < 0.001, partial eta squared = 0.291). Females had higher post-stress corticosterone levels than males at all ages (2 months, t = 2.32, P = 0.030; 4 months, t = 7.76, P < 0.001; 10 months, t = 5.07, P < 0.001). Among females, post-stress corticosterone levels at 10 months (t = 2.87, P = 0.008) and 4 months (t = 5.11, P < 0.001) were higher than at 2 months of age, but levels at 4 and 10 months did not differ (P = 0.768). Among males, post-stress corticosterone was higher at 2 months vs. 4 months of age (t = 6.19, P < 0.001); no other time points differed. No other interactions were significant (age*genotype, P = 0.290; age*sex*genotype, P = 0.779; sex*genotype, P = 0.150). Post-stress corticosterone data over time shown in Figure 7E.
After visual inspection of data, we performed focused 2-way ANOVA analyses (genotype by sex) for post-stress corticosterone levels at each age separately. We chose to do so because in this mouse model of AD, amyloid beta deposition and neuropathology should be pronounced by 10 months of age, but not necessarily by 2 and 4 months of age. Additionally, based on the glucocorticoid cascade hypothesis, we would expect more pronounced HPA axis responsiveness at 10 months of age vs the two earlier ages (Sapolsky et al., 1986). At 2 months of age, neither genotype (P = 0.212), sex (P = 0.160), or their interaction (P = 0.391) impacted post-stressor corticosterone levels. At 4 months of age, females had higher post-stress corticosterone than did males (F1, 31 = 49.65, P < 0.0001, partial eta squared = 0.616), but genotype (P = 0.076) and sex * genotype interactions (P = 0.468) did not impact outcomes. At 10 months of age, transgenic mice had more pronounced post-stress corticosterone than did non-transgenic mice (F1, 28 = 11.12, P = 0.002, partial eta squared = 0.284), and females had higher corticosterone than did males (F 1, 28 = 24.72, P < 0.0001, partial eta squared = 0.469). However, there was no interaction of sex and genotype (P = 0.818).
3.4.2. Post-Stress Ghrelin Levels
At 10 months of age (range: 295–306 days), post-stress acyl ghrelin concentration was not affected by genotype (F1,27 = 0.164, P = 0.689, partial eta squared = 0.006) or by sex (F1,27 = 0.981, P = 0.331, partial eta squared = 0.035), nor was there an interaction (sex *genotype, F 1, 27 = 0.110, P = 0.743, partial eta squared = 0.004).
3.5. Adrenal, Spleen, and Thymus mass
First, we assessed body mass using an ANOVA with sex and genotype as fixed factors. Genotypes did not differ in final body mass measurement (F1,28 = 0.127, P = 0.724, partial eta squared < 0.005), nor was there a genotype*sex interaction (F1,28 = 0.036, P = 0.851, partial eta squared = 0.001), but overall, as expected, males were heavier than females (mean ± SEM; males: 40.47 ± 1.59 g; females: 26.16 ± 0.77 g; F1,28 = 60.34, P < 0.001, partial eta squared = 0.683).
Right and left adrenal gland masses were significantly correlated (r = 0.80, P < 0.001, N = 22) and therefore a combination variable (right + left) was used for adrenal mass. We did not have full organ datasets for all animals (22 animals had full adrenal data; 18 had adrenal, spleen and thymus data). We ran two analyses. In the initial analysis with combined adrenal mass, genotype and sex were both included as fixed factors and body mass was used as a covariate. Effect of genotype was not significant this analysis and was removed from the model to increase available degrees of freedom; sex and body mass were both significant and were retained for the below analysis.
We then ran one MANCOVA with all organ masses included from 7 female mice and 9 male mice (results here mirrored findings in the above adrenal analysis); data passed Box’s and Levene’s tests. The following single analysis (all organs) revealed that body mass was significant in the analysis (F3,11 = 24.74, P < 0.001, Wilks’ Lambda = 0.129, partial eta squared = 0.87) and that females had higher body-mass-corrected combined organ mass (F3,11 = 29.03, P < 0.001, Wilks’ Lambda = 0.122, partial eta squared = 0.89). For individual organs, females had heavier adrenals (F 1,13 = 75.49, P < 0.001, partial eta squared = 0.85), thymus (F1,13 = 25.62, P < 0.001, partial eta squared = 0.66), and spleen (F1,13 = 36.57, P < 0.001, partial eta squared = 0.74).
3.6. Amyloid Beta Analysis
All transgenic mice had detectable levels of amyloid beta 40 and 42 in brain homogenates; as expected, none of the tested non-transgenic mice had detectable human amyloid beta. Overall, females had higher amyloid beta load than did males (F4, 9 = 6.478, Wilks’ Lambda = 0.258, P = 0.010; Figure 8). In both cortex and hippocampus, female transgenic mice had higher concentrations of amyloid beta 40 and 42 than did male transgenic mice (R cortex Aβ 40 F1,12 = 7.23, P = 0.020, partial eta squared = 0.376; R hippocampus Aβ 40 F1,12 = 19.73, P = 0.001, partial eta squared = 0.622; R cortex Aβ 42 F1,12 = 5.89, P = 0.032, partial eta squared = 0.329; R hippocampus Aβ 42 F1,12 = 5.11, P = 0.043. partial eta squared = 0.299).
Figure 8.
