Abstract
Background
Vivascope 2500 ex vivo confocal microscopy (EVCM) is an emerging optical imaging device that allows nuclear level resolution of freshly excised tissues. EVCM provides, rapid real‐time pathological examination in many subspecialties of pathology including skin, prostate, breast, liver, etc. In contrast to traditional time‐consuming frozen sectioning and histological analysis.
Aims
To evaluate the current state of EVCM utilization.
Materials and Methods
This study highlights the advantages, limitations, and prospects of EVCM in skin pathology.
Results
Our findings demonstrate that EVCM is a promising adjunctive tool to assess margins in Mohs surgery and to provide rapid, accurate diagnosis of cutaneous tumors, infectious and inflammatory diseases.
Conclusion
EVCM is a revolutionary device that can be used as an adjunct to paraffin‐fixed, hematoxylin and eosin‐stained slides and frozen sectioning. Additional refinements are required before EVCM can be used as an alternative to frozen sectioning or traditional tissue processing.
Keywords: fluorescence confocal microscope, frozen sectioning, fusion confocal microscope, pseudo staining, reflectance confocal microscopy
1. INTRODUCTION
Vivascope 2500 ex vivo confocal microscopy (EVCM) is an innovative device that allows rapid, real‐time, quasi histologic, microscopic examination of excised cutaneous tissue within a few minutes without frozen sectioning or traditional tissue processing, embedding and sectioning. The device uses two diode lasers to visualize excised tissue biopsies in three different individualized modes: reflectance, fluorescence, and pseudocolor. Reflectance mode uses a 785‐nm wavelength to visualize subcellular structures by their differences in refractive indices. Fluorescence mode uses a 488‐nm wavelength to activate cellular fluorochromes to see cellular microstructures. 1 Visualization in pseudocolor mode emulates a digital hematoxylin and eosin stain, is comparable to the use of traditional hematoxylin & eosin (H&E) stains. However, the depth of light penetration is limited to 200 μm, which allow adequate visualization of the papillary dermis.
1.1. Imaging acquisition protocol
Histologic examination utilizes staining protocols to visualize various cellular structures of interest. Common staining agents used for different laser types and tissue types are summarized in (Table 1). 2 The staining protocol for freshly excised tissues involves immersion of the sample in acetic acid for 30 s. This process induces brightening of the nuclei by compaction of chromatin and whitening of the epithelium (Figure 1). Next, excised tissues were dipped in 0.6 mM acridine orange (AO) solution for 30 s. AO is a dye selective for DNA and RNA and induces nuclei brightening and enhances the contrast in fluorescence mode by selectively highlighting nucleic acids. Studies have shown that staining with AO does not affect subsequent immuno‐histologic, molecular examination and frozen sectioning. 3 , 4 , 5 , 6
TABLE 1.
Different staining protocols for ex vivo. CM, reflectance confocal microscopy; FCM, fluorescence confocal microscopy; M‐CM, multimodal confocal microscopy. Adapted from Malvehy et al 2 .
| Staining agent | Laser | Sample |
|---|---|---|
| Aluminum chloride, acetic acid, citric acid | RCM | Skin tumors |
| Acridine orange, methylene blue, and toluidine blue, fluorescein, Nile blue, or Patent Blue V | FCM | Skin tumors |
| Combinations | Laser | Sample |
| Methylene blue + toluidine blue | M‐CM | Skin cancer |
| Fluorescence proflavine + acetic acid + toluidine blue | FCM | BCC |
| Acetic acid + Acridine orange | M‐CM | BCC |
| Acridine orange + ethidium bromide | 3‐color FCM | BCC |
| Immunostaining | Laser | Sample |
| FITC‐labeled S‐100A10, Melan‐A, and anti‐Ber‐EP4 antibodies, NPs10@D1_ICF_Alexa647_ DOTAGA Fe3 + | FCM | No‐melanocytic and melanocytic tumors |
| Fluorescent‐labeled IgG and C3 antibodies | M‐CM | Bullous pemphigoid |
| IgG, IgM, IgA, C3, and Fibrinogen | M‐CM | Cutaneous vasculitis |
FIGURE 1.
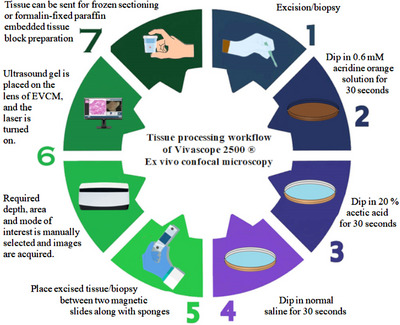
Tissue processing workflow of Vivascope 2500 ex vivo confocal microscopy.
