Abstract
Adjuvant chemotherapy recommendations for ER+/HER2− early-stage breast cancers (eBC) involve integrating prognostic and predictive information which rely on physician judgment; this can lead to discordant recommendations. In this study we aim to evaluate whether Oncotype DX improves confidence and agreement among oncologists in adjuvant chemotherapy recommendations. We randomly select 30 patients with ER+/HER2− eBC and recurrence score (RS) available from an institutional database. We ask 16 breast oncologists with varying years of clinical practice in Italy and the US to provide recommendation for the addition of chemotherapy to endocrine therapy and their degree of confidence in the recommendation twice; first, based on clinicopathologic features only (pre-RS), and then with RS result (post-RS). Pre-RS, the average rate of chemotherapy recommendation is 50.8% and is higher among junior (62% vs 44%; p < 0.001), but similar by country. Oncologists are uncertain in 39% of cases and recommendations are discordant in 27% of cases (interobserver agreement K 0.47). Post-RS, 30% of physicians change recommendation, uncertainty in recommendation decreases to 5.6%, and discordance decreases to 7% (interobserver agreement K 0.85). Interpretation of clinicopathologic features alone to recommend adjuvant chemotherapy results in 1 out of 4 discordant recommendations and relatively high physician uncertainty. Oncotype DX results decrease discordancy to 1 out of 15, and reduce physician uncertainty. Genomic assay results reduce subjectivity in adjuvant chemotherapy recommendations for ER +/HER2− eBC.
Subject terms: Breast cancer, Breast cancer
Introduction
Adjuvant therapy decision-making for patients with early breast cancer is based on several considerations, including estimation of risk of recurrence, expected benefit from various components of treatment, patients’ preferences, and probabilities of short- and long-term toxicities.
Among women with estrogen receptor-positive/human epidermal growth factor receptor 2-negative (ER+/HER2−) early breast cancer, adjuvant endocrine therapy is highly effective1. The addition of chemotherapy has also demonstrated a reduction in the risk of recurrence and death in selected patients2,3 and, more recently, the CDK4/6 inhibitor abemaciclib has been shown to further reduce the risk of recurrence in high-risk patients4. In modern oncology one size does not fit all and optimal treatment tailoring to minimize both overtreatment and undertreatment without compromising survival rate has been (and still is) a major focus of research.
Advances in our understanding of the molecular biology of breast cancer have led to the development of genomic assays that help identify patients who can be safely spared adjuvant chemotherapy5. Among genomic assays, the 21-gene recurrence score (RS) assay (Oncotype DX) is one of the most widely used. Oncotype DX provides prognostic information independent of clinicopathologic features and predicts chemotherapy benefit in patients with node-negative and node-positive (1–3 nodes) ER+/HER2− early breast cancer6–8. The clinical utility of Oncotype DX has been prospectively evaluated in the large West German Study Group PlanB, TAILORx, and RxPONDER randomized clinical trials9–11, achieving a level of evidence and a category of recommendation of IA3,12. More recently, an effort to provide an estimation of the absolute chemotherapy benefit expected in individual patients led to the development of the RSClin tool that combines prognostic information from tumor size, grade and age with RS to provide a more accurate prognostic risk estimate13. Multiple studies showed that the use of the Oncotype DX assay results leads to changes in treatment recommendations by physicians leading to decrease in adjuvant chemotherapy prescription14–16. The use of the assay is cost-effective under most circumstances17, and it increases physician and patient confidence in treatment recommendations18,19. It is usually implied that genomic assays also reduce unwarranted subjectivity in treatment recommendations. However, to what extent RS results impact concordance of physician recommendations has not been studied in the past.
The goal of this study is to use real-life case histories to assess how physician confidence in adjuvant chemotherapy recommendation and concordance in the recommendations change by the RS results, and if physicians’ years of experience or country of practice have an effect on RS interpretation.
Results
Among 30 patients included in the analysis, median age was 50.5 years (range 30–75) and 40% were premenopausal. Most patients (83%) had invasive ductal carcinoma; half had a primary tumor >20 mm in size (pT2); 27% had grade 3 tumors, 90% a Ki67 level >20%, and 40% a PgR expression ≤20% (Table 1).