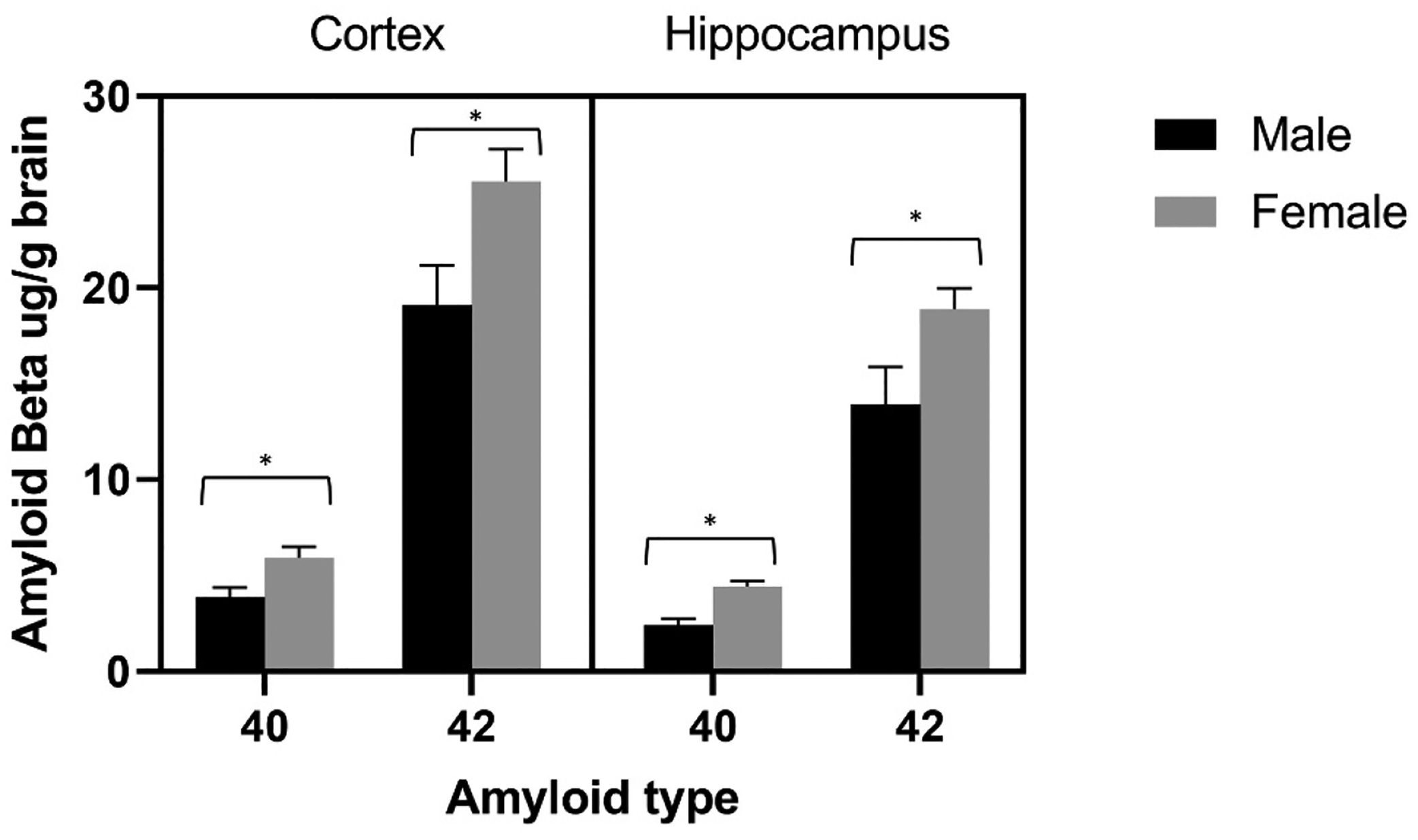
Mean ± SEM amyloid beta 40 and 42, expressed as ug/g of brain tissue, in the right cortex and right hippocampus of male and female transgenic mice at 10 months of age. Females had higher amyloid that did males at each measure. *P< 0.05
3.7. Correlations and Exploratory Analyses
In addition to above analyses, we calculated Pearson’s correlations to determine if hormone concentrations (baseline corticosterone and post-stress corticosterone and ghrelin) were related to object recognition performance and anxiety-like behaviors. Furthermore, we analyzed whether, among transgenic animals, these variables were correlated with amyloid beta 40 and 42 concentrations. We found several significant correlations among our variables, but relationships were not consistent (for full correlation table, see Supplemental File 1 and Supplemental Table 3). These correlational results should be interpreted cautiously as we made many comparisons. Given we were interested in exploring a priori predictions, and in generating information for future hypothesis testing, we did not perform alpha correction. Lastly, these analyses were conducted on the full dataset (combined genotypes) to check whether there are any robust, overall trends related to our a priori predictions (Table 1). Due to sample size constraints, data for males and females were combined in these analyses resulting in correlations being driven by sex-related differences when present in one and/or both genotypes. In addition, we also conducted exploratory analyses within specific genotype groups to look for trends related to sex or genotypes (see Supplemental File 1).
In relation to our predictions in Table 1, we found the following outcomes. Overall, corticosterone concentration and anxiety-like behaviors were not consistently related, and most significant relationships point to higher corticosterone concentrations being associated with lower, not higher, anxiety-like behavior. Post-stress ghrelin concentration was not correlated with any behavioral or physiological measure. No measure of object recognition performance was related to corticosterone level at any time point. Post-stress object recognition performance at both time points (7 and 8.75 months of age) was correlated with a few measures of anxiety-like behavior, however directionality was not consistent. At 7 months of age, better memory performance was associated with lower anxiety-like behavior. However, at 8.75 months of age, higher memory scores at that time were associated with markers of higher anxiety. Within transgenic animals, amyloid beta 40 and 42 concentrations were all significantly and positively correlated with one another. Importantly, these correlations were very strong with a range that is rarely seen in biological systems (~0.8–0.9). There were only a few significant correlations of amyloid beta 40 or 42 with other variables and they were not for the same measures.
Lastly, to look for validity and reliability of behavioral tests, we assessed the correlation among variables within and between anxiety tasks. Lightdark test outcomes suggest time in the dark is consistent across test conditions and ages, and that dark preference at a young age could help predict preference for the dark at older ages. However, the duration of time in the center of the open field at first baseline (1.5 months of age) was not significantly related to time in the center during any of the other open field tests, suggesting this marker of behavior is not as consistent over time or can be specifically affected by novelty at the first testing. When comparing between light dark and open field tests, we would expect duration in the dark to be negatively related to time spent in the center of the open field. Across several, but not all comparisons, increased duration of time in the dark was significantly correlated with decreased duration in the center of the open field. In general, it seems these variables have predictive validity and are measuring similar constructs of anxiety-like behavior, but this relationship may be more robust when open field is conducted following exposure to stress.
