Summary
Background
Low rates of postnatal retention in HIV care and viral suppression have been reported in women living with HIV (WLWH) despite viral suppression at delivery. At the same time, postpartum follow-up is of crucial importance in light of the increasing support offered in many resource-rich countries including Switzerland to WLWH choosing to breastfeed their infant, if optimal scenario criteria are met.
Methods
We longitudinally investigated retention in HIV care, viral suppression, and infant follow-up in a prospective multicentre HIV cohort study of WLWH in the optimal scenario who had a live birth between January 2000 and December 2018. Risk factors for adverse outcomes in the first year postpartum were assessed using logistic and proportional hazard models.
Findings
Overall, WLWH were retained in HIV care for at least six months after 94.2% of the deliveries (694/737). Late start of combination antiretroviral therapy (cART) during the third trimester was found to be the main risk factor for failure of retention in HIV care (crude odds ratio [OR] 3.91; 95% confidence interval [CI], 1.50–10.22; p = 0.005). Among mothers on cART until at least one year after delivery, 4.4% (26/591) experienced viral failure, with illicit drugs use being the most important risk factor (hazard ratio [HR], 13.2; 95% CI, 2.35–73.6; p = 0.003). The main risk factors for not following the recommendations regarding infant follow-up was maternal depression (OR, 3.52; 95% CI, 1.18–10.52; p = 0.024).
Interpretation
Although the results are reassuring, several modifiable risk factors for adverse postpartum outcome, such as late treatment initiation and depression, were identified. These factors should be addressed in HIV care of all WLWH, especially those opting to breastfeed in resource-rich countries.
Funding
This study has been financed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant #201369), by SHCS project 850 and by the SHCS research foundation.
Keywords: Postpartum period, Retention in HIV care, Postpartum HIV care engagement, Viral suppression, Breastfeeding, Infant follow-up
Research in context.
Evidence before this study
Despite major advances in antiretroviral treatment, low rates of postnatal retention in HIV care and viral suppression have been reported in women living with HIV (WLWH). As an increasing number of resource-rich countries recognize the importance of supporting WLWH with a strong wish to breastfeed their infant, postpartum follow-up becomes crucial to avoid vertical HIV transmission. We searched PubMed for studies published in English between January 1st, 2010 and December 1st, 2022 using following search terms: “HIV” AND “postpartum period” AND (“retention in HIV care” OR “lost to follow up” OR “viral load” OR “viral rebound”). We identified several studies evaluating retention in HIV care and viral suppression during the postpartum period in WLWH, but none of them focused specifically on the outcome of women with fully suppressed plasma viral loads (pVL) at delivery. Additionally, we couldn't identify any study combining maternal and infant follow-up information.
Added value of this study
This study focused on a clearly defined subgroup of pregnant WLWH with regular antenatal HIV care and fully suppressed HIV pVL. Results confirm that most WLWH meeting these criteria also achieved the outcomes that are essential to support breastfeeding in resource-rich settings. Additionally, several modifiable risk factors for adverse postpartum outcomes were identified, which can be addressed when providing HIV care to all WLWH, particularly those opting to breastfeed in resource-rich countries. The results provide direct implications for the care of pregnant WLWH and their offspring and contribute to the ongoing public health discussions regarding the provision of support for WLWH who choose to breastfeed their infant in resource-rich countries.
Implications of all the available evidence
Antenatal HIV care engagement is essential for avoiding unfavourable postpartum outcomes. Factors such as late treatment initiation, illicit drug use and depression should be addressed to enhance adherence to cART and retention in HIV care during the postpartum period among WLWH and their offspring.
Introduction
The postpartum period is a vulnerable phase characterized by irregular sleep and elevated risk for mood disorders. Even if HIV is suppressed during pregnancy and the majority of pregnant women in resource-rich countries show viral suppression at delivery,1 low rates of postnatal retention in HIV care and viral suppression have been described.2, 3, 4 These findings fuel existing doubts about the safety of breastfeeding in women living with HIV (WLWH), as in the event of elevated maternal HIV plasma viral load (pVL) despite combination antiretroviral therapy (cART), the risk for vertical transmission of HIV might be increased. Current Swiss recommendations for medical care of WLWH and their offspring, published on December 10, 2018,5 do not actively recommend breastfeeding. However, mothers with a strong wish to breastfeed, are supported in a shared decision making process after weighting the pros and cons but only if certain criteria are met. These criteria define an “optimal scenario” under the following conditions: the pregnant woman (i) is under regular clinical care and (ii) has a suppressed HIV pVL of <50 RNA copies/ml ideally throughout pregnancy, but at least at the last two consecutive measurements before birth (minimal interval of four weeks and the last measurement within four weeks before delivery). This new approach strengthens the autonomy of the mother6 and allows for an appropriate support of women in this situation to ensure the best medical care for both mother and child. In fact, in the first three months after delivery pVL monitoring is intensified in women opting to breastfeed (i.e. monthly). In a recently published study describing the successful implementation of the Swiss recommendations for medical care of WLWH and their offspring,7 61% of the mothers fulfilling the criteria of the optimal scenario decided to breastfeed their infant. The median duration of breastfeeding was 6.3 months (range 0.7–25.7, IQR 2.5–11.1). The assumption of a very low risk of HIV transmission through breastfeeding in this setting is based mainly on the results of the PROMISE trial in southern Africa.8,9 When related to the relevant exposure risk of HIV transmission (i.e. the frequency of breastfeeding episodes), these results are comparable to the results of the PARTNER Study,10 which provided compelling evidence to support the U = U (undetectable equals untransmittable) campaign on sexual HIV transmission.11,12 However, before U = U can be applied without any doubt to breastfeeding, many unknowns remain and all clinical guidelines from high-income countries still advice against breastfeeding with HIV, even though they recognize the need of supporting women who choose to breastfeed.13,14 Although the question “Does U = U apply to breastfeeding?” will probably not be answered for some time, insights into the challenges of postpartum care over time are an invaluable support for the management of WLWH wishing to breastfeed.
In this study, we investigated retention in HIV care, viral suppression, and infant follow-up during the postpartum period in WLWH fulfilling “optimal scenario” criteria. Although the risk of vertical transmission wasn't addressed in the presented analysis, the outcomes assessed here are of crucial importance to maternal and child's health given the increasing support for breastfeeding in WLWH in many resource-rich countries.
