Abstract
Introduction
Female pelvic floor disorders (PFDs) include clinical conditions such as urinary and fecal incontinence and pelvic organ prolapse. Disease-specific questionnaires like the Pelvic Floor Distress Inventory-20 (PFDI-20) have facilitated pelvic floor disorder assessment. We aimed to investigate the prevalence of pelvic floor disorders in Japanese women after different modes of delivery and the association of pelvic floor disorders with epidural anesthesia.
Material and methods
We included 212 women who gave birth at our institution. The PFDI-20 questionnaire (validated in Japanese) was used to evaluate the symptoms of pelvic floor disorders 6-15 months postpartum.
Results
Out of the 212 postpartum women who participated in this study, 156 (73.6%) had symptoms of pelvic floor disorder; the most prevalent symptom was urinary distress inventory in 114 (53.8%) women [79 (37.3%) of them experienced urine leakage related to increased abdominal pressure]. A comparison of the epidural and non-epidural groups to determine the association between pelvic floor disorder and delivery mode revealed a higher disease burden score of 8.67 points in the epidural group.
Conclusion
The prevalence of pelvic floor disorder symptoms is relatively high, affecting 156 (73.6%) of 212 women. Accurate diagnosis of women and appropriate and regular follow-up until improvement of their symptoms are crucial. Furthermore, healthcare workers should advise pregnant women on whether to choose vaginal delivery with or without anesthesia. To the best of our knowledge, our study is the first to investigate postpartum pelvic floor disorder in Japan.
Keywords: urinary incontinence, prevalence, pelvic floor distress inventory-20 (pfdi-20), pelvic floor disorders, japanese postpartum woman, epidural delivery
Introduction
Female pelvic floor disorders (PFD) comprise various clinical conditions, such as urinary and fecal incontinence and pelvic organ prolapse. PFD assessment has been facilitated by the development of disease-specific questionnaires, such as the Pelvic Floor Distress Inventory-20 (PFDI-20). The PFDI-20 is a 20-item questionnaire divided into scores on three scales and evaluating the pelvic organ prolapse distress inventory (POPDI), colorectal anal distress inventory (CRADI), and urinary distress inventory (UDI) [1].
Pregnancy and labor are known risk factors for PFD [1]. Among the 196 surveyed Australian women, PFD was prevalent at six months postpartum in 97%, according to the PFDI-20 survey [2]. In a study conducted in Israel, 117 women were investigated during late pregnancy and puerperium. PFD assessment at three months postpartum showed POPDI in 12.8%, CRADI in 20.7%, and UDI in 15.8% of them. Despite these, mixed trends of spontaneous recovery following childbirth have been reported [3]. Additionally, there are mixed trends regarding the prevalence and recovery period. We hypothesized that symptoms of PFD would persist following the point at which the woman becomes more active (such as returning to her normal daily routine) or as the infant’s weight increases, compared to the early postpartum period, like three months after delivery. The prevalence of PFD after delivery and the recovery in Japanese women are unclear; thus, we investigated symptoms of PFD during the postpartum period, with a focus on those occurring later.
Anim-Somuah et al. reported that epidural anesthesia was associated with a prolonged second stage of labor [4]. Vaginal delivery with epidural anesthesia is associated with an increased risk for instrumental delivery [5]. Moreover, a prolonged second stage of labor and forceps delivery may injure pelvic floor muscles, causing PFD in postpartum women [6-7]. Epidural anesthesia is associated with urinary incontinence immediately after delivery but not at three and 12 months postpartum [8]. Therefore, the hypothesis that epidural anesthesia affects pelvic floor function is reasonable. In contrast, epidural anesthesia reportedly relaxes the pelvic floor muscles, reduces indirect and direct trauma to the pelvic floor, and prevents the development of symptoms related to pelvic floor trauma [9]. Therefore, the association of PFD risk with the use of epidural anesthesia in labor is controversial. Thus, we analyzed the data to reveal the association between the mode of delivery and the prevalence of PFD symptoms. However, data on the association of PFD prevalence with epidural anesthesia in postpartum Japanese women are scarce. Therefore, we conducted this study to further investigate this association and the prevalence of PFD symptoms in Japanese women six to fifteen months after delivery.
This article was previously presented as a meeting abstract at the 74th Annual Congress of the Japan Society of Obstetrics and Gynecology on August 5-7, 2022, and the 73rd Annual Congress of the Japan Society of Obstetrics and Gynecology on April 22-25 (Sunday) 2021, separately. This article was previously posted to the Research Square preprint server on June 1, 2022.