Table 1.
Patient and disease baseline characteristics.
| Characteristic | Overall | Recurrence score categories | |
|---|---|---|---|
| 0–25 (%) | 26–100 (%) | ||
| Age | |||
| Median - yr | 50.5 | ||
| Range -yr | 30–75 | ||
| Menopausal status | |||
| Pre-/perimenopausal | 12 (40) | 7 (58) | 5 (42) |
| Postmenopausal | 18 (60) | 13 (72) | 5 (28) |
| Histology | |||
| Ductal | 25 (83) | 16 (64) | 9 (36) |
| Lobular | 4 (13) | 3 (75) | 1 (25) |
| Mucinous | 1 (4) | 1 (100) | 0 |
| Primary tumor | |||
| T1c | 15 (50) | 9 (60) | 6 (40) |
| T2 | 15 (50) | 11 (73) | 4 (27) |
| Grade | |||
| G2 | 22 (73) | 17 (77) | 5 (23) |
| G3 | 8 (27) | 3 (37) | 5 (63) |
| Ki67 | |||
| ≤20 | 3 (10) | 2 (67) | 1 (33) |
| 21–30 | 19 (63) | 15 (79) | 4 (21) |
| >30 | 8 (27) | 3 (37) | 5 (63) |
| PgR | |||
| ≤20 | 12 (40) | 6 (50) | 6 (50) |
| >20 | 18 (60) | 14 (78) | 4 (22) |
Based on the clinicopathologic characteristics only, the average rate of chemotherapy recommendation was 51% (range 26.7–76.7%). Chemotherapy prescription rate was higher among Junior than Senior physicians (62% vs 44%; p < 0.001; chi-square test), but similar by country (US 50%, Italy 51%). Comparing pre- and post-RS chemotherapy recommendations by the same physician, overall 30% of recommendations (142 of 480) were changed by the assay result, with no significant differences by experience or country (28% vs 33% for Junior and Senior, respectively; 27% vs 33% for Italy and US, respectively) (Supplementary Fig. 1). Across the group of physicians, treatment recommendations for individual patients changed from 6 to 15 of the 30 patients that were assessed. In 20% of cases (94 of 480), the change in treatment led to chemotherapy omission, with a range of 1–11 out of 30 recommendations across oncologists. In 10% (48 of 480), the change led to the addition of chemotherapy to adjuvant endocrine therapy, with a range of 0–7 out of 30 recommendations given by each oncologist.
Confidence
Oncologists providing their recommendations pre-RS results were uncertain, fairly certain, and absolutely certain in 39%, 44%, and 17% of the cases, respectively. Uncertainty was significantly higher among US oncologists than among Italian oncologists (48% vs 32%, p 0.0008; chi-square test), while there were no significant differences between Junior and Senior (36% vs 41%, p 0.28) (Fig. 1).
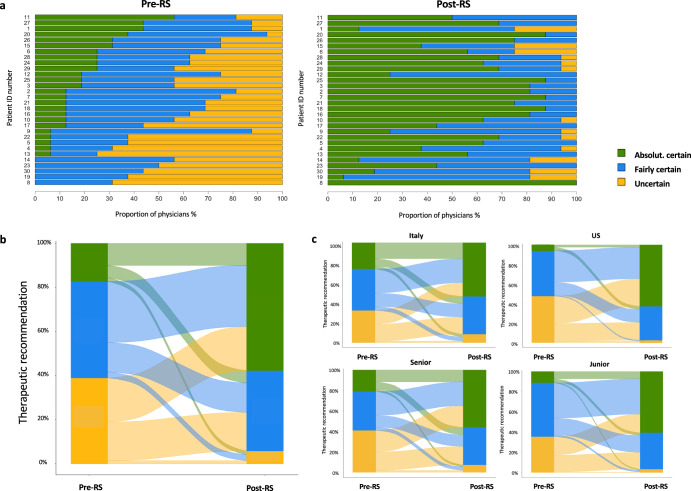
Fig. 1. Change of confidence in the therapeutic decision after RS.
a Confidence in the therapeutic decision pre- and post- RS results for each case. b Change of confidence in therapeutic recommendations pre- and post- RS results in the overall population. c Change of confidence in therapeutic recommendations pre- and post- RS results by oncologist country and experience. In (b and c), Y axes indicate the degree of confidence for all the therapeutic recommendations provided by each oncologist for each patient. Alluvial plots describe the change of confidence for each individual therapeutic recommendation pre- and post- RS in the overall population (b) or by oncologist country and experience (c).
