Abstract
A true aneurysm of the celiac artery is a rare form of a visceral aneurysm, constituting ∼4% of visceral aneurysms. Mortality in ruptured cases is high; thus, early recognition and treatment are crucial. Recent guidelines suggest endovascular therapy; however, numerous complications are associated with endoluminal treatment. Open repair in select cases, using an individualized strategy fit for the patient's anatomy still provides excellent early and long-term results. Our patient was treated with open surgical resection and end-to-end anastomosis of the celiac and common hepatic arteries. A 43-month follow-up computed tomography angiogram revealed excellent hepatic artery patency and no pseudoaneurysm formation.
Keywords: Aneurysm, Celiac artery, Endovascular, Stent graft
Celiac artery aneurysms (CAAs) are rare, although the frequency of the diagnosis of CAAs has been reported.1, 2, 3 Endovascular therapy (EVT) should be considered first-line treatment because of the resulting better early survival.4 However, the surgical techniques and perioperative care advances have decreased open surgical morbidity and mortality. We report the case of a 57-year-old White man with a symptomatic CAA, who was treated by open surgical repair, with aneurysm resection, end-to-end anastomosis of the celiac and common hepatic arteries, and suture ligation of the splenic and left gastric arteries. At 43 months of follow-up, the patient was in excellent general condition, and computed tomography angiography (CTA) revealed no pseudoaneurysm formation and good patency of the common hepatic artery.
Case report
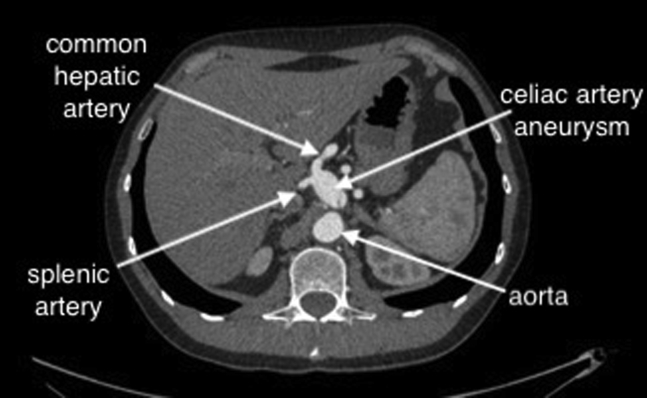
A 57-year-old man with epigastric discomfort was referred to our outpatient clinic to manage a CAA, measuring 25 mm in diameter. The patient was examined by a gastroenterologist, and other etiologies for the epigastric pain were excluded before vascular consultation. CTA revealed the aneurysm originating 6 mm from the celiac artery origin from the aorta. The CAA extended into bifurcation into the common hepatic and splenic arteries (Fig 1). Because of the risk of rupture, the patient was offered surgical repair. Endovascular therapy was not chosen because the short neck of the CAA raised concerns regarding the long-term outcome. The patient was in good general condition, without comorbidities and, thus, was offered open repair. We planned to resect and either anastomose the approximated common hepatic artery if intraoperative mobilization would suffice or perform a bypass with the great saphenous vein if the arterial mobilization would not be sufficient, with ligation of the proximal part of the splenic and left gastric arteries. The risk of stomach ischemia was low, because the collateralization was very good with uncompromised hepatic flow through the right gastric and right gastroepiploic arteries. The risk of ischemia would be increased in patients with compromised circulation, including patients with atherosclerosis, heart failure, or small vessel disease, such as those with diabetes or renal insufficiency. The present study was retrospective, and the requirement for ethics approval was waived. The patient provided written informed consent for the report of his case details and imaging studies.
Fig 1.
Preoperative computed tomography angiogram.