Methods
Study design, study population, and data collection
The Swiss HIV Cohort Study (SHCS) is a systematic longitudinal cohort study enrolling people living with HIV (PLWH) in Switzerland. Since 1988 more than 21,000 PLWH have been prospectively enrolled and continuously followed at seven cohort centres, affiliated hospitals, and private practices throughout Switzerland.15 The Swiss Mother and Child HIV Cohort Study (MoCHiV) is a substudy of the SHCS and prospectively collects data on pregnant WLWH, their infants, as well as children living with HIV irrespective of the mother's HIV status.
All WLWH included in the SHCS and MoCHiV who had a live birth between January 2000 (i.e. broad introduction of cART) and December 2018 (i.e. publication of the new Swiss recommendations for medical care of WLWH and their offspring) were eligible for inclusion in the study together with their offspring. Deliveries with no data during the whole pregnancy were excluded from the analysis. Both studies (SHCS and MoCHiV) were approved by local ethics committees to which the participating centres are affiliated and written informed consent was obtained from all participants for each study.
Data collection, outcome variables, and definitions
Information on social and demographic characteristics, HIV diagnosis and treatment, pregnancy, infant follow-up, substance use, and depression were extracted from the SHCS and MoCHiV databases. The following maternal outcomes were assessed during the postpartum period (defined as the first 12 months after delivery) in women fulfilling the “optimal scenario” criteria as defined above: rate of treatment discontinuation (defined as interruption of cART for more than seven days), rate of viral suppression (defined as pVL measurement <50 RNA copies/ml) at 90 and 180 days after delivery, rate of viral failure (defined as two consecutive pVL measurements >50 RNA copies/ml or one pVL measurement >1000 RNA copies/ml), rate of postpartum HIV care engagement (defined as ≥1 HIV pVL test within 90 days of delivery) and rate of retention in HIV care (defined as ≥1 HIV pVL test within 180 days of delivery). Additionally, the rate of correct infant follow-up (defined as ≥1 HIV pVL test in the first six months of life and exclusion of HIV infection age at 18–24 months by a negative antibody screening test as described in the current Swiss recommendations5) was assessed as only outcome in the new born children. For all outcomes, the proportion of failure and where appropriate, their duration as well as the associated risk factors were determined. Among the risk factors, use of any illicit drugs was defined as use of at least one of either heroin, cocaine, cannabis or other drugs. Consumption of any substance was defined as use of either alcohol, illicit drugs, and/or smoking with available information for at least two of these substances. The presence of depression, whether the diagnosis was made by a psychiatrist or another physician and which diagnostic tool was used was recorded during each follow-up visit. The SHCS does not request the use of a specific diagnostic tool for the diagnosis of depression; psychiatrists in Switzerland use the Diagnostic and Statistical Manual of Mental Disorders (DSM) whereas Infectious Diseases specialists at SHCS centers and HIV care physicians in private practice use clinical screening questions such as the two questions screening.16 The definition of the “optimal scenario” described above was modified for the analysis in this study extending the maximal time period for the last pVL measurement before delivery to 90 days in order to better reflect real life situation, since a last follow-up visit within four weeks of birth is not always possible in clinical practice and has been more systematically performed only in recent years. Additionally, a third consecutive pVL measurement <50 RNA copies/ml was required if the minimal interval of four weeks between the last two measurements was not met.
Statistical analysis
Continuous data were described using median and interquatile range (IQR), and categorical data were described using frequency and percentage. Kaplan–Meier curves were used to graphically represent the time course of viral suppression and recovery from viral failure, as well as the association between potential risk factors and the rate of viral suppression. Risk factors for viral failure were assessed by using univariable Cox proportional hazard models. Hazard ratios (HRs) with 95% confidence interval (CI) were reported.
Risk factors for ART discontinuation, for failure of postpartum retention in HIV care and correct infant follow-up (all treated as binary outcomes, regardless of their timing) were assessed using univariable and multivariable logistic regression models. Crude (ORs) and adjusted odds ratios (aORs) with 95% CI were reported, respectively. To account for non-independence of successive deliveries from the same mother, all models included mother identity as cluster term; confidence intervals (CI) and p-values were derived from robust standard error estimates. Deliveries of twins or triplets (n = 12) were treated as a single delivery and siblings were pooled into one observation.
Many missing values occurred for some of the risk factors, mostly because in previous years (i.e. during the first part of our study period) these variables were not yet part of the study protocol or because individual data were not collected by treating physicians. This pattern of missing information was considered to be at random with respect to the risk factors themselves. A smaller part of the missing values (substance use and depression during more recent pregnancies) may not have been missing at random, e.g. due to refusal of mothers to provide information. To account for this possibility, we included missing values as a separate category in all univariable analyses. For multivariable analyses, we pooled substance use (alcohol, illicit drugs and/or smoking) into a single co-variable with “missing” as a separate category. The other covariables with missing values were excluded from the main multivariable analysis because they were strongly correlated with year of delivery (year of HIV diagnosis and time since HIV diagnosis), with substance use (depression) or with gestational age at viral suppression (start of cART), respectively. Multivariable models including time since HIV diagnosis and depression are presented as sensitivity analysis in Supplementary Table S2.
Two-tailed p values below 0.05 were considered statistically significant. All statistical analysis were performed using R (version 4.0.2). Regression models for clustered data were fitted with functions coxph in R package survival (version 3.1–12) and function geeglm in R package geepack (version 1.3.3).
Role of the funding source
The funder of the study had no role in study design, data analysis, data interpretation, writing of the report, or decision to submit the paper for publication. Data collection was made possible within the framework of the SHCS.