Materials and methods
This was a retrospective cohort study of women who gave birth between July 2018 and May 2019 at the Kameda Medical Center, Chiba Prefecture, Japan, where an average of 600 deliveries are made annually.
All applicants were informed and enrolled by mail, which included an informed consent form. Hence, informed consent was received from all participants before the initiation of the study.
PFD was evaluated using the PFDI-20 questionnaire, which consists of 20 condition-specific measures of pelvic symptoms. It has three subscales: the Pelvic Organ Prolapse Distress Inventory with six items (POPDI-6), the Colorectal-Anal Distress Inventory with eight items (CRADI-8), and the Urinary Distress Inventory with six items (UDI-6).
Each question relates to the presence of an individual symptom. If the symptom is present, the responder scores it on a 4-point scale to indicate the extent of inconvenience the symptom elicits; a score of 1 is "not at all", and a score of 4 is "quite a bit."
POPDI-6, CRADI-8, and UDI-6 are each scored similarly. First, subscale scores for each scale are obtained by taking the mean value of all items answered within each subscale. These subscale scores are determined by multiplying each scale’s mean score by 25, which gives each subscale score a range of 0-100. As a result, adding up the subscale scores (which range from 0 to 300) yields the overall scale score. A high score indicates more symptoms and higher levels of inconvenience caused by the symptoms. The questionnaire was based on the validated Japanese version of the PFDI-20 [10]. The questionnaire was digitized as a web form without any modifications to the original form.
Participants were sent a questionnaire along with an explanation document about the PFDI-20 six to fifteen months after delivery. The target study population was 6-15 months postpartum in patients who gave birth at the Kameda Medical Center. The inclusion criteria for the study were non-preterm deliveries, singleton pregnancies, and fluency in Japanese. Individuals who either did not respond or did not respond well to the questionnaire were excluded from the analysis.
We divided all women into three groups: the cesarean section, epidural (vaginal delivery with epidural anesthesia), and non-epidural groups. Furthermore, in the cesarean-section group, emergency cesarean-section was divided into four groups: emergency cesarean-section, with epidural in labor, without epidural in labor, and not in labor. Epidural anesthesia was mainly indicated based on the patient’s demand and in cases where it was required because of medical issues (e.g., hypertensive disorders of pregnancy).
The PFDI-20 questionnaire was answered at 6-15 months postpartum, reflecting the respondent’s state during that period. A "yes" on the questionnaire represented a positive result, indicating the occurrence of PFD. Study identification numbers were assigned to each woman, and their medical information was obtained from the center’s medical records.
Statistical analyses
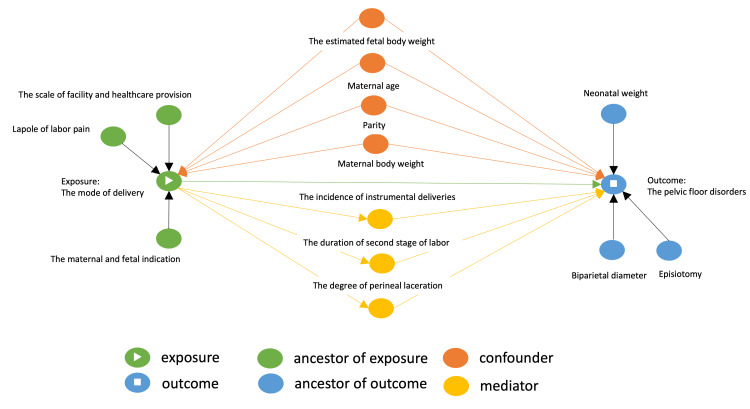
We obtained data on age, parity, maternal body weight before the pregnancy, mode of delivery, and the result of delivery mode. Continuous variables with a normal distribution are presented as mean and median values. We compared maternal, fetal, and neonatal characteristics with PFDI-20 (POPDI, CRADI, and UDI) in each group. Continuous variables were compared using the one-way ANOVA, and categorical variables were evaluated using the chi-squared test. A linear regression model was constructed for the PFDI-20 (POPDI, CRADI, and UDI), with adjustments for confounding factors (age, parity, and pre-pregnancy body weight). All confounding factors used were derived from the findings of previous studies. Causal-directed acyclic graphs (DAGs) were used to identify potential interplay among variables [11]. Supplementary Material Data 1 shows the DAGs used in this study.
