Abstract
Objectives:
Generalization (or near-transfer) effects of an intervention to tasks not explicitly trained are the most desirable intervention outcomes. However, they are rarely reported and even more rarely explained. One hypothesis for generalization effects is that the tasks improved share the same brain function/computation with the intervention task. We tested this hypothesis in the present study of transcranial direct current stimulation (tDCS) over the left inferior frontal gyrus (IFG) that is claimed to be involved in selective semantic retrieval of information from the temporal lobes.
Materials and Methods:
In the present study, we examined whether tDCS over the left IFG a group of patients with primary progressive aphasia (PPA), paired with a lexical/semantic retrieval intervention (oral and written naming), may specifically improve semantic fluency, a non-trained near-transfer task that relies on selective semantic retrieval, in patients with primary progressive aphasia (PPA).
Results:
Semantic fluency improved significantly more in the active tDCS vs. sham tDCS condition immediately after and two weeks post-treatment. This improvement was marginally significant two months post-treatment. We also found that the active tDCS effect was specific to tasks that require this IFG computation (selective semantic retrieval) but not to other tasks that may require different computations of the frontal lobes.
Conclusions:
We provided interventional evidence that the left IFG is critical for selective semantic retrieval, and tDCS over the left IFG may have a near-transfer effect on tasks that depend on the same computation, even if they are not specifically trained.
Keywords: primary progressive aphasia, transcranial direct current stimulation (tDCS), inferior frontal gyrus (IFG), semantic retrieval, verbal fluency
1. Introduction
The distinction between function and task has been at the heart of cognitive neuroscience from the early days of functional neuroimaging. Most brain areas, although they may specialize in certain functions (computations), are usually involved in several tasks (for a review, see Price [1]). This is an even more pertinent consideration in the era of network neuroscience, since it is now understood that although a whole network may be involved in a task, each area involved performs a specific function/computation. For example, Hickok and Poeppel [2] have described the network of speech production with all the areas needed to produce speech (e.g., posterior superior temporal gyrus [STG], inferior frontal gyrus opercularis [IFG op], supramarginal gyrus [SMG] etc.) while describing the exact function/computation performed in each area (e.g., the particular computation of the posterior STG is to compute an acoustic analysis of sounds, the left IFG opercularis is to plan the articulation of speech sounds, the SMG holds in temporary phonological storage of the sequence of sounds, etc.). This important understanding has two predictions that are relevant for aphasia concerning where the language network(s), and possibly other networks, are disrupted: (1) performance in all tasks that require the computation/function of a lesioned area will be impaired, and (2) rehabilitation must either target to improve the function of the lesioned area, or the performance of the whole network (or alternative but relevant networks), where other areas may compensate for the loss of function in the lesioned area. Good examples of neural rehabilitation are the reperfusion studies in post-stroke aphasia that showed how a reperfused area improved performance in all previously-impaired tasks that involved the computation of that area and were previously impaired [3,4]. This process may explain generalization, or ‘near-transfer’ effects, as they are usually referred to in aphasia rehabilitation, i.e., improvement in tasks that are not explicitly trained but are relevant to the treatment task because they rely on the same computation.
Generalization effects of an intervention in tasks that were not explicitly trained during the intervention are one of the most desirable intervention outcomes. However, they are rarely reported and even more rarely explained. One hypothesis for the existence of generalization effects is that the tasks that improved along with the intervention task share the same brain function/computation. A critical way to test this hypothesis is neuromodulation studies (either transcranial direct current stimulation of transcranial magnetic stimulation) that involve the stimulation of specific brain areas and their computations thereafter. In the context of neuro-rehabilitation in aphasia, this hypothesis allows for the exciting possibility that if we were able to modulate the neural function of a particular area, then we would potentially modulate all the tasks in which it is involved. In the present study we provide evidence of favoring this hypothesis in a transcranial direct current stimulation (tDCS) study in a neurodegenerative condition involving language, namely in primary progressive aphasia (PPA). We show how tDCS targeting the left inferior frontal gyrus (IFG), paired with a lexical retrieval treatment (written/oral naming), enhanced performance in other lexical retrieval tasks (e.g., semantic fluency) not explicitly trained but that do depend on the computation of the stimulated area, as several previous studies have shown [5–10] and we will discuss later.
Primary progressive aphasia, a term coined by Mesulam in his seminal paper in 1982 [11], is a neurodegenerative syndrome encompassing different pathologies, affecting individuals early in life (as early as late 40s), in which language abilities gradually deteriorate [12–15], while other cognitive functions remain relatively intact, at least in the early years of the condition. There are only symptomatic treatment options but no disease-modifying treatments for PPA. According to the recent consensus, there are 3 main PPA variants with different language manifestations [14], and the task of semantic fluency has been shown to be impaired in all 3 PPA variants [16–18], although performance was numerically worse in the semantic variant (svPPA). Semantic fluency is a neuropsychological task in which the participant is asked to generate words that belong to specific semantic categories, such as fruits or animals [19]. The task of semantic fluency relies on the function of selective semantic retrieval. Lesion studies in post-stroke aphasia have already shown that semantic fluency performance depends on semantic representations stored in temporal regions, as well as on executive functions of retrieval operating in frontal regions [20,21]. Semantic fluency is also impaired in healthy aging [22], as well as in neurodegenerative conditions, such as, Parkinson’s Disease and Alzheimer’s disease [23–25]. For PPA, in particular, two previous studies [18,26] and a recent one from our group [17], correlated verbal fluency performance to brain atrophy and hypometabolism in the temporal lobe in logopenic variant (lvPPA) and svPPA [17,18,26] and in the frontal lobe in non-fluent/agrammatic variant (nfvPPA) [17,26].
Functional MRI (fMRI) studies have shown that selective semantic retrieval is regulated by the left IFG triangularis (Brodmanns’ area [BA] 45/47) that retrieves semantic information stored in the temporal brain regions in healthy controls [6,27–30]. Further fMRI and DTI studies have shown that the left IFG triangularis is directly and monosynaptically connected to the temporal lobes via the extreme capsule fasciculus, and these pathways are critical for lexical/semantic access [31–33]. Other studies have provided similar evidence for the adjacent and larger (and therefore usually difficult to distinguish from the extreme capsule) uncinate fasciculus, especially for proper names [34–36]. Overall, all these studies suggests that these pathway(s) are critical for selection of meaning or lexical items from the temporal lobes.
Causal evidence, however, for the role of a particular area for a particular computation comes only from evaluating the computation before and after a temporary lesions or evaluating the computation before and after intervention to improve the function of an area. Temporary lesions can be caused by in vivo electrical stimulation mostly during epilepsy surgery [see [37] for a review on language functions] or with implanted subdural grids, or by inhibitory transcranial magnetic stimulation (TMS). Inhibitory (low frequency or single pulse) TMS has been traditionally used to make claims on the involvement of specific anatomical areas in certain functions and tasks in heathy controls, based on impaired function associated with inhibition of an area.
Improvement in a function can be brought about by restoring blood flow to a focal area of hypoperfusion (see for examples [3,38]) or with excitatory (high frequency) TMS or anodal tDCS. High frequency TMS and anodal tDCS have been used to enhance intervention outcomes in post-stroke aphasia as well as PPA [39–48]. Evidence for clinical efficacy is mostly shown for tDCS. It has been shown that tDCS may benefit language performance in post-stroke aphasia [49–55] and more recently tDCS is emerging as a potentially helpful adjunct to intervention in PPA (see recent reviews [56–59]). In the tDCS literature, many studies show transfer to untrained items/words. We and three other independent groups have shown that tDCS over the left IFG augments behavioral treatment outcomes in oral and written naming [39–46]. However, as mentioned above, generalization of treatment to other tasks may the most desirable outcome of an intervention but the most rarely reported, particularly in neurodegenerative disorders where performance deteriorates over time.
