Abstract
Purpose
Myocardial injury is common in hypertensive patients with 2019 coronavirus disease (COVID-19). Immune dysregulation could be associated to cardiac injury in these patients, but the underlying mechanism has not been fully elucidated.
Methods
All patients were selected prospectively from a multicenter registry of adults hospitalized with confirmed COVID-19. Cases had hypertension and myocardial injury, defined by troponin levels above the 99th percentile upper reference limit, and controls were hypertensive patients with no myocardial injury. Biomarkers and immune cell subsets were quantified and compared between the two groups. A multiple logistic regression model was used to analyze the associations of clinical and immune variables with myocardial injury.
Results
The sample comprised 193 patients divided into two groups: 47 cases and 146 controls. Relative to controls, cases had lower total lymphocyte count, percentage of T lymphocytes, CD8+CD38+ mean fluorescence intensity (MFI), and percentage of CD8+ human leukocyte antigen DR isotope (HLA-DR)+ CD38–cells and higher percentage of natural killer lymphocytes, natural killer group 2A (NKG2A)+ MFI, percentage of CD8+CD38+cells, CD8+HLA-DR+MFI, CD8+NKG2A+MFI, and percentage of CD8+HLA-DR–CD38+cells. On multivariate regression, the CD8+HLA-DR+MFI, CD8+CD38+MFI, and total lymphocyte count were associated significantly with myocardial injury.
Conclusion
Our findings suggest that lymphopenia, CD8+CD38+MFI, and CD8+HLA-DR+MFI are immune biomarkers of myocardial injury in hypertensive patients with COVID-19. The immune signature described here may aid in understanding the mechanisms underlying myocardial injury in these patients. The study data might open a new window for improvement in the treatment of hypertensive patients with COVID-19 and myocardial injury.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-023-01523-6.
Keywords: Troponin, myocardial injury, hypertension, immune system, COVID-19
Introduction
The 2019 coronavirus disease (COVID-19) pandemic has been the deadliest viral outbreak to occur in decades, causing more than 6 million deaths worldwide as of October 2022 [1]. At the beginning of the pandemic, the respiratory tract was identified as the principal target of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but it is currently known that other major organs, such as the heart, can be affected [2]. Cardiac comorbidities, particularly hypertension, are highly prevalent in patients hospitalized with COVID-19 and are related directly to the risk of clinical disease progression [3, 4].
Cardiac injury, defined as significant cardiac troponin elevation, is the most frequently reported cardiac abnormality in patients hospitalized with COVID-19, with a prevalence ranging from 7 to 44% [5–7]. Our group and others have identified the troponin level as an important risk predictor for these patients, capable of discriminating between those with low and increased mortality rates. Diverse forms and mechanisms of myocardial injury, including acute myocardial ischemia due to plaque rupture, oxygen supply/demand imbalance (types 1 and 2 infarction), non-ischemic injuries such as myocarditis, direct virus-mediated injury, and immune system dysregulation, have been described in patients with COVID-19 [8, 9]. Myocardial injury is more prevalent in older patients and those with less oxygen saturation, diabetes, hypertension, or other cardiovascular comorbidities.
Among the comorbidities associated with COVID-19 and myocardial injury, hypertension is particularly important due to its high prevalence and global burden, but the mechanism of this association and the reasons for clinical progression have not been elucidated clearly [10, 11]. Considering that myocardial injury due to direct SARS-CoV-2 infection is rare and that the immune system is often dysregulated in patients with COVID-19, and cytokine storm and lymphopenia are important immune markers of progression to severe disease [12–14], we investigated the immune profile of hypertensive patients with COVID-19 and myocardial injury aiming to obtain a better understanding of the immune dysregulation and to provide new insights on the mechanism of myocardial injury in hypertensive patients with this disease.
Methods
Patients included in this case-control study were selected prospectively from a multicenter registry of adult patients hospitalized with confirmed COVID-19 diagnoses in 10 tertiary hospitals in Brazil between November 2020 and December 2021. SARS-CoV-2 infection was confirmed by real‐time reverse-transcription polymerase chain reaction of nasopharyngeal and/or oropharyngeal swab samples. Cases were hypertensive patients with myocardial injury, defined by troponin levels exceeding the 99th percentile upper reference limit, and controls were hypertensive patients without myocardial injury. Blood samples were collected in the first week of hospitalization. The protocol was approved by the institutional review boards and ethics committees at participating sites (CAAE#34035120.1.0000.5249). All patients provided written informed consent before enrollment.
Trained investigators collected demographic, clinical, and laboratory data using the standardized form from the International Severe Acute Respiratory and Emerging Infection Consortium/World Health Organization Clinical Characterization Protocol [15]. Data were collected from electronic medical records and entered into electronic case-report forms using the Research Electronic Data Capture platform (Vanderbilt University, Nashville, TN, USA). Clinical data included data on comorbidities, complications, and treatment. Laboratory tests were performed throughout hospitalization according to local clinical practice. The patients were followed prospectively until hospital discharge or in-hospital death. They were categorized according to age (< 50, 50–69, and ≥ 70 years), sex, body mass index (BMI), oxygen saturation (> 93% and ≤ 93%), and the presence of diabetes and cardiac and other comorbidities.
