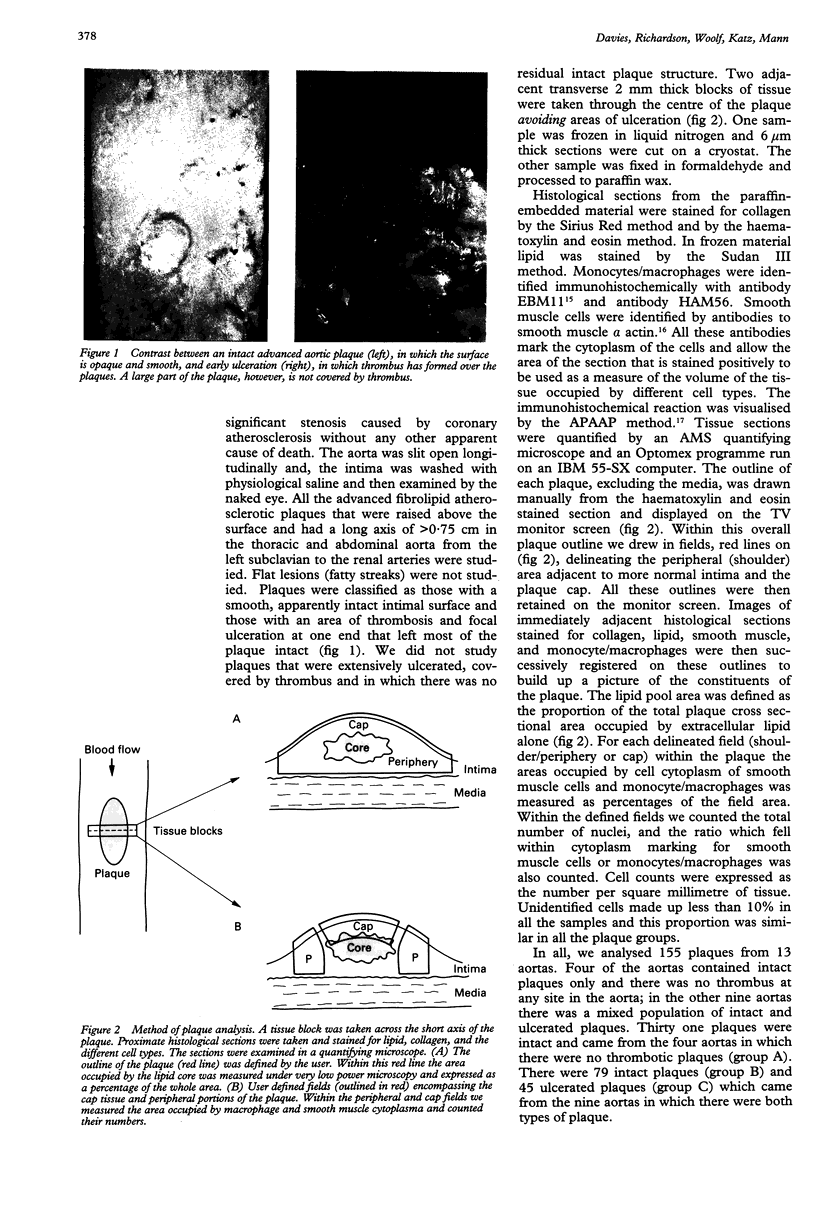
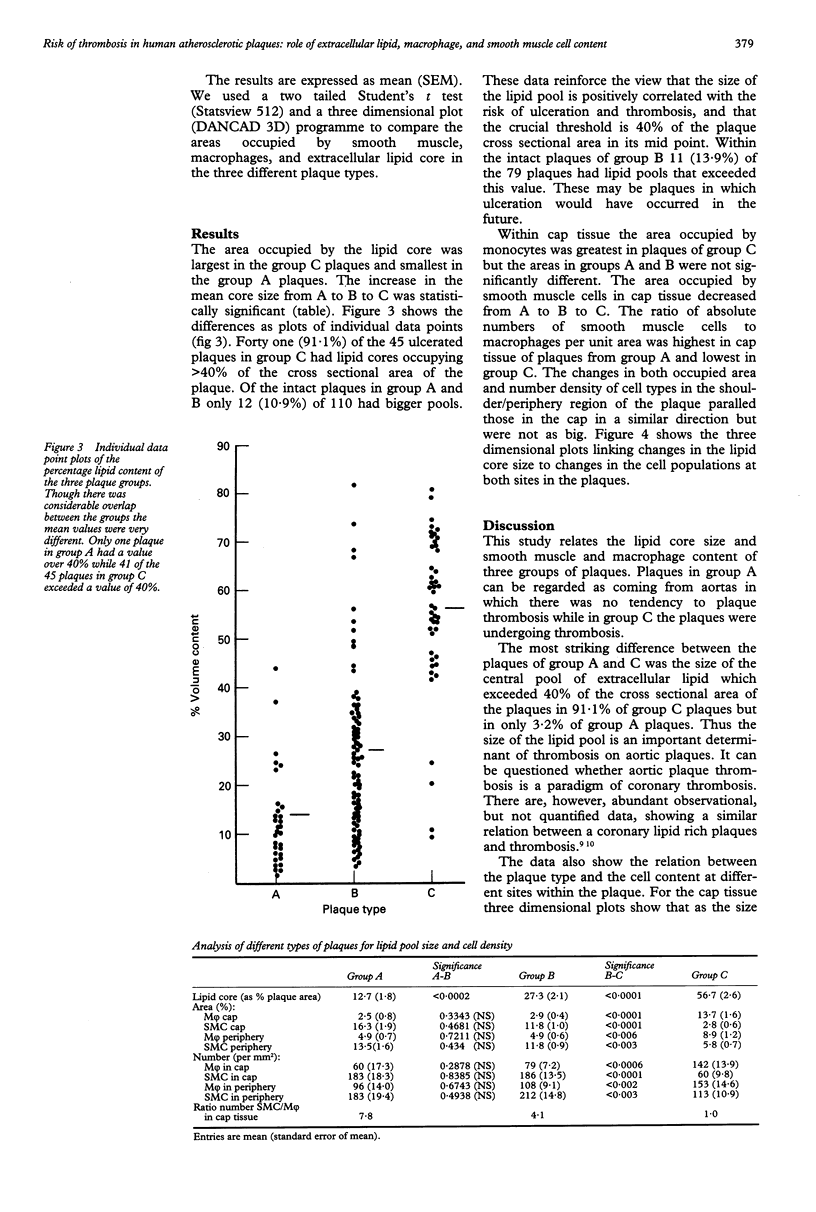
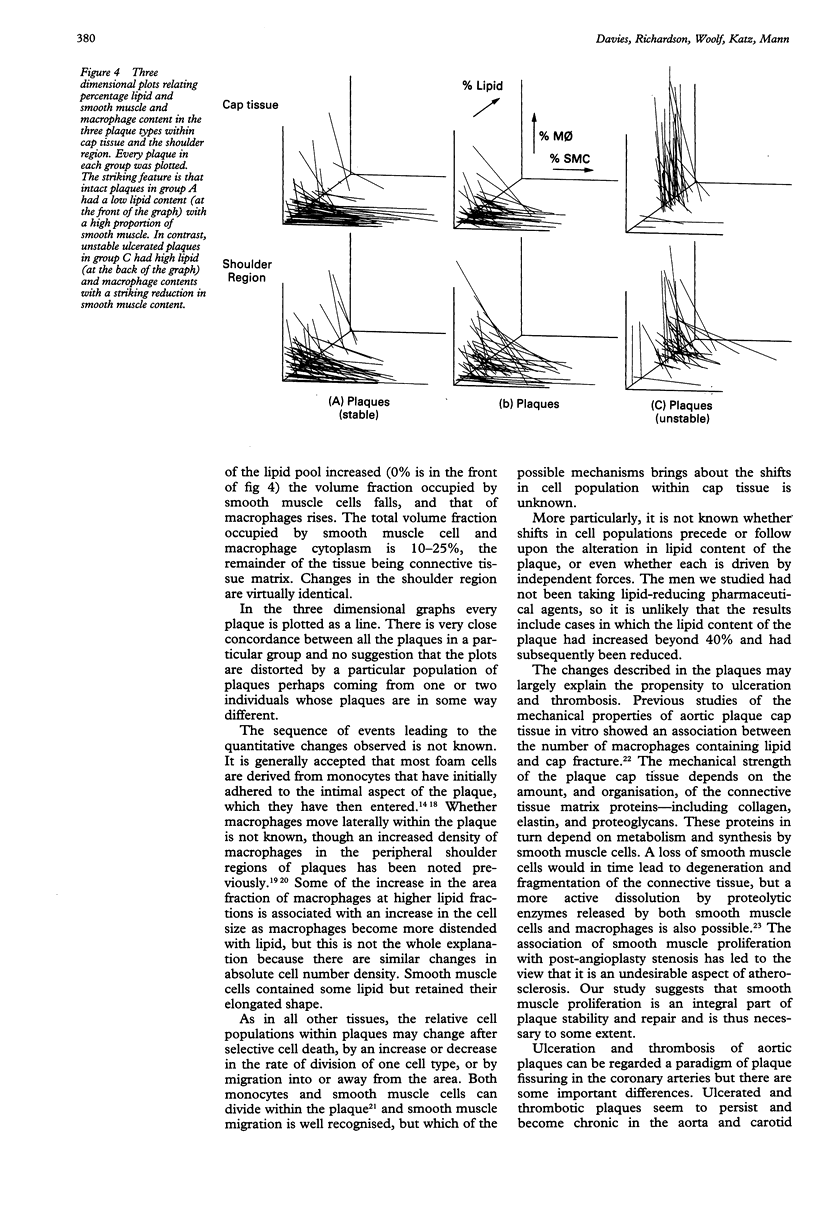
Abstract
OBJECTIVE--To assess the size of the lipid pool and the number of smooth muscle cells and monocyte/macrophages in human aortic plaques that were intact and to compare the results with those in aortic plaques undergoing ulceration and thrombosis. DESIGN--The lipid pool was measured as a percentage of the total cross sectional area of the plaque. Immunohistochemistry was used to identify cell types (monocytes/macrophages (M phi) by EBM11 and HAM56, smooth muscle cells by alpha actin). The area of the tissue occupied by each cell type was measured by quantitative microscopy in the peripheral (shoulder) area of the plaque and the plaque cap. Absolute counts of each cell type were expressed as the ratio of SMC:M phi. MATERIAL--Aortas were obtained at necropsy from men aged less than 69 years who died suddenly (within 6 hours of the onset of symptoms) of ischaemic heart disease. 155 plaques from 13 aortas were studied. Four aortas showed intact plaques only (group A, n = 31). Nine aortas showed both intact plaques (group B, n = 79) and plaques that were undergoing thrombosis (group C, n = 45). RESULTS--In 41 (91.1%) of the 45 plaques undergoing thrombosis (group C) lipid pools occupied more than 40% of the cross sectional area of the plaque. Only 12 (10.9%) of the 110 intact plaques (groups A + B) had lipid pools of this size. The mean size of the lipid pool in plaques of groups A, B, and C was 12.7%, 27.3% and 56.7% respectively. Compared with intact plaques those undergoing thrombosis contained a smaller volume of smooth muscle cells (2.8% v 11.8%) and