Given the small number of tDCS studies in PPA that report generalization effects to untrained tasks (only 3) [46,60,61], we refer to each one separately. Cotelli and colleagues [46] have shown that anodal tDCS over the left dorsolateral prefrontal cortex improved naming accuracy in 16 patients with nfvPPA PPA and significant improvement was also found in self-assessments of speech production in functional communication scales [46]. In another recent study, Gervits and colleagues described six people with PPA who narrated wordless children’s books while undergoing 10 sessions of active tDCS over the left frontal cortex (centered at F7) [60]. Category (semantic) fluency was found to improve significantly compared to sham tDCS at follow-up intervals. Roncero and colleagues [61] also found transfer effects to untrained picture-naming items and Digit Span (forward and backward) [62] after active tDCS over the left inferior parietal cortex in 10 patients with PPA [61]. Therefore, there is encouraging preliminary evidence of transfer to other tasks. Of note, another two studies directly targeted semantic fluency as the training task and found that a single-session active tDCS over the left IFG improved semantic fluency in healthy controls showing its causal involvement in selective semantic retrieval [63,64].
However, the basis of such a generalization is not clear. Here we tested the hypothesis that generalization or ‘near-transfer’ effects documented in the rehabilitation literature are due to the same neural computation performed by the brain area. We tested this hypothesis by measuring the additional tDCS effect in tasks that depend on the computations of the stimulated area in individuals with PPA. We hypothesized that if the function of the left IFG (and in particular of the pars triangularis that seemed to be mostly modulated in our tDCS study, see [65,66]) is selective semantic retrieval of information stored in the temporal lobes [6,28,67], then tDCS over the left IFG would also improve performance in other, non-trained tasks that involve the function of active selective semantic retrieval, such as semantic fluency.
Critically, we compared the tDCS effects in verbal fluency that involves the left IFG with other frontal tasks that do not involve the left IFG, i.e., tasks that usually implicate the adjacent, left middle frontal areas. Such tasks depend on the basic computation(s) of the left middle frontal gyrus (MFG) (e.g., monitoring). The only such tasks that were available for the present cohort, and included the Digit Span, Trail Making Test [68], and letter fluency [19]. Additionally, we tested improvement in a general mood task Patient Health Questionnaire-9 (PHQ-9) [69] to exclude the possibility that stimulation effects were due to a general improvement in mood (a ‘feeling good’ effect). We tested our hypothesis in a large cohort of patients with PPA who had participated in a previous tDCS trial.
2. Materials and Methods
2.1. Participants
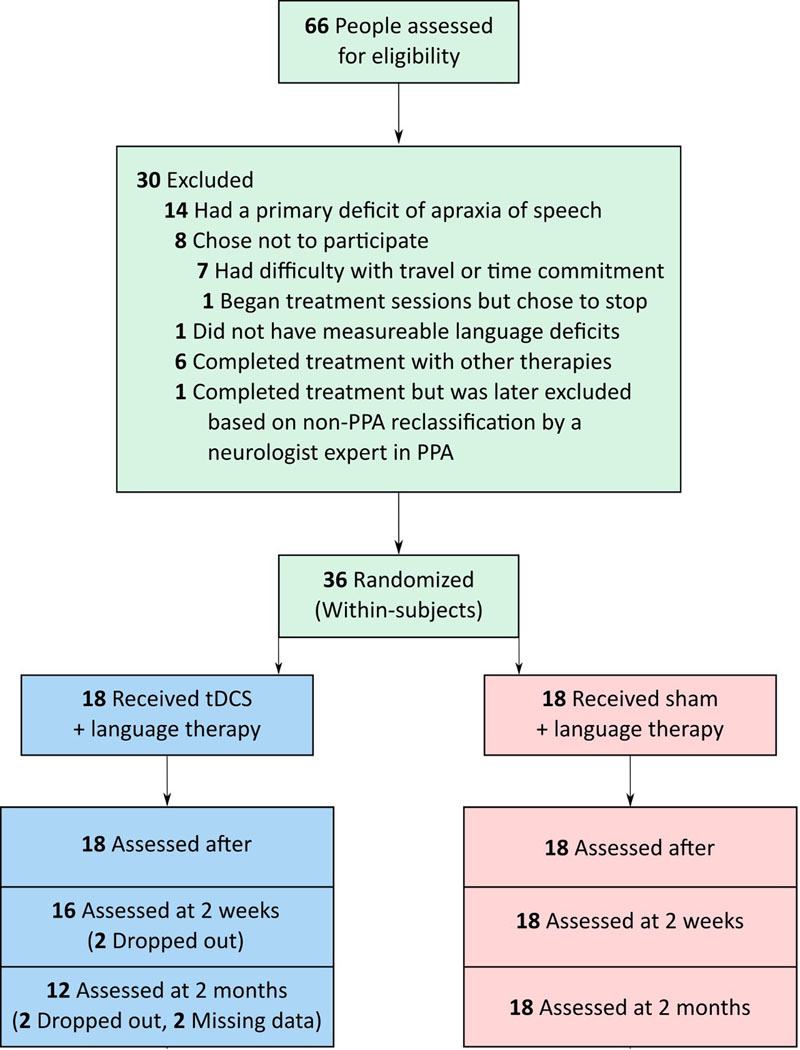
Thirty-six patients with PPA participated in this study (17 female): 14 with lvPPA, 13 with nfvPPA and 9 with svPPA. All were right-handed, native English speakers, between 50 and 80 years old, and diagnosed based on clinical assessment, neuropsychological and language testing, and MRI, according to consensus criteria [14]. Informed consent was obtained from participants or their spouses. All data were acquired in compliance with the Johns Hopkins Hospital Institutional Review Board (NA_00071337) and this study was registered on clinicaltrials.gov (NCT02606422). Figure 1 shows the participants recruited and their randomization to active tDCS or sham tDCS condition. Each PPA variant group was matched on sex, age, education, years post onset of symptoms, overall Frontotemporal Lobar Degeneration (FTLD)-modified Clinical Dementia Rating scale (FTD-CDR) [70] score and language severity measures (Tables 1A, 1B).
Figure 1.
Participants recruited and randomization to active tDCS or sham tDCS.
Table 1A.
Means and standard deviations of demographic variables and baseline semantic fluency scores grouped by first-phase condition (n=36).
| active tDCS first | Sham tDCS first | F(1, 34) | p-value | |
|---|---|---|---|---|
| Sex | 9 F, 9 M | 8 F, 10 M | * | 1.000 |
| Variant | 7 L, 6 N, 5 S | 7 L, 7 N, 4 S | * | 0.500 |
| Age (years) | 66.17 (7.49) | 69.72 (5.42) | 2.66 | 0.113 |
| Years post symptom onset | 5.17 (3.40) | 4.72 (2.55) | 0.20 | 0.660 |
| Language severity (FTD-CDR) | 1.92 (0.90) | 1.83 (0.71) | 0.10 | 0.759 |
| Total severity (FTD-CDR) | 6.89 (4.53) | 7.53 (4.66) | 0.17 | 0.679 |
| Sessions in phase 1 | 12.72 (2.11) | 11.06 (1.63) | 7.05 | 0.012 |
| Baseline semantic fluency | 14.50 (11.17) | 11.81 (7.49) | 0.72 | 0.400 |
Fisher’s exact test used. FTD-CDR, Frontotemporal Dementia Clinical Dementia Rating Scale sum of boxes. F, female; M, male. L, logopenic; N, nonfluent; S semantic.
Table 1B.
