Abstract
Background
Orthostatic intolerance (OI) is a core diagnostic criterion in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). The majority of ME/CFS patients have no evidence of hypotension or postural orthostatic tachycardia syndrome (POTS) during head-up tilt, but do show a significantly larger reduction in stroke volume index (SVI) when upright compared to controls. Theoretically a reduction in SVI should be accompanied by a compensatory increase in heart rate (HR). When there is an incomplete compensatory increase in HR, this is considered chronotropic incompetence. This study explored the relationship between HR and SVI to determine whether chronotropic incompetence was present during tilt testing in ME/CFS patients.
Methods
From a database of individuals who had undergone tilt testing with Doppler measurements for SVI both supine and end-tilt, we selected ME/CFS patients and healthy controls (HC) who had no evidence of POTS or hypotension during the test. To determine the relation between the HR increase and SVI decrease during the tilt test in patients, we calculated the 95% prediction intervals of this relation in HC. Chronotropic incompetence in patients was defined as a HR increase below the lower limit of the 95th % prediction interval of the HR increase in HC.
Results
We compared 362 ME/CFS patients with 52 HC. At end-tilt, tilt lasting for 15 (4) min, ME/CFS patients had a significantly lower SVI (22 (4) vs. 27 (4) ml/m2; p < 0.0001) and a higher HR (87 (11) vs. 78 (15) bpm; p < 0.0001) compared to HC. There was a similar relationship between HR and SVI between ME/CFS patients and HC in the supine position. During tilt ME/CFS patients had a lower HR for a given SVI; 37% had an inadequate HR increase. Chronotropic incompetence was more common in more severely affected ME/CFS patients.
Conclusion
These novel findings represent the first description of orthostatic chronotropic incompetence during tilt testing in ME/CFS patients.
Keywords: Orthostatic intolerance, Tilt table testing, ME/CFS, Stroke volume index, Cardiac index, Chronotropic incompetence
Highlights
-
•
Adults with ME/CFS experience a 3-fold greater reduction in cerebral blood flow during end-tilt tilt compared to healthy controls, confirming orthostatic intolerance.
-
•
During tilt testing we found that in 134/362 (37%) patients with ME/CFS without POTS or hypotension, the heart rate increase was below the lower limit of the 95% prediction interval of the heart rate increase of controls, indicative of orthostatic chronotropic incompetence.
-
•
These novel findings represent the first description of orthostatic chronotropic incompetence during tilt testing, confirming another abnormality in the circulatory response to upright posture in ME/CFS.
1. Introduction
Orthostatic intolerance (OI) is defined as a clinical condition in which symptoms—like dizziness, fatigue, pain, and memory and concentration problems—worsen upon assuming and maintaining upright posture and are ameliorated by recumbency (Gerrity et al., 2002). OI is a core feature of myalgic encephalomyelitis/chronic fatigue syndrome (Institute of Medicine Iom, 2015). Based on heart rate (HR) and blood pressure (BP) changes during orthostatic stress tests, a variety of hemodynamic abnormalities can be diagnosed, including classical or delayed orthostatic hypotension (cOH, dOH), postural orthostatic tachycardia syndrome (POTS), and syncope (Fedorowski et al., 2009, Sheldon et al., 2015, Shen et al., 2017).
In earlier work, using extracranial Doppler measurements of blood flow through the internal carotid and vertebral arteries, we calculated cerebral blood flow (CBF) supine and upright (van Campen et al., 2020b). Compared to supine values, adults with ME/CFS experienced a highly significant mean 26% reduction in CBF at the end of 30 min of head-up tilt compared to a 7% reduction in healthy controls. Based on the mean and 2 standard deviations of the healthy controls, the lower limit of normal was set at a CBF reduction of 13%. Using this criterion, 90% of ME/CFS patients met criteria for reduced CBF during upright tilt. Within the ME/CFS group, 28% developed POTS, 14% dOH, and 58% had neither excessive tachycardia nor hypotension, indicating heterogeneity within the ME/CFS population. Understanding the pathophysiologic differences between these groups will be important for ensuring effective treatment.
In a previous study, focusing on the patients with neither tachycardia nor hypotension during head-up tilt, we observed a similar increase in HR in both ME/CFS patients and in healthy controls. However, there was a large and significant mean group difference in stroke volume index (SVI) in those with ME/CFS (percent SVI reductions of 35 (9)% in patients and 28 (10)% in healthy controls: p < 0.0001) (van Campen and Visser, 2018a). Theoretically, any decrease in stroke volume should be compensated by a concordant increase in HR. The absence of an adequate HR compensation would be consistent with chronotropic incompetence, a phenomenon that has been reported in response to exercise in ME/CFS (Inbar et al., 2001, Vanness et al., 2003, Davenport et al., 2019, Cook et al., 2022), but to our knowledge has not been examined in response to orthostatic stress. Based on the observation of a larger SVI reduction in combination with a similar increase in HR of ME/CFS patients versus healthy controls during a tilt test and based on the observation that chronotropic incompetence was described in ME/CFS patients during exercise, we hypothesized that chronotropic incompetence is also present during a tilt test in ME/CFS patients. Therefore, in this study we examined in greater detail the relationship between HR and SVI in individual patients, as we have previously described in ME/CFS patients at rest (van Campen and Visser, 2022). Our results identify an attenuated HR response to upright tilt that is consistent with chronotropic incompetence.
2. Patient, material and methods
Eligible patients and controls were drawn from the database of individuals who had participated in studies conducted at the Stichting CardioZorg, an outpatient cardiology clinic specializing in the care of those with ME/CFS. We included individuals who had been seen between October 2012 to December 2020 and who underwent a head-up tilt table test. During the first visit, we determined by history taking of the treating physician whether patients satisfied the criteria for CFS (Fukuda et al., 1994) and ME (Carruthers et al., 2011), taking the exclusion criteria into account. No other illnesses provided a sufficient alternative explanation of the symptomatology. Patients were selected for analysis if the tilt test Doppler data for SVI and CI were available in both supine and tilted positions. Patients were excluded from the analysis if they were being treated with drugs influencing HR or BP at the time of the tilt testing. For comparison healthy controls were also studied. Healthy controls were recruited from three sources: (a) announcements on ME/CFS patient advocacy websites, (b) posters in the medical clinic’s office building, and (c) healthy acquaintances of the ME/CFS participants. Healthy controls were also asked for daily life OI symptoms. Subjects in whom no abnormalities were documented during evaluation in our clinic for syncope or other cardiologic diseases, were not eligible to be considered healthy controls. To improve the hemodynamic similarity between ME/CFS cases and healthy controls, we elected to include only those from each group who had no evidence of postural orthostatic tachycardia syndrome (POTS) or orthostatic hypotension (OH)during the test.
The study was carried out in accordance with the Declaration of Helsinki. All ME/CFS participants and healthy controls gave informed, written consent. The study was approved by the medical ethics committee of the Slotervaart Hospital, Amsterdam, for healthy controls P1450 and for ME/CFS patients P1736.
2.1. Tilt test protocol
Measurements were performed as described previously (van Campen et al., 2018b, van Campen et al., 2020b). Briefly, all participants were positioned supine for 20 min before being tilted head-up to 70 degrees for a maximum of 30 min. The tilt duration was determined by patient wellbeing and symptomatology; with increasing severity of symptoms patients were tilted back either at their request or at our discretion to avoid syncope. As the aim of the tilt test was quantification of orthostatic intolerance, not provocation of syncope, this approach did not interfere with that goal but led to a shorter tilt duration (15 min in the ME/CFS group). Because healthy controls tolerated upright tilt longer than the patients, to provide a closer comparison to the ME/CFS patient tilt duration, for controls we used the mid-tilt image acquisition, which was started at 13 (3) min post onset of tilt and ended at 17 (4) min post onset of tilt. After the Doppler data acquisition during the upright phase of the tilt test, patients were tilted back to the horizontal position. HR, systolic, and diastolic blood pressures (SBP, DBP) were continuously recorded by finger plethysmography (Eeftinck Schattenkerk et al., 2009, Martina et al., 2012). After the test, HR and BP’s were extracted from the device and imported into an Excel spreadsheet.
