Abstract
Brucellosis is a zoonotic infection that commonly affects cattle in Ethiopia, causing significant negative economic impact. A cross-sectional study was carried out between November 2020 and November 2021 in southwest Ethiopia to determine the seroprevalence of brucellosis and its associated risk factors in cattle herds. Blood samples were taken from 461 randomly selected cattle to test for the presence of Brucella antibodies using the Rose Bengal Plate test, with positive serum confirmed through the complement fixation test. A multivariable random effect logistic regression analysis was used to identify potential risk factors for Brucella seropositivity. The study found 7.14% (95% CI: 4.44–9.01) seroprevalence at the animal level and 12.23% (95% CI: 6.52–16.05) at the herd level based on the complement fixation test. Age (OR = 6.9, 95%CI: 1.83–15.97), herd size (OR = 3.66, 95%CI: 1.39–9.61), introducing new animals (OR = 2.72, 95%CI: 1.17–6.29), management system (OR = 12.2, 95%CI: 1.53–26.80), species composition (OR = 4.24, 95%CI: 1.51–11.91), and abortion (OR = 7.1, 95%CI: 1.93–15.39) were found to be associated with Brucella seropositivity. The analysis also revealed two risk factors for Brucella infection at the herd level, including herd size (OR = 3.4, 95% CI: 1.05–10.68) and species composition (OR = 3.1, 95% CI: 1.20–7.88). The presence of Brucella antibodies in cattle highlights the need for increased awareness and measures to mitigate the identified risk factors of the disease to prevent its spread. Furthermore, further studies are necessary to investigate the zoonotic transmission of brucellosis to humans and its role in cattle reproduction disorders in the study area.
Keywords: Brucellosis, Epidemiology, Cattle, Southwest Ethiopia
1. Introduction
Bovine brucellosis is a widespread disease in several countries, affecting both human and animal populations, and leading to substantial economic losses [[1], [2], [3]]. The main reason for the negative effect of the disease on cattle breeding in these countries is the low reproductive efficiency and occurrence of abortions [[4], [5], [6], [7]]. Brucella abortus and B. melitensis are the primary causes of bovine brucellosis, while B. suis is a less common pathogen that can cause late-term abortions, retained fetal membranes, and infertility in subsequent pregnancies [8,9]. The transmission of Brucella is usually through direct contact with an infected animal or fetus or indirect contact with contaminated objects [10]. Various risk factors for bovine brucellosis have been identified, including herd size, cattle age and sex, management practices, interaction with wildlife, environmental factors, and the use of different cattle breeds [3,[11], [12], [13]].
Despite Ethiopia having one of the largest cattle populations in Africa, this valuable resource is not being optimally utilized due to a range of constraints affecting cattle production [14]. Key factors such as animal diseases, poor genetics, inadequate animal health services, nutritional deficiencies, and management issues have all contributed to this situation [15,16]. One significant animal disease that affects both cattle and humans is brucellosis, which has a high seroprevalence in areas where people live in close proximity to livestock [17,18]. Various authors [4,[19], [20], [21]] have conducted serological evaluations to determine the prevalence of Brucella infections in Ethiopian cattle across different regions of the country.
Several studies conducted in different areas of the country [[22], [23], [24], [25]] have reported a significant economic burden caused by brucellosis in cattle due to abortion and other reproductive issues. However, these studies did not provide adequate information on the risk factors contributing to the development and spread of the disease across various agro-ecologies. Understanding the epidemiology of brucellosis in southwest Ethiopia is crucial to designing effective control measures. Hence, this study aimed to determine the seroprevalence of Brucella infection at both the herd and individual animal levels and to identify the associated risk factors in different agro-ecologies in southwest Ethiopia.
2. Materials and methods
2.1. Description of study areas
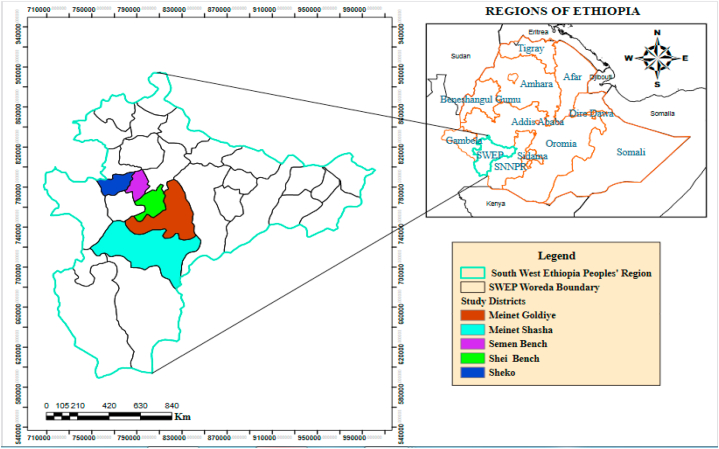
The study was carried out in the Southwest Ethiopia, specifically in the Bench-Sheko (Semen-Bench, Shei Bench, and Sheko) and West Omo (Meinet Shasha and Goldiya) zones, which are represented in Fig. 1. These zones are located between latitudes 5°.88′ and 7°.21′ N and longitudes 34°.88′ and 36°.14′ E and are situated at altitudes ranging from 500 to 3000 m above sea level. The annual temperature in the zones varies from 15.1 °C to 27 °C, with an average rainfall of 400–2000 mm per year. In Ethiopian agro-ecology, the zones are classified as lowland (1500 m above sea level), mid-altitude (1500–2300 m above sea level), and highland (>2300 m above sea level) [26]. Thus, the selected districts were grouped into three agro-ecological zones: lowland (Meinet Shasha), mid-altitude (Sheko and Shei-Bench), and highland (Semen Bench and Meinet Goldiya). The study area has an estimated 71,047 goats, 73,384 sheep, and 1,596,803 cattle [14], with the Zebu and Sheko breeds being the most common among the cattle, and a few Holstein-Friesian crosses. The management practices considered for inclusion in the research areas were extensive (for crop-livestock production) and semi-intensive (for urban production).
Fig. 1.
Map of study areas.
2.2. Study design and population
A cross-sectional study was carried out from November 2020 to November 2021 to determine the seroprevalence and risk factors associated with Brucella infection in female cattle aged six months and above. The study was conducted in the Bench-Sheko and West Omo zones' districts, including Semen Bench, Shei Bench, Sheko, Meinet Goldiya, and Meinet Shasha. The study population was diverse in terms of age, body condition, breed, agro-ecology, and management system. Notably, the cattle used in this study were unvaccinated against brucellosis.
