Abstract
Background:
Doxorubicin is a widely used agent in the treatment of cancer, but the cardiotoxicity associated with this drug limits its potential for use. The cardioprotective effects of dapagliflozin, an antidiabetic drug, have the potential to counteract the cardiotoxic effect of doxorubicin therapy. In our study, we aimed to investigate the protective effect of dapagliflozin from possible doxorubicin-induced cardiotoxicity.
Methods:
A total of 40 male Wistar albino rats were divided into 4 groups consisting of 10 each (control = 10, dapagliflozin = 10, doxorubicin = 10, doxorubicin + dapagliflozin = 10). Meanwhile, doxorubicin and doxorubicin + dapagliflozin groups received a total dose of 15 mg/kg doxorubicin intraperitoneally, dapagliflozin and doxorubicin + dapagliflozin groups were gavaged daily with 10 mg/kg dapagliflozin. At the sixth week of the study, rats were examined by echocardiography and electrocardiogram. Furthermore, histopathological method was used to evaluate the level of cardiotoxicity.
Results:
Ejection fraction decreased by 15% in the doxorubicin group, and this reduction in ejection fraction was alleviated in the doxorubicin + dapagliflozin group. In addition, a 65% increase in QRS duration was observed in the group given doxorubicin, while an increase of 7% was observed in doxorubicin + dapagliflozin group. Corrected QT duration increased by 12% in the doxorubicin group, compared to 2% in doxorubicin + dapagliflozin group. Meanwhile, sarco-myolysis, inflammatory cell infiltration, and necrotic changes were examined heavily in doxorubicin group, they were minimal in doxorubicin+dapagliflozin group.
Conclusion:
Our study showed that dapagliflozin has the potential to reduce the effects of doxorubicin-induced cardiotoxicity.
Keywords: Doxorubicin, cardiotoxicity, dapaglifozin
Highlights
In our study, a 15% decrease in ejection fraction resulting from doxorubicin (DOX) cardiotoxicity and a significant increase in ejection fraction after our treatment with dapagliflozin (DAPA) were observed.
In our study, a 65% increase in QRS duration was observed in electrocardiogram resulting from DOX cardiotoxicity, while this increase was observed very minimally in our treatment group with DAPA.
In the tissue histology observed in our study, such as sarcomyolysis, inflammation cell infiltration, and necrotic changes as a result of cardiotoxicity of DOX, these changes were minimal in our DAPA treatment group.
During our study, a significant weight loss was observed, especially in our DOX and DOX + DAPA groups. It is essential to use DAPA, especially considering that it has a weight loss effect besides weight loss, which is one of the most important side effects of chemotherapy treatment.
Introduction
There has been a significant decrease in cancer-related mortality in recent years. Despite the development of new chemotherapeutic agents for cancer treatment, doxorubicin (DOX) is still one of the most widely used anticancer drugs.1-4 Doxorubicin is associated with anthracycline-induced cardiomyopathy (AIC) in up to 26% of patients and can progress to severe left ventricular (LV) failure. This form of AIC is generally considered irreversible, as 45% of chemotherapy patients who develop LV failure do not improve with standard heart failure treatment.5 Free oxygen radicals, membrane lipid peroxidation, and mitochondrial damage are considered responsible for DOX-induced cardiotoxicity.6,7 The chemical structure of DOX causes the formation of free radicals and increases oxidative stress, which results in cell damage.6,8 There are clinical predictors of AIC. Despite conventional heart failure treatment and AIC blocking agents such as dexrazoxane, AIC is still highly prevalent and associated with high mortality and morbidity.9 Therefore, there is a need for AIC preventive treatment strategies.
Dapagliflozin (DAPA), a sodium-glucose cotransporter 2 (SGLT2) inhibitor, is a class of glucose-lowering agents and is used to treat patients with type 2 diabetes. Besides reducing glucose reabsorption, DAPA has also shown protective effects on cardiovascular diseases. The DAPA-HF study demonstrated that DAPA reduced primary combined outcomes, including cardiovascular mortality and heart failure, in patients with type 2 diabetes.10 The cardioprotective effects of DAPA have been demonstrated in patients with diabetic cardiomyopathy, heart failure with preserved ejection fraction (EF), and heart failure with low EF.10,11 Dapagliflozin shows anti-oxidative properties by reducing cytosolic and mitochondrial free oxygen radical production and changing Ca2+ dynamics, thus exerting protective effects against cell damage under oxidative stress conditions.12,13
Theoretically, DAPA has the potential to inhibit DOX-induced AIC. This study aimed to compare the possible cardioprotective effects of DAPA on AIC in rats.
Methods
Animal Model
Forty male Wistar albino rats were purchased from Süleyman Demirel University Experimental Animals and Medical Research Practice and Research Center (Isparta, Turkey) and all of the animals were housed in the same place from starting of the experiment to end of the experiment. All animals were kept in the temperature-controlled experiment center (22-24) and on 12-h light/dark cycle. All of the animals were fed an ad libitum diet during the experiment. All animals were used according to Universal Declaration of Animal Rights and ethic was approved by the Süleyman Demirel University Animal Experiments Local Ethics Committee.