Lastly, the excised tissues were dipped in normal saline to remove excess dye. Excised tissue were placed between two sponges held in place by magnetic slides to provide tissue flattening and stability during scanning.
Ultrasound gel was placed on the lens of EVCM, and the laser was turned on. Required depth, area, and mode of interest were manually selected, and images were acquired. The time required to scan a tissue sample of 8 × 8 mm is approximately 50 s. Scanning time is dependent upon the size of the excised tissue, as the size of the tissue increases so does the time needed for scanning. According to technical specifications provided by CaliberID, scanning time is around 2:10 min for a tissue sample of 16 × 12 mm. The microscope stage has a maximum range of 20 × 20 mm to image specimens up to 2 cm2, and it would require 4:25 min of scan time. 3 Scanned images allow magnification of 550×, which is equivalent to traditional histology 3 (Table 2). In contrast to in vivo confocal microscopy, EVCM allows vertical scanning of tissues, enabling visualization of all skin layers. 4
TABLE 2.
Technical aspects of VivaScope 2500 M‐G4 ex vivo confocal microscopy.
| Technical features | |
|---|---|
| Vertical optical resolution | <5.0 μm at middle of field of view |
| Horizontal optical resolution | <1.25 μm at middle of field of view |
| Maximum imaging depth | 200 μm |
| Maximum sample size | 25 × 25 mm |
| Image resolution |
1024 × 1024 pixels (single field of view), or 0.5 μm/pixel |
| Imaging wavelength |
488 nm (blue) 785 nm (near‐infrared) |
| Optical operating power |
100–120 V, 50–60 Hz 220–240 V, 50 Hz |
| Dimensions of Scan Head (L × W × H) | 25 × 52.5 × 25 cm |
| Magnification | 550x |
| Imaging types | Single frame picture, blocks/mosaics, cube, movie/stack |
| Objective |
Caliber I.D. StableView 38× magnification numerical aperture of 0.85 water immersion |
| Imaging modes |
Reflectance confocal mode (RCM) Fluorescnece confocal mode (FCM) Fusion mode (RCM+ FCM) Pseudo hematoxylin and eosin (digital or pseudo staining) |
| Scan time |
16 × 12 mm, around 2:10 min 20 × 20 mm, around 4:25 min |
1.2. Technical advantages
EVCM combines a small form factor for ease of intraoperative transportation with efficient sample processing. The time to view excised tissue is 5 min in EVCM compared to 45 to 52 min with frozen sectioning. 2 In Mohs surgeries, EVCM reduces procedure time by approximately one third and reduces histotechnicians time spent on frozen sectioning, hence reducing operating cost. 6 Digital images can be sent directly to the dermatopathologist for diagnosis or consultation, further improving workflow efficiency. This extemporaneous diagnostic capability of EVCM makes it a valuable tool for Mohs surgeries and in life threatening conditions, such as invasive fungal infections like mucormycosis that require prompt pathologic diagnosis. 7 EVCM also increases opportunities for telepathology and teaching as images can be electronically protected and distributed, without the need to send physical slides. Scanned images can be retained forever without the fear of fading or occupying physical space, a problem commonly encountered in physical histopathology slides. Technical advantages of EVCM are summarized in (Table 3).
TABLE 3.
Advantages and limitations of Vivascope 2500 ex vivo confocal microscopy.