Results
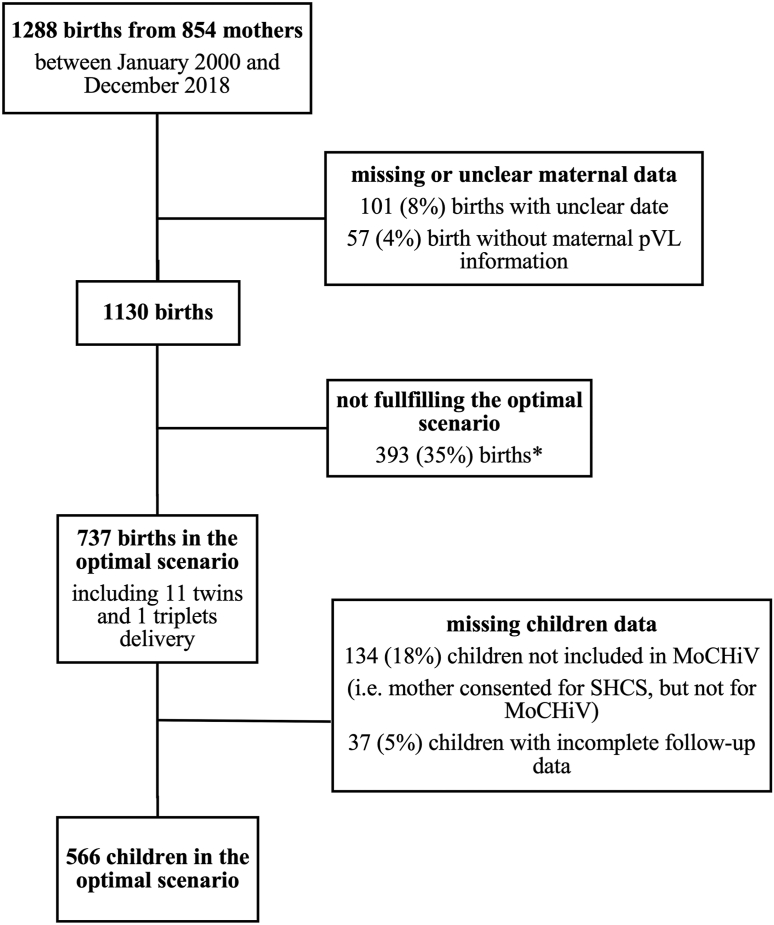
Over the 19-year study period 1288 live births in 854 mothers were reported within the SHCS and MoCHiV. After excluding deliveries with missing or incomplete data and selecting for cases in the “optimal scenario”, we included a total of 737 deliveries in 586 mothers in the analysis. Of the 737 neonates, 603 were followed up in MoCHiV. Of those, 566 children had complete follow-up information and 553 were included in the offspring outcome analysis after pooling siblings from twins (11) and triplets (1) deliveries into one observation (Fig. 1). Clinical and demographic characteristics of the patients are presented in Table 1.
Fig. 1.
Flowchart of deliveries and patient selection ∗of which in 303 of the cases maternal pVL was not suppressed (i.e. <50 copies/ml) at the last two or three consecutive measurements before delivery, in 26 of the cases the last maternal pVL measurement was performed more than 90 days before delivery and in 64 of the cases both conditions were present. Abbreviations: pVL, plasma viral load; MoCHiV, Swiss Mother and Child HIV Cohort Study; SHCS, Swiss HIV Cohort Study.
Table 1.
Patients characteristics.
| Mothers | Value | n |
|---|---|---|
| Age at delivery in years, median (IQR) | 33 (29–37) | 737 |
| Ethnicity, n (%) | 737 | |
| White | 256 (34.7%) | – |
| Black | 416 (56.4%) | – |
| Asian | 43 (5.8%) | – |
| Hispano-american | 19 (2.6%) | – |
| Other | 3 (0.4%) | – |
| Parity, n (%) | 737 | |
| 1 | 449 (60.9%) | – |
| 2 | 210 (28.5%) | – |
| 3+ | 78 (10.6%) | – |
| Year of delivery, n (%) | 737 | |
| 2000–2005 | 148 (20.1%) | – |
| 2006–2010 | 220 (29.9%) | – |
| 2011–2015 | 245 (33.2%) | – |
| 2016–2018 | 124 (16.8%) | – |
| Time since HIV diagnosis | 654 | |
| Days before delivery, median (IQR) | 2308 (1008–3832) | – |
| <5 years, n (%) | 268 (42.0%) | – |
| 5–10 years | 193 (30.3%) | – |
| >10 years | 193 (30.3%) | – |
| CD4 count at delivery in cells/mm3, median (IQR) | 512 (375–680) | 513 |
| Time of cART start, n (%) | 737 | |
| Before pregnancy | 541 (73.4%) | – |
| 1st trimester | 33 (4.5%) | – |
| 2nd trimester | 133 (18%) | – |
| 3rd trimester | 27 (3.7%) | – |
| Time of first test with pVL <50 copies/ml during pregnancy, n (%) | 737 | |
| 1st trimester | 464 (63.0%) | – |
| 2nd trimester | 167 (22.7%) | – |
| 3rd trimester | 106 (14.4%) | – |
| Children | Value | n |
| Male sex | 326 (54.1%) | 603 |
Abbreviations: cART, combination antiretroviral therapy; IQR, interquartile range; pVL, plasma viral load.
Treatment discontinuation and viral suppression in the postpartum period
Overall, mothers remained on cART until at least one year after delivery following 81.5% of the deliveries (598/734). For three deliveries treatment end date was missing. The majority (128/136, 94.1%) of treatment discontinuations occurred before 2016 when the international guidelines did not yet recommend universal cART initiation for all PLWH irrespectively of their CD4 counts. In line with this observation, most treatment discontinuations occurred either at delivery (38/136, 28%) or early after delivery (50% within 50 days) and in 93 women (68.4%) the reason for interrupting treatment was either patient's or physician's wish, probably reflecting current recommendations at the time of discontinuation. Correspondingly, significant risk factors for treatment discontinuation during the postpartum period in multivariable logistic regression analysis were year of delivery (decreasing over time; aOR 0.22 per 10 years later delivery; 95% CI, 0.12–0.41; p < 0.001) and later gestational age (GA) at the first HIV pVL test documenting viral suppression during pregnancy (aOR, 2.32 per trimester; 95% CI, 1.77–3.05; p < 0.001) as shown in Table 2. Univariable logistic regression analysis for this outcome is shown in Supplementary Table S1.
Table 2.