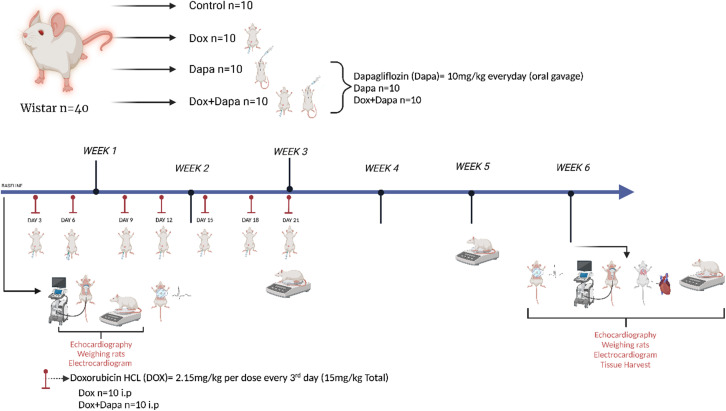
This study was organized according to a previously established model of cardiotoxicity study design. Rats were intraperitoneally applied DOX (2.15 mg/kg) every 3 days for 21 days (15 mg/kg total) (n = 20). Following the first dose of DOX, DAPA (n = 10) and DOX + DAPA (n = 10) groups were administered. Dapagliflozin (10 mg/kg in normal saline) daily by gavage for the duration of study. This dose of DAPA was decided according to the previous study which successfully managed DAPA in rats (Figure 1).
Figure 1.
Study design.
Experimental Design
Ejection fraction, fractional shortening (FS), diastolic, and systolic functions were evaluated with the 2-dimensional Philips Lumify echocardiography system with a 3.5-MHz transducer (Koninklijke Philips N.V. Amsterdam, Netherlands) in all animals at baseline (n = 40). All animals were evaluated before the first chemotherapy and DAPA dose. At 6 weeks following the beginning of the experiment, all animals’ heart functions were evaluated with 2-dimensional echocardiography along with histopathologic evaluation like inflammatory cell infiltration, sarcomyolysis, and necrotic findings.
Electrocardiogram
Electrocardiograph machine (Fukuda Denshi Co. Ltd, Tokyo, Japan) was used while performing electrocardiogram (ECG). Electrodes were located at the right wrist, sternum, right ankle, and left ankle of the anesthetized rats. Electrocardiogram was performed at baseline and at the end of 6 weeks.
Histopathological Examination
The heart tissues taken from animals were fixed with a 10% buffered formaldehyde solution. Before tissue processing, the samples were washed overnight with running tap water, dehydrated in graded ethanol, cleared with xylene, and embedded in paraffin. About 4-5 μm thick sections were taken from the paraffin blocks and stained with hematoxylin and eosin. Histopathological examination and scoring were performed with a light microscope. According to the degree of the findings, a modified semiquantitative scoring was performed as (0): no finding, (+1): low level of finding, (+2): moderate finding, (+3): severe finding.14
Statistical Analysis
Statistical analyses were performed with the Statistical Package for Social Sciences software, version 26.0 (IBM Inc., Armonk, NY). Descriptive statistics were presented as mean ± SD. The conformity of the measurements to the normal distribution was tested with Kolmogorov–Smirnov method. Since the data were normally distributed, 1-way analysis of variance (ANOVA) was used for comparisons between groups, and paired t-test was used for comparisons before and after treatment. Rats were weighed over 4 periods; hence, 2-way repeated measures ANOVA was used to compare intragroup and repeated measurements. Tukey’s HSD was chosen as the pairwise comparison test in multiple group analyses. Pairwise comparison results were shown using the same exponential letters for significant groups. The homogeneity of variances was checked with the mean-based Levene test. Interobserver agreement values for ECG measurements were calculated using the intraclass correlation coefficient (ICC). For a type I error value of 5%, P < .05 was considered statistically significant in all analyses.
