Abstract
This study aimed to increase the encapsulation efficiency (EE%) of liposomes loaded with green tea polyphenols (GTP), by optimizing with response surface methodology (RSM), characterizing the obtained particles, and modeling their release under conventional heating and pulsed electric fields. GTP-loaded liposomes were prepared under conditions of Lecithin/Tween 80 (4:1, 1:1, and 1:4), cholesterol (0, 30, and 50%), and chitosan as coating (0, 0.05, and 0.1%). Particles were characterized by size, polydispersity index, ζ-potential, electrical conductivity, and optical microscopy. The release kinetics was modeled at a temperature of 60 °C and an electric field of 5.88 kV/cm. The optimal manufacturing conditions of GTP liposomes (ratio of lecithin/Tween 80 of 1:1, cholesterol 50%, and chitosan 0.1%) showed an EE% of 60.89% with a particle diameter of 513.75 nm, polydispersity index of 0.21, ζ-potential of 33.67 mV, and electrical conductivity of 0.14 mS/cm. Optical microscopy verified layering in the liposomes. The kinetic study revealed that the samples with chitosan were more stable to conventional heating, and those with higher cholesterol content were more stable to pulsed electric fields. However, in both treatments, the model with the best fit was the Peppas model. The results of the study allow us to give an indication of the knowledge of the behavior of liposomes under conditions of thermal and non-thermal treatments, helping the development of new functional ingredients based on liposomes for processed foods.
Keywords: Liposome, Chitosan, Cholesterol, Optimization, Release kinetics, Pulsed electric field
Introduction
Liposomes are efficient encapsulation systems for different biocomposites due to their physical and chemical properties (Shishir et al., 2019, Mohammadi et al., 2021). These encapsulation systems are characterized by being spherical microscopic vesicles formed by one or multiple concentric layers of phospholipids, which allows them to house both hydrophilic components on the external surface and inside the liposomes and hydrophobic components between the phospholipid tails of the liposomes, liposome membrane (Garavand et al., 2019). In addition, they provide greater absorption in the body due to their chemical structure that is similar to epithelial cells (Srinivasan et al., 2019). Another of their properties is that they allow the incorporation of a higher concentration of naturally insoluble substances and mask some sensory properties, particularly undesirable flavors and aromas (Ahmadi et al., 2022). Recently, studies have focused on using natural extracts to develop functional ingredients that promote health. Green tea (Camellia sinensis) is a herb consumed for its beneficial health properties associated with its high polyphenol content (Reygaert, 2017). Green tea polyphenols (GTP) represent 40% of dry weight, main catechins as epigallocatechin-3-gallate, epigallocatechin, epicatechin-3-gallate, and epicatechin (Xing et al., 2019). Some beneficial health properties include antioxidant, anti-inflammatory, antimicrobial, and immunomodulatory activity (Musial et al., 2020). They have even been used as a complementary measure to prevent COVID-19 (Tallei et al., 2021) by applying a 600 to 900 mg/day dose of epigallocatechin-3-gallate (Menegazzi et al., 2020). In the food industry, GTP has had a high interest both for its functional properties for human nutrition and for preserving food; for example, Rashidinejad et al. (2014) used GTP as an antioxidant to prolong the shelf life of cheeses. However, the applications of GTP in food are limited by its low stability to environmental conditions such as oxygen, light, heat, and humidity, to processing conditions due to its thermosensitivity, to storage conditions, related to the temperature and the oxygen barrier, and to gastrointestinal conditions such as acid–base pH and enzymatic activity (Reygaert, 2017). In this sense, encapsulation is a promising technique for the food industry to fortify and complement foods (Ephrem et al., 2018). Therefore, the development of liposomes as a means of GTP loading could help to overcome these limitations (Gibis et al., 2014); in fact, GTP has been successfully encapsulated previously, in liposomes, as well as in various biopolymers (Belščak- Cvitanović et al., 2015).
From an operational point of view, one of the most critical challenges in applying liposomes in the food industry is their relative instability in aqueous dispersions (Nakhaei et al., 2021); this can lead to unwanted effects such as reduced encapsulation efficiency (EE%) (Toh & Chiu, 2013), oxidation, hydrolysis (Frenzel & Steffen-Heins, 2015), membrane binding, and aggregation (Rahdar et al., 2019). Recently, Jara-Quijada et al. (2022) reported that the modification of the membrane surface is an important factor in the design of liposomes. Cholesterol has been shown to provide stability to liposomes (Nakhaei et al., 2021), such as regulation of permeability, elasticity, rigidity, membrane strength, matter transport (Ermilova & Lyubartsev, 2019), thermal stability (Jovanović et al., 2018), and electrical (Kotnik et al., 2019). On the other hand, the use of chitosan for the development of liposomes (Tian et al., 2019) has shown that it provides non-toxic and odorless (Bakshi et al., 2020), antimicrobial, and easily degradable properties (Zhou et al., 2018), increases hardness and mechanical, thermal, and chemical stability (Mazloomi et al., 2020), and improves the properties of liposome particles, including their EE% (Ahmadi et al., 2022).
Liposome optimization and characterization have been useful tools to develop more efficient liposomes (Jahanfar et al., 2021). Statistical optimization allows one to examine the effect of one factor at a time on a dependent variable or by using multivariate statistical techniques, such as response surface methodology (RSM) (Baş & Boyacı, 2007), where RSM is used to optimize the levels of different parameters simultaneously, offering information on the interaction or quadratic effects of the independent variables on the dependent variables (Nwabueze, 2010). The characterization of the particles allows us to evaluate the behavior and stability of the liposomes. Nakhaei et al. (2021) mention that stability can be evaluated by the size of the particles, the rigidity, and the charge of the membrane, among other properties. Some studies have determined that the aggregation phenomenon is generated by the accumulation of particles and can be affected by the size and distribution of the particles (Hamadou et al., 2020). The use of mathematical models of release kinetics is another useful tool to study the behavior of liposomes in relation to environmental conditions, such as gastrointestinal processing (Lu and Ten Hagen, 2020). Some studies have modeled the release of grape seed extract (Davidov-Pardo et al., 2013), as nanoparticle-encapsulated hesperetin (Fathi et al., 2013) in gastric and intestinal solutions. However, there are few investigations related to the study of release in thermal and non-thermal processes.
Pulsed electric fields (PEF) have been used in the food industry as a non-thermal technology to increase the shelf life of foods and beverages (Kersh et al., 2023). This technology is based on the application of high intensity electric fields, in the form of very short pulses, causing an increase in the size of the cell membrane pores, generating cell electroporation (Vignali et al., 2022). PEF, being a non-thermal technology, allows us to preserve important biocomponents, as well as the color and flavor of food, which normally degrade with heat (Wibowo et al., 2019). In the medical field, PEF is employed as an adjunctive method for the delivery of liposomal drugs to cells by electroporation of cell membranes and liposomes (Caramazza et al., 2020). This application has potential in studies of food processing that incorporate liposomes in the formulation of foods, as well as in the controlled release that could exist when subjected to this technology.
However, there are few investigations dedicated to the study of the release behavior of liposomes under heat treatment conditions and even less under non-thermal conditions, considering the new trends worldwide and the sustainable development goals that demand the use of environmentally friendly technologies such as ultrasound, ultraviolet light, electric fields, among others. Knowing the influence of the structural components of a liposome and how they affect its stability in food processing by thermal and non-thermal technologies presents a new field of interest for the future development of new ingredients based on liposomes.