| Features | Advantages | Limitations/Disadvantages |
|---|---|---|
| General |
‐Small form factor ‐Easy to move or transport ‐No lost images ‐No fading of stored images ‐Quick access to previous scans/images |
‐Limited availability of EVCM due to disruption in supply chain |
| User Experience/ Reporting |
‐Technical aspect is easy to learn ‐Three different modes of scanning/viewing (reflectance, fluorescence, pseudo color) ‐Fusion mode (combination of reflectance and fluorescence modes) ‐Magnification |
‐Uneven and thick sectioning of fresh tissues makes scanning difficult ‐Lack of standardized staining protocol ‐Suboptimal anchorage of magnetic slides on stage may result in movement during scanning that may alter image quality |
| Telepathology |
‐No need for physical slide transfer ‐Short turnaround time for second consultation. ‐Quick on demand access to saved images. ‐Suitable for understaffed/remote areas. ‐Ability to consult specialist of your choice from around the globe |
‐Tissue blocks not available for additional stains/molecular tests ‐Overuse of second opinion ‐Medico‐legal repercussions due to restricted workup that is not the gold standard ‐Reimbursement issues when consulting specialists from abroad |
| Teaching |
‐Modern appeal to attract students ‐Digital images readily available ‐Self‐study and remote learning |
‐Learning curve |
| Cost/Efficiency Improvements |
‐Decreases operating time and cost ‐Evaluation of all types of skin conditions (e.g., benign and malignant skin tumors, inflammatory diseases, wound healing, and parasitic infections) ‐Expedites Mohs surgery/skin pathology |
‐Initial cost to buy/lease EVCM ‐Storage of glass slides is still deemed necessary by law ‐Maintaining traditional equipment in addition to EVCM |
1.3. Application of EVCM for skin cancers
Unlike in vivo confocal microscopy, EVCM allows for seamless scanning and viewing in three different individual modes: reflectance, fluorescence or pseudocolor (Figure 2). In addition, a fourth fusion mode combines both reflectance and fluorescence modes. Reflectance mode in EVCM is similar to in vivo confocal microscopy, allowing visualization of cytoplasm and stroma. These modes allow EVCM to generate precise and efficient histopathologic correlation for clinical application into various dermatological conditions including skin cancers, inflammatory skin diseases, and cutaneous infections.
FIGURE 2.
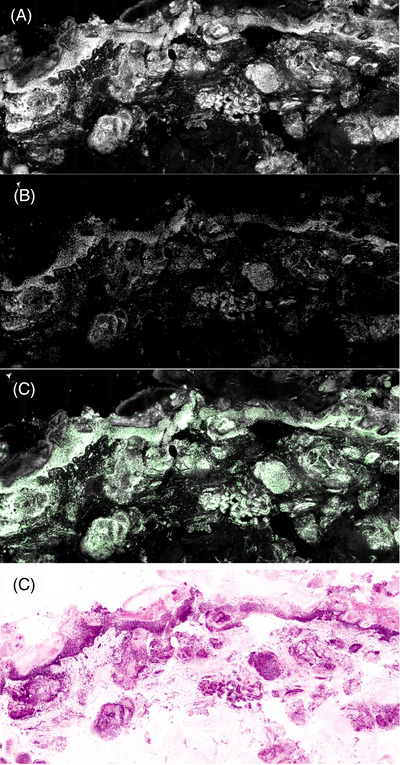
Modes of ex vivo confocal Microscopy. (A) Reflectance mode. (B) Fluorescence mode. (C) Fusion mode (fluorescence and reflectance). (D) Pseudo staining.
1.4. Basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin tumor evaluated using EVCM, with good diagnostic concordance between EVCM and conventional histology. 8 , 9 Diagnostic criteria for BCC in confocal mode include nuclear crowding, peripheral palisading, clefting, increased nuclear‐to‐cytoplasmic ratio, and nuclear pleomorphism. 2 Subtyping of BCC is also possible using EVCM. 2 Bennassar et al. scanned margins of 80 prospectively collected BCCs with EVCM. They reported a sensitivity of 88 % and specificity of 99 % in detecting residual BCC. 10 Using EVCM Espinasae et al. studied margins of 42 samples of BCCs with a sensitivity and specificity of 100%. 11 Perez‐Anker et al. used different modes of EVCM to study features of excised BCCs using double staining protocol and concluded that fluorescence mode was better for visualizing nuclei characteristics of BCCs and reflectance mode was more effective in visualizing stroma and cytoplasm. Fusion mode enabled identification of new features such as plump and dendritic cells in BCC. 12
1.5. Squamous cell carcinoma
EVCM has also been used to study squamous cell carcinoma (SCC), 13 , 14 Fluorescent EVCM has been used to study features and grade cutaneous SCC. In a study of 13 SCCs, assessment of margins between frozen histopathologic sections and fluorescent EVCM agreed in 41of 43 mosaics. One margin that was positive in histopathology but negative in fluorescent EVCM was obtained from a patient with SCC in situ. This study highlights the fact that detecting SCC is possible with fluorescence EVCM but detecting SCC in situ may be challenging. 13 SCC can be identified by the presence of keratinocytes with densely packed and irregularly distributed nuclei, 13 and the fluorescence mode can be used to grade the degree of differentiation. 14 and provide helpful information on invasion.