Results
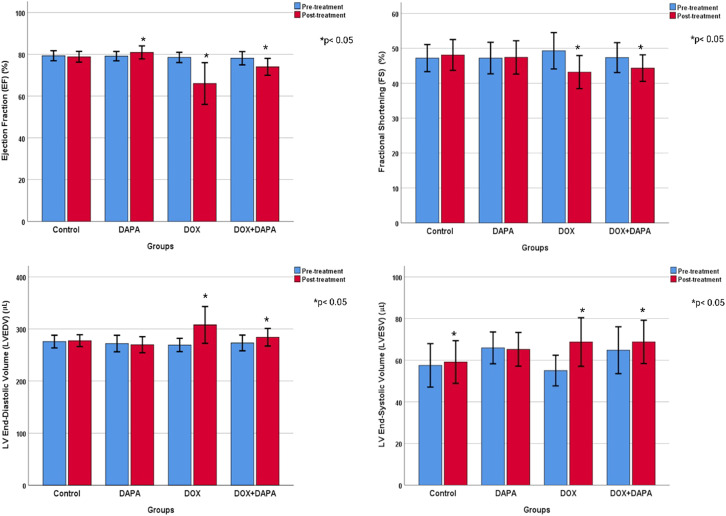
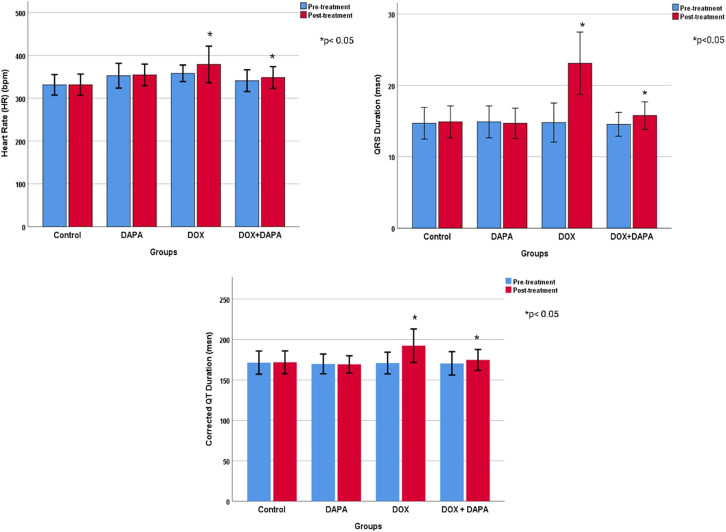
Cardiac measurements of animals were made by 2 observers. Interobserver agreement values were quite high (ICC > 0.932). Ejection fraction values were not different between study groups before treatment but differed significantly after treatment (P < .001). Ejection fraction values decreased significantly in the DOX group compared to the other groups (66.00 ± 9.98% vs. 78.80 ± 2.57%, 80.90 ± 3.10%, and 74.00 ± 4.03%, respectively). Fractional shortening before and after treatment did not differ significantly between the groups. Diastolic blood pressure (DBP) was not different before the treatment, but the mean value (307.70 ± 35.32 mm Hg) measured in the DOX group after the treatment was significantly higher than the control and DAPA groups (P = .003). Systolic blood pressure (SBP) (P = .019), on the other hand, was significantly lower in the DOX group before treatment but did not differ significantly between the groups after treatment. Heart rate values differed significantly after treatment (P = .014). The heart rate was the lowest in the control group and significantly higher in the DOX group (378.90 ± 42.82 beats/min) compared to the other groups. Corrected QT interval and PR waves did not differ significantly between the study groups. Yet, after the treatment, the QR wave was significantly elevated in the DOX group (23.10 ± 4.38) (P < .001) (Table 1).
Table 1.
Cardiac and ECG Measurements of Animals According to Study Groups
| Controla | DAPAb | DOXc | DAPA + DOXd | P groups | P measures | |
|---|---|---|---|---|---|---|
| First weight measurement, g | 272.00 ± 36.72 | 299.60 ± 16.95 | 287.90 ± 17.51 | 287.10 ± 17.40 | <.001*
a–c a–d |
<.001* |
| Second weight measurement, g | 301.40 ± 42.25 | 301.50 ± 15.23 | 273.40 ± 18.31 | 242.10 ± 21.08 | ||
| Third weight measurement, g | 338.20 ± 43.37 | 325.00 ± 21.53 | 280.00 ± 21.00 | 265.33 ± 23.21 | ||
| Fourth weight measurement, g | 338.10 ± 41.49 | 325.00 ± 22.45 | 275.50 ± 24.22 | 275.88 ± 21.24 | ||
| Ejection fraction, baseline, % | 79.30 ± 2.40 | 79.10 ± 2.23 | 78.50 ± 2.46 | 78.00 ± 3.01 | <.001*
a–c |
<.001* |
| Ejection fraction, week 6, % | 78.80 ± 2.57 | 80.90 ± 3.10 | 66.00 ± 9.98 | 74.00 ± 4.03 | ||
| Fractional shortening baseline, % | 47.20 ± 3.88 | 47.20 ± 4.51 | 49.30 ± 5.20 | 46.70 ± 4.49 | .764 | <.001* |
| Fractional shortening week 6, % | 48.10 ± 4.40 | 47.40 ± 4.76 | 43.20 ± 4.73 | 44.33 ± 3.80 | ||
| End-diastolic volume baseline, μL | 275.70 ± 12.18 | 271.90 ± 15.83 | 269.20 ± 12.73 | 278.80 ± 22.92 | .141 | <.001* |
| End-diastolic volume week 6, μL | 277.50 ± 11.22a | 269.65 ± 15.53b | 307.70 ± 35.32a.b | 284.11 ± 16.91 | ||
| End-systolic volume baseline, μL | 57.50 ± 10.42 | 65.90 ± 7.63 | 55.00 ± 7.36a | 66.30 ± 11.64a | .205 | <.001* |
| End-systolic volume week 6, μL | 59.10 ± 10.25 | 65.20 ± 8.09 | 68.70 ± 11.64 | 68.77 ± 10.38 | ||
| Heart rate baseline, bpm | 331.30 ± 24.09 | 352.60 ± 28.96 | 358.10 ± 19.30 | 345.90 ± 28.51 | .026*
a–c |
.014 |
| Heart rate week 6, bpm | 331.70 ± 24.77a | 354.40 ± 25.25 | 378.90 ± 42.82a | 348.33 ± 25.42 | ||
| QTc baseline, ms | 171.40 ± 19.96 | 169.80 ± 17.11 | 170.90 ± 18.71 | 171.30 ± 17.99 | .525 | <.001* |
| QTc week 6, ms | 171.80 ± 19.67 | 169.20 ± 14.92 | 192.30 ± 28.91 | 174.77 ± 16.72 | ||
| QRS baseline, ms | 14.70 ± 2.21 | 14.90 ± 2.23 | 14.80 ± 2.74 | 15.00 ± 2.10 | .001*
a–c |
<.001* |
| QRS week 6, ms | 14.90 ± 2.23a | 14.70 ± 2.11b | 23.10 ± 4.38a.b.c | 15.77 ± 1.92c |
Data are given as mean ± SD.