Oncologists providing their recommendations post-RS were uncertain, fairly certain, and absolutely certain in 6%, 36%, and 58% of the cases, respectively. Uncertainty was significantly higher among Italian oncologists than among US oncologists (9% vs 2%, p 0.008; chi-square test), while numerical differences between Senior and Junior were not significant (7% vs 3%, p 0.11; chi-square test) (Fig. 1). Overall, 82% and 79% of the post-RS recommendations given with uncertainty were provided by Italian and Senior oncologists, respectively.
Post-RS, confidence increased and decreased in 65% and 10%, respectively (p < 0.001; chi-square test). The confidence increase was significantly higher among US oncologists compared to Italian oncologists (79% US vs 55% Italy, p < 0.00001; chi-square test), while no significant differences in the confidence increase were observed between Senior and Junior (Supplementary Fig. 2). Confidence decrease was almost confined to Italian oncologists (14% Italian vs 4% US). Interestingly, in 11.5% of the cases the recommendation pre-RS was given with absolute/fair certainty, and— after getting RS result—the recommendation was changed again with absolute/fair certainty.
Agreement
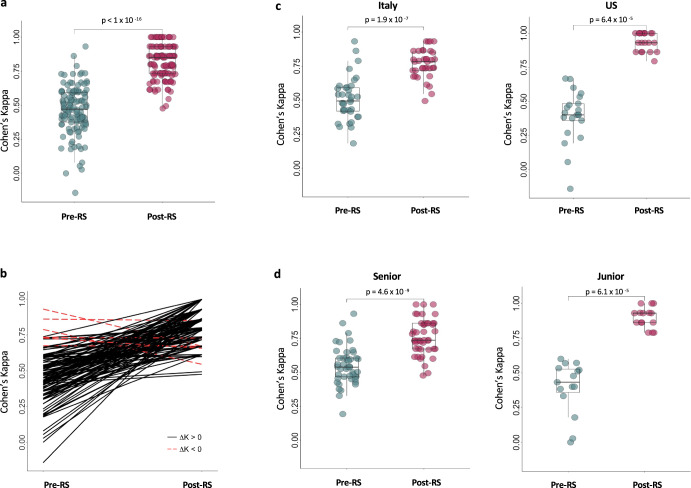
The interobserver agreement on chemotherapy recommendation pre-RS was only moderate (K 0.47, range −0.14–0.93; FK 0.46), corresponding to 27% (53.3–3.3%) discordant recommendations. Pre-RS agreement was significantly lower among Junior compared to Senior (Junior K 0.39 vs Senior K 0.54; Wilcoxon p value: 0.008) and among US oncologists compared to Italian (US K 0.39 vs Italian K 0.51; Wilcoxon p value: 0.04) (Fig. 2). Post-RS, the interobserver agreement was near perfect (K 0.85, range 0.47–1.00), corresponding to only 7% (26.7–0%) discordant recommendations. Post-RS agreement was significantly higher in US (US K 0.94 vs Italian K 0.77; Wilcoxon p value < 0.001) and Junior oncologists (Junior K 0.90 vs Senior K 0.76; Wilcoxon p value < 0.001) (Fig. 2). The intraobserver agreement pre- and post-RS was only fair (K 0.40; range 0.05–0.60). Results of agreement comparison using Fleiss’ kappa were similar.
Fig. 2. Overall agreement in the therapeutic indication given pre- and post-RS.
a Cohen’s kappa coefficients for pairwise inter-observer agreement on therapeutic recommendation pre- and post-RS result. b Change in Cohen’s kappa pre- and post-RS results for each pairwise comparison. c, d Cohen’s kappa coefficients for pairwise inter-observer agreement pre- and post-RS result by oncologist country (c) and experience (d). In (a, c and d), each dot represents the result of a pairwise comparison between two oncologists for every patient. The p values (Wilcoxon test) refer to the comparison between the overall agreement pre-RS and post-RS. The horizontal lines in the boxes denote the first quartile, median, and third quartile. The boundaries of the whiskers are based on the 1.5 × interquartile values.