To fulfill the criteria of a normal HR and BP response in all participants, the HR increase during tilt testing had to be less than 30 bpm, and the reductions in BP had to be less than 20 mm Hg systolic, and less than 10 mm Hg diastolic, in accordance with the definitions of POTS and OH in consensus statements (Fedorowski et al., 2009, Freeman et al., 2011, Sheldon et al., 2015).
2.2. Doppler echocardiographic measurements
Time velocity integral (VTI) frames were obtained in the resting supine position and while upright in the final minutes of the 30-minute tilt. The aortic VTI was measured using a continuous wave Doppler pencil probe connected to a Vivid I machine (GE, Hoevelaken, NL) with the transducer positioned in the suprasternal notch. A 2 MHz frequency range, non-imaging pencil probe was used with a 17 mm footprint. A maximal Doppler signal was assumed to be the optimal flow alignment. At least 2 frames of 6 s were obtained. Echo Doppler recordings were stored digitally. The VTI was measured off-line by manual tracing of at least 6 cardiac cycles, using the GE EchoPac post-processing software. This was performed by one operator (CMCvC). The outflow tract was manually drawn just below the valve insertion in the parasternal long-axis view of a previously made echocardiogram and the cross-sectional area calculated. As the outflow tract is not circular but ellipsoid with the long axis median to lateral and the short axis anterior to posterior, we used the data of Maes et al. (2017) to correct for the overestimation by the circular shape of the ellipsoid ventricular outflow tract calculation. In their study the overestimation of the outflow tract area, using the circular calculation by transthoracic echocardiography, was 24.5%. Therefore, we reduced the outflow tract area by 25%. SVI was calculated by the equation: corrected left ventricular outflow tract cross-sectional area times the aortic VTI, divided by the body surface area (BSA; DuBois formula). SVI’s of the separate cardiac cycles were averaged. CI was calculated as: HR times SVI.
2.3. Extracranial Doppler: cerebral blood flow measurements
Measurements were performed as described previously (van Campen et al., 2020b, van Campen et al., 2018a). Internal carotid artery and vertebral artery Doppler flow velocity frames were acquired by one operator (FCV), using a Vivid-I system (GE Healthcare, Hoevelaken, the Netherlands) equipped with a 6–13 MHz linear transducer. Frames were recorded in the supine position approximately 8 min before the onset of the tilt period and while upright at the end of the tilt period. Blood flow of the internal carotid and vertebral arteries was calculated offline by an investigator (CMCvC) who was unaware of the patient severity status. Blood flow in each vessel was calculated from the mean blood flow velocities times the vessel cross-sectional area and expressed in ml/minute. Flow in the individual arteries was calculated in 3–6 cardiac cycles and data were averaged. Total CBF was calculated by adding the flow of the four arteries.
2.4. Determination of chronotropic incompetence
For this study, in the absence of a published definition of chronotropic incompetence during tilt testing, we conservatively classified patients as having chronotropic incompetence if they had a HR below the lower 95th percentile of the prediction limits for HR change for a given SVI change between supine and standing in healthy controls.
2.5. International Consensus Criteria (ICC) severity of disease
To classify ME/CFS severity, we used the ICC criteria. Mild severity required an approximate 50% reduction in pre-illness activity level. Moderate severity required patients to be mostly housebound. Severe patients were mostly bedridden and very severe patients were totally bedridden and needed help with basic functions (Carruthers et al., 2011). Very severe patients were excluded because they were unable to tolerate tilt testing. We have confirmed that the ICC severity classification is valid and correlates with objective measures of physical function, including number of steps per day and exercise capacity on cardiopulmonary exercise testing (van Campen et al., 2020a).
2.6. Statistical analysis
Data were analyzed using the statistical package of Graphpad Prism version 6.05 (Graphpad software, La Jolla, California, USA). All continuous data were tested for normal distribution using the D′Agostino & Pearson omnibus normality test and by visual inspection of the histograms. Data were presented as mean and standard deviation (SD) or as median with the interquartile range (IQR) where appropriate. Nominal data were compared using the Chi-square test. Baseline characteristics and hemodynamic data of groups were compared using Students t-test for unpaired data or by the Wilcoxon signed ranks test, where appropriate. The HR and SVI were correlated for patients and controls in both supine and upright positions, using linear regression. The slopes of the regression lines were compared using the statistical package of the Graphpad Prism software. The 95% prediction limits of a HR change for a given SVI change in healthy controls were calculated using Excel. These calculations of the upper and lower limits of the healthy controls formed the basis for the determination whether the HR increase in patients were within limits of the HR increase of healthy controls. The calculations are presented in appendix A. We also performed a regression analysis of the change between supine and standing for SVI versus the change in HR in both ME/CFS patients and in healthy controls. Due to the number of comparisons, we choose a conservative p-value of < 0.01 to be statistically significant.
3. Results
3.1. Participants
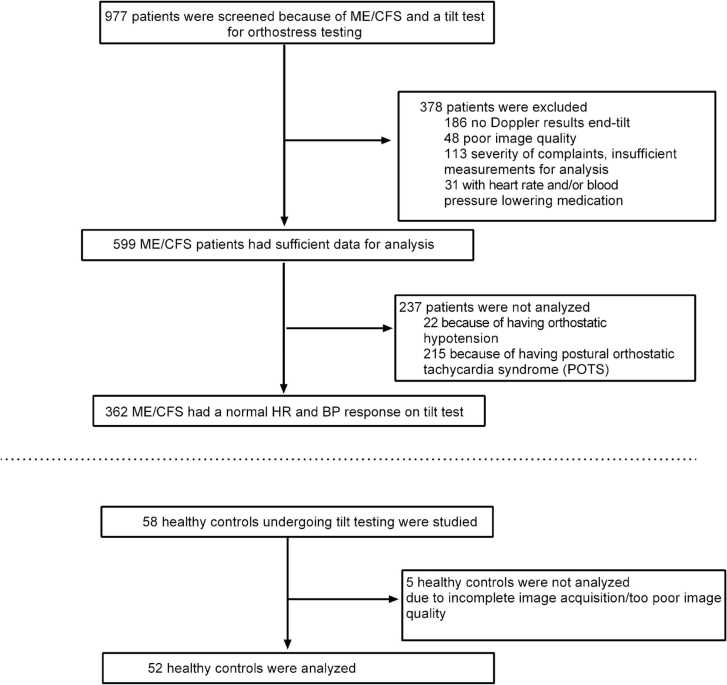
Fig. 1 illustrates the selection of patients for this study. Of the 977 patients evaluated in the Stichting CardioZorg between 2012 and 2020, 378 were excluded for the following reasons: 186 had no hemodynamic echo Doppler data during the standing period of the tilt test, 48 were excluded due to poor image quality, 113 due to the rapidity and severity of symptom exacerbations that made acquisition of Doppler data impossible and 31 because of the use of HR and/or BP lowering medication at the time of the tilt test. Of the 599 who had adequate image data for analysis, 237 others were not analyzed, 22 with orthostatic hypotension and 215 with POTS. This left 362 ME/CFS patients (55 male, 307 female) with no evidence of POTS or hypotension on tilt testing for analysis. From the database, we identified 58 healthy controls who had participated in studies, 52 of whom had complete hemodynamic and Doppler data of sufficient quality during the supine and upright positions to include in the analysis.
Fig. 1.
Subject flow. Legend to Fig. 1: ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome; HC: healthy controls.
3.2. Demographic and hemodynamic results
Table 1 displays the baseline characteristics of the study population. No significant differences between ME/CFS patients and healthy controls were identified for age, sex, height, weight, or BMI. All patients fulfilled the CFS criteria and 261 had typical ME (72%), 101 (28%) had atypical ME. Using the ICC definition of disease severity, 117 patients were graded as having mild, 198 patients as moderate and 47 patients as severe ME/CFS. Tilt duration in patients was slightly shorter than in healthy controls, with a mean tilt duration of 15 (4) minutes versus 17 (5) in healthy controls (mid-tilt duration).
Table 1.
