Abstract
Background
The availability of high-quality patient-reported outcome (PRO) data is crucial to guiding shared decision-making in the context of locally recurrent rectal cancer (LRRC), where potential treatment benefits must be balanced against the impact of both the disease and treatment on PROs, such as quality of life. This review aimed to identify the patient-reported outcome measures (PROMs) currently being reported in LRRC and to appraise the methodological quality of studies using these measures.
Methods
PubMed, Embase and CINAHL databases were searched, including studies published up until 14th September 2022. Studies in adults with LRRC reporting PROMS as a primary or secondary outcome measure were included. Data were extracted concerning the methodological quality of the reporting of PROMs using criteria informed by the CONSORT-PRO checklist and the psychometric properties of the PROMs identified using the COSMIN Risk of Bias checklist.
Results
Thirty-five studies including 1914 patients with LRRC were identified. None of the studies included in the review met all eleven criteria for the quality of reporting of PROMs. Seventeen PROMs and two clinician-reported outcome measures were identified, none of which have been validated for use in patients with LRRC.
Conclusions
None of the PROMs which are currently being used to report PROs in LRRC have been validated for use in this cohort of patients. Future studies in this disease area should focus on utilising PROMs that have undergone a robust development process including patients with LRRC, to produce data which is high quality, accurate and relevant.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-023-13388-5.
The availability of high-quality studies reporting patient-reported outcome (PRO) data utilising robustly developed patient-reported outcome measures (PROMs), offer several advantages to patient care, including their utility within shared decision-making discussions. Baseline PRO data has been shown to act as a prognostic factor for overall survival in cancer patients,1 including those with advanced malignancy.2,3 Integrating PROs into clinical care to monitor adverse effects of cancer treatment can also enhance patient quality of life,4 and has even been reported to improve survival.5,6 The interest in utilising PROMs from both a clinical and academic standpoint continues to grow given the potential utility of these outcome measures, including in patients with locally recurrent rectal cancer (LRRC). The inclusion of patient-reported outcomes (PROs) is particularly important in the context of advanced malignancy such as LRRC. LRRC can lead to debilitating symptoms such as pain, bleeding/discharge from the rectum, pelvic sepsis, urinary symptoms, lower limb symptoms and impaired sexual function. Surgical resection represents the only curative treatment option for patients with LRRC, with 5-year survival rates of 42.4% - 63% reported by specialist tertiary centres.7–11 Exenterative surgery has evolved, with ultra-radical techniques developed in recent years, which can offer potential cure to patients with LRRC, such as high sacrectomy and extended lateral pelvic sidewall excision (ELSiE), are generally accompanied by significant morbidity.12–14 In this context, balancing the patients’ existing symptoms, the potential survival benefits to be gained from treatment and their impact on PROs, is essential to enabling patients to make informed decisions regarding their care.
However, it is crucial that the methodological quality of the studies reporting PROs and the PROMs used are sufficient to produce valid and reliable results, particularly in complex disease settings. Validity is the degree to which a PROM measures the construct it purports to measure.15 In a clinical context, such as in measuring health-related quality of life (HrQoL) in patients with LRRC, a PROM can only be considered valid if there is evidence that it has been developed with input from patients with LRRC and provides a comprehensive assessment of HrQoL as the construct of interest, meaning that all aspects of HrQoL that are relevant to patients with LRRC are included. PROMs can be designed as disease-specific or generic, for instance, a generic PROM measures concepts which are broadly relevant to the population, whereas disease-specific PROMs measure concepts specific to a group of patients with a particular condition. To be considered valid in a specific group of patients, both disease-specific and generic PROMs should be shown to have content validity in the group of patients they have been designed for.
The existing evidence concerning PROs in LRRC possesses several limitations from a methodological standpoint, this includes heterogeneity in relation to the groups of patients included, with outcomes frequently reported in combined cohorts of patients with primary and recurrent disease,16–19 and heterogeneity in comparator groups. In addition to significant variability in the PROMs used and timing of PROM assessment.16–19 The majority of existing studies are retrospective in nature18 and the evidence is generally low in quality.16–20 Denys et al.’s review focused on patient-centred outcomes following pelvic exenteration for colorectal cancer, including both primary and recurrent disease, also found that the impact of urinary complications, discomfort or pain on sitting and functional disability are inadequately represented in the PROMs currently being used.19
This review sought to evaluate the methodological quality of the existing evidence concerning PROs in LRRC, utilising a systematic approach. The specific aims of the review were to identify the PROMs currently being used to report outcomes in patients with LRRC and to examine the methodological quality of the studies against criteria informed by the Consolidated Standards of Reporting Trials- Patient Reported Outcome (CONSORT-PRO) extension,21,22 and the psychometric properties of the PROMs identified using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) Risk of Bias checklist.23,24
Methods
This systematic review was conducted using a pre-specified protocol in keeping with Cochrane guidelines,25 and reported in line with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist.26 The review was registered on the international prospective register of systematic reviews, PROSPERO (reference: CRD42022332577).
Eligibility Criteria
Studies in adults (aged ≥ 18) with LRRC that included PROMs as a primary or secondary outcome measure were included. Studies in patients with LRRC undergoing any form of treatment with curative or palliative intent, were eligible for inclusion. Studies in patients with a history of only local excision for primary rectal cancer who developed a regrowth or recurrence were excluded. Only studies published in the English language were considered. Case reports, conference abstracts, study protocols, reviews and letters were excluded.
Information Sources
The search was undertaken using the PubMed, Embase and CINAHL databases, including studies published from 1966 (PubMed), 1980 (Embase) and 1981 (CINAHL) up until 14th September 2022. The search strategy can be found in the supplementary material. Reference searching was also undertaken to identify additional studies. Studies describing the psychometric properties of the PROMs identified from this search were retrieved from citations and through manual searching to enable evaluation of the psychometric properties of the PROMs identified.
Selection Process
Titles and abstracts of studies retrieved were exported to EndNote X9 (Clarivate Analytics, Philadelphia, USA) and duplicates removed. The titles and abstracts were uploaded to Rayyan online software and screened for relevance by two authors (NM and ER). The full text for potentially eligible studies were retrieved and assessed, any queries regarding the eligibility of a study were resolved through discussion with senior authors.
Data collection process
Data concerning the characteristics of the studies included and the quality of the reporting of PROMs against criteria informed by the CONSORT-PRO checklist were extracted independently by authors NM and ER into Excel®. The COSMIN Risk of Bias checklist23 was completed using the Excel® template available from the COSMIN website27 independently by authors NM and FH. Any differences in data extraction or ratings were discussed with senior authors to reach consensus.
Data Items
Quality of Reporting of PROMs
There are currently no checklists available via the Enhancing the QUAlity and Transparency Of health Research (EQUATOR) network regarding the inclusion of PRO data for observational studies. The CONSORT-PRO extension was developed to promote transparent reporting of trials including PROs as primary or secondary outcomes; facilitating the interpretation or PRO results for use in clinical practice.22 The CONSORT-PRO checklist was used to inform the evaluation of studies identified in relation to how the findings were reported and whether the methodology of the study and the PROMs used were sufficient to capture significant and meaningful findings.