Biomarker Quantification
Plasma samples were separated after centrifugation and then frozen and stored at − 20 °C until analysis. Circulating levels of cytokines were measured using the MILLIPLEX MAP human cytokine/chemokine magnetic bead panel (#HCYTMAG-60 K-PX29; Merck Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. The C-reactive protein level was measured by latex-enhanced immunoturbidimetric assay. Cytokines not detected in > 50% of the patient samples were excluded from further analyses.
Peripheral Blood Mononuclear Cell Isolation
Blood samples collected into ethylenediaminetetraacetic acid (EDTA) tubes (BD Vacutainer® spray-coated K2EDTA tube; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) were centrifuged at 1500 × g for 15 min at 21 °C. Lymphocytes and monocytes were quantified by photometry using an automized ABX Micros 60 system (Horiba Medical, Montpellier, France). To obtain peripheral blood mononuclear cells (PBMCs), density gradient centrifugation (Ficoll-Paque; GE Healthcare, Piscataway, NJ, USA) was performed, as previously described [14].
Flow Cytometry
To quantify the immune cell populations in the PBMCs, 1 × 106 cells were stained with various combinations of fluorophore-conjugated antibodies, as previously described [14]. The following profiles were quantified: total monocytes, total lymphocytes, B lymphocytes, T lymphocytes, natural killer (NK) cells, CD4+ T cells, CD8+ T cells, CD4/CD8 cell ratio, CD8+ CD38+ T cells [percentage and CD38 mean fluorescence intensity (MFI)], CD8+ human leukocyte antigen DR isotope (HLA-DR)+ T cells (percentage and HLA-DR MFI), CD8+ natural killer group 2A (NKG2A)+ T cells (percentage and NKG2A MFI), CD8+ HLA-DR+ CD38– T cells, CD8+ HLA-DR+ CD38+ T cells, CD8+ HLA-DR– CD38+ T cells, and CD8+ HLA-DR– CD38– T cells. The approximate membrane expression (MFI) of the antigens HLA-DR and CD38, important markers of T-cell activation, and of NKG2A, an inhibitory T-cell receptor, was evaluated to improve the understanding of lymphocyte properties.
Statistical Analysis
Categorical variables, including continuous clinical variables categorized according to traditional cut-off points (age, oxygen saturation, and BMI), were characterized as proportions. Fisher’s exact test was used to compare the frequencies of clinical variables between groups with and without myocardial injury. Continuous variables were described as medians, means, and standard deviations, and compared between cases and controls using the Mann–Whitney test. To assess the magnitude of variable associations, Cohen’s effect sizes (d values) were calculated. Pearson correlation (r) was performed to assess correlations among cytokines and immune cell subsets. Immune marker data were log transformed and standardized, and multivariate forward automatic stepwise logistic regression analysis was performed to identify variables associated with myocardial injury. Variables showing significant associations were dichotomized using 90% sensitivity cut-off points, and receiver operating characteristic (ROC) curves were used to assess their value in discriminating between cases and controls. To assess correlations between baseline clinical and selected immune biomarkers according to the presence or absence of myocardial injury, phi (φ) correlation coefficients were calculated. To quantify the associations of the selected clinical and immune variables with myocardial injury, a multiple logistic regression model was used and adjusted, and unadjusted odds ratios and 95% confidence intervals were calculated. All analyses were performed using SPSS software (version 24.0; IBM Corporation, Armonk, NY, USA).
Results
In total, 193 hypertensive patients with COVID-19 were enrolled in this study: 47 cases with myocardial injury and 146 controls without myocardial injury. The median time of the blood collection was 3.0 and 2.0 days after hospitalization for the patients with and without myocardial injury, respectively. The cases were predominantly male, but the sex distribution did not differ significantly between groups. The cases were older than controls, had lower oxygen saturation values, and were more overweight but not obese. In addition, larger proportions of the cases were diabetic; had cardiac comorbidities, including chronic heart disease, heart failure, and coronary and valvular disease; and had other comorbidities, including asthma, chronic pulmonary disease, and chronic kidney disease (Table 1).
Table 1.
