Abstract
AIM
To elucidate the relationship between macular sensitivity and time in range (TIR) obtained from continuous glucose monitoring (CGM) measures in diabetic patients with or without diabetic retinopathy (DR).
METHODS
This was a cross-sectional study including 100 eyes of non-DR patients and 60 eyes of DR patients. An advanced microperimetry was used to quantitate the retinal mean sensitivity (MS) and fixation stability in central macula. TIR of 3.9-10.0 mmol/L was evaluated with CGM. Pearson coefficient analysis and multiple linear regression analysis were used to assess the correlation between TIR and retinal sensitivity.
RESULTS
In a comparison of non-DR patients, significant differences (P<0.05) were found in HbA1c, TIR, coefficient of variation (CV), standard deviation of blood glucose (SDBG) and mean amplitude of glucose excursion (MAGE) values in DR patients. Besides, those DR patients had significantly poor best-corrected visual acuity (BCVA, logMAR, P=0.001). In terms of microperimetry parameters, retinal mean sensitivity (MS) and the percentages of fixation points located within 2° and 4° diameter circles were significantly decreased in the DR group (P<0.001, P<0.001, P=0.02, respectively). The bivariate contour ellipse area (BCEA) encompassing 68.2%, 95.4%, 99.6% of fixation points were all significantly increased in the DR group (P=0.01, P=0.006, P=0.01, respectively). Correlation analysis showed that MS were significantly correlated with HbA1c (P=0.01). TIR was positively correlated with MS (r=0.23, P=0.01). SDBG was negatively correlated with MS (r=-0.24, P=0.01) but there was no correlation between CV and MAGE with MS (P>0.05). A multivariable linear regression analysis was performed to prove that TIR and SDBG were both independent risk factors for MS reduction in the DR group.
CONCLUSION
TIR is correlated with retinal MS reduction in DR patients, suggesting a useful option for evaluating DR progression.
Keywords: diabetic retinopathy, time in range, microperimetry, continuous glucose monitoring
INTRODUTION
Diabetic retinopathy (DR) is a microvascular complication of diabetes that can go undetected until irreversible damage and even blindness has appeared[1]–[2]. DR is the leading cause of vision loss in working-age adults and the number of patients with DR around the world will continue to increase due to the rapidly rising diabetes mellitus (DM) population, which would climb up to 700 million by 2045[3]. Published studies have demonstrated that blood glucose fluctuation was associated with diabetic nephropathy or retinopathy[4]. HbA1c has been confirmed as the “golden standard” for the management of glycaemic control. However, there is also several limitations of HbA1c for the optimal glucose control, in which hypoglycaemia, hyperglycaemia and glycaemic fluctuations could not be captured[5].
Increasing evidence shows that continuous glucose monitoring (CGM) could improve glycemic control and decrease risk of hypoglycemia[6]. Time in range (TIR) of glucose is one of the CGM related indicators. It is defined as the proportion of time that an individual's glucose level spends within desired target range (usually 3.9–10.0 mmol/L), which provides valid information for assessing the frequency or severity of hypoglycemia or hyperglycemia improved within given time[7]. A survey derived from capillary blood glucose (CBG) monitoring data in the Diabetes Control and Complications Trial (DCCT) estimated TIR as an important metric to assess development of DR and proteinuria[8]. Despite the key role of TIR in reflecting blood glucose management, there is still lack of clinical valid evidence on the relationship between TIR and diabetic microvascular complications.
In most studies, a reduction in thickness of the nerve fiber layer is obvious in patients that do not have diabetic macular edema (DME), suggesting that significant neural degeneration occurs before a clinically apparent fluid accumulation. Furthermore, DR-associated retinal neurodegeneration might occur before any detectable microcirculatory abnormalities in ophthalmic examinations[9].