*Significant at the .05 level according to two-way repeated measure ANOVA.
a,b,c,dThe letters denote the significant pairwise study groups according to Dunnet t (2-sided) post-hoc test.
bpm, beat per minute; DAPA, dapagliflozin; DOX, doxorubicin; QTc, corrected QT interval.
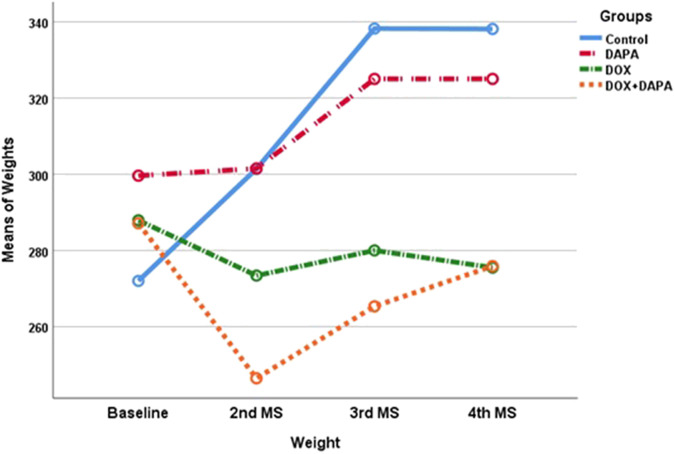
Cardiac measurements and ECG results of animals before and after treatment were compared (Table 1). The weights of the rats were measured in 4 different periods. Overall, there was a significant increase in weight gain over time. However, weight gains were not similar between groups. In the control group, basal weight was significantly lower than the measurements taken at other times (P < .001). The basal weight was also significantly lower in the DAPA-treated group than the mean weight in the post-treatment period (P < .001). However, there was no significant weight gain in the DOX and DOX + DAPA-treated groups. Rats’ weights decreased in the DOX group, whereas they first decreased and then increased and approached the basal level in the DOX + DAPA group. Nevertheless, the 2-way analysis also revealed a significant difference between study groups (P < .001). The overall mean weight was significantly higher in the control group than in the DOX and DOX + DAPA groups and was significantly higher in the DAPA group than in the DOX and DOX + DAPA groups (Figure 2). The EF value increased by 1% in the post-treatment period in rats in the DAPA study group, but decreased in other groups in the post-treatment period (P < .001). The EF value is significantly lower in DOX group then controls (P < .001). Fractional shortening values were likely different in the post-treatment period in both DOX and DOX + DAPA groups (P < .001), but there is no significant difference between the study groups as FS. End-DBP and -SBP were not significantly different between the study groups, however the volumes significantly increased in the post-treatment period in both DOX and DOX + DAPA groups (P < .001). Heart rate values were significantly higher in the post-treatment period in both DOX and DOX + DAPA groups (P = .014), and the values of both DOX and DOX + DAPA groups were likely higher than controls (P = .026). All QTc and QRS signals were elevated after 6 weeks (P < .001). Moreover, QRS mean was significantly higher in DOX group than control group (P = .001) whereas QTc signals were not different between the groups (Figures 3 and 4).
Figure 2.
Echocardiographic findings of groups before and after treatment.
Figure 3.
Electrocardiographic findings of groups before and after treatment.
Figure 4.
Weight changes in the study process of groups. MS, measurements.