Therefore, this study aimed to increase the EE% of GTP-loaded liposomes by optimizing liposome formulations, characterizing the obtained particles, and modeling GTP release in a model food under conventional heating and pulsed electric fields.
Materials and Methods
Materials and Reagents
Green tea leaves were acquired in the local market in Chillán, Chile. The leaves were stored in a desiccator at room temperature for 24 h. Ethanol, gallic acid, Folin-Ciocalteu reagent, anhydrous sodium carbonate, l-α-lecithin (from soybean), Tween 80, cholesterol, and low molecular weight chitosan were analytical grade (Sigma-Aldrich, Santiago, Chile).
Liposome Manufacturing
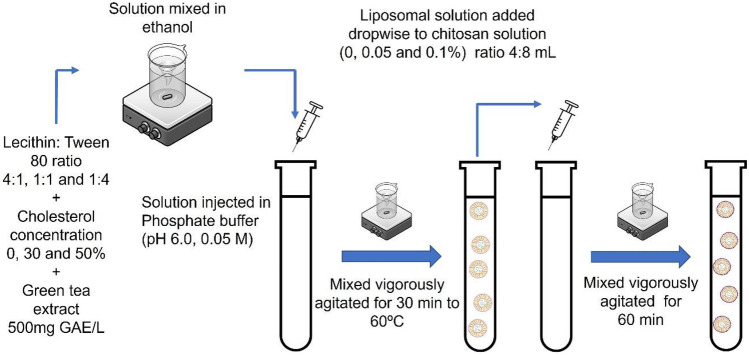
Liposome preparation was performed according to the ethanol injection methodology proposed by Zou et al. (2014). Green tea extract (500 mg GAE/L, obtained by pulsed electric fields, in another investigation), lecithin:Tween 80 ratios (4:1, 1:1, and 1:4), and cholesterol concentrations (0, 30, and 50%) were dissolved in ethanol. The chitosan solution (0, 0.05, and 0.1%) was obtained by dissolving a suitable amount of chitosan in distilled water containing 0.5% (v/v) acetic acid. The solution was injected into phosphate buffer (pH 6.0, 0.05 M) at a 1:1 ratio. The mixture was vigorously shaken at 60 °C for 30 min. A 4-mL liposomal solution was added dropwise to 8 mL of chitosan solution, which was mixed for 60 min (Fig. 1) (Jiao et al., 2018).
Fig. 1.
Schematic overview of the method to prepare liposomes
Total Polyphenol Content (TPC)
The total polyphenol content was determined using the Folin-Ciocalteu method proposed by Waterhouse (2002). The samples were duly diluted (1:10); 100 μL was taken and combined with 7900 μL of water, 500 μL of Folin-Ciocalteu reagent, and 1500 μL of 20% anhydrous sodium carbonate; the mixture was incubated for 2 h, in the dark. The absorbance of the mixture was measured at 760 nm with a UV/vis spectrophotometer (T70, Oasis Scientific Inc., Greenville, SC, USA). A gallic acid curve was used as a standard (50 to 500 mg/L). The equation of the line was y = 0.0011x + 0.0095 with an R2 = 0.9992. Results were expressed as milligrams of gallic acid equivalents (mg GAE)/L of the samples.
Encapsulation Efficiency (EE%)
An extract of 500 mg GAE/L was used as a base; after the encapsulation of the liposomes, it was allowed to precipitate, and the supernatant was taken from which the TPC was determined. The encapsulation efficiency was determined indirectly, considering that the supernatants contain the extract not loaded in the liposomes (Katouzian & Taheri, 2021). The EE% was calculated according to Eq. 1 from the ratio between polyphenols loaded in the liposome (TPC loaded) and the content of polyphenols in the initial solution (TPC initial).
| 1 |
Experimental Design and Optimization
The experiments were evaluated using the response surface methodology. Lecithin factors:Tween 80 (4:1, 1:1, 1:4), cholesterol concentration (0, 30, and 50%), and chitosan (0, 0.05, and 0.1%) were identified, as A, B, and C, respectively. The relationship between factors and levels was used to identify the optimal conditions that maximize EE%. Using the model represented by Eq. 2, the 27 GTP liposome formulations were optimized.
| 2 |
where β0 is a constant, β1, β2, and β3 are linear coefficients, β12, β13, and β23 are cross product coefficients, β11, β22, and β33 are quadratic coefficients, and ε is a residual.
Physicochemical Properties
The liposome’s average particle size, polydispersity index, ζ-potential, and electrical conductivity were determined by dynamic light scattering with a Zetasizer Nano ZS laser diffractometer (Malvern Instruments Ltd., Malvern, UK). Liposomes were diluted 1/1000 in a disposable DTS1070 zeta cuvette to determine particle properties.
Optical Microscopy
The morphology and detection of layers in the liposomes were observed by optical microscopy (Gibis et al., 2014). An amount of 10 μL of the sample was taken, placed on a glass slide, and viewed at 1000 × magnification (oil immersion). Structures with mean diameters > 100 nm have been detected.
Release Behavior of Optimal Liposome
The kinetic study of release in aqueous food simulant (AFS) was carried out according to the method proposed by Katouzian and Taheri (2021) with some modifications. For the release study of liposomes, 10% (v/v) ethanol and distilled water were obtained to mimic AFS conditions. A total of 10 mL of liposomal solution was immersed in a vessel up to 100 mL with the aqueous simulant and stirred using a magnetic stirrer at 1000 rpm at room temperature.
The samples were subjected to two treatments, one group under conventional heating in a water bath (Memmert WNB7 water bath, Memmert GmbH & Co. KG, Schwabach, Germany) at 60 °C by 5, 10, 20, 30, and 40 min and another group under pulsed electric field conditions (PEF) in a PEF equipment (Energy Pulse Systems, Lisbon, Portugal) with an energy modulator (Marx Epulsus-PM1-10 modulator) in a batch parallel plate treatment chamber composed of stainless steel electrodes with an electrode area of 58.81 cm2, a gap of 1.7 cm, and a treated volume of 100 mL. An electric field of 5.88 kV/cm of square wave, a pulse width of 100 μs, a frequency of 1 Hz, and number of pulse 10 were applied by 1000 to 5000 μs. Approximately 3 mL of the solution was withdrawn, and the TPC was measured. The results were expressed as % released.
After obtaining the kinetics, the release behavior was evaluated according to the mathematical models presented in Table 1. The data adjustment to each kinetic model was made from the calculation of the root mean square error (RMSE) according to Eq. 3.
| 3 |
Table 1.
Mathematical models used to describe the release kinetics
| Model | Mathematical equation | |
|---|---|---|
| Zero-order | (4) | |
| First-order | (5) | |
| Higuchi | (6) | |
| Peppas | (7) | |
| Weibull | (8) |
Letter C is the % of polyphenols released in the medium, k and n are the kinetic constants of the model, and t is time
Letter C is the % of polyphenols released in the medium, k and n are the kinetic constants of the model, and t is time.
Statistical Analysis
The results were submitted to analysis of variance (ANOVA) and test of least significant difference (LSD) using the Statgraphics Centurion XVI software (Statpoint Technologies Inc., Warrenton, VG, USA) to obtain the statistical analysis of the data from the study. All experiments were performed in triplicate, and the results were reported as mean ± standard deviation.