PROM Psychometric Properties
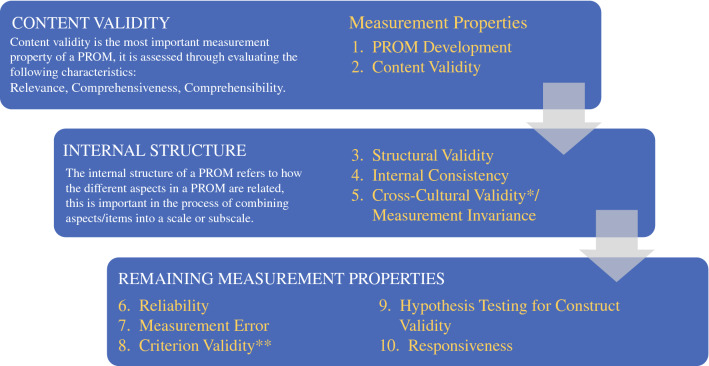
The psychometric properties of the PROMs identified were evaluated using the COSMIN Risk of Bias checklist. The COSMIN Risk of Bias checklist for systematic reviews was developed to assess risk of bias of studies on measurement properties of PROMs,23 this information can be used to identify the most appropriate PROM for a specific purpose or study. There are ten criteria (see Figure 1), PROM development and content validity are the first to be assessed, if a PROM is deemed to have insufficient content validity, it should not undergo further assessment. Once sufficient evidence for content validity has been identified, the internal structure and remaining measurement properties are assessed. Studies are qualitatively summarised to give an overall rating of sufficient (+), insufficient (-), inconsistent (±), or indeterminate (?) for each measurement property.28 The quality of the evidence is rated using a modified Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.29
Fig. 1.
Summary of the COSMIN Risk of Bias Checklist. *Cross-cultural validity was not assessed in this review as the search strategy was not deemed suitable for identifying all studies describing this psychometric property. **The COSMIN panel determined that no gold standard exists for PROMs30 and therefore criterion validity was not assessed in this review.
Risk of Bias Assessment
Risk of bias was assessed using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool,31 and the revised tool to assess Risk of Bias in randomised trials (RoB 2).32
Data Synthesis
A basic descriptive analysis was undertaken to report the number of patients included in the studies identified and the proportion of patients with LRRC and who contributed to assessments with PROMs.
Results
Study Selection
A total of 1475 references were identified; 147 duplicates and 5 animal studies were removed. Abstracts were screened for 1323 references and the full text for 56 references were retrieved. Thirty-one eligible references were included from the search strategy in addition to 4 references identified through manual searching (see Figure 2).
Fig. 2.
PRISMA flow diagram
Study Characteristics
A summary of the characteristics of the studies is presented in Table 1, including a total of 1914 patients with LRRC across the 35 studies included, of which PROM data was reported for 1104 (57.7%) patients. Twenty-one (63.6%) of the studies identified were published in the last decade. The studies were conducted mostly in Europe (n = 18, 51.4%), Australia (n = 13, 37.1%) or the USA (n = 4, 11.4%), with one study conducted in China (2.9%). Twenty-six (74.3%) studies recruited patients from a single centre. The majority were prospective cohort studies (n = 19, 54.3%) in addition to cross-sectional (n = 7, 20.0%), case-control (n = 5, 14.3%), retrospective cohort (n = 2, 5.7%) and randomised studies (n = 2, 5.7%). Eight (22.9%) of the studies identified included only patients with LRRC, in addition to two (5.7%) case control studies comparing patients with LRRC to other cohorts, with sample sizes of patients with LRRC ranging from 12 to 117 patients. The other 23 (69.7%) studies included combined cohorts of patients with primary and recurrent pelvic disease including LRRC, with sample sizes ranging from 12 to 710 patients in total. Median number of PROM assessments was two (IQR 1). In the 19 prospective, longitudinal studies identified, median follow-up was 12 months (IQR 15) the longest follow-up time point was 8 years.33
Table 1.
Summary of studies identified
| Country | Type of study | Primary outcome(s) | Total no patients | Patients included | Total no with LRRC | Total no with LRRC with PRO data | PROM data for LRRC | Inclusion of comparative group | Timing of PROM assessment | PROMs used | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Huang 2022 |
Australia | Prospective cohort | QoL | 271 | PE 2008-2019 | 160 | 150 | Yes | LARC vs LRRC | Baseline, 6, 12 months |
FACT-C SF-36 |
|
Westerduin 2021 |
Netherlands, Belgium, and France | Cross-sectional | QoL | 52 | Redo anastomosis 2007-2017 | 2 | 2 | No | Control group of 118 patients undergoing TME surgery for rectal cancer | Cross-sectional |
LARS EORTC-C30 EORTC-CR29 |
|
Alahmadi 2021 |
Australia | Prospective cohort | QoL, Survival, Post-operative complications | 710 |
PE 1994-2019 |
235 | Not known | No | Elderly (>65) vs younger patients undergoing PE | Baseline, 6, 12, 18, 24, 30, 36, 48, 60 months |
FACT-C SF-36 |
|
McCarthy 2020 |
Australia | Cross-sectional | QoL, lower limb motor, bowel, bladder, and sexual function | 256 |
PE with sacrectomy 2008-2015 |
111 | 11 | No | PE and sacrectomy vs PE only | Cross-sectional |
SF-36 EORTC-C30 & CR29 MSTS LEFS SHIM FSFI |
|
Van Ramshort 2020 |
Australia | Prospective cohort | Flap-related complications | 87 |
PE with VRAM reconstruction 2003-2016 |
30 | Not known | No | PE with VRAM vs PE no VRAM | Baseline, 6, 12, 18, 24 months |
FACT-C SF-36 |
|
Denost 2020 |
France Australia |
Prospective cohort | Surgical resection rate | 154 | LARC or LRRC 2015-2017 | 105 | Not known | No | PE vs no PE | 6, 12 months |
SF-36 Distress thermometer Scale |
|
Smith 2020 |
UK | Prospective cohort | Local control | 30 |
SBRT for LRRC 2015-2019 |
30 | 30 | Yes | No | Baseline 1, 3, 6 months, then 6 monthly intervals |
EQ-5D EQ-VAS |
|
Brown 2019 |
Australia | Prospective cohort | Survival, function, QoL | 68 | Sciatic and femoral nerve resection 1994-2018 | 33 | Not known | No | Complete vs partial sciatic or femoral nerve resection | Baseline, 6, 12 months |
FACT-C SF-36 |
|
Steffens 2018 |
Australia | Prospective cohort | Survival, QoL | 515 |
PE 1994-2016 (PE 2008-2016 for QoL study) |
181 | 119 | No | No | Baseline, 6, 12, 18, 24, 30, 36, 48, 60 months |
FACT-C SF-36 |
|
Lim 2018 |
Australia | Prospective cohort | Post-operative pain, pre-operative opiate use, post-operative pain | 99 |
PE 2013-2014 |
51 | 42 | Yes | No | Days 1, 2, 3 and 7 | VNRS |
|
Choy 2017 |
Australia | Prospective cohort | QoL | 117 | LRRC referred for PE 2008-2013 | 117 | 101 | Yes | No | Baseline, 1, 3, 6, 9, 12 months |
AQOL SF6D FACT-C |
|
Quyn 2016 |
Australia | Prospective cohort | QoL, morbidity, survival | 39 | Palliative PE 1995-2015 | 30 | Not known | No | No | Baseline, 1, 3, 6, 9, 12 months |
AQOL SF-36 |
|
Cameron 2016 |
Norway | Prospective cohort | Severity of symptoms | 51 | Palliative pelvic radio-therapy 2009-2015 | 12 | Not known | No | No | Baseline, completion of radiotherapy, 6, 12 weeks |