Maternal age and pre-pregnancy body weight reportedly influence the mode of delivery due to maternal obstetrical complications [12-13]. Moreover, a previous mode of delivery affects the choice of delivery mode in future pregnancies; for example, the rate of vaginal birth after a cesarean procedure is reduced by approximately 10-30% in Japan [14]. Factors such as these influence the PFD [15]; therefore, we considered these factors to be confounding factors. Statistical significance was set at p<0.05, and all statistical analyses were conducted using R version 3.6.3 on July 22, 2011, at 23:54:00 (R Foundation for Statistical Computing, Vienna, Austria).
We assumed that any missing data was completely missing at random; hence, we conducted all analyses using the available data.
Ethics statement
This study was approved by the institutional ethical review board of our hospital on August 28th, 2019 (approval ID: 19-052).
Results
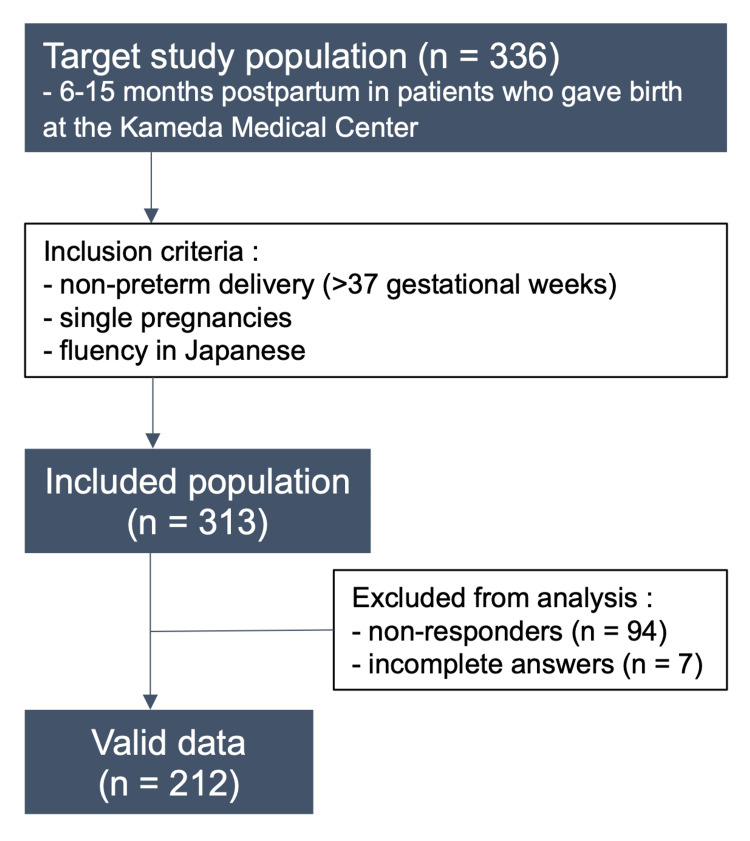
Out of 336 women included in the study, 313 met the inclusion criteria. However, 101 women were excluded from the study due to no or incomplete responses. Ultimately, 212 postpartum women were analyzed (Figure 1).
Figure 1. Study flow chart.
Maternal, fetal, and neonatal characteristics (Table 1) revealed that the mode of delivery included emergency cesarean sections in 14 (6.6%) women, elective cesarean sections in 30 (14.2%), vaginal deliveries with epidural anesthesia in 21 (9.9%), and vaginal deliveries without epidural anesthesia in 147 (69.3%) women.
Table 1. Maternal, fetal, and neonatal characteristics of the study groups.
SD: standard deviation. †The duration of labor in emergency cesarean section group in labor.