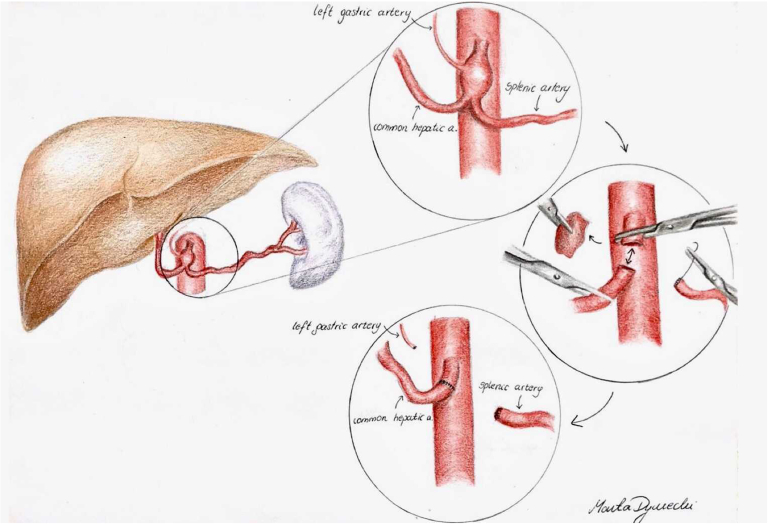
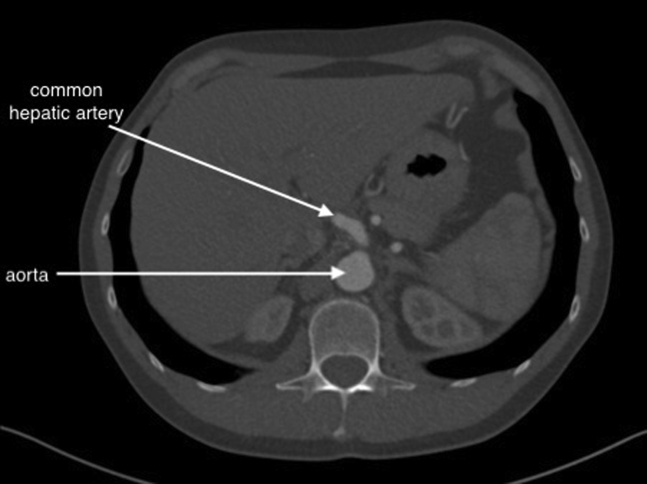
With the patient under general anesthesia, a chevron incision was performed in the upper abdomen. A Condor GoldLine retractor (Condor Medtec GmbH) was used to facilitate exposure. The lesser omentum was opened, and the CAA was exposed. The arcuate ligament of the diaphragm and celiac ganglion were cut, allowing for exposure of the aorta above and below the celiac artery trunk to facilitate secure clamping. The proximal part of the common hepatic, splenic, and left gastric arteries were exposed. After systemic heparinization, aortic side clamping was used, and the dissected parts of the common hepatic, splenic, and left gastric arteries were clamped. The CAA was resected in its entirety. The common hepatic artery was sufficiently mobilized and approximated to the proximal stump of the celiac trunk to facilitate a tension-free anastomosis. A direct end-to-end anastomosis was performed with running Surgipro 5-0 suture (Medtronic; Fig 2). The splenic and left gastric arteries were suture ligated in the proximal sections. The procedure lasted for 150 minutes. The postoperative period was uneventful. The patient returned to oral feeding on the first postoperative day and was discharged home on day 4. He received low-molecular-weight heparin at a prophylactic dose for 10 postoperative days. Because the patient did not have atherosclerosis or coronary artery disease, he was not prescribed antiplatelet or statin therapy. CTA was performed at his 43-month follow-up visit. No pseudoaneurysm formation was found, and flow to the hepatic artery was preserved (Fig 3).
Fig 2.
Schematic of surgery performed.
Fig 3.
Follow-up computed tomography angiogram.