EORTC-C30 BPI |
|
Pellino 2015 |
Italy | Case-control | QoL | 116 | LRRC 2002-2011 | 45 | 40 | Yes | Control group of patients with primary rectal cancer and R0 resection | Baseline, 12, 36 months | EORTC-C30 |
|
Li 2015 |
China | Prospective cohort | Pain | 31 | LRRC 2009-2013 | 31 | 31 | Yes | No | Baseline, 1 week, 1, 3, 6 months | VAS (pain) |
|
Thaysen 2014 |
Denmark | Case-control | QoL | 180 |
PE 2001-2008 |
62 | 62 | No | Compared to population norms and a group undergoing standard rectal cancer surgery. | Baseline, 3, 6, 12, 18, 24 months |
EORTC-C30 & CR38 SF-36 |
|
Beaton 2014 |
Australia | Cross-sectional | Morbidity, QoL | 31 | PE 1996-2007 | 17 | 17 | No | Comparison of low, normal and high BMI | Cross-sectional | FACT-C |
|
Pusceddu 2013 |
Italy | Prospective cohort | Pain | 12 | LRRC with severe pain not responding to chemo-radiotherapy 2006-2010 | 12 | 12 | Yes | No | Baseline, 1, 3, 6, 12, 22 months | VAS (pain) |
|
Traa 2013 |
Netherlands | Prospective cohort | QoL, sexual function | 439 | LARC and LRRC 2000-2010 | 67 | 67 | Yes | Population norms vs LARC vs LRRC | Cross-sectional |
EORTC-C30 & CR38 |
|
Holman 2013 |
Netherlands | Cross-sectional | Flap-related complications, function following vaginal reconstruction, QoL | 51 | VRAM for LARC or LRRC 1994-2010 | 18 | Not known | No | Patients with LARC and LRRC undergoing VRAM reconstruction vs patients not undergoing reconstruction. | Cross-sectional |
EORTC-C30 & CR38 |
|
Brændengen 2011 |
Norway | Cross-sectional | Morbidity, sexual function | 207 | Non-resectable LARC or LRRC undergoing pre-op radiotherapy or chemoradiotherapy 1996-2003 | 7 | 5 | Yes | Patients receiving chemoradiotherapy vs those receiving radiotherapy | Cross-sectional |
EORTC-C30 IIEF SVQ LENT SOMA St. Marks’s FI score |
|
Haapamaki 2011 |
Sweden | Cross-sectional | Physical function, QoL | 19 | Extralevator APER with gluteus maximus flap 2005-2007 | 1 | 1 | No | No | Cross-sectional |
EQ-5D EQ-VAS VAS |
|
You 2011 |
USA | Prospective cohort | Survival, QoL, Pain | 105 | LRRC 1997-2007 | 105 | 54 | Yes | Curative treatment surgery vs non-curative surgery and non-surgical treatment | Baseline, 3, 6, 9, 12, 24, 36, 60, 96 months |
FACT-C BPI |
|
Austin 2010 |
Australia | Case-control | QoL | 44 |
PE 1996-2007 |
20 | 20 | Yes | Patients undergoing PE vs patients with rectal cancer undergoing LAR or APER vs population norms | Cross-sectional |
FACT-C SF-36 |
|
Zoucas 2010 |
Sweden | Prospective cohort | Morbidity, survival, QoL | 85 | PE 2003-2008 | 20 | Not known | No | No | 4, 16 months | EORTC-C30 |
|
Palmer 2008 |
Sweden | Case-control | QoL | 142 | LARC or LRRC 1991-2003 | 13 | 13 | No | LARC and LRRC vs TME surgery alone and population norms | Cross-sectional |
EORTC-C30 & CR38 |
|
Miner 2003 |
USA | Prospective cohort | Morbidity, survival, QoL | 105 |
LRRC 1997-1999 |
105 | 105 | Yes | Palliative versus non-palliative treatment | Not specified | Not specified |
|
Mannaerts 2002 |
Netherlands | Prospective cohort | Functional outcome | 121 | LARC or LRRC 1994-1999 | 66 | 39 | Yes | LARC vs LRRC | 6 months pre-treatment, median 14 months post-treatment | Questionnaire devised for the study including questions from the anal incontinence scale and MSKCC Sphincter Function Scale |
|
Esanaola 2002 |
USA | Prospective cohort | Pain, QoL | 45 | LRRC 1999-2000 | 45 | 45 | Yes | Non-operative palliation vs resection | Cross-sectional |
FACT-C BPI |
|
Camilleri-Brennan 2001 |
UK | Cross-sectional | QoL | 75 | LRRC 1992-1997 | 13 | 13 | No | LRRC vs patients with primary rectal cancer who did not develop recurrence | Cross-sectional |
EORTC-C30 & CR38 |
|
Mannaerts 2001 |
Netherlands | Retrospective cohort | Urological function | 121 | LARC or LRRC 1994-1999 | 66 | 39 | Yes | LARC vs LRRC | Cross-sectional | Not specified |
|
Guren 2001 |
Norway | Case-control | QoL | 37 | Patients undergoing urinary diversion for LARC or LRRC since 1991 | 12 | 12 | No | Patients undergoing urinary diversion vs patients who did not undergo urinary diversion vs population norms | Cross-sectional |
EORTC-C30 & CR38 & BLM30 (6 items only) |
|
Trotter 1996 |
Australia | Randomised study | Disease progression, toxicity, QoL | 73 | LRRC or primary inoperable rectal cancer 1985-1991 | 64 | 64 | No | Microwave therapy combined with external beam radiotherapy vs standard external beam radiotherapy | Weekly during treatment and then every 4 weeks | Spitzer |
|
Scheithauer 1993 |
Austria | Randomised study | Survival, QoL | 36 | Inoperable metastatic or recurrent colorectal cancer 1988-1989 | Not known | Not known | No | Patients receiving chemotherapy vs best supportive care vs healthy volunteers | Baseline, every 2 months | FLIC |
|
Wanebo 1987 |
USA | Retrospective cohort | Morbidity, mortality, survival, QoL | 28 | LRRC | 28 | 10 | Yes | No | Cross-sectional | Not specified |
QoL – quality of life, PROM – patient-reported outcome measure, PE - pelvic exenteration, LRRC – locally recurrent rectal cancer, LARC – locally advanced rectal cancer, FACT-C - Functional Assessment of Cancer Therapy – Colorectal Measure, SF-36 – 36-Item Short Form Survey, TME – total mesorectal excision, LARS – Low Anterior Resection Syndrome score, EORTC-C30 – European Organisation for Research and Treatment of Cancer Core Measure, EORTC-CR29/CR38 – European Organisation for Research and Treatment of Cancer Colorectal Module, MSTS – Musculoskeletal Tumour Society Score, LEFS – Lower Extremity Functional Scale, SHIM – Sexual Health Inventory for Men, FSFI – Female Sexual Function Index, VRAM - Vertical Rectus Abdominis Myocutaneous flap, SBRT – Stereotactic Body Radiotherapy, EQ-5D – EuroQoL measure of health-related quality of life, EQ-VAS – EuroQoL Visual Analogue Scale, VNRS – Verbal Numerical Rating Scale, SF6D – Short Form Six-Dimension, AQOL – Assessment of Quality of Life, BPI – Brief Pain Inventory, R0 – Complete Surgical Resection, VAS – Visual Analogue Scale, BMI – Body Mass Index, IIEF – International Index of Erectile Function, SVQ – Sexual function – Vaginal changes Questionnaire, LENT-SOMA – Late Effects of Normal Tissue – Subjective , Objective, Management and Analytic, St. Mark’s FI Score – St. Mark’s Faecal Incontinence Score, APER – Abdominoperineal Excision of the Rectum, LAR – Low Anterior Resection, MSKCC – Memorial Sloan Kettering Cancer Center, EORTC-BLM30 – European Organisation for Research and Treatment of Cancer Muscle Invasive Bladder Cancer Measure, FLIC – Functional Living Index – Cancer.