An illustrative case
A few cases accounted for the highest discrepancy and uncertainty. Among these, one offers the opportunity to investigate potential clinicopathologic characteristics associated with uncertainty and discordancy. Patient number 19 was a 41-year-old premenopausal woman diagnosed with a node-negative invasive ductal carcinoma of 2.1 cm. Biological characteristics were: Grade 2, ER 90%, PgR 80%, Ki67 26%. Before knowing the RS result, oncologists split into two groups: half (4 Italy, 3 Junior and 1 Senior; 4 US, 2 Junior and 2 Senior) recommended the addition of chemotherapy to adjuvant endocrine therapy, the other half recommended endocrine therapy alone. In providing these recommendations, 63% of the oncologists were uncertain (5 Italy, 1 Junior and 4 Senior; 5 US, 2 Junior and 3 Senior) and 37% were fairly certain. No one was absolutely certain. After knowing the RS result of 24, most of the oncologists (88%) recommended the addition of chemotherapy to adjuvant endocrine therapy and uncertainty decreased to 19% (3 Senior, 2 Italy and 1 US). Among the 8 oncologists who changed their recommendation, the confidence increased in 4 cases and remained stable in 4.
Discussion
Decisions around adding chemotherapy to adjuvant endocrine therapy in patients with ER+/HER2− early breast cancer can be challenging.
While recommending endocrine therapy is straightforward for ER positive cancers, there are no clinicopathologic features clearly and independently predictive of chemotherapy sensitivity. Hence, physician recommendations to add adjuvant chemotherapy to endocrine therapy, or not, is based on subjective estimates of risk of distant recurrence and presumptions about chemotherapy responsiveness.
Due to the subjective weighting of clinicopathologic features that collectively determine risk of recurrence, different oncologists often provide different adjuvant chemotherapy recommendations for the same cases, and are also well aware of the uncertainty in their decisions. Moreover, some of the clinicopathologic features are subject to technical reproducibility issues, intralaboratory and interlaboratory, increasing the uncertainty for chemotherapy recommendations. Guidelines on the management of early breast cancer provide general recommendations and guidance, but allow room for physician judgement and patient preference. This is especially true for cases with controversial clinicopathologic characteristics that may fall in a “gray zone” for chemotherapy recommendations.
Genomic signatures such as Oncotype DX, despite being affected by reproducibility issues related to intratumor heterogeneity, provide complementary information to clinicopathologic prognostic variables and may aid clinicians in more accurately identifying patients with good outcomes for whom chemotherapy can be safely omitted13. Indeed, a plethora of studies demonstrated that the use of genomic signatures can lead to a decrease of chemotherapy recommendation in up to 50% of cases19–28, and some studies showed that physicians’ confidence in treatment recommendations may improve with the use of these signatures19–21,23.
Discordant adjuvant chemotherapy recommendations for the same case by different physicians is commonly encountered in routine practice and is well documented in the literature. This is distressing for patients, generally undermines trust in the health care system and results in unwarranted variance in practice. Undoubtedly, it also results in under- and overtreatment of some patients. It is assumed by practice guidelines that the use of genomic assays would reduce heterogeneity in practice and variation in treatment recommendations. However, how genomic test results affect concordance in physician treatment recommendations has not been studied. We show that Oncotype DX assay results significantly decreased discordant adjuvant chemotherapy recommendations for real cases even among a group of academic breast cancer experts. In the absence of the RS result, on average 1 out of 4 patients received discordant recommendations, while discordant recommendations were observed in only 1 out of 15 patients with RS available.
Of note, all participating oncologists in the study belonged to tertiary institutions for breast cancer treatment, and with RS results the interobserver agreement significantly increased irrespective of oncologists’ country and experience.