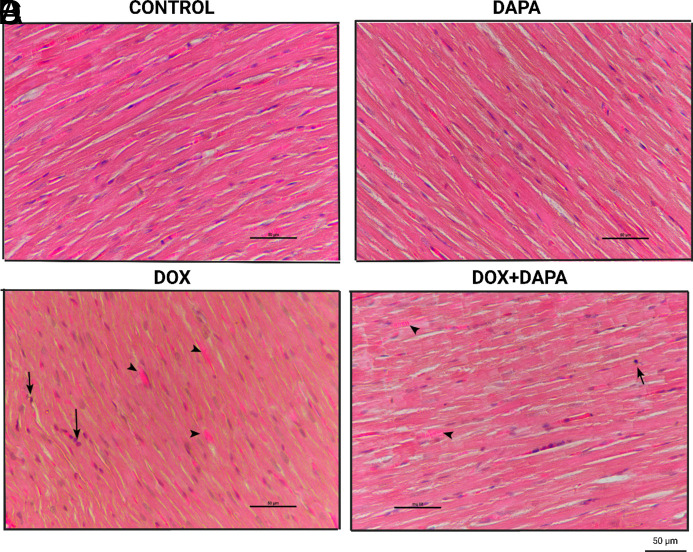
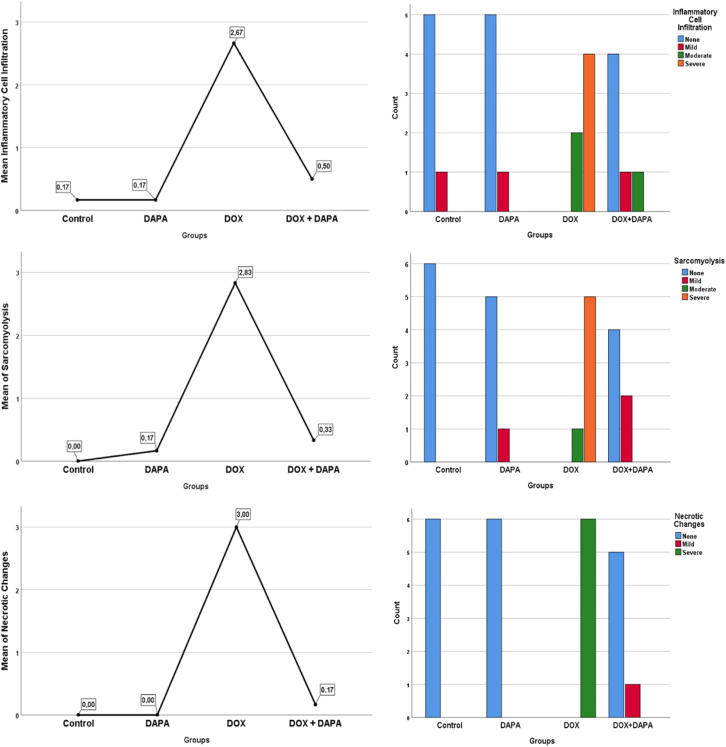
In histochemical evaluations, intracellular inflammatory filtration evaluation was found to be significantly higher in the DOX group (P < .001) (Figure 5). The general filtration value of the group was found to be “severe.” Similarly, sarcomyolysis and necrotic changes were significantly higher and “severe” in the DOX group (P < .001). While no positive results were found in the control and DAPA groups, low and moderate results were obtained in the DOX + DAPA group (Figures 5 and 6, Table 2).
Figure 5.
Representative pathohistological images of groups. Representative images (hematoxylin and eosin) of inflammatory cell infiltration, sarcomyolysis, and necrotic changes for each group at 6 weeks following initiation of chemotherapy. Heart tissues of control and DAPA groups were with normal histological appearance (A, B). Inflammatory cell infiltration (arrow), sarcomyolysis (arrowhead), and necrotic changes (loss of transverse striations and diffuse eosinophilia) are seen in DOX group (C). Inflammatory cell infiltration (arrow) and sarcomyolysis (arrowhead) are rarely seen in DOX + DAPA group (D). DAPA, dapagliflozin; DOX, doxorubicin.
Figure 6.
Pathohistological findings of the groups.
Table 2.
Histochemical Evaluations Across Study Groups
| Measurements | Control | DAPA | DOX | DOX + DAPA | P |
|---|---|---|---|---|---|
| Mean ± SD (Median; Minimum–Maximum) | |||||
| Inflammatory cell infiltration |
|
|
|
0.50 ± 0.83c
(0; 0-2) |
.001* |
| Sarcomyolysis |
|
|
2.83 ± 0.40a,b,c
(3; 1-3) |
0.33 ± 0.51c
(0; 0-1) |
<.001* |
| Necrotic changes |
|
|
3.00 ± 0.00a,b,c
(3; 3-3) |
0.17 ± 0.40c
(0; 0-1) |
<.001* |
Data are given as mean ± SD, number, or median (interquartile range).
DAPA, dapagliflozin; DOX, doxorubicin.
a,b,cThe same superscript letters denote the significant pairwise comparison in each row according to Kruskal–Wallis critical difference post-hoc test.