Baseline characteristics of healthy controls (HC) and ME/CFS patients.
| HC (n = 52) | ME/CFS (n = 362) | p-value | |
|---|---|---|---|
| Male/femalea | 10/42 | 55/307 | 0.42 |
| Age (years) | 39 (15) | 42 (12) | 0.15 |
| Height (cm) | 173 (9) | 171 (9) | 0.12 |
| Weight (kg) | 74 (15) | 75 (18) | 0.66 |
| BMI (kg/m2) | 24.5 (4.3) | 25.3 (5.6) | 0.20 |
| BSA (m2) | 1.87 (0.21) | 1.86 (0.22) | 0.82 |
| Patients fulfilling CFS criteria | NA | 362 (100%) | |
| Patients with typical/atypical ME | NA | 262/101 (72/28%) | |
| Disease duration (years) # | NA | 11.5 (6–20) |
Chi-square 2 × 2 analysis; # Median (IQR); BMI: body mass index: BSA: body surface area (formula duBois); HC: healthy controls; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome. A p-value of < 0.01 is considered statistically significant.
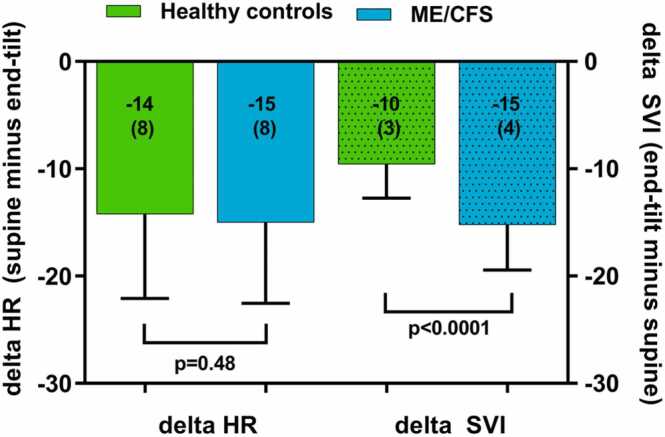
Table 2 shows the hemodynamic head-up tilt test results of healthy controls and ME/CFS patients. End-tilt HR was significantly higher than supine HR both in healthy controls and in ME/CFS patients (both p < 0.0001 with paired t-test). Supine HR and end-tilt HR were significantly higher in ME/CFS patients than in healthy controls (both p < 0.0001). SVI at end-tilt was significantly lower than supine SVI in healthy controls and in ME/CFS patients (both p < 0.0001 with paired t-test). End-tilt SVI was significantly higher in healthy controls than in ME/CFS patients (p < 0.0001). Supine CI was significantly higher than end-tilt CI both in healthy controls and in ME/CFS patients (both p < 0.0001 with paired t-test). Supine CI was higher in ME/CFS patients than in healthy controls (p < 0.0001) and end-tilt CI was significantly lower in ME/CFS patients than in healthy controls (p = 0.0004). Supine and end-tilt SBP were not different in healthy controls and ME/CFS patients. End-tilt DBP was lower in healthy controls than in ME/CFS patients (p = 0.002). End-tilt CBF was significantly lower than supine CBF in ME/CFS patients (p < 0.0001), and ME/CFS patients had significantly lower end-tilt CBF compared to healthy controls. Fig. 2 shows the graphical representation of the change in HR (supine minus end-tilt) and the change in SVI (end-tilt minus supine). The change in stroke volume index was significantly larger in ME/CFS patients than in healthy controls (p < 0.0001).
Table 2.
Hemodynamic results of head-up tilt testing of healthy controls (HC) and ME/CFS patients.
| HC (n = 52) | ME/CFS (n = 362) | Unpaired t-test | |
|---|---|---|---|
| HR supine (bpm) | 64 (10) | 72 (11) | < 0.0001 |
| HR end-tilt (bpm) | 78 (15) | 87 (11) | < 0.0001 |
| SBP supine (mmHg) | 134 (15) | 138 (18) | 0.17 |
| SBP end-tilt (mmHg) | 127 (14) | 135 (19) | 0.008 |
| DBP supine (mmHg) | 79 (7) | 80 (10) | 0.49 |
| DBP end-tilt (mmHg) | 81 (8) | 86 (11) | 0.002 |
| SVI supine (ml/m2) | 37 (5) | 37 (6) | 0.31 |
| SVI end-tilt (ml/m2) | 27 (4) | 22 (4) | < 0.0001 |
| CI supine (L/min/m2) | 2.30 (0.35) | 2.58 (0.43) | < 0.0001 |
| CI end-tilt (L/min/m2) | 2.04 (0.28) | 1.86 (0.34) | 0.0004 |
| CBF supine (ml/min) | 622 (82) | 612 (100) | 0.50 |
| CBF end-tilt (ml/min) | 584 (80) | 451 (89) | < 0.0001 |
CBF: cerebral blood flow; CI: cardiac index; DBP: diastolic blood pressure; end-tilt: upright measurements just before tilting the subject back to supine; HC: healthy controls; HR: heart rate; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome; SBP: systolic blood pressure; SVI: stroke volume index. A p-value of < 0.01 is considered significant.
Fig. 2.
Change in heart rate and stroke volume index in healthy controls and ME/CFS patients. Legend to Fig. 2: delta HR: change in heart rate during the tilt (supine minus end-tilt); delta SVI: stroke volume index decrease during the tilt (end-tilt minus supine); ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome.
3.3. Correlations between HR and SVI
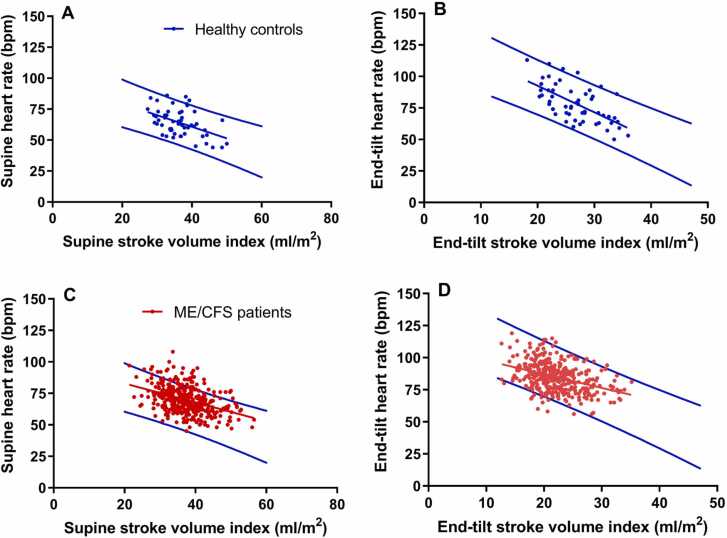
Fig. 3 shows the correlation between SVI and HR in the supine and end-tilt positions in healthy controls and ME/CFS patients. All regression lines were significant showing that with increasing SVI there is a decrease in HR. Fig. 3A shows the supine SVI-HR relation and the 95% prediction values of healthy controls, Fig. 3B shows the regression data at the end-tilt in this group. Fig. 3C shows the regression line of supine data in ME/CFS patients and the 95% prediction intervals of the healthy controls, and Fig. 3D the SVI-HR regression data at end-tilt in ME/CFS patients. Table 3 shows the statistical comparison between the slopes of the regression lines, as determined by GraphPad Prism. The slopes of the supine SVI-HR regression lines were not different between healthy controls and ME/CFS patients. At end-tilt the slope of the regression line in healthy controls was higher than in ME/CFS patients: p = 0.0063. In healthy controls the slope of the SVI-HR regression line at end-tilt was significantly higher (p = 0.0095) than the slope of the supine SVI-HR relation. In ME/CFS patients the slope of the SVI-HR regression line was not different between supine and end-tilt: p = 0.045 (considering a p value <0.01 to be significant).
Fig. 3.
Correlations between heart rate and stroke volume index supine and at end-tilt in healthy controls and ME/CFS patients and the 95% prediction values of the heart rate of healthy controls. Legend to Fig. 3: The blue lines indicate the lower and upper limit values of the 95% prediction intervals of the heart rate supine and end-tilt of healthy controls. The middle blue line in Figs. A and B is the regression line in healthy controls, the red line in Figures CD is the regression line in ME/CFS patients. ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome.