a larger volume of monocyte/macrophages (13.7% v 2.9%) in the plaque cap. The ratio of the number of smooth muscle cells to monocytes/macrophages was 7.8 in group A plaques, 4.1 in group B plaques, and 1.0 in group C plaques. This gradient was the result of an absolute increase in monocyte/macrophages and an absolute decrease in smooth muscle cells. CONCLUSIONS--In the aorta ulceration and thrombosis were characteristic of plaques with a high proportion of their volume occupied by extracellular lipid, and in which there was a shift toward a preponderance of monocyte/macrophages compared with smooth muscle cells in the cap.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarenco P., Duyckaerts C., Tzourio C., Hénin D., Bousser M. G., Hauw J. J. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992 Jan 23;326(4):221–225. doi: 10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- Ambrose J. A., Winters S. L., Arora R. R., Haft J. I., Goldstein J., Rentrop K. P., Gorlin R., Fuster V. Coronary angiographic morphology in myocardial infarction: a link between the pathogenesis of unstable angina and myocardial infarction. J Am Coll Cardiol. 1985 Dec;6(6):1233–1238. doi: 10.1016/s0735-1097(85)80207-2. [DOI] [PubMed] [Google Scholar]
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Monocytic origin of foam cells in human atherosclerotic plaques. Atherosclerosis. 1984 Dec;53(3):265–271. doi: 10.1016/0021-9150(84)90127-8. [DOI] [PubMed] [Google Scholar]
- Armstrong M. L., Heistad D. D., Megan M. B., Lopez J. A., Harrison D. G. Reversibility of atherosclerosis. Cardiovasc Clin. 1990;20(3):113–126. [PubMed] [Google Scholar]