Microperimetry offers a possibility to record assessment of retinal sensitivity and the location and stability of fixation[10]–[11]. It has been reported that retinal sensitivities are decreased compared to control subjects in type 2 diabetes patients without DME[12]. In this aspect, microperimetry has been performed successfully to characterize central defects in DME[13]–[15], which allows precise mapping of the central visual field and accurate measurement of correlations between structural and functional abnormalities[16]. Therefore, association of TIR outcomes with neuro-retinal degeneration in DR patients will be warranted.
In the present study, we aim to investigate whether glucose variability might correlate with the progression of neuro-retinal degeneration in DR patients.
SUBJECTS AND METHODS
Ethical Approval
Each patient provided informed consent in accordance with the Declaration of Helsinki, and the study was approved by the local ethics committee (No.2019KY150).
Subjects
This was a cross-sectional study involving 160 eyes of 160 type 2 diabetes mellitus (T2DM) patients who were hospitalized in the Department of Endocrinology at Nanjing Drum Tower Hospital between February 2019 and July 2021. The study population was divided into two groups: DR (n=60) and diabetic non-retinopathy (non-DR; n=100).
All patients and subjects underwent a complete ophthalmic examination, including distance best-corrected visual acuity (BCVA) by using the logarithm of the minimum angle of resolution (logMAR), intraocular pressure (IOP), slit-lamp biomicroscopy, indirect fundus ophthalmoscopy, and color fundus photographs. Exclusion criteria included denial of formal consent, other ocular diseases such as significant media opacities, glaucoma, and macula disorders, poor fixation, and any history of retinal surgery or treatment.
Continuous Glucose Monitoring Parameters
A retrospective CGM system (Medtronic Inc., Northridge, CA, USA) was used to monitor subcutaneous interstitial glucose for three consecutive days. Patients had blood glucose regularly detected for no less than 21 times. TIR was defined as the percentage of time during a 24h period when the target glucose was in the range of 3.9–10.0 mmol/L. A number of metrics concerning glycemic variability (GV) including standard deviation of blood glucose (SDBG) coefficient of variation (CV), and mean amplitude of glucose excursion (MAGE) were calculated during the three-day CGM period. CV=SDBG/the mean of the corresponding glucose readings (%).
Microperimetry
All subjects underwent microperimetry with dilated pupils, and the contralateral eyes were patched during the tests. The test was based on the 4-2 threshold staircase method using the standard Goldmann III stimulus, and the white background was set at a luminance of 31.4 asb. The study included a fixation target consisting of a red ring, 1° in diameter, and 45 stimulation points. Central retinal mean sensitivity (MS) was evaluated within the central 2° and 10°, covering approximately 1 and 3 mm of the central retina area respectively (Figure 1). Fixation stability was evaluated by classification as stable, relatively unstable, or unstable, and the MS was expressed in decibels. The bivariate contour ellipse area (BCEA) value provided a quantitative measure of fixation stability in the area of eccentric preferred retinal locus (PRL). BCEA is constructed by plotting the position of each fixation on Cartesian axes and calculating the area of an ellipse encompassing given percentage of fixation points (68.2%, 95.4%, and 99.6%). The test is based on the SDs of the horizontal and vertical eye movements during fixation[17].
Figure 1. The microperimetry examination.
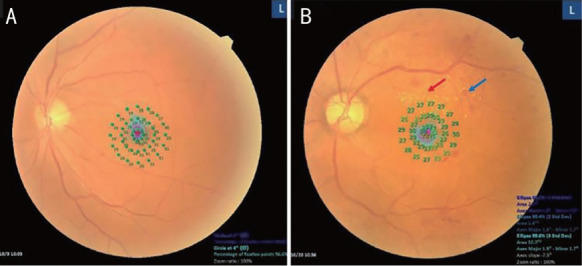
A: A photograph of an eye with non-DR combining with central retinal MS results; B: A photograph of an eye with DR showing exudates (red arrows) and haemorrhages (blue arrows) combining with central retinal MS results. DR: Diabetic retinopathy. MS: Mean sensitivity.