2.3. Sampling procedure and sample size determination
Due to the lack of previous study on brucellosis in cattle in the study areas, we used the formula provided by Thrusfield [27] to determine the required sample size for this study. A desired absolute precision of 5% and a 95% confidence interval of the estimated prevalence of 50% were used in the calculation, resulting in a requirement of 384 cattle. However, to account for non-response or missing values and avoid bias, we oversampled by 10%–20%, as suggested by Naing et al. [28]. Therefore, we used a total of 461 cattle for this study. We employed a multistage sampling procedure, with random selection of sampling units at each stage [29]. The study zones were purposefully selected because they had high incidences of Brucella infection and a large cattle population (unpublished data). Districts, kebeles, villages, and herds were selected using random sampling techniques. Specifically, Semen Bench, Shei Bench, Sheko, Meinet Shasha, and Meinet Goldiya were chosen by lottery method from a total of fifteen districts in the zones. From these districts, 28 kebeles were selected using a proportional simple sampling technique. Based on the number of villages in the kebeles, 84 villages were then randomly selected using a proportional simple random selection method. Finally, a proportional simple random sampling method was used to select 191 herds based on the number of herds present in each village. For the sampling of individual cattle within each herd, a simple random method was used, with the number of animals sampled from each herd varying according to the number of cattle present.
2.4. Blood sample collection
Using a sterile needle and a vacutainer tube, approximately 10 ml of blood was collected from the jugular vein of each cattle. The vacutainer tubes were labeled with the corresponding identification of each animal. The blood samples were allowed to stand overnight at room temperature for the serum to separate. The obtained serum was decanted into cryovials, which were labeled with the animal codes. The serum samples were then stored at −20 °C [30] in the Mizan Regional Veterinary Laboratory until they were transported to the National Veterinary Institute in Debrezeite. The serum samples were kept in an ice box during transportation for serological analysis.
2.5. Serological tests
The presence of Brucella agglutinins in serum samples was assessed using the Rose Bengal Plate Test (RBPT) (Veterinary Laboratories Agency, New Haw, Addlestone, Surrey, KT153, UK), following the recommendations of the OIE [31]. Prior to testing, both serum samples and antigens were removed from refrigeration and left at room temperature for 30 min, as per the guidelines. To perform the test, 30 μL of serum was dispensed onto a plate, followed by the addition of 30 μL of RBPT antigen. An applicator stick was used to thoroughly mix the serum and antigen, which were then manually rocked on the plate for approximately 4 min. Positive and negative controls were included and interpreted based on the degree of agglutination, with bright lighting or a magnifying glass used as necessary for micro agglutination. Results were reported using the classification system described by Dohoo et al. [29], with a score of 0 indicating no agglutination, + indicating agglutination visible only with magnification, ++ indicating fine agglutination, and +++ indicating coarse clumping. Serums that showed no agglutination were considered negative, while those with a score of +, ++, or +++ were considered positive.
The complement fixation test (CFT), along with B. abortus antigen S99 and control sera (positive and negative), was employed to confirm all positive sera (RBPT). The Veterinary Laboratories Agency in New Haw, Addlestone, Surrey, KT153, UK was used for this purpose. The test sera were prepared in two-fold dilutions (1:5, 1:10, 1:20, and 1:40) using a standard antigen dilution of 1:10, and were added to Brucella antigen, guinea pig complement, and 3% sensitized sheep red blood cells in standard 96-well U-bottom microliter plates. The reagent was prepared and tested by titration using OIE-recommended protocols [30]. The plates were centrifuged at 2500 rpm for 5 min at 4 °C, and then incubated at 37 °C for 30 min with agitation. A strong reaction, more than 75% complement fixation (3+) at 1:5 dilution, or at least 50% complement fixation (2+) at 1:10 dilution and above, was considered positive, while lack of fixation/complete hemolysis was considered negative [31]. For serial interpretation, cattle were considered positive if they were seropositive on both the RBPT and the CFT. Combining RBPT and CFT in serial is recommended to eliminate false-positive serological cross-reactions and increase test specificity [29].
2.6. Data collection
Information regarding putative risk factors for cattle brucellosis was recorded using the MS Excel Spreadsheet 2010 program. For each individual, factors such as agro-ecology, age, breed, body condition, parity, pregnancy status, history of abortions, retained fetal membranes, herd size, introduction of new animals into a herd, accessibility to wild animals, management system, and species composition (cattle mixed with sheep and/or goats) were recorded. To categorize the body condition score of cattle, a system based on the appearance of ribs and vertebral spinous processes was used. Scores of 1 and 2 were categorized as poor, 3 as medium, and scores of 4 and 5 as good [32]. The three herd size categories used were small (<15 heads of cattle), medium (15–30 heads of cattle), and large (>30 heads of cattle). The livestock management system was classified as either extensive or semi-intensive, based on criteria established by Richard [33]. Cattle were divided into three age groups (<3, 3–6, and >6 years) based on the understanding that the age at first calving for cattle in tropical conditions ranged from two to three years [34]. Cattle were categorized as nulliparous, monoparous, and pluriparous, based on the number of offspring they had [35]. Abortion was defined as the termination of pregnancy between 45 and 260 days gestation [36,37].
2.7. Data analysis
The collected data were analyzed using STATA version 14.0 (Stata Corp. College Station, TX, USA) for Windows. Individual animals were considered positive for brucellosis if they tested RBPT + or CFT+. Similarly, herds with at least one seropositive animal were considered positive for brucellosis. The herd level seroprevalence was calculated by dividing the number of herds with at least one infected animal and positive RBPT and CFT results by the total number of herds sampled. To determine the apparent seroprevalence of brucellosis, the proportion of RBPT and CFT seropositive samples was divided by the total number of tested animals. To estimate the true prevalence of brucellosis in cattle, the sensitivity and specificity estimates for RBPT and CFT tests predicted by EFSA [38] were imputed in the Rogan and Gladen formula [39]: TP= (AP + CSes −1)/(CSes + CSps −1), where TP represents true prevalence, AP represents apparent prevalence, CSes represents the combined sensitivity of the test series (SeRBPT × SeCFT), and CSps represents the combined specificity of the test series (1-(1-spRBPT) × (1-spCFT)). The animal level seroprevalence was then determined after adjusting for sample weighing. The 95% confidence interval (CI) for each seroprevalence was calculated using the binomial exact method in Epitools.
A logistic regression model was used to investigate the association between seroprevalence and brucellosis risk factors. Univariable random effects logistic regression methods were used to screen multiple variables associated with brucellosis. The herd was used as a random effect to explain the likelihood of cattle grouping in herds and herd size variation. Variables with P ≤ 0.25 in univariable analysis were included in the multivariable logistic model. A backward elimination procedure was used for further variable selection. The risk factors associated with brucellosis were identified using a multivariable random effects logistic regression model, and their strength of association was assessed using adjusted odds ratios (OR). Cross-product terms were used to examine interaction effects, and the collinear matrix index was used to check for multiple-collinearity before building the final model. The validity and predictive power of the model were evaluated using the Hosmer-Lemeshow test and ROC curve. Significance was set at P ≤ 0.05 with a confidence level (CL) of 95% for all analyses.
3. Results
3.1. Animal-level and herd-level true seroprevalence of brucellosis
The RBPT and CFT examinations showed that 7.16% and 6.72% of the cattle were positive for Brucella antibodies, respectively. The Semen Bench district had the highest prevalence of Brucella antibody (9.09%), while the Meinet Shasha district had the lowest prevalence (4.55%). The true seroprevalence of Brucella antibody positivity for all animals, according to CFT, was 7.14%. However, the Sheko district had the highest herd level seroprevalence (21.05%), while the Meinet Goldiya district had the lowest (4.88%). The overall true seroprevalence was 12.23%. A statistically significant difference (P < 0.05) was found between study areas at both the animal and herd levels (Table 1).