Means and standard deviations of demographic variables and baseline semantic fluency scores grouped by PPA variant (n=36).
| lvPPA | nfvPPA | svPPA | F(2,33) | p-value | |
|---|---|---|---|---|---|
| Sex | 7 F, 7 M | 5 F, 8 M | 5 F, 4 M | * | 0.800 |
| First-phase condition | 7 s, 7 t | 7 s, 6 t | 4 s, 5 t | * | 1.000 |
| Age (years) | 66.29 (8.11) | 69.77 (6.00) | 67.89 (4.96) | 0.91 | 0.412 |
| Years post symptom onset | 4.82 (3.33) | 4.65 (2.66) | 5.56 (3.08) | 0.25 | 0.780 |
| Language severity (FTD-CDR) | 1.57 (0.83) | 2.04 (0.72) | 2.11 (0.78) | 1.76 | 0.188 |
| Total FTD-CDR | 6.18 (3.76) | 7.85 (4.19) | 7.89 (6.17) | 0.57 | 0.571 |
| Sessions in phase 1 | 11.93 (2.02) | 11.85 (1.91) | 11.89 (2.47) | 0.01 | 0.990 |
| Baseline semantic fluency | 17.50 (10.97) | 12.08 (8.38) | 7.94 (5.15) | 3.30 | 0.049 |
Fisher’s exact test used. FTD-CDR, Frontotemporal Dementia Clinical Rating Scale sum of boxes [70]. F, female; M, male. s, sham tDCS; t, active tDCS.
2.2. Overall design
We used a within-subjects, double-blind, crossover design with two experimental conditions: speech-language intervention plus conventional anodal tDCS over the left IFG, and speech-language intervention plus sham tDCS. Each condition lasted approximately 12 consecutive weekday sessions; the two phases were separated by a 2-month wash-out period. Evaluations—consisting of a set of trained and untrained items of the same task, as well as extensive neuropsychological and neurolinguistic assessments—occurred immediately before, immediately after, two weeks after, and two months after each treatment phase. Participants, speech-language therapists, and examiners were blind to the experimental condition. Randomization to assign to first arm of treatment (active tDCS or sham tDCS) was performed after initial evaluation before beginning of treatment within each variant because the flow of cases per variant could not be predicted. We used an independent randomization procedure based on an algorithm that assigns each condition in 50% of times.
2.3. tDCS methods
Each daily intervention session lasted one hour. For both active tDCS and sham conditions, two 5 cm x 5 cm, non-metallic, conductive, rubber electrodes covered with saline-soaked sponges were placed over the right cheek (cathodal electrode) and the left IFG centered at F7 of the EEG 10–20 electrode position (anodal electrode) [71]. The electrodes were hooked up to a Soterix 1×1 Clinical Trials device, which elicited a tingling sensation on the scalp as it ramped up within 30 seconds, to deliver current at an intensity of 2 mA (estimated current density 0.08 mA/cm2; estimated total charge 0.096 C/cm2). In the active tDCS condition, current was delivered for 20 minutes for a daily maximum of 2.4 Coulombs; in the sham condition, current ramped up to 2 mA over a 30 sec interval and immediately ramped down to elicit the same tingling sensation on the scalp that is experienced in the non-sham (20-min) condition, a procedure that has been shown to blind participants to treatment condition [72]. Stimulation started at the beginning of each intervention session and lasted for 20 min whereas speech-language intervention continued for a full session, i.e., 25 additional minutes, for a total of 45–50 min of a regular speech-language intervention session. Twice during each session, participants rated their level of pain with the Wong-Baker FACES Pain Rating Scale (www.WongBakerFACES.org).
2.4. Language intervention
The language intervention protocol was based on studies that have successfully treated written language production. We adapted the basic design of a spell-study-spell procedure [73] to a lexical retrieval, oral and written naming paradigm [74] to simultaneously target orthography, phonology and semantics. Although the intervention focused on written naming and spelling, this particular paradigm gave us the flexibility to accommodate the deficit in each variant (e.g., semantics in svPPA, phonological paraphasias in lvPPA or apraxia of speech [AOS] errors in nfvPPA). The exact steps are described in previous publications of the overall trial results [42].
2.5. Language/cognitive tasks evaluated in the present study: Verbal fluency (letter and semantic), Digit Span, Trail A and B, PHQ-9.
Participants were evaluated with a series of standardized language and cognitive assessments. For the semantic fluency task, participants were instructed to name as many fruits, or animals, or vegetables as possible, administered separately in the order listed here, in one minute per category [19,75]. Scores used in the present analysis were calculated by adding the number of words generated in all three categories. Performance was assessed before, immediately after, two weeks after, and two months after each phase.
Behavioral tasks that involve less selective semantic retrieval and depend less on left IFG, including Digit Span forward and backward, letter fluency, Trail A&B, and Patient Health Questionnaire −9 (PHQ-9). In the Digit Span forward task, participants were instructed to repeat an increasing number of digits in the exact order presented by the examiner. In the Digit Span backward task, participants were instructed repeat an increasing number of digits in the order opposite of that presented by the examiner. In the FAS or letter fluency task, participants were instructed name as many words as possible that start with the letters ‘F’, ‘A’, or ‘S’, administered separately in the order listed here, in one minute per letter. In the Trail A task, participants were instructed to trace the numbers from 1–25 depicted in different parts of a page as quickly and as accurately as possible. We had also administered the Trail B task, in which participants were instructed to alternate between numbers and letters depicted in different parts of a page, as quickly and as accurately as possible, but many participants found the task too difficult/could not complete the task and the data are not included.
2.7. Statistical analyses
2.7.1. Evaluation of tDCS effects
We evaluated the effect of tDCS immediately after, at two weeks, and at two months post the intervention using the first-phase data only, in order to rid the estimation of any possible impact of carryover effects (see details in Section 2.7.3). In fact, we focus on the first-phase data throughout the statistical analysis for a similar reason, to eliminate possible treatment-phase interaction due to possibly insufficient wash-out period.
The additional active tDCS effect compared to sham was evaluated as the average treatment effect (ATE), δ(T vs S) = E[Y|T = 1] - E[Y|T = 0], where Y is the change in semantic fluency scores from baseline and T is the treatment assignment indicator (valued as 1 for active tDCS, 0 for sham). We assumed that (Yi, Ti), i = 1, …, n, is an independent and identically distributed (IID) sample, and that the actual observed Yi is one of the two potential outcomes, YiT = 1 and YiT = 0, depending on the treatment assignment, i.e., Yi = Ti YiT = 1 + (1 - Ti) YiT = 0. These potential outcomes are defined as the changes that would have been observed if the i-th subject had been assigned to active tDCS or sham, was observed. Treatment randomization guarantees that the preassigned treatment variable, Ti, is independent from the subsequent potential outcomes, YiT = 1 and YiT = 0, and that the assignment probability is constant and positive. The following baseline covariates were used to improve the efficiency of statistical tests: baseline semantic fluency [76,77], PPA variant, number of treatment sessions, sex, age, years post onset of symptoms, and total FTD-CDR severity and language severity measures.
The estimation of ATE was conducted using the Targeted Minimum Loss-Based Estimation (TMLE) method [78]. We chose the method mainly for its advantage of flexibility in selecting the covariates automatically (based on cross validations), which reduced subjective modeling choices of the researchers. Meanwhile, TMLE achieves the desired properties as other commonly practiced methods. For example, TMLE guarantees the estimation consistency and asymptotic normality in randomized trials as analysis of covariances (ANCOVA) estimators, as well as the doubly robust local efficiency as augmented inverse propensity score weighting (AIPW) estimators. The TMLE R package [79] has been made available, which can be directly applied for the aforementioned ATE estimation problem. The estimator configuration details and the simulation evidence supporting the choice of method are provided in the supplementary materials (Appendix A). Estimations of the ATE are reported as the effect sizes [80] adjusted by the first-phase before-after sham group standard deviation (SD= 3.00). The standard errors, Z-test statistics, p-values, and 95% confidence intervals are reported.
To handle missing observations in semantic fluency due to dropouts at two weeks post (two dropouts) and two months post (six dropouts), we assumed Missing at Random (MAR), that is, given the knowledge of the observed semantic fluency values and the series of observed baseline covariates, whether a subject happened to drop out was assumed to have no impact on the unobserved semantic fluency values. TMLE can be applied to handle such missingness in the outcome [78], and can be directly implemented with the TMLE R package [79].