4. DISCUSSION
Alzheimer’s Disease is increasingly recognized as a whole-body disease that results in several dynamic and reciprocal changes in cognition, HPA axis function, emotionality, and overall physiology. Our study provides important information on the trajectory of HPA axis function, anxiety-like behavior, and recognition memory during development of Aβ amyloidosis in a commonly used mouse model of AD, APPswe/PS1dE9 mice. These data represent an integrative approach to determining how AD pathophysiology can impact multiple aspects of organismal physiology and behavior during aging, as well as provide insight into the stability of those impacts overtime. Overall, we found partial support for several of our initial predictions, details of those results are discussed below.
HPA axis activity and responsiveness
Consistent with the glucocorticoid cascade hypothesis, we found that baseline corticosterone levels increased with age. For both genotypes, baseline corticosterone increased from 2 to 4 months of age but did not increase from 4 to 10 months, suggesting age-related changes may be more related to reproductive maturation and not to transition to a middle age. Females had higher baseline corticosterone than males after 2 months of age, and this is consistent with reports that female rats have higher corticosterone levels, at least partially due to interaction from reproductive hormones (Seale et al., 2004). At each age, exposure to predator odor increased corticosterone, demonstrating activation of the HPA axis and confirming that our manipulations with predator odors can be considered as a valid stressor. Post-stress corticosterone did not consistently increase with age, but females had higher post-stress corticosterone than males, and sexes differed in their post-stress corticosterone pattern across ages. Females showed the lowest post-stress corticosterone at 2 months and corticosterone response increased with age, whereas males had their highest post-stress corticosterone at 2 months with post-stress corticosterone decreasing with age. Thus, it appears corticosterone regulation of female mice is more altered by aging. These sex differences in corticosterone responsiveness mirror human data in which females had greater age-associated increases in glucocorticoids compared to males (Otte et al., 2005).
We predicted transgenic mice would have higher baseline and stress-induced corticosterone than non-transgenic mice, and that higher corticosterone would be associated with worse memory performance. Overall, transgenic mice did have higher post-stress corticosterone levels than non-transgenic mice, suggesting the HPA axis of transgenic mice is more stress-responsive than that of non-transgenics. However, baseline corticosterone did not differ by genotype. This finding is consistent with a previous study using APPswe/PS1dE9 mice, in which 7.5-month-old mice did not differ in baseline corticosterone but transgenic males had higher corticosterone following a 20-min restraint stress; females were not tested (Sierksma et al., 2014). Interestingly, exploratory analyses suggest genotype differences in post-stressor corticosterone develop sometime between 4–10 months of age, a time that would correspond with substantial deposition of amyloid beta. In this line of transgenic animals, amyloid beta deposition begins around 4–6 months of age, is pronounced after 9 months of age, and females accumulate higher burdens than males (GarciaAlloza et al., 2006; Melnikova et al., 2016; Savonenko et al., 2005; Wang et al., 2003). A longitudinal study of amyloid beta and corticosterone in this model would be informative to determine if sex and genotype differences hold across older ages, and to determine if sex by genotype interactions become apparent at any point. Thus, having a more refined time series of corticosterone over a longer time span in this transgenic line would be beneficial. Lastly, no measure of baseline or post-stress corticosterone was correlated with memory performance in any instance of the novel object recognition task. This outcome is like our recent findings from aging humans. In that study, baseline cortisol was not a strong predictor of cognitive outcomes, and in several cases, higher cortisol related to better cognitive performance (Harris et al., 2022).
Anxiety-like behavior interpretations and limitations
We predicted that baseline and post-stress anxiety-like behavior would increase with age; that transgenic mice would display higher baseline and post-stressor anxiety-like behavior than non-transgenic mice; and that females would be more anxious than males. In contrast to our predictions, baseline anxiety-like behavior did not change robustly with age nor were there pronounced genotype or sex effects. However, some notable patterns in line with our predictions emerged. In the light dark test, the only difference observed was an increase in transitions (decreased anxiety-like behavior) at 3.5 months vs. 1.5 months of age, suggesting lower anxiety in early adulthood; no other ages differed and no other measures in the light dark test changed. In the open field test, the variable that is commonly interpreted as a measure of anxiety (time spent in the center) did not change with age, but mice seemed to decrease exploratory behavior as they aged, and this was impacted by sex and genotype. Overall, mice spent more time moving at 1.5 months vs. other ages. These effects were especially pronounced for transgenic female mice as their distance traveled decreased with age, suggesting higher anxiety-like behavior at older ages in this group, and transgenic males traveled farther than did transgenic females at 3.5 and 5.5 months, suggesting that at older ages transgenic females are more anxious than their male transgenic counterparts. This outcome could be due to anxiety phenotype, or due to changes in locomotor performance with age and/or with habituation to the testing paradigm over time.
A previous comparison of impact of age class (2–12 months) on behavior in C57Bl/6J mice found that locomotor behavior to novel environment does tend to decrease with age (Shoji et al., 2016). However, the difference between transgenic males and females was due to decreased distance traveled in females and a trend for increased distance traveled in males. Thus, the effect was not driven by a general decrease over time in all animals with a bigger drop in females, but from sex-dependent differences, suggesting animals were not simply and uniformly habituating to the testing apparatus. Lastly, open field tests were spaced by breaks of approximately 1 month, providing a substantial washout period for any memory of the testing arena. In a study of C57Bl/6J male mice, repeated testing in the light dark test every 2–3 days for a total of 6 sessions found no evidence of habituation, nor were there signs of habituation in a subset of mice tested 1, 3, 5, or 7 days apart (Blumstein and Crawley, 1983), suggesting 20–30 days between tests would not impact behavior, but there could be strain, sex, or test paradigm differences that influence propensity to habituation.
Based on previous literature and the robust impact of predators on prey physiology and behavior (Harris and Carr, 2016), we hypothesized that predator odor would increase anxiety levels and exacerbate genotype and sex differences. This outcome was partially supported. In the light dark test, repeated exposure to predator odor increased anxiety. Overall, mice spent more time in the dark and made fewer transitions after the second predator odor exposure when mice were mature adults (5 months of age) vs. at 2.5 months of age; the latency to enter dark portion of the arena trended towards a shorter latency at second exposure. Within transgenic mice, females were more anxious than males (fewer transitions and shorter latency to enter dark). Within males, however, transgenic mice were less anxious than non-transgenic (longer latency to enter dark). In the open field test, exploratory behavior findings mirrored baseline data in that duration moving decreased with time, but in contrast to our predictions, transgenic mice appeared to less anxious after the second exposure (increased proportion in the center). It does appear that two transgenic female mice were driving this outcome, and the result should be interpreted cautiously; however, when those two mice were removed the interaction remained significant.