Risk of Bias
Risk of bias was high overall, with 32 (91.4%) studies highly or seriously biased (see supplementary Figures 1 and 2).
Results of Individual Studies
Quality of Reporting of PROMs
The assessment of the studies identified against criteria informed by the CONSORT-PRO checklist are illustrated in Figure 3. None of the studies included in the review met all eleven criteria for the quality of reporting of PROMs, with an overall median of 5.8 (58.3%) criteria. The least reported criteria were defining the PROM of interest (n = 3, 8.6%), describing the statistical approach to missing PRO data (n = 6, 17.1%), and detailing a PRO hypothesis (n = 6, 17.1%). The most commonly met criterion was the identification of a PRO as a primary or secondary outcome (n = 35, 100.0%).
Fig. 3.
Quality of Reporting of PROMS in LRRC
Characteristics of the PROMs Identified
Seventeen PROMs and two clinician-reported outcome measures (MSTS and Spitzer) were identified. The most commonly reported PROMs were the EORTC QLQ-C30 (n = 12, 32.3%),34,36,45,46,48,51–53,56,57,61,63 the SF-36 (n = 11, 31.4%),7,35–38,40,41,43,44,48,55 the FACT-C (n = 10, 28.6%)7,33,35,37,40,41,43,49,55,60 and the EORTC QLQ-CR29 (formerly CR38) (n = 8, 22.9%).34,36,48,51,52,57,61,63
Four of the PROMs identified were specific to patients with cancer (see Table 2), however, there were no disease-specific PROMs for patients with LRRC. The cancer-specific measures included the EORTC QLQ-C30 which is a measure of QoL in patients with cancer and the Functional Living Index – Cancer (FLIC) is a measure of functional state in adult patients with cancer. Two measures which are cancer-site specific were also identified; the EORTC-QLQ CR29 and FACT-C which are both measures of QoL in patients with primary colorectal cancer.
Table 2.
Summary of cancer-specific measures identified
| Measure | Patient-reported outcome | Target population | No of Items | Scales | No of languages/Dialogues | Total no of studies identified using this PROM | Studies identified using this PROM |
|---|---|---|---|---|---|---|---|
|
European Organisation for Research and Treatment of Cancer Core Measure (EORTC QLQ-C30) |
QoL | Patients with cancer | 30 |
Functional scales: - Physical - Role - Cognitive - Emotional - Social Symptom scales: - Fatigue - Pain - Nausea and vomiting Global health status |
11767 | 12 | 34,36,45,46,48,51–53,56,57,61,63 |
| Functional Living Index – Cancer (FLIC) | Functional state | Patients with cancer | 22 |
Psychological Physical Symptoms Family Social |
1568 | 1 | 65 |
|
European Organisation for Research and Treatment of Cancer Colorectal Module (EORTC QLQ-CR29, formerly EORTC QLQ-CR38) |
QoL | Patients with primary colorectal cancer | 29 |
Urinary frequency Blood or mucus in stools Stool frequency Body image |
6669 | 8 | 34,36,48,51,52,57,61,63 |
|
Functional Assessment of Cancer Therapy – Colorectal Measure (FACT-C) |
QoL | Patients with primary colorectal cancer | 36 |
Emotional Well-Being Social Well-Being Functional Well-Being Physical Well-Being Colorectal Cancer Subscale |
4070 | 10 | 7,33,35,37,40,41,43,49,55,60 |
Seven PROMs which relate to forms of function or functional limitations were identified (Table 3), including bowel function, physical function, and sexual function. The Low Anterior Resection Syndrome (LARS) score is a measure to assess bowel dysfunction following low anterior resection for rectal cancer and the St. Mark’s Faecal Incontinence Score for adult patients with faecal incontinence. The Lower Extremity Functional Scale (LEFS) is a measure of lower extremity physical function designed for patients with lower extremity orthopaedic conditions. Four of the measures identified were measures of sexual function, including the Sexual Health Inventory for Men (SHIM) and the International Index of Erectile Function (IIEF) which are measures of erectile dysfunction developed for use in male patients with a history of erectile dysfunction and the Female Sexual Function Index (FSFI) measure of sexual function for female patients with a history of sexual arousal disorder and the Sexual function – Vaginal changes Questionnaire (SVQ) measure of sexual and vaginal problems developed for patients with a history of gynaecological cancer.
Table 3.
Summary of measures related to function or functional limitations
| Measure | Patient-reported outcome | Target population | No of items | Scales | No of languages/Dialogues | Total no of studies identified using this PROM | Studies identified using this PROM |
|---|---|---|---|---|---|---|---|
| Low Anterior Resection Syndrome (LARS) score | Low Anterior Resection Syndrome | Patients who have undergone low anterior resection for rectal cancer | 5 | N/A | 2471 | 1 | 34 |
|
Lower Extremity Functional Scale (LEFS) |
Lower extremity physical function | Patients with lower extremity orthopaedic conditions | 20 | N/A | 1468 | 1 | 36 |
| Sexual Health Inventory for Men (SHIM) | Erectile dysfunction | Male patients with erectile dysfunction | 5 | N/A | 968 | 1 | 36 |
| International Index of Erectile Function (IIEF) | Erectile dysfunction | Male patients with erectile dysfunction | 15 |
Erectile function Orgasmic function Sexual desire Intercourse satisfaction Overall satisfaction |
8868 | 1 | 53 |
|
Female Sexual Function Index (FSFI) |
Sexual function | Female patients with sexual arousal disorder | 19 |
Desire Arousal Lubrication Orgasm Satisfaction pain |
5268 | 1 | 42 |
| Sexual function – Vaginal changes Questionnaire (SVQ) | Sexual and vaginal problems | Gynaecological cancer patients | 20 core items (7 additional items for use in follow-up) |
Intimacy Sexual interest Global sexual satisfaction Vaginal changes Sexual functioning |
Not known | 1 | 53 |
| St. Mark’s Faecal Incontinence Score | Faecal incontinence | Adult patients with faecal incontinence | 7 | N/A | Not known | 1 | 53 |
Six of the PROMs identified were generic measures (see Table 4), including three measures of QoL for use in adult patients; the 36-Item Short Form Survey (SF-36), EuroQoL (EQ-5D) and Assessment of Quality of Life (AQOL-4D), two measure of pain intensity; the Verbal Numerical Rating Scale (VNRS) and Visual Analogue Scale (VAS), and finally one measure of pain, the Brief Pain Inventory (BPI).