Baseline characteristics of hypertensive patients hospitalized for COVID-19 with and without myocardial injury
| Characteristics | Myocardial injury | Fisher’s exact test p-value | |||
|---|---|---|---|---|---|
| No (n = 146) | Yes (n = 47) | ||||
| n | (%) | n | (%) | ||
| Sex | |||||
| Male | 96 | (65.8) | 35 | (74.5) | 0.288 |
| Female | 50 | (34.2) | 12 | (25.5) | |
| Age | |||||
| < 50 years | 56 | (38.4) | 4 | (8.5) | < 0.001 |
| 50 to 69.9 years | 77 | (52.7) | 17 | (36.2) | |
| ≥ 70 years | 13 | (8.9) | 26 | (55.3) | |
| Oxygen Saturation | |||||
| > 93% | 123 | (84.2) | 28 | (59.6) | 0.001 |
| ≤ 93% | 23 | (15.8) | 19 | (40.4) | |
| BMI (kg/m2) | |||||
| < 25 | 13 | (8.9) | 9 | (19.1) | 0.002 |
| 25 a 29.9 | 57 | (39.0) | 23 | (48.9) | |
| ≥ 30.0 | 76 | (52.1) | 10 | (21.3) | |
| Diabetes | |||||
| No | 109 | (74.7) | 24 | (51.1) | 0.004 |
| Yes | 37 | (25.3) | 23 | (48.9) | |
| Cardiac comorbiditiesa | |||||
| No | 136 | (93.2) | 34 | (72.3) | < 0.001 |
| Yes | 10 | (6.8) | 13 | (27.7) | |
| Other comorbiditiesb | |||||
| No | 142 | (97.3) | 38 | (80.9) | 0.001 |
| Yes | 4 | (2.7) | 9 | (19.1) | |
aChronic heart disease, heart failure, and coronary and valvular disease
bChronic lung disease, asthma, and chronic kidney disease
COVID-19, 2019 coronavirus disease; BMI, body mass index
Immune Signatures of Hypertensive Patients With and Without Myocardial Injury
Relative to the controls, the cases had higher IL-ra, IFN-γ, MIP-1α, MIP-1β, IL-17A, IL-12 (p70), TNF-α, and TNF-β levels and lower EGF, eotaxin, MCP-1, and VEGF levels (Fig. 1). A large effect size was observed for EGF (d = –1.02; Table S1).
Fig. 1.
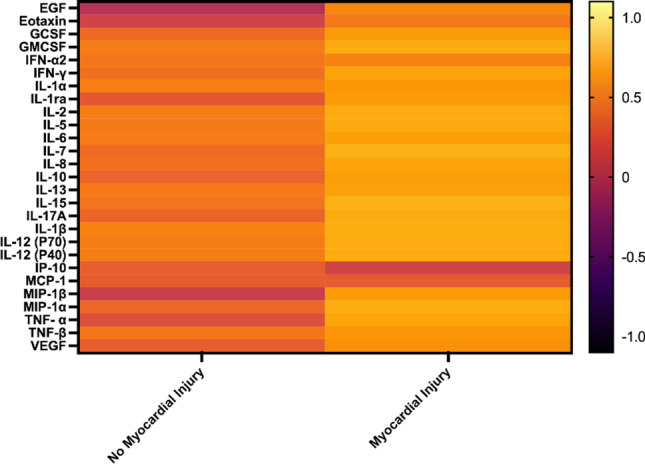
Log transformed and standardized mean cytokine values in hypertensive patients with Covid-19 with and without myocardial injury. Color scale bar shows a range of log transformed and standardized mean cytokine values. The orange-yellow colors represent higher levels, and dark colors represent lower levels
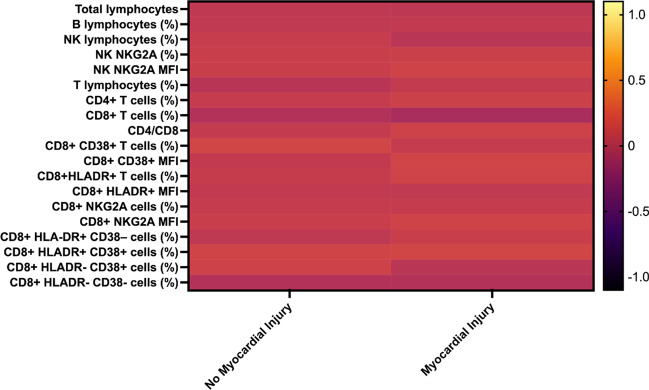
The total lymphocyte count, percentage of T lymphocytes, CD8+ CD38+ MFI, and percentage of CD8+ HLA-DR+ CD38– cells were lower in patients with than in those without myocardial injury. The percentage of NK lymphocytes, NK NKG2A MFI, percentage of CD8+ CD38+ cells, CD8+ HLA-DR MFI, CD8+ NKG2A MFI, and percentage of CD8+ HLA-DR– CD38+ cells were increased in patients with myocardial injury (Fig. 2). Effect sizes were largest for the CD8+ CD38+ MFI (d = –0.86), CD8+ NKG2A MFI (d = 0.96), and CD8+ HLA-DR MFI (d = 2.41; Table S2).
Fig. 2.