- Bang N. U. Leeches, snakes, ticks, and vampire bats in today's cardiovascular drug development. Circulation. 1991 Jul;84(1):436–438. doi: 10.1161/01.cir.84.1.436. [DOI] [PubMed] [Google Scholar]
- Brown G., Albers J. J., Fisher L. D., Schaefer S. M., Lin J. T., Kaplan C., Zhao X. Q., Bisson B. D., Fitzpatrick V. F., Dodge H. T. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990 Nov 8;323(19):1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Thomas A. C. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985 Apr;53(4):363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation. 1985 Apr;71(4):699–708. doi: 10.1161/01.cir.71.4.699. [DOI] [PubMed] [Google Scholar]
- Forrester J. S., Litvack F., Grundfest W., Hickey A. A perspective of coronary disease seen through the arteries of living man. Circulation. 1987 Mar;75(3):505–513. doi: 10.1161/01.cir.75.3.505. [DOI] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992 Jan 23;326(4):242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992 Jan 30;326(5):310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Henney A. M., Wakeley P. R., Davies M. J., Foster K., Hembry R., Murphy G., Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8154–8158. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Kelly P. M., Bliss E., Morton J. A., Burns J., McGee J. O. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988 May;41(5):510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. M., Bliss E., Morton J. A., Burns J., McGee J. O. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988 May;41(5):510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. C., Fallon J. T. Significance of the angiographic morphology of localized coronary stenoses: histopathologic correlations. Circulation. 