Discussion
A true CAA is a rare condition, with an estimated incidence of 0.005% to 0.01%.2 Atherosclerosis, medial degeneration, injuries, connective tissue disorders, vasculitis, and infections are the leading causes of CAAs.5,6 In addition, 15% to 20% of cases are complicated by rupture, with a mortality rate of ≤80%.7 In the present case, the patient was referred to our department because of an ultrasound finding of a CAA, after the exclusion of all other causes of epigastric pain. No other intra-abdominal aneurysms were revealed by CTA.
It has been recommended to treat symptomatic CAAs >2 cm, with EVT considered as first-line treatment.1,4 However, to the best of our knowledge, the open surgical and endovascular strategies for this type of aneurysm have not yet been compared in any study. Aneurysm isolation, sac exclusion with a covered stent or coil packing, and the use of liquid embolic agents can be considered.6 Good long-term results can be obtained using modern peripheral stent grafts.8 However, specific anatomic criteria must be met to obtain success with EVT, and compromising these criteria can lead to treatment failure.9 The limitations of EVT include the risk of contrast toxicity, access-related injury, and end-organ embolization.6 A significantly higher reintervention rate after EVT has been reported, raising concerns for the long-term durability.1 The lack of a proximal landing zone makes EVT for CAAs inadequate significantly often.10 Treatment failure such as persistent sac perfusion, coil migration, and sac recanalization have been observed, with an early reperfusion rate as high as 10.3%.6 Even an initially successful EVT procedure can eventually fail owing to possible recanalization and sac reperfusion, which can lead to late rupture.11 For our patient, stent grafting was not an option, because the 6-mm proximal landing zone was thought to be too short for secure endograft fixation. Surgical repair was considered because of these technical issues and because our patient was in good health.
Shumaker and Siderys12 reported the first resection with concomitant revascularization of a CAA in 1958. Advances in surgical treatment and perioperative care have decreased the morbidity and mortality of open surgical repair in well-selected cases to levels previously reserved for EVT. Also, open surgical repair of CAAs yields excellent long-term results.4,13,14 If surgical treatment is considered, either resection with celiac artery ligation or reconstruction can be considered. Simple celiac artery ligation has been reported in ≤35% of cases, is well tolerated in most cases, and is an especially viable option for ruptured cases.7 For elective cases, revascularization is suggested.13 When arterial reconstruction is required, bypass surgery from the aorta using a prosthetic vascular graft or autologous vein graft is often necessary.3 The performance of aortic anastomosis in the supraceliac aorta is the challenging step of the procedure. The use of a sutureless anastomotic device (PAS-port; Cardica) has been reported to facilitate aortic anastomosis.15 Aneurysmectomy with primary end-to-end anastomosis has also been reported.16,17 In our case, no conduit was used, because we performed a primary end-to-end anastomosis of a short stump of healthy celiac artery to a mobilized part of the common hepatic artery. This made the procedure safer and less demanding, with no need for an aortic anastomosis. The chance for good long-term patency of the repaired common hepatic artery was also improved, because early and mid-term vein graft occlusions have been reported in CAA repair.13 A primary end-to-end anastomosis also decreases the risk of late pseudoaneurysm formation, because vein grafts can degenerate with time, and prosthetic grafts are prone to infection. CTA follow-up at 43 months confirmed our assumption, revealing excellent patency and no pseudoaneurysm formation. A brief literature review and comparison of operative methods are included in Tables I and II.8,13,14,16,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33
Table I.