Results and Discussion
Optimization Model
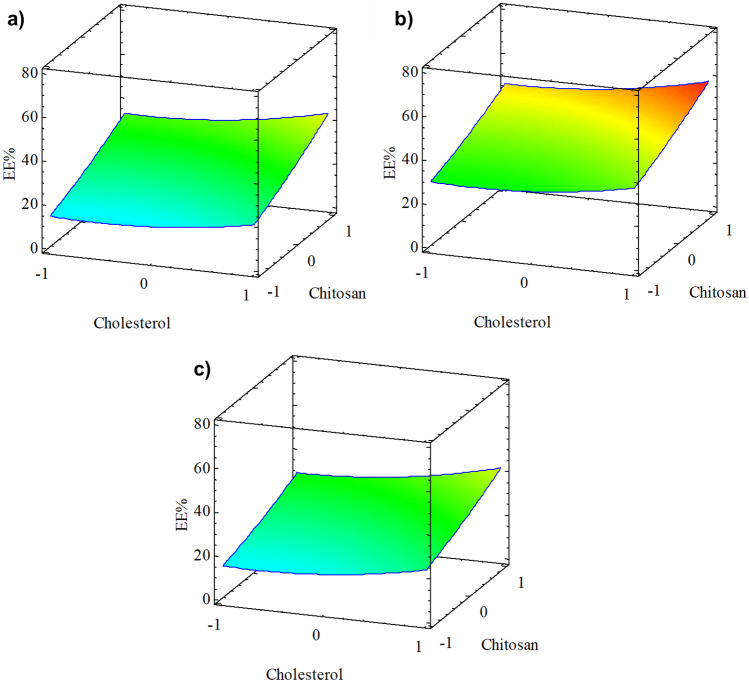
Encapsulation efficiency is an important factor in liposome design. Experimentally, the value of EE% obtained was 60.89 ± 0.39% using lecithin:Tween 80 ratio of 1:1, 50% cholesterol, and 0.1% chitosan. Figure 2 shows 3D surface graphs obtained from the RSM statistical model for the proportion of lecithin:Tween 80, cholesterol, and chitosan at their levels. The optimal value of the regression model was 58.07%.
Fig. 2.
Response surface for EE% optimization of GTP-loaded liposome formulations: a lecithin:Tween 80 4:1, b lecithin:Tween 80 1:1, and c lecithin:Tween 80 1:4
The GTP-loaded liposome formulations were optimized using a statistical model obtained by RSM. Equation 4 expresses the empirical relationship between the factors (A, B, and C) and the response variable (EE%), considering the linear and quadratic effects that significantly improve the model (p < 0.05) to obtain the highest performance for variable EE%. When analyzing the second-order multiple regression model with the experimental data, it was determined that the regression model had a correlation coefficient of 0.925 with this experiment. Table 2 shows the analysis of variance (ANOVA) used in the optimization.
| 4 |
Table 2.
Analysis of variance for the GTP liposome optimization model
| Source | Sum of squares | Degrees of freedom | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| A: Lecithin:Tween 80 | 1.10014 | 1 | 1.10014 | 0.07 | 0.8011a |
| B: Cholesterol | 370.827 | 1 | 370.827 | 22.07 | 0.0002b |
| C: Chitosan | 1699.25 | 1 | 1699.25 | 101.15 | 0.0000b |
| AA | 1340.92 | 1 | 1340.92 | 79.82 | 0.0000b |
| AB | 3.61901 | 1 | 3.61901 | 0.22 | 0.6484a |
| AC | 14.6744 | 1 | 14.6744 | 0.87 | 0.3631a |
| BB | 55.5307 | 1 | 55.5307 | 3.31 | 0.0867a |
| BC | 12.2816 | 1 | 12.2816 | 0.73 | 0.4044a |
| CC | 7.8052 | 1 | 7.8052 | 0.46 | 0.5047a |
| Total error | 285.592 | 17 | 16.7995 | ||
| Total | 3791.6 | 26 |
Results are mean ± standard deviation
Different letters in the row indicate that values differ significantly (p < 0.05)
Results are mean ± standard deviation. Different letters in the row indicate that values differ significantly (p < 0.05).
Figure 3 shows the Pareto diagram and the graph of significant effects. Figure 3a shows that chitosan followed by cholesterol had a significant effect (p < 0.05) on the lecithin:Tween 80 ratio in the EE% obtained. Figure 3b shows the main effects graph where a linear relationship is observed between the chitosan content and the EE%, similar to cholesterol. However, only the lecithin:Tween 80 1:1 ratio significantly affected EE%.
Fig. 3.
a Standardized Pareto chart. b Main effect plot for encapsulation efficiency (EE%)
The EE% reported in the present study was similar to the findings of Ahmadi et al. (2022) and ranged from 51 to 76%. On the contrary, our EE% values were higher than those indicated by Hamadou et al. (2020), who reached 52.09% with marine phospholipids. Some investigations have shown the interaction of cholesterol and chitosan in the liposomal membrane favors the encapsulation of polyphenols and other bioactive compounds. Nakhaei et al. (2021) indicated higher EE% when the cholesterol concentration increased; this could be because cholesterol can change the mobility of phospholipids in the membrane, thus improving their stability. Liu et al. (2015) showed that chitosan coats the liposome surface and fills the membrane spaces in the hydrophobic zone through van der Waals forces, hydrogen bonding, and electrostatic interactions during encapsulation. Gibis et al. (2016) encapsulated grape seed polyphenols in chitosan-coated liposomes and obtained 99.5% and 88.2% for coated and uncoated liposomes, respectively; EE% increased when chitosan was incorporated into the liposomes. Liu et al. (2015) reported similar results for chitosan-coated liposomes. Souza et al. (2014) reported an encapsulation efficiency of 96.13% in lecithin/chitosan 0.2% nanocapsules. Therefore, adding both chitosan and cholesterol improves EE%.
Physicochemical Properties
GTP-loaded liposomes with different wall materials were prepared and characterized. The physicochemical properties of each liposome formulation are shown in Table 3.
Table 3.