Multivariable logistic regression analysis for the risk of maternal cART discontinuation and failure of correct infant follow-up.
| Variable and unit | Risk of maternal cART discontinuation (n = 734) |
Risk of failure in correct infant follow-up (n = 533) |
||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Time of delivery (per 10 years) | 0.22 (0.12–0.41) | <0.001 | 0.47 (0.28–0.78) | 0.004 |
| Mother age at delivery (per 10 years) | 0.76 (0.51–1.13) | 0.172 | 0.91 (0.63–1.31) | 0.606 |
| Ethnicity: black vs. white | 0.92 (0.58–1.46) | 0.721 | 1.46 (0.97–2.19) | 0.071 |
| Parity: per additional child from 1 to 3+ | 1.11 (0.70–1.76) | 0.649 | 0.85 (0.55–1.30) | 0.446 |
| Any substance usea: yes vs. no | 1.82 (0.93–3.56) | 0.079 | 1.79 (0.94–3.41) | 0.078 |
| Any substance usea: missing vs. no | 1.50 (0.82–2.76) | 0.188 | 2.05 (1.15–3.66) | 0.015 |
| Gestational age at first pVL <50 copies/ml during pregnancy (per trimester) | 2.32 (1.77–3.05) | <0.001 | 0.77 (0.61–0.97) | 0.026 |
Bold font indicates statistically significant p values. Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; OR, odds ratio; pVL, plasma viral load.
Consumption of any substance defined as use of either alcohol, illicit drugs and or smoking with available information for at least two of these substances.
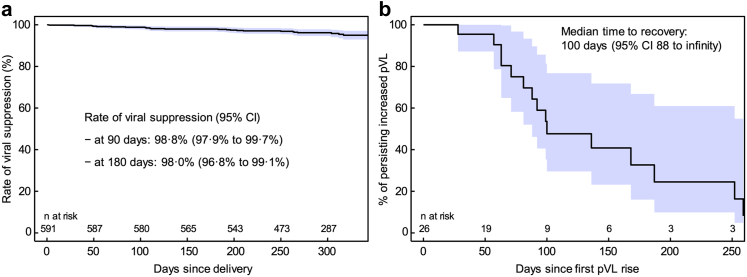
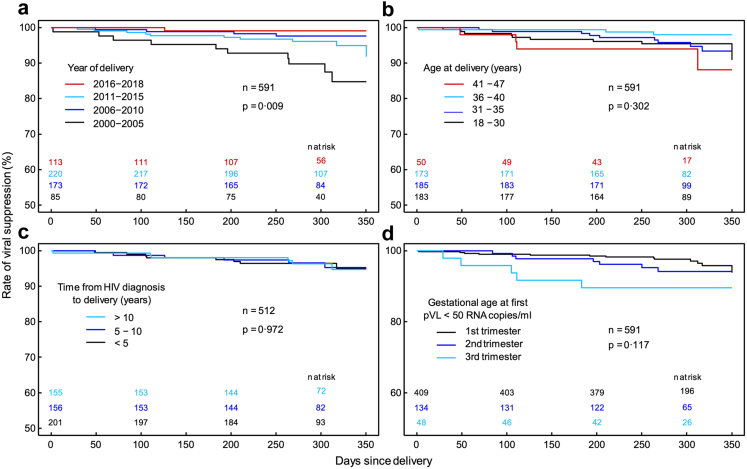
Virological data were available in 591 of the 598 cases where the mother remained under cART until at least one year after delivery. Of these, 26 (4.4%) experienced viral failure during the postpartum period. The rate of viral suppression at 90 and 180 days after delivery was 98.8% (95% CI, 97.9–99.7%) and 98% (95% CI, 96.8–99.1%), respectively (Fig. 2a). If viral failure occurred, re-suppression was rather slow; within 100 days only 50% of mothers were virally suppressed again (Fig. 2b). Factors that were found to be associated with increased risk for viral failure during the postpartum period in univariable Cox regression analysis were late cART start during third trimester (HR, 8.18; 95% CI, 2.47–27.0; p = 0.001), late viral suppression only achieved from third trimester (HR, 3.92; 95% CI, 1.51–10.2; p < 0.001), illicit drugs use (HR, 13.2; 95% CI, 2.35–73.6; p = 0.003), and smoking (HR, 4.21; 95% CI, 1.62–11.0; p = 0.003). The lowest risk for viral failure was found for most recent deliveries from 2016 onward (HR 0.07; 95% CI, 0.01–0.52; p = 0.010) and no association between viral failure and maternal age at delivery or time since HIV diagnosis could be shown (Table 3). Confirmation of these findings in multivariable analysis was not possible due to the low number of events. Kaplan–Meier curves in Fig. 3 graphically depict the rate of viral suppression for the most important factors and show a lower rate of viral suppression during postpartum period for deliveries between 2000 and 2005 and among mothers reaching viral suppression only during third trimester.
Fig. 2.
Kaplan–Meier curves showing (a) the time course of viral suppression rate from the date of delivery up to the last pVL test in the postpartum period and (b) the time course of recovery from viral failure. 95% CI (shaded area) are based on simple non-parametric survival fits and do not take into account possible dependencies of multiple births from the same mother. Abbreviations: CI, confidence interval; pVL, plasma viral load.
Table 3.
Univariable regression analysis for the risk of viral failure, failure of retention in HIV care and failure in correct infant follow-up.