Endovascular repair of celiac artery aneurysms (CAAs)
| Investigator | Patients, no. | Observation time, months | CAA obliteration rate, % | Patency rate, % | Type of operation |
|---|---|---|---|---|---|
| Gabelmann et al,18 2002 | 2 | 51 (1 patient lost to follow up) | 100 | – | Coil embolization |
| Tulsyan et al,19 2007 | 3 | 16 (mean) | 66.7 | – | Coil/glue embolization |
| Waldenberger et al,20 2007 | 3 | 21 (mean) | 100 | – | Coil embolization |
| Etezadi et al,21 2011 | 4 | 45 (mean) | 100 | 100 | Coil embolization |
| Carrafiello et al,22 2011 | 1 | 12 | 100 | 100 | Self-expanding stent |
| Zhang et al,23 2016 | 5 | 19 (mean) | 100 | 100 | Stent graft |
| Venturini et al,8 2018 | 3 | 12 (mean) | 100 | 100 | Stent graft |
| Xia et al,24 2019 | 3 | 24 (mean) | 100 | 100 | Covered stent |
| Yuan et al,25 2020 | 4 | 34 (mean) | 100 | 100 | Covered and uncovered stents |
| Dwivedi et al,26 2021 | 1 | 12 | 100 | 100 | Stent graft and coil embolization |
Table II.
Open repair of Celiac Artery Aneurysm
| Investigator | Patients, No. | Observation, months | Postoperative complications | Early mortality, % | Long-term success, % | Type of operation |
|---|---|---|---|---|---|---|
| Stone et al,13 2002 | 8 | 99 (mean) | In 2 patients, ventral herniation of abdominal wound developed; in another 2 patients, saphenous vein conduits were occluded at 1 and 6 months of follow-up | 0 | 78 | Aneurysmectomy plus revascularization or prosthetic graft |
| Sessa et al,27 2004 | 5 | no info | 1 Thrombosis (nonruptured aneurysm); 1 pulmonary embolism and death (ruptured aneurysm) | 20 | 60 | No information |
| D'Ayala et al,28 2004 | 1 | 5 | – | 0 | 100 | Aneurysmectomy plus prosthetic graft |
| Taberkant et al,29 2006 | 1 | 18 | – | 0 | 100 | Aneurysmectomy plus revascularization |
| Lipari et al,30 2006 | 2 | 19 (mean) | Pulmonary thickening with pleural effusion | 0 | 100 | No information |
| Popov et al,31 2007 | 4 | 1 (mean) | 1 Patient died during surgery of hemorrhagic shock; 2 patients had prolonged intestinal ischemia and diarrhea | 25 | 75 | No information |
| Pulli et al,16 2008 | 3 | 82 (mean) | – | 0 | 100 | Aneurysmectomy plus prosthetic graft |
| Vasconcelos et al,32 2010 | 3 | 10 (mean) | – | 0 | 100 | Aneurysmectomy plus revascularization or prosthetic graft |
| Higashiyama et al,14 2011 | 1 | 37 | – | 0 | 100 | Aneurysmectomy plus revascularization |
| Uchida et al,15 2017 | 1 | 6 | – | 0 | 100 | Aneurysmectomy plus revascularization |
| Azimi-Ghomi et al,33 2017 | 1 | 3 | – | 0 | 100 | Aneurysmectomy plus prosthetic graft |
Conclusions
The morphology of the aneurysm and that mobilization of the common hepatic artery was possible made this procedure safer and less demanding than a bypass from the supraceliac aorta. By offering the patient open repair, all possible late complications of EVT were avoided.