Statistical Analysis
Statistical analyses were conducted using SPSS for Windows statistical software (ver. 17.0; SPSS Inc., Chicago, IL, USA). Data are presented as means±SD for continuous variables and as percentages for categorical variables. The mean values of TIR, SDBG, CV, MAGE, MS and BCEA did not show a Gaussian distribution, so the Mann-Whitney U test was used for the comparisons. Qualitative analyses of the stability of fixation were expressed as absolute and relative percentages. Pearson coefficient analysis was used to assess the correlation between HbA1c and retinal sensitivity. Multiple linear regression analysis was used to evaluate the relationships between CGM and microperimetry parameters. A value of P<0.05 was interpreted as statistically significant.
RESULTS
The baseline characteristics of participants enrolled in the study are summarized in Table 1. No significant difference was found in age, sex distribution, systolic blood pressure, diastolic blood pressure and IOP. The patients with DR had a longer history of diabetes and increased HbA1c (P<0.05).
Table 1. Baseline characteristics of participants.
| Parameters | Non-DR group (n=100) | DR group (n=60) | P |
| Age (y) | 52.45±3.27 | 54.50±4.02 | 0.7 |
| Gender (male/female) | 55/45 | 28/32 | 0.33 |
| History of diabetes (y) | 18.29±7.38 | 22.2±7.45 | 0.002a |
| SBP (mm Hg) | 120.9±6.89 | 121.4±6.28 | 0.67 |
| DBP (mm Hg) | 79.06±6.09 | 81.26±6.17 | 0.61 |
| HbA1c (%) | 8.43±1.18 | 9.57±1.37 | 0.006a |
| SDBG (mmol/L) | 2.02±0.59 | 2.64±0.67 | <0.001a |
| CV (%) | 24.28±4.66 | 26.15±5.35 | 0.03a |
| MAGE (mmol/L) | 5.38±1.34 | 5.93±1.34 | 0.02a |
| TIR (%) | 67.64±13.47 | 56.53±14.32 | <0.001a |
| BCVA (logMAR) | 0.06±0.04 | 0.27±0.09 | 0.001a |
| IOP (mm Hg) | 17.01±0.69 | 16.31±0.75 | 0.49 |
DR: Diabetic retinopathy; Non-DR: Type 2 diabetic patients without diabetic retinopathy; BCVA: Best-corrected visual acuity; IOP: Intraocular pressure; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; SDBG: Standard deviation of blood glucose; CV: Coefficient of variation; MAGE: Mean amplitude of glucose excursion. aStatistically significant.
All the CGM parameters derived from CBG data showed statistically significance between the non-DR group and the DR group. The DR group had lower TIR levels and higher SDBG, CV and MAGE levels. The ratio of SD in the DR group was 2.64±0.67 vs 2.02±0.59 mmol/L in the non-DR group (P<0.001), CV (%) was 26.15±5.35 vs 24.28±4.66 (P=0.03), HbA1c was 9.57%±1.37% vs 8.43%±1.18% (P=0.006), MAGE was 5.93±1.34 vs 5.38±1.34 mmol/L (P=0.02), and TIR was 56.53%±14.32% vs 67.64%±13.47% (P<0.001).
Table 2 listed all the automatically calculation of microperimetry parameters in the two groups. Compared to the control group, the MS in the central 10° macular area was significantly decreased in the DR group (24.86±4.23 vs 28.56±1.38 dB; P<0.001). Similar significant differences were observed in the fixation stability 2° (82.52±7.07 vs 76.73±9.15 dB; P<0.001) and in the fixation stability 4° (82.93±8.35 vs 79.26±8.83 dB; P=0.02). BCEAs encompassing 68.2%, 95.4%, 99.6% of fixation points in DR group were significantly increased than the non-DR group (P=0.01, P=0.006, P=0.01). In the non-DR group, fixation was stable in 56 eyes (56%), relatively unstable in 19 eyes (19%), and unstable in 25 eyes (25%). In the DR group, fixation was stable in 40 eyes (67%), and relatively unstable in 20 eyes (33%).