Table 3.
Differences in the stroke volume index – heart rate correlation between healthy controls and ME/CFS patients and between supine and end-tilt.
| Correlations supine healthy controls versus ME/CFS patients |
Slope difference | |||
|---|---|---|---|---|
| Healthy Controls | ME/CFS patients | p-value | ||
| Correlation supine | R and p-value | Correlation supine | R and p-value | |
| Y = −0,9769 *X + 99,18 | 0.52; < 0.0001 | Y = −0,7510 *X + 97,86 | 0.42; < 0.0001 | 0.39 |
| Correlations end-tilt healthy controls versus ME/CFS patients | ||||
| Correlation end-tilt | R and p-value | Correlation end-tilt | R and p-value | |
| Y = −1968 *X + 130,7 | 0.68; < 0.0001 | Y = −1066 *X + 108,4 | 0.39; < 0.0001 | 0.0063 |
| Correlations healthy controls: supine versus end-tilt |
Slope difference | |||
|---|---|---|---|---|
| Supine | End-tilt | p-value | ||
| Correlation | R and p-value | Correlation | R and p-value | |
| Y = −0,9769 *X + 99,18 | 0.52; < 0.0001 | Y = −1968 *X + 130,7 | 0.68; < 0.0001 | 0.0095 |
| Correlations ME/CFS patients: supine versus end-tilt | ||||
| correlation | R and p-value | correlation | R and p-value | |
| Y = −0,7510 *X + 97,86 | 0.42; < 0.0001 | Y = −1066 *X + 108,4 | 0.39; < 0.0001 | 0.045 |
ME/CFS: myalgic encephalomyelitis. X = stroke volume index, Y = heart rate; A p-value of < 0.01 is considered statistically significant.
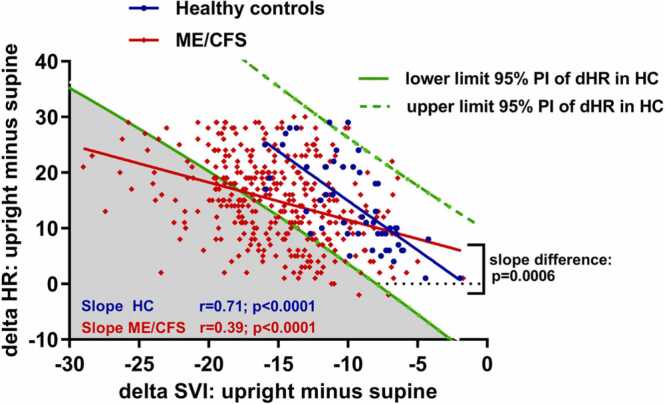
Fig. 4 shows the correlations of the delta HR (difference in HR end-tilt minus supine) versus the delta SVI (SVI end-tilt minus supine) between healthy controls and ME/CFS patients. Within-group correlations were both highly significant (p < 0.0001). However, the slope of the relation between changes in SVI versus the change in HR was significantly lower in ME/CFS patients than in healthy controls: p < 0.0006. This indicates that for a given change in SVI the increase in heart rate is lower in ME/CFS patients than in controls.
Fig. 4.
Correlations of the delta heart rate and the delta stroke volume index of healthy controls and ME/CFS patients. Legend to Fig. 4: The 95% prediction intervals (PI) of the relation of the stroke volume index decrease during the tilt versus the heart rate increase during the tilt in healthy controls, indicated by the two green lines. The blue line indicates the regression line of the relation SVI decrease versus the heart rate increase in healthy controls, the red line the same regression in ME/CFS patients. The gray area shows the ME/CFS patients with an abnormally low HR increase for the decrease in stroke volume index, meeting the study definition of chronotropic incompetence. Delta HR: heart rate increase during the tilt; delta SVI: stroke volume index decrease during the tilt; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome.
Taking the 95% prediction intervals of the HR increase of healthy controls as the normal HR increase to a given SVI reduction, 134 of 362 ME/CFS patients (37%) had a HR change below the lower 95th percentile prediction interval for a given change in SVI, consistent with an inadequate chronotropic response. When using the lower 80% prediction values, as used in cardiopulmonary stress tests (Inbar et al., 2001, Vanness et al., 2003, Davenport et al., 2019, Cook et al., 2022) the number of patients with chronotropic incompetence increased to 206 of 362 (57%). The distinction between patients with and without chronotropic incompetence could not be derived from the single supine or the end-tilt data of patients but only when measuring the change in HR and SVI (data not shown).
3.4. Differences between ME/CFS patients with and without chronotropic incompetence
We explored whether there were any differences between ME/CFS patients who did or did not met the study definition for chronotropic incompetence during tilt testing. Table 4 shows that the only difference in baseline characteristics was that the group with chronotropic incompetence was more likely to meet the ICC classification for severe disease.
Table 4.
Comparison of baseline characteristics ME/CFS patients with and without orthostatic chronotropic incompetence.
| Chronotropic Incompetence + n = 134 |
Chronotropic Incompetence - n = 228 |
p-value | |
|---|---|---|---|
| Male/femalea | 19/115 (14/86%) | 36/192 (16/84%) | 0.68 |
| Age (years) | 43 (12) | 41 (12) | 0.08 |
| Height (cm) | 172 (9) | 171 (8) | 0.35 |
| Weight (kg) | 73 (17) | 75 (18) | 0.32 |
| BMI (kg/m2) | 24.9 (5.2) | 25.9 (5.8) | 0.11 |
| BSA (m2) | 1.85 (0.23) | 1.86 (0.21) | 0.81 |
| Disease duration (years)b | 11 (6–20) | 12 (6.25–20) | 0.50 |
| Severity (mild/moderate/severe)a | 34/73/27 (25/54/20%) |
83/125/20 (36/55/9%) |
0.003 |
Chi-square 2 × 2 or 3 × 2 analysis;
Median (IQR); BMI: body mass index: BSA: body surface area (formula duBois); ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome. A p-value of < 0.01 is considered statistically significant.
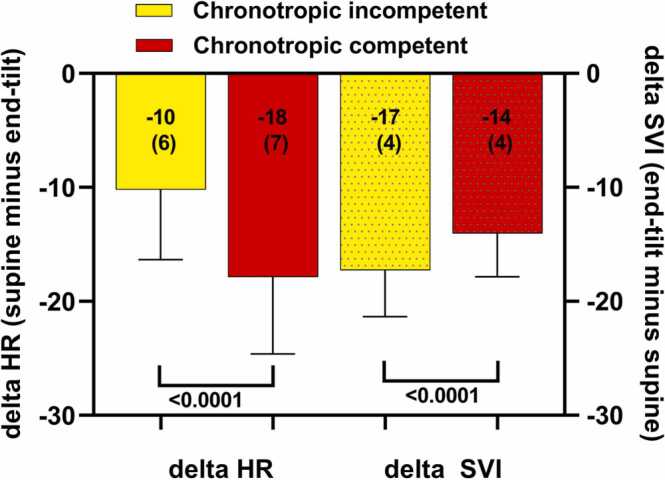
Table 5 shows the results of the hemodynamics of the ME/CFS patients with and without chronotropic incompetence. End-tilt HR was significantly higher than supine HR in both groups (both p < 0.0001 with paired t-test), and end-tilt HR was significantly higher in the group without chronotropic incompetence than in the group with chronotropic incompetence (p < 0.0001). Supine SVI and end-tilt SVI were significantly higher in the patients with chronotropic incompetence compared to the patients without chronotropic incompetence (both p < 0.0001). End-tilt SVI was significantly lower than supine SVI in both groups (both p < 0.0001 with paired t-test). Supine CI was significantly higher in patients with chronotropic incompetence than patients without (p < 0.0001). In both patient groups end-tilt CI was significantly lower than supine CI (both p < 0.0001 with paired t-test). SBP was not significantly different. End-tilt CBF was significantly lower than supine CBF in both groups (both p < 0.0001 with paired t-test). Fig. 5 shows the graphical representation of the change in HR (supine minus end-tilt) and the change in SVI (end-tilt minus supine) in patients with and without chronotropic incompetence. As expected, the change in HR was significantly larger in patients without chronotropic incompetence than in patients with chronotropic incompetence: p < 0.0001. In contrast, the stroke volume index reduction during the tilt was larger in patients with chronotropic incompetence than in patients without: p < 0.0001). In a post-hoc analysis of the one-way ANOVA of the supine CI of healthy controls, and of patients with and without chronotropic incompetence, the supine CI of healthy controls was not different from the CI of patients without chronotropic incompetence (p = 0.045), but in both groups supine CI was significantly lower compared to the patients with chronotropic incompetence (both p < 0.0001). Patients with chronotropic incompetence had a shorter tilt duration than patients without chronotropic incompetence: 14 (5) min versus 16 (5) min (p = 0.0014).