2.7.2. Tasks depending in non-semantic retrieval computations
Behavioral tasks that do not involve selective semantic retrieval including Digit Span forward and backward, letter fluency, Trail A, and (PHQ-9), are also analyzed as comparisons to confirm that the generalization depends on this specific left IFG computation. The estimates, standard errors, test statistics, p-values, and 95% confidence intervals are reported for the additional tDCS effects on Before-After changes. Details of estimator configuration are listed in Appendix A.
2.7.3. Evidence and sensitivity analysis of potentially unbalanced carryover effects
Data evidence shows that there exists a marginally significant treatment by phase interaction (see Appendix C Table 2, where the first-phase “2month” time point is identical to the “Before” time point of the second phase per study design, and hence the “Before-2month” subsection analyzes the possibly unbalanced carryover effects of the two treatment groups; estimated effect size of treatment by phase interaction 1.08, SE = 0.64, 95% CI [−0.17, 2.33], p = 0.091). This marginally significant result shows possible positive treatment by phase interaction, as the 95% CI is largely overlapping with the positive line. Therefore, throughout the main analysis, we focus on first-phase data to rid possible impact from unbalanced carryover effects, as we had seen in the main trial[42].
Moreover, in Appendix D a sensitivity analysis of the second phase data is conducted ignoring carryover effects. The estimations under this unsupported assumption are biased, which confirms the impact of carryover effects on the second-phase data and justifies the method choice of first-phase-only data analysis[81,82].
3. Results
3.1. tDCS tolerability
Some participants reported tingling, itching, or discomfort from the stimulation, but no episodes of intolerability or adverse effects occurred. The maximum reported Wong-Baker FACES pain rating scale for each daily session was averaged across sessions and participants, with a tDCS mean pain rating of 2.21 (standard deviation 2.48, range 0–10) and a sham mean rating of 2.14 (standard deviation 2.13, range 0–10).
3.2. tDCS effects on spelling accuracy
Previously, in the same patient cohort, we found that active tDCS over the left IFG resulted in larger and longer-retained gains in letter accuracy for spelling trained items (words treated in intervention) and untrained items (words not treated in intervention as a measure of generalization to other items in the same task) as compared to sham [42,83]. This additional improvement with active tDCS was retained marginally significant up to 2 months. We refer to the previous study only as background information here. In the present paper, we extend these findings to semantic fluency as a measure of treatment generalization.
3.3. tDCS effects on semantic fluency
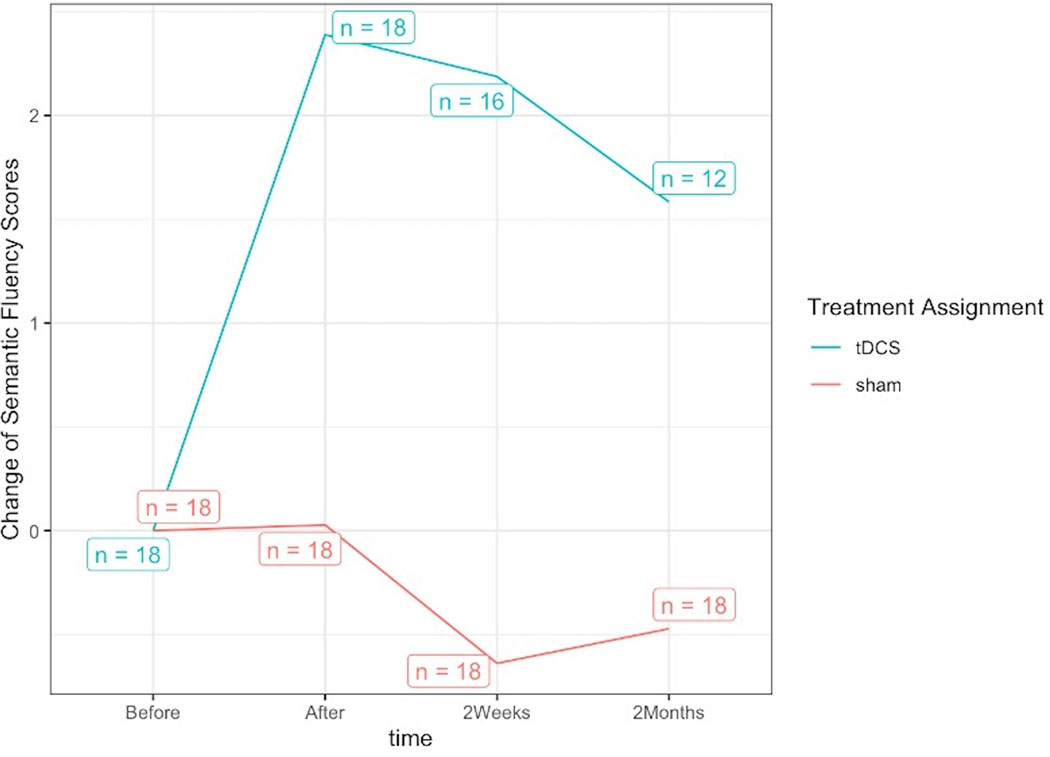
We present the additional tDCS effects (active over sham) immediately after, two weeks and two months post-intervention from the first phase of intervention only, to avoid possible carryover effects (see Figure 2 for observed group average levels). Semantic fluency scores ranged from 0–46 words. Scores were generated by the sum of fruits, animals, and vegetables generated in one minute each. All statistical analyses were performed the changes from the baseline in the summed scores of the three semantic categories.
Figure 2.
Observed group average levels of changes in semantic fluency (shown on the ordinate, y-axis) for both the active tDCS and sham conditions. Time points: baseline (Before), immediately after intervention (After), two weeks post intervention (2Weeks) and two months post intervention (2Months) shown in the abscissa (x-axis); the statistical sample (number of patients) is indicated in boxes for both sham and active tDCS. Please refer to results in Section 3.3.1 for estimation and inference adjusting for confounders and missingness at random, where additional tDCS effect was confirmed to be significant immediately after and two weeks post intervention, and marginally significant two months post intervention.
3.3.1. Evaluation of semantic fluency immediately after, two weeks post, and two months post the intervention
Restricting to the first-phase data, from before to after the intervention, the effect of active tDCS vs. sham was significant, and the effect size was estimated as 1.35 (95% CI [0.30, 2.41], SE = 0.54, = 2.52, p = 0.012*) adjusted for all the following covariates (baseline semantic fluency, PPA variant, number of treatment sessions, sex, age, years post onset of symptoms, and total FTD-CDR severity and language severity measures) as listed in Section 2.7.1. Thus, active tDCS showed an additional effect that is 1.35 times the standard deviation of the sham group’s change (SD=3) in the semantic fluency score.
Significant additional tDCS effect on semantic fluency was also confirmed two weeks post the intervention (effect size estimation 1.56, 95% CI: [0.40, 2.73], SE: 0.59, = 2.64, p = 0.008*) but only marginally at two months post- intervention (effect size estimation 1.08, 95% CI: [−0.17, 2.33], SE: 0.64, = 1.69, p = 0.091) (see Appendix C for more detailed reports).
3.3.2. Effects in tasks that do not depend on IFG computations
Importantly, the effects were specific to selective semantic retrieval, i.e., there were no additional effects of tDCS on After-Before changes other cognitive tasks asks that do not depend on the left IFG, e.g., Digit Span forward (95% CI [−0.23, 0.38], Estimate = 0.08, SE = 0.15, Z = 0.49, p = 0.625), Digit Span backward (95% CI [−0.74, 0.36], Estimate = −0.19, SE = 0.28, Z = −0.68 p = 0.495), FAS (95% CI [−0.47, 0.34], Estimate = −0.07, SE = 0.21, Z = −0.32, p = 0.748), Trail A (95% CI [−0.78, 1.39], Estimate = 0.30, SE = 0.49, t(10.07) = −0.62, p = 0.551); nor was there any additional tDCS effect of any index of general improvement, e.g., in a mood task (PHQ-9) (95% CI [−43.6, 41.0], Estimate = −1.29, SE = 3.45, t(1.02) = 0.37, p = 0.772).