Table 2. Microperimetry measurements in each group.
| Parameters | Non-DR group (n=100) | DR group (n=60) | P |
| MS | 28.56±1.38 | 24.86±4.23 | <0.001a |
| FS, 2° | 82.52±7.07 | 76.73±9.15 | <0.001a |
| FS, 4° | 82.93±8.35 | 79.26±8.83 | 0.02a |
| BCEA, 68.2 deg2 | 6.13±13.42 | 6.83±2.69 | 0.01a |
| BCEA, 95.4 deg2 | 11.13±4.17 | 13.25±4.83 | 0.006a |
| BCEA, 99.6 deg2 | 28.03±11.8 | 33.33±14.65 | 0.01a |
DR: Diabetic retinopathy; Non-DR: Type 2 diabetic patients without diabetic retinopathy; FS: Fixation stability; MS: Mean sensitivity; BCEA: Bivariate contour ellipse area. aStatistically significant.
3×3 mm2
The results of the correlation analysis between HbA1c and microperimetry parameters suggested that only MS was significantly correlated with HbA1c level (r=-0.22, P=0.01). In terms of other microperimetry parameters, there was no significant correlation among the HbA1c, fixation stability and BCEAs.
Considering that MS is a sensitive and accurate indicator to reflect early retinal functional changes under glucose stress, we performed a Pearson correlation analysis between the MS value and CGM variables. CGM parameters, including SDBG (r=-0.24, P=0.01) and TIR (r=0.23, P=0.01), were strongly associated with MS. Multivariate linear regression further indicated an association between the MS value and SDBG/TIR variables. The other CGM parameters showed no significant association with the MS value (P>0.05; Table 3).
Table 3. Linear regression analysis evaluating association between the CGM variables and the MS value.
| Variables | Pearson correlation |
Multivariate Linear regression |
||||
| r | 95%CI | P | β | 95%CI | P | |
| SDBG (mmol/L) | -0.24 | -0.39 to -0.07 | 0.01a | 0.41 | -1.80 to -0.18 | 0.01a |
| CV (%) | -0.08 | -0.25 to 0.08 | 0.33 | 0.06 | -0.13 to 0.09 | 0.74 |
| MAGE (mmol/L) | -0.04 | -0.21 to 0.12 | 0.60 | 0.20 | -0.41 to 0.39 | 0.96 |
| TIR (%) | 0.23 | 0.07 to 0.38 | 0.01a | 0.02 | 0.01 to 0.08 | 0.02a |
CGM: Continuous glucose monitoring; MS: Mean sensitivity; SDBG: Standard deviation of blood glucose; CV: Coefficient of variation; MAGE: Mean amplitude of glucose excursion; TIR: Time in range. aStatistically significant.
DISCUSSION
In this study, we found that both MS value and FS (2° and 4°) was significantly lower in the DR group compared to the non-DR group. We also focused on four indicators for GV assessment, namely TIR, SDBG, CV, and MAGE. All CGM parameters were highly different between two groups of subjects. Additionally, there was a significant negative correlation between the HbA1c level and the MS value. Furthermore, we found CGM parameters including SDBG and TIR were closely correlated with MS decrease in DR group.
Microperimetry allows real-time assessment of the retina and can determine the retinal light sensitivity in certain areas, which provides more information on retinal functions. Previous studies using microperimetry reported a decrease in the MS in diabetic patients without DR[18]–[19]. A reduction in MS was also found in both type 1 and type 2 diabetic patients without DME[20]. Consistent with these previous studies, we found a significant decrease in the MS in DR group. In addition, we measured the MS using the MP-3, which is one of the latest generation microperimeters. For many years, MS measurements were performed using the MP-1, whose procedure were meant to be interrupted and restarted several times by examinee eye blink or rotation. Additionally such eye movements would resulted unreliable data. In contrast, the MP-3 device features an automatic eye-tracking system and an improved dynamic range of between 0–34 dB. In addition, this device can register the eye position 25 times/s, thus facilitating the experimenter. In patients with retinitis pigmentosa, the MP-3 test estimates retinal sensitivity more accurately than the Humphrey field analyzer[21]. Thus, estimates produced by the MP-3 are more reliable, and better for assessing visual function in diabetic patients without DR.