1.6. Melanoma
In a pilot study involving melanocytic lesions, EVCM was able to identify various malignant melanomas subtypes (n = 6) based on presence of inflammatory cells, consumption of dermoepidermal junction (DEJ), proliferation of atypical melanocytes and transepidermal melanocytic migration. Atypical nests and atypical melanocytes correlate with high specificity and sensitivity for diagnosing malignant lesions. In this study, EVCM was able to make effective histologic correlation in other melanocytic lesions such as junctional, compound, dermal, Spitz, and dysplastic nevi. 3 Hartman et al. correlated the thickness in malignant melanomas using traditional histological approach of Breslow's thickness and compared it with thickness acquired via ruler tool in EVCM. Based on the findings of the pilot study, they concluded that there was only a slight difference observed in the correlation curve of EVCM. Eight out of 10 patients were inside the 95% confidence interval. Only one patient was outside the confidence interval. 15
1.7. Autoimmune blistering diseases
A study in Germany has identified basement membrane fluorescence (BMF) in skin sections of patients with bullous pemphigoid using EVCM. EVCM showed an overall performance of 65.3 % in identifying BMF in reflectance and fluorescence mode using fluorescent labeled IgG and C3 antibodies. Although the sensitivity of EVCM in detecting BMF was low (C3: 45.5 %, IgG: 50%) but it showed a high specificity (C3: 90 %, IgG: 100%). EVCM showed a high positive predictive value of 100% in IgG staining and 93.75 % in C3 staining. Based on this study, EVCM can be used to detect concurrent changes in histology as well as immunofluorescence in the same session in patients with bullous pemphigoid. 4
1.8. Fungal infections
EVCM can also be particularly useful for the fast and precise identification of a fungus such as mucor. 7 or hair dermatophytes. 16 EVCM has been effective in detecting hair dermatophytosis in cases of tinea capitis and tinea barbae. EVCM showed presence of conidia around the proximal part of hair shaft that appeared as dispersed hyper‐reflective roundish homogenous structures. 16 EVCM has been used to observe dermatophytes in nail plates in cases of onychomycosis, appearing as reflective and typical linear shape of hyphae. 17 Laclercq et al. described two cases of mucormycosis that were diagnosed with EVCM. Fungi appeared as hyperreflective structures in reflective mode and exhibited high fluorescence in the fluorescent mode. Direct mycological examination was performed in both cases to confirm the diagnosis. 7
1.9. Viral infections
Fluorescent EVCM has the potential to detect viral infections as well. Cinotti et al. has demonstrated that EVCM can detect viral cytopathic effects of Herpes Simplex Virus (HSV) 1. In this study, roof samples from six vesicles that were clinically suspicious for HSV infection were incubated for 20 min with anti HSV‐1 and anti HSV‐2 monoclonal antibodies. The samples were positive for fluorescent after incubation with HSV‐1 antibodies under fluorescent mode of EVCM indicating a positive test. Fluorescence mode can also detect HSV in necrotic or inflamed tissue which would not be possible under reflectance mode. Reflectance mode showed large acantholytic keratinocytes, intraepidermal cavity, and erosion, all suggesting HSV‐1 infection. 18
1.10. Limitations
The technical aspect of obtaining an ex vivo microscopy image, requires training and experience to obtain adequate imaging for histopathological exam. For example, a smoothly cut tissue surface is crucial for quality image sampling. Any irregularities in tissue surface can result in artifacts during the imaging process. Experience is also required for tissue mounting to ensure complete contact without any air bubbles, which can result in artifact formation. Inability to flatten tissue can obscure adequate evaluation of the entire specimen, resulting in false‐negative results (Figure 3).
FIGURE 3.
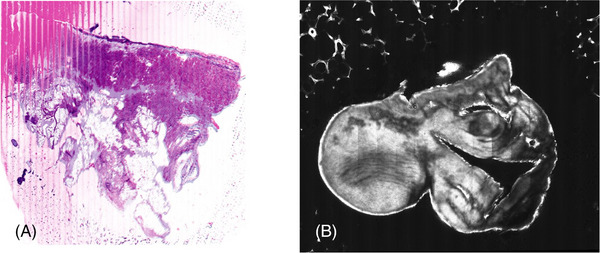
Limitations/Disadvantages. (A) Insufficient anchorage of the slide resulting in line formation, pink linear distortion on the left side and loss of focus while scanning. (B) Insufficient flattening of tissue resulting in poor image resolution on the right side.