4. Discussion
In the present study we evaluated the effects of anodal tDCS to left IFG on an untrained (during treatment) task, semantic fluency, to provide interventional evidence on the role of the left IFG in selective semantic retrieval. Previously, we [42] and others [40,46,61] have found positive effects of active tDCS of the left IFG in PPA, especially in lexical retrieval tasks, such as oral and written naming (see reviews [57–59]). Here, we tested the hypothesis that if the left IFG (triangularis, BA 45) subserves the function of selective semantic retrieval, and especially of selection of some aspect or subset of available information among competing alternatives [6,8,84,85], such as semantic category, then tDCS over this area will improve performance on a task of semantic selection, such as semantic verbal fluency, even if not explicitly trained. We found that performance in semantic fluency improved significantly more in the active tDCS condition than in sham. The significant additional improvement was maintained two weeks post-treatment and was marginally significant two months post-treatment. Importantly, the effects were specific to selective semantic retrieval, i.e., there were no effects of tDCS on other cognitive tasks asks that do not depend on semantic retrieval functions of the left IFG, i.e., involve other frontal functions such as monitoring and working memory (e.g., Digit Span forward and backward and Trail A); nor was there any tDCS effect of any index of general improvement in a mood task (e.g., PHQ-9). The present findings based on the largest, to date, cohort of tDCS in PPA demonstrate that generalization effects of tDCS to untrained tasks depend on the particular computation of the stimulated area. The present study also allows specific predictions about not only which tasks will improve but also which patients will benefit from tDCS over the left IFG. Below, we discuss the present results in the realm of generalization (or ‘near-transfer’ effects) in aphasia rehabilitation in general.
The present findings align well with those of other tDCS studies in healthy aging and post-stroke aphasia that have found that tDCS facilitates transfer of intervention effects to untrained but related tasks (near-transfer effects). For example, Marangolo and colleagues [86] report that oral naming improved after AOS training and tDCS over the left IFG in post-stroke aphasia, and Meinzer and colleagues [87], report that in mild cognitive impairment (MCI), oral naming improved after stimulation over the left motor cortex (M1). With regard to tDCS studies in PPA, in particular, our study aligns with previous tDCS transfer effects on verbal fluency in smaller studies that targeted the left IFG and trained a variety of other language tasks, ranging from generic tasks such as story-telling [60] to oral naming [61,88]. The present study thus confirms in a large group of patients with adequate power that stimulation over the left IFG improves selective semantic retrieval even if not explicitly trained.
Previous imaging studies have extensively documented the role of the left IFG in selective semantic retrieval of verbal information stored in long-term memory in the temporal lobe [6,8,27,89]. In a systematic review of fMRI studies related to semantic fluency, Costafreda and colleagues [90], by distinguishing between phonological and semantic fluency, supported the association between semantic fluency and brain activation (BOLD signal) in the anterior and ventral portions of the left IFG [90]. Thompson-Schill and colleagues argued that the left IFG triangularis is important for the selection of some aspect or subset of available information among competing alternatives, e.g., semantic category [6,67]. Other studies argued for differential roles of subdivisions within the left IFG: the pars opercularis (BA 44) for phonologically-cued retrieval, and the pars triangularis (BA 45) for strategic semantically-cued retrieval [9,84,91]. Furthermore, several studies have shown that the extreme capsule, a bundle connecting the left IFG with left temporal areas, is important for semantic processing and comprehension [92–94]. Although this bundle is sometimes difficult to detect and many times considered as part of the uncinate, in vivo tracing studies in humans and the macaque have shown that the uncinate connects prefrontal regions to anterior temporal and involves emotional regulation [95]. The structural white matter connections between semantic control (the left IFG) and semantic processing areas (the anterior, inferior, and middle temporal and fusiform gyri) may allow the left IFG to act as the neural substrate of selective retrieval of categorical information stored in temporal areas. Furthermore, in a recent study, we have also identified the white-matter integrity of the bundle connecting the left frontal areas to temporal areas was a significant predictor of tDCS in written naming intervention [96]. Overall, the present findings provide interventional support for a neural theory of the roles of the left IFG.
An important consideration is that some of the participants in our study had atrophy in the stimulated area. Patients with nfvPPA usually present with atrophy of the left IFG [14]. In our underpowered exploratory analysis of variants (see Appendix B) we found that this patient group benefited most from tDCS treatment. Given that we [65] and others [97,98] have not found a correlation between functional connectivity and atrophy, we would like to entertain the following 3 accounts for the present results: (1) tDCS may be more beneficial on atrophied (but still viable) tissue by means of modulating baseline function; (2) tDCS may have greater therapeutic effect when other network areas remain intact. People with nfvPPA, for example, have non-atrophied temporal regions [14]. Lesion studies suggested that the left temporal cortex stores information about semantic categories, whereas frontal areas are important in retrieving this information [21,99]. We also found that left inferior temporal gyrus (ITG) is associated with storage of lexical characteristics of both nouns and verbs in PPA [100,101]. With regard to verbal fluency in PPA, a previous study [18], as well as a recent one from our group [17] where we controlled for dementia severity, found that atrophy in the anterior and inferior left temporal regions as well as in right frontal regions was associated with impairment of semantic fluency. Yet, the question of which areas or networks predict the treatment effects (behavioral and neuromodulatory) are for a subsequent imaging study to determine; (3) other brain areas may mediate the response of tDCS over the left IFG, since we stimulated with large electrodes (2×2 inches). Although possible, we have not found evidence for this hypothesis in any of our previous imaging studies that focused on the areas that were modulated with our large patches. On the contrary, when we searched in the language and the default mode networks [66], or in the whole brain [65], we found that the only the left IFG, and in particular the IFG triangularis, was modulated by tDCS in our trial, and this modulation was significantly correlated with behavioral outcomes in lexical retrieval. Finally, we cannot exclude the possibility that other remote areas such as the right IFG may mediate the effects of tDCS over the left IFG given their homotopic structural and functional connectivity. This is, however, the topic for a subsequent imaging study.
Importantly, in the present study, when we tested the effects of tDCS on tasks associated with computations of other frontal areas (and the left MFG in particular) we have found no evidence of any modulation of these tasks by tDCS. We found no evidence of modulation of (1) Digit Span forward or backward, which rely partially on monitoring and working memory respectively, along with verbal production, (2) Trail-making Part A, which relies on monitoring, or (3) letter fluency (FAS), which relies on frontal areas for searching and monitoring [9,21,90,102]. None of these tasks showed any beneficial effect of tDCS. Therefore, we could conclude that the effects we see are specific to the stimulated area, the left IFG, and are not mediated by executive control areas or their functions. The effects on selective semantic retrieval presented here also cannot be attributed to a general ‘feeling good’ improvement that could have resulted after stimulation of the left MFG, as other neuromodulation studies have shown [103]. A note on other language tasks such as sentence comprehension: sentence comprehension tasks may also involve the left IFG along with other dorsal and ventral areas, depending on semantic or syntactic task requirements [92,104]. Such tasks have sub-scores that may relate to semantic retrieval to different degrees. Given the compositionality and complexity of such tasks, we did not deem it appropriate to use them as contrasts here. However, they do deserve further thorough investigation, and thus are reported elsewhere [105].
The present results align also with previous studies on the neural basis of tDCS effects, both from our group and others. Meinzer and colleagues in healthy aging [87,106], MCI [107], and our group in PPA [65,66], found that although we stimulated a large area including the left IFG, and paired with lexical retrieval tasks, tDCS modulated specifically the left IFG. We had hypothesized then that this was because the behavioral treatment task (oral/written naming/spelling targeting lexical/semantic retrieval) was paired with the function of the stimulated area (selective semantic retrieval). The present study provides additional evidence for this hypothesis by showing generalization to another task (semantic fluency task) that targets the same function, the selective semantic retrieval. Thus the present results confirm a principle of neuromodulation, the ‘functional pairing’ introduced by Bikson and colleagues that states that: tDCS is effective only when the area of stimulation is paired with the task it performs [108]. The present results would add a precision adjustment in this principle: tDCS is effective only when the area of stimulation is paired with a task that depends on the same computations that the area performs.