Log transformed and standardized mean immune cell subsets values in hypertensive patients with Covid-19 with and without myocardial injury. Color scale bar shows a range of log transformed and standardized mean cytokine values. The orange-yellow colors represent higher levels, and dark colors represent lower levels
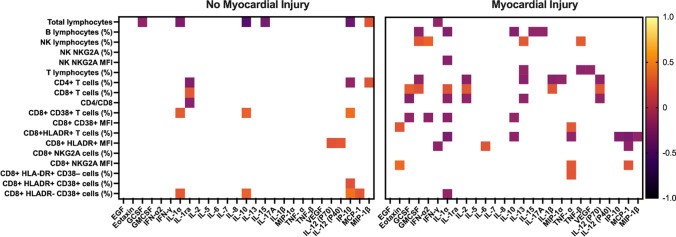
We explored the Pearson correlation between the total cytokines and the different cell subsets in both groups and noticed significant correlations in patients with and without myocardial injury. Although most correlations were weak, significant correlations were more frequent in patients with myocardial injury, and IL-1α was the cytokine that presented the higher number of correlations with the cell subsets studied in this group. In addition, we found moderate correlations between total lymphocyte count with IL-10 and IP-10 in patients with no myocardial injury (-0.512 and -0.408, respectively; Fig. 3).
Fig. 3.
Heat map of correlations among log transformed and standardized immune cell subsets and cytokines in hypertensive patients with Covid-19 with and without myocardial injury. Color scale bar shows a range of correlation coefficients (r). The orange color represents a positive correlation, decreasing to dark colors bar, which represents a negative correlation
Best Immune Biomarkers for the Prediction of Myocardial Injury in Hypertensive Patients with COVID-19
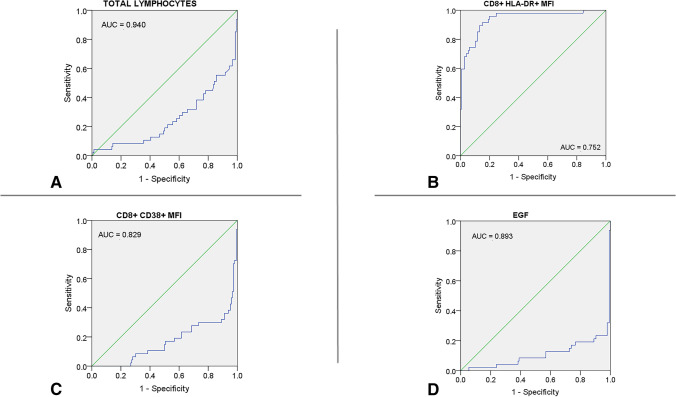
Four variables were associated with myocardial injury in the final logistic regression model: the CD8+ HLA-DR MFI (β = 2.62, p < 0.001), EGF concentration (β = –1.15, p = 0.014), CD8+ CD38 MFI (β = –2.18, p = 0.002), and total lymphocyte count (β = –1.39, p = 0.004; Table S3). Areas under ROC curves were 0.940 for CD8+ HLA-DR MFI > 1800, 0.893 for EGF concentration < 196 pg/mL, 0.829 for CD8+ CD38 MFI < 4.700, and 0.752 for total lymphocyte count < 1740 (all p < 0.001; Fig. 4). The binary associations of these biomarkers in patients with and without myocardial injury are illustrated in Fig. S1.
Fig. 4.
Receiver operating characteristic curves for immune markers associated with myocardial injury in hypertensive patients with COVID-19. A, Total lymphocytes; B, CD8+ HLA-DR+ MFI; C, CD8+ CD38+ MFI; D, EGF. COVID-19, 2019 coronavirus disease; AUC, area under the curve; HLA-DR, human leukocyte antigen DR isotope; MFI, mean fluorescence intensity; EGF, epidermal growth factor
Among patients with myocardial injury, significant correlations were detected between age ≥ 70 years and cardiac comorbidities (φ = 0.556, p < 0.001), diabetes and total lymphocyte count < 740 (φ = –0.352, p = 0.015), and CD8+ HLA-DR MFI > 1800 and EGF level < 196 pg/mL (φ = 0.389, p = 0.007). Among patients without myocardial injury, significant correlations were observed between diabetes and oxygen saturation ≤ 93% (φ = 0.180, p = 0.029), diabetes and cardiac comorbidities (φ = 0.216, p = 0.009), and age ≥ 70 years and non-cardiac comorbidities (φ = 0.242, p = 0.003; Table S4).
In a univariate logistic model, myocardial injury was associated significantly with age ≥ 70 years, oxygen saturation ≤ 93%, BMI < 30 kg/m2, diabetes, cardiac comorbidities, other comorbidities, and the four biomarkers selected by logistic regression. After adjustment, only three biomarkers remained significantly associated with myocardial injury: the CD8+ HLA-DR MFI [adjusted odds ratio (aOR) = 462.78], CD8+ CD38 MFI (aOR = 35.14), and total lymphocytes (aOR = 39.99; Table 2; Fig. 5).
Table 2.