1982 Aug;66(2):316–320. doi: 10.1161/01.cir.66.2.316. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Satomura K., Miyamoto A., Arakawa K., Shibuya T., Arai T., Kurita A., Nakamura H., Ambrose J. A. Angioscopic evaluation of coronary-artery thrombi in acute coronary syndromes. N Engl J Med. 1992 Jan 30;326(5):287–291. doi: 10.1056/NEJM199201303260502. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Satomura K., Miyamoto A., Arakawa K., Shibuya T., Arai T., Kurita A., Nakamura H., Ambrose J. A. Angioscopic evaluation of coronary-artery thrombi in acute coronary syndromes. N Engl J Med. 1992 Jan 30;326(5):287–291. doi: 10.1056/NEJM199201303260502. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Satomura K., Miyamoto A., Arakawa K., Shibuya T., Arai T., Kurita A., Nakamura H., Ambrose J. A. Angioscopic evaluation of coronary-artery thrombi in acute coronary syndromes. N Engl J Med. 1992 Jan 30;326(5):287–291. doi: 10.1056/NEJM199201303260502. [DOI] [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M., Bond M. G., Waugh D., Prack M., Sawyer J. K. Physicochemical and histological changes in the arterial wall of nonhuman primates during progression and regression of atherosclerosis. J Clin Invest. 1984 Jun;73(6):1590–1605. doi: 10.1172/JCI111366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy R. E., Devaney K., Kissling G. Characteristics of the plaque under a coronary thrombus. Virchows Arch A Pathol Anat Histopathol. 1985;405(4):411–427. doi: 10.1007/BF00737168. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Cell biology of arterial proteoglycans. Arteriosclerosis. 1989 Jan-Feb;9(1):1–20. doi: 10.1161/01.atv.9.1.1. [DOI] [PubMed] [Google Scholar]