| Characteristics | Overall n = 212 | Cesarean section n = 44 | Vaginal delivery with epidural anesthesia n = 21 | Vaginal delivery without epidural anesthesia n = 147 | p-value |
| Age (mean (SD)) | 33.00 (4.73) | 34.59 (4.07) | 32.52 (4.53) | 32.60 (4.87) | 0.043 |
| Parity (mean (SD)) | 0.68 (0.81) | 1.05 (0.94) | 0.57 (0.75) | 0.59 (0.76) | 0.004 |
| Pre-pregnancy body weight (kg) (mean (SD)) | 49.49 (16.79) | 46.19 (18.44) | 44.27 (22.66) | 51.23 (15.06) | 0.07 |
| Duration of labor (min) | |||||
| 1st stage (mean (SD)) | 499.01 (384.79) | 475.63 (348.68)† | 926.73 (738.98) | 438.62 (220.87) | 0.001 |
| 2nd stage (mean (SD)) | 60.97 (95.52) | 51.22 (61.10)† | 114.36 (297.81) | 92.71 (79.29) | 0.026 |
| Outcome of delivery (%) | <0.001 | ||||
| Natural (n (%)) | 133 (62.7) | 0 | 11 (52.4) | 122 (83.0) | |
| Instrumental (n (%)) | 35 (16.5) | 0 | 10 (47.6) | 25 (17.0) | |
| Vacuum (n (%)) | 33 (15.6) | 0 | 10 (47.6) | 23 (15.6) | |
| Forceps (n (%)) | 2 (0.94) | 0 | 0 | 2 (0.94) | |
| Cesarean section (n (%)) | 44 (20.8) | 44 (100.0) | 0 | 0 | |
| Emergency cesarean section (n (%)) | 14 (6.6) | 14 (/44=31.8) | 0 | 0 | |
| With epidural in labor (n (%)) | 2 (0.94) | 2 (/44=4.5) | 0 | 0 | |
| Without epidural in labor (n (%)) | 9 (4.3) | 9 (/44=20.5) | 0 | 0 | |
| Not in labor (n (%)) | 3 (1.4) | 3 (/44=6.8) | 0 | 0 | |
| Perineal laceration | <0.001 | ||||
| None (n (%)) | 99 (46.7) | 44 (100.0) | 2 (9.5) | 53 (36.1) | |
| 1st (n (%)) | 11 (5.2) | 0 | 0 | 11 (7.5) | |
| 2nd (n (%)) | 98 (46.2) | 0 | 19 (90.5) | 79 (53.7) | |
| 3rd (n (%)) | 4 (1.9) | 0 | 0 | 4 (2.7) | |
| Neonatal weight (mean (SD)) | 2988.87 (364.89) | 2949.82 (427.61) | 3027.14 (346.34) | 2995.09 (348.47) | 0.68 |
The outcomes of delivery were spontaneous for 133 (62.7%) women, instrumental for 35 (16.5%), and cesarean section for 44 (20.8%). The degree of perineal laceration included none for 99 (46.7%) women, a first degree for 11 (5.2%), a second degree for 98 (46.2%), and a third degree for 4 (1.9%).
Among 212 postpartum women, 156 (73.6%) presented with PFD symptoms. Broken down into three groups, 59 women had POPDI (27.8%), 111 had CRADI (52.4%), and 114 had UDI (53.8%). The most prevalent PFD symptom was UDI in 114 (53.8%) women, of whom 79 (37.3%) experienced urine leakage related to increased abdominal pressure. The prevalence of PFD-20 items is shown in Supplementary Material Data 2.
The prevalence of PFD symptoms was statistically significant (p = 0.037) for all groups: cesarean section group, 61.4% (n = 27); epidural group, 90.5% (n = 19); and non-epidural group, 74.8% (n = 110) (Table 2).
Table 2. Prevalence of each section of the PFDI-20 symptoms among the three groups.
PFDI-20: Pelvic Floor Distress Inventory-20; POPDI: pelvic organ prolapse distress inventory; CRADI: colorectal anal distress inventory; UDI: urinary distress inventory
| PFDI | Cesarean section | Vaginal delivery with epidural anesthesia | Vaginal delivery without epidural anesthesia | p-value |
| Overall (n = 212) | n = 44 (%) | n = 21 (%) | n = 147 (%) | |
| PFDI-20 | 27 (61.4) | 19 (90.5) | 110 (74.8) | 0.037 |
| POPDI | 10 (22.7) | 8 (38.1) | 41 (27.9) | 0.433 |
| CRADI | 18 (40.9) | 15 (71.4) | 78 (53.1) | 0.067 |
| UDI | 19 (43.2) | 14 (66.7) | 81 (55.1) | 0.174 |
Multivariable regression for pelvic floor symptom burden score showed the following results: the epidural group had an 8.67-point higher symptom burden score (estimate, 8.67 [0.03 to 17.3]) and the cesarean section group had a 7.61-point lower score (estimate, −7.61 [−14.2 to −1.03]), as compared to the non-epidural group, regarding the association between PFD and delivery mode (Table 3).
Table 3. Multivariate regression analysis of disease burden scores (PFDI-20, each section).