Overall, these data suggest that baseline anxiety-like behavioral measures are not consistently impacted by age, but that post-stress anxiety-like behaviors change over time. These changes could be due to aging or to sensitization from repeated exposures. Notably, female transgenic mice seem to differ from transgenic males as they experienced higher anxiety-like behavior in the light dark test after stressor exposure, and in the open field under baseline conditions they traveled less distance over time than transgenic males. In summary, genotype and stressor exposure do seem to impact anxiety-like behavior, but effects are subtle, and seem to be most apparent in transgenic females.
We did not find robust differences in measures of anxiety-like behavior in our study. We did see that behavioral tests were anxiogenic in the classical light-avoidance sense as mice spent most of their time in the dark (light dark test) or around the edges of the open field arena. Repeated measures designs provide higher power than do cross-sectional designs, but they also have limitations as animals are exposed to more than one experimental manipulation, and order of presentation and past exposure cannot always be controlled or randomized. Thus, it is possible that habituation or experience with the experimenters or the arenas impacted our results. Interestingly, repeated (3 times over 5 days), single, or no open field testing in C57BL/6J mice did not have differential impacts on aspects of HPA axis function and anxiety-like behavior (Bodden et al., 2018), but we cannot be certain that repeated testing did not impact our results. On the positive side, due to our repeated design, we were able to assess within-animal changes and to relate physiology and behavior over time. Further, repeated measures designs seem more likely to model the within-individual changes that occur in people with AD. Lastly, we should also note that we chose to use different predator odors (wolf, bobcat, fox) over the course of the study. This was done to decrease chances of habituation, although, predator odor should remain an honest cue of threat (Harris and Carr, 2016). We have previously found that urine from multiple predators (e.g., bobcat, wolf, coyote, mountain lion, fox; all from Maine Outdoor Solutions) significantly elevates plasma corticosterone in mice (Peromyscus californicus; Chauke et al., 2011; Harris et al. 2012, 2013; Harris and Saltzman, 2013a,b), as we saw in this study, but we cannot rule out that there might be slightly different behavioral responses to the different odors.
Stability and consistency of anxiety-like behavior
We were also able to correlate anxiety-like behaviors across ages and testing conditions. When analyzing all mice together we found that duration of time in the dark was positively correlated across trials, whereas duration of time in the center of the open field was not as consistently related and duration of time in the dark was not always inversely related to duration of time spent in the center of the open field. This result mirrors other findings from our lab where we aimed to validate baseline light-dark preference anxiety tests for Xenopus laevis (Coleman et al., 2019), suggesting this lack of correlation is not an isolated occurrence (see discussion section of Colman et al., 2019 for more). In a study of behavior across age in C57BL/J6 mice, compared to young mice, older mice showed increased anxiety-like behavior in the light dark test, but decreased anxiety-like behavior in the elevated plus maze, and equivocal differences in behavior in the open field (Shoji et al., 2016).Thus, these anxiety tests using similar tasks (e.g., light/open area avoidance) may provide different information. Additionally, a study comparing various inbred strains of mice found that genotype significantly impacts measures of baseline anxiety-like behavior in various tests, including the open field, and that similar anxiety tests may be measuring different constructs of behavior (Trullas and Skolnick, 1993). However, here we did find a negative relationship between duration in the dark, under baseline or post-stress conditions, and duration in the center of the open field was most pronounced when open field tests were conducted after stressor exposure. These findings suggest light dark may be a more robust and generalizable test to determine baseline anxiety-like behaviors as compared to open field. The open field and light dark tests are both unconditioned, ethological conflict tests that rely on innate light-dark preferences (Bourin et al., 2007), but the light dark test has more pronounced contrast between arena areas.
Overall, mice in our study generally spent at or slightly less than chance levels of time in the center of the open field, suggesting they did not show a strong preference for the borders. The open field used here was relatively small, although within the scope of arenas used in other studies, and thus the behavioral choice between center and border area may have seemed less stark, especially under undisturbed conditions. However, situational- or experience-related factors could render anxiety tests potentially more or less sensitive as both light dark and open field tests are generally thought to be reflective of state, vs. trait, anxiety-like behavior. Thus, when animals were primed with a stressful situation (here a predator cue) this experience perhaps made the center vs. the periphery choice more relevant and more like behavioral choices made when animals were faced with highly contrasting light and dark spaces. Overall, the open field test may be more informative as an anxiety measure when animals are primed with stress prior to testing.
Object recognition, corticosterone, ghrelin, and amyloid beta: relationships among variables
None of our predictions on relations between object recognition performance, ghrelin levels and organ masses were robustly supported by our data. Genotypes did not differ in post-stressor ghrelin levels, nor did sexes differ. Post-stress ghrelin concentration was not correlated with any behavioral or physiological measure. No measure of object recognition performance was related to corticosterone level at any time point. Corticosterone concentrations were correlated with some open field and light dark variables, but the general trend was for increased corticosterone to be associated with lower, not higher, anxiety-like behavior. Interestingly, we recently found a similar relationship between higher baseline cortisol and lower anxiety in a study of aged humans (Harris et al., 2019). We found mixed results for the relationship between memory performance and anxiety-like behavior: at 7 months better memory performance was associated with lower anxiety-like behavior and at 8.75 months the opposite was found. Genotype did not impact organ masses. Female and male mice differed on several measures, and overall, females had higher corticosterone, lower body mass, higher body-mass-corrected organ mass, and within transgenic mice, higher amyloid beta concentrations than males. The lack of robust significant correlations among corticosterone, ghrelin, memory, anxiety-like behavior, or amyloid beta, suggested that outcomes are not strongly related on the individual level. Detection of more subtle correlations will undoubtedly require much higher sample sizes than are commonly used.
Levels of amyloid beta 40 and 42 were highly and positively correlated, and overall physiological variables showed stronger correlations than did behavioral variables. Even though all transgenic mice had high levels of amyloid beta 40 and 42 in the hippocampus and cortex, they did not perform worse than non-transgenic mice on the object recognition task at the ages tested, nor did we see any correlation between object recognition performance at either age and amyloid beta concentrations. Baseline and post-stress object recognition performance did not decrease with age. As expected from previous research (Melnikova et al., 2016), female transgenic mice did have higher amyloid beta than did male transgenic mice. However, we did not find sex by genotype interactions in memory outcomes, suggesting that the higher amyloid load in females did not translate to sex differences in memory recognition.