Measurements of average particle diameter, polydispersity index, ζ-potential, and electrical conductivity of different liposome formulations loaded with green tea extract
| N° | Lecithin:Tween 80 ratio | Cholesterol (%) | Chitosan (%) | Average particle diameter (nm) | Polydispersity index |
ζ-Potential (mV) |
Electrical conductivity (mS/cm) |
|---|---|---|---|---|---|---|---|
| 1 | 4:1 | 0 | 0 | 135.75 ± 2.73a | 0.63 ± 0.02a | − 27.63 ± 0.15a | 0.30 ± 0.01a |
| 2 | 4:1 | 0 | 0.05 | 221.85 ± 3.97b | 0.61 ± 0.01a | 22.10 ± 0.26b | 0.34 ± 0.02b |
| 3 | 4:1 | 0 | 0.1 | 440.98 ± 8.71c | 0.58 ± 0.01b | 32.27 ± 0.15c | 0.35 ± 0.01bc |
| 4 | 4:1 | 30 | 0 | 226.41 ± 3.72b | 0.55 ± 0.02c | − 30.70 ± 0.70f | 0.14 ± 0.01de |
| 5 | 4:1 | 30 | 0.05 | 340.41 ± 4.14d | 0.49 ± 0.00de | 27.23 ± 0.67 g | 0.23 ± 0.01 fg |
| 6 | 4:1 | 30 | 0.1 | 482.98 ± 8.01e | 0.46 ± 0.01f | 35.53 ± 0.59i | 0.24 ± 0.00gh |
| 7 | 4:1 | 50 | 0 | 250.48 ± 1.35f | 0.43 ± 0.01 g | − 42.23 ± 0.60j | 0.10 ± 0.00i |
| 8 | 4:1 | 50 | 0.05 | 424.66 ± 6.58 h | 0.39 ± 0.01hi | 23.60 ± 0.89 k | 0.15 ± 0.00e |
| 9 | 4:1 | 50 | 0.1 | 495.01 ± 4.16i | 0.35 ± 0.01jkl | 33.20 ± 0.62de | 0.17 ± 0.01f |
| 10 | 1:1 | 0 | 0 | 145.78 ± 2.25j | 0.38 ± 0.01hij | − 41.40 ± 0.92j | 0.30 ± 0.01a |
| 11 | 1:1 | 0 | 0.05 | 242.91 ± 1.91 g | 0.36 ± 0.02ijk | 23.23 ± 0.85 k | 0.35 ± 0.00bc |
| 12 | 1:1 | 0 | 0.1 | 495.11 ± 3.82i | 0.34 ± 0.01 lm | 28.67 ± 0.67 h | 0.37 ± 0.00d |
| 13 | 1:1 | 30 | 0 | 247.48 ± 2.91 fg | 0.33 ± 0.01 lm | − 46.93 ± 0.57 l | 0.14 ± 0.01de |
| 14 | 1:1 | 30 | 0.05 | 396.53 ± 1.73 k | 0.33 ± 0.01 m | 25.50 ± 0.26 m | 0.22 ± 0.00 fg |
| 15 | 1:1 | 30 | 0.1 | 504.22 ± 5.02 l | 0.30 ± 0.02o | 32.57 ± 0.15 cd | 0.23 ± 0.01gh |
| 16 | 1:1 | 50 | 0 | 262.85 ± 2.85 m | 0.27 ± 0.02p | − 49.23 ± 0.65n | 0.09 ± 0.00i |
| 17 | 1:1 | 50 | 0.05 | 483.11 ± 6.72e | 0.23 ± 0.00q | 25.37 ± 0.21 m | 0.13 ± 0.00d |
| 18 | 1:1 | 50 | 0.1 | 513.75 ± 2.00n | 0.21 ± 0.01q | 33.67 ± 0.23e | 0.14 ± 0.02de |
| 19 | 1:4 | 0 | 0 | 135.41 ± 2.72a | 0.53 ± 0.01c | − 22.70 ± 0.46o | 0.32 ± 0.00j |
| 20 | 1:4 | 0 | 0.05 | 187.08 ± 5.09o | 0.49 ± 0.02d | 22.10 ± 0.26b | 0.36 ± 0.01 cd |
| 21 | 1:4 | 0 | 0.1 | 474.58 ± 3.22p | 0.47 ± 0.03ef | 25.77 ± 0.57 m | 0.39 ± 0.01 k |
| 22 | 1:4 | 30 | 0 | 213.58 ± 4.87q | 0.43 ± 0.02 g | − 23.10 ± 0.70o | 0.21 ± 0.00 h |
| 23 | 1:4 | 30 | 0.05 | 336.75 ± 6.71d | 0.41 ± 0.01gh | 27.83 ± 0.51gh | 0.24 ± 0.01i |
| 24 | 1:4 | 30 | 0.1 | 460.05 ± 5.83r | 0.38 ± 0.01hi | 35.83 ± 0.40i | 0.27 ± 0.00 l |
| 25 | 1:4 | 50 | 0 | 250.25 ± 3.20 fg | 0.37 ± 0.01hij | − 30.90 ± 0.60f | 0.13 ± 0.00d |
| 26 | 1:4 | 50 | 0.05 | 393.91 ± 2.54 k | 0.35 ± 0.01klm | 27.90 ± 0.50gh | 0.15 ± 0.01e |
| 27 | 1:4 | 50 | 0.1 | 474.68 ± 2.65p | 0.34 ± 0.00 lm | 35.93 ± 0.42i | 0.17 ± 0.00f |
Results are mean ± standard deviation
Different letters in the same row indicate that values differ significantly (p < 0.05)
The device was set to a mean viscosity of 1.0200 and a mean refractive index of 1.335
Results are mean ± standard deviation. Different letters in the same row indicate that values differ significantly (p < 0.05). The device was set to a mean viscosity of 1.0200 and a mean refractive index of 1.335.
The results showed an average particle size mean from 135.41 ± 2.72 to 513.75 ± 2.00 nm, a polydispersity index from 0.21 ± 0.01 to 0.63 ± 0.02, ζ-potential from − 49.23 ± 0.65 to + 35.93 ± 0.0.0.42 mV, and electrical conductivity from 0.0 ± 0.42 to 0.39 ± 0.01 mS/cm. Incorporating cholesterol and chitosan in the liposomal membrane affected liposomes’ physicochemical characteristics. Some researchers reported sizes similar to those obtained in the present study, such as Ghorbanzade et al. (2017) for sunflower oil liposomes (409.00 nm) and Hamadou et al. (2020) for marine phospholipid liposomes (236.72 nm). These authors reported that the main factors in the liposomes’ size are encapsulated polyphenols’ content, the type of phospholipid, and the coating material. In general, the liposomes developed in this investigation with increased cholesterol and chitosan reached a larger size, due to chemical interactions between the membrane phospholipids, cholesterol, and chitosan (Liu et al., 2015; Nakhaei et al., 2021). The PDI decreased when increasing the cholesterol and chitosan contents in the liposomes. Jiao et al. (2018) reported similar behavior for uncoated and chitosan-coated liposomes with values of 0.31 ± 0.01 to 0.18 ± 0.01, respectively. Nakhaei et al. (2021) explain that this behavior is given by the rearrangement effect generated by the cholesterol molecules in the structure of the liposomes. The cholesterol tail at C17 intermingles with the hydrophobic fatty acyl chains, while the sterol hydroxyl group binds to the hydrophilic head group of phospholipids, thus increasing the cohesion of the membrane (Giulimondi et al., 2019). The ζ-potential in the present study indicated that the GTP-loaded liposomes had a negative surface charge, which decreased with increasing cholesterol; the opposite effect occurred when chitosan was incorporated, which changed the surface charge of the liposome to positive. A high zeta potential (> 30 mV or < − 30 mV) is stable due to the high repulsive electrostatic forces between the particles; this was related to what was reported by Hamadou et al. (2020), Jiao et al. (2018), and Gibis et al. (2016) with the incorporation of cholesterol and chitosan in liposomes. Ahmadi et al. (2022) indicated that liposomal systems with high surface charges, either positive or negative, have greater stability due to overcoming aggregation, which is associated with the ratio of phospholipids and cholesterol in the liposome membrane. Zou et al. (2014) showed that a higher negative charge potential value ζ also favors the stability of liposomes. Jiao et al. (2018) found that the ζ-potential of liposomes changed from negative to positive values after coating them with chitosan. This behavior is related to the results obtained in this study, indicating greater stability due to the high electrostatic charge given by the interaction of chitosan with the liposomal membrane (Liu et al., 2015). The electrical conductivity obtained in the present GTP-loaded liposome study decreased when the phospholipid membrane cholesterol content increased. However, when the chitosan content increased, the electrical conductivity also increased. Caramazza et al. (2020) reported electrical conductivity similar to this study in egg phosphatidylcholine liposomes. Katouzian and Taheri (2021) reported an increase in electrical conductivity when incorporating chitosan in chitosan-coated liposomes, which is related to the results obtained in this study. Jara-Quijada et al. (2022) mentioned that the electrical conductivity of liposomes mainly depends on their membrane composition, biological nature of phospholipids, phospholipid polarity, asymmetric membranes, cholesterol content, and surfactant content.