PFDI-20: Pelvic Floor Distress Inventory-20; POPDI: pelvic floor disorders distress inventory; CRADI: colorectal anal distress inventory; UDI: urinary distress inventory.
| PFDI-20 | POPDI | CRADI | UDI | |||||
| Coefficient | 95%CI | Coefficient | 95%CI | Coefficient | 95%CI | Coefficient | 95%CI | |
| Age | 0.42 | −0.13 to 0.98 | 0.13 | −0.080 to 0.33 | 0.14 | −0.096 to 0.37 | 0.16 | −0.15 to 0.46 |
| Parity | 0.72 | −2.54 to 3.99 | 0.15 | −1.07 to 1.36 | 0.081 | −1.29 to 1.45 | 0.50 | −1.30 to 2.29 |
| Pre-pregnancy body weight | −0.042 | −0.20 to 0.11 | −0.046 | −0.10 to 0.011 | −0.022 | −0.086 to 0.043 | 0.025 | −0.059 to 0.11 |
| Exposure | ||||||||
| Vaginal delivery without epidural anesthesia | Reference | Reference | Reference | Reference | ||||
| Cesarean section | −7.61 | −14.19 to −1.03 | −1.20 | −3.65 to 1.26 | −2.29 | −5.05 to 0.47 | −4.12 | −7.74 to −0.52 |
| Vaginal delivery with epidural anesthesia | 8.67 | 0.034~17.30 | 4.32 | 1.10~7.53 | 3.39 | −0.23 to 7.01 | 0.96 | −3.78 to 5.69 |
Discussion
This study showed the prevalence of PFD symptoms in Japanese women in a large, single, rural center. It was relatively high, affecting 156 (73.6%) of the 212 women surveyed. The affected women were divided into three groups: 27.8% were affected by POPDI, 52.4% by CRADI, and 53.8% by UDI. The most prevalent PFD symptom was UDI in 114 (53.8%) women, of whom 79 (37.3%) experienced urine leakage associated with increased abdominal pressure. In the cesarean section group (n=44), PFD symptoms were prevalent in 27 (64.1%) women, including primipara and multipara, suggesting a high prevalence of PFD symptoms in the cesarean section group (Table 2).
A previous study reported that higher scores were recorded on the PFDI-20 (p=0.003) questionnaire in late pregnancy and the early postpartum period rather than in early pregnancy; hence, postpartum PFD is usually associated with delivery as a risk factor [16].
Limited data are available on the prevalence of PFD symptoms in Japanese women; hence, it is important to assess their prevalence.
PFD is widespread in the immediate postpartum period; it tends to resolve six months later; however, it persists in a considerable number of cases [17]. It is remarkable that our results show high rates of prevalence of PFD symptoms even 6-15 months postpartum, as their prevalence in other countries is relatively variable. In Australia, PFDI prevalence was seen in 97% (n = 196) of the patients who answered the questionnaire, with 9% of them affected by POPDI, 79% by CRADI, and 82% by UDI [2]. Conversely, a study on Israeli women showed that 12.8% of surveyed women were affected by POPDI, 20.7% by CRADI, and 15.8% by UDI at three months postpartum [3].
Our results regarding the prevalence of PFD symptoms in Japanese women contradicted those of previous studies [2,3]. We hypothesized several reasons for this. The first reason could be due to the differences in modes of delivery. The Australian study only enrolled patients who delivered vaginally, excluding those who underwent cesarean sections, which would explain the relatively higher prevalence of PFD in their patients. Our study demonstrated a lower prevalence of PFD symptoms with cesarean section than with vaginal delivery. The second reason involves the timing of the study period. Women at three months postpartum may still be less active in their daily routines and may be bearing lesser loads on their bodies due to the lighter body weight of their infants, thereby exerting a lesser burden on their pelvic floor muscles. This would explain the lower prevalence of PFD in the Israeli study [3].
Furthermore, our result would have a higher prevalence than other studies due to our methodology, wherein any response to the questionnaire, even the lowest possible score of 1, would be considered indicative of a positive symptom. We believe that individuals who are currently presenting symptoms, regardless of the severity of their scores, may still be at risk for the development of their symptoms in the future; thus, our result would be significant in this aspect [18].
To date, no prominent factor has been identified for these differences. However, these differences could arise from the type of perinatal management and the timing of the study. Therefore, more studies exploring these factors should be conducted in the future.
Regarding the association between PFD and the use of epidural anesthesia during labor, a higher disease burden score of 8.67 points (estimate: 8.67 [0.03 to 17.3]) was observed in the epidural group compared with the non-epidural group (Table 3).