Acknowledgments
The data supporting the findings of this study are available from University Clinical Centre, Gdansk, but restrictions apply to the availability of these data, which are not publicly available. Data are, however, available from the authors on reasonable request and with permission from the University Clinical Centre, Gdansk.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Barrionuevo P., Malas M.B., Nejim B., et al. A systematic review and meta-analysis of the management of visceral artery aneurysms. J Vasc Surg. 2019;70:1694–1699. doi: 10.1016/j.jvs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 2.McMullan D.M., McBride M., Livesay J.J., Dougherty K.G., Krajcer Z. Celiac artery aneurysm: a case report. Tex Heart J. 2006;33:235–240. [PMC free article] [PubMed] [Google Scholar]
- 3.Obara H., Kentaro M., Inoue M., Kitagawa Y. Current management strategies for visceral artery aneurysms: an overview. Surg Today. 2020;50:38–49. doi: 10.1007/s00595-019-01898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaer R.A., Abularrage C.J., Coleman D.M., et al. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg. 2020;72:3S–39S. doi: 10.1016/j.jvs.2020.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Badea R. Splanchnic artery aneurysms: the diagnostic contribution of ultrasonography in correlation with other imaging methods. J Gastrointestin Liver Dis. 2008;17:101–105. doi: 10.1007/s11749-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 6.Hemp J.H., Sabri S.S. Endovascular management of visceral arterial aneurysms. Tech Vasc Interv Radiol. 2015;18:14–23. doi: 10.1053/j.tvir.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Graham L.M., Stanley J.C., Whitehouse W.M., et al. Celiac artery aneurysms: Historic (1745-1949) versus contemporary (1950-1984) differences in etiology and clinical importance. J Vasc Surg. 1985;2:757–764. doi: 10.1067/mva.1985.avs0020757. [DOI] [PubMed] [Google Scholar]
- 8.Venturini M., Marra P., Colombo M., et al. Endovascular repair of 40 visceral artery aneurysms and pseudoaneurysms with the Viabahn stent-graft: technical aspects, clinical outcome and mid-term patency. Cardiovasc Inter Rad. 2018;41:385–397. doi: 10.1007/s00270-017-1844-5. [DOI] [PubMed] [Google Scholar]
- 9.Laganà D., Carrafiello G., Mangini M., et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol. 2006;59:104–111. doi: 10.1016/j.ejrad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Chadha M., Ahuja C. Visceral artery aneurysms: diagnosis and Percutaneous management. Semin Intervent Rad. 2009;26:196–206. doi: 10.1055/s-0029-1225670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onohara T., Okadome K., Mii S., Yasumori K., Muto Y., Sugimachi K. Rupture of embolised coeliac artery pseudoaneurysm into the stomach: is coil embolisation an effective treatment for coeliac anastomotic pseudoaneurysm? Eur J Vasc Surg. 1992;6:330–332. doi: 10.1016/s0950-821x(05)80329-9. [DOI] [PubMed] [Google Scholar]
- 12.Shumacker H.B., Siderys H. Excisional treatment of aneurysm of celiac artery. Ann Surg. 1958;148:885–889. doi: 10.1097/00000658-195812000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone W.M., Abbas M.A., Gloviczki P., Fowl R.J., Cherry K.J. Celiac arterial aneurysms: a critical reappraisal of a rare entity. Arch Surg-chicago. 2002;137:670–674. doi: 10.1001/archsurg.137.6.670. [DOI] [PubMed] [Google Scholar]
- 14.Higashiyama H., Yamagami K., Fujimoto K., et al. Open surgical repair using a reimplantation technique for a large celiac artery aneurysm anomalously arising from the celiomesenteric trunk. J Vasc Surg. 2011;54:1805–1807. doi: 10.1016/j.jvs.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Uchida T., Hamasaki A., Kuroda Y., et al. Surgical repair of a celiac artery aneurysm using a sutureless proximal anastomosis device. J Vasc Surg Cases Innovative Tech. 2017;3:221–224. doi: 10.1016/j.jvscit.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulli R., Dorigo W., Troisi N., et al. Surgical treatment of visceral artery aneurysms: a 25-year experience. J Vasc Surg. 2008;48:334–342. doi: 10.1016/j.jvs.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Stanley J.C., Wakefield T.W., Graham L.M., et al. Clinical importance and management of splanchnic artery aneurysms. J Vasc Surg. 1986;3:836–840. [PubMed] [Google Scholar]