Univariate and multivariate logistic regression results for the prediction of myocardial injury in hypertensive patients with COVID-19
| Characteristics | Univariate model | Multivariate model | |||
|---|---|---|---|---|---|
| OR | p-value | aOR | 95% CI | p-value | |
| Sex | |||||
| Male | 1 | 1 | |||
| Female | 0.66 | 0.268 | 1.43 | 0.25—8.21 | 0.689 |
| Age | < 0.001 | 0.129 | |||
| 28 to 49 years | 1 | 1 | |||
| 50 to 69 years | 3.09 | 0.053 | 0.84 | 0.08—8.36 | 0.881 |
| 70 to 97 years | 28.00 | < 0.001 | 5.71 | 0.48—67.3 | 0.166 |
| Oxygen Saturation | |||||
| > 93% | 1 | 1 | |||
| ≤ 93% | 3.63 | 0.001 | 3.41 | 0.53—21.7 | 0.194 |
| BMI (kg/m2) | 0.004 | 0.057 | |||
| ≤ 24,9 | 1 | 1 | |||
| 25 to 29.9 | 0.58 | 0.279 | 9.08 | 0.73—112.6 | 0.086 |
| ≥ 30 (obese) | 0.19 | 0.002 | 1.10 | 0.06—18.6 | 0.945 |
| Diabetes | |||||
| No | 1 | 1 | |||
| Yes | 2.82 | 0.003 | 6.04 | 0.97—37.7 | 0.054 |
| Cardiac comorbidities | |||||
| No | 1 | 1 | |||
| Yes | 5.20 | < 0.001 | 5.54 | 0.66—46.7 | 0.116 |
| Other comorbidities | |||||
| No | 1 | 1 | |||
| Yes | 8.41 | 0.001 | 4.68 | 0.26—82.9 | 0.292 |
| HLADR MFI > 1790 | |||||
| No | 1 | 1 | |||
| Yes | 47.30 | < 0.001 | 462.78 | 37.1—5.771 | < 0.001 |
| EGF < 196 | |||||
| No | 1 | 1 | |||
| Yes | 14.16 | < 0,001 | 3.56 | 0.57—22.3 | 0.175 |
| CD38 MFI < 4700 | |||||
| No | 1 | 1 | |||
| Yes | 6.69 | 0.001 | 35.14 | 2.66—464.5 | 0.007 |
| Total lymphocytes < 1740 | |||||
| No | 1 | 1 | |||
| Yes | 4.65 | 0.002 | 39.99 | 2.51—636.3 | 0.009 |
COVID-19, 2019 coronavirus disease; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; BMI, body mass index; HLA-DR, human leukocyte antigen DR isotope; MFI, mean fluorescence intensity; EGF, epidermal growth factor
Fig. 5.
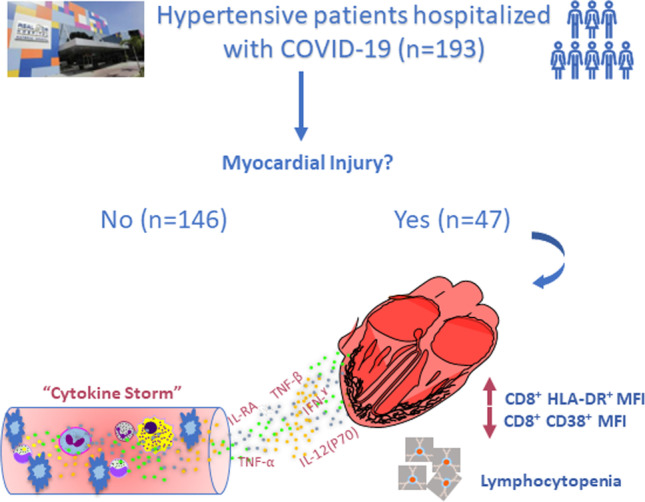
Schematic illustration of the main findings: immune biomarkers associated with myocardial injury in hypertensive patients hospitalized with COVID-19
Discussion
Hypertension is among the most common comorbidities associated with worse clinical prognosis in patients with COVID-19 [16]. In this study, we examined the immunological signature of myocardial injury in hypertensive patients with COVID-19 in detail. We found that myocardial injury was associated strongly with immune derangement, reflected by differential circulating cytokine levels and immune cellular subset expression and activation. In addition, we identified the CD8+ HLA-DR+ MFI, CD8+ CD38+ MFI, and total lymphocyte count as biomarkers associated with myocardial injury in this population. These data provide new insight on mechanisms of myocardial injury in hypertensive patients with COVID-19.
Cytokine storms have been associated extensively with COVID-19 progression [12]. In a previous work, we demonstrated the relevance of IL-12 (p70) and IL-10, together with clinical measures obtained at admission, as biomarkers of the increased risk of COVID-19 progression in hypertensive patients [13]. In the present study, the IL-12 (p70) level was higher in hypertensive patients with COVID-19 and myocardial injury than in those without injury, and the IL-10 level showed a clear, albeit nonsignificant, increasing trend. These findings confirm that these cytokines not only play an important role in disease progression, but also are associated with myocardial injury. The level of IFN-γ, a cytokine classically related to viral infection, was also higher in patients with myocardial injury. IFN-γ elevation is a known independent mortality risk factor in patients with COVID-19 [17]. Although IL-6 and IL-1β elevations have frequently been associated with progression to severe COVID-19 [13, 18, 19], the levels of these cytokines were highly variable in patients with myocardial injury in this study; thus, additional research with larger samples is needed to determine whether these are markers of myocardial injury. Consistent with the characterization of TNF-α as an independent predictor of survival in patients with COVID-19 [20], we observed TNF-α elevation in patients with myocardial injury. Although high circulating levels of EGF have been consistently associated with COVID-19 severity [21, 22], these levels were lower in patients with than in those without myocardial injury in this study, and this variable had a larger effect size than did the other cytokine variables studied. Overall, the data from this study consistently support the hypothesis that cytokines are involved in myocardial injury in hypertensive patients with COVID-19.