Evidence has confirmed that HbA1c value is a well-established metric to predict the progression of diabetes complications, including DR or diabetic nephropathy and cardiovascular events[22]. However, contrary to expectation, in a few studies limitations of the measurement of HbA1c for lack of accuracy affected with many factors including anaemia, pregnancy, hemoglobinopathy ethnicity were observed[23]–[24]. For the record, research has increased in recent years as CGM has become more popular to assess overall glycemic control. A recent study evaluated 18 randomized controlled trials (RCTs) comprising type 1 and type 2 diabetes patients and recognized TIR as a critical indicator correlated with HbA1c value[25]. Besides, Beck et al[26] demonstrated similar clinical connection between effects of TIR with HbA1c levels, which derived from 4 RCTs in type 1 diabetes patients. Therefore, TIR has been accepted as a meaningful indicator to assess risk for diabetic vascular complications. A study conducted in China based on a large sample size found that TIR could be regarded as a measure to reveal diabetic cardiovascular events[27]. Additionally, research data including a large sample size from China also found that TIR is closely related to the risk of DR[28]. Using the data from DCCT, Beck et al[8] found a strong association of TIR with the risk of development or progression of retinopathy.
Extensive effort has been made to exploring the morphological characteristics of DR[29], but the pivotal role of visual function changes, and their relationships with pathological variations in the early stage of the disease, have not been thoroughly investigated. Previous summarization of the relation among different functional changes in the anatomical features of DR patients showed that inner retinal layer thickness changes correlated with alterations in retinal sensitivity in non-DR patients[19]. There were only very small differences in the ganglion cell layer (GCL) and GCL-inner plexiform layer (IPL) thicknesses, and in retinal sensitivity, when comparing the non-DR and control group. Hatef et al[30] reported that macula sensitivity increased with retinal thickness (for thicknesses ≤280 µm). In contrast, the macula sensitivity decreased with increases in retinal thickness (for thicknesses >280 µm). In branch retinal vein occlusion patients, capillary non-perfusion in the superficial and deep layers could be translated into retinal sensitivity reductions by using microperimetry[31]. Therefore, retinal sensitivity examinations are essential for evaluating the status of the entire macular.
To the best of our knowledge, few studies have been conducted to analyze the relation between TIR and diabetic retinal sensitivity reductions. It is expected that TIR can be used to assess various changes in the functional features of non-DR patients by microperimetry. Our study could help to understand whether neurodegenerative damage is the milestone in the progression of DR.
The major limitation of this study was that it was a single-center, cross-sectional trial with a small sample size. It is therefore necessary to conduct a follow-up study to confirm our findings. Another limitation was the use of a central fovea area of 3×3 mm2, which may be limited in terms of its ability to reveal early microvascular changes.
In conclusion, the results of our study confirmed a significant association of TIR with the functional damage in the early stage of DR. Microperimetry may be a sensitive and physiologically relevant tool to detect early changes in diabetic patients. The value of TIR might be assumed as an outcome metric in future studies. A compelling study are needed to elucidate the relationship between TIR and DR.
Acknowledgments
Authors' contributions: Collection of data (Zhu DD, Wu X, Cheng XX), preparation of the manuscript (Zhu DD), and supervision (Ding N). All the authors read and approved the final manuscript.
Conflicts of Interest: Zhu DD, None; Wu X, None; Cheng XX, None; Ding N, None.