Limitations and Future Considerations
We based our methods on commonly used protocols and test parameters, and clearly reported our methods and data; however, these results should be interpreted with some caution, and our study is not without limitations. It is possible that our objects chosen for recognition tests in the 6- and 7-month trials were not different enough (steel cube and glass marble). This would explain why we obtained low recognition index values across animal groups at these time points. The objects used in the 8- and 8.75-month trial were quite different (glass shell and Duplo block) and overall mice exhibited higher recognition index values. Overall, our experiences with object recognition task suggest that sensitivity of this task to genotype and age effects are not as high as expected from published literature. We hope that publication of negative data using this task will correct these expectations.
It should also be noted that our animals were pair housed in cages with corncob bedding and had free access to food and water. They were also handled at least once per week. Different housing and pairing conditions can influence various experimental outcomes in rodents (Becker et al., 2016; Prendergast et al., 2014), including measures of HPA axis function and behavior (Bartolomucci et al., 2003; Butler-Struben et al., 2022; Harris, 2020; Kalliokoski et al., 2013; Trainor et al., 2013). Housing condition and environmental factors also matter for studies on AD mice. For example, prolonged exposure to an enriched environment, meant to provide cognitive stimulation, led to increased baseline corticosterone and elevated brain amyloid beta in aged, APPswe/PS1dE9 male mice (Stuart et al., 2017) and elevated brain amyloid beta in APPswe/PS1dE9 female mice (Jankowsky et al., 2003; corticosterone was not assessed in that study), compared to their standard housing counterparts. Additionally, in TgCRND8 transgenic mice, which express human amyloid beta, exposure to an enriched social environment resulted in higher brain amyloid load and no change in baseline corticosterone compared to individually housed controls (Lewejohann et al., 2009). However, neither cognitive ability nor emotionality were assessed in those studies. Another study on APPswe/PS1dE9 female mice found environmental enrichment improved cognitive performance but also resulted in higher amyloid burden (Jankowsky et al., 2005), suggesting environmental variables are important and may alter the relationship between amyloid beta and cognition. These studies also highlight the need for accurate reporting of housing, husbandry, and environmental variables in all studies (e.g., by following ARRIVE guidelines; https://arriveguidelines.org/arrive-guidelines), as these parameters can influence repeatability.
Conclusions
Our study provides novel information on HPA axis function over time in both male and female transgenic mice, and suggests at the individual level, amyloid beta, corticosterone, and behavior are not strongly or consistently correlated. Our data suggest that despite accumulating amyloid beta loads in the hippocampus and cortex, male and female APPswePS1dE9 transgenic mice do not differ robustly from their non-transgenic littermates in physiological and behavioral measures studied here, but there are subtle differences. Transgenic mice did not differ in basal levels of corticosterone but had higher post-stressor corticosterone than did non-transgenic mice. Female transgenic mice showed the most consistent anxiety-like outcomes and appear to be more anxious than transgenic males, but this was not seen consistently across all test variables and ages. Each of the behavioral tests used here are incredibly common (Cryan and Holmes, 2005; Griebel and Holmes, 2013), but often employed with slightly differing methodological details (Antunes and Biala, 2012; Borsini et al., 2002; Bourin and Hascoët, 2003). Thus, we should be careful when interpreting and comparing data from studies where methodology differs (Butler-Struben et al., 2022; Harris, 2020), especially when clinically relevant conclusions are being drawn from animal models (Belzung and Lemoine, 2011; Smith, 2022). Understanding how amyloid beta load relates to environmental factors and behavioral outcomes is critical, as is understanding how the outcomes and type of analysis can impact interpretation of behavioral data. Future studies addressing time course and relationship among amyloid beta, HPA axis function, housing condition, and emotional and cognitive behavior in both sexes can add important information to our knowledge of how these variables interact to impact functional, behavioral outcomes.
Supplementary Material
Highlights.
Consistent with glucocorticoid cascade hypothesis, baseline cort increased with age
Overall, tg mice had higher post-stressor, but not baseline, cort than non-tg mice
Across genotypes, females had higher (baseline and post-stress) cort than males
Tg females had higher Aβ and showed more anxiety-like behavior than tg males
No robust correlations, suggesting outcomes are not related on the individual level
ACKNOWLEDGEMENTS
This research was conducted at Texas Tech University and was supported by funds from the National Institutes of Health and from TTU. Main project funding was supported by NIH R15AG048447 (PIs P.L Soto & B.N. Harris, Co-I A. Savonenko). B. Roberts was a Plains Bridges to the Baccalaureate Program fellow and her effort was supported by NIH 5R25GM83730-2 (PI Jaclyn Cañas-Carrell). Open access costs and some of A. Savonenko’s effort was supported by NIH RO1 AG055974. Funds provided to G. DiMarco from TTU CALUE (now TrUE) were used for some of the hormone analysis; G. DiMarco and B. Roberts were also supported by the TTU Center for the Integration of STEM Education and Research (CISER) scholars program. We would like to thank Dr. Laci Alexander, Dr. Kamaleshwar Singh, and Marisol Alonzo for their assistance with the Plains Bridges program, and Dr. Jessica Spott and Levi Johnson for their help and support of undergraduate researchers. We thank undergraduate students Kara Foster, Brittany Gaither, Amber Loya, Michal Ibarra, and Emily Stephens for their support in data collection. Our work would not be possible without our wonderful vivarium technicians – Veronica Vasquez and Michael Chandler – and we thank them for their dedication. We extend our appreciation to Dr. Tatiana Melnikova (JHU) for providing demonstration on brain homogenization and preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- Albarran-Zeckler RG, Sun Y, Smith RG, 2011. Physiological roles revealed by ghrelin and ghrelin receptor deficient mice. Peptides 32, 2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M, Biala G, 2012. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process 13, 93–110. 10.1007/s10339-011-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE, Parmigiani S, 2003. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology 28, 540–558. [DOI] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, Liang JW, 2016. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol. Sex Differ 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Lemoine M, 2011. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol. Mood Anxiety Disord 1, 1–14. 10.1186/2045-5380-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein LK, Crawley JN, 1983. Further characterization of a simple, automated exploratory model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav 18, 37–40. [DOI] [PubMed] [Google Scholar]