Sartore et al. concluded that the use of epidural anesthesia is not associated with symptoms related to perineal trauma and pelvic floor muscle weakness [19]. We adjusted for the anesthetic method used and the duration of labor during analysis, but the results remained unchanged; the epidural group still showed a higher risk for PFD. The measurement tools and methodologies used for assessing pelvic floor function in Sartore’s study were dissimilar from those used in this study, explaining the variations between the two studies.
Epidural anesthesia is related to a prolonged second stage of labor [4]. Vaginal delivery with epidural anesthesia is associated with a higher risk of instrumental vaginal deliveries compared with labor without epidural anesthesia [5]. Therefore, epidural anesthesia is believed to harm pelvic floor function. The results of these studies are not contradictory to ours.
Interpreting the association between PFD and delivery mode, a lower disease burden score (estimate, −7.61 (−14.2 to −1.03)) was observed in the cesarean group compared with the non-epidural group (Table 3). This indicates that, in the short term, cesarean sections are associated with a lower incidence of PFD in postpartum women compared with vaginal delivery, whether non-epidural or epidural. Furthermore, Baud et al. revealed that women were more likely to report urinary incontinence after vaginal delivery than after cesarean sections at six years postpartum. In contrast, women more frequently reported symptoms of sexual dysfunction, especially dyspareunia, after cesarean sections than after vaginal deliveries. Therefore, the advantages, disadvantages, and long-term consequences of the delivery mode should always be considered [20].
Thus, based on the results of our study, we recommend that cesarean delivery not be chosen over vaginal-based delivery solely based on the lower risk of PFD associated with cesarean deliveries.
Our study demonstrated a high prevalence of PFD symptoms in women at six to fifteen months postpartum. Therefore, an accurate diagnosis of PFD in postpartum women, along with appropriate and regular follow-up until improvement of their symptoms, is crucial.
Regular pelvic floor muscle training in postpartum women (n=50) showed significant improvements in pelvic floor function as per the PFDI-20 (mean change of −41.8; p<0.001) [21]. Therefore, healthcare workers should consider early identification of symptoms to provide care and thereby reduce PFD development. This would be more crucial in the epidural group, which has a higher prevalence of PFD symptoms. A study reported that in women with relatively mild pelvic floor symptoms, an improvement of 13.5 points in the PFDI-20 score can be considered clinically relevant [22]. This minimal yet significant change can be used for clinical trial planning and evaluation of treatment or intervention effects. Hence, our result of 8.67 points could be considered a clinical risk. Healthcare workers should therefore inform pregnant women of the possible increase in the risk of pelvic floor disorders with anesthesia.
This study has several limitations. First, the PFD-20 is an evaluation of symptoms, not of pathology. Hence, even though the prevalence of UDI was high, it cannot be equated with the severity of stress urinary incontinence. Second, this study was based on data from a single center; therefore, the composition of the catchment population and resources may potentially limit the generalizability of our results in Japan. Third, a low response rate of 67.7% raised suspicions that non-responders probably had no or few PFD symptoms. Fourth, regarding analytic limitations, we considered instrument delivery and perineal laceration as mediators; we could not separate the effect of these mediators from the methods of delivery. Furthermore, factors that affect the prognosis of PFD, such as episiotomy, birth weight, and fetal biparietal diameter, were considered predictors, not confounders. However, we believe that this did not affect the study's results. Additionally, since this was an observational study, we were not able to adjust for unmeasured confounders. Therefore, the effect of epidurals on PFDI may have been biased. For example, the estimated fetal body weight could have affected the implementation of both epidurals and PFDI, which could not be investigated in this study. More factors need to be prospectively investigated in future studies.
Conclusions
In conclusion, the results of this study suggest that pelvic floor disorders are highly prevalent in Japanese women after giving birth. The use of the PFDI-20 questionnaire has facilitated the assessment of these conditions and revealed urinary distress as the most common symptom among postpartum women. Additionally, the study found an association between epidural anesthesia and a higher disease burden score, indicating a potential role for this intervention in the development of pelvic floor disorders.
Appendices
Supplementary Material data 1: The causal directed acyclic graphs (DAGs)
Figure 2. The causal directed acyclic graphs (DAGs).
Supplementary material data 2: The prevalence of each PFDI-20 question
Table 4. The prevalence of each PFDI-20 question.