Table 5.
Hemodynamic results of ME/CFS patients with and without orthostatic chronotropic incompetence.
| Chronotropic Incompetence + Group 1 n = 134 | Chronotropic incompetence -Group 2 n = 228 | Unpaired t-test | |
|---|---|---|---|
| HR supine (bpm) | 72 (11) | 73 (11) | 0.50 |
| HR end-tilt (bpm) | 82 (9) | 90 (11) | < 0.0001 |
| SBP supine (mmHg) | 139 (19) | 138 (18) | 0.61 |
| SBP end-tilt (mmHg) | 137 (21) | 133 (18) | 0.03 |
| DBP supine (mmHg) | 80 (11) | 80 (10) | 1.00 |
| DBP end-tilt (mmHg) | 87 (12) | 86 (11) | 0.69 |
| SVI supine (ml/m2) | 41 (6) | 35 (5) | < 0.0001 |
| SVI end-tilt (ml/m2) | 23 (4) | 21 (4) | < 0.0001 |
| CI supine (L/min/m2) | 2.80 (0.40) | 2.46 (0.40) | < 0.0001 |
| CI end-tilt (L/min/m2) | 1.86 (0.32) | 1.87 (0.35) | 0.81 |
| CBF supine (ml/min) | 622 (97) | 606 (101) | 0.13 |
| CBF end-tilt (ml/min) | 438 (83) | 458 (92) | 0.04 |
CBF: cerebral blood flow; CI: cardiac index; DBP: diastolic blood pressure; HR: heart rate; ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome; SBP: systolic blood pressure; SVI: stroke volume index. A p-value of < 0.01 is considered significant.
Fig. 5.
Change in heart rate and stroke volume index in ME/CFS patients with and without chronotropic incompetence. Legend to Fig. 5: delta HR: heart rate change during the tilt (supine minus end-tilt); delta SVI: stroke volume index change during the tilt.
4. Discussion
In this study we examined the SVI-HR relation both at rest, in the supine position, and during a head-up tilt test. This relation was established in ME/CFS patients without evidence of POTS or OH during the tilt test, and in healthy controls without POTS or OH. In 1965 Ross et al. established the relation between HR and stroke volume in a pacing study in healthy controls: increasing the HR by right atrial pacing led to a decrease in stroke volume with an unchanged cardiac output (Ross et al., 1965). Although the methodology in the present study was different from the study of Ross et al. (orthostatic stress vs. atrial pacing), we also found an inverse and significant relation between SVI and HR, both at rest and during the tilt in both patients and healthy controls. The inverse relation between SVI and HR at rest in healthy controls and in ME/CFS patients has already been described by us in a previous paper (van Campen and Visser, 2022). In the present study we extended this observation, showing that the change/decrease in SVI from supine to end-tilt was accompanied by an increase in HR both in patients and healthy controls. However, based on the 95% prediction intervals of the HR change for the SVI change in the healthy controls, we found that 37% of the ME/CFS patients showed a lower than predicted HR increase during the tilt. This inadequate increase in HR in these patients can be regarded as chronotropic incompetence. These novel findings represent the first description of chronotropic incompetence during tilt table testing in this subset of ME/CFS patients, and are consistent with the findings of chronotropic incompetence in ME/CFS during cardiopulmonary exercise testing (Inbar et al., 2001, Vanness et al., 2003, Davenport et al., 2019, Cook et al., 2022). Of note, the presence or absence of chronotropic incompetence could not be determined by the resting or the end-tilt SVI-HR data, but only when the changes were analyzed. The mechanisms of the chronotropic incompetence in a subset of the ME/CFS population are unclear but are unlikely to be due to myocardial dysfunction or sick sinus syndrome as these would have been identified by an electrocardiogram or echocardiogram. Other mechanisms for chronotropic incompetence proposed in the exercise literature include insensitivity of beta-adrenergic receptors due to down-regulation or blocking by antibodies, insufficient epinephrine or norepinephrine release, excessive vagal tone, or resetting of the baroreceptors (Camm and Fei, 1996a, Camm and Fei, 1996b, Davenport et al., 2019). However, other central mechanisms are possible, such as a change in brainstem cardiac nuclei function, or cellular mechanisms like the synergistic action of cortisol and catecholamines, or altered calcium regulation in cardiac muscle, and altered expression of other agents from catecholamine-activated second messenger systems. It remains to be determined whether the patients with chronotropic incompetence during the tilt test are also the patients that show chronotropic incompetence during cardiopulmonary exercise testing. Furthermore, the association of chronotropic incompetence with severe ME/CFS raises the question whether chronotropic incompetence has a causal role or is the consequence of severe disease.
In the present study ME/CFS patients showed a significantly larger SVI decrease during the tilt test than the healthy controls. In healthy controls a large number of studies have reported the effect of orthostatic stress on stroke volumes, showing large differences between studies. Decrease in stroke volumes ranged from 11% in the elderly (Youde et al., 2003) to a decrease of 63% in healthy young women (Nwosu et al., 1994, Zaidi et al., 2000, Shoemaker et al., 2001, Freitas et al., 2007, Tahvanainen et al., 2009a, Tahvanainen et al., 2009b, Chaudhari et al., 2012). Among the methodologic factors that influence the decrease in stroke volume during tilt testing in healthy controls are age, gender, training status, fluid filling status, used technique of measurement, the angle of tilting and tilt duration. Our healthy control stroke volumes fall within this spectrum of stroke volume changes during the tilt. The larger decrease in SVI during tilt testing has been previously described in ME/CFS patients by us and others (Lamanca et al., 1999, Timmers et al., 2002, Wyller et al., 2007, van Campen and Visser, 2018a). Possible mechanisms to consider for the larger SVI changes in ME/CFS might be hypovolemia (Hurwitz et al., 2010), impaired muscle pump, capacitance vessel distention and impaired venous return or venous pooling (Joseph et al., 2021, van Campen et al., 2021a). Another mechanism to consider is that the larger SVI reduction is related to contractility abnormalities in ME/CFS patients. Studies have suggested that a reduced SVI is caused by an abnormal cardiac function (Miwa and Fujita, 2009, Hollingsworth et al., 2012). However, cardiac function is dependent on preload, contractility and afterload, following the family of Frank-Starling curves. These curves imply that a reduction in preload results in a reduction of stroke volume. This is a normal phenomenon and not indicating cardiac dysfunction. Moreover, the presence of hypovolemia lowers the preload-stroke volume curves, while retaining the relation between preload and stroke volume. Oppositely, catecholamines lift the preload-stroke volume curve upwards while retaining the preload-stroke volume relation. The larger than normal SVI reduction during the tilt may therefore be explained by a larger preload reduction in these patients and is not suggestive of an intrinsic cardiac dysfunction. Moreover, multiple studies have shown that ME/CFS patients may have a reduced blood volume (Hurwitz et al., 2010, van Campen et al., 2018a, van Campen and Visser, 2018b), lowering the stroke volume for a given preload. Indeed, Hurwitz et al. calculated that more than 90% of the stroke volume abnormalities could be attributed to the lower blood volumes. We cannot rule out that intrinsic cardiac contractile abnormalities are present in these patients but further (complicated) studies are needed.