Table 4.
Summary of generic measures identified
| Measure | Patient-Reported Outcome | Target Population | No of Items | Scales | No of Languages/ Dialogues | Total no of studies identified using this PROM | Studies identified using this PROM |
|---|---|---|---|---|---|---|---|
|
36-Item Short Form Survey (SF-36) including the Short Form Six-Dimension (SF6D) |
QoL | Adult patients | 36 |
Energy/ vitality Physical functioning Bodily pain General health perceptions Physical role functioning Emotional role functioning Social role functioning Mental health |
2 available via RAND,72 191 listed on ePROVIDE68 | 11 | 34–38,40,41,43,44,48,55 |
|
EuroQoL (EQ-5D) including the Visual Analogue Scale (EQ-VAS) |
QoL | Adult patients | 5 |
Mobility Self-care Usual activities Pain/ discomfort Anxiety/ depression |
18373 | 2 | 39,54 |
| Verbal Numerical Rating Scale (VNRS) | Pain Intensity | Adult patients | 10-point scale | N/A | Not known | 1 | 42 |
| Visual Analogue Scale (VAS) | Pain Intensity | Adult patients | 100mm line | N/A | Not known | 3 | 47,50,54 |
| Assessment of Quality of Life (AQOL-4D) | QoL | Adult patients | 15 |
Illness Independent living Social relationships Physical senses Psychological wellbeing |
774 | 2 | 43,44 |
| Brief Pain Inventory (BPI) | Pain | Adult patients | 11 |
Pain intensity Pain interference |
5375 | 3 | 33,45,60 |
The three remaining measures included (see Table 5), were not patient-reported but clinician reported. Those included the Late Effects of Normal Tissue – Subjective, Objective, Management, and Analytic (LENT-SOMA) scoring system for late effects of radiotherapy, including a subjective scale to be completed by patients with the remainder being completed by clinicians. The Spitzer is a clinician-reported measure of QoL for patients with cancer or other chronic diseases and the Musculoskeletal Tumour Society Score (MSTS) is a clinician-reported measure of physical function for patients with musculoskeletal neoplasms.
Table 5.
Summary of other measures identified
| Measure | Patient-reported Outcome | Target population | No of items | Scales | No of languages/Dialogues | Total no of studies identified using this PROM | Studies identified using this PROM |
|---|---|---|---|---|---|---|---|
| Late Effects of Normal Tissue – Subjective, Objective, Management, and Analytic (LENT-SOMA) scales | Late effects of radiotherapy | Adult patients who have received radiotherapy | 5 (for subjective rectum scale) |
Tenesmus Mucosal loss Sphincter control Stool frequency Pain |
Not known | 1 | 53 |
|
Spitzer *designed to be used as a clinician-reported outcome measure |
QoL | Patients with cancer or other chronic diseases | 5 |
Activity Daily life Health perceptions Social support Behaviour |
568 | 1 | 64 |
|
Musculoskeletal Tumour Society Score (MSTS) *designed to be used as a clinician-reported outcome measure |
Physical function | Patients with musculoskeletal neoplasms | 6 |
Pain Function Emotional acceptance Criteria specific to the lower extremity: - Use of supports - Walking - Gait Criteria specific to the upper extremity: - Hand positioning - Manual dexterity - Lifting ability |
Not known | 1 | 36 |
PROM Psychometric Properties
The psychometric properties were only assessed for PROMs and not the LENT-SOMA or the clinician-reported outcome measures, Spitzer and MSTS.
Content Validity
None of the PROMs identified were developed specifically for patients with LRRC (Tables 2, 3, 4 and 5) and no studies were identified in which the psychometric properties of these PROMs were evaluated in patients with LRRC.
Internal Structure and Remaining Measurement Properties
Content validity is the most important measurement property of a PROM and therefore full review is not advised if a PROM does not meet criteria for content validity.
Discussion
There has been an expansion in PROMs reporting in LRRC, with several papers (n = 21, 63.6%) published in the last decade. However, despite this increase, these studies are methodologically limited due to the use of non-validated measures used to assess PROs in this cohort of patients. This systematic review did not identify a disease-specific PROM available for use in LRRC and none of the PROMs identified met the COSMIN criteria for content validity in the context of LRRC. The most used PROMS in LRRC were the FACT-C (n = 10, 28.6%), SF-36 (n = 11, 31.4%) EORTC QLQ-C30 (n = 12, 34.3%) and CR29 (n = 8, 22.9%), none of which have demonstrated content validity specifically for patients with LRRC.
Overall, the findings build on the existing evidence16–19 of variable methodological quality of reporting of PROMs within small sample sizes and mixed disease cohorts. This review focuses specifically on the methodological quality of PRO reporting using criteria informed by the CONSORT-PRO checklist; common weaknesses were identified in several domains, including defining the PRO of interest, describing the statistical approach to missing data and stating PRO-specific limitations and implications for generalisability. These results were comparable to those reported in Efficace et al.’s pooled analysis of randomised cancer trials utilising CONSORT-PRO,76 though methods of PRO data collection had higher levels of reporting in this current review. Ultimately, the key limitation identified is the lack of input from patients with LRRC in the PROMs currently being used, with none demonstrating content validity for use in this context. Content validity is the most important measurement property of a PROM; for PROMs to give meaningful results in LRRC, it is essential that they are relevant to patients with LRRC and present a comprehensive assessment of the construct of interest. Without addressing the lack of an appropriate PROM for use in patients with LRRC, the impact of addressing issues such as heterogeneity in the groups of patients included, the comparator groups used, and the timing of PROM assessment, is likely to be limited.
Harji et al. reported the development of the Locally Recurrent Rectal Cancer – Quality of Life (LRRC-QoL) conceptual framework through undertaking a systematic review and qualitative focus groups to identify the HrQoL issues relevant to patients with LRRC.18,77 The themes identified were symptoms, sexual function, psychological impact, role and social functioning, future perspective and healthcare service utilisation and delivery. Nineteen (54.3%) of the studies identified in this review have been published since this work,35–51 using a median of two PROMS, with the EORTC QLQ-CR29 and FACT-C most used. The EORTC QLQ-CR29 and FACT-C have also both demonstrated robust psychometric properties, including content validity, in patients with primary colorectal cancer.78,79 When compared with the LRRC-QoL conceptual framework,77 the EORTC QLQ-CR29 covers 50% of the LRRC-specific domains, including symptoms, sexual function, and psychological impact. It does not however cover the domains of role functioning, or future perspective. The FACT-C covers 66.6% of the LRRC-specific domains identified in the LRRC-QoL conceptual framework including symptoms, psychological impact, role functioning, and future perspective, it does not cover sexual function. Neither the EORTC QLQ-CR29 or FACT-C cover issues relating to healthcare services, self-efficacy and body image, future plans, disease re-recurrence, gynaecological or locomotor symptoms. The evidence identified reporting outcomes utilising these PROMs should not be completely disregarded, as the EORTC QLQ-CR29 and FACT-C capture a proportion of the issues relevant to patients with LRRC. However, it should be interpreted with caution, as they are unlikely to capture the full scope and complexity of the range of issues patients with LRRC experience.18,77
A number of PROMs which measure issues relevant to patients with LRRC were identified in this review; urinary and sexual function were evaluated using specific questionnaires for this purpose by two studies,36,53 however, other questionnaires, such as the EORTC QLQ-CR29, also contain items concerning sexual and urinary function. No specific PROMs concerning stoma-related quality of life were used in the studies identified, despite being relevant to patients with LRRC.77 However, PROMs such as the EORTC QLQ-CR29 and FACT-C contain items specifically for patients with stomas. The increasing number of PROMs currently being used in LRRC reflects the lack of an existing disease-specific measure which adequately reports all the PROs relevant to this cohort of patients. The trend to include several PROMs is likely to reflect the greater understanding of the wider issues which affect patients with LRRC. However, the measures identified in this review are not valid for use in patients with LRRC and therefore this is not a psychometrically robust approach to addressing the lack of a LRRC disease-specific measure. Additionally, this approach potentially increases the burden of participation for patients, without sufficient methodological justification.