Table 1.
The distribution of cattle brucellosis seroprevalence at the animal and herd levels in the study areas.
| Animal-level |
Herd-level |
|||
|---|---|---|---|---|
| Study areas | Total cattle tested | Seroprevalence % (95%CI) | Total herds tested | Seroprevalence % (95%CI) |
| Shey Bench | 118 | 8.47 (3.45–13.50) | 44 | 15.91 (5.10–26.72) |
| Sheko | 171 | 6.43 (2.76–10.11) | 38 | 21.05 (8.09–34.01) |
| Semen Bench | 44 | 9.09 (0.60–17.59) | 22 | 9.09 (2.92–21.10) |
| Meinet Goldiya | 62 | 4.84 (0.50–10.18) | 41 | 4.88 (1.72–11.47) |
| Meinet Shasha | 66 | 4.55 (0.48–9.57) | 46 | 6.52 (0.61–13.66) |
| Overall | 461 | 7.14 (4.44–9.01) | 191 | 12.23 (6.52–16.05) |
CI: Confidence Interval.
3.2. Herd-level potential risk factors of brucellosis
In the final multivariable logistic regression model, herd size and species composition were found to have a significant effect (P < 0.05) on the herd-level seroprevalence of cattle brucellosis in both univariable and multivariable analyses. However, the analysis showed that there was no significant association (P > 0.05) between the herd-level seroprevalence of brucellosis and factors such as agro-ecology, management system, accessibility to wild animals, or introducing new animals, as shown in Table 2.
Table 2.
Analysis of potential risk factors for brucellosis at the herd level using univariate and multivariate methods in southwest Ethiopia.
| Variables |
Category |
Total herd examined |
Total herd positive (%) |
Univariable |
Multivariable |
||
|---|---|---|---|---|---|---|---|
| Crude OR (CI 95%) | P-value | Adjusted OR (CI 95%) | P-value | ||||
| Agro-ecology | 0.810 | ||||||
| Lowland | 57 | 12.28 | – | – | – | – | |
| Mid-land | 52 | 13.46 | 0.88 (0.29–2.71) | 0.682 | |||
| Highland | 81 | 9.88 | 1.3 (0.43–3.67) | 0.525 | |||
| Herd size | 0.016 | 0.036 | |||||
| Small | 88 | 19.32 | – | – | |||
| Medium | 41 | 2.44 | 9.6 (1.23–74.67) | 0.031 | 6.8 (0.84–14.42) | 0.072 | |
| Large | 62 | 6.45 | 3.5 (1.11–10.89) | 0.033 | 3.4 (1.05–10.68) | 0.041 | |
| Species composition | Only cattle | 52 | 23.08 | – | – | – | – |
| Mixed with sheep and/or goats | 139 | 7.19 | 3.9 (1.56–9.63) | 0.004 | 3.1 (1.20–7.88) | 0.020 | |
| Management system | Semi-intensive | 38 | 18.42 | – | – | ||
| Extensive | 153 | 9.80 | 2.1 (0.78–5.53) | 0.143 | |||
| Introducing new animals into a herd | No | 99 | 16.16 | – | – | ||
| Yes | 92 | 6.52 | 2.8 (1.03–7.40) | 0.043 | |||
| Accessibility to wild animal | No | 178 | 11.80 | – | – | ||
| Yes | 13 | 7.69 | 1.6 (0.20–12.98) | 0.657 | |||
OR: Odds Ratio; CI: Confidence Interval.
3.3. Animal-level potential risk factors of brucellosis
The results of the study indicated a significant association between age groups and the prevalence of brucellosis (P < 0.05). Older animals had a significantly higher chance of being seropositive, with an odds ratio (OR) of 6.6, compared to their younger counterparts. The study also found a significant association (P < 0.05) between herd size and seroprevalence of brucellosis, with cattle from larger herds having an OR of 4.3 for Brucella seropositivity compared to those from smaller herds. Furthermore, the study showed a statistically significant association (P < 0.05) between the management system and Brucella seropositivity, with cattle under extensive management having an OR of 8.4 for Brucella seropositivity compared to those under semi-intensive management. Introducing new animals into herds was found to significantly increase the risk of brucellosis, with an OR of 2.8. Animals that had contact with sheep and/or goats were found to have a significantly higher risk of contracting brucellosis (P < 0.05), with an OR of 4.2 for those with close contact compared to those with less or no contact. The study also found a significant association (P < 0.05) between brucellosis seropositivity and a history of abortion in cattle. However, univariable analysis showed that body condition, parity, pregnancy status, breed, agro-ecology, cattle access to wild animals, and retained fetal membrane were not significantly associated (P > 0.05) with the seroprevalence of brucellosis (Table 3).
Table 3.
Possible risk factors for cattle in the study areas based on a single-variable logistic regression analysis.
| Variables | Category | Total cattle examined | Total cattle positivity (%) | Crude OR (95%CI) | P-value |
|---|---|---|---|---|---|
| Age | 0.003 | ||||
| <3 years | 138 | 3 (2.22) | – | – | |
| 3–6 years | 188 | 10 (5.32) | 2.7 (1.19–5.98) | 0.017 | |
| >6 years | 135 | 18 (13.04) | 6.6 (1.90–22.97) | 0.003 | |
| Breed | Local | 440 | 29 (6.59) | – | – |
| Cross | 21 | 2 (9.52) | 1.5 (0.33–6.72) | 0.602 | |
| BCS | 0.477 | ||||
| Poor | 89 | 4 (4.49) | – | – | |
| Medium | 219 | 14 (6.39) | 1.4 (0.62–2.98) | 0.443 | |
| Good | 153 | 13 (8.50) | 1.2 (0.62–6.25) | 0.248 | |
| Herd size | 0.002 | ||||
| Small | 231 | 9 (3.90) | – | – | |
| Medium | 129 | 7 (5.43) | 3.0 (1.19–7.77) | 0.020 | |
| Large | 101 | 15 (14.85) | 4.3 (1.82–10.20) | 0.001 | |
| Species composition | Only cattle | 198 | 5 (2.53) | – | – |
| Mixed with sheep and/or goats | 263 | 26 (9.89) | 4.2 (1.60–11.24) | 0.004 | |
| Management system | Extensive | 366 | 30 (8.20) | 8.4 (1.13–22.35) | 0.038 |
| Semi-intensive | 95 | 1 (1.05) | |||
| Introducing new animals into a herd | Yes | 176 | 19 (10.80) | 2.8 (1.30–5.82) | 0.008 |
| No | 285 | 12 (4.21) | – | – | |
| Accessibility to wild animal | Yes | 16 | 1 (6.25) | 1.2 (1.30–8.49) | 0.939 |
| No | 445 | 30 (6.74) | – | – | |
| Agro-ecology | 0.634 | ||||
| Lowland | 55 | 2 (3.64) | 1.0 (0.47–2.13) | 0.989 | |
| Mid-land | 210 | 15 (7.14) | 2.0 (0.45–9.25) | 0.356 | |
| Highland | 196 | 14 (7.14) | |||
| Parity | 0.951 | ||||
| Nulliparous | 110 | 8 (7.27) | 0.9 (0.36–2.10) | 0.760 | |
| Monoparous | 101 | 7 (6.93) | 0.92 (0.37–2.30) | 0.856 | |
| Pluriparous | 250 | 16 (6.40) | – | – | |
| 0.353 | |||||
| Pregnancy status | After 5 months | 93 | 8 (8.60) | 2.6 (0.59–11.26) | 0.208 |
| Before 5 months | 70 | 2 (2.86) | 0.8 (0.34–1.88) | 0.618 | |
| None pregnant | 298 | 21 (7.05) | – | – | |
| History of abortion | Yes | 104 | 12 (11.54) | 9.5 (1.27–20.15) | 0.028 |
| No | 357 | 19 (5.32) | – | – | |
| Retained placenta | Yes | 19 | 1 | 1.3 (0.17–10.16) | 0.796 |
| No | 442 | 30 | – | – |
OR: Odds Ratio; CI: Confidence Interval, BCS: Body condition score.
No multicollinearity or significant interactions among the variables were detected. The model was deemed suitable for the data, as indicated by a Hosmer-Lemeshow goodness-of-fit value (χ2 = 8.620, P = 0.375). The accuracy of the model was confirmed by the ROC curve (AUC = 0.857, 95%CI: 0.79–0.92). Based on the multivariable logistic regression analysis, various factors, including age, herd size, species composition, management system, introduction of new animals, and a history of abortion in cattle, were associated with the occurrence of brucellosis in cattle (Table 4).
Table 4.
Analysis of factors associated with Brucella seropositivity in study areas using multivariable logistic regression.
| Variables | Category | Total cattle examined | Total cattle positivity (%) | Adjusted OR (95%CI) | P-value |
|---|---|---|---|---|---|
| Age | 0.002 | ||||
| <3 years | 138 | 3 (2.22) | – | – | |
| 3–6 years | 188 | 10 (5.32) | 3.5 (1.43–8.44) | 0.006 | |
| >6 years | 135 | 18 (13.04) | 6.9 (1.83–15.97) | 0.004 | |
| Herd size | 0.018 | ||||
| Small | 231 | 9 (3.90) | – | – | |
| Medium | 129 | 7 (5.43) | 2.91 (1.04–8.16) | 0.042 | |
| Large | 101 | 15 (14.85) | 3.66 (1.39–9.61) | 0.008 | |
| Species composition | Only cattle | 198 | 5 (2.53) | – | – |
| Mixed with sheep and/or goats | 263 | 26 (9.89) | 4.24 (1.51–11.91) | 0.006 | |
| Management system | Semi-intensive | 95 | 1 (1.05) | – | – |
| Extensive | 366 | 30 (8.20) | 12.2 (1.53–26.80) | 0.018 | |
| Introducing of new animal | No | 285 | 12 (4.21) | – | – |
| Yes | 176 | 19 (10.80) | 2.72 (1.17–6.29) | 0.020 | |
| History of abortion | No | 357 | 19 (5.32) | – | – |
| Yes | 104 | 12 (11.54) | 7.1 (1.93–15.39) | 0.031 |
OR: Odds Ratio; CI: Confidence Interval.
4. Discussion
This study revealed that various factors at the individual animal level, such as age, species composition, herd size, management practices, introduction of new animals, and history of abortion, can affect the prevalence of brucellosis in cattle. Moreover, the study found that herd size and species composition significantly influence the prevalence of Brucella infection at the herd level. The high prevalence of Brucella infection in cattle in the study areas causes severe economic losses due to reproductive disorders such as abortion and infertility, and also poses a significant risk to public health. Based on serological evidence, this study provides epidemiological data on Brucella infection in cattle in southwest Ethiopia. Brucellosis in cattle is a zoonotic disease that causes significant economic loss in the country [22,40]. As the first study in the selected areas to provide information on the epidemiology of brucellosis, this research could inform the application of appropriate management techniques to control and prevent the disease in cattle.
In the present study, the overall seroprevalence of Brucella antibodies at the animal level was found to be 7.14%. This aligns with previous reports by Mekonen et al. [12] and Eyob et al. [41] who reported seroprevalence of 6.10% and 9.87%, respectively, in Western Tigray and Asella, Ethiopia. Similar findings were also reported in other African countries such as Ghana (6.6%) by Kubuafaor et al. [42] and Chad (6.6%) by Schelling et al. [43]. However, compared to earlier studies conducted in the country, such as in Assela (14.1%) [44] and Borana (10.6%) [45], the prevalence reported in this study is lower. However, higher seroprevalence have been observed in other African countries, including Togo (41%) [46] and Uganda (46.8%) [47]. The current study's seroprevalence results were higher than those reported in central Ethiopia (2.9%) [48], Sidama zone (1.7%) [4], Addis Ababa dairy farms (1.5%) [49], and Arsi zone (2.6%) [50]. The variation in seroprevalence across different study areas and countries could be attributed to various factors, including environmental conditions, management strategies, farming practices, sources of replacement animals, farmer education levels, hygienic practices on the farms, and accessibility of maternity pens during calving [9].
The herd-level seroprevalence in this study (12.23%) is similar to that reported by previous studies, such as Tsegaye et al. [50] (9.5% in Aris zone), Robi and Gelalcha [22] (11.6% in the Jimma zone), Asmare et al. [4] (13.7% in Sidama zone), Etefa et al. [25] (12.3% in Jimma zone), and Alhaji et al. [51] (9.7% in Nigeria). However, it is lower than the findings reported by Megersa et al. [45] in southern and eastern Ethiopia (26.1%), and Mekonen et al. [12] in western Tigray (24.1%). Other studies have reported even higher herd-level seroprevalence, such as 62% in Zambia [52], 55.6% in Uganda [53], and 43.3% in Ethiopia [18]. The difference in results could be due to variations in the prevalence of the disease at the overall animal level and the herd size at the time of the study. Moreover, the study found that the size of the herd was a risk factor for Brucella seropositivity at the herd level, with larger herds having approximately three times higher odds (OR = 3.4) of Brucella seropositivity than smaller herds. This finding is consistent with [45], who reported that large herds in southern and eastern Ethiopia were at the highest risk of acquiring Brucella infection at the herd level.
The study revealed that age was significantly associated with the risk of being seropositive for Brucella infection. Animals over six years old were found to have a significantly higher risk (OR = 6.9) of contracting the infection compared to their younger counterparts. This finding is in line with several studies conducted in Ethiopia [13,18,22,45,[54], [55], [56]] and elsewhere [3,57,58], which also identified age as a major risk factor for Brucella infection in cattle. This study supports the findings of Radostits et al. [9], which suggest that younger animals tend to be more resistant to infection and recover from it more frequently. This is likely due to age-related increases in the concentration of sex hormones and erythritol, which promote bacterial growth and multiplication, making older animals more susceptible to brucellosis [9].
Based on this study, introducing a new animal to a herd increases the risk of brucellosis threefold (OR = 2.72). This association between Brucella serostatus and the adding new animals to the herd was found to be significant. These results are consistent with previous studies [51,59] which also revealed a statistically significant association between Brucella antibody seropositivity and the introduction of new animals into the herd. Moreover, Stringer et al. [60] and Cardenas et al. [61] found that introducing new animals from unknown areas was a significant risk factor for Brucella infection in cattle, further supporting these findings.
In the present study, a significant difference in the prevalence of Brucella antibodies was observed between herd sizes, with larger herds being almost four times more likely (OR = 3.66) to be seropositive. Previous studies have identified herd size as a critical factor in the transmission of Brucella among susceptible and infected animals [62]. This is mainly due to the higher availability of positive animals in larger herds compared to smaller ones [63]. Previous researchers have suggested that the likelihood of exposure and maintenance of Brucella after an abortion increases with larger herd sizes due to increased contact in shared feeding and watering areas, facilitating the spread of Brucella organisms [3,4,13,20,22,64]. In contrast to this finding, Kebede et al. [56] reported that there was no association between herd size and brucellosis. The variation in reports across different regions of Ethiopia and other countries may be due to several factors, including agro-ecology, animal breed, and management systems.
This study has found that cattle kept in households with a mix of goats, sheep, and/or cattle have a significantly higher likelihood of testing positive for Brucella (OR = 4.24) compared to those kept with only cattle. This is likely due to the increased chance of Brucella transmission between species when they are herded together. It is important to note that Brucella is not limited to a particular host species; Brucella melitensis has been found in cattle as well [65]. This suggests that mixing animal species could have contributed to the spread of the pathogen from small ruminants to cattle. Moreover, combining too many animal species into one herd can lead to higher contact and density between animals, increasing the risk of exposure to the Brucella organism and infection [66]. The results of this study align with previous studies conducted in Ethiopia [22,45]. These studies demonstrated that in Ethiopia, the combination of sheep and/or goats with cattle increased the likelihood of Brucella seropositivity in cattle. Similar findings were reported in studies from Eritrea [62], Jordan [63], and Malaysia [67], indicating that mixing sheep and/or goats with cattle poses a risk for Brucella transmission among various animal species. However, these findings contradict the results of Elabdin et al. [68], who found no significant association between Brucella seropositivity and the presence of sheep and/or goats with cattle in Sudan. These differences may be attributed to variations in the environment, animal breeds, and management practices. The prevalence of Brucella infections at the herd level was found to be influenced by the species present, with herds containing sheep and/or goats exhibiting a greater likelihood of seropositivity. Multiple studies have reported that the practice of herding sheep, goats, and cattle is associated with an increased risk of Brucella infection seropositivity [22,45,51,63,66].
The study demonstrated a significant (P < 0.05) association between the management system and the seropositivity of brucellosis in cattle. Cattle managed extensively were 12.2 times more likely to be infected with Brucella (OR = 12.2) than those managed semi-intensively. The higher risk of infection among extensively managed cattle could be attributed to poor husbandry practices and the increased likelihood of contact with diseased animals during co-grazing. In addition, a comprehensive management system can also increase the likelihood of contact with infected or carrier animals. The findings of this study are consistent with a previous study [69] that reported an increased risk of Brucella transmission in cattle that share a common pasture.
The current study found significant association between Brucella seroprevalence in female cattle and their history of abortion. Cattle with a prior history of abortion were found to have a 7.1 times higher likelihood of being Brucella seropositive compared to those without such history. This finding suggests association between the prevalence of Brucella pathogen and cattle abortion. Our study aligns with previous research conducted in Ethiopia [20,21,25,50], as well as with the findings of Alhaji et al. [51], Sagamiko et al. [70], and Derdour et al. [71] which reported an association between brucellosis and a history of cattle abortion. However, this finding differs from the results of Asmare et al. [72], Shabbir et al. [73], and Asmare [74] which did not find a significant association between brucellosis and cattle abortion. The differences in findings may be attributed to variations in agro-ecologies, management systems, and environmental conditions in the study areas that could facilitate the spread of various causes of abortion [9].
Brucellosis serology has a limitation in that the tests used worldwide detect antibodies against s-LPS epitopes that are shared by other Brucella species and other organisms, leading to cross-reactions, such as with Yersinia enterocolitica O:9 [75,76]. To maximize specificity, it is recommended to use two tests serially in epidemiologic studies [30,77]. Dohoo et al. [29] suggest that serial testing has a higher presumed specificity compared to the individual specificities of each test since test specificities are conditionally independent of one another. Utilizing series testing in populations with diseases can increase specificity and positive predictive value, but there is a potential for false negatives and missing true positive cases [45]. The combination of RBPT, CFT, and c-ELISA is expected to reduce misclassification and increase the chance of detecting antibodies against brucellosis when present in a given serum. However, Mainar-Jaime et al. [78] argue that serial testing using pairs of specificity-correlated serological testing (RBPT, CFT, c-ELISA) has lower specificity than expected when applied to disease-free populations. This can lead to an increased proportion of non-infected animals being classified as seropositive. Dohoo et al. [79] point out that the choice of test cut-offs has different diagnostic goals depending on the context, such as a screening situation versus a confirmatory diagnostic situation. In this study, using a high cut-off point may increase the specificity of the test, but it may also have the shortcoming of missing positive cases. Therefore, it is important to consider the context when choosing the cut-off point.
5. Conclusion
The findings of the study indicate that Brucella antibodies are prevalent among cattle in the southwest Ethiopia. Although the presence of antibodies does not necessarily indicate active infection, these results suggest that brucellosis may be present in the study area. The study also identified several risk factors for Brucella seropositivity at the animal level, including age, management practices, introduction of new animals, and instances of abortion. Moreover, the study found that herd size and species composition were associated with Brucella seropositivity at both the individual and herd levels. The presence of brucellosis in milk-producing animals poses a significant risk to human health. Therefore, it is crucial to implement effective control measures and increase public awareness of the zoonotic transmission of brucellosis. It is also important to conduct further research on the zoonotic transmission of brucellosis to humans, as well as its potential role as a cause of reproductive disorders in cattle in the area.
Funding statement
This work was supported by the Ethiopian Institute of Agricultural Research (EIAR), Ethiopia.
Ethics consideration and clearance
Ethiopian Institute of Agricultural Research (EIAR) undertook all techniques following the experimentation standards and practices that were confirmed by the committee on the welfare of animals and ethical issues, which were in line with the global standards for animal welfare with the reference number EIAR-2662/2010.
Author contribution statement
Dereje Tulu Robi: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Beksisa Urge: Contributed reagents, materials, analysis tools or data.
Ararsa Bogale: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Melkam Aleme: Analyzed and interpreted the data.
Shiferaw Temteme: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors claim to have no competing interests.