One of the striking differences between healthy controls and ME/CFS patients is the significantly larger decline in CBF in ME/CFS patients compared to healthy controls. This observation has been previously published (van Campen et al., 2020b). In healthy controls there is a significant relation between cardiac output changes and CBF changes (Meng et al., 2015). The relation between decreases in CI and decreases CBF in ME/CFS patients need to be studied further in the future.
Patients showing chronotropic incompetence have a higher supine CI with a higher SVI and a similar supine HR compared to patients without chronotropic incompetence and healthy controls. The mechanism is unknown, but may be related to the disease severity, hypothesizing that in severe patients the chronic inflammatory reaction (Klimas and Koneru, 2007) is larger than in mildly or moderately diseased patients. This inflammatory reaction may lead to vasodilation, a lower peripheral resistance and consequently a higher cardiac output. Another explanation may be that oxygen extraction is lower in ME/CFS patients than in healthy controls. The reduced oxygen extraction was demonstrated by Joseph et al. during cardiopulmonary stress testing in ME/CFS patients (Joseph et al., 2021). The authors showed that in ME/CFS patients with the highest increase of systemic flow during exercise, peak oxygen consumption was lowest and hypothesized that impaired oxygen delivery due to endothelial dysfunction was the underlying mechanism. It is conceivable that the reduced oxygen delivery (or reduced oxygen extraction) is already present at rest in severe ME/CFS patients leading to a higher CI at rest to maintain normal oxygen consumption. This hypothesis is strengthened by our study showing that severe ME/CFS patients have the lowest peak oxygen consumption compared to mildly and moderately affected patients (van Campen et al., 2020a). The relation between resting cardiac output and oxygen consumption in severe patients’ needs to be studied in future. As supine HR was similar between patients with and without chronotropic incompetence, we performed a post hoc analysis to determine whether the results would change by matching patients on the similarity of the supine HR. The supine CI remained abnormal, and the overall results did not change. The high SVI in the chronotropic incompetence patients argue against the hypothesis that the heart is small in ME/CFS patients (Miwa and Fujita, 2011). Further studies can address whether ME/CFS patients with chronotropic incompetence during tilt testing respond differently to certain classes of medications. For example, for the treatment of orthostatic intolerance in chronotropic incompetent patients, beta-blockers, ivabradine and pyridostigmine bromide might be less appropriate, and fludrocortisone and midodrine might be preferable.
In the present study we excluded patients and healthy controls using HR and BP lowering drugs, but did not exclude patients with so far undetected high BP. The inclusion of patients with undetected high BP may have increased the SBP and DBP in the patient group, leading to an absence of differences with healthy controls. The BP in ME/CFS patients is still a matter of debate: Newton et al. found a lower BP during 24 hr. BP recordings in ME/CFS patients compared to controls (Newton et al., 2009). In contrast, Van de Luit et al. found similar blood pressures in ME/CFS patients and controls, however BP oscillations were significantly larger in ME/CFS patients compared to controls (van de Luit et al., 1998). Moreover, the authors found a significantly higher prevalence of nighttime hypotension in the ME/CFS population compared to healthy controls. Finally, Duprez et al. found no differences in BP during 24 hr. BP recordings of ME/CFS patients compared to healthy controls, but found a systematically higher HR in patients (Duprez et al., 1998). All in all these data of small studies suggest that BP is lower in ME/CFS than in healthy controls, but measurements may be influenced by a higher adrenergic state (Freeman and Komaroff, 1997; Frith et al., 2012; Sisto et al., 1995), whether or not associated with e.g. nervous expectation for the tilt test, the presence of fibromyalgia, hypermobility syndrome, and a hypovolemic state. All these conditions (undetected high BP, higher circadian BP variation, hyperadrenergic states, nervousness, fibromyalgia, hypermobility, and hypovolemia) may have led to absence of differences between patients and healthy controls.
4.1. Strengths
The strengths of this study include the relatively large sample size for both the ME/CFS patients and healthy controls, the comprehensive assessment of hemodynamic changes, and the consistency of the study methods over time in a single clinic. We used a conservative definition of chronotropic incompetence: in exercise studies chronotropic incompetence has been defined variously using HR values less than 80–95% of the maximal predicted HR (Davenport et al., 2019, Cook et al., 2022). We used a lower limit of the 95% prediction interval of the HR change of healthy controls during tilt testing. Using the lower limit of the 80% prediction interval increased the number of ME/CFS patients meeting the definition of chronotropic incompetence to 57%. The assessment of the changes in SVI vs. the changes in HR is new and may also be applicable to other patient populations.
4.2. Limitations
We excluded patients with orthostatic hypotension, postural orthostatic tachycardia syndrome and vasovagal syncope, to ensure differences seen were not the result of a different hemodynamic response to orthostatic stress. Biased referral of patients with more severe orthostatic intolerance symptoms might have influenced the prevalence of chronotropic incompetence in our sample. The clinical profile of the 378 excluded patients was comparable to the ones analyzed (data not shown), however we do not know whether this group of patients would have had a different hemodynamic profile. The tilt duration was slightly, but significantly shorter in ME/CFS patients than in healthy controls (15 vs. 17 min), and patients with chronotropic incompetence had a shorter tilt duration than patients without (14 vs. 16 min). As the major hemodynamic changes occur within the first couple of minutes after onset of tilt, we are confident that there is minimal influence of the tilt duration between minimally 14 and maximally 17 min on the assessed hemodynamics.
5. Conclusions
In the present study an inverse relation between HR and stroke volume was found during tilt testing both in healthy controls and in ME/CFS patients without tachycardia or hypotension. Taking the lower limits of the 95% prediction interval of changes in HR vs. the changes in SVI in healthy controls as the reference value, 37% of ME/CFS patients have a lower HR increase during the tilt, indicative of chronotropic incompetence. This is the first description of chronotropic incompetence during tilt testing. These findings contribute to the growing body of literature on circulatory abnormalities in ME/CFS. Given the potential of ME/CFS to follow SARS-CoV-2 (van Campen et al., 2021b), these observations are relevant to the growing population of post COVID-19 patients in whom chronotropic incompetence has been identified during exercise testing (Jimeno-Almazan et al., 2021).
Ethics statement
The ethics statement is also included in the methodology. The study was carried out in accordance with the Declaration of Helsinki. All ME/CFS participants and HEALTHY CONTROLS gave informed, written consent. The study was approved by the medical ethics committee of the Slotervaart Hospital, Amsterdam, for healthy controls P1450 and for ME/CFS patients P1736.
CRediT authorship contribution statement
C. (Linda) M.C. van Campen, Peter C. Rowe and Frans C. Visser conceived the study, C. (Linda) M.C. van Campen and Frans C. Visser collected the data, C. (Linda) M.C. van Campen performed the primary data analysis and Frans C. Visser, Freek W.A. Verheugt and Peter C. Rowe performed secondary data analyses. All authors were involved in the drafting and review of the manuscript.
Funding
This study was not funded.
Acknowledgement
Dr Rowe is supported by the Sunshine Natural Wellbeing Foundation Professorship.
Data availability statement
This manuscript/research paper does not include datasets of the following: Structural/crystallographic data for both macromolecular structures and small molecules; Protein and nucleic acid sequence data (this includes RNASeq data); Functional genomics and molecular interactions/proteomics/metabolomics data; Computational models; Genetics data (genetic polymorphisms; genotype data). Therefore data availability is not applicable to this study.
Disclosure
No authors have any financial interest to disclose.