Optical Microscopy
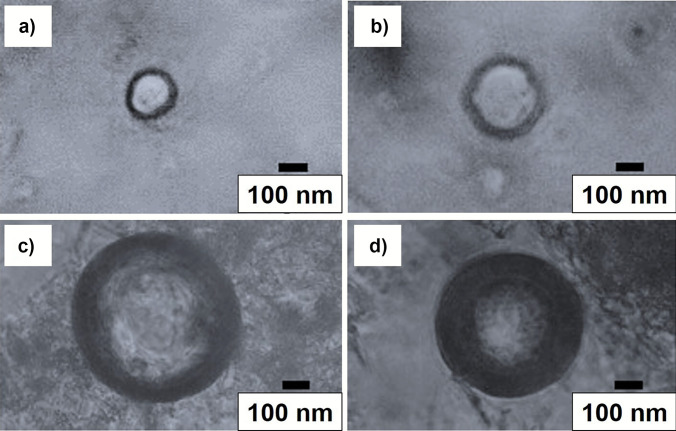
Images were obtained by optical microscopy to verify the formation of the layers visually. Figure 4 shows capture and structure schematics made to GTP-loaded liposomes (formulation 10), GTP-loaded liposomes + 50% cholesterol (formulation 16), GTP-loaded liposomes + 0.1% chitosan (formulation 12), and liposomes loaded with GTP + 50% cholesterol + 0.1% chitosan (formulation 18). In all cases, a hemispherical geometry was observed. The captures of formulations 10 and 16, with formulations 12 and 18, did not show differences in shape and size. Based on the size of the liposomes and the number of layers, it was determined that the liposomes represented in Fig. 4a and b correspond to large unilamellar liposomes. In contrast, those in Fig. 4c and d correspond to giant multilamellar liposomes, according to Ajeeshkumar et al. (2021). The images verified the incorporation of cholesterol into the phospholipid membrane (formulations 16 and 18) and the aggregation of chitosan on the liposome surface (formulations 12 and 18).
Fig. 4.
Images obtained from GTP-loaded liposomes for lecithin:Tween 80 (1:1) ratio. a GTP-loaded liposomes (formulation 10), b GTP-loaded liposomes + 50% cholesterol (formulation 16), c GTP-loaded liposomes + 0.1% chitosan (formulation 12), and d GTP-loaded liposomes + 50% cholesterol + 0.1% chitosan (formulation 18)
Release Kinetics and Modeling
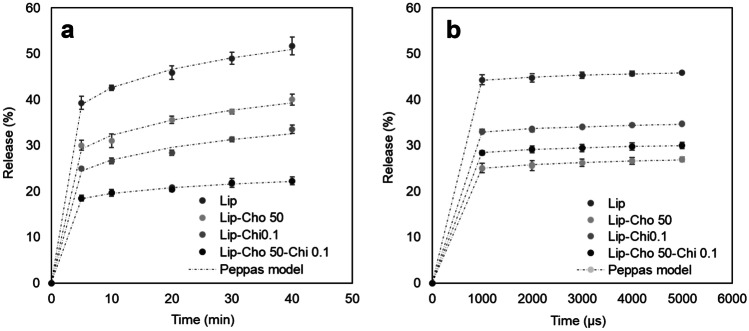
During thermal and non-thermal treatments, food cells undergo structural alterations that generally cause leakage and expulsion of the internal material; the same behavior has been demonstrated in liposomes. Therefore, it is important to study the stability of nanostructures. Figure 5 presents the release kinetics of polyphenols under conventional heating conditions and pulsed electric fields from liposomes loaded with GTP with cholesterol, chitosan, and their mixture in their highest ratio.
Fig. 5.
In vitro release profile of the GTP-loaded liposomes in an aqueous food simulant, treated by a conventional heating and b pulsed electric fields
Figure 5a shows the release kinetics of polyphenols in conventional heating at 60 °C for up to 40 min, simulating a conventional pasteurization process. The result shows that GTP-loaded liposomes had the highest release of polyphenols (51.74 ± 1.93%), followed by liposomes with 50% cholesterol (40.05 ± 1.19%), 0.1% chitosan (33.56 ± 0.91%), and from the mixture of cholesterol 50% plus chitosan 0.1% (22.29 ± 0.83%). The results indicate that incorporating cholesterol and chitosan in the liposomes provides greater stability to conventional heating of uncoated liposomes. It is also observed that the liposomes with 50% cholesterol plus 0.1% chitosan have a lower variation of polyphenols released between 5 and 40 min, unlike the other liposomes. Wechtersbach et al. (2012) studied the effects of cholesterol on the membrane of dipalmitoylphosphatidylcholine (DPPC) liposomes. Studies under heating conditions at 60 °C reported a lower release of ascorbic acid in liposomes with cholesterol (26%) compared to liposomes without cholesterol (81%). Researchers report the stabilizing effect of cholesterol due to the interaction of the carbon bonds it has with the phospholipid membrane. Liu et al. (2015) studied the effects of chitosan on curcumin-loaded liposomes. About the release of curcumin at 60 °C from uncoated and chitosan-coated liposomes, a behavior similar to that reported in the study was reported, reaching a release at 40 min of around 50 and 20%, respectively. The researchers report that the chitosan molecule forms a network interacting with the liposome membrane, changing the structure and the transition phase, and granting greater thermal stability.
Figure 5b shows the release kinetics of polyphenols for treating pulsed electric fields of 5.88 kV/cm in a time up to 5000 μs. The results show that the liposomes loaded with GTP presented the highest release of polyphenols (45.92 ± 0.29%), followed by liposomes coated with chitosan 0.1% (34.74 ± 0.19%), liposomes with cholesterol 50% with chitosan 0.1% (30.02 ± 0.71%), and liposomes with 50% cholesterol (26.99 ± 0.50%). An almost constant release over time was also observed for all treatments. Karal et al. (2020) studied the influence of cholesterol (0 to 40%) on the electroporation of dioleoylphosphatidylglycerol (DOPG) and dioleoylphosphatidylcholine (DOPC) membranes. The researchers reported that higher cholesterol content in the phospholipid membrane provided greater stability under electric field conditions, thus limiting the current flow and reducing electrical conductivity, which is consistent with the results of this study. Casciola et al. (2014) studied the electroporation of lipid bilayers at different cholesterol contents (20 to 50%) using molecular dynamics (MD) simulation. The results indicated that the increase in cholesterol concentration in the membrane requires an increase in voltage (almost double) to generate membrane pores and resealing time. The researchers explain that cholesterol provides greater stability to the phospholipid bilayer by locating it in the inner part of the membranes, thus avoiding the formation of pores. Li et al. (2020) studied the influence of the application of electric fields on the hydrolysis of chitosan. The researchers indicated that the electric fields generate a rearrangement of the structure of the molecule and, depending on the electric field, a cut of the chitosan chains can be generated, generating a destabilization of the coating in the liposomes, which could justify the increase of the mass transfer into liposomes with chitosan.