| Factor | Viral failure |
Retention in HIV care |
Infant follow-up |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI)a | p-value | n | OR (95% CI)b | p-value | n | OR (95% CI)b | p-value | |
| Year of delivery | |||||||||
| 2000–2005 | 85 | ref | – | 148 | ref | – | 127 | ref | – |
| 2006–2010 | 173 | 0.17 (0.06–0.54) | 0.002 | 220 | 1.91 (0.68–5.35) | 0.216 | 150 | 0.57 (0.35–0.94) | 0.026 |
| 2011–2015 | 220 | 0.35 (0.14–0.88) | 0.025 | 245 | 1.83 (0.61–5.47) | 0.279 | 186 | 0.48 (0.29–0.77) | 0.003 |
| 2016–2018 | 113 | 0.07 (0.01–0.52) | 0.010 | 124 | 2.34 (0.74–7.37) | 0.146 | 90 | 0.51 (0.29–0.89) | 0.019 |
| Age of mother | |||||||||
| ≤30 years | 183 | ref | – | 241 | ref | – | 188 | ref | – |
| 31–35 years | 185 | 0.94 (0.34–2.62) | 0.912 | 235 | 1.27 (0.54–2.99) | 0.582 | 177 | 0.73 (0.48–1.13) | 0.162 |
| 36–40 years | 173 | 0.47 (0.15–1.53) | 0.212 | 206 | 1.63 (0.68–3.93) | 0.276 | 155 | 1.09 (0.70–1.71) | 0.700 |
| >40 years | 50 | 1.83 (0.56–6.02) | 0.320 | 55 | 0.50 (0.08–3.10) | 0.451 | 33 | 0.59 (0.24–1.42) | 0.237 |
| Parity | |||||||||
| 1 | 350 | ref | – | 449 | ref | – | 330 | ref | – |
| 2 | 172 | 0.94 (0.39–2.29) | 0.894 | 210 | 1.92 (1.02–3.63) | 0.043 | 164 | 0.89 (0.60–1.31) | 0.552 |
| 3+ | 69 | 0.98 (0.29–3.38) | 0.976 | 78 | 1.85 (0.74–4.61) | 0.184 | 59 | 1.00 (0.56–1.80) | 0.991 |
| Ethnicity | |||||||||
| white | 203 | ref | – | 256 | ref | – | 193 | ref | – |
| other | 388 | 1.18 (0.50–2.78) | 0.698 | 481 | 1.03 (0.52–2.03) | 0.942 | 360 | 1.17 (0.80–1.72) | 0.422 |
| Time since HIV diagnosis | |||||||||
| <5 years | 201 | ref | – | 270 | ref | – | 215 | ref | – |
| 5–10 years | 156 | 1.08 (0.37–3.12) | 0.887 | 193 | 3.03 (1.31–7.01) | 0.009 | 143 | 0.95 (0.60–1.48) | 0.806 |
| >10 years | 155 | 0.96 (0.32–2.91) | 0.941 | 178 | 3.27 (1.26–8.44) | 0.014 | 112 | 0.98 (0.60–1.61) | 0.937 |
| missing | 79 | 1.58 (0.49–5.07) | 0.443 | 96 | 1.62 (0.47–5.64) | 0.444 | 83 | 0.61 (0.34–1.12) | 0.114 |
| Year of HIV diagnosis | |||||||||
| ≤2000 | 156 | ref | – | 207 | ref | – | 140 | ref | – |
| 2001–2010 | 278 | 0.85 (0.33–2.19) | 0.733 | 346 | 1.13 (0.55–2.34) | 0.731 | 261 | 0.55 (0.36–0.84) | 0.006 |
| >2010 | 78 | 0.26 (0.03–2.05) | 0.201 | 88 | 0.19 (0.02–1.45) | 0.107 | 69 | 0.64 (0.35–1.19) | 0.157 |
| missing | 79 | 1.26 (0.42–3.79) | 0.685 | 96 | 0.70 (0.22–2.21) | 0.538 | 83 | 0.43 (0.23–0.80) | 0.007 |
| Alcohol consumption>once a week | |||||||||
| no | 243 | ref | – | 284 | ref | – | 208 | ref | – |
| yes | 48 | 0.78 (0.09–6.50) | 0.819 | 58 | 0.61 (0.13–2.76) | 0.520 | 35 | 0.98 (0.43–2.23) | 0.968 |
| missing | 300 | 2.21 (0.89–5.46) | 0.087 | 395 | 1.15 (0.60–2.20) | 0.684 | 310 | 1.81 (1.23–2.67) | 0.003 |
| Any illicit drug usec | |||||||||
| no | 245 | ref | – | 278 | ref | – | 195 | ref | – |
| yes | 11 | 13.2 (2.35–73.6) | 0.003 | 15 | 1.37 (0.17–11.28) | 0.767 | 12 | 2.23 (0.68–7.38) | 0.187 |
| missing | 335 | 2.96 (1.08-8.15) | 0.035d | 444 | 1.26 (0.65–2.46) | 0.487 | 346 | 1.89 (1.28–2.80) | 0.001 |
| Smoking | |||||||||
| no | 295 | ref | – | 363 | ref | – | 275 | ref | – |
| yes | 59 | 4.21 (1.62–11.0) | 0.003 | 86 | 3.49 (1.39–8.75) | 0.008 | 62 | 2.10 (1.20–3.67) | 0.009 |
| missing | 237 | 1.23 (0.46–3.29) | 0.686 | 288 | 2.01 (0.97–4.15) | 0.059 | 216 | 1.23 (0.84–1.81) | 0.294 |
| Any substance usee | |||||||||
| no | 209 | ref | – | 240 | ref | – | 176 | ref | – |
| yes | 82 | 1.76 (0.42–7.43) | 0.442 | 102 | 0.86 (0.28–2.60) | 0.782 | 67 | 1.61 (0.87–2.99) | 0.13 |
| missing | 300 | 2.73 (0.99–7.52) | 0.053 | 395 | 1.17 (0.59–2.32) | 0.646 | 310 | 2.09 (1.37–3.19) | <0.001 |
| Depression | |||||||||
| no | 203 | ref | – | 226 | ref | – | 163 | ref | – |
| yes | 18 | 2.68 (0.30–23.8) | 0.377 | 20 | 1.33 (0.18–9.53) | 0.778 | 15 | 3.52 (1.18–10.52) | 0.024 |
| missing | 370 | 2.33 (0.85–6.37) | 0.098 | 491 | 1.66 (0.79–3.49) | 0.179 | 375 | 2.30 (1.50–3.54) | <0.001d |
| Start of cART | |||||||||
| first | 514 | ref | – | 572 | ref | – | 421 | ref | – |
| second | 71 | 1.97 (0.72–5.44) | 0.189 | 133 | 1.07 (0.47–2.47) | 0.871 | 107 | 0.87 (0.56–1.36) | 0.544 |
| third | 5 | 8.18 (2.47–27.0) | 0.001 | 28 | 3.91 (1.50–10.22) | 0.005 | 22 | 0.92 (0.36–2.37) | 0.861 |
| missing | 1 | 4 | 3 | ||||||
| Gestational age at first pVL <50 copies/ml during pregnancy | |||||||||
| first | 409 | ref | – | 464 | ref | – | 347 | ref | – |
| second | 134 | 1.64 (0.72–3.77) | 0.240 | 167 | 1.12 (0.54–2.35) | 0.754 | 119 | 1.01 (0.65–1.55) | 0.972 |
| third | 48 | 3.92 (1.51–10.2) | 0.005 | 106 | 1.31 (0.56–3.08) | 0.533 | 87 | 0.68 (0.40–1.16) | 0.156 |
Bold font indicates statistically significant p values. Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; HR, hazard ratio; OR, odds ratio; pVL, plasma viral load.