For traditional formalin‐fixed paraffin‐embedded tissue block preparation, the tissue must be cut thinly for formalin and H&E stains to penetrate, and processing will take approximately 8 to 20 h. 2 While ex vivo microscopic imaging requires tissues to be submerged in contrast agent (acridine orange) for 30 s. However, further research for optimal staining reagents in different tissue types is needed to minimize artifacts and maximize scanning quality during imaging. With the exception of intensive reflection by AO stained pigmented structures, such as hair shafts, melanocytes, and melanophages which produced blurry, futile EVCM images, Schüürmann et al. reported minimal differences between EVCM images obtained via the Vivascope 2500 and H&E slides. 19
Although EVCM can visualize the cellular nucleus, the nucleolus cannot be visualized, which omits another clue to atypia or dysplasia. EVCM's pseudocoloring feature mimics H&E images, but the pseudocolored cytoplasm lacks details. Due to the lack of cytologic details using EVCM, hair follicles and eccrine glands or sebaceous glands may be confused with BCC islands, leading to false‐positive results in the diagnosis of BCC. 2 Therefore, better staining reagents, along with optimization of the staining and processing protocols are crucial to address this issue. Although, EVCM can be useful for the expedited identification of a fungus such as mucor. 7 or hair dermatophytes, 16 limited studies on its use and effectiveness in this domain prevent its adaptation in the clinical setting.
While the lack of specificity of this device is another limitation of this device, it can be addressed using specific antibodies conjugated with any fluorescent agent that can be excited by a wavelength of 488 or 658 nm to help identify a specific pathogen. Large‐scale EVCM studies on various skin lesions, including melanocytic, nonmelanocytic, and inflammatory conditions, are required to help expand our understanding and better correlate with conventional histology to allow broader clinical application of this novel EVCM technology. The limitations of EVCM are further summarized in Table 3.
2. DISCUSSION
Presently, EVCM is being used as an adjunct diagnostic modality in many clinical practices and research settings in different subspecialties of pathology such as skin pathology, uro‐pathology, gastrointestinal pathology, and gynecologic pathology, etc. In skin pathology, EVCM has been effective in histological correlation and detecting tumors such as BCC, SCC and melanoma. EVCM has shown promising results in detecting BMF, cutaneous fungal, and viral infections.
The availability of various EVCM tools allows for tailoring of EVCM based on the size of the specimen. For instance, the small and compact model can be used for skin specimens, whereas the larger model or device with bigger scanning surface area can be used for whole tissue mounting of bigger specimens such as the prostate, breast, colon, or lungs. The EVCM imaging process is much more expedited than that of frozen sectioning. Further research on contrast agents can improve image quality and rendering. Newer tissue flattening devices can be introduced to enable complete visualization of the margins while limiting artifacts formation. EVCM can also be modified and enhanced to allow 3D imaging and artificial intelligence can be incorporated into the EVCM imaging platform to help characterize different disease entities. Further research is also being performed on EVCM's potential in evaluating intraoperative tumor margins and smaller biopsies, such as core needle biopsies (CNB) and endoscopic biopsies. Frozen section analysis is not recommended for assessment of CNB and endoscopic biopsy specimens because of the fear that small tissue may get lost in the cryostat during frozen sectioning. Therefore, ex vivo microscopy is an appealing option for CNB and endoscopic biopsy specimens for rapid bedside evaluation of small tissue samples. EVCM is therefore advantageous for situations with small tissue samples because it requires a little tissue preparation and does not cause any loss of tissue, or damage during immediate assessment 20 .
3. CONCLUSION
EVCM is a revolutionary device that can be used as an adjunct to paraffin‐fixed, H&E stained histological slides and frozen sectioning. More refinements need to be made in EVCM before it can be used as an alternative to frozen sectioning or traditional tissue processing. Our manuscript demonstrates that EVCM has many promising applications (Table 4). Despite encouraging results, more multicenter studies need to be performed in dermatology and other pathology subspecialties to broaden available data and uncover the true potential of this innovative device.
TABLE 4.