There are limitations related to the evidence included in this review, notably, most of the studies identified have a high risk of bias (n = 32, 91.4%) and their findings should generally be interpreted with caution. They also present a predominately Western perspective of PROs in LRRC and demonstrate a lack of multi-centre, international reporting of PROs in LRRC. Furthermore, 13 (37.1%) of the studies identified were conducted within a single centre, reporting cohorts of patients which may potentially overlap. It was not possible to assess the availability and quality of translated PROMs in this review, however, to further the success of initiatives such as the PelvEx collaborative in advancing international outcome reporting in this cohort of patients80 and integrating PRO data, it is essential that PROMs undergo a rigorous process of cross-cultural adaption.
There are several approaches which could be employed to address the lack of PROMs with content validity for patients with LRRC. It is possible to demonstrate the content validity of existing PROMS specifically for LRRC, however, given the narrow breadth of relevant HrQoL issues captured by existing measures, this approach will require significant revision to make these measures applicable to LRRC.77 Employing a modular approach to PROM assessment to LRRC is an alternative approach, provided both the core cancer and site-specific measures are appropriately revised and validated for use in LRRC. Development of a new disease-specific PROMs for use in patients with LRRC, to capture concerns that are specific to patients with LRRC which can be used to more accurately monitor the impact of particular treatments on PROs such as HrQoL is likely to be the most realistic and valid approach.81 The development of the LRRC-QoL PROM will build on the development of the LRRC-QoL conceptual framework.77 The LRRC-QoL is the first disease-specific PROM developed for use in patients with LRRC82 and has been designed to be used in combination with EORTC QLQ-C30, in a modular fashion, which would allow comparison across patient groups. Recruitment to a study to externally validate the LRRC-QoL for use internationally is currently underway (ISRCTN13692671) and includes a robust cross-cultural adaptation process to produce versions of the LRRC-QoL for use in several countries.
Conclusion
This systematic review highlights key methodological issues in the current state of reporting of PROs in LRRC, finding that none of the PROMs currently being used in LRRC are able to provide meaningful results within this context. Future studies in this disease area should focus on utilising PROMs that have undergone a robust development process with the inclusion of patients with LRRC, to ensure high quality, accurate results which are relevant to this patient group. The development of a disease-specific PROM for patients with LRRC or undertaking content validity studies of existing PROMs are approaches which could be employed to enable this, in addition to undertaking cross-cultural adaptation to enable international reporting of outcomes. Greater emphasis should also be placed on the way in which PROMs data are reported and analysed, particularly in defining the PRO of interest and in handling missing PROM data, to ensure that results are reliable.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Funding for this work was provided by Bowel Research UK and Pelican Cancer Foundation.
Disclosures
Prof. Galina Velikova, Honoraria: Pfizer, Novartis, Eisai, Advisory boards: Consultancy fees from AstraZeneca, Roche, Novartis, Pfizer, Seagen, Eisai, Sanofi Institutional grant from Pfizer.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol. 2009;27(34):5816–5822. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coates A, Porzsolt F, Osoba D. Quality of life in oncology practice: Prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer. 1997;33(7):1025–1030. doi: 10.1016/s0959-8049(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 3.Maisey NR, Norman A, Watson M, Allen MJ, Hill ME, Cunningham D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer. 2002;38(10):1351–1357. doi: 10.1016/s0959-8049(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 4.Absolom K, Warrington L, Hudson E, et al. Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol. 2021;39(7):734–747. doi: 10.1200/JCO.20.02015. [DOI] [PubMed] [Google Scholar]
- 5.Denis F, Basch E, Septans A-L, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA. 2019;321(3):306–307. doi: 10.1001/jama.2018.18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Steffens D, Koh CE, Young JM, Solomon MJ. Differences in surgical outcomes and quality of life outcomes in pelvic exenteration between locally advanced versus locally recurrent rectal cancer. Dis Colon Rectum. 2022;65(12):1475–1482. doi: 10.1097/DCR.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 8.Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14(2):447–454. doi: 10.1245/s10434-006-9256-9. [DOI] [PubMed] [Google Scholar]
- 9.Hagemans JAW, van Rees JM, Alberda WJ, et al. Locally recurrent rectal cancer; long-term outcome of curative surgical and non-surgical treatment of 447 consecutive patients in a tertiary referral centre. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2019:S0748-7983(0719)30917-30915 [DOI] [PubMed]
- 10.Westberg K, Palmer G, Hjern F, Johansson H, Holm T, Martling A. Management and prognosis of locally recurrent rectal cancer—a national population-based study. Eur J Surg Oncol : J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2018;44(1):100–107. doi: 10.1016/j.ejso.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 11.You YN, Roses RE, Chang GJ, et al. Multimodality salvage of recurrent disease after local excision for rectal cancer. Dis Colon Rectum. 2012;55(12):1213–1219. doi: 10.1097/DCR.0b013e318270837f. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal Dis. 2011;13(7):732–742. doi: 10.1111/j.1463-1318.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 13.Heriot AG, Byrne CM, Lee P, et al. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51(3):284–291. doi: 10.1007/s10350-007-9152-9. [DOI] [PubMed] [Google Scholar]
- 14.Harris CA, Solomon MJ, Heriot AG, et al. The outcomes and patterns of treatment failure after surgery for locally recurrent rectal cancer. Ann Surg. 2016;264(2):323–329. doi: 10.1097/SLA.0000000000001524. [DOI] [PubMed] [Google Scholar]
- 15.Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Thaysen HV, Jess P, Laurberg S. Health-related quality of life after surgery for primary advanced rectal cancer and recurrent rectal cancer—a review. Colorectal Dis Off J Assoc Coloproctol Great Br Irel. 2012;14:797–803. doi: 10.1111/j.1463-1318.2011.02668.x. [DOI] [PubMed] [Google Scholar]
- 17.Glyn T, Frizelle F. Quality of life outcomes in patients undergoing surgery for locally recurrent rectal cancer. Semin Colon Rectal Surg. 2020;31(3):100767. [Google Scholar]
- 18.Harji DP, Griffiths B, Velikova G, Sagar PM, Brown J. Systematic review of health-related quality of life issues in locally recurrent rectal cancer. J Surg Oncol. 2015;111:431–438. doi: 10.1002/jso.23832. [DOI] [PubMed] [Google Scholar]
- 19.Denys A, van Nieuwenhove Y, Van de putte D, et al. Patient-reported outcomes after pelvic exenteration for colorectal cancer: A systematic review. Colorectal Dis. 2021;24(4):353–368. doi: 10.1111/codi.16028. [DOI] [PubMed] [Google Scholar]
- 20.Collaborative TP. Palliative pelvic exenteration: a systematic review of patient-centered outcomes. Eur J Surg Oncol. 2019;45(10):1787–1795. doi: 10.1016/j.ejso.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 23.Mokkink LB, de Vet HCW, Prinsen CAC, et al. COSMIN risk of Bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1171–1179. doi: 10.1007/s11136-017-1765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terwee CB, Prinsen CAC, Chiarotto A, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res. 2018;27(5):1159–1170. doi: 10.1007/s11136-018-1829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, ed. 2nd Edition ed: Cochrane 2022; 2022: www.training.cochrane.org/handbook.