*Significant at the .05 level according to two-way Repeated Measure ANOVA
Discussion
The pathophysiology of AIC has not yet been clearly elucidated, but the prevailing hypothesis is that DOX alters the redox balance in more than one way. Ultimately, this process results in remodeling and impaired cardiac function.15 Renin angiotensin system-mediated apoptosis has been reported as one of the main mechanisms of DOX-induced cardiotoxicity in cardiomyocytes.16,17 Accordingly, therapeutic strategies to prevent or alleviate DOX-induced cardiotoxicity have focused on direct targeting of reactive oxygen species (ROS) or inhibition of key pathways in the remodeling process. Several preclinical studies have reported that inhibition of the renin-angiotensin-aldosterone system by both angiotensin-converting enzyme inhibitors and angiotensin receptor blockers reduces oxidative stress and histological evidence of cardiotoxicity during anthracycline therapy.18,19 The fact that dexrazoxane is an iron chelator that binds free iron or removes iron from the DOX–iron complex, thus preventing oxygen free radical formation, may explain how dexrazoxane reduces some of the cardiotoxic effects of anthracyclines.20 However, dexrazoxane increases the risk of infection and may cause secondary malignant neoplasms. It can also reduce the metabolic efficiency of DOX and is an expensive medication.21 Therefore, many ongoing studies trying to discover new agents against DOX-induced cardiotoxicity focus on interfering with oxidative stress, inflammation, and apoptosis.22
Empagliflozin, Cardiovascular Outcomes, Mortality in Type 2 Diabetes (EMPA-REG), Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes (CANVAS), and Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA-HF) clinical trials have shown cardioprotective effects of SGLT2 inhibitors in reducing cardiovascular adverse events, including heart failure hospitalizations and cardiovascular mortality.23 Animal models of cardiomyopathy have shown that DAPA improves cardiac morphology and position, including cardiac hypertrophy, fibrosis, and heart failure, as well as both systolic and diastolic LV function.24,25
Zaibi et al12 found that DAPA reduces hydrogen peroxide-induced cell damage in proximal tubule cells via reducing cytosolic ROS and mitochondrial superoxide ROS production. Dapagliflozin also altered the dynamics of Ca2+ influx through the oxidative-sensitive Transient Receptor Potential Cation Channel Subfamily M Member 2 (TRPM2) Ca2+ channels.12 Evidence from the SGLT2 inhibitor study of Hazem et al26 could explain DAPA’s protective effects against DOX cardiotoxicity detected in our study. Dapagliflozin treatment increased the levels of antioxidant enzymes catalase and superoxide dismutase in liver tissue.26
Inflammation and secondary cardiac fibrosis are considered one of the main pathophysiological mechanisms of DOX-induced cardiotoxicity.16 Studies with SGLT2 inhibitors have also documented the protective effects of this molecule on inflammation and fibrosis. Zhang et al27 showed that the use of DAPA in rats reduced inflammation by decreasing TGF-β1/Smad expression in cardiac tissue. The use of DAPA also reduced myocardial fibrosis, myocardial collagen synthesis, and myocardial hypertrophy induced by Angiotensin II infusion. Another study showed that DAPA inhibited myocardial fibrosis through inhibition of SGK1 and epithelial sodium channel protein found in both myocardial tissue and fibrosis-induced cardiomyoblast cells. They also reported that DAPA showed an anti-inflammatory effect and improved mitochondrial degradation.28
In our study, the chemotherapy group that received DAPA exhibited less deterioration in parameters such as QRS duration, QT duration, and basal heart rate that predict arrhythmogenic events compared to the group that did not receive DAPA. Studies in the literature also show that DAPA has protective effects against arrhythmia. In the DAPA-HF study, the group receiving DAPA had a significantly lower incidence of severe ventricular arrhythmias (5.9% vs. 7.4%) and a 21% relative risk reduction compared to the placebo group.29 Another meta-analysis also reported a significant reduction in the incidence of atrial arrhythmias with an overall prevalence of approximately 1% with SGLT2 inhibitor therapy.30 Studies at the cellular level have shown that SGLT2 inhibitors have a regulatory and stabilizing effect on cardiac electrophysiological changes. One study showed that empagliflozin reduced the late sodium channel current in cardiomyocytes in mice with heart failure or sodium channel mutation, but not in healthy murine cardiac myocytes.31 The decrease in late sodium channel current contributes to less prolongation of cardiac action potential duration (APD) and may protect against arrhythmias associated with prolonged action potentials.31 Another study found that DAPA improved ventricular repolarization by increasing mitochondrial stability.32
A research conducted by Barış et al33 showed that empagliflozin, another SGLT-2 inhibitor drug, has cardioprotective effects from AIC on rat models.33 In this research, it has been found that empagliflozin has protective effects on reduction of EF and potential arrhythmic events after DOX injection, thus supporting our research. In a research conducted by Belen et al34 shows that DAPA has protective effects on increasing cardiac injury parameters TNF-a, pro-BNP, troponin T and plasma FGF-21 levels after DOX injection.
Our study revealed the protective effects of DAPA on cardiotoxicity. However, loss of appetite, weight loss, and cachexia are serious problems in cancer patients receiving chemotherapy with DOX. In our study, the group that received DOX + DAPA lost more weight than the group that received only DOX during chemotherapy. Even if weight loss recovers after chemotherapy, this may be an issue to consider in clinical practice.
Study Limitations
First, since we were not able to examine the parameters of diastolic dysfunction in rats, we could not evaluate the possibility of diastolic dysfunction in our model and the effects of DAPA on these functions. Second, DOX was injected intraperitoneally in our study, causing direct liver damage. This is not the routine clinical use of DOX, and its intravenous use may yield different results than our findings. Finally, since healthy rats were used in our study, it is unclear whether a cardioprotective effect can be achieved in rats with cancer.