Lymphopenia is one of the most important and frequently encountered immune signatures of COVID-19 and progression to severe disease [23, 24]. We found marked lymphopenia, particularly of T lymphocytes, in patients with myocardial injury. We previously proposed lymphopenia as a biomarker of COVID-19 progression risk in hypertensive patients [13, 14] and the present results demonstrate that it is also an important predictor of myocardial injury in these patients. Similarly, immunologic dysregulation with lymphopenia was described in children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19, and the presence of myocardial injury was also reported in these patients [25]. These findings allow us to speculate that the lymphopenia and the cytokine storm play a vital role in the underlying mechanism of myocardial injury in COVID-19 in adults and pediatric patients.
We observed a moderate negative correlation between lymphopenia with IL-10 and IP-10 in patients with no myocardial injury. Lymphopenia and higher levels of both IL-10 and IP-10 have been associated with the severity progression of patients with COVID-19 [26, 27] and, considering that all our patients were hypertensive, it is interesting to remark that a previous study reported higher levels of IP-10 as the strongest independent predictor of increased systolic blood pressure in patients with essential hypertension [28]. In addition, IL-1α was the cytokine that presented the highest number of correlations with the different cell subsets studied in patients with myocardial injury. It is well known that IL-1 has a marked enhancing effect on antigen-specific CD8 + T cell expansion, differentiation, migration to the periphery, and memory [29]. The association between IL-1α and CD8 + T cells activation has also been described in other diseases, like cancer [30]. The higher frequency of correlations observed in patients with myocardial injury compared to patients without myocardial injury reinforces the hypothesis that innate and adaptative immune interplay is involved in the mechanism underlying cardiac injury.
Consistent with the finding that T-lymphocyte activation markers are increased in patients with COVID-19 with worse clinical outcomes [31, 32], we observed a greater percentage of CD8 + CD38 + T cells and greater CD8 + HLA-DR+ MFI in patients with myocardial injury. This T-lymphocyte activation (reflected by CD38–HLA-DR co-expression) is consistent with observed antiviral responses to other infections [21, 33, 34]. Furthermore and similarly, increased percentages of CD8+ CD38+ T lymphocytes predict faster human immunodeficiency virus-1 disease progression and more intensive depletion of CD4+ T lymphocytes in infected patients [35].
Our group previously proposed the CD8+ NKG2A+ MFI at the time of hospital admission, in combination with several clinical variables, as a robust biomarker associated with a high risk of COVID-19 progression in hypertensive patients [14]. In the mentioned study we reported an increase in both CD8 + HLA-DR+ MFI, and CD8+ NKG2A+ MFI on admission, highlighting the dysregulated immune response with increased activation and at the same time exhaustion of cytotoxic T cells. In fact, some studies suggest that exhaustion could be a consequence of the overactivation of CD8 + T cells [36]. This phenomenon of activation and exhaustion concomitantly with immune activation of T cells has been described in other viral infections such as HIV [37].
In addition, our present finding of an inverse relationship between HLADR and CD38 expression has been previously described in HIV infection. Among other studies, Hua et al. (3) have reported that although HIV-specific CD8 + T cells usually express similar levels of two activation markers, they may exhibit a differential expression, with low CD38 and high HLA-DR expression, particularly in HIV-infected patients who exhibit spontaneous viral control. They suggest that this differential CD8 + activation profile is related to a different cytotoxic capacity in HIV-infected patients (3). Together, these findings suggest the presence of diverse CD8 + T cell activation mechanisms that may involve either HLA-DR or CD38, which may be related to different phenotypes.
In the present study, the CD8+ NKG2A+ MFI was greater in patients with myocardial injury, confirming the relevance of this parameter not only for clinical COVID-19 progression but also for myocardial injury. Collectively, the changes observed in several cell subsets studied help to define the immune signature of hypertensive patients with COVID-19 and myocardial injury. The multivariable analysis performed demonstrated the value of lymphopenia, the CD8 + CD38+ MFI, and the CD8+ HLA-DR+ MFI as independent predictors of myocardial injury, even after adjustment for selected clinical and immune markers, highlighting the robustness of our findings.