REFERENCES
- 1.Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12(8):1322–1325. doi: 10.1111/jdi.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fekadu SA, Akalu Y, Gela YY, Diress M, Getnet M, Dagnew B, Belsti Y. Factors associated with diabetic retinopathy screening and regular eye checkup practice among diabetic patients attending Felege Hiwot Specialized Hospital. Int J Ophthalmol. 2022;15(11):1829–1836. doi: 10.18240/ijo.2022.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045. Ophthalmology. 2021;128(11):1580–1591. doi: 10.1016/j.ophtha.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Yapanis M, James S, Craig ME, O'Neal D, Ekinci EI. Complications of diabetes and metrics of glycemic management derived from continuous glucose monitoring. J Clin Endocrinol Metab. 2022;107(6):e2221–e2236. doi: 10.1210/clinem/dgac034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HU, Park SP, Kim YK. Long-term HbA1c variability and the development and progression of diabetic retinopathy in subjects with type 2 diabetes. Sci Rep. 2021;11:4731. doi: 10.1038/s41598-021-84150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254–2263. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 7.Bergenstal RM, Bailey TS, Rodbard D, Ziemen M, Guo HL, Muehlen-Bartmer I, Ahmann AJ. Comparison of insulin glargine 300 units/mL and 100 units/mL in adults with type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care. 2017;40(4):554–560. doi: 10.2337/dc16-0684. [DOI] [PubMed] [Google Scholar]
- 8.Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li ZM, Brown AS, Close KL. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simó R, Simó-Servat O, Bogdanov P, Hernández C. Diabetic retinopathy: role of neurodegeneration and therapeutic perspectives. Asia Pac J Ophthalmol. 2022;11(2):160–167. doi: 10.1097/APO.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 10.Qian TW, Xu X, Liu XY, Yen M, Zhou H, Mao MM, Cai HT, Shen HQ, Xu X, Gong YY, Yu SQ. Efficacy of MP-3 microperimeter biofeedback fixation training for low vision rehabilitation in patients with maculopathy. BMC Ophthalmol. 2022;22(1):197. doi: 10.1186/s12886-022-02419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang CH, Gong B, Huang C, Shu XW, Chen TY, Chen X, Wu CL, Wang Y. Morphological and functional changes in the macular area in diabetic macular edema after a single intravitreal injection of aflibercept. Int J Ophthalmol. 2023;16(1):88–94. doi: 10.18240/ijo.2023.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso Plasencia M, Abreu González R, Gómez Culebras MA. Structure–function correlation using OCT angiography and microperimetry in diabetic retinopathy. Clin Ophthalmol. 2019;13:2181–2188. doi: 10.2147/OPTH.S220877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vujosevic S, Midena E, Pilotto E, Radin PP, Chiesa L, Cavarzeran F. Diabetic macular edema: correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci. 2006;47(7):3044–3051. doi: 10.1167/iovs.05-1141. [DOI] [PubMed] [Google Scholar]
- 14.Mokrane A, Zureik A, Bonnin S, Erginay A, Lavia C, Gaudric A, Tadayoni R, Couturier A. Retinal sensitivity correlates with the superficial vessel density and inner layer thickness in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2021;62(14):28. doi: 10.1167/iovs.62.14.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos AR, Raimundo M, Alves D, Lopes M, Pestana S, Figueira J, Cunha-Vaz J, Silva R. Microperimetry and mfERG as functional measurements in diabetic macular oedema undergoing intravitreal ranibizumab treatment. Eye (Lond) 2021;35(5):1384–1392. doi: 10.1038/s41433-020-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah VA, Chalam KV. Values for macular perimetry using the MP-1 microperimeter in normal subjects. Ophthalmic Res. 2008;41(1):9–13. doi: 10.1159/000162111. [DOI] [PubMed] [Google Scholar]