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.COSMIN. COSMIN Tools. 2021; https://www.cosmin.nl/tools/guideline-conducting-systematic-review-outcome-measures/?portfolioCats=19. Accessed 28.04.2021, 2021.
- 28.Terwee CB, Mokkink LB, Knol DL, Ostelo RWJG, Bouter LM, de Vet HCW. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res. 2012;21(4):651–657. doi: 10.1007/s11136-011-9960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GRADE Handbook. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013; https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 11/01/2022, 2022.
- 30.Mokkink LB, Terwee CB, Knol DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol. 2010;10:22. doi: 10.1186/1471-2288-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 33.You YN, Habiba H, Chang GJ, Rodriguez-bigas MA, Skibber JM. Prognostic value of quality of life and pain in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2011;18(4):989–996. doi: 10.1245/s10434-010-1218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westerduin E, Elfeki H, Frontali A, et al. Functional outcomes and quality of life after redo anastomosis in patients with rectal cancer—an international multicenter comparative cohort study. Dis Colon Rectum. 2021;64(7):822–832. doi: 10.1097/DCR.0000000000002025. [DOI] [PubMed] [Google Scholar]
- 35.Alahmadi R, Steffens D, Solomon MJ, Lee PJ, Austin KKS, Koh CE. Elderly patients have better quality of life but worse survival following pelvic exenteration: a 25-year single-center experience. Ann Surg Oncol. 2021;28:5226–5235. doi: 10.1245/s10434-021-09685-6. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy ASE, Solomon MJ, Koh CE, Firouzbakht A, Jackson SA, Steffens D. Quality of life and functional outcomes following pelvic exenteration and sacrectomy. Colorectal Dis. 2020;22(5):521–528. doi: 10.1111/codi.14925. [DOI] [PubMed] [Google Scholar]
- 37.van Ramshorst GH, Young JM, Solomon MJ. Complications and impact on quality of life of vertical rectus abdominis myocutaneous flaps for reconstruction in pelvic exenteration surgery. Dis Colon Rectum. 2020;63(9):1225–1233. doi: 10.1097/DCR.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 38.Denost Q, Solomon M, Tuech J-J, et al. International variation in managing locally advanced or recurrent rectal cancer: prospective benchmark analysis. Br J Surg. 2020;107(13):1846–1854. doi: 10.1002/bjs.11854. [DOI] [PubMed] [Google Scholar]
- 39.Smith T, O’Cathail SM, Silverman S, et al. Stereotactic body radiation therapy reirradiation for locally recurrent rectal cancer: outcomes and toxicity. Adv Radiat Oncol. 2020;5(6):1311–1319. doi: 10.1016/j.adro.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown KGM, Solomon MJ, Lau YC, Steffens D, Austin KKS, Lee PJ. Sciatic and femoral nerve resection during extended radical surgery for advanced pelvic tumours: long-term survival, functional, and quality-of-life outcomes. Ann Surg. 2021;273(5):982–988. doi: 10.1097/SLA.0000000000003390. [DOI] [PubMed] [Google Scholar]
- 41.Steffens D, Solomon MJ, Young JM, et al. Cohort study of long-term survival and quality of life following pelvic exenteration. BJS Open. 2018;2(5):328–335. doi: 10.1002/bjs5.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim S-YJ, Koh EC, Liu JH, Solomon SHM, Johnstone SHC. The price we pay for radical curative pelvic exenterations: prevalence and management of pain. Dis Colon Rectum. 2018;61(3):314–319. doi: 10.1097/DCR.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 43.Choy I, Young JM, Badgery-Parker T, et al. Baseline quality of life predicts pelvic exenteration outcome. ANZ J Surg. 2017;87(11):935–939. doi: 10.1111/ans.13419. [DOI] [PubMed] [Google Scholar]
- 44.Quyn JA, Solomon JM, Lee MP, Badgery-Parker MT, Masya ML, Young MJ. Palliative pelvic exenteration: clinical outcomes and quality of life. Dis Colon Rectum. 2016;59(11):1005–1010. doi: 10.1097/DCR.0000000000000679. [DOI] [PubMed] [Google Scholar]
- 45.Cameron MG, Kersten C, Vistad I, et al. Palliative pelvic radiotherapy for symptomatic rectal cancer – a prospective multicenter study. Acta Oncol. 2016;55(12):1400–1407. doi: 10.1080/0284186X.2016.1191666. [DOI] [PubMed] [Google Scholar]
- 46.Pellino G, Sciaudone G, Candilio G, Selvaggi F. Effect of surgery on health-related quality of life of patients with locally recurrent rectal cancer. Dis Colon Rectum. 2015;58(8):753–761. doi: 10.1097/DCR.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Ye X, Yang X, et al. Microwave ablation as palliative treatment of locally recurrent colorectal cancer. Indian J Cancer. 2015;52(Suppl 2):e61–63. doi: 10.4103/0019-509X.172515. [DOI] [PubMed] [Google Scholar]
- 48.Thaysen HV, Jess P, Rasmussen PC, Nielsen MB, Laurberg S. Health-related quality of life after surgery for advanced and recurrent rectal cancer—a nationwide prospective study. Colorectal Dis. 2014;16(7):O223–O233. doi: 10.1111/codi.12551. [DOI] [PubMed] [Google Scholar]
- 49.Beaton J, Carey S, Solomon MJ, Tan K-K, Young J. Preoperative body mass index, 30-day postoperative morbidity, length of stay and quality of life in patients undergoing pelvic exenteration surgery for recurrent and locally-advanced rectal cancer. Ann Coloproctol. 2014;30(2):83–87. doi: 10.3393/ac.2014.30.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pusceddu C, Sotgia B, Melis L, Fele RM, Meloni GB. Painful pelvic recurrence of rectal cancer: percutaneous radiofrequency ablation treatment. Abdom Imaging. 2013;38(6):1225–1233. doi: 10.1007/s00261-013-0012-x. [DOI] [PubMed] [Google Scholar]
- 51.Traa MJ, Orsini RG, Oudsten BLD, et al. Measuring the health-related quality of life and sexual functioning of patients with rectal cancer: does type of treatment matter? Int J Cancer. 2014;134(4):979–987. doi: 10.1002/ijc.28430. [DOI] [PubMed] [Google Scholar]
- 52.Holman FA, Martijnse IS, Traa MJ, et al. Dynamic article: vaginal and perineal reconstruction using rectus abdominis myocutaneous flap in surgery for locally advanced rectum carcinoma and locally recurrent rectum carcinoma. Dis Colon Rectum. 2013;56(2):175–185. doi: 10.1097/DCR.0b013e31827a267c. [DOI] [PubMed] [Google Scholar]