Regarding the possible role of lymphocytes in the mechanisms underlying myocardial injury, several works have highlighted the presence of non-specific sub-epicardial inflammatory infiltrate in cardiac samples from patients with COVID-19 and myocardial injury [38, 39]. Most of these reports describe atypical cardiac histological patterns, with few cardiac samples fulfilling the classical histological criteria for myocarditis [38]. These findings could be explained by the association of myocardial damage with larger percentages of activated CD8+ T cells, as found in this study. In support to this hypothesis, the essential role of T cells in the mechanisms underlying viral myocarditis has been demonstrated [40]. Additionally, we observed an increase in the level of NKG2A, a marker of T-cell exhaustion, in association with increased T-cell activation, reflecting T-cell dysregulation. Taken together, these data provide a new perspective on the role played by these important immune cells in the main mechanism of myocardial injury in hypertensive patients with COVID-19, offering potential new therapeutic targets.
This study has some limitations. The interval between symptom onset and hospital admission varied among the enrolled patients, although all patients were included in the study in the first week of hospitalization (the median blood collection time interval was 3.0 and 2.0 days after hospitalization for groups with and without myocardial injury, respectively). Other limitation of this study was the lack of data regarding some additional confounding factors such as previous medications or smoking history. In addition, large degrees of variability in a few immune markers together with the presence of some outliers may have reduced the sensitivity of some analyses. However, we log transformed and standardized the immune markers to control for such variability and calculated Cohen’s effect sizes to assess the clinical significance of our findings.
Our data reveal immune markers directly involved in the main mechanisms of myocardial injury in hypertensive patients with COVID-19. Based on these findings, we propose lymphopenia, the CD8+ CD38+ MFI, and the CD8+ HLA-DR+ MFI as remarkable immune biomarkers of myocardial injury in this context.
Conclusion
The immune signatures described in the present work may further our understanding of the immune contribution to the mechanisms underlying myocardial injury in hypertensive patients with COVID-19, opening a new avenue for the improvement of the treatment of hypertensive patients with COVID-19 and myocardial injury.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are very grateful to the staff and research assistants at the D’Or Institute for Research and Education and Rede D’Or hospitals who dedicated their time to support this study.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Renata Moll-Bernardes, Ronir Raggio Luiz, and Emiliano Medei. The first draft of the manuscript was written by Renata Moll-Bernardes and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.”
Funding
This work was supported by intramural grants from D’Or Institute for Research and Education; FAPERJ (nos. E-26/210.155/2020, E-26/203.169/2017, E-26/010.000149/2020, E-26/210.191/2020, and E-26/210.253/2020); SEI-260003/002718/2020; CNPq (no. 310681/2018–9); CAPES; FINEP; and Serrapilheira Institute.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Local Ethics Committee (CAAE#34035120.1.0000.5249) in June 25th, 2020. All patients provided written informed consent before enrollment.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World HO. World Health Organization. WHO corona disease (COVID-19) dashboard.World Health Organization. 2020 Available at https://covid19.who.int. 2022 (Accessed October 25, 2022).
- 2.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382(25):e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Feldman A, D'Andrea Saba Arruda G, et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–The BRACE CORONA Trial. Am Heart J. 2020;226:49–59. doi: 10.1016/j.ahj.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majure DT, Gruberg L, Saba SG, Kvasnovsky C, Hirsch JS, Jauhar R, et al. Usefulness of Elevated Troponin to Predict Death in Patients With COVID-19 and Myocardial Injury. Am J Cardiol. 2021;138:100–106. doi: 10.1016/j.amjcard.2020.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aikawa T, Takagi H, Ishikawa K, Kuno T. Myocardial injury characterized by elevated cardiac troponin and in-hospital mortality of COVID-19: An insight from a meta-analysis. J Med Virol. 2021;93(1):51. doi: 10.1002/jmv.26108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siripanthong B, Asatryan B, Hanff TC, Chatha SR, Khanji MY, Ricci F, et al. The pathogenesis and long-term consequences of COVID-19 cardiac injury. Basic Transl Sci. 2022;7(3_Part_1):294–308. doi: 10.1016/j.jacbts.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visco V, Vitale C, Rispoli A, Izzo C, Virtuoso N, Ferruzzi GJ, et al. Post-COVID-19 Syndrome: Involvement and Interactions between respiratory, cardiovascular and nervous systems. J Clin Med. 2022;11(3):524. doi: 10.3390/jcm11030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Guan B, Su T, Liu W, Chen M, Waleed KB, et al. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106(15):1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng X, Rathinasabapathy A, Liu D, Zha L, Liu X, Tang Y, et al. Association of cardiac injury with hypertension in hospitalized patients with COVID-19 in China. Sci Rep. 2021;11(1):1–10. doi: 10.1038/s41598-021-01796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moll-Bernardes R, De Sousa AS, Macedo AV, Lopes RD, Vera N, Maia LC, et al. IL-10 and IL-12 (P70) levels predict the risk of COVID-19 progression in hypertensive patients: Insights from the BRACE-CORONA trial. Frontiers in Cardiovascular Medicine. 