- 17.Sahli E, Altinbay D, Bingol Kiziltunc P, Idil A. Effectiveness of low vision rehabilitation using microperimetric acoustic biofeedback training in patients with central scotoma. Curr Eye Res. 2021;46(5):731–738. doi: 10.1080/02713683.2020.1833348. [DOI] [PubMed] [Google Scholar]
- 18.Nittala MG, Gella L, Raman R, Sharma T. Measuring retinal sensitivity with the microperimeter in patients with diabetes. Retina. 2012;32(7):1302–1309. doi: 10.1097/IAE.0b013e3182365a24. [DOI] [PubMed] [Google Scholar]
- 19.Montesano G, Gervasoni A, Ferri P, Allegrini D, Migliavacca L, De Cillà S, Rossetti L. Structure-function relationship in early diabetic retinopathy: a spatial correlation analysis with OCT and microperimetry. Eye (Lond) 2017;31(6):931–939. doi: 10.1038/eye.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JF, Zhang JX, Zhang CY, Zhang JT, Gu LM, Luo DW, Qiu QH. Diabetic macular edema: current understanding, molecular mechanisms and therapeutic implications. Cells. 2022;11(21):3362. doi: 10.3390/cells11213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahina Y, Kitano M, Hashimoto Y, Yanagisawa M, Murata H, Inoue T, Obata R, Asaoka R. The structure-function relationship measured with optical coherence tomography and a microperimeter with auto-tracking: the MP-3, in patients with retinitis pigmentosa. Sci Rep. 2017;7(1):15766. doi: 10.1038/s41598-017-16143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hietala K, Wadén J, Forsblom C, Harjutsalo V, Kytö J, Summanen P, Groop PH, on behalf of the FinnDiane Study Group HbA1c variability is associated with an increased risk of retinopathy requiring laser treatment in type 1 diabetes. Diabetologia. 2013;56(4):737–745. doi: 10.1007/s00125-012-2816-6. [DOI] [PubMed] [Google Scholar]
- 23.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bomholt T, Feldt-Rasmussen B, Butt R, Borg R, Sarwary MH, Elung-Jensen T, Almdal T, Knop FK, Nørgaard K, Ranjan AG, Larsson A, Rix M, Hornum M. Hemoglobin A1c and fructosamine evaluated in patients with type 2 diabetes receiving peritoneal dialysis using long-term continuous glucose monitoring. Nephron. 2022;146(2):146–152. doi: 10.1159/000519493. [DOI] [PubMed] [Google Scholar]
- 25.Vigersky R A, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81–85. doi: 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 26.Beck RW, Bergenstal RM, Cheng PY, Kollman C, Carlson AL, Johnson ML, Rodbard D. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614–626. doi: 10.1177/1932296818822496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu JY, Ma XJ, Shen Y, Wu QA, Wang R, Zhang L, Mo YF, Lu W, Zhu W, Bao YQ, Vigersky RA, Jia WP, Zhou JA. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22(2):72–78. doi: 10.1089/dia.2019.0251. [DOI] [PubMed] [Google Scholar]
- 28.Lu JY, Ma XJ, Zhou J, Zhang L, Mo YF, Ying LW, Lu W, Zhu W, Bao YQ, Vigersky RA, Jia WP. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370–2376. doi: 10.2337/dc18-1131. [DOI] [PubMed] [Google Scholar]
- 29.Sun ZH, Yang DW, Tang ZQ, Ng DS, Cheung CY. Optical coherence tomography angiography in diabetic retinopathy: an updated review. Eye (Lond) 2021;35(1):149–161. doi: 10.1038/s41433-020-01233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatef E, Colantuoni E, Wang JM, Ibrahim M, Shulman M, Adhi F, Sepah YJ, Channa R, Khwaja A, Nguyen QD, Do DV. The relationship between macular sensitivity and retinal thickness in eyes with diabetic macular edema. Am J Ophthalmol. 2011;152(3):400–405.e2. doi: 10.1016/j.ajo.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Wei PY, Liu CC, Zhang YZ, Yang L. Evaluation of retinal sensitivity and microstructure in areas of capillary nonperfusion of eyes with branch retinal vein occlusion. BMC Ophthalmol. 2021;21(1):331. doi: 10.1186/s12886-021-02089-w. [DOI] [PMC free article] [PubMed] [Google Scholar]