- Bodden C, Siestrup S, Palme R, Kaiser S, Sachser N, Richter SH, 2018. Evidence-based severity assessment: Impact of repeated versus single open-field testing on welfare in C57BL/6J mice. Behav. Brain Res 336, 261–268. https://doi.org/ 10.1016/j.bbr.2017.08.029 [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D, 2002. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl). 163, 121–141. 10.1007/s00213-002-1155-6 [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoët M, 2003. The mouse light/dark box test. Eur. J. Pharmacol 463, 55–65. 10.1016/S0014-2999(03)01274-3 [DOI] [PubMed] [Google Scholar]
- Bourin M, Petit-Demoulière B, Nic Dhonnchadha B, Hascöet M, 2007. Animal models of anxiety in mice. Fundam. Clin. Pharmacol 21, 567–574. https://doi.org/ 10.1111/j.1472-8206.2007.00526.x [DOI] [PubMed] [Google Scholar]
- Bu XL, Xiang Y, Jin WS, Wang J, Shen LL, Huang ZL, Zhang K, Liu YH, Zeng F, Liu JH, Sun HL, Zhuang ZQ, Chen SH, Yao XQ, Giunta B, Shan YC, Tan J, Chen XW, Dong ZF, Zhou HD, Zhou XF, Song W, Wang YJ, 2018. Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol. Psychiatry 23, 1948–1956. 10.1038/mp.2017.204 [DOI] [PubMed] [Google Scholar]
- Burton C, Campbell P, Jordan K, Strauss V, Mallen C, 2013. The association of anxiety and depression with future dementia diagnosis: A case-control study in primary care. Fam. Pract 30, 25–30. 10.1093/fampra/cms044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Struben HM, Kentner AC, Trainor BC, 2022. What’s wrong with my experiment?: The impact of hidden variables on neuropsychopharmacology research. Neuropsychopharmacology 47, 1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini VP, Monzón ME, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR, 2002. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem. Biophys. Res. Commun 299, 739–743. [DOI] [PubMed] [Google Scholar]
- Carnes M, Smith JC, Kalin NH, Bauwens SF, 1983. The dexamethasone suppression test in demented outpatients with and without depression. Psychiatry Res. 9, 337–344. [DOI] [PubMed] [Google Scholar]
- Caruso A, Nicoletti F, Mango D, Saidi A, Orlando R, Scaccianoce S, 2018. Stress as risk factor for Alzheimer’s disease. Pharmacol. Res 132, 130–134. 10.1016/J.PHRS.2018.04.017 [DOI] [PubMed] [Google Scholar]
- Chauke M, Malisch JL, Robinson C, de Jong TR, Saltzman W 2011. Effects of reproductive status on behavioral and endocrine responses to acute stress in a biparental rodent, the California mouse (Peromyscus californicus). Horm. Behav 60, 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RB, Aguirre K, Spiegel HP, Pecos C, Carr JA, Harris BN, 2019. The plus maze and scototaxis test are not valid behavioral assays for anxiety assessment in the South African clawed frog. J. Comp. Physiol A 205, 567–582. [DOI] [PubMed] [Google Scholar]
- Conrad CD, 2008. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci 19, 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A, 2005. Model organisms: The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov 4, 775–790. 10.1038/nrd1825 [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Ph D, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC, 2006. Plasma Cortisol and Progression of Dementia in DAT Subjects. Am. J. Psychiatry 163, 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong TR, Harris BN, Perea-Rodriguez JP, Saltzman W, 2013. Physiological and neuroendocrine responses to chronic variable stress in male California mice (Peromyscus californicus): Influence of social environment and paternal state. Psychoneuroendocrinology 38, 2023–2033. 10.1016/j.psyneuen.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon M, Mcrae T, Tsai J, George A, Marcus D, Freedman M, Wolf A, Mcewen B, 1988. Abnormal cortisol response in Alzheimer’s disease linked to hippocampal atrophy. Lancet 332, 391–392. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, 2006. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci 9, 381–388. [DOI] [PubMed] [Google Scholar]
- Dong H, Csernansky JG, 2019. Editorial_ Stress and its impact on Alzheimer’s Disease | Enhanced Reader. Neurobiol. Stress 100167. 10.1016/j.ynstr.2019.100167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Csernansky JG, 2009. Effects of stress and stress hormones on amyloid-β protein and plaque deposition. J. Alzheimer’s Dis 18, 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG, 2008. Corticosterone and related receptor expression are associated with increased β-amyloid plaques in isolated Tg2576 mice. Neuroscience 155, 154–163. 10.1016/j.neuroscience.2008.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahete MD, Córdoba-Chacón J, Kineman RD, Luque RM, Castaño JP, 2011. Role of ghrelin system in neuroprotection and cognitive functions: Implications in Alzheimer’s disease. Peptides 32, 2225–2228. https://doi.org/ 10.1016/j.peptides.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Fischer CE, Iaboni A, 2017. Neuropsychiatric symptoms in mild cognitive impairment: an update on prevalence, mechanisms, and clinical significance. Can. J. Psychiatry 62, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Huang Changquan, Zhao K, Ma L, Qiu X, Zhang L, Xiu Y, Chen L, Lu W, Huang Chunxia, Tang Y, Xiao Q, 2013. Depression as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Int. J. Geriatr. Psychiatry 28, 441–449. 10.1002/gps.3845 [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP, 2006. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis 24, 516–524. 10.1016/j.nbd.2006.08.017 [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA, 2007. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage 35, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson B, Leger M, Piercy C, Adamson L, Harte M, Neill JC, 2015. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res 285, 176–193. [DOI] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM, 2006. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer’s disease. J. Neurosci 26, 9047–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holmes A, 2013. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov 12, 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, 2020. Stress hypothesis overload: 131 hypotheses exploring the role of stress in tradeoffs, transitions, and health. Gen. Comp. Endocrinol 288, 113355. [DOI] [PubMed] [Google Scholar]