The present findings allow us to look at generalization (near-transfer) effects in aphasia rehabilitation studies without neuromodulation, in a different way, informed by neural theory. In this context, generalization to other tasks, not trained during intervention would only be expected if these tasks share the same neural computation(s) with the trained task(s). Having this principle in mind, investigations of generalization may become more efficient because of a hypothesis-driven point of departure. Also, from a methodological point of view, there should be fewer tasks to compare to and fewer multiple comparisons to correct for.
A possible question that can be raised is why tDCS over the left IFG did not affect letter fluency since there are studies on people with aphasia and healthy participants showing that left IFG tDCS often boosted both semantic and letter fluency task performance [109]. Indeed, tDCS over the left IFG may boost both semantic and letter fluency performance but this may happen for different reasons. Indeed, both letter and semantic fluency involve selective lexical retrieval. However, the criterion for selection is different. Semantic selective retrieval seems to be a specific function of the left IFG triangularis as seminal studies by Thompson-Schill and colleagues [6] have eloquently shown. Letter fluency, in contrast to semantic fluency, is considered a more prefrontal task [110] than semantic fluency. We have even confirmed the involvement of the dorsolateral prefrontal cortex in letter but not in semantic fluency in a highly overlapping PPA cohort [17]. Searching with a strategy for which you have to keep in mind the sequence of letters in the alphabet and monitor them, involves both monitoring of letter sequences (and therefore the dorsolateral prefrontal cortex, DLPFC) and lexical representations (and therefore the temporal gyrus). In contrast, searching with a strategy for which you have to select amongst semantic alternatives seems to involve a semantic selection criterion (and therefore the left IFG triangularis) and lexical representations (and therefore the temporal gyrus). Furthermore, the extreme capsule connects the left IFG triangularis with the anterior temporal lobe which is considered the neural substrate of semantic representations [84].
Then, the question becomes why tDCS over the left IFG may have boosted both letter and semantic fluency and not only semantic fluency in other studies. We would like to offer two explanations: (1) The IFG and the DLPFC are connected with each other both functionally and structurally and since the current in tDCS is more diffuse, the DLPFC may get activated. Given the principle of ‘functional targeting’ where only active cells and networks are modulated by tDCS [108], it could be the case that our behavioral task primed only semantic selective retrieval and not any lexical retrieval. (2) It may also be the case that generalization in both letter and semantic fluency occurs in montages with a cathode in the Right DLPFC. Since all the areas in the same electrical loop may get activated, activation of the DLPFC is more probable in montages that include prefrontal areas. In our montage (anode over the left IFG and extracephalic cathode over the right cheek) we tried to make stimulation more focal by avoiding the creation of such loops with other brain areas of the left or the right hemisphere.
The limitations of the present study lie mostly in the lack of power for investigation of stimulation effects in each variant separately. Although this is the largest study to-date, at least to our knowledge, looking systematically at generalization effects of tDCS, these effects would need to be replicated in a larger multi-site study in the future. A future study should also associate these findings in the brains of these patients, and, in particular, with functional connectivity or gamma aminobutyric acid (GABA) changes that we and others have previously found to be modulated by tDCS [65,66,106,111,112].
5. Conclusions
The present tDCS study provides interventional evidence that the left IFG is a critical area for selective semantic retrieval even in neurodegenerative conditions such as PPA. The combination of tDCS over the left IFG with a lexical/semantic retrieval intervention will affect additional selective semantic retrieval tasks but not other frontal tasks that depend on different frontal computations such as monitoring, which depends on different frontal and prefrontal areas. Further work determining correlated functional connectivity changes and, most importantly, biomarkers of these generalization effects, is warranted and needed.
Supplementary Material
Acknowledgements
We would like to thank our participants and referring physicians for their dedication and interest in our study.
Funding
This work was supported by grants from the Science of Learning Institute at Johns Hopkins University and by the National Institutes of Health (National Institute of Deafness and Communication Disorders; NIDCD) through award R01 DC014475 and R01 AG06881to KT. AH was supported by NIH (NIDCD) through awards R01 DC03681, R01 DC011317 and P50 DC014664.
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
Ethics Statement
All data were acquired in compliance with the Johns Hopkins Hospital Institutional Review Board (NA_00071337). All participants were informed about and signed the consent form for the study.
REFERENCES
- [1].Price CJ. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage 2012;62:816–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 2007;8:393–402. 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- [3].Hillis AE, Kane A, Tuffiash E, Ulatowski JA, Barker PB, Beauchamp NJ, et al. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain Lang 2001;79:495–510. [DOI] [PubMed] [Google Scholar]
- [4].Hillis AE, Barker PB, Beauchamp NJ, Winters BD, Mirski M, Wityk RJ. Restoring blood pressure reperfused Wernicke’s area and improved language. Neurology 2001;56:670–2. [DOI] [PubMed] [Google Scholar]
- [5].Selective activation of the ventrolateral prefrontal cortex in the human brain during active retrieval processing - Cadoret - 2001 - European Journal of Neuroscience - Wiley Online Library n.d. 10.1046/j.0953-816x.2001.01737.x (accessed February 11, 2019). [DOI] [PubMed]
- [6].Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci 1997;94:14792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proc Natl Acad Sci 1998;95:15855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol 2005;15:219–24. 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- [9].Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—The roles of Brodmann areas 44 and 45. NeuroImage 2004;22:42–56. 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- [10].Heim S, Eickhoff SB, Amunts K. Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? NeuroImage 2008;40:1362–8. [DOI] [PubMed] [Google Scholar]
- [11].Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–8. [DOI] [PubMed] [Google Scholar]
- [12].Mesulam MM. Primary progressive aphasia. Ann Neurol 2001;49:425–32. [PubMed] [Google Scholar]
- [13].Mesulam M-M. Primary Progressive Aphasia — A Language-Based Dementia. N Engl J Med 2003;349:1535–42. 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- [14].Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grossman M. Progressive aphasic syndromes: clinical and theoretical advances. Curr Opin Neurol 2002;15:409–13. [DOI] [PubMed] [Google Scholar]
- [16].Wilson SM, Ogar JM, Laluz V, Growdon M, Jang J, Glenn S, et al. Automated MRI-based classification of primary progressive aphasia variants. NeuroImage 2009;47:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Riello M, Frangakis C, Ficek B, Webster KT, Faria AV, John Desmond, et al. Neural correlates of phonemic and semantic fluency in primary progressive aphasia independent from language and cognitive severity. Rev n.d. [Google Scholar]
- [18].Libon DJ, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, et al. Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology 2009;73:535–42. 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lezak M, Loring D, Howieson D. Neuropsychological Assessment. vol. 4. New York: Oxford University Press; 2004. [Google Scholar]
- [20].Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology 1998;12:259–67. 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- [21].Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc 2006;12:896–900. [DOI] [PubMed] [Google Scholar]
- [22].Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol 2014;5:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Henry JD, Crawford JR. Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc 2004;10:608–22. [DOI] [PubMed] [Google Scholar]