Univariable Cox regression analysis.
Univariable logistic regression analysis.
Use of any illicit drugs defined as use of at least one of either heroin, cocaine, cannabis or other drugs.
Significance of the missing values probably reflects the influence of year of delivery as the most missing values occurred in previous years when the variable was not yet part of the study protocol.
Use of any substance defined as use of either alcohol, drugs and or smoking with available information for at least two of these substances.
Fig. 3.
Kaplan–Meier curves showing the time course of viral suppression rate from the date of delivery up to the last pVL test in the postpartum period for (a) year of delivery, (b) age at delivery, (c) time from HIV diagnosis to delivery and (d) gestational age at viral suppression. The significance of associations is given by p-values from robust score tests based on Cox regression including mother identity as cluster term. Numbers at risk are given in total (n) as well as for individual groups and times. Abbreviation: pVL, plasma viral load.
Engagement and retention in HIV care
The rate of HIV care engagement and retention in HIV care in our cohort was 81.8% (603/737) and 94.2% (694/737), respectively. Overall, after only 16 deliveries (2.2%) the mother was lost to follow-up (LTFUP) with no HIV pVL measurements within 12 months. In univariable logistic regression analysis, factors associated with higher rates of failure in HIV care retention were time from HIV diagnosis to delivery between five and ten years and of more than ten years (OR 3.03; 95% CI, 1.31–7.01; p = 0.009 and OR 3.27; 95% CI, 1.26–8.44; p = 0.014, respectively), smoking (OR 3.49; 95% CI, 1.39–8.75; p = 0.008), and late start of cART during third trimester (OR 3.91; 95% CI, 1.50–10.22; p = 0.005) as shown in Table 3. Confirmation of these findings in multivariable analysis was not possible due to the low number of events.
Infant follow-up
Of the 553 children with complete follow-up information included in the analysis, 535 (96.7%) had ≥1 HIV RNA test in the first 6 months of life and 381 (68.9%) had additional definitive exclusion of vertical HIV infection by serology no later than 24 months after delivery, fulfilling the criteria for correct infant follow-up. In univariable logistic regression analysis, smoking (OR, 2.10; 95% CI, 1.20–3.67; p = 0.009) and depression (OR, 3.52; 95% CI, 1.18–10.52; p = 0.024) were associated with increased risk of failure in correct infant follow-up, whereas delivery after 2005 was significantly associated with lower risk for this outcome as shown in Table 3. In multivariable logistic regression analysis, the decreasing risk of failure in correct infant follow-up over time could be confirmed (aOR, 0.47 per 10 years; 95% CI, 0.28–0.78; p = 0.004) and later GA at viral suppression was as well significantly associated with lower risk for this outcome (aOR, 0.77 per trimester; 95% CI, 0.61–0.97; p = 0.026) (Table 2).
Discussion
In a well-defined cohort of WLWH in Switzerland with impeccable antenatal HIV care, the majority maintained viral suppression and was retained in HIV care in the year after giving birth. Only 2.2% of the mothers were LTFU with no HIV pVL measurements within 12 months after delivery. The relatively high rate of treatment discontinuation in our cohort (18.5%) can be explained with the change of the international treatment guidelines over time. In fact, the majority of treatment discontinuations occurred not only at times when the international guidelines did not yet recommend universal cART initiation for all PLWH irrespectively of their CD4 counts, but also either at delivery or early thereafter and the reason for interrupting treatment was either patient's or physician's wish, probably reflecting current recommendations at the time of discontinuation.
In contrast to our findings, low rates of postnatal HIV care retention and viral suppression in WLWH have been reported in several studies in high,1, 2, 3,17,18 middle19 and low20 income countries. In a previous Swiss study3 it has also been shown that between 1996 and 2011 34% of women had a delayed visit (defined as >180 days with no visit after delivery) in the first year after giving birth and 12% were LTFU. Not achieving undetectable HIV plasma viral load (pVL) at delivery was significantly associated with being LTFU (aOR 2.42; 95% CI, 1.21–4.85; p = 0.017). Based on these findings and on the results from other studies,17,18 the “optimal scenario” criteria were defined in the current Swiss recommendations where WLWH with a strong wish to breastfeed are supported during the postpartum period. In our study these criteria were validated for the first time focusing on a clear defined subgroup of pregnant WLWH with regular antenatal HIV care and fully suppressed HIV pVL. Our data confirm that the majority of WLWH who meet these criteria also achieve the outcomes we consider essential to support breastfeeding for WLWH in resource-rich settings. The importance of antenatal HIV care engagement to avoid adverse postpartum outcome is further supported by results of previous studies.2,21 Although the results are reassuring, we have identified some modifiable risk factors that allow for improvement: illicit drugs use and smoking, were associated with an increased risk of postpartum viral failure. This is in line with the results of a recent study in PLWH.22 The relationship between late viral suppression and late start of cART during pregnancy and adverse postpartum HIV outcome is consistent with the results of previous studies.2,17,21,23 Remarkably, in our analysis we found that longer time since HIV diagnosis was associated with adverse postpartum HIV care outcome. This finding is in contrast to previously published studies reporting that women diagnosed with HIV shortly before pregnancy were less likely to remain in care.2,24 This observation is probably explained by the fact that in our cohort the proportion of late cART start during third trimester, a risk factor for adverse postpartum HIV care outcome, substantially decreased over time and only 6% of WLHW diagnosed less than 5 years prior delivery started cART in the third trimester. Another possible explanation is that experienced WLWH become less worried about adverse outcome over time and are used to less frequent follow-up. At the same time, delivery in more recent years was clearly associated with decreased risk of postpartum viral failure in our cohort, most likely reflecting improved availability, tolerability, and efficacy of cART over the years, and with decreased risk of failure in correct infant follow-up, probably due to better information of the mothers and to an improved interdisciplinary approach during antenatal care in recent years. In our analysis, depression was associated with higher risk for failure in correct infant follow-up, but not for viral failure or failure of retention in HIV care. Furthermore, we found no association between maternal factors such as age, parity, and ethnicity and postpartum HIV care outcome as it was the case in other studies.2,18
Despite the unique setting of SHCS and MoCHiV providing high quality prospectively collected data on pregnant WLWH and their infants, there are several limitations to our analysis. First, important variables such as maternal socioeconomic status were not considered in the analysis limiting generalizability of our findings to countries with similar sociodemographic profiles and health insurance policies. Second, we did not perform a comparison with non-pregnant WLWH as a control group. Although this was not strictly necessary for the aim of our study, such a comparison would have strengthened our results. Third, not all WLWH in Switzerland are included in the SHCS and in MoCHiV (estimated coverage of 71% of all patients on cART in Switzerland15) and those that refused to participate to the study might be less adherent to treatment and to follow-up. Fourth, despite a quite large sample size multivariable analysis of risk factors for viral failure and failure of HIV care retention was not possible due to the small number of events. And finally, the definition of HIV care retention and engagement in HIV care in the literature is heterogeneous challenging comparison between published studies. Nevertheless, the high rates of HIV care retention observed in our study are consistent with the high rate of viral suppression and we are confident that they well represent the current situation in our cohort of WLWH.