Our study also showed that the use of Oncotype DX significantly reduced the degree of physicians’ uncertainty about the role of adjuvant chemotherapy. Pre-RS, the degree of confidence was lower among US oncologists compared to Italian ones, but post-RS we observed exactly the opposite: uncertainty was lower among US oncologists and was almost confined to Italian ones. This indicates that US physicians had greater “trust” in the RS result than their Italian counterparts. This may be due to greater availability of the assay and larger number of studies conducted with Oncotype DX in the USA. Oncotype DX has been commercially available in US since 2004, and its use among US oncologists has progressively increased29. In Italy, Oncotype DX and other genomic tests are only being reimbursed by the National Health Service since May 202130, and their use at a national level is currently quite scattered.
Notably, we showed that the use of Oncotype DX significantly increased confidence and agreement among Junior oncologists. In this group, the agreement pre-RS was only fair (FK 0.39) and significantly lower compared to Senior. Post-RS, the agreement was near perfect (FK 0.93). These data suggest that in less experienced oncologists the role of Oncotype DX in aiding decision making may be even more important.
The illustrative clinical case we presented offers the opportunity to investigate the clinicopathologic characteristics associated with higher uncertainty and discordancy. Young age, premenopausal status and controversial biological characteristics (high hormone receptors expression and intermediate/high proliferation) had split the participating oncologists exactly in half when asked for the addition or not of adjuvant chemotherapy, and none of them provided the recommendation with absolute certainty. After knowing the RS result, 88% of the oncologists converged to the addition of adjuvant chemotherapy and only 3 remained uncertain in providing their recommendation. This example points out the uncertainties around the optimal adjuvant therapy in young women with low genomic risk, where some of the benefit observed with chemotherapy might be due to its endocrine effect of ovarian function suppression, and highlights the importance of the careful interpretation of genomic test results within the scope of a comprehensive evaluation that includes also the clinicopathologic variables.
The most peculiar and novel element of our study is that Oncotype Dx, besides its recognized and established role in fine-tuning treatment recommendations, reduces the differences in treatment choice among oncologists, who often give discordant recommendations in the absence of a genomic test. This aspect is highly valuable, as it guarantees homogeneous treatments to patients across different institutions. Such a result is even more valuable in an era in which patients may ask for a second opinion. One could argue that tools other than oncotype, such as online available risk calculators, might be also useful in reducing discordancy in adjuvant therapy recommendations if widely used by clinicians. However, since none of these tools are recommended by Guidelines to tailor adjuvant therapy decisions, they are not widely employed in clinical practice and no studies have investigated their role in this context.
In conclusion, we showed that Oncotype DX significantly increased physicians’ confidence in adjuvant chemotherapy recommendations and agreement among oncologists in providing adiuvant treatment recommendations for patients with ER+/HER2− early breast cancer. In our opinion, these are two highly relevant and underappreciated benefits of using genomic tests in routine practice. These results add to a large body of literature that supports the use of genomic assays to determine adjuvant chemotherapy use and encourage a broader implementation of these assays in clinical practice.
Materials and methods
Clinical cases
The study used real-world data retrieved from the institutional database of San Raffaele Hospital in Milan, Italy. Among patients with ER+/HER2− early breast cancer who underwent breast surgery at our institution, we identified those with stage pT1c-2, node-negative, grade 2/3, Ki67 of at least 15%, and RS available. Among patients with the above characteristics, we randomly selected 30 cases.
Procedures
Within our network of collaborating colleagues, we identified 16 breast oncologists with different years of clinical practice experience in Italy and US: 10 seniors, defined as oncologists with at least 15 years of experience (Italy, n = 6; US, n = 4), and 6 juniors, defined as oncologists within 2 years from the end of fellowship (Italy, n = 3; US, n = 3). Each participant was contacted by email and received a file containing the clinicopathologic features of the 30 patients: age, menopausal status, tumor histotype, tumor size, grade, ER, PgR and Ki67 levels (Supplementary Table 1). The study was conducted between January and March 2020.
Participants had to provide recommendations if adjuvant chemotherapy was indicated or not, twice; first, based on clinicopathologic features only (pre-RS), and then (about 2 weeks later) with the RS results available (post-RS). Each pre- and post-RS adjuvant chemotherapy recommendation had to be annotated with three levels of confidence; absolutely certain, fairly certain, or uncertain.