Appendix A
The calculations of the 95% prediction intervals of the HR change for a given SVI change in healthy controls are presented in the Excel spreadsheat. We used the formula: ŷ0 + /- tα/2,df=n-2 * s.e, where s.e. = Syx√(1 + 1/n + (x0 – x)2/SSx). The use of Excel formula to calculate the 95% prediction intervals (upper and lower limits of normal) in HC are explained on the website: https://www.statology.org/prediction-interval-excel/. The calculation of the 95% prediction intervals of healthy controls are shown in the Excel file. In column A the SVI decrease of the 52 HC are shown, in column B the corresponding HR increase are given. The parts of the calculation for the upper and lower limits are presented in cells D3-D7, and in cells D10-D12. In cell D8 a value of the SVI change is entered, leading to a calculation of the lower and upper limit of normal of the HR increase in cells D16 and D17. The calculated LL and UL of a normal heart rate increase in healthy controls are then value pasted to cells G16 and G17. Subsequently, the values of SVI decreases of − 29 to + 4 are calculated and value pasted in cells H16,H17 to cells AO16, AO17. The calculations in healthy controls were validated using the 95% prediction values of Statgraph Prism, showing that the Excel calculated values were identical to the Prism derived values. To compare the HR increase in patients relative to the HR increase of healthy controls -for a given SVI decrease- the SVI decrease and HR increase are entered in colums E and F, below cells E19 and F19. The HR increase in these patients were compared with the HR increase of healthy controls. In patient 1 the HR increase for a given SVI decrease was within the normal limits of healthy controls. In patient 2 the expected HR increase was lower than than the lower limit of healthy controls. In this patient the HR increase should be at least 27 bpm while in this patient the HR increase was 13 bpm. This is indicative of a chronotropic incompetence. To use another prediction interval (e.g. 80%) alfa/2 in cell E10 can be changed (eg 0,10).
References
- Camm A.J., Fei L. Chronotropic incompetence--Part I: Normal regulation of the heart rate. Clin. Cardiol. 1996;19:424–428. doi: 10.1002/clc.4960190518. [DOI] [PubMed] [Google Scholar]
- Camm A.J., Fei L. Chronotropic incompetence--Part II: Clinical implications. Clin. Cardiol. 1996;19:503–508. doi: 10.1002/clc.4960190612. [DOI] [PubMed] [Google Scholar]
- Carruthers B.M., Van De Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T., Staines D., Powles A.C., Speight N., Vallings R., Bateman L., Baumgarten-Austrheim B., Bell D.S., Carlo-Stella N., Chia J., Darragh A., Jo D., Lewis D., Light A.R., Marshall-Gradisbik S., Mena I., Mikovits J.A., Miwa K., Murovska M., Pall M.L., Stevens S. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari S.A., Sacerdote A., Bahtiyar G. 1-alpha hydroxylation defect in postural orthostatic tachycardia syndrome: remission with calcitriol supplementation. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5730. bcr0220125730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D.B., Vanriper S., Dougherty R.J., Lindheimer J.B., Falvo M.J., Chen Y., Lin J.S., Unger E.R., Group M.S. Cardiopulmonary, metabolic, and perceptual responses during exercise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Multi-site Clinical Assessment of ME/CFS (MCAM) sub-study. PLoS One. 2022;17 doi: 10.1371/journal.pone.0265315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport T.E., Lehnen M., Stevens S.R., Vanness J.M., Stevens J., Snell C.R. Chronotropic intolerance: an overlooked determinant of symptoms and activity limitation in myalgic encephalomyelitis/chronic fatigue syndrome? Front Pedia. 2019;7:82. doi: 10.3389/fped.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez D.A., De Buyzere M.L., Drieghe B., Vanhaverbeke F., Taes Y., Michielsen W., Clement D.L. Long- and short-term blood pressure and RR-interval variability and psychosomatic distress in chronic fatigue syndrome. Clin. Sci. (Lond. ) 1998;94:57–63. doi: 10.1042/cs0940057. [DOI] [PubMed] [Google Scholar]
- Eeftinck Schattenkerk D.W., Van Lieshout J.J., Van Den Meiracker A.H., Wesseling K.R., Blanc S., Wieling W., Van Montfrans G.A., Settels J.J., Wesseling K.H., Westerhof B.E. Nexfin noninvasive continuous blood pressure validated against Riva-Rocci/Korotkoff. Am. J. Hypertens. 2009;22:378–383. doi: 10.1038/ajh.2008.368. [DOI] [PubMed] [Google Scholar]
- Fedorowski A., Burri P., Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J. Hypertens. 2009;27:976–982. doi: 10.1097/hjh.0b013e3283279860. [DOI] [PubMed] [Google Scholar]
- Freeman R., Wieling W., Axelrod F.B., Benditt D.G., Benarroch E., Biaggioni I., Cheshire W.P., Chelimsky T., Cortelli P., Gibbons C.H., Goldstein D.S., Hainsworth R., Hilz M.J., Jacob G., Kaufmann H., Jordan J., Lipsitz L.A., Levine B.D., Low P.A., Mathias C., Raj S.R., Robertson D., Sandroni P., Schatz I.J., Schondorf R., Stewart J.M., Van Dijk J.G. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Freitas J., Santos R., Azevedo E., Carvalho M., Boomsma F., Meiracker A., Falcao De Freitas A., Abreu-Lima C. Hemodynamic, autonomic and neurohormonal behaviour of familial amyloidotic polyneuropathy and neurally mediated syncope patients during supine and orthostatic stress. Int J. Cardiol. 2007;116:242–248. doi: 10.1016/j.ijcard.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Straus S.E., Hickie I., Sharpe M.C., Dobbins J.G., Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Gerrity T.R., Bates J., Bell D.S., Chrousos G., Furst G., Hedrick T., Hurwitz B., Kula R.W., Levine S.M., Moore R.C., Schondorf R. Chronic fatigue syndrome: what role does the autonomic nervous system play in the pathophysiology of this complex illness? Neuroimmunomodulation. 2002;10:134–141. doi: 10.1159/000067176. [DOI] [PubMed] [Google Scholar]
- Hollingsworth K.G., Hodgson T., Macgowan G.A., Blamire A.M., Newton J.L. Impaired cardiac function in chronic fatigue syndrome measured using magnetic resonance cardiac tagging. J. Intern Med. 2012;271:264–270. doi: 10.1111/j.1365-2796.2011.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz B.E., Coryell V.T., Parker M., Martin P., Laperriere A., Klimas N.G., Sfakianakis G.N., Bilsker M.S. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin. Sci. (Lond. ) 2010;118:125–135. doi: 10.1042/CS20090055. [DOI] [PubMed] [Google Scholar]
- Inbar O., Dlin R., Rotstein A., Whipp B.J. Physiological responses to incremental exercise in patients with chronic fatigue syndrome. Med Sci. Sports Exerc. 2001;33:1463–1470. doi: 10.1097/00005768-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (Iom) The National Academies Press,; Washington DC: 2015. Beyond Mayalgic Encephalomyelitis/chronic Fatigue Syndrome: Redefining an Illness. [PubMed] [Google Scholar]
- Jimeno-Almazan A., Pallares J.G., Buendia-Romero A., Martinez-Cava A., Courel-Ibanez J. Chronotropic Incompetence in Non-Hospitalized Patients with Post-COVID-19 Syndrome. J. Clin. Med. 2021:10. doi: 10.3390/jcm10225434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph P., Arevalo C., Oliveira R.K.F., Faria-Urbina M., Felsenstein D., Oaklander A.L., Systrom D.M. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160:642–651. doi: 10.1016/j.chest.2021.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas N.G., Koneru A.O. Chronic fatigue syndrome: inflammation, immune function, and neuroendocrine interactions. Curr. Rheuma Rep. 2007;9:482–487. doi: 10.1007/s11926-007-0078-y. [DOI] [PubMed] [Google Scholar]