- 53.Brændengen M, Tveit KM, Bruheim K, Cvancarova M, Berglund Å, Glimelius B. Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: results from a randomized phase III study. Int J Radiat Oncol, Biol, Phys. 2011;81(4):1017–1024. doi: 10.1016/j.ijrobp.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Haapamäki MM, Pihlgren V, Lundberg O, Sandzén B, Rutegård J. Physical performance and quality of life after extended abdominoperineal excision of rectum and reconstruction of the pelvic floor with gluteus maximus flap. Dis Colon Rectum. 2011;54(1):101–106. doi: 10.1007/DCR.0b013e3181fce26e. [DOI] [PubMed] [Google Scholar]
- 55.Austin KKS, Young JM, Solomon MJ. Quality of life of survivors after pelvic exenteration for rectal cancer. Dis Colon Rectum. 2010;53(8):1121–1126. doi: 10.1007/DCR.0b013e3181e10c46. [DOI] [PubMed] [Google Scholar]
- 56.Zoucas E, Frederiksen S, Lydrup M-L, Månsson W, Gustafson P, Alberius P. Pelvic exenteration for advanced and recurrent malignancy. World J Surg. 2010;34(9):2177–2184. doi: 10.1007/s00268-010-0637-7. [DOI] [PubMed] [Google Scholar]
- 57.Palmer G, Martling A, Lagergren P, Cedermark B, Holm T. Quality of life after potentially curative treatment for locally advanced rectal cancer. Ann Surg Oncol. 2008;15(11):3109–3117. doi: 10.1245/s10434-008-0112-y. [DOI] [PubMed] [Google Scholar]
- 58.Miner TJ, Jaques DP, Paty PB, Guillem JG, Wong WD. Symptom control in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2003;10(1):72–79. doi: 10.1245/aso.2003.03.040. [DOI] [PubMed] [Google Scholar]
- 59.Mannaerts GHH, Rutten HJT, Martijn H, Hanssens PEJ, Wiggers T. Effects on functional outcome after IORT-containing multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2002;54(4):1082–1088. doi: 10.1016/s0360-3016(02)03012-2. [DOI] [PubMed] [Google Scholar]
- 60.Esnaola NF, Cantor SB, Johnson ML, et al. Pain and quality of life after treatment in patients with locally recurrent rectal cancer. J Clin Oncol : Off J Am Soc Clin Oncol. 2002;20(21):4361–4367. doi: 10.1200/JCO.2002.02.121. [DOI] [PubMed] [Google Scholar]
- 61.Camilleri-Brennan J, Steele RJC. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol (EJSO). 2001;27(4):349–353. doi: 10.1053/ejso.2001.1115. [DOI] [PubMed] [Google Scholar]
- 62.Mannaerts GHH, Schijven MP, Hendrikx A, Martijn H, Rutten HJT, Wiggers T. Urologic and sexual morbidity following multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Eur J Surg Oncol (EJSO). 2001;27(3):265–272. doi: 10.1053/ejso.2000.1099. [DOI] [PubMed] [Google Scholar]
- 63.Guren MG, Wiig JN, Dueland S, et al. Quality of life in patients with urinary diversion after operation for locally advanced rectal cancer. Eur J Surg Oncol (EJSO). 2001;27(7):645–651. doi: 10.1053/ejso.2001.1195. [DOI] [PubMed] [Google Scholar]
- 64.Trotter JM, Edis AJ, Blackwell JB, et al. Adjuvant VHF therapy in locally recurrent and primary unresectable rectal cancer. Australas Radiol. 1996;40(3):298–305. doi: 10.1111/j.1440-1673.1996.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 65.Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ (Clinical Research ed.) 1993;306(6880):752–755. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wanebo HJ, Gaker DL, Whitehill R, Morgan RF, Constable WC. Pelvic recurrence of rectal cancer. Options for curative resection. Ann Surg. 1987;205(5):482–495. doi: 10.1097/00000658-198705000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Life EQo. Quality of Life of Cancer Patients Translations. Questionnaireshttps://qol.eortc.org/questionnaire/eortc-qlq-c30/. Accessed 19.05.2022, 2022.
- 68.ePROVIDE™. Instrumentshttps://eprovide.mapi-trust.org/advanced-search. Accessed 19.05.2022, 2022.
- 69.Life EQo. Colorectal. Questionnaireshttps://qol.eortc.org/questionnaire/qlq-cr29/. Accessed 19.05.2022, 2022.
- 70.FACIT. FACT-C Languages. 2021; https://www.facit.org/measure-languages/FACT-C-Languages. Accessed 19.05.2022, 2022.
- 71.ESCP. The LARS Score - Therese Juul https://www.escp.eu.com/news/focus-on/beyond-colorectal-cancer/1579-lars-score. Accessed 19.09.2022, 2022.
- 72.RAND. 36-Item Short Form Survey (SF-36). RAND Medical Outcomes Study 2022; https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html. Accessed 19.05.2022, 2022.
- 73.EuroQol. EQ-5D-5L / Self-complete version on paper. EQ-5D-5L 2022; https://euroqol.org/eq-5d-instruments/eq-5d-5l-available-modes-of-administration/self-complete-on-paper/. Accessed 19.05.2022, 2022.
- 74.Life AAoQo. AQoL Available Languages. 2014; http://www.aqol.com.au/index.php/aqol-translations. Accessed 19.05.2022, 2022.
- 75.Center MAC. The Brief Pain Inventory. Symptom Assessment Tools 2022; https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Accessed 19.05.2022, 2022.
- 76.Efficace F, Fayers P, Pusic A, et al. Quality of patient-reported outcome reporting across cancer randomized controlled trials according to the CONSORT patient-reported outcome extension: a pooled analysis of 557 trials. Cancer. 2015;121(18):3335–3342. doi: 10.1002/cncr.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harji DP, Koh C, Solomon M, Velikova G, Sagar PM, Brown J. Development of a conceptual framework of health-related quality of life in locally recurrent rectal cancer. Colorectal Dis : Off J Assoc Coloproctol Great Br Irel. 2015;17(11):954–964. doi: 10.1111/codi.12944. [DOI] [PubMed] [Google Scholar]
- 78.Ward W, Hahn E, Mo F, Hernandez L, Tulsky D, Cella D. Reliability and validity of the functional assessment of cancer therapy-colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8(3):181–195. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- 79.Whistance RN, Conroy T, Chie W, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer. 2009;45(17):3017–3026. doi: 10.1016/j.ejca.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 80.Collaborative TP. Factors affecting outcomes following pelvic exenteration for locally recurrent rectal cancer. J Br Surg . 2018;105(6):650–657. doi: 10.1002/bjs.10734. [DOI] [PubMed] [Google Scholar]
- 81.Pfennings LEMA, van der Ploeg HM, Cohen L, Polman CH. A comparison of responsiveness indices in multiple sclerosis patients. Qual Life Res. 1999;8(6):481–489. doi: 10.1023/a:1008971904852. [DOI] [PubMed] [Google Scholar]
- 82.Harji DP. The Development and Validation of a Patient-Reported Outcome Measure of Health-Related Quality of Life in Locally Recurrent Rectal Cancer, University of Leeds; 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.