Chemotherapy recommendation pre-RS was given according to ESMO/ASCO guidelines (depending on country); participants could use (but this was not mandatory) a clinical risk score calculator to help in decision making.
Study objectives
The primary objective of the study was to assess both oncologists’ degree of confidence and intra- and inter-oncologist agreement in adjuvant chemotherapy treatment recommendation pre- and post-RS.
Statistics
Descriptive statistics were used to summarize patient and tumor characteristics. Each patient was assigned an identification (ID) number. McNemar’s test was used to assess whether the proportion of patients for whom chemotherapy was recommended changed from pre- to post‐RS. Intra- and inter-observer agreement was assessed by Cohen’s kappa (K) in all possible pairwise comparisons between two oncologists, the Fleiss’ kappa (FK) agreement was used to measure overall agreement in recommendations pre- and post-RS.
Ethics
The study was conducted in accordance with the Declaration of Helsinki as revised in 2013. All participants signed informed consent to allow use of routine surgical pathology specimens for Oncotype DX testing. The 30 patients included in this study were randomly selected among the cohort of patients participating at the observational PONDx study31. The protocol was approved by the Institutional Review Board of San Raffaele Hospital.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors received support from the Associazione Italiana per la Ricerca sul Cancro (IG2018eID21787 project grant to G.B.) and Fondazione Michelangelo (grants to G.B.).
Author contributions
Concept and design: L.L., G.V., G.B. Acquisition, analysis, or interpretation of data: L.L., G.V., M.G., G.C., M.C.M., J.F., O.O., J.C., L.D.M., F.P., F.M., C.V., L.G., S.G., A.R., L.S., O.D.G., S.C., L.P., A.G., C.C., G.B. Drafting of the paper: L.L., G.V., A.G., C.C., G.B. Critical revision of the paper for important intellectual content: L.L., G.V., M.G., G.C., M.C.M., J.F., O.O., J.C., L.D.M., F.P., F.M., C.V., L.G., S.G., A.R., L.S., O.D.G., S.C., L.P., A.G., C.C., G.B. Statistical analysis: L.L., G.V., G.B. Final approval of the paper for submission: L.L., G.V., M.G., G.C., M.C.M., J.F., O.O., J.C., L.D.M., F.P., F.M., C.V., L.G., S.G., A.R., L.S., O.D.G., S.C., L.P., A.G., C.C., G.B. L.L. and G.V. contributed as co-first authors. A.G., C.C. and G.B. contributed as co-senior authors.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
L.L. has served on the advisory boards for: Lilly, Exact Sciences, AstraZeneca and Daiichi Sankyo; has received consulting fee from: Exact Sciences; honoraria for speakers’ bureaus from: Gilead, Exact Sciences and EISAI; support for travel, accommodations, expenses from: Lilly and Gilead. G.V. has served on the advisory boards for Gilead; has received honoraria for speakers’ bureaus from Novartis, Lilly; support for travel, accommodations, expenses from: Lilly and Pfizer. M.G. has served on the advisory boards for AstraZeneca, Daichii Sankyo, Exact Sciences, Lilly, MSD, Novartis, Pfizer, Roche, Seagen; has received travel grants from Roche, Celgene, Pfizer and research funding (to the institution) from Novartis and AstraZeneca. G.C. has received honoraria for speaker’s engagement from: Roche, Seagen, Novartis, Lilly, Pfizer, BMS, Merck; honoraria for providing consultancy from: Roche, Seagen; honoraria for participating in Advisory Board: Roche, Lilly, Pfizer, Foundation Medicine, Seagen, Novartis, Astra Zeneca, Daichii Sankyo; honoraria for writing engagement from: Novartis, BMS; honoraria for participation in Ellipsis Scientific Affairs Group; Institutional research funding for conducting phase I and II clinical trials: Pfizer, Roche, Novartis, Sanofi, Celgene, Servier, Orion, AstraZeneca, Seattle Genetics, AbbVie, Tesaro, BMS, Merck Serono, Merck Sharp Dome, Janssen-Cilag, Philogen, Bayer, Medivation, Medimmune. M.C.M. has received consulting fee from: Pfizer, Genentech, Astra Zeneca, Lilly, Novartis, Exact Sciences. L.D.M. has received personal fees from Eli Lilly, Novartis, Roche, MSD, Pfizer, Exact Sciences, Pierre Fabre, Daiichi Sankyo, Astra