- Lamanca J.J., Peckerman A., Walker J., Kesil W., Cook S., Taylor A., Natelson B.H. Cardiovascular response during head-up tilt in chronic fatigue syndrome. Clin. Physiol. 1999;19:111–120. doi: 10.1046/j.1365-2281.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- Maes F., Pierard S., De Meester C., Boulif J., Amzulescu M., Vancraeynest D., Pouleur A.-C., Pasquet A., Gerber B., Vanoverschelde J.-L. Impact of left ventricular outflow tract ellipticity on the grading of aortic stenosis in patients with normal ejection fraction. J. Cardiovasc. Magn. Reson. 2017;19:37. doi: 10.1186/s12968-017-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina J.R., Westerhof B.E., Van Goudoever J., De Beaumont E.M., Truijen J., Kim Y.S., Immink R.V., Jobsis D.A., Hollmann M.W., Lahpor J.R., De Mol B.A., Van Lieshout J.J. Noninvasive continuous arterial blood pressure monitoring with Nexfin(R) Anesthesiology. 2012;116:1092–1103. doi: 10.1097/ALN.0b013e31824f94ed. [DOI] [PubMed] [Google Scholar]
- Meng L., Hou W., Chui J., Han R., Gelb A.W. Cardiac output and cerebral blood flow: the integrated regulation of brain perfusion in adult humans. Anesthesiology. 2015;123:1198–1208. doi: 10.1097/ALN.0000000000000872. [DOI] [PubMed] [Google Scholar]
- Miwa K., Fujita M. Cardiovascular dysfunction with low cardiac output due to a small heart in patients with chronic fatigue syndrome. Intern Med. 2009;48:1849–1854. doi: 10.2169/internalmedicine.48.2347. [DOI] [PubMed] [Google Scholar]
- Miwa K., Fujita M. Small heart with low cardiac output for orthostatic intolerance in patients with chronic fatigue syndrome. Clin. Cardiol. 2011;34:782–786. doi: 10.1002/clc.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton J.L., Sheth A., Shin J., Pairman J., Wilton K., Burt J.A., Jones D.E. Lower ambulatory blood pressure in chronic fatigue syndrome. Psychosom. Med. 2009;71:361–365. doi: 10.1097/PSY.0b013e31819ccd2a. [DOI] [PubMed] [Google Scholar]
- Nwosu E.A., Rahko P.S., Hanson P., Grogan E.W., Jr. Hemodynamic and volumetric response of the normal left ventricle to upright tilt testing. Am. Heart J. 1994;128:106–113. doi: 10.1016/0002-8703(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Ross J., Jr., Linhart J.W., Brauwald E. Effects of changing heart rate in man by electrical stimulation of the right atrium. studies at rest, during exercise, and with isoproterenol. Circulation. 1965;32:549–558. doi: 10.1161/01.cir.32.4.549. [DOI] [PubMed] [Google Scholar]
- Sheldon R.S., Grubb B.P., 2nd, Olshansky B., Shen W.K., Calkins H., Brignole M., Raj S.R., Krahn A.D., Morillo C.A., Stewart J.M., Sutton R., Sandroni P., Friday K.J., Hachul D.T., Cohen M.I., Lau D.H., Mayuga K.A., Moak J.P., Sandhu R.K., Kanjwal K. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W.K., Sheldon R.S., Benditt D.G., Cohen M.I., Forman D.E., Goldberger Z.D., Grubb B.P., Hamdan M.H., Krahn A.D., Link M.S., Olshansky B., Raj S.R., Sandhu R.K., Sorajja D., Sun B.C., Yancy C.W. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2017;70:620–663. doi: 10.1016/j.jacc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Shoemaker J.K., Hogeman C.S., Khan M., Kimmerly D.S., Sinoway L.I. Gender affects sympathetic and hemodynamic response to postural stress. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2028–H2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- Tahvanainen A., Koskela J., Tikkakoski A., Lahtela J., Leskinen M., Kahonen M., Nieminen T., Koobi T., Mustonen J., Porsti I. Analysis of cardiovascular responses to passive head-up tilt using continuous pulse wave analysis and impedance cardiography. Scand. J. Clin. Lab Invest. 2009;69:128–137. doi: 10.1080/00365510802439098. [DOI] [PubMed] [Google Scholar]
- Tahvanainen A., Leskinen M., Koskela J., Ilveskoski E., Nordhausen K., Oja H., Kahonen M., Koobi T., Mustonen J., Porsti I. Ageing and cardiovascular responses to head-up tilt in healthy subjects. Atherosclerosis. 2009;207:445–451. doi: 10.1016/j.atherosclerosis.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Timmers H.J., Wieling W., Soetekouw P.M., Bleijenberg G., Van Der Meer J.W., Lenders J.W. Hemodynamic and neurohumoral responses to head-up tilt in patients with chronic fatigue syndrome. Clin. Auton. Res. 2002;12:273–280. doi: 10.1007/s10286-002-0014-1. [DOI] [PubMed] [Google Scholar]
- van Campen C.(L.)M.C., Rowe P.C., Visser F.C. Blood Volume Status in ME/CFS Correlates With the Presence or Absence of Orthostatic Symptoms: Preliminary Results. Front Pedia. 2018;6:4. doi: 10.3389/fped.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen C.(L.)M.C., Rowe P.C., Visser F.C. Validation of the Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Other Measures than History: Activity Bracelet, Cardiopulmonary Exercise Testing and a Validated Activity Questionnaire: SF-36. Healthcare. 2020;8:16. doi: 10.3390/healthcare8030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen C.(L.)M.C., Rowe P.C., Visser F.C. The Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients with Joint Hypermobility Show Larger Cerebral Blood Flow Reductions during Orthostatic Stress Testing Than Patients without Hypermobility: A Case Control Study. Med. Res. Arch. 2021:9. [Google Scholar]
- van Campen C.L.M.C., Rowe P.C., Visser F.C. Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Med. (Kaunas. ) 2021:58. doi: 10.3390/medicina58010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen C.(L.)M.C., Verheugt F.W.A., Rowe P.C., Visser F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pr. 2020;5:50–58. doi: 10.1016/j.cnp.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen C.(L.)M.C., Verheugt F.W.A., Visser F.C. Cerebral blood flow changes during tilt table testing in healthy volunteers, as assessed by Doppler imaging of the carotid and vertebral arteries. Clin. Neurophysiol. Pr. 2018;3:91–95. doi: 10.1016/j.cnp.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen C.(L.)M.C., Visser F.C. The abnormal Cardiac Index and Stroke Volume Index changes during a normal Tilt Table Test in ME/CFS patients compared to healthy volunteers, are not related to deconditioning. J. Thromb. Circ. 2018:1–8. [Google Scholar]
- van Campen C.(L.)M.C., Visser F.C. Blood volume status in patients with chronic fatigue syndrome: relation to complaints. Int. J. Clin. Med. 2018;9:809–819. [Google Scholar]
- van Campen C.(L.)M.C., Visser F.C. The higher resting heart rate in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients compared to healthy controls: relation with stroke volumes. Med. Res. Arch. 2022:10. [Google Scholar]
- van de Luit L., van der Meulen J., Cleophas T.J., Zwinderman A.H. Amplified amplitudes of circadian rhythms and nighttime hypotension in patients with chronic fatigue syndrome: improvement by inopamil but not by melatonin. Angiology. 1998;49:903–908. doi: 10.1177/000331979804901105. [DOI] [PubMed] [Google Scholar]
- Vanness J.M., Snell C.R., Strayer D.R., Dempsey L., Stevens S.R. Subclassifying chronic fatigue syndrome through exercise testing. Med Sci. Sports Exerc. 2003;35:908–913. doi: 10.1249/01.MSS.0000069510.58763.E8. [DOI] [PubMed] [Google Scholar]
- Wyller V.B., Saul J.P., Amlie J.P., Thaulow E. Sympathetic predominance of cardiovascular regulation during mild orthostatic stress in adolescents with chronic fatigue. Clin. Physiol. Funct. Imaging. 2007;27:231–238. doi: 10.1111/j.1475-097X.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- Youde J., Panerai R., Gillies C., Potter J. Reproducibility of circulatory changes to head-up tilt in healthy elderly subjects. Age Ageing. 2003;32:375–381. doi: 10.1093/ageing/32.4.375. [DOI] [PubMed] [Google Scholar]
- Zaidi A., Benitez D., Gaydecki P.A., Vohra A., Fitzpatrick A.P. Haemodynamic effects of increasing angle of head up tilt. Heart. 2000;83:181–184. doi: 10.1136/heart.83.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript/research paper does not include datasets of the following: Structural/crystallographic data for both macromolecular structures and small molecules; Protein and nucleic acid sequence data (this includes RNASeq data); Functional genomics and molecular interactions/proteomics/metabolomics data; Computational models; Genetics data (genetic polymorphisms; genotype data). Therefore data availability is not applicable to this study.