Conclusion
Our study showed the protective effect of DAPA against LV remodeling and dysfunction in a DOX-induced cardiomyopathy model. Dapagliflozin treatment was also shown to reduce the deterioration in DOX-dependent arrhythmia parameters. Future research should focus on the cardiac function protection properties of DAPA in the setting of DOX-induced cardiotoxicity in large animal models and cancer patients.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Süleyman Demirel University Animal Experiments Local Ethics Committee (approval number: 02.12.2021 - 11/02).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.U., K.G., A.P., M.S., A.K., M.S.K.; Design – S.U., K.G., A.P., M.S., A.K., M.S.K.; Supervision – S.U., K.G., A.P., M.S., A.K., M.S.K.; Funding – S.U., K.G., A.P., M.S., A.K., M.S.K.; Materials – S.U., K.G., A.P., M.S., A.K., M.S.K.; Data Collection and/or Processing – S.U., K.G., A.P., M.S., A.K., M.S.K.; Analysis and/or Interpretation – S.U., K.G., A.P., M.S., A.K., M.S.K.; Literature Review – S.U., K.G., A.P., M.S., A.K., M.S.K.; Writing – S.U., K.G., A.P., M.S., A.K., M.S.K.; Critical Review – S.U., K.G., A.P., M.S., A.K., M.S.K.
Declaration of Interests: The authors declare that they have no competing interest.
Funding: This work was supported by a grant from TUBITAK (Scientific and Technological Research Council of Turkey) 2209-A Research Project Support Programme for Undergraduate Students.
References
- 1. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231 2247. ( 10.1016/j.jacc.2009.02.050) [DOI] [PubMed] [Google Scholar]
- 2. Alıcı H, Balakan O, Ercan S, Çakıcı M, Yavuz F, Davutoğlu V. Evaluation of early subclinical cardiotoxicity of chemotherapy in breast cancer. Anatol J Cardiol. 2015;15(1):56 60. ( 10.5152/akd.2014.5185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peruzzi M, Palazzoni G, Biondi-Zoccai G, Lotrionte M. Cardiotoxicity due to chemotherapy for breast cancer: the dark side of the moon. Anatol J Cardiol. 2015;15(1):61 62. ( 10.5152/akd.2014.14271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozbay B, Şimşek E, Kemal H, Cakar B, Yavuzgil O. Anthracycline Chemotherapy-Induced Electro-Mechanical Changes: Strain Echocardiography Combined with Repolarization Parameters on Electrocardiography to Predict Early Cardiotoxicity. Turk Kardiyol Dern Ars. 2022;50(7):478 484. ( 10.5543/tkda.2022.22359) [DOI] [PubMed] [Google Scholar]
- 5. Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213 220. ( 10.1016/j.jacc.2009.03.095) [DOI] [PubMed] [Google Scholar]
- 6. Saad SY, Najjar TA, Al-Rikabi AC. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res. 2001;43(3):211 218. ( 10.1006/phrs.2000.0769) [DOI] [PubMed] [Google Scholar]
- 7. Özdoğru İ. Anthracycline-induced cardiotoxicity. Turk Kardiyol Dern Ars. 2014;42(3):274 276. [PubMed] [Google Scholar]
- 8. Argun M, Üzüm K, Sönmez MF, et al. Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anatol J Cardiol. 2016;16(4):234 241. ( 10.5152/akd.2015.6185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyon AR, López-Fernández T, Couch LS, et al. ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J. 2022:ehac244. ( 10.1093/eurheartj/ehac244) [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995 2008. ( 10.1056/NEJMoa1911303) [DOI] [PubMed] [Google Scholar]
- 11. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089 1098. ( 10.1056/NEJMoa2206286) [DOI] [PubMed] [Google Scholar]
- 12. Zaibi N, Li P, Xu SZ. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS One. 2021;16(2):e0247234. ( 10.1371/journal.pone.0247234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balcıoğlu AS, Çelik E, Şahin M, Göçer K, Aksu E, Aykan AÇ. Dapagliflozin improves cardiac autonomic function Measures in Type 2 diabetic patients with cardiac autonomic neuropathy. Anatol J Cardiol. 2022;26(11):832 840. ( 10.5152/AnatolJCardiol.2022.1934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savran M, Ascı H, Armagan İ, et al. Thymoquinone could be protective against valproic acid-induced testicular toxicity by antioxidant and anti-inflammatory mechanisms. Andrologia. 2020;52(7):e13623. ( 10.1111/and.13623) [DOI] [PubMed] [Google Scholar]
- 15. Rochette L, Guenancia C, Gudjoncik A, et al. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci. 2015;36(6):326 348. ( 10.1016/j.tips.2015.03.005) [DOI] [PubMed] [Google Scholar]
- 16. Menna P, Paz OG, Chello M, Covino E, Salvatorelli E, Minotti G. Anthracycline cardiotoxicity. Expert Opin Drug Saf. 2012;11(suppl 1):S21 S36. ( 10.1517/14740338.2011.589834) [DOI] [PubMed] [Google Scholar]