The fit of the release models and their kinetic parameters are presented in Table 4. Compared to the other models, the RMSE results show a better fit of the kinetics for both treatments to the Peppas model. The Peppas model is one of the most applied non-linear regression models to interpret diffusion profiles (Jain & Jain, 2016). The Peppas model has previously been used successfully to describe the kinetics of drug release from liposomes (Haghiralsadat et al., 2018). In this equation, C represents fractional drug permeate, t is time, K is the transport constant (time−1), and n is the transport exponent (dimensionless). The release constant K mainly provides information on the drug formulation, such as the structural characteristics of the nanocarriers. At the same time, n is important because it relates to the drug release mechanism (Wu et al., 2019). The values, particularly of n in the kinetics of the Peppas model, show a higher speed in the samples treated by conventional heating. However, a decrease in the rate related to the incorporation of cholesterol and chitosan into the liposomes can be observed in both treatments. It is worth mentioning for future research the realization of more detailed studies with the interactions of the liposome materials and how these influence the behavior of liposomes in food systems treated by these technologies or others.
Table 4.
Root means square error (RMSE), correlation coefficients (R2), and other coefficients obtained from fitting with release mathematical models for GTP-loaded liposomes
| Formulation | Mathematical model | Conventional heating | Pulsed electric fields | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RMSE | R2 | k | n | RMSE | R2 | k | n | ||
| Lip | Zero-order | 25.166 | 0.534 | 0.910 | 24.326 | 0.460 | 0.007 | ||
| First-order | 41.144 | 0.966 | 0.008 | 40.383 | 0.943 | 0.000 | |||
| Higuchi | 29.947 | 0.992 | 2.974 | 39.203 | 0.964 | 0.043 | |||
| Peppas | 0.531 | 0.980 | 31.744 | 0.129 | 0.106 | 0.958 | 37.900 | 0.023 | |
| Weibull | 41.664 | 0.982 | 3.441 | 0.035 | 41.038 | 0.958 | 3.610 | 0.006 | |
| Lip-Cho 50 | Zero-order | 18.753 | 0.560 | 0.715 | 13.658 | 0.492 | 0.004 | ||
| First-order | 31.231 | 0.965 | 0.008 | 22.986 | 0.960 | 0.000 | |||
| Higuchi | 21.812 | 0.979 | 2.523 | 21.472 | 0.969 | 0.049 | |||
| Peppas | 0.727 | 0.952 | 23.344 | 0.142 | 0.137 | 0.951 | 18.466 | 0.045 | |
| Weibull | 31.709 | 0.954 | 3.126 | 0.042 | 23.550 | 0.952 | 2.875 | 0.015 | |
| Lip-Chi 0.1 | Zero-order | 15.598 | 0.561 | 0.595 | 18.071 | 0.472 | 0.005 | ||
| First-order | 25.856 | 0.983 | 0.008 | 30.132 | 0.965 | 0.000 | |||
| Higuchi | 18.225 | 0.971 | 2.081 | 28.854 | 0.957 | 0.044 | |||
| Peppas | 0.684 | 0.938 | 19.548 | 0.139 | 0.158 | 0.923 | 26.600 | 0.031 | |
| Weibull | 26.319 | 0.944 | 2.944 | 0.043 | 30.750 | 0.924 | 3.251 | 0.009 | |
| Lip-Cho 50-Chi 0.1 | Zero-order | 11.976 | 0.464 | 0.376 | 15.614 | 0.473 | 0.004 | ||
| First-order | 17.997 | 0.851 | 0.005 | 25.927 | 0.930 | 0.000 | |||
| Higuchi | 15.096 | 0.888 | 0.927 | 24.877 | 0.960 | 0.040 | |||
| Peppas | 0.407 | 0.887 | 15.970 | 0.089 | 0.094 | 0.966 | 22.642 | 0.033 | |
| Weibull | 18.719 | 0.886 | 2.717 | 0.032 | 26.311 | 0.966 | 3.085 | 0.010 | |
Conclusions
The present study reveals the use of liposomes as vehicles to deliver polyphenols stable to environmental and processing conditions. This study sought to optimize, characterize, and evaluate the release kinetics of different cholesterol- and chitosan-containing liposome formulations under conventional heating and pulsed electric field conditions. The results showed that the encapsulation efficiency was optimal with a higher cholesterol and chitosan content. The physicochemical properties revealed that the incorporation of cholesterol and chitosan increased the size of the particles. Regarding the surface charge, the liposomes loaded with GTP and cholesterol presented a negative surface charge and a lower conductivity, unlike those coated with chitosan, which presented a positive surface charge and a higher conductivity. Light microscopy confirmed the formation of spherical liposomes and the formation of layers. The release of polyphenols from the liposomes subjected to conventional heating and pulsed electric fields was determined, and it was observed that the mathematical model that best fitted was the Peppas model. It was also observed that the liposomes with chitosan showed greater stability against heat treatments, while the liposomes containing cholesterol showed greater stability against pulsed electric fields. This study provided information on the design of liposomes for thermal and non-thermal treatments. These findings are relevant for the development of more efficient and stable liposome-based encapsulation systems under food processing conditions, by thermal and non-thermal technologies. The results contribute to fundamental knowledge about the behavior of liposomes and their ability to protect and release bioactive compounds under certain processing conditions. With a better understanding of the factors that affect the efficiency of encapsulation and release of polyphenols, more effective liposome-based functional ingredients can be developed to improve the quality and nutritional value of processed foods.
Acknowledgements
Author Jara-Quijada acknowledges funding through the Postgraduate Directorate research grant and the Food Engineering Doctoral Program support at the Universidad del Bio-Bio, Chile.
Author Contribution
Erick Jara-Quijada: conceptualization, methodology, investigation, software, writing—original draft data curation, funding acquisition. Mario Pérez-Won: supervision, funding acquisition, writing—review, and editing. Gipsy Tabilo-Munizaga: writing—review and editing. Roberto Lemus-Mondaca: writing—review and editing. Luis González-Cavieres: writing—original draft. Anais Palma-Acevedo: writing—original draft. Carolina Herrera-Lavados: writing—original draft.
Funding
The study was supported by the research scholarship of the Postgraduate Direction of the University of Bio Bio.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article. The raw/derived data supporting the findings of this study are available from the corresponding author at request.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Erick Jara-Quijada, Email: eajara@ubiobio.cl.
Mario Pérez-Won, Email: mperez@ubiobio.cl.