zeneca, Seagen, Eisai, Ipsen and Gilead, F.P. has received honoraria for speakers’ bureaus/consultancy/advisory board from: Amgen, Exact Sciences, Pierre-Fabre, Gilead, Pfizer, Celgene, GSK, Daiichi Sankyo, Ipsen, Seagen, Takeda, Eli Lilly, MSD, Novartis, AstraZeneca, Roche, Eisai, Viatris; research funding from AstraZeneca, Roche, Eisai. F.M. has received personal fees from Novartis, Astra Zeneca, Daiichi Sankyo, Seagen, MSD, Pfizer, Roche and PUMA. C.V. has served on the advisory boards for Novartis; has received travel grants from: Lilly, Novartis, Istituto Gentili, Roche, Pfizer; research grants from Roche. L.P. has received consulting fees and honoraria from Pfizer, Astra Zeneca, Merck, Novartis, Bristol-Myers Squibb, Genentech, Eisai, Pieris, Immunomedics, Seattle Genetics, Clovis, Syndax, H3Bio, and Daiichi. A.G. has served on the advisory boards for Pfizer. C.C. has received honoraria for speakers’ bureaus/consultancy/advisory board from: Pfizer, Roche, Novartis, Lilly, MSD, Seagen, Gilead, Daiichi Sankyo, AstraZeneca. G.B. has received consulting fee from Roche, AstraZeneca, Novartis, MSD, Sanofi, Daiichi Sankyo, and Exact Sciences; honoraria for speakers’ bureaus from Roche, Pfizer, Astra- Zeneca, Lilly, Novartis, Neopharm Israel, MSD, Chugai, Daiichi Sankyo, EISAI, and Exact Sciences; support for travel, accommodations, expenses from Roche, Pfizer, and AstraZeneca; is co-inventor of ‘European patent Application N. 12195182.6 and 12196177.5 titled “PDL-1 expression in anti-HER2 therapy” -Roche- Issued (no compensation provided); has served on the advisory boards for Pfizer, Roche, Daiichi Sankyo, Lilly, MSD, Novartis, AstraZeneca, Genomic Health, EISAI, Gilead, Seagen.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Luca Licata, Giulia Viale.
These authors jointly supervised this work: Antonio Giordano, Carmen Criscitiello, Giampaolo Bianchini.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-023-00559-6.
References
- 1.Group, E. B. C. T. C. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (Lond. Engl.) 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Henry NL, et al. Role of Patient and Disease Factors in Adjuvant Systemic Therapy Decision Making for Early-Stage, Operable Breast Cancer: Update of the ASCO Endorsement of the Cancer Care Ontario Guideline. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2019;37:1965–1977. doi: 10.1200/JCO.19.00948. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 4.Harbeck N, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021;32:1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 6.Paik S, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitz U, et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res. Treat. 2017;165:573–583. doi: 10.1007/s10549-017-4358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparano JA, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalinsky K, et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N. Engl. J. Med. 2021;385:2336–2347. doi: 10.1056/NEJMoa2108873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andre F, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: ASCO Clinical Practice Guideline Update-Integration of Results From TAILORx. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2019;37:1956–1964. doi: 10.1200/JCO.19.00945. [DOI] [PubMed] [Google Scholar]
- 13.Sparano JA, et al. Development and Validation of a Tool Integrating the 21-Gene Recurrence Score and Clinical-Pathological Features to Individualize Prognosis and Prediction of Chemotherapy Benefit in Early Breast Cancer. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2021;39:557–564. doi: 10.1200/JCO.20.03007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurian AW, et al. Recent Trends in Chemotherapy Use and Oncologists’ Treatment Recommendations for Early-Stage Breast Cancer. J. Natl Cancer Inst. 2018;110:493–500. doi: 10.1093/jnci/djx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly CM, et al. Utility of oncotype DX risk estimates in clinically intermediate risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer. 2010;116:5161–5167. doi: 10.1002/cncr.25269. [DOI] [PubMed] [Google Scholar]