- 17. Dindaş F, Güngör H, Ekici M, et al. Angiotensin receptor-neprilysin inhibition by sacubitril/valsartan attenuates doxorubicin-induced cardiotoxicity in a pretreatment mice model by interfering with oxidative stress, inflammation, and caspase 3 apoptotic pathway. Anatol J Cardiol. 2021;25(11):821 828. ( 10.5152/AnatolJCardiol.2021.356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cadeddu C, Piras A, Mantovani G, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J. 2010;160(3):487.e1 487.e7. ( 10.1016/j.ahj.2010.05.037) [DOI] [PubMed] [Google Scholar]
- 19. Arozal W, Watanabe K, Veeraveedu PT, et al. Effect of telmisartan in limiting the cardiotoxic effect of daunorubicin in rats. J Pharm Pharmacol. 2010;62(12):1776 1783. ( 10.1111/j.2042-7158.2010.01196.x) [DOI] [PubMed] [Google Scholar]
- 20. Hasinoff BB. The interaction of the cardioprotective agent ICRF-187 ((+)-1, 2-bis (3, 5-dioxopiperazinyl-1-yl) propane); its hydrolysis product (ICRF-198); and other chelating agents with the Fe (III) and Cu (II) complexes of adriamycin. Agents Actions. 1989;26(3-4):378 385. ( 10.1007/BF01967305) [DOI] [PubMed] [Google Scholar]
- 21. Wang XY, Yang CT, Zheng DD, et al. Hydrogen sulfide protects H9c2 cells against doxorubicin-induced cardiotoxicity through inhibition of endoplasmic reticulum stress. Mol Cell Biochem. 2012;363(1-2):419 426. ( 10.1007/s11010-011-1194-6) [DOI] [PubMed] [Google Scholar]
- 22. Gülgün M, Fidancı K, Genç FA, Kesik V. Natriuretic peptide and cardiac troponin levels in doxorubicin-induced cardiotoxicity. Anatol J Cardiol. 2016;16(4):299. ( 10.14744/AnatolJCardiol.2016.7001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117 2128. ( 10.1056/NEJMoa1504720) [DOI] [PubMed] [Google Scholar]
- 24. Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium–glucose co-transporter 2 inhibitors. Cardiovasc Diabetol. 2018;17(1):101. ( 10.1186/s12933-018-0745-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Çavuşoğlu Y, Altay H, Cahn A, et al. Sodium glucose co-transporter 2 inhibitors in heart failure therapy. Turk Kardiyol Dern Ars. 2020;48(3):330 354. ( 10.5543/tkda.2020.74332) [DOI] [PubMed] [Google Scholar]
- 26. Hazem RM, Ibrahim AZ, Ali DA, Moustafa YM. Dapagliflozin improves steatohepatitis in diabetic rats via inhibition of oxidative stress and inflammation. Int Immunopharmacol. 2022;104:108503. ( 10.1016/j.intimp.2021.108503) [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Lin X, Chu Y, et al. Dapagliflozin: a sodium-glucose cotransporter 2 inhibitor, attenuates angiotensin II-induced cardiac fibrotic remodeling by regulating TGFβ1/Smad signaling. Cardiovasc Diabetol. 2021;20(1):121. ( 10.1186/s12933-021-01312-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee SG, Kim D, Lee JJ, et al. Dapagliflozin attenuates diabetes-induced diastolic dysfunction and cardiac fibrosis by regulating SGK1 signaling. BMC Med. 2022;20(1):309. ( 10.1186/s12916-022-02485-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42(36):3727 3738. ( 10.1093/eurheartj/ehab560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandes GC, Fernandes A, Cardoso R, et al. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: A meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021;18(7):1098 1105. ( 10.1016/j.hrthm.2021.03.028) [DOI] [PubMed] [Google Scholar]
- 31. Hegyi B, Mira Hernandez J, Shen EY, Habibi NR, Bossuyt J, Bers DM. Empagliflozin reverses late Na(+) current enhancement and cardiomyocyte proarrhythmia in a translational murine model of heart failure with preserved ejection fraction. Circulation. 2022;145(13):1029 1031. ( 10.1161/CIRCULATIONAHA.121.057237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Durak A, Olgar Y, Degirmenci S, Akkus E, Tuncay E, Turan B. A SGLT2 inhibitor dapagliflozin suppresses prolonged ventricular-repolarization through augmentation of mitochondrial function in insulin-resistant metabolic syndrome rats. Cardiovasc Diabetol. 2018;17(1):144. ( 10.1186/s12933-018-0790-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barış VÖ, Dinçsoy AB, Gedikli E, Zırh S, Müftüoğlu S, Erdem A. Empagliflozin significantly prevents the doxorubicin-induced acute cardiotoxicity via non-antioxidant pathways. Cardiovasc Toxicol. 2021;21(9):747 758. ( 10.1007/s12012-021-09665-y) [DOI] [PubMed] [Google Scholar]
- 34. Belen E, Canbolat IP, Yigittürk G, et al. Cardio-protective effect of dapagliflozin against doxorubicin induced cardiomyopathy in rats. Eur Rev Med Pharmacol Sci. 2022;26(12):4403 4408. ( 10.26355/eurrev_202206_29079) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a