Selected studies highlighting the importance of ex vivo reflectance confocal microscopy in skin pathology.
| Study (Year) | Result/Significance |
|---|---|
| Cinotti et al. (2013) | Detected dermatophytes in nail plates |
| Bennassar et al. (2014) | Sn 88 %; Sp of 99 % in detecting residual BCC |
| Cinotti et al. (2014) | Detect viral cytopathic effects of HSV 1 |
| Cinotti et al. (2015) | detected hair dermatophytosis in tinea capitis and tinea barbae |
| Laclercq et al. (2015) | Detected murcormycosis |
| Longo et al. (2015) | In a study of 13 SCCs, fluorescent EVCM was accurate in 41of 43 mosaics |
| Hartmann et al. (2016) | Correlated the thickness in malignant melanoma via ruler tool in EVCM. Eight of 10 patients were inside 95% CI |
| Espinasae et al. (2016) | In 42 lesions of BCC; Sn, Sp, PPV and NPV in FCM were 100% |
| Hartmann et al. (2017) | Identified various malignant melanomas subtypes (n = 6), correlated EVCM and histopathologic features of junctional, compound, dermal, Spitz and dysplastic nevi |
| Perez‐Anker et al. (2019) | Using Fusion mode discovered new features in BCCs such as plump and dendritic cells |
| Bağcı et al. (2019) |
Identified basement membrane fluorescence in patients with bullous pemphigoid (Sn C3: 45.5 %, IgG: 50%) (Sp C3: 90 %, IgG: 100%) PPV of 100% in IgG staining and 93.75 % in C3 staining |
Abbreviations: BCC; basal cell carcinoma; CI, confidence interval; EVCM, ex vivo confocal microscopy; HSV; herpes simplex virus; NPV, negative predictive value; PPV, positive predictive value; Sn, sensitivity; Sp, specificity; SSC, squamous cell carcinoma.
3.1. Plain language summary
An important component of making a diagnosis in dermatology is confirmed by histopathological exam, but the traditional processing of tissues can result in significant delays in diagnosis. Vivascope 2500 EVCM is an imaging device that captures both histologic and confocal microscopy images for efficient diagnosis. Prior technologies available provided in vivo imaging for real‐time diagnosis but could not provide histologic confirmation. EVCM offers physicians the ability to quickly acquire digital pathology, by eliminating time‐consuming tissue processing. This study aims to summarize and highlight the advantages, limitations, and the future of EVCM in skin pathology. Our manuscript demonstrates that EVCM is a promising adjunctive tool for applications to Mohs surgery and to provide rapid differentials of cutaneous malignancies, as well as infectious and inflammatory diseases.
CONFLICT OF INTEREST STATEMENT
Babar Rao serves as a consultant for Caliber I.D. (Rochester, NY). Other authors have no conflict of interest to disclose.
FUNDING INFORMATION
The authors received no specific funding for this work.
ETHICS STATEMENT
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Razi S, Ouellette S, Khan S, Oh KS, Truong TM, Rao BK. Role of VivaScope 2500 ex vivo confocal microscopy in skin pathology: Advantages, limitations, and future prospects. Skin Res Technol. 2023;29:1–7. 10.1111/srt.13388
DATA AVAILABILITY STATEMENT
Data supporting the research are indicated in the references.
REFERENCES
- 1. Titze U, Sievert K‐D, Titze B, et al. Ex vivo fluorescence confocal microscopy in specimens of the liver: a proof‐of‐concept study. Cancers. 2022;14(3):590. doi: 10.3390/cancers14030590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malvehy J, Pérez‐Anker J, Toll A, et al. Ex vivo confocal microscopy: revolution in fast pathology in dermatology. Br J Dermatol. 2020;183(6):1011‐1025. [DOI] [PubMed] [Google Scholar]
- 3. Hartmann D, Ruini C, Mathemeier L, et al. Identification of ex‐vivo confocal laser scanning microscopic features of melanocytic lesions and their histological correlates. J Biophotonics. 2017;10:128‐142. [DOI] [PubMed] [Google Scholar]
- 4. Bağcı IS, Aoki R, Krammer S, et al. Ex vivo confocal laser scanning microscopy for bullous pemphigoid diagnostics: New Era in direct immunofluorescence? J Eur Acad Dermatol Venereol. 2019;33(11):2123‐2130. doi: 10.1111/jdv.15767 [DOI] [PubMed] [Google Scholar]