- Harris BN, Carr JA, 2016. The role of the hypothalamus-pituitary-adrenal/interrenal axis in mediating predator-avoidance trade-offs. Gen. Comp. Endocrinol 230, 110–142. [DOI] [PubMed] [Google Scholar]
- Harris BN, Cooke JT, Littlefield AK, Tucker CA, Campbell CM, King KS 2022. Relations among CRFR1 and FKBP5 genotype, cortisol, and cognitive function in aging humans: A Project FRONTIER study. Phys. Behav 254, 113884. [DOI] [PubMed] [Google Scholar]
- Harris BN, Hohman ZP, Campbell CM, King KS, Tucker CA, 2019. FAAH genotype, CRFR1 genotype, and cortisol interact to predict anxiety in an aging, rural Hispanic population: A Project FRONTIER study. Neurobiol. Stress 10, 100154. https://doi.org/ 10.1016/j.ynstr.2019.100154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, Saltzman W, 2013a. Effects of aging on hypothalamic-pituitary-adrenal (HPA) axis activity and reactivity in virgin male and female California mice (Peromyscus californicus). Gen. Comp. Endocrinol 186, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, Saltzman W 2013b. Effect of reproductive status on hypothalamic–pituitary–adrenal (HPA) activity and reactivity in male California mice (Peromyscus californicus). Phys. Behav 112, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, De Jong TR, Yang V, Saltzman W 2013. Chronic variable stress in fathers alters paternal and social behavior but not pup development in the biparental California mouse (Peromyscus californicus). Horm. Behav 64, 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, Saltzman W, De Jong TR, Milnes MR, 2012. Hypothalamic–pituitary–adrenal (HPA) axis function in the California mouse (Peromyscus californicus): changes in baseline activity, reactivity, and fecal excretion of glucocorticoids across the diurnal cycle. Gen. Comp. Endocrinol 179, 436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I, 1997. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol. Aging 18, 285–289. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, 2007. Prolonged stress will induce Alzheimer’s disease in elderly people by increased release of homocysteic acid. Med. Hypotheses 69, 1135–1139. [DOI] [PubMed] [Google Scholar]
- Hendrickx JO, De Moudt S, Calus E, Martinet W, Guns P-JDF, Roth L, De Deyn PP, Van Dam D, De Meyer GRY, 2021. Serum Corticosterone and Insulin Resistance as Early Biomarkers in the hAPP23 Overexpressing Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 10.3390/ijms22136656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Het S, Ramlow G, Wolf OT, 2005. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 30, 771–784. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Epperson CN, 2019. Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biol. Psychiatry 86, 421–432. 10.1016/j.biopsych.2019.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail Z, Gatchel J, Bateman DR, Barcelos-Ferreira R, Cantillon M, Jaeger J, Donovan NJ, Mortby ME, 2018. Affective and emotional dysregulation as pre-dementia risk markers: exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int. psychogeriatrics 30, 185–196. [DOI] [PubMed] [Google Scholar]
- Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, Agüera-Ortiz L, Sweet R, Miller D, Lyketsos CG, 2016. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimer’s Dement. 12, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 14, 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, 2004. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet 13, 159–170. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV, 2005. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neurosci 25, 5217–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Xu G, Fromholt D, Gonzales V, Borchelt DR, 2003. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol 62, 1220–1227. [DOI] [PubMed] [Google Scholar]
- Janus C, Westaway D, 2001. Transgenic mouse models of Alzheimer’s disease. Physiol. Behav 73, 873–886. 10.1016/S0031-9384(01)00524-8 [DOI] [PubMed] [Google Scholar]
- Johansson L, Guo X, Waern M, Östling S, Gustafson D, Bengtsson C, Skoog I, 2010. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain 133, 2217–2224. [DOI] [PubMed] [Google Scholar]
- Johansson L, Skoog I, Gustafson DR, Olesen PJ, Waern M, Bengtsson C, Björkelund C, Pantoni L, Simoni M, Lissner L, 2012. Midlife psychological distress associated with late-life brain atrophy and white matter lesions: a 32-year population study of women. Psychosom. Med. 74, 120–125. [DOI] [PubMed] [Google Scholar]
- Joshi YB, Praticò D, 2013. Stress and HPA axis dysfunction in Alzheimer’s disease, in: Studies on Alzheimer’s Disease. Springer, pp. 159–165. [Google Scholar]
- Justice NJ, 2018. The relationship between stress and Alzheimer’s disease. Neurobiol. Stress 8, 127–133. 10.1016/j.ynstr.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski O, Jacobsen KR, Darusman HS, Henriksen T, Weimann A, Poulsen HE, Hau J, Abelson KSP, 2013. Mice do not habituate to metabolism cage housing–a three week study of male BALB/c mice. PLoS One 8, e58460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristenssson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, de la Cour CD, Håkanson R, Lindström E, 2006. Acute psychological stress raises plasma ghrelin in the rat. Regul. Pept 134, 114–117. [DOI] [PubMed] [Google Scholar]
- Landfield P, Blalock E, Chen K-C, Porter N, 2007. A New Glucocorticoid Hypothesis of Brain Aging: Implications for Alzheimers Disease. Curr. Alzheimer Res 4, 205–212. 10.2174/156720507780362083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, 2018. Sex as an important biological variable in biomedical research. BMB Rep. 51, 167–173. 10.5483/BMBRep.2018.51.4.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewejohann L, Reefmann N, Widmann P, Ambrée O, Herring A, Keyvani K, Paulus W, Sachser N, 2009. Transgenic Alzheimer mice in a semi-naturalistic environment: More plaques, yet not compromised in daily life. Behav. Brain Res 201, 99–102. 10.1016/j.bbr.2009.01.037 [DOI] [PubMed] [Google Scholar]
- Mahgoub N, Alexopoulos GS, 2016. Amyloid Hypothesis: Is There a Role for Antiamyloid Treatment in Late-Life Depression? Am. J. Geriatr. Psychiatry 24, 239–247. https://doi.org/ 10.1016/j.jagp.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I, Gomes S, Costa RO, Otvos L, Oliveira CR, Resende R, Pereira CMF, 2013. Leptin and ghrelin prevent hippocampal dysfunction induced by Aβ oligomers. Neuroscience 241, 41–51. [DOI] [PubMed] [Google Scholar]