PFDI-20: Pelvic Floor Distress Inventory-20; POPDI: pelvic floor disorders distress inventory; CRADI: colorectal anal distress inventory; UDI: urinary distress inventory.
| PFDI | Cesarean section | Vaginal delivery with epidural anesthesia | Vaginal delivery without epidural anesthesia | p-value |
| n (%) | 44 (%) | 21 (%) | 147 (%) | |
| POPDI_Q1 | 3 (6.8) | 3 (14.3) | 8 (5.4) | 0.311 |
| POPDI_Q2 | 3 (6.8) | 4 (19.0) | 20 (13.6) | 0.326 |
| POPDI_Q3 | 2 (4.5) | 3 (14.3) | 6 (4.1) | 0.140 |
| POPDI_Q4 | 3 (6.8) | 3 (14.3) | 7 (4.8) | 0.230 |
| POPDI_Q5 | 3 (6.8) | 2 (9.5) | 14 (9.5) | 0.855 |
| POPDI_Q6 | 0 (0.0) | 1 (4.8) | 0 (0.0) | 0.010 |
| CRADI_Q7 | 4 (9.1) | 6 (28.6) | 25 (17.0) | 0.135 |
| CRADI_Q8 | 7 (15.9) | 9 (42.9) | 33 (22.4) | 0.052 |
| CRADI_Q9 | 0 (0.0) | 0 (0.0) | 3 (2.0) | 0.510 |
| CRADI_Q10 | 0 (0.0) | 0 (0.0) | 7 (4.8) | 0.202 |
| CRADI_Q11 | 8 (18.2) | 5 (23.8) | 30 (20.4) | 0.868 |
| CRADI_Q12 | 4 (9.1) | 5 (23.8) | 13 (8.8) | 0.104 |
| CRADI_Q13 | 7 (15.9) | 3 (14.3) | 27 (18.4) | 0.859 |
| CRADI_Q14 | 3 (6.8) | 3 (14.3) | 7 (4.8) | 0.230 |
| UDI_Q15 | 3 (6.8) | 7 (33.3) | 21 (14.3) | 0.018 |
| UDI_Q16 | 2 (4.5) | 1 (4.8) | 13 (8.8) | 0.561 |
| UDI_Q17 | 8 (18.2) | 9 (42.9) | 62 (42.2) | 0.013 |
| UDI_Q18 | 6 (13.6) | 2 (9.5) | 29 (19.7) | 0.389 |
| UDI_Q19 | 3 (6.8) | 0 (0.0) | 2 (1.4) | 0.084 |
| UDI_Q20 | 6 (13.6) | 2 (9.5) | 12 (8.2) | 0.552 |
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. The institutional ethical review board of Kameda Medical Center issued approval 19-052. This study was approved by the institutional ethical review board of our hospital on August 28, 2019 (approval ID: 19-052)
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Questionnaires to evaluate pelvic floor dysfunction in the postpartum period: a systematic review. Zuchelo LT, Bezerra IM, Da Silva AT, et al. Int J Womens Health. 2018;10:409–424. doi: 10.2147/IJWH.S164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perineal outcome and the risk of pelvic floor dysfunction: a cohort study of primiparous women. Rikard-Bell J, Iyer J, Rane A. Aust N Z J Obstet Gynaecol. 2014;54:371–376. doi: 10.1111/ajo.12222. [DOI] [PubMed] [Google Scholar]
- 3.Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Yohay D, Weintraub AY, Mauer-Perry N, Peri C, Kafri R, Yohay Z, Bashiri A. Eur J Obstet Gynecol Reprod Biol. 2016;200:35–39. doi: 10.1016/j.ejogrb.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Epidural versus non-epidural or no analgesia in labour. Anim-Somuah M, Smyth RM, Jones L. Cochrane Database Syst Rev. 2011:0. doi: 10.1002/14651858.CD000331.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Effect of epidural vs parenteral opioid analgesia on the progress of labor: a meta-analysis. Halpern SH, Leighton BL, Ohlsson A, Barrett JF, Rice A. JAMA. 1998;280:2105–2110. doi: 10.1001/jama.280.24.2105. [DOI] [PubMed] [Google Scholar]
- 6.Contribution of the second stage of labour to pelvic floor dysfunction: a prospective cohort comparison of nulliparous women. Rogers RG, Leeman LM, Borders N, et al. BJOG. 2014;121:1145–1153. doi: 10.1111/1471-0528.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postpartum impairment of pelvic floor muscle function: factors involved and association with prolapse. Diez-Itza I, Arrue M, Ibañez L, Paredes J, Murgiondo A, Sarasqueta C. Int Urogynecol J. 2011;22:1505–1511. doi: 10.1007/s00192-011-1484-2. [DOI] [PubMed] [Google Scholar]
- 8.The effects of epidural analgesia on labor, maternal, and neonatal outcomes: a systematic review. Leighton BL, Halpern SH. Am J Obstet Gynecol. 2002;186:0–77. doi: 10.1067/mob.2002.121813. [DOI] [PubMed] [Google Scholar]