2021;8:702507. doi: 10.3389/fcvm.2021.702507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moll-Bernardes R, Fortier SC, Sousa AS, Lopes RD, Vera N, Conde L, et al. NKG2A Expression among CD8 Cells Is Associated with COVID-19 Progression in Hypertensive Patients: Insights from the BRACE CORONA Randomized Trial. J Clin Med. 2022;11(13):3713. doi: 10.3390/jcm11133713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhvlediani T, Ali SM, Angus DC, Arabi YM, Ashraf S, Baillie JK, et al. Global outbreak research: harmony not hegemony. Lancet Infect Dis. 2020;20(7):770–772. doi: 10.1016/S1473-3099(20)30440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, Wang J, Liu F, Liu J, Cao G, Yang C, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43(8):824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadotti AC, de Castro DM, Telles JP, Wind R, Goes M, Ossoski RGC, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289:198171. doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Xu W, Li W, Huang C, Chen L. Dynamics of cytokines and lymphocyte subsets associated with the poor prognosis of severe COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(23):12536–12544. doi: 10.26355/eurrev_202012_24051. [DOI] [PubMed] [Google Scholar]
- 19.Jin M, Shi N, Wang M, Shi C, Lu S, Chang Q, et al. CD45: a critical regulator in immune cells to predict severe and non-severe COVID-19 patients. Aging (Albany NY) 2020;12(20):19867. doi: 10.18632/aging.103941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hue S, Beldi-Ferchiou A, Bendib I, Surenaud M, Fourati S, Frapard T, et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(11):1509–1519. doi: 10.1164/rccm.202005-1885OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angioni R, Sanchez-Rodriguez R, Munari F, Bertoldi N, Arcidiacono D, Cavinato S, et al. Age-severity matched cytokine profiling reveals specific signatures in Covid-19 patients. Cell Death Dis. 2020;11(11):1–12. doi: 10.1038/s41419-020-03151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol Lett. 2020;225:31. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8(1):1–10. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caro-Paton GDL, de Azagra-Garde AM, Garcia-Salido A, Cabrero-Hernandez M, Tamariz A, Nieto-Moro M. Shock and myocardial injury in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection: what we know. Case series and review of the literature. J Intensive Care Med. 2021;36(4):392–403. doi: 10.1177/0885066620969350. [DOI] [PubMed] [Google Scholar]
- 26.Haroun RA-H, Osman WH, Eessa AM. Interferon-γ-induced protein 10 (IP-10) and serum amyloid A (SAA) are excellent biomarkers for the prediction of COVID-19 progression and severity. Life Sci. 2021;269:119019. doi: 10.1016/j.lfs.2021.119019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann J, Prezzemolo T, Vanderbeke L, Roca CP, Gerbaux M, Janssens S, et al. Increased IL-10-producing regulatory T cells are characteristic of severe cases of COVID-19. Clin Transl Immunol. 2020;9(11):e1204. doi: 10.1002/cti2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stumpf C, Auer C, Yilmaz A, Lewczuk P, Klinghammer L, Schneider M, et al. Serum levels of the Th1 chemoattractant interferon-gamma-inducible protein (IP) 10 are elevated in patients with essential hypertension. Hypertens Res. 2011;34(4):484–488. doi: 10.1038/hr.2010.258. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med. 2013;210(3):491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udagawa K, Niki Y, Kikuchi T, Fukuhara Y, Takeda Y, Miyamoto T, et al. Overexpression of Interleukin-1α Suppresses Liver Metastasis of Lymphoma: Implications for Antitumor Effects of CD8+ T-cells. J Histochem Cytochem. 2021;69(4):245–255. doi: 10.1369/0022155421991634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horenstein AL, Faini AC, Malavasi F. CD38 in the age of COVID-19: a medical perspective. Physiol Rev. 2021;101(4):1457–1486. doi: 10.1152/physrev.00046.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeidler JD, Kashyap S, Hogan KA, Chini EN. Implications of the NADase CD38 in COVID pathophysiology. Physiol Rev. 2022;102(1):339–341. doi: 10.1152/physrev.00007.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McElroy AK, Akondy RS, Davis CW, Ellebedy AH, Mehta AK, Kraft CS, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci. 2015;112(15):4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ndhlovu ZM, Kamya P, Mewalal N, Kløverpris HN, Nkosi T, Pretorius K, et al. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impact viral set point. Immunity. 2015;43(3):591–604. doi: 10.1016/j.immuni.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. JAIDS J Acquir Immune Defic Syndr. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 36.Christensen E, Jørgensen MJ, Nore KG, Dahl TB, Yang K, Ranheim T, et al. Critical COVID-19 is associated with distinct leukocyte phenotypes and transcriptome patterns. J Intern Med. 2021;290(3):677–692. doi: 10.1111/joim.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 2010;54(5):447–54. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dal Ferro M, Bussani R, Paldino A, Nuzzi V, Collesi C, Zentilin L, et al. SARS-CoV-2, myocardial injury and inflammation: insights from a large clinical and autopsy study. Clin Res Cardiol. 2021;110(11):1822–1831. doi: 10.1007/s00392-021-01910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tschöpe C, Ammirati E, Bozkurt B, Caforio AL, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18(3):169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.