- Melnikova T, Park D, Becker L, Lee D, Cho E, Sayyida N, Tian J, Bandeen-Roche K, Borchelt DR, Savonenko AV, 2016. Sex-related dimorphism in dentate gyrus atrophy and behavioral phenotypes in an inducible tTa: APPsi transgenic model of Alzheimer’s disease. Neurobiol. Dis 96, 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA, 2014. A ghrelin–growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol. Psychiatry 19, 1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TP, Taylor J, Rogerson S, Mauricio M, Kennedy Q, Schatzberg A, Tinklenberg J, Yesavage J, 1998. Cognitive and noncognitive symptoms in dementia patients: relationship to cortisol and dehydroepiandrosterone. Int. Psychogeriatrics 10, 85–96. [DOI] [PubMed] [Google Scholar]
- Moon M, Choi JG, Nam DW, Hong H-S, Choi Y-J, Oh MS, Mook-Jung I, 2011. Ghrelin ameliorates cognitive dysfunction and neurodegeneration in intrahippocampal amyloid-β 1–42 oligomer-injected mice. J. Alzheimer’s Dis 23, 147–159. [DOI] [PubMed] [Google Scholar]
- Morris JK, Honea RA, Vidoni ED, Swerdlow RH, Burns JM, 2014. Is Alzheimer’s disease a systemic disease? Biochim. Biophys. Acta - Mol. Basis Dis 10.1016/j.bbadis.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC, 2005. A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology 30, 80–91. 10.1016/j.psyneuen.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Pedrós I, Petrov D, Allgaier M, Sureda F, Barroso E, Beas-Zarate C, Auladell C, Pallàs M, Vázquez-Carrera M, Casadesús G, 2014. Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer’s disease. Biochim. Biophys. Acta (BBA)-Molecular Basis Dis 1842, 1556–1566. [DOI] [PubMed] [Google Scholar]
- Petrov D, Pedrós I, Artiach G, Sureda FX, Barroso E, Pallas M, Casadesús G, Beas-Zarate C, Carro E, Ferrer I, 2015. High-fat dietinduced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim. Biophys. Acta (BBA)-Molecular Basis Dis 1852, 1687–1699. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I, 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev 40, 1–5. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol 463, 3–33. 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- Raskind M, Peskind E, Rivard M-F, Veith R, Barnes R, 1982. Dexamethasone suppression test and cortisol circadian rhythm in primary degenerative dementia. Am. J. Psychiatry [DOI] [PubMed] [Google Scholar]
- Reich N, Hölscher C, 2020. Acylated Ghrelin as a Multi-Targeted Therapy for Alzheimer’s and Parkinson’s Disease. Front. Neurosci 14, 614828. 10.3389/fnins.2020.614828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LS, Gannon OJ, Thomas MA, Salinero AE, Abi-Ghanem C, Poitelon Y, Belin S, Zuloaga KL, 2020. Role of sex and high-fat diet in metabolic and hypothalamic disturbances in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neuroinflammation 17, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, Martin B, Mattson MP, 2012. 3xTgAD mice exhibit altered behavior and elevated Aβ after chronic mild social stress. Neurobiol. Aging 33, 830.e1–830.e12. 10.1016/j.neurobiolaging.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS, 1986. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr. Rev 7, 284–301. 10.1210/edrv-7-3-284 [DOI] [PubMed] [Google Scholar]
- Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MPF, Price DL, Tang F, Markowska AL, Borchelt DR, 2005. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: Relationships to β-amyloid deposition and neurotransmitter abnormalities. Neurobiol. Dis 18, 602–617. 10.1016/j.nbd.2004.10.022 [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL, 2004. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J. Neuroendocrinol 16, 989–998. [DOI] [PubMed] [Google Scholar]
- Selye H, 1936. A syndrome produced by diverse nocuous agents. Nature 138, 32. [DOI] [PubMed] [Google Scholar]
- Shoji H, Takao K, Hattori S, Miyakawa T, 2016. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol. Brain 9, 11. 10.1186/s13041-016-0191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierksma ASR, Van Den Hove DLA, Pfau F, Philippens M, Bruno O, Fedele E, Ricciarelli R, Steinbusch HWM, Vanmierlo T, Prickaerts J, 2014. Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology 77, 120–130. 10.1016/j.neuropharm.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Smith LN 2022. What’s Right and Wrong in Preclinical Science: A Matter of Principled Investigation. Front. Behav. Neurosci 16. 805661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart KE, King AE, Fernandez-Martos CM, Summers MJ, Vickers JC, 2017. Environmental novelty exacerbates stress hormones and Aβ pathology in an Alzheimer’s model. Sci. Rep 7, 1–7. 10.1038/s41598-017-03016-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W, Bleiler L, 2012. 2012 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 8, 131–168. 10.1016/j.jalz.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ, 2013. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm. Behav 63, 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, Skolnick P, 1993. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl). 111, 323–331. [DOI] [PubMed] [Google Scholar]
- Wang H-X, Wahlberg M, Karp A, Winblad B, Fratiglioni L, 2012. Psychosocial stress at work is associated with increased dementia risk in late life. Alzheimer’s Dement. 8, 114–120. [DOI] [PubMed] [Google Scholar]
- Wang J, Tanila H, Puoliväli J, Kadish I, Van Groen T, 2003. Gender differences in the amount and deposition of amyloidβ in APPswe and PS1 double transgenic mice. Neurobiol. Dis 14, 318–327. 10.1016/j.nbd.2003.08.009 [DOI] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ, 2014. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet 5, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MF, Vobach S, Olsson K, Svetlik D, Risser RC, 1997. Cortisol secretion and Alzheimer’s disease progression. Biol. Psychiatry 42, 1030–1038. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA, 2006. Chronic Psychological Distress and Risk of Alzheimer’s Disease in Old Age. Neuroepidemiology 27, 143–153. 10.1159/000095761 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA, 2011. Vulnerability to stress, anxiety, and development of dementia in old age. Am. J. Geriatr. Psychiatry 19, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA, 2003. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology 61, 1479 LP – 1485. 10.1212/01.WNL.0000096167.56734.59 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA, 2007. Chronic distress and incidence of mild cognitive impairment. Neurology 68, 2085 LP – 2092. 10.1212/01.wnl.0000264930.97061.82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