- 18.Gabelmann A., Görich J., Merkle E.M. Endovascular treatment of visceral artery aneurysms. J Endovascular Ther. 2002;9:38–47. doi: 10.1177/152660280200900108. [DOI] [PubMed] [Google Scholar]
- 19.Tulsyan N., Kashyap V.S., Greenberg R.K., et al. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276–283. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 20.Waldenberger P., Bendix N., Petersen J., Tauscher T., Glodny B. Clinical outcome of endovascular therapeutic occlusion of the celiac artery. J Vasc Surg. 2007;46:655–661. doi: 10.1016/j.jvs.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 21.Etezadi V., Gandhi R.T., Benenati J.F., et al. Endovascular treatment of visceral and renal artery aneurysms. J Vasc Interv Radiol. 2011;22:1246–1253. doi: 10.1016/j.jvir.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Carrafiello G., Rivolta N., Annoni M., Fontana F., Piffaretti G. Endovascular repair of a celiac trunk aneurysm with a new multilayer stent. J Vasc Surg. 2011;54:1148–1150. doi: 10.1016/j.jvs.2011.03.274. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W., Fu Y.F., Wei P.L., et al. Endovascular repair of celiac artery aneurysm with the Use of stent grafts. J Vasc Interv Radiol. 2016;27:514–518. doi: 10.1016/j.jvir.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Xia F.F., Fan Z.Q., Huo X.B., Fu Y.F., Xu Y.S. Endovascular stent repair of celiac arterial aneurysm. Medicine. 2019;98:e18203. doi: 10.1097/MD.0000000000018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan F.K., Xi H.L., Qin R.H., et al. Endovascular treatment with stenting of celiac artery aneurysms. Medicine. 2020;99:e23448. doi: 10.1097/MD.0000000000023448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dwivedi A., Wayne E., Sangroula D., Sigdel A. Endovascular treatment of Giant celiac artery aneurysm in Behcet’s disease. Vasc Endovasc Surg. 2021;55:398–401. doi: 10.1177/1538574420975906. [DOI] [PubMed] [Google Scholar]
- 27.Sessa C., Tinelli G., Porcu P., et al. Treatment of visceral artery aneurysms: description of a retrospective series of 42 aneurysms in 34 patients. Ann Vasc Surg. 2004;18:695–703. doi: 10.1007/s10016-004-0112-8. [DOI] [PubMed] [Google Scholar]
- 28.D’Ayala M., Deitch J.S., deGraft-Johnson J., et al. Giant celiac artery aneurysm with associated visceral occlusive disease. Vascular. 2004;12:390–393. doi: 10.1258/rsmvasc.12.6.390. [DOI] [PubMed] [Google Scholar]
- 29.Taberkant M., Elkaoui H., Bouchentouf M., et al. Anévrysme du tronc cœliaque À propos d’un cas. J Mal Vasc. 2006;31:284–286. doi: 10.1016/s0398-0499(06)76628-8. [DOI] [PubMed] [Google Scholar]
- 30.Lipari G., Migliara B., Cavallini A., et al. Traitement des anévrismes du tronc cœliaque : expérience personnelle et revue de la littérature. J Mal Vascul. 2006;31:72–75. doi: 10.1016/s0398-0499(06)76521-0. [DOI] [PubMed] [Google Scholar]
- 31.Popov B., Sagic R., Ilijevski N., et al. Treatment of visceral artery aneurysms: Retrospective study of 35 cases. Vasa. 2007;36:191–198. doi: 10.1024/0301-1526.36.3.191. [DOI] [PubMed] [Google Scholar]
- 32.Vasconcelos L., Garcia A.C., Castro J.S. e., Castro J.A. e., Capitão L.M. Celiac artery aneurysms. Ann Vasc Surg. 2010;24:554.e17–554.e22. doi: 10.1016/j.avsg.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Azimi-Ghomi O., Khan K., Ulloa K. Celiac artery aneurysm diagnosis and repair in the postpartum female. J Surg Case Rep. 2017;2017:rjx010. doi: 10.1093/jscr/rjx010. [DOI] [PMC free article] [PubMed] [Google Scholar]