In conclusion, our results indicate that among mothers living with HIV who meet the “optimal scenario” criteria, good adherence to cART and retention in HIV care is achievable. These findings are reassuring in view of the growing number of mothers with a strong wish to breastfeed during the vulnerable postpartum period. In addition, several modifiable factors such as delayed treatment initiation during pregnancy, illicit drug use and depression were identified as risk factors for adverse postpartum outcome and should be considered in the decision-making process on breastfeeding and addressed in HIV care for WLWH and in future studies.
Contributors
PP and CK contributed to the study concept and design; PP, CK, KAP, BMT, CR, DLB, NW, PAC, KD contributed to data acquisition; SG, PP, CK directly accesses and verified the data and contributed to data analysis and interpretation; PP and CK drafted the manuscript; All authors contributed to the review and editing of the manuscript; All authors had full access to all the dataset and had final responsibility for the decision to submit for publication.
Data sharing statement
Individual de-identified study data and the study protocol can be made available upon request to the corresponding author (paolo.paioni@kispi.uzh.ch). The data can be requested from the date of publication until December 31, 2023. A detailed explanation of the purpose for the request as well as a study protocol, if applicable, should be presented. The final decision about data release will be taken by the scientific board of the SHCS.
Declaration of interests
BMT received payments from Effik AG, and Pierre-Favre Pharma for her participation to advisory boards. The institution of EB received honoraria from Gilead Sciences, ViiV Healthcare, MSD, Pfizer AG, Ely Lilly, and Astra Zeneca for his participation to advisory boards, and travel grants from Gilead Sciences, ViiV Healthcare, MSD, and Pfizer AG. DLB received honoraria from Gilead Sciences, ViiV Healthcare, and Merck for his participation to advisory boards and for lectures, payments from Abbvie for lectures, and travel grants from Gilead Sciences. RK received research grants from Gilead Sciences, the Swiss National Science Foundation (SNSF) and the National Institutes of Health (NIH) unrelated to this work. CP received honoraria from Effik AG for his participation to advisory boards. The authors have no other conflicts of interest to disclose.
Acknowledgements
We would like to thank the children and their parents who contributed to this study participating in the Swiss Mother and Child HIV Cohort Study (MoCHiV) and the Swiss HIV Cohort Study (SHCS). This study has been financed within the framework of the Swiss HIV Cohort Study (SHCS project 850), supported by the Swiss National Science Foundation (grant #201369).
Members of the Swiss HIV Cohort Study and the Swiss Mother and Child HIV Cohort Study.
Abela I, Aebi-Popp K, Anagnostopoulos A, Battegay M, Baumann M, Bernasconi E, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Crisinel PA, Darling K, Duppenthaler A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Francini K, Furrer H, Fux CA, Günthard HF (President of the SHCS), Hachfeld A, Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Jackson-Perry D (patient representatives), Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Kapfhammer E, Keiser O, Klimkait T, Kohns M, Kottanattu L, Kouyos RD, Kovari H, Kusejko K (Head of Data Centre), Labhardt N, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nemeth J, Nicca D, Notter J, Paioni P, Pantaleo G, Perreau M, Polli Ch, Rauch A (Chairman of the Scientific Board), Salazar-Vizcaya L, Schmid P, Speck R, Stöckle M (Chairman of the Clinical and Laboratory Committee), Tarr P, Thanh Lecompte M, Trkola A, Wagner N, Wandeler G, Weisser M, Yerly S.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100656.
Contributor Information
Paolo Paioni, Email: paolo.paioni@kispi.uzh.ch.