- 16.Dzimitrowicz H, et al. Impacts of Early Guideline-Directed 21-Gene Recurrence Score Testing on Adjuvant Therapy Decision Making. J. Oncol. Pr. 2017;13:e1012–e1020. doi: 10.1200/JOP.2017.022731. [DOI] [PubMed] [Google Scholar]
- 17.Wang SY, et al. Cost-Effectiveness Analyses of the 21-Gene Assay in Breast Cancer: Systematic Review and Critical Appraisal. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2018;36:1619–1627. doi: 10.1200/JCO.2017.76.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gligorov J, et al. Prospective Clinical Utility Study of the Use of the 21-Gene Assay in Adjuvant Clinical Decision Making in Women With Estrogen Receptor-Positive Early Invasive Breast Cancer: Results From the SWITCH Study. Oncologist. 2015;20:873–879. doi: 10.1634/theoncologist.2014-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albanell J, et al. Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur. J. Cancer. 2016;66:104–113. doi: 10.1016/j.ejca.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Soliman H, et al. MammaPrint guides treatment decisions in breast Cancer: results of the IMPACt trial. BMC Cancer. 2020;20:81. doi: 10.1186/s12885-020-6534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martín M, et al. Prospective study of the impact of the Prosigna assay on adjuvant clinical decision-making in unselected patients with estrogen receptor positive, human epidermal growth factor receptor negative, node negative early-stage breast cancer. Curr. Med. Res. Opin. 2015;31:1129–1137. doi: 10.1185/03007995.2015.1037730. [DOI] [PubMed] [Google Scholar]
- 22.Levine MN, et al. Prospective Evaluation of the 21-Gene Recurrence Score Assay for Breast Cancer Decision-Making in Ontario. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2016;34:1065–1071. doi: 10.1200/JCO.2015.62.8503. [DOI] [PubMed] [Google Scholar]
- 23.Lo SS, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 24.Oratz R, et al. Physician survey of the effect of the 21-gene recurrence score assay results on treatment recommendations for patients with lymph node-positive, estrogen receptor-positive breast cancer. J. Oncol. Pr. 2011;7:94–99. doi: 10.1200/JOP.2010.000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dieci MV, et al. Impact of 21-Gene Breast Cancer Assay on Treatment Decision for Patients with T1-T3, N0-N1, Estrogen Receptor-Positive/Human Epidermal Growth Receptor 2-Negative Breast Cancer: Final Results of the Prospective Multicenter ROXANE Study. Oncologist. 2019;24:1424–1431. doi: 10.1634/theoncologist.2019-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuchel A, et al. The impact of the 21-gene assay on adjuvant treatment decisions in oestrogen receptor-positive early breast cancer: a prospective study. Br. J. Cancer. 2016;114:731–736. doi: 10.1038/bjc.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuerstlein R, et al. Strong impact of MammaPrint and BluePrint on treatment decisions in luminal early breast cancer: results of the WSG-PRIMe study. Breast Cancer Res. Treat. 2019;175:389–399. doi: 10.1007/s10549-018-05075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penault-Llorca F, et al. Decision of adjuvant chemotherapy in intermediate risk luminal breast cancer patients: A prospective multicenter trial assessing the clinical and psychological impact of EndoPredict® (EpClin) use (UCBG 2-14) Breast (Edinb. Scotl.) 2020;49:132–140. doi: 10.1016/j.breast.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinan MA, et al. Initial Trends in the Use of the 21-Gene Recurrence Score Assay for Patients With Breast Cancer in the Medicare Population, 2005-2009. JAMA Oncol. 2015;1:158–166. doi: 10.1001/jamaoncol.2015.43. [DOI] [PubMed] [Google Scholar]
- 30.Salute, M. D. Modalita’ di riparto e requisiti di utilizzo del fondo per i test genomici ormonoresponsivo per il carcinoma mammario in stadio precoce., https://www.gazzettaufficiale.it/eli/id/2021/07/07/21A04069/sg (2021).
- 31.Cognetti F, et al. PONDx: real-life utilization and decision impact of the 21-gene assay on clinical practice in Italy. NPJ Breast Cancer. 2021;7:47. doi: 10.1038/s41523-021-00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.