References
- Ahmadi E, Elhamirad AH, Mollania N, Saeidi Asl MR, Pedramnia A. Incorporation of white tea extract in nano-liposomes: Optimization, characterization, and stability. Journal of the Science of Food and Agriculture. 2022;102(5):2050–2060. doi: 10.1002/jsfa.11544. [DOI] [PubMed] [Google Scholar]
- Ajeeshkumar KK, Aneesh PA, Raju N, Suseela M, Ravishankar CN, Benjakul S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Comprehensive Reviews in Food Science and Food Safety. 2021;20(2):1280–1306. doi: 10.1111/1541-4337.12725. [DOI] [PubMed] [Google Scholar]
- Bakshi PS, Selvakumar D, Kadirvelu K, Kumar NS. Chitosan as an environment friendly biomaterial–A review on recent modifications and applications. International Journal of Biological Macromolecules. 2020;150:1072–1083. doi: 10.1016/j.ijbiomac.2019.10.113. [DOI] [PubMed] [Google Scholar]
- Baş D, Boyacı IH. Modeling and optimization I: Usability of response surface methodology. Journal of Food Engineering. 2007;78(3):836–845. doi: 10.1016/j.jfoodeng.2005.11.024. [DOI] [Google Scholar]
- Belščak-Cvitanović A, Lević S, Kalušević A, Špoljarić I, Đorđević V, Komes D, Nedović V. Efficiency assessment of natural biopolymers as encapsulants of green tea (Camellia sinensis L.) bioactive compounds by spray drying. Food and Bioprocess Technology. 2015;8:2444–2460. doi: 10.1007/s11947-015-1592-y. [DOI] [Google Scholar]
- Caramazza L, Nardoni M, De Angelis A, Paolicelli P, Liberti M, Apollonio F, Petralito S. Proof-of-concept of electrical activation of liposome nanocarriers: From dry to wet experiments. Frontiers in Bioengineering and Biotechnology. 2020;8:819. doi: 10.3389/fbioe.2020.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola M, Bonhenry D, Liberti M, Apollonio F, Tarek M. A molecular dynamic study of cholesterol rich lipid membranes: Comparison of electroporation protocols. Bioelectrochemistry. 2014;100:11–17. doi: 10.1016/j.bioelechem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Davidov-Pardo G, Arozarena I, Marín-Arroyo MR. Optimization of a wall material formulation to microencapsulate a grape seed extract using a mixture design of experiments. Food and Bioprocess Technology. 2013;6:941–951. doi: 10.1007/s11947-012-0848-z. [DOI] [Google Scholar]
- Ephrem E, Najjar A, Charcosset C, Greige-Gerges H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: An overview. Journal of Functional Foods. 2018;48:65–84. doi: 10.1016/j.jff.2018.06.021. [DOI] [Google Scholar]
- Ermilova I, Lyubartsev AP. Cholesterol in phospholipid bilayers: Positions and orientations inside membranes with different unsaturation degrees. Soft Matter. 2019;15(1):78–93. doi: 10.1039/c8sm01937a. [DOI] [PubMed] [Google Scholar]
- Fathi M, Varshosaz J, Mohebbi M, Shahidi F. Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: Preparation, characterization, and modeling. Food and Bioprocess Technology. 2013;6:1464–1475. doi: 10.1007/s11947-012-0845-2. [DOI] [Google Scholar]
- Frenzel M, Steffen-Heins A. Whey protein coating increases bilayer rigidity and stability of liposomes in food-like matrices. Food Chemistry. 2015;173:1090–1099. doi: 10.1016/j.foodchem.2014.10.076. [DOI] [PubMed] [Google Scholar]
- Garavand F, Rahaee S, Vahedikia N, Jafari SM. Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends in Food Science & Technology. 2019;89:26–44. doi: 10.1016/j.tifs.2019.05.005. [DOI] [Google Scholar]
- Ghorbanzade T, Jafari SM, Akhavan S, Hadavi R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chemistry. 2017;216:146–152. doi: 10.1016/j.foodchem.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Gibis M, Ruedt C, Weiss J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Research International. 2016;88:105–113. doi: 10.1016/j.foodres.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Gibis M, Zeeb B, Weiss J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocolloids. 2014;38:28–39. doi: 10.1016/j.foodhyd.2013.11.014. [DOI] [Google Scholar]
- Giulimondi F, Digiacomo L, Pozzi D, Palchetti S, Vulpis E, Capriotti AL, et al. Interplay of protein corona and immune cells controls blood residency of liposomes. Nature Communications. 2019;10:3686–3711. doi: 10.1038/s41467-019-11642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghiralsadat, F., Amoabediny, G., Helder, M. N., Naderinezhad, S., Sheikhha, M. H., Forouzanfar, T., & Zandieh-Doulabi, B. (2018). A comprehensive mathematical model of drug release kinetics from nano-liposomes, derived from optimization studies of cationic PEGylated liposomal doxorubicin formulations for drug-gene delivery. Artificial Cells, Nanomedicine, and Biotechnology, 46(1), 169–177. 10.1080/21691401.2017.1304403 [DOI] [PubMed]
- Hamadou, A. H., Huang, W. C., Xue, C., & Mao, X. (2020). Formulation of vitamin C encapsulation in marine phospholipids nanoliposomes: Characterization and stability evaluation during long term storage. LWT - Food Science and Technology, 127, 109439. 10.1016/j.lwt.2020.109439
- Jahanfar, S., Ghavami, M., Khosravi-Darani, K., & Jahadi, M. (2021). Liposomal green tea extract: Optimization and physicochemical characterization. Journal of Applied Biotechnology Reports, 8(1). 10.30491/jabr.2020.231423.1228
- Jain A, Jain SK. In vitro release kinetics model fitting of liposomes: An insight. Chemistry and Physics of Lipids. 2016;201:28–40. doi: 10.1016/j.chemphyslip.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Jara-Quijada E, Pérez-Won M, Tabilo-Munizaga G, González-Cavieres L, Lemus-Mondaca R. An overview focusing on food liposomes and their stability to electric fields. Food Engineering Reviews. 2022;14(2):292–306. doi: 10.1007/s12393-022-09306-2. [DOI] [Google Scholar]
- Jiao Z, Wang X, Yin Y, Xia J, Mei Y. Preparation and evaluation of a chitosan-coated antioxidant liposome containing vitamin C and folic acid. Journal of Microencapsulation. 2018;35(3):272–280. doi: 10.1080/02652048.2018.1467509. [DOI] [PubMed] [Google Scholar]
- Jovanović, A. A., Balanč, B. D., Ota, A., Ahlin Grabnar, P., Djordjević, V. B., Šavikin, K. P., & Poklar Ulrih, N. (2018). Comparative effects of cholesterol and β‐sitosterol on the liposome membrane characteristics. European Journal of Lipid Science and Technology, 120(9), 1800039. 10.1002/ejlt.201800039
- Karal MAS, Ahamed MK, Mokta NA, Ahmed M, Ahammed S. Influence of cholesterol on electroporation in lipid membranes of giant vesicles. European Biophysics Journal. 2020;49:361–370. doi: 10.1007/s00249-020-01443-y. [DOI] [PubMed] [Google Scholar]
- Katouzian I, Taheri RA. Preparation, characterization and release behavior of chitosan-coated nanoliposomes (chitosomes) containing olive leaf extract optimized by response surface methodology. Journal of Food Science and Technology. 2021;58:3430–3443. doi: 10.1007/s13197-021-04972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh DME, Hammad G, Donia MS, Farag MA. A comprehensive review on grape juice beverage in context to its processing and composition with future perspectives to maximize its value. Food and Bioprocess Technology. 2023;16(1):1–23. doi: 10.1007/s11947-022-02858-5. [DOI] [Google Scholar]
- Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane electroporation and electropermeabilization: Mechanisms and models. Annual Review of Biophysics. 2019;48:63–91. doi: 10.1146/annurev-biophys-052118-115451. [DOI] [PubMed] [Google Scholar]
- Li, D. D., Tao, Y., Shi, Y. N., Han, Y. B., Yang, N., & Xu, X. M. (2020). Effect of re-acetylation on the acid hydrolysis of chitosan under an induced electric field. Food Chemistry, 309, 125767. 10.1016/j.foodchem.2019.125767 [DOI] [PubMed]
- Liu Y, Liu D, Zhu L, Gan Q, Le X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Research International. 2015;74:97–105. doi: 10.1016/j.foodres.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Lu T, Ten Hagen TL. A novel kinetic model to describe the ultra-fast triggered release of thermosensitive liposomal drug delivery systems. Journal of Controlled Release. 2020;324:669–678. doi: 10.1016/j.jconrel.2020.05.047. [DOI] [PubMed] [Google Scholar]
- Mazloomi, S. N., Mahoonak, A. S., Ghorbani, M., & Houshmand, G. (2020). Physicochemical properties of chitosan-coated nanoliposome loaded with orange seed protein hydrolysate. Journal of Food Engineering, 280, 109976. 10.1016/j.jfoodeng.2020.109976
- Menegazzi, M., Campagnari, R., Bertoldi, M., Crupi, R., Di Paola, R., & Cuzzocrea, S. (2020). Protective effect of epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled immune activation: Could such a scenario be helpful to counteract COVID-19?. International Journal of Molecular Sciences, 21(14), 5171. 10.3390/ijms21145171 [DOI] [PMC free article] [PubMed]
- Mohammadi A, Jafari SM, Mahoonak AS, Ghorbani M. Liposomal/nanoliposomal encapsulation of food-relevant enzymes and their application in the food industry. Food and Bioprocess Technology. 2021;14:23–38. doi: 10.1007/s11947-020-02513-x. [DOI] [Google Scholar]
- Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green tea catechins. International Journal of Molecular Sciences. 2020;21(5):1744. doi: 10.3390/ijms21051744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhaei, P., Margiana, R., Bokov, D. O., Abdelbasset, W. K., Jadidi Kouhbanani, M. A., Varma, R. S., ... & Beheshtkhoo, N. (2021). Liposomes: Structure, biomedical applications, and stability parameters with emphasis on cholesterol. Frontiers in Bioengineering and Biotechnology, 748. 10.3389/fbioe.2021.705886 [DOI] [PMC free article] [PubMed] [Retracted]
- Nwabueze TU. Basic steps in adapting response surface methodology as mathematical modelling for bioprocess optimisation in the food systems. International Journal of Food Science & Technology. 2010;45(9):1768–1776. doi: 10.1111/j.1365-2621.2010.02256.x. [DOI] [Google Scholar]
- Rahdar, A., Sayyadi, K., Sayyadi, J., & Yaghobi, Z. (2019). Nano-gels: A versatile nano-carrier platform for drug delivery systems: A mini review. Nanomedicine Research Journal, 4(1), 1–9. 10.22034/nmrj.2019.01.001
- Rashidinejad A, Birch EJ, Sun-Waterhouse D, Everett DW. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chemistry. 2014;156:176–183. doi: 10.1016/j.foodchem.2014.01.115. [DOI] [PubMed] [Google Scholar]
- Reygaert WC. An update on the health benefits of green tea. Beverages. 2017;3:6. doi: 10.3390/beverages3010006. [DOI] [Google Scholar]
- Shishir MRI, Karim N, Gowd V, Zheng X, Chen W. Liposomal delivery of natural product: A promising approach in health research. Trends in Food Science & Technology. 2019;85:177–200. doi: 10.1016/j.tifs.2019.01.013. [DOI] [Google Scholar]
- Souza MP, Vaz AF, Correia MT, Cerqueira MA, Vicente AA, Carneiro-da-Cunha MG. Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food and Bioprocess Technology. 2014;7:1149–1159. doi: 10.1007/s11947-013-1160-2. [DOI] [Google Scholar]
- Srinivasan, V., Chavan, S., Jain, U., & Tarwadi, K. (2019). Liposomes for nanodelivery systems in food products. Nanoscience for Sustainable Agriculture, 627–638. 10.1007/978-3-319-97852-9_24
- Tallei, T. E., Niode, N. J., Idroes, R., Zidan, B. M., Mitra, S., Celik, I., & Capasso, R. (2021). A comprehensive review of the potential use of green tea polyphenols in the management of COVID-19. Evidence-Based Complementary and Alternative Medicine, 2021. 10.1155/2021/7170736 [DOI] [PMC free article] [PubMed]
- Tian, M., Han, J., Ye, A., Liu, W., Xu, X., Yao, Y., & Zhou, W. (2019). Structural characterization and biological fate of lactoferrin‐loaded liposomes during simulated infant digestion. Journal of the Science of Food and Agriculture, 99(6), 2677–2684. 10.1002/jsfa.9435 [DOI] [PubMed]
- Toh, M. R., & Chiu, G. N. (2013). Liposomes as sterile preparations and limitations of sterilisation techniques in liposomal manufacturing. Asian Journal of Pharmaceutical Sciences, 8(2), 88–95. 10.1016/j.ajps.2013.07.011
- Vignali G, Gozzi M, Pelacci M, Stefanini R. Non-conventional stabilization for fruit and vegetable juices: Overview, technological constraints, and energy cost comparison. Food and Bioprocess Technology. 2022;15(8):1729–1747. doi: 10.1007/s11947-022-02772-w. [DOI] [Google Scholar]
- Waterhouse AL. Determination of total phenolics. Current Protocols in Food Analytical Chemistry. 2002;6(1):I1–1. [Google Scholar]
- Wechtersbach L, Ulrih NP, Cigić B. Liposomal stabilization of ascorbic acid in model systems and in food matrices. LWT - Food Science and Technology. 2012;45(1):43–49. doi: 10.1016/j.lwt.2011.07.025. [DOI] [Google Scholar]
- Wibowo, S., Essel, E. A., De Man, S., Bernaert, N., Van Droogenbroeck, B., Grauwet, T., & Hendrickx, M. (2019). Comparing the impact of high pressure, pulsed electric field and thermal pasteurization on quality attributes of cloudy apple juice using targeted and untargeted analyses. Innovative Food Science & Emerging Technologies, 54, 64–77. 10.1016/j.ifset.2019.03.004
- Wu, I. Y., Bala, S., Škalko-Basnet, N., & Di Cagno, M. P. (2019). Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. European Journal of Pharmaceutical Sciences, 138, 105026. 10.1016/j.ejps.2019.105026 [DOI] [PubMed]
- Xing L, Zhang H, Qi R, Tsao R, Mine Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. Journal of Agricultural and Food Chemistry. 2019;67(4):1029–1043. doi: 10.1021/acs.jafc.8b06146. [DOI] [PubMed] [Google Scholar]
- Zhou, F., Xu, T., Zhao, Y., Song, H., Zhang, L., Wu, X., & Lu, B. (2018). Chitosan-coated liposomes as delivery systems for improving the stability and oral bioavailability of acteoside. Food Hydrocolloids, 83, 17–24. 10.1016/j.foodhyd.2018.04.040
- Zou, L. Q., Liu, W., Liu, W. L., Liang, R. H., Li, T., Liu, C. M., & Liu, Z. (2014). Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. Journal of Agricultural and Food Chemistry, 62(4), 934–941. 10.1021/jf402886s [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article. The raw/derived data supporting the findings of this study are available from the corresponding author at request.