- [24].Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia 2004;42:1212–22. [DOI] [PubMed] [Google Scholar]
- [25].Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology 2004;18:284. [DOI] [PubMed] [Google Scholar]
- [26].Laisney M, Matuszewski V, Mézenge F, Belliard S, de la Sayette V, Eustache F, et al. The underlying mechanisms of verbal fluency deficit in frontotemporal dementia and semantic dementia. J Neurol 2009;256:1083. [DOI] [PubMed] [Google Scholar]
- [27].Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci 2011;15:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Petrides M. Broca’s area in the human and the non-human primate brain. Broca’s Reg 2006:31–46. [Google Scholar]
- [29].Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J Neurosci 2011;31:16949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tyler LK, Marslen-Wilson WD, Randall B, Wright P, Devereux BJ, Zhuang J, et al. Left inferior frontal cortex and syntax: function, structure and behaviour in patients with left hemisphere damage. Brain 2011;134:415–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci Off J Soc Neurosci 2008;28:11435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J Neurosci Off J Soc Neurosci 2011;31:16949–57. 10.1523/JNEUROSCI.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci 2008;105:18035–40. 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Duffau H, Gatignol P, Moritz-Gasser S, Mandonnet E. Is the left uncinate fasciculus essential for language? J Neurol 2009;256:382. [DOI] [PubMed] [Google Scholar]
- [35].Harvey DY, Wei T, Ellmore TM, Hamilton AC, Schnur TT. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia 2013;51:789–801. 10.1016/j.neuropsychologia.2013.01.028. [DOI] [PubMed] [Google Scholar]
- [36].Papagno C, Miracapillo C, Casarotti A, Romero Lauro LJ, Castellano A, Falini A, et al. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain 2011;134:405–14. [DOI] [PubMed] [Google Scholar]
- [37].Rofes A, Mandonnet E, de Aguiar V, Rapp B, Tsapkini K, Miceli G. Language processing from the perspective of electrical stimulation mapping. Cogn Neuropsychol 2018:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hillis AE, Kleinman JT, Newhart M, Heidler-Gary J, Gottesman R, Barker PB, et al. Restoring cerebral blood flow reveals neural regions critical for naming. J Neurosci Off J Soc Neurosci 2006;26:8069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Roncero C, Service E, De Caro M, Thiel A, Probst S, Chertkow H. Maximizing the treatment benefits of tDCS in neurodegenerative anomia. Front Neurosci 2019;13:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McConathey EM, White NC, Gervits F, Ash S, Coslett H, Grossman M, et al. Baseline performance predicts tdcs-mediated improvements in language symptoms in primary progressive aphasia. Front Hum Neurosci 2017;11:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gervits F, Ash S, Coslett HB, Rascovsky K, Grossman M, Hamilton R. Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain Lang 2016;162:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, et al. Electrical brain stimulation in different variants of primary progressive aphasia: a randomized clinical trial. Alzheimers Dement Transl Res Clin Interv 2018;4:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fenner AS, Webster KT, Ficek BN, Frangakis CE, Tsapkini K. Written Verb Naming Improves After tDCS Over the Left IFG in Primary Progressive Aphasia. Front Psychol 2019;10. 10.3389/fpsyg.2019.01396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Themistocleous C, Webster K, Tsapkini K. Effects of tDCS on Sound Duration in Patients with Apraxia of Speech in Primary Progressive Aphasia. Brain Sci 2021;11:335. 10.3390/brainsci11030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cotelli M, Fertonani A, Miozzo A, Rosini S, Manenti R, Padovani A, et al. Anomia training and brain stimulation in chronic aphasia. Neuropsychol Rehabil 2011;21:717–41. [DOI] [PubMed] [Google Scholar]
- [46].Cotelli M, Manenti R, Petesi M, Brambilla M, Cosseddu M, Zanetti O, et al. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimers Dis JAD 2014;39:799–808. [DOI] [PubMed] [Google Scholar]
- [47].Price AR, Peelle JE, Bonner MF, Grossman M, Hamilton RH. Causal Evidence for a Mechanism of Semantic Integration in the Angular Gyrus as Revealed by High-Definition Transcranial Direct Current Stimulation. J Neurosci 2016;36:3829–38. 10.1523/JNEUROSCI.3120-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chesters J, Möttönen R, Watkins KE. Transcranial direct current stimulation over left inferior frontal cortex improves speech fluency in adults who stutter. Brain 2018;141:1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke J Cereb Circ 2010;41:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chrysikou EG, Hamilton RH. Noninvasive brain stimulation in the treatment of aphasia: Exploring interhemispheric relationships and their implications for neurorehabilitation. Restor Neurol Neurosci 2011. [DOI] [PubMed] [Google Scholar]
- [51].Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, et al. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. J Cogn Neurosci 2011;23:2309–23. [DOI] [PubMed] [Google Scholar]
- [52].Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke J Cereb Circ 2011;42:819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kang EK, Kim YK, Sohn HM, Cohen LG, Paik NJ. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restor Neurol Neurosci 2011;29:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Marangolo P, Marinelli CV, Bonifazi S, Fiori V, Ceravolo MG, Provinciali L, et al. Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behav Brain Res 2011;225:498–504. [DOI] [PubMed] [Google Scholar]
- [55].Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, et al. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry 2008;79:451–3. [DOI] [PubMed] [Google Scholar]
- [56].Tippett DC, Hillis AE, Tsapkini K. Treatment of Primary Progressive Aphasia. Curr Treat Options Neurol 2015;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cotelli M, Manenti R, Ferrari C, Gobbi E, Macis A, Cappa SF. Effectiveness of language training and non-invasive brain stimulation on oral and written naming performance in Primary Progressive Aphasia: A meta-analysis and systematic review. Neurosci Biobehav Rev 2020;108:498–525. 10.1016/j.neubiorev.2019.12.003. [DOI] [PubMed] [Google Scholar]
- [58].Nissim NR, Moberg PJ, Hamilton RH. Efficacy of Noninvasive Brain Stimulation (tDCS or TMS) Paired with Language Therapy in the Treatment of Primary Progressive Aphasia: An Exploratory Meta-Analysis. Brain Sci 2020;10:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Coemans S, Struys E, Vandenborre D, Wilssens I, Engelborghs S, Paquier P, et al. A Systematic Review of Transcranial Direct Current Stimulation in Primary Progressive Aphasia: Methodological Considerations. Front Aging Neurosci 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gervits F, Ash S, Diloyan M, Morgan B, Coslett H, Grossman M, et al. Transcranial direct current stimulation for the treatment of primary progressive aphasia. Neurology 2015;84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Roncero C, Kniefel H, Service E, Thiel A, Probst S, Chertkow H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimers Dement Transl Res Clin Interv 2017;3:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wechsler D. Manual for the Wechsler adult intelligence scale-revised (WAIS-R). San Antonio TX Psychol Corp 1981. [Google Scholar]
- [63].Cattaneo Z, Pisoni A, Papagno C. Transcranial direct current stimulation over Broca’s region improves phonemic and semantic fluency in healthy individuals. Neuroscience 2011;183:64–70. [DOI] [PubMed] [Google Scholar]
- [64].Penolazzi B, Pastore M, Mondini S. Electrode montage dependent effects of transcranial direct current stimulation on semantic fluency. Behav Brain Res 2013;248:129–35. 10.1016/j.bbr.2013.04.007. [DOI] [PubMed] [Google Scholar]
- [65].Tao Y, Ficek B, Wang Z, Rapp B, Tsapkini K. Selective Functional Network Changes Following tDCS-Augmented Language Treatment in Primary Progressive Aphasia. Front Aging Neurosci 2021;13:378. 10.3389/fnagi.2021.681043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ficek BN, Wang Z, Zhao Y, Webster KT, Desmond JE, Hillis AE, et al. The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage Clin 2018. 10.1016/j.nicl.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Thompson-Schill SL, Aguirre GK, D’Esposito M, Farah MJ. A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia 1999;37:671–6. [DOI] [PubMed] [Google Scholar]