- 9.Epidural anaesthesia and avoidance of postpartum stress urinary incontinence. Schuessler B, Hesse U, Dimpfl T, Anthuber C. Lancet Lond Engl. 1988;1:762. doi: 10.1016/s0140-6736(88)91561-9. [DOI] [PubMed] [Google Scholar]
- 10.Reliability and validity of the Japanese version of the pelvic floor distress inventory-short form 20. Yoshida M, Murayama R, Ota E, Nakata M, Kozuma S, Homma Y. Int Urogynecol J. 2013;24:1039–1046. doi: 10.1007/s00192-012-1962-1. [DOI] [PubMed] [Google Scholar]
- 11.Causal directed acyclic graphs. Lipsky AM, Greenland S. JAMA. 2022;327:1083–1084. doi: 10.1001/jama.2022.1816. [DOI] [PubMed] [Google Scholar]
- 12.Caesarean section by maternal age group among singleton deliveries and primiparous Japanese women: a secondary analysis of the WHO Global Survey on Maternal and Perinatal Health. Yoshioka-Maeda K, Ota E, Ganchimeg T, Kuroda M, Mori R. BMC Pregnancy Childbirth. 2016;16:39. doi: 10.1186/s12884-016-0830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pregnancy outcomes based on pre-pregnancy body mass index in Japanese women. Enomoto K, Aoki S, Toma R, Fujiwara K, Sakamaki K, Hirahara F. PLoS One. 2016;11:0. doi: 10.1371/journal.pone.0157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reasons Why some Japanese pregnant women choose trial of labor after cesarean. Suzuki S, Ikeda M. J Clin Med Res. 2015;7:641–642. doi: 10.14740/jocmr2214w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A population-based survey of the prevalence, potential risk factors, and symptom-specific bother of lower urinary tract symptoms in adult Chinese women. Zhang L, Zhu L, Xu T, et al. Eur Urol. 2015;68:97–112. doi: 10.1016/j.eururo.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Pelvic floor changes in the first term pregnancy and postpartum period. Wu E, Kuehl TJ, Gendron JM, White W, Yandell PM. Int Urogynecol J. 2021;32:1897–1905. doi: 10.1007/s00192-020-04456-5. [DOI] [PubMed] [Google Scholar]
- 17.Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Thompson JF, Roberts CL, Currie M, Ellwood DA. Birth. 2002;29:83–94. doi: 10.1046/j.1523-536x.2002.00167.x. [DOI] [PubMed] [Google Scholar]
- 18.The demographics of pelvic floor disorders: current observations and future projections. Luber KM, Boero S, Choe JY. Am J Obstet Gynecol. 2001;184:1496–1501. doi: 10.1067/mob.2001.114868. [DOI] [PubMed] [Google Scholar]
- 19.Effects of epidural analgesia during labor on pelvic floor function after vaginal delivery. Sartore A, Pregazzi R, Bortoli P, Grimaldi E, Ricci G, Guaschino S. Acta Obstet Gynecol Scand. 2003;82:143–146. doi: 10.1034/j.1600-0412.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 20.Comparison of pelvic floor dysfunction 6 years after uncomplicated vaginal versus elective cesarean deliveries: a cross-sectional study. Baud D, Sichitiu J, Lombardi V, De Rham M, Meyer S, Vial Y, Achtari C. Sci Rep. 2020;10:21509. doi: 10.1038/s41598-020-78625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Impact of pelvic floor muscle training in the postpartum period. Gagnon LH, Boucher J, Robert M. Int Urogynecol J. 2016;27:255–260. doi: 10.1007/s00192-015-2822-6. [DOI] [PubMed] [Google Scholar]
- 22.Minimal important change in the pelvic floor distress inventory-20 among women opting for conservative prolapse treatment. Wiegersma M, Panman CM, Berger MY, De Vet HC, Kollen BJ, Dekker JH. Am J Obstet Gynecol. 2017;216:397. doi: 10.1016/j.ajog.2016.10.010. [DOI] [PubMed] [Google Scholar]