Swiss HIV Cohort Study (SHCS) and the Swiss Mother and Child HIV Cohort Study (MoCHiV):
I. Abela, K. Aebi-Popp, A. Anagnostopoulos, M. Battegay, M. Baumann, E. Bernasconi, D.L. Braun, H.C. Bucher, A. Calmy, M. Cavassini, A. Ciuffi, P.A. Crisinel, K. Darling, A. Duppenthaler, G. Dollenmaier, M. Egger, L. Elzi, J. Fehr, J. Fellay, K. Francini, H. Furrer, C.A. Fux, H.F. Günthard, A. Hachfeld, D. Haerry, B. Hasse, H.H. Hirsch, M. Hoffmann, I. Hösli, M. Huber, D. Jackson-Perry, C.R. Kahlert, L. Kaiser, E. Kapfhammer, O. Keiser, T. Klimkait, M. Kohns, L. Kottanattu, R.D. Kouyos, H. Kovari, K. Kusejko, N. Labhardt, B. Martinez de Tejada, C. Marzolini, K.J. Metzner, N. Müller, J. Nemeth, D. Nicca, J. Notter, P. Paioni, G. Pantaleo, M. Perreau, Ch Polli, A. Rauch, L. Salazar-Vizcaya, P. Schmid, R. Speck, M. Stöckle, P. Tarr, M. Thanh Lecompte, A. Trkola, N. Wagner, G. Wandeler, M. Weisser, and S. Yerly
Appendix A. Supplementary data
References
- 1.Meade C.M., Badell M., Hackett S., et al. HIV care continuum among postpartum women living with HIV in Atlanta. Infect Dis Obstet Gynecol. 2019;2019 doi: 10.1155/2019/8161495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams J.W., Brady K.A., Michael Y.L., Yehia B.R., Momplaisir F.M. Postpartum engagement in HIV care: an important predictor of long-term retention in care and viral suppression. Clin Infect Dis. 2015;61(12):1880–1887. doi: 10.1093/cid/civ678. [DOI] [PubMed] [Google Scholar]
- 3.Aebi-Popp K., Kouyos R., Bertisch B., et al. Postnatal retention in HIV care: insight from the Swiss HIV Cohort Study over a 15-year observational period. HIV Med. 2016;17(4):280–288. doi: 10.1111/hiv.12299. [DOI] [PubMed] [Google Scholar]
- 4.Swain C.A., Smith L.C., Nash D., et al. Postpartum loss to HIV care and HIV viral suppression among previously diagnosed HIV-infected women with a live birth in New York state. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0160775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Empfehlungen der Eidgenössischen Kommission für sexuelle Gesundheit (EKSG) für die medizinische Versorgung von HIV-infizierten Frauen und ihren Kindern. Bull Swiss Fed Off Publ Health. 2018;50:10–22. [Google Scholar]
- 6.Kahlert C., Aebi-Popp K., Bernasconi E., et al. Is breastfeeding an equipoise option in effectively treated HIV-infected mothers in a high-income setting? Swiss Med Wkly. 2018;148 doi: 10.4414/smw.2018.14648. [DOI] [PubMed] [Google Scholar]
- 7.Crisinel P.A., Kusejko K., Kahlert C.R., et al. Successful implementation of new Swiss recommendations on breastfeeding of infants born to women living with HIV. Eur J Obstet Gynecol Reprod Biol. 2023;283:86–89. doi: 10.1016/j.ejogrb.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Flynn P.M., Taha T.E., Cababasay M., et al. Prevention of HIV-1 transmission through breastfeeding: efficacy and safety of maternal antiretroviral therapy versus infant nevirapine prophylaxis for duration of breastfeeding in HIV-1-Infected women with high CD4 cell count (IMPAACT PROMISE): a randomized, open-label, clinical trial. J Acquir Immune Defic Syndr. 2018;77(4):383–392. doi: 10.1097/QAI.0000000000001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn P.M., Taha T.E., Cababasay M., et al. Association of maternal viral load and CD4 count with perinatal HIV-1 transmission risk during breastfeeding in the PROMISE postpartum component. J Acquir Immune Defic Syndr. 2021;88(2):206–213. doi: 10.1097/QAI.0000000000002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodger A.J., Cambiano V., Bruun T., et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428–2438. doi: 10.1016/S0140-6736(19)30418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waitt C., Low N., Van de Perre P., Lyons F., Loutfy M., Aebi-Popp K. Does U=U for breastfeeding mothers and infants? Breastfeeding by mothers on effective treatment for HIV infection in high-income settings. Lancet HIV. 2018;5(9):e531–e536. doi: 10.1016/S2352-3018(18)30098-5. [DOI] [PubMed] [Google Scholar]
- 12.Behrens G.M.N., Aebi-Popp K., Babiker A. Close to zero, but not zero: what is an acceptable HIV transmission risk through breastfeeding? J Acquir Immune Defic Syndr. 2022;89(4):e42. doi: 10.1097/QAI.0000000000002887. [DOI] [PubMed] [Google Scholar]
- 13.Ryom L., De Miguel R., Cotter A.G., et al. Major revision version 11.0 of the European AIDS clinical society guidelines 2021. HIV Med. 2022;23(8):849–858. doi: 10.1111/hiv.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins D., Blott M., Clayden P., et al. Guidelines for the management of HIV infection in pregnant women and the prevention of mother-to-child transmission of HIV. HIV Med. 2005;6(Suppl 2):107–148. doi: 10.1111/j.1468-1293.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 15.Scherrer A.U., Traytel A., Braun D.L., et al. Cohort profile update: the Swiss HIV cohort study (SHCS) Int J Epidemiol. 2022;51(1):33–34j. doi: 10.1093/ije/dyab141. [DOI] [PubMed] [Google Scholar]
- 16.Whooley M.A., Avins A.L., Miranda J., Browner W.S. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12(7):439–445. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adhikari E.H., Yule C.S., Roberts S.W., et al. Factors associated with postpartum loss to follow-up and detectable viremia after delivery among pregnant women living with HIV. AIDS Patient Care STDS. 2019;33(1):14–20. doi: 10.1089/apc.2018.0117. [DOI] [PubMed] [Google Scholar]
- 18.Chen J.S., Pence B.W., Rahangdale L., et al. Postpartum HIV care continuum outcomes in the southeastern USA. AIDS. 2019;33(4):637–644. doi: 10.1097/QAD.0000000000002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onoya D., Sineke T., Brennan A.T., Long L., Fox M.P. Timing of pregnancy, postpartum risk of virologic failure and loss to follow-up among HIV-positive women. AIDS. 2017;31(11):1593–1602. doi: 10.1097/QAD.0000000000001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakyi K.S., Lartey M.Y., Kennedy C.E., et al. Barriers to maternal retention in HIV care in Ghana: key differences during pregnancy and the postpartum period. BMC Pregnancy Childbirth. 2020;20(1):398. doi: 10.1186/s12884-020-03067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momplaisir F.M., Storm D.S., Nkwihoreze H., Jayeola O., Jemmott J.B. Improving postpartum retention in care for women living with HIV in the United States. AIDS. 2018;32(2):133–142. doi: 10.1097/QAD.0000000000001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones T.P.W., Lampe F.C., Arenas-Pinto A., et al. Alcohol, smoking, recreational drug use and association with virological outcomes among people living with HIV: cross-sectional and longitudinal analyses. HIV Med. 2022;23(3):209–226. doi: 10.1111/hiv.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huntington S., Thorne C., Newell M.L., et al. The risk of viral rebound in the year after delivery in women remaining on antiretroviral therapy. AIDS. 2015;29(17):2269–2278. doi: 10.1097/QAD.0000000000000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavallo I.K., Kakehasi F.M., Andrade B.A., et al. Predictors of postpartum viral load rebound in a cohort of HIV-infected Brazilian women. Int J Gynaecol Obstet. 2010;108(2):111–114. doi: 10.1016/j.ijgo.2009.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.