Acknowledgments
The farmers who participated in this research were all gratefully acknowledged by the authors. The authors also acknowledge the logistical and financial assistance provided by the Ethiopian Institute of Agricultural Research. In addition, we appreciate the help that the Bench-Sheko and West Omo zone offices gave us while we were working in the field.
References
- 1.Jergefa T., Kelay B., Bekana M., Teshale S., Gustafson H., Kindah H. Epidemiological study of bovine brucellosis in three agro-ecological areas of central Oromiya, Ethiopia. Review in Scientific and Technical of the Office International des Epizooties. 2009;28:933–943. doi: 10.20506/rst.28.3.1939. [DOI] [PubMed] [Google Scholar]
- 2.Mai H.M., Irons P.C., Kabir J., Thompson P.N. A large seroprevalence survey of brucellosis in cattle herds under diverse production systems in northern Nigeria. BMC Vet. Res. 2012;8:1–14. doi: 10.1186/1746-6148-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matope G., Bhebe E., Muma J.B., Lund A., Skjerve E. Herd level factors for Brucella seropositivity in cattle reared in smallholder dairy farms in Zimbabwe. Prev. Vet. Med. 2010;94:213–221. doi: 10.1016/j.prevetmed.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Asmare K., Asfaw Y., Gelaye E., Ayelet G. Brucellosis in extensive management system of Zebu cattle in Sidama zone, southern Ethiopia. Afr. J. Agric. Res. 2010;5:257–263. [Google Scholar]
- 5.Ducrotoya M., Bertub W.J., Matopec G., Cadmusd S., Conde-Álvareze R., Gusib A.M., et al. Brucellosis in Sub-Saharan Africa: current challenges for management, diagnosis and control. Acta Trop. 2017;165:179–193. doi: 10.1016/j.actatropica.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Franc K.A., Krecek R.C., Hasler B.N., Arenas-Gamboa A.M. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary ac- tion. BMC Publ. Health. 2018;18:125. doi: 10.1186/s12889-017-5016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ndazigaruye G., Mushonga B., Kandiwa E., Samkange A., Segwagwe B.E. Prevalence and risk factors for brucellosis seropositivity in cattle in Nyagatare District, Eastern Province, Rwanda. J. S. Afr. Vet. Assoc. 2018;89(0) doi: 10.4102/jsava.v89i0.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szulowski K., Iwaniak W., Weiner M., Złotnicka J. Brucella suis biovar 2 isolations from cattle in Poland. Ann. Agric. Environ. Med. 2013;20:672–675. [PubMed] [Google Scholar]
- 9.Radostits O.M., Gay C.C., Hinchcliff K.W., Constable P.D. tenth ed. W.B., Saunders; London: 2007. Veterinary Medicine. A Text Book of Diseases of Cattle, Sheep, Pigs, Goats and Horses; pp. 963–985. [Google Scholar]
- 10.Acha N.P., Szyfres B. third ed. Pan. Amer. Health; Organization Washington, D.C., USA: 2001. Brucellosis in Zoonosis and Communicable Diseases Common to Humans and Animals; pp. 40–62. [Google Scholar]
- 11.Muma J.B., Samui K.L., Oloya J., Munyeme M., Skjerve E. Risk factors for brucellosis in indigenous cattle reared in livestock–wildlife interface areas of Zambia. Prev. Vet. Med. 2007;80:306–317. doi: 10.1016/j.prevetmed.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Mekonen H., Kalayou S., Kyule M. Serological survey of bovine brucellosis in Barka and Orado breeds (Bos indicus) of western Tigray, Ethiopia. Prev. Vet. Med. 2010;94:28–35. doi: 10.1016/j.prevetmed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Tolosa T., Bezabih D., Regassa F. Study on seroprevalence of bovine brucellosis, and abortion and associated risk factor. Bull. Anim. Health Prod. Afr. 2010;58:236–247. [Google Scholar]
- 14.CSA Livestock and livestock characteristics, agricultural sample survey. Addis Ababa, Ethiopia. Stat. Bull. 2021;2(589):13–140. [Google Scholar]
- 15.Kebede H., Melaku A., Kebede E. Constraints in animal health service delivery and sustainable improvement alternatives in North Gondar, Ethiopia. Onderstepoort J. Vet. Res. 2014;81:10. doi: 10.4102/ojvr.v81i1.713. [DOI] [PubMed] [Google Scholar]
- 16.Welay G.M., Tedla D.G., Teklu G.G., Weldearegay S.K., Shibeshi M.B., Kidane H.H., et al. A preliminary survey of major diseases of ruminants and management practices in Western Tigray province, northern Ethiopia. BMC Vet. Res. 2018;14:293. doi: 10.1186/s12917-018-1621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim N., Belihu K., Lobago F., Bekana M. Sero-prevalence of bovine brucellosis and its risk factors in Jimma zone of Oromia Region, South-western Ethiopia. Trop. Anim. Health Prod. 2010;42:35–40. doi: 10.1007/s11250-009-9382-z. [DOI] [PubMed] [Google Scholar]
- 18.Berhe G., Belihu K., Asfaw Y. Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray region of Ethiopia. Int. J. Appl. Res. Vet. Med. 2007;5:65–71. [Google Scholar]
- 19.Tolosa T., Regassa F., Belihu K. Seroprevalence study of bovine brucellosis in extensive management system in selected sites of Jimma zone, western Ethiopia. Bull. Anim. Health Prod. Afr. 2008;56:25–37. [Google Scholar]
- 20.Haileselassie M., Kalayou S., Kyule M., Asfaha M., Belihu K. Effect of Brucella infection on reproduction conditions of female breeding cattle and its public health significance in western Tigray, northern Ethiopia. Vet. Med. Int. 2011;201:7. doi: 10.4061/2011/354943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adugna K.E., Agga G.E., Zewde G. Seroepidemiological survey of bovine brucellosis in cattle under a traditional production system in western Ethiopia. Review in Scientific and Technical of the Office International des Epizooties. 2013;32:1–20. doi: 10.20506/rst.32.2.2218. [DOI] [PubMed] [Google Scholar]
- 22.Robi D.T., Gelalcha B.D. Epidemiological investigation of brucellosis in breeding female cattle under the traditional production system of Jimma zone in Ethiopia. Vet. Anim. Sci. 2020;9 doi: 10.1016/j.vas.2020.100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bifo H., Gugsa G., Kifleyohannes T., Abebe E., Ahmed M. Sero-prevalence and associated risk factors of bovine brucellosis in sendafa, oromia special zone surrounding Addis Ababa, Ethiopia. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0238212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenea T., Megersa B. Bovine brucellosis: seroepidemiology and herder's knowledge, attitude and practices in Bench Maji zone, southern Ethiopia. Ethiop. Vet. J. 2021;25(1):23–42. [Google Scholar]
- 25.Etefa M., Kabeta T., Merga D., Debelo M. Cross-sectional study of seroprevalence and associated risk factors of bovine brucellosis in selected districts of Jimma zone, south western oromia, Ethiopia. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/9549942. Article ID 9549942, 13 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libeau G., Prehaud C., Lancelot R., Colas F., Guerre L., Bishop D., Diallo A. Development of a competitive ELISA for detecting antibodies to the peste des petits ruminant virus using a recombinant nucleoprotein. Res. Vet. Sci. 1995;58:50–55. doi: 10.1016/0034-5288(95)90088-8. [DOI] [PubMed] [Google Scholar]
- 27.Thrusfield M. third ed. Blackwell Publishing; England: 2005. Veterinary Epidemiology; pp. 345–543. [Google Scholar]
- 28.Naing L., Winn T., Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006;1:9–14. [Google Scholar]
- 29.Dohoo I., Martin W., Stryhn H. second ed. AVC, Charlottetown, Prince Edward Island; 2009. Veterinary Epidemiologic Research; pp. 239–249. [Google Scholar]
- 30.OIE . vol. 2. World Organization of Animal Health; 2009. pp. 5–35. (Manual of Diagnostic Tests and Vaccines for Terrestrial Animals). [Google Scholar]
- 31.OIE . fifth ed. Office International des Epizootics; Paris: 2004. Bovine Brucellosis. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; pp. 409–438. [Google Scholar]
- 32.Nicholson M., Butterworth M. International Livestock Center for Africa, Addis Ababa; Ethiopia: 1986. A Guide to Condition Scoring of Zebu Cattle; pp. 3–29. [Google Scholar]
- 33.Richard W. Macmillan Press London; 1993. Dairying. Tropical Agriculturalist. 1st; pp. 43–48. [Google Scholar]
- 34.Wathes D., Brickell J., Bourne N., Swali A. Cheng Z. Factors influencing heifer survival and fertility. J. Anim. Sci. 2008;2:1135–1143. doi: 10.1017/S1751731108002322. [DOI] [PubMed] [Google Scholar]
- 35.Markusfeld N.O. Epidemiology of bovine abortions in Israeli dairy herds. Prev. Vet. Med. 1997;31:245–255. doi: 10.1016/s0167-5877(96)01142-7. [DOI] [PubMed] [Google Scholar]
- 36.Peter A.T. Abortions in dairy cows: new insights and economic impact. Adv. Dairy Technol. 2000;12:233. [Google Scholar]
- 37.Tulu D., Deresa B., Begna F. Case-control study on risk factors associated with brucellosis in aborted cattle of Jimma zone, Ethiopia. Iran. J. Vet. Sci. Technol. 2019;11:27–36. [Google Scholar]
- 38.EFSA (European Food Safety Authority) Scientific opinion on performance of brucellosis diagnostic methods for bovines, sheep, and goats. EFSA J. 2006;432:1–44. [Google Scholar]
- 39.Boukary A.R., Saegerman C.A., batih E., Fretin D., Bada R.A., Deken R., et al. Seroprevalence and potential risk factors for Brucella spp. infection in traditional cattle, sheep and goats reared in urban, per urban and rural areas of Niger. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deresa B., Tulu D., Deressa F.B. Epidemiological investigation of cattle abortion and its association with brucellosis in Jimma zone, Ethiopia. Vet Med (Auckl) 2020;11:87–98. doi: 10.2147/VMRR.S266350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eyob E., Hani S., Diriba L., Birhanu A. Prevalence and risk analysis of bovine brucellosis in Asella organized dairy farm, Oromia Regional State, southeast Ethiopia. J. Vet. Med. Anim. Health. 2018;10(10):245–249. [Google Scholar]
- 42.Kubuafaor D.K., Awumbila B., Akanmori B.D. Seroprevalence of brucellosis in cattle and humans in the Akwapim-south district of Ghana: public health implication. Acta Trop. 2000;76:45–48. doi: 10.1016/s0001-706x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 43.Schelling E., Dinguimbaye C., Daoud S., Nicoletti J., Boertin P., Tanner M., Zinnstag J. Brucellosis and Q-fever seroprevalence of nomadic pastoralists and their livestock in Chad. Prev. Vet. Med. 2003;61:279–293. doi: 10.1016/j.prevetmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Deselegn T.B., Gangwar S.K. Seroprevalence study of bovine brucellosis in Assela government dairy farm of Oromia Regional State, Ethiopia. Int. J. Sci. Nat. 2011;2:692–697. [Google Scholar]
- 45.Megersa B., Biffa D., Abunna F., Regassa A., Godfroid J., Skjerve E. Seroprevalence of brucellosis and its contribution to abortion in cattle, camel, and goat kept under pastoral management in Borana, Ethiopia. Trop. Anim. Health Prod. 2011;43:651–656. doi: 10.1007/s11250-010-9748-2. [DOI] [PubMed] [Google Scholar]
- 46.Domingo A.M. Current status of some zoonoses in Togo. Acta Trop. 2000;76:65–67. doi: 10.1016/s0001-706x(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 47.Kungu J.M., Okwee A.J., Ayebazibwe C., Okech S.G., Erume J. Sero-prevalence and risk factors for brucellosis in cattle in Gulu and Amuru districts, Northern Uganda. Afr. J. Anim. Biomed. Sci. 2010;5:1819–4214. [Google Scholar]
- 48.Teshale S., Kindahl H., Bekana M., Kelay B., Jergefa T. Epidemiological study of bovine brucellosis in three agro ecological areas of central Oromia Ethiopia. Review in Scientific and Technical of the Office International des Epizooties. 2009;28:933–943. doi: 10.20506/rst.28.3.1939. [DOI] [PubMed] [Google Scholar]
- 49.Tesfaye D., Speybroeck N., Deken R.D., Thys E. Economic burden of bovine trypanosomosis in three villages of Metekel zone, Northwest Ethiopia. Trop. Anim. Health Prod. 2011;44:873–879. doi: 10.1007/s11250-011-9981-3. [DOI] [PubMed] [Google Scholar]
- 50.Tsegaye Y., Kyule M., Lobago F. Seroprevalence and risk factors of bovine brucellosis in Arsi zone, oromia regional state, Ethiopia. Am. Sci. Res. J. Eng. Technol. Sci. 2016;24:16–25. [Google Scholar]
- 51.Alhaji N.B., Wungak Y.S., Bertu W.J. Serological survey of bovine brucellosis in Fulani nomadic cattle breeds (Bos indicus) of North-central Nigeria: potential risk factors and zoonotic implications. Acta Trop. 2016;153:28–35. doi: 10.1016/j.actatropica.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Samui K.L., Oloya J., Munyeme M., Skjerve E. Risk factors for brucellosis in indigenous cattle reared in livestock-wildlife interface areas of Zambia. Prev. Vet. Med. 2007;80:306–317. doi: 10.1016/j.prevetmed.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Faye B., Castel V., Lesnoff M., Rutabinda D., Dhalwa J. Tuberculosis and brucellosis prevalence survey on dairy cattle in Mbarara milk basin in Uganda. Prev. Vet. Med. 2005;67:267–281. doi: 10.1016/j.prevetmed.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Mussie H., Tesfu K., Yilkal A. Seroprevalence study of bovine brucellosis in bahir dar milk shed, northwestern amhara region. Ethiop. Vet. J. 2007;11:42–49. [Google Scholar]
- 55.Asgedom H., Damena D., Duguma R. vol. 5. Springer Plus; 2016. pp. 1–8. (Seroprevalence of Bovine Brucellosis and Associated Risk Factors in and Around Alage District, Ethiopia). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kebede T., Ejeta G., Ameni G. Seroprevalence of bovine brucellosis in smallholder farms in central Ethiopia (Wuchale-Jida district) Rev. Med. Vet. (Toulouse) 2008;159:3–9. [Google Scholar]
- 57.Kushwaha N., Rajora V.S., Mohan A., Upadhyay A.K., Kumar R. Comparison of serological tests for detection of Brucella antibodies in cattle of an organized dairy farm. Indian J. Anim. Res. 2016;50:69–74. [Google Scholar]
- 58.Ali S., Akhter S., Neubauer H., Melzer F., Khan I., Nji A.E., Eladawy H., Irfan M., Muhammad A., Waqas A.M., Umar S., Ali Q., Naeem I.M., Mahmood A., Ahmed H. Seroprevalence and risk factors associated with bovine brucellosis in the Potohar Plateau, Pakistan. BMC Res. Notes. 2017;10:1–11. doi: 10.1186/s13104-017-2394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asmare K., Sibhat B., Molla W., Ayelet J., Shiferaw J., Martin E., Skjerve E., Godfroid J. The status of bovine brucellosis in Ethiopia with special emphasis on exotic and cross bred cattle in dairy and breeding farms. Acta Trop. 2013;126:186–192. doi: 10.1016/j.actatropica.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Stringer L.A., Guitian F.J., Abernethy D.A., Honhold N.H., Menzies F.D. Risk associated with animals moved from herds infected with brucellosis in Northern Ireland. Prev. Vet. Med. 2008;84:72–84. doi: 10.1016/j.prevetmed.2007.11.005. 2008. [DOI] [PubMed] [Google Scholar]
- 61.Cardenas L., Pena M., Melo O., Casal J. Risk factors for new bovine brucellosis infections in Colombian herds. BMC Vet. Res. 2019;15:81. doi: 10.1186/s12917-019-1825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Omer M.K., Skjerve E., Woldehiwet Z., Holstad G. Risk factors for Brucella spp. infection in dairy cattle farms in Asmara, State of Eritrea. Prev. Vet. Med. 2000;46:257–265. doi: 10.1016/s0167-5877(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 63.Al-Majali A.M., Talafha A.Q., Ababneh M.M., Ababneh M.M. Seroprevalence and risk factors for bovine brucellosis in Jordan. J. Vet. Sci. 2009;10:61–65. doi: 10.4142/jvs.2009.10.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terefe Y., Girma S., Mekonnen N., Asrade B. Brucellosis and associated risk factors in dairy cattle of eastern Ethiopia. Trop. Anim. Health Prod. 2017;49:599. doi: 10.1007/s11250-017-1242-7. [DOI] [PubMed] [Google Scholar]
- 65.Smits H.L. Brucellosis in pastoral and confined livestock: prevention and vaccination. Review in Scientific and Technical of the Office International des Epizooties. 2013;32:219–228. doi: 10.20506/rst.32.1.2200. [DOI] [PubMed] [Google Scholar]
- 66.Kaou H., Zaki M.M., Shimaa A., Nasr A. Epidemiology of brucellosis among farm animals. Nat. Sci. 2010;8:190–197. [Google Scholar]
- 67.Anka M.S., Hassan L., Khairani-Bejo S., Zainal M.A., Mohamad R.B., Salleh A., Adzhar A. Case-control study of risk factors for bovine brucellosis seropositivity in peninsular Malaysia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elabdin S.Z., Angara E., Elfadil A., Sanousi E., Ibrahim A. Prevalence and risk factors of ruminants brucellosis in Jabel Aolia Locality, Sudan. Sudan J. Sci. Technol. 2014;15:60–72. [Google Scholar]
- 69.McDermott J.J., Arimi S.M. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet. Microbiol. 2002;90:111–134. doi: 10.1016/s0378-1135(02)00249-3. [DOI] [PubMed] [Google Scholar]
- 70.Sagamiko F.D., Muma J.B., Karimuribo E.D., Mwanza A.M., Sindato C., Hang'Ombe B.M. Sero-prevalence of Bovine Brucellosis and associated risk factors in mbeya region, Southern highlands of Tanzania. Acta Trop. 2018;178:169–175. doi: 10.1016/j.actatropica.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 71.Derdour S.Y., Hafsi F., Azzag N., Tennah S., Laamari A., China B., Ghalmi F. Prevalence of the main infectious causes of abortion in dairy cattle in Algeria. J. Vet. Res. 2017;61:337–343. doi: 10.1515/jvetres-2017-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asmare K., Regassa F., Robertson L.J., Martin A.D., Skjerve E. Reproductive disorders in relation to Neospora caninum, Brucella spp. and bovine viral diarrhoea virus sero status in breeding and dairy farms of central and southern Ethiopia. Epidemiol. Infect. 2012:1–9. doi: 10.1017/S0950268812002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shabbir M.Z., Khalid R.K., Freitas D.M., et al. Serological evidence of selected abortifacients in a dairy herd with history of abortion. Pak. Vet. J. 2013;33(1):19–22. [Google Scholar]
- 74.Asmare K. Neospora caninum versus Brucella spp. exposure among dairy cattle in Ethiopia: a case control study. Trop. Anim. Health Prod. 2014;46:961–966. doi: 10.1007/s11250-014-0599-0. [DOI] [PubMed] [Google Scholar]
- 75.Munoz P.M., Marin C.M., Monreal D., Gonzalez D., Garin-Bastuji B., Diaz R., et al. Efficacy of several serological tests and antigens for diagnosis of Bovine Brucellosis in the presence of false-positive serological results due to Yersinia enterocolitica O:9. Clin. Diagn. Lab. Immunol. 2005;12(1):141–151. doi: 10.1128/CDLI.12.1.141-151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corbel, M. J. (2006). In W. H. O. Press (Ed.). Geneva: World Health Organization.
- 77.Godfroid J., Nielsen K., Saegerman C. Diagnosis of brucellosis in livestock and wildlife. Croat. Med. J. 2010;51:296–305. doi: 10.3325/cmj.2010.51.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mainar-Jaime R.C., Munoz P.M., de Miguel M.J., Grillo M.J., Marin C.M., Moriyon I., et al. Specificity dependence between serological tests for diagnosing bovine brucellosis in Brucella-free farms showing false positive serological reactions due to Yersinia enterocolitica O: 9. Can. Vet. J. = La revue ve- terinaire canadienne. 2005;46(10):913–916. [PMC free article] [PubMed] [Google Scholar]
- 79.Dohoo I., Martin S.W., Stryhn H. AVC Inc; 2003. pp. 34–56. (Veterinary Epidemiologic Research. Charlottetown, Price Edward's Island). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.