- [68].Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004;19:203–14. [DOI] [PubMed] [Google Scholar]
- [69].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008;131:2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Homan RW. The 10–20 Electrode System and Cerebral Location. Am J EEG Technol 1988;28:269–79. 10.1080/00029238.1988.11080272. [DOI] [Google Scholar]
- [72].Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 2006;117:845–50. [DOI] [PubMed] [Google Scholar]
- [73].Rapp B, Glucroft B. The benefits and protective effects of behavioural treatment for dysgraphia in a case of primary progressive aphasia. Aphasiology 2009;23:236–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Beeson PM, Egnor H. Combining treatment for written and spoken naming. J Int Neuropsychol Soc JINS 2006;12:816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Benton AL, Sivan AB, deS Hamsher K, Spreen O. Contributions to neuropsychological assessment: A clinical manual. Oxford University Press, USA; 1994. [Google Scholar]
- [76].DeMarco AT, Turkeltaub PE. Functional anomaly mapping reveals local and distant dysfunction caused by brain lesions. BioRxiv 2018:464248. 10.1101/464248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lazar RM, Minzer B, Antoniello D, Festa JR, Krakauer JW, Marshall RS. Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke J Cereb Circ 2010;41:1485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].van der Laan M, Rose S. Targeted Learning: Prediction and Causal Inference for Observational and Experimental Data. New York: Springer; 2011. [Google Scholar]
- [79].Gruber S, Laan M van der. tmle: An R Package for Targeted Maximum Likelihood Estimation. J Stat Softw 2012;51:1–35. 10.18637/jss.v051.i13.23504300 [DOI] [Google Scholar]
- [80].Glass GV, McGaw B, Smith ML. Meta-analysis in social science research. Beverly Hills CA Sage; 1981. [Google Scholar]
- [81].The performance of the two-stage analysis of two-treatment, two-period crossover trials - Freeman - 1989 - Statistics in Medicine - Wiley Online Library n.d. 10.1002/sim.4780081202?casa_token=JBhv7voUpLkAAAAA:71UZRxa8W2-Y0pFAcTh9s-9OZFBalzsKZOaHkhEmkW3k3K7iEdGZ7WPc0WWyczVH4NYNl7ZB4PvuNQ (accessed August 28, 2022). [DOI] [PubMed]
- [82].Li T, Yu T, Hawkins BS, Dickersin K. Design, Analysis, and Reporting of Crossover Trials for Inclusion in a Meta-Analysis. PLOS ONE 2015;10:e0133023. 10.1371/journal.pone.0133023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tsapkini K, Frangakis C, Gomez Y, Davis C, Hillis AE. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology 2014;28(:1112–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Petrides M. Neuroanatomy of Language Regions of the Human Brain. New York, NY: Academic Press; 2014. [Google Scholar]
- [85].Nozari N, Hepner CR. To select or to wait? The importance of criterion setting in debates of competitive lexical selection. Cogn Neuropsychol 2019;36:193–207. [DOI] [PubMed] [Google Scholar]
- [86].Marangolo P, Marinelli CV, Bonifazi S, Fiori V, Ceravolo MG, Provinciali L, et al. Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behav Brain Res 2011;225:498–504. [DOI] [PubMed] [Google Scholar]
- [87].Meinzer M, Lindenberg R, Antonenko D, Flaisch T, Flöel A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J Neurosci 2013;33:12470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hosseini M, McConathey EM, Ungrady M, Grossman M, Coslett HB, Hamilton RH. Proceedings #10: Transcranial Direct Current Stimulation Mediates Improvements in Verbal Fluency for Patients with Primary Progressive Aphasia. Brain Stimul Basic Transl Clin Res Neuromodulation 2019;12:e69–71. 10.1016/j.brs.2018.12.179. [DOI] [Google Scholar]
- [89].Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci 1995;769:85–96. [DOI] [PubMed] [Google Scholar]
- [90].Costafreda SG, Fu CHY, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Hum Brain Mapp 2006;27:799–810. 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Katzev M, Tüscher O, Hennig J, Weiller C, Kaller CP. Revisiting the Functional Specialization of Left Inferior Frontal Gyrus in Phonological and Semantic Fluency: The Crucial Role of Task Demands and Individual Ability. J Neurosci 2013;33:7837–45. 10.1523/JNEUROSCI.3147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci 2008;105:18035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci 2009;13:175–81. [DOI] [PubMed] [Google Scholar]
- [94].Tyler LK, Marslen-Wilson WD, Randall B, Wright P, Devereux BJ, Zhuang J, et al. Left inferior frontal cortex and syntax: function, structure and behaviour in patients with left hemisphere damage. Brain J Neurol 2011;134:415–31. 10.1093/brain/awq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Petrides M, Pandya DN. Distinct Parietal and Temporal Pathways to the Homologues of Broca’s Area in the Monkey. PLOS Biol 2009;7:e1000170. 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhao Y, Ficek B, Webster K, Frangakis C, Caffo B, Hillis AE, et al. White Matter Integrity Predicts Electrical Stimulation (tDCS) and Language Therapy Effects in Primary Progressive Aphasia. Neurorehabil Neural Repair 2021;35:44–57. 10.1177/1545968320971741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Agosta F, Galantucci S, Canu E, Cappa SF, Magnani G, Franceschi M, et al. Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: a DT MRI study and a literature review. Brain Lang 2013;127:157–66. [DOI] [PubMed] [Google Scholar]
- [98].Mandelli ML, Vilaplana E, Brown JA, Hubbard HI, Binney RJ, Attygalle S, et al. Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain 2016;139:2778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mummery CJ, Patterson K, Wise RJ, Vandenberghe R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain J Neurol 1999;122 ( Pt 1):61–73. [DOI] [PubMed] [Google Scholar]
- [100].Race DS, Tsapkini K, Crinion J, Newhart M, Davis C, Gomez Y, et al. An area essential for linking word meanings to word forms: evidence from primary progressive aphasia. Brain Lang 2013;127:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Riello M, Faria AV, Ficek B, Webster K, Onyike CU, Desmond J, et al. The Role of Language Severity and Education in Explaining Performance on Object and Action Naming in Primary Progressive Aphasia. Front Aging Neurosci 2018;10. 10.3389/fnagi.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology 2006;20:529–38. [DOI] [PubMed] [Google Scholar]
- [103].Brunoni AR, Valiengo L, Baccaro A, Zanao TA, Oliveira JF de, Vieira GP, et al. r vs. ELectrical Current Therapy for Treating Depression Clinical Trial–SELECT TDCS: design, rationale and objectives. Contemp Clin Trials 2011;32:90–8. [DOI] [PubMed] [Google Scholar]
- [104].Friederici AD. The neuroanatomical pathway model of Language: Syntactic and semantic networks. Neurobiol. Lang, Elsevier; 2015, p. 349–56. [Google Scholar]
- [105].Peristeri E, Wang Z, Herrmann O, Caffo B, Frangakis C, Tsapkini K. Transcranial direct current stimulation over the left inferior frontal gyrus improves sentence comprehension. MedRxiv 2020. 10.1101/2020.09.08.20190744. [DOI] [Google Scholar]
- [106].Meinzer M, Antonenko D, Lindenberg R, Hetzer S, Ulm L, Avirame K, et al. Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J Neurosci Off J Soc Neurosci 2012;32:1859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Meinzer M, Lindenberg R, Sieg MM, Nachtigall L, Ulm L, Flöel A. Transcranial direct current stimulation of the primary motor cortex improves word-retrieval in older adults. Front Aging Neurosci 2014;6:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bikson M, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci 2013;7. 10.3389/fnhum.2013.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Pisoni A, Mattavelli G, Papagno C, Rosanova M, Casali AG, Romero Lauro LJ. Cognitive Enhancement Induced by Anodal tDCS Drives Circuit-Specific Cortical Plasticity. Cereb Cortex N Y N 1991 2018;28:1132–40. 10.1093/cercor/bhx021. [DOI] [PubMed] [Google Scholar]
- [110].Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology 2006;20:529. [DOI] [PubMed] [Google Scholar]
- [111].Harris AD, Wang Z, Ficek B, Webster K, Edden RAE, Tsapkini K. Reductions in GABA following a tDCS-language intervention for primary progressive aphasia. Neurobiol Aging 2019;79:75–82. 10.1016/j.neurobiolaging.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Meinzer M, Lindenberg R, Darkow R, Ulm L, Copland D, Flöel A. Transcranial direct current stimulation and simultaneous functional magnetic resonance imaging. JoVE J Vis Exp 2014:e51730–e51730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.