- 5. Gareau DS, Li Y, Huang B, Eastman Z, Nehal KS, Rajadhyaksha M. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Longo C, Rajadhyaksha M, Ragazzi M, et al. Evaluating ex vivo fluorescence confocal microscopy images of basal cell carcinomas in Mohs excised tissue. Br J Dermatol. 2014;171:561–570. [DOI] [PubMed] [Google Scholar]
- 7. Leclercq A, Cinotti E, Labeille B, Perrot JL, Cambazard F. ex vivoconfocal microscopy: a new diagnostic technique for mucormycosis. Skin Res Technol. 2015;22(2):203‐207. doi: 10.1111/srt.12251 [DOI] [PubMed] [Google Scholar]
- 8. Karen JK, Gareau DS, Dusza SW, Tudisco M, Rajadhyaksha M, Nehal KS Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160(6):1242‐1250. doi: 10.1111/j.1365-2133.2009.09141.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Longo C, Pampena R, Bombonato C, et al. Diagnostic accuracy of ex vivo fluorescence confocal microscopy in Mohs surgery of basal cell carcinomas: a prospective study on 753 margins. Br J Dermatol. 2019;180(6):1473‐1480. doi: 10.1111/bjd.17507. PMID: 30512198. [DOI] [PubMed] [Google Scholar]
- 10. Bennàssar A, Vilata A, Puig S, Malvehy J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170(2):360‐365. doi: 10.1111/bjd.12671 [DOI] [PubMed] [Google Scholar]
- 11. Espinasse M, Cinotti E, Grivet D, et al. ‘En face’ ex vivo reflectance confocal microscopy to help the surgery of basal cell carcinoma of the eyelid. Clin Experiment Ophthalmol. 2017;45(5):442‐447. doi: 10.1111/ceo.12904 [DOI] [PubMed] [Google Scholar]
- 12. Pérez‐Anker J, Ribero S, Yélamos O, et al. Basal cell carcinoma characterization using fusion ex vivo confocal microscopy: a promising change in conventional skin histopathology. Br J Dermatol. 2019;182(2):468‐476. doi: 10.1111/bjd.18239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartmann D, Krammer S, Bachmann MR, et al. Ex vivo confocal microscopy features of cutaneous squamous cell carcinoma. J Biophotonics. 2018;11(4):e201700318. doi: 10.1002/jbio.201700318. PMID: 29227042. [DOI] [PubMed] [Google Scholar]
- 14. Longo C, Ragazzi M, Gardini S, et al. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2015;73(2):321‐322. doi: 10.1016/j.jaad.2015.04.027 [DOI] [PubMed] [Google Scholar]
- 15. Hartmann D, Krammer S, Ruini C, Ruzicka T, von Braunmühl T. Correlation of histological and ex‐vivo confocal tumor thickness in malignant melanoma. Lasers Med Sci. 2016;31(5):921‐927. doi: 10.1007/s10103-016-1936-5 [DOI] [PubMed] [Google Scholar]
- 16. Cinotti E, Perrot JL, Labeille B, Raberin H, Flori P, Cambazard F. Hair dermatophytosis diagnosed by reflectance confocal microscopy: six cases. J Eur Acad Dermatol Venereol. 2015;29(11):2257‐2259. doi: 10.1111/jdv.12557 [DOI] [PubMed] [Google Scholar]
- 17. Cinotti E, Fouilloux B, Perrot JL, Labeille B, Douchet C, Cambazard F. Confocal microscopy for healthy and pathological nail. J Eur Acad Dermatol Venereol. 2013;28(7):853‐858. doi: 10.1111/jdv.12330 [DOI] [PubMed] [Google Scholar]
- 18. Cinotti E, Perrot JL, Labeille B, et al. First identification of the herpes simplex virus by skin‐dedicated ex vivo fluorescence confocal microscopy during herpetic skin infections. Clin Exp Dermatol. 2014;40(4):421‐425. doi: 10.1111/ced.12546 [DOI] [PubMed] [Google Scholar]
- 19. Schüürmann M, Stecher MM, Paasch U, Simon JC, Grunewald S. Evaluation of digital staining for ex vivo confocal laser scanning microscopy. J Eur Acad Dermatol Venereol. 2020;34(7):1496‐1499. doi: 10.1111/jdv.16085. PMID: 31732988. [DOI] [PubMed] [Google Scholar]
- 20. Krishnamurthy S, Ban K, Shaw K, et al. Confocal fluorescence microscopy platform suitable for rapid evaluation of small fragments of tissue in surgical pathology practice. Arch Pathol Lab Med. 2019;143 (3):305‐313. doi: 10.5858/arpa.2018-0352